Controlling Oxygen Mobility in Ruddlesden–Popper Oxides
Abstract
:1. Introduction
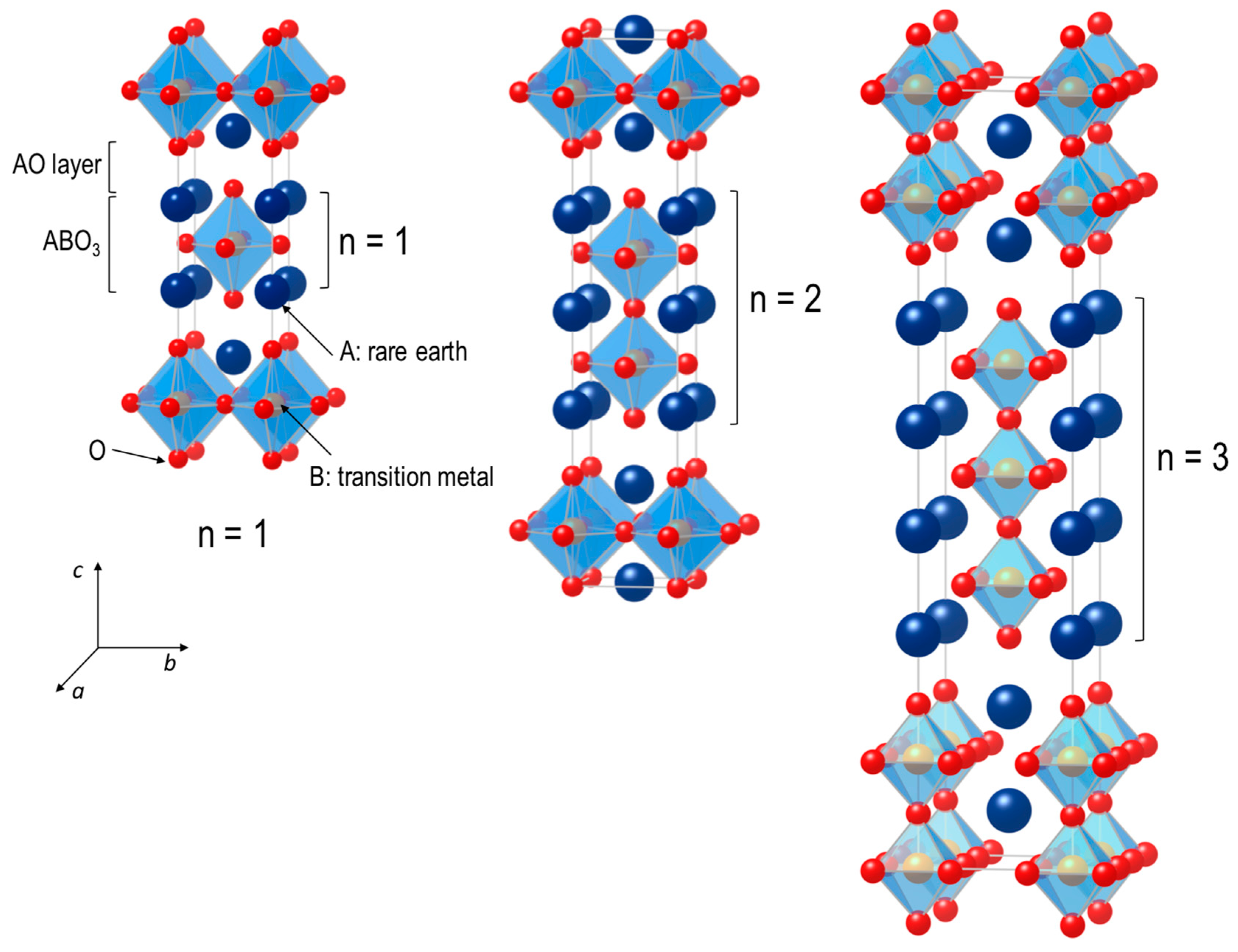
2. Oxygen Migration Mechanisms in Ruddlesden–Popper Oxides
2.1. Oxygen-Excess RP Oxides
2.2. Oxygen-Deficient RP Oxides
3. Control of Oxygen Migration in RP Oxides
3.1. Influence of Cation Substitutions on Oxygen Migration
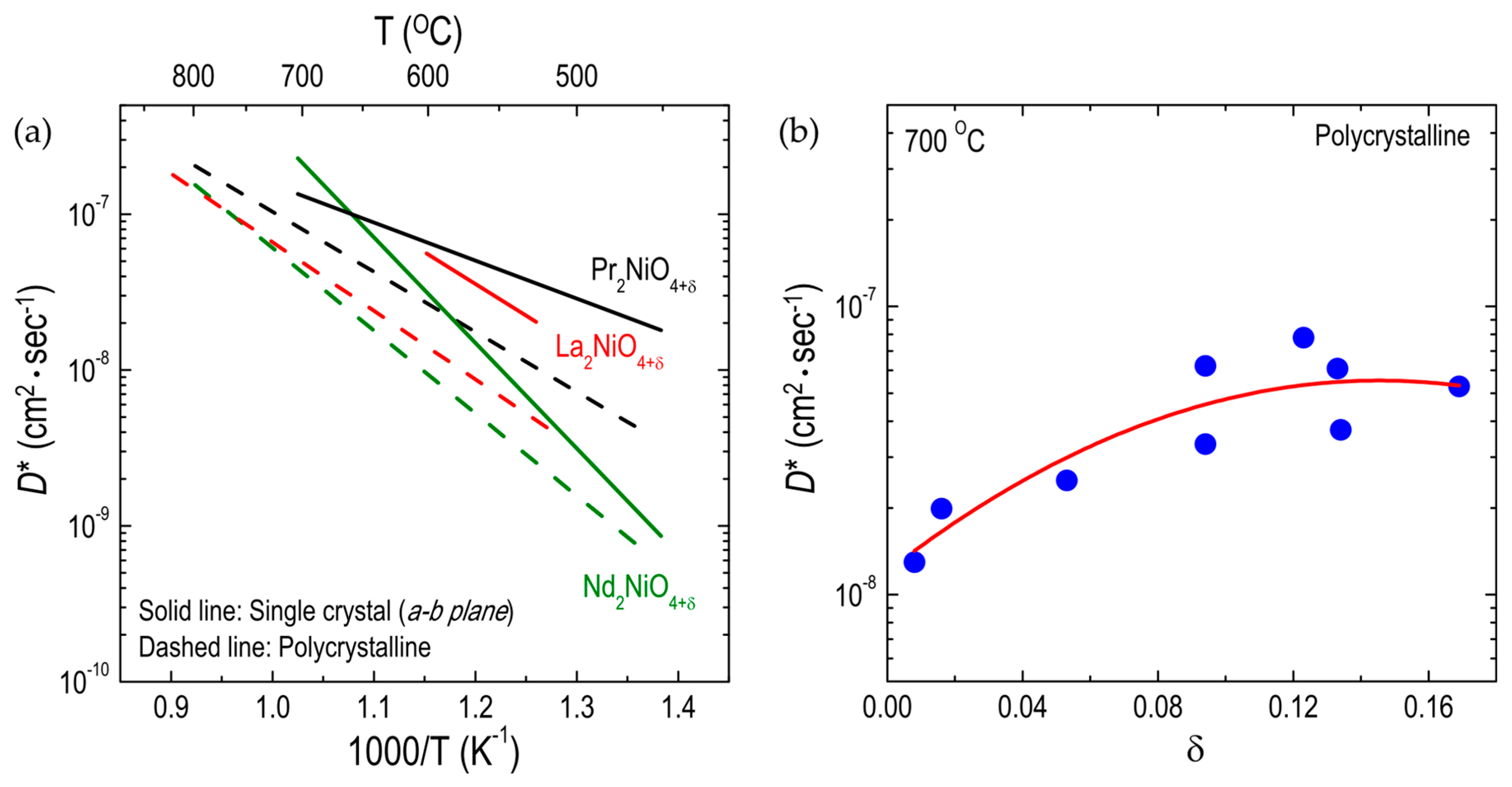
3.1.1. Effect of Substitutions on the A-Site
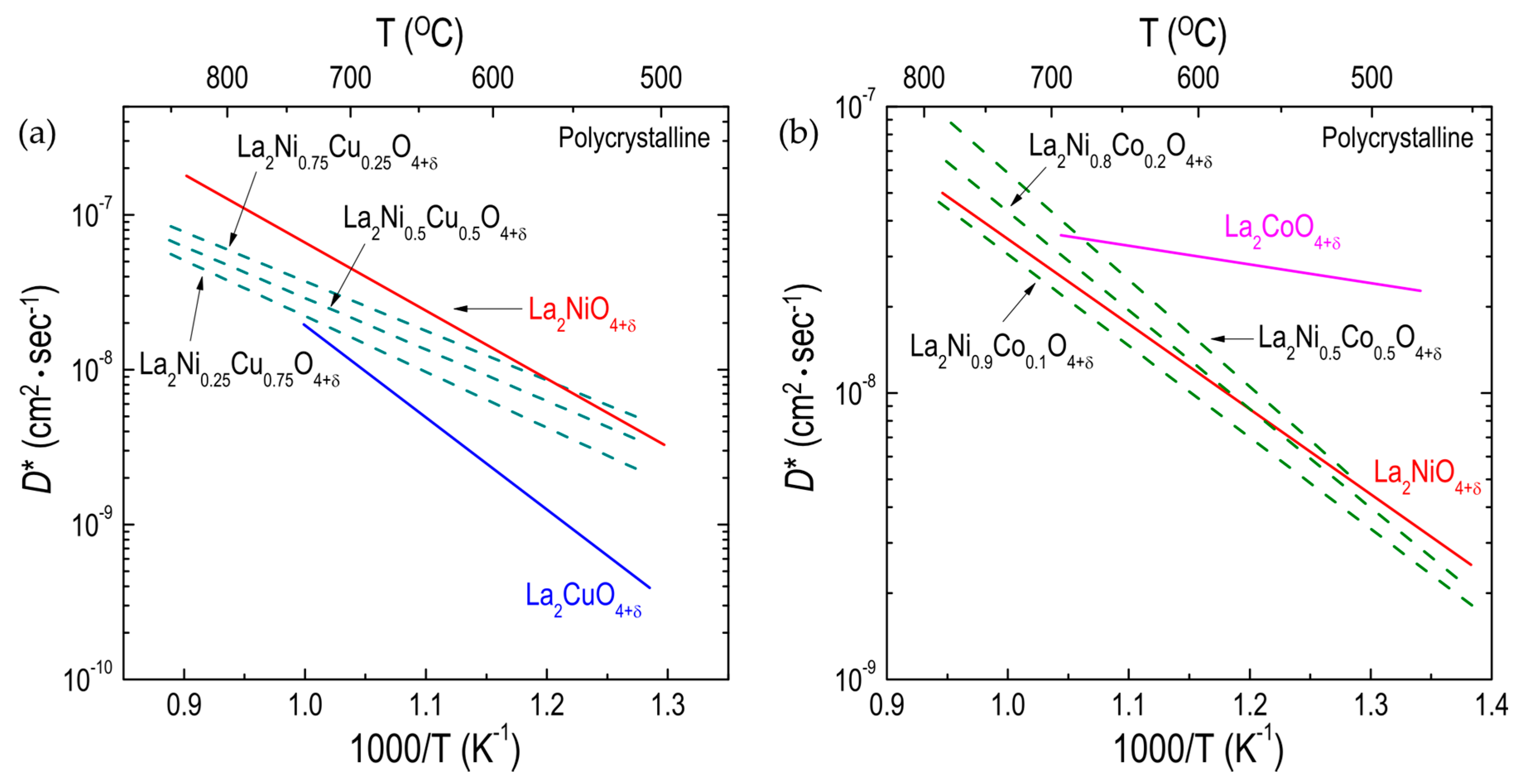
3.1.2. Effect of Substitutions on the B-Site
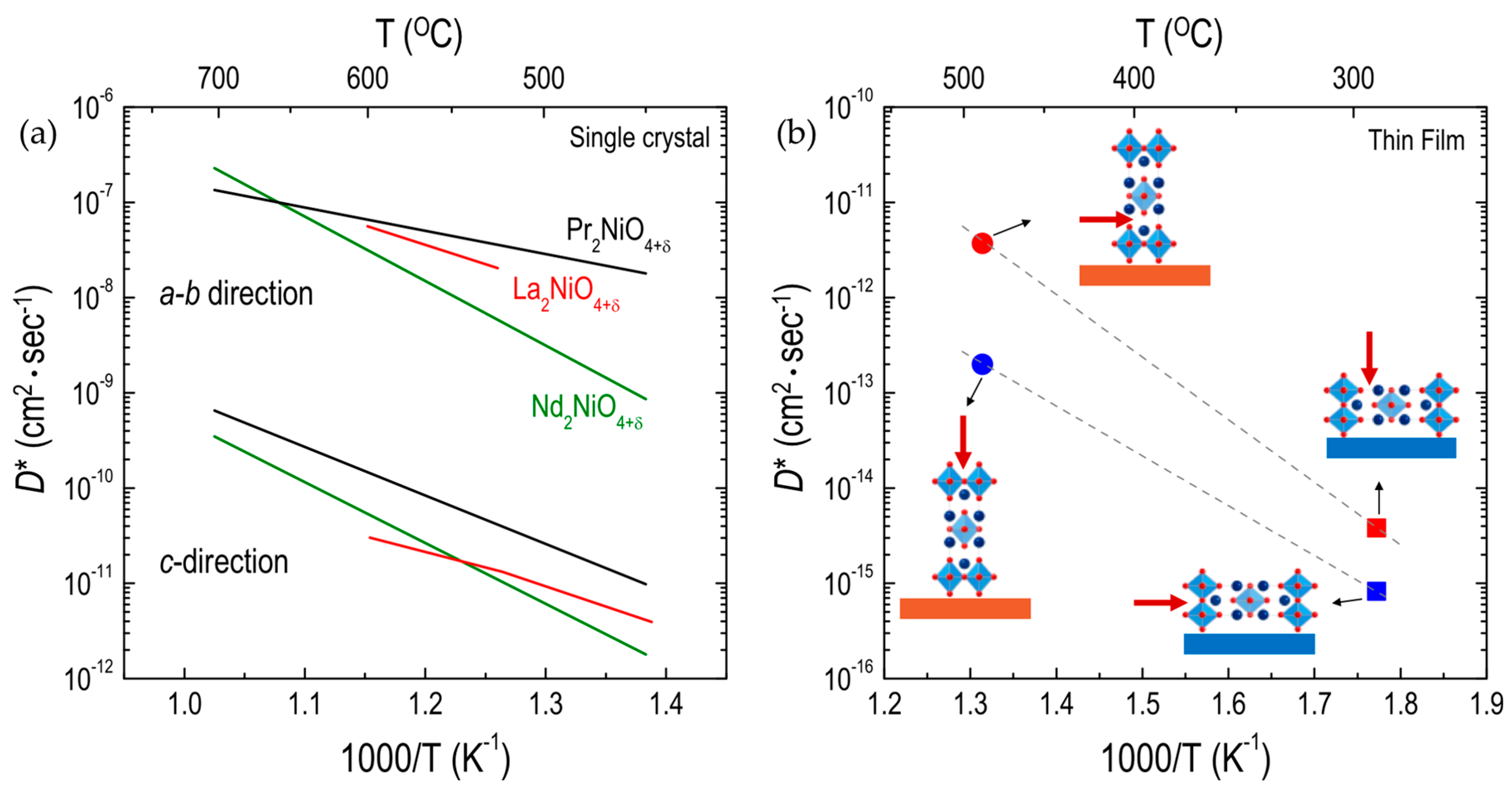
3.2. Influence of Crystallographic Orientation on Oxygen Migration
3.3. Influence of Strain on Oxygen Migration
4. Control of Oxygen Stability in RP Oxides
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4843. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, A.S.; Guo, R.Y.; Roy, R. The perovskite structure—A review of its role in ceramic science and technology. Mater. Res. Innov. 2000, 4, 3–26. [Google Scholar] [CrossRef]
- Voorhoeve, R.J.H.; Johnson, D.W.; Remeika, J.P.; Gallagher, P.K. Perovskite oxides: Materials science in catalysis. Science 1977, 195, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1−xSrxCoO3−δ electrodes. Solid State Ion. 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Berenov, A.V.; Atkinson, A.; Kilner, J.A.; Bucher, E.; Sitte, W. Oxygen tracer diffusion and surface exchange kinetics in La0.6Sr0.4CoO3−δ. Solid State Ion. 2010, 181, 819–826. [Google Scholar] [CrossRef]
- Crumlin, E.J.; Ahn, S.J.; Lee, D.; Mutoro, E.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Oxygen electrocatalysis on epitaxial La0.6Sr0.4CoO3−δ perovskite thin films for solid oxide fuel cells. J. Electrochem. Soc. 2012, 159, F219–F225. [Google Scholar] [CrossRef]
- Kubicek, M.; Cai, Z.H.; Ma, W.; Yildiz, B.; Hutter, H.; Fleig, J. Tensile lattice strain accelerates oxygen surface exchange and diffusion in La1−xSrxCoO3−δ thin films. ACS Nano 2013, 7, 3276–3286. [Google Scholar] [CrossRef] [PubMed]
- La O’, G.J.; Ahn, S.J.; Crumlin, E.; Orikasa, Y.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Catalytic activity enhancement for oxygen reduction on epitaxial perovskite thin films for solid oxide fuel cells. Angew. Chem. Int. Ed. 2010, 49, 5344–5347. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Lee, Y.-L.; Grimaud, A.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Enhanced oxygen surface exchange kinetics and stability on epitaxial La0.8Sr0.2CoO3−δ thin films by La0.8Sr0.2MnO3−δ decoration. J. Phys. Chem. C 2014, 118, 14326–14334. [Google Scholar] [CrossRef]
- Mizusaki, J. Nonstoichiometry, diffusion, and electrical properties of perovskite-type oxide electrode materials. Solid State Ion. 1992, 52, 79–91. [Google Scholar] [CrossRef]
- Van der Haar, L.M.; den Otter, M.W.; Morskate, M.; Bouwmeester, H.J.M.; Verweij, H. Chemical diffusion and oxygen surface transfer of La1−xSrxCoO3−δ studied with electrical conductivity relaxation. J. Electrochem. Soc. 2002, 149, J41–J46. [Google Scholar] [CrossRef]
- Van Doorn, R.H.E.; Burggraaf, A.J. Structural aspects of the ionic conductivity of La1−xSrxCoO3−δ. Solid State Ion. 2000, 128, 65–78. [Google Scholar] [CrossRef]
- Baumann, F.S.; Fleig, J.; Habermeier, H.U.; Maier, J. Impedance spectroscopic study on well-defined (La,Sr)(Co,Fe)O3−δ model electrodes. Solid State Ion. 2006, 177, 1071–1081. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M.; Kruidhof, H.; Burggraaf, A.J. Importance of the surface exchange kinetics as rate-limiting step in oxygen permeation through mixed-conducting oxides. Solid State Ion. 1994, 72, 185–194. [Google Scholar] [CrossRef]
- Carter, S.; Selcuk, A.; Chater, R.J.; Kajda, J.; Kilner, J.A.; Steele, B.C.H. Oxygen transport in selected nonstoichiometric perovskite-structure oxides. Solid State Ion. 1992, 53, 597–605. [Google Scholar] [CrossRef]
- Esquirol, A.; Brandon, N.P.; Kilner, J.A.; Mogensen, M. Electrochemical characterization of La0.6Sr0.4Co0.2Fe0.8O3 cathodes for intermediate-temperature SOFCs. J. Electrochem. Soc. 2004, 151, A1847–A1855. [Google Scholar] [CrossRef]
- Geary, T.C.; Lee, D.; Shao-Horn, Y.; Adler, S.B. Nonlinear impedance analysis of La0.6Sr0.4Co0.2Fe0.8O3−δ thin film oxygen electrodes. J. Electrochem. Soc. 2016, 163, F1107–F1114. [Google Scholar] [CrossRef]
- Ingram, B.J.; Eastman, J.A.; Chang, K.C.; Kim, S.K.; Fister, T.T.; Perret, E.; You, H.; Baldo, P.M.; Fuoss, P.H. In situ X-ray studies of oxygen surface exchange behavior in thin film La0.6Sr0.4Co0.2Fe0.8O3−δ. Appl. Phys. Lett. 2012, 101, 051603. [Google Scholar] [CrossRef]
- Jiang, S.P. A comparison of O2 reduction reactions on porous (La,Sr)MnO3 and (La,Sr)(Co,Fe)O3 electrodes. Solid State Ion. 2002, 146, 1–22. [Google Scholar] [CrossRef]
- Katsuki, M.; Wang, S.; Dokiya, M.; Hashimoto, T. High temperature properties of La0.6Sr0.4Co0.8Fe0.2O3−δ oxygen nonstoichiometry and chemical diffusion constant. Solid State Ion. 2003, 156, 453–461. [Google Scholar] [CrossRef]
- Kuhn, M.; Fukuda, Y.; Hashimoto, S.; Sato, K.; Yashiro, K.; Mizusaki, J. Oxygen nonstoichiometry and thermo-chemical stability of perovskite-type La0.6Sr0.4Co1−yFeyO3−δ (y = 0, 0.2, 0.4, 0.5, 0.6, 0.8, 1) materials. J. Electrochem. Soc. 2013, 160, F34–F42. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.-L.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Oxygen surface exchange kinetics and stability of (La,Sr)2CoO4±δ/La1−xSrxMO3−δ (M = Co and Fe) hetero-interfaces at intermediate temperatures. J. Mater. Chem. A 2015, 3, 2144–2157. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.-L.; Wang, X.R.; Morgan, D.; Shao-Horn, Y. Enhancement of oxygen surface exchange on epitaxial La0.6Sr0.4Co0.2Fe0.8O3−δ thin films using advanced heterostructured oxide interface engineering. MRS Commun. 2016, 6, 204–209. [Google Scholar] [CrossRef]
- Lynch, M.E.; Yang, L.; Qin, W.T.; Choi, J.J.; Liu, M.F.; Blinn, K.; Liu, M.L. Enhancement of La0.6Sr0.4Co0.2Fe0.8O3−δ durability and surface electrocatalytic activity by La0.85Sr0.15MnO3±δ investigated using a new test electrode platform. Energy Environ. Sci. 2011, 4, 2249–2258. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Bae, J.M. Properties of La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF) double layer cathodes on gadolinium-doped cerium oxide (CGO) electrolytes—II. Role of oxygen exchange and diffusion. Solid State Ion. 1998, 106, 255–261. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1−xSrxCo1−yFeyO3. Part 1. The system La0.8Sr0.8Co1−yFeyO3. Solid State Ion. 1995, 76, 259–271. [Google Scholar] [CrossRef]
- Al Daroukh, M.; Vashook, V.V.; Ullmann, H.; Tietz, F.; Raj, I.A. Oxides of the AMO3 and A2MO4-type: Structural stability, electrical conductivity and thermal expansion. Solid State Ion. 2003, 158, 141–150. [Google Scholar] [CrossRef]
- Chen, X.Y.; Yu, J.S.; Adler, S.B. Thermal and chemical expansion of Sr-doped lanthanum cobalt oxide (La1−xSrxCoO3−δ). Chem. Mater. 2005, 17, 4537–4546. [Google Scholar] [CrossRef]
- Hashimoto, S.; Fukuda, Y.; Kuhn, M.; Sato, K.; Yashiro, K.; Mizusaki, J. Thermal and chemical lattice expansibility of La0.6Sr0.4Co1−yFeyO3−δ (y = 0.2, 0.4, 0.6 and 0.8). Solid State Ion. 2011, 186, 37–43. [Google Scholar] [CrossRef]
- Mastin, J.; Einarsrud, M.A.; Grande, T. Structural and thermal properties of La1−xSrxCoO3−δ. Chem. Mater. 2006, 18, 6047–6053. [Google Scholar] [CrossRef]
- Benson, S.J.; Waller, D.; Kilner, J.A. Degradation of La0.6Sr0.4Co0.8Fe0.2O3−δ in carbon dioxide and water atmospheres. J. Electrochem. Soc. 1999, 146, 1305–1309. [Google Scholar] [CrossRef]
- Ding, H.P.; Virkar, A.V.; Liu, M.L.; Liu, F. Suppression of Sr surface segregation in La1−xSrxCo1−yFeyO3−δ: A first principles study. Phys. Chem. Chem. Phys. 2013, 15, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.; Gostovic, D.; Wachsman, E.D. Mechanism of La0.6Sr0.4Co0.2Fe0.8O3 cathode degradation. J. Mater. Res. 2012, 27, 1992–1999. [Google Scholar] [CrossRef]
- Simner, S.P.; Anderson, M.D.; Engelhard, M.H.; Stevenson, J.W. Degradation mechanisms of La-Sr-Co-Fe-O3 SOFC cathodes. Electrochem. Solid State Lett. 2006, 9, A478–A481. [Google Scholar] [CrossRef]
- Baledent, V.; Fauque, B.; Sidis, Y.; Christensen, N.B.; Pailhes, S.; Conder, K.; Pomjakushina, E.; Mesot, J.; Bourges, P. Two-dimensional orbital-like magnetic order in the high-temperature La2−xSrxCuO4 superconductor. Phys. Rev. Lett. 2010, 105, 4. [Google Scholar] [CrossRef] [PubMed]
- Cava, R.J.; Batlogg, B.; Palstra, T.T.; Krajewski, J.J.; Peck, W.F.; Ramirez, A.P.; Rupp, L.W. Magnetic and electrical properties of La2−xSrxNiO4±δ. Phys. Rev. B 1991, 43, 1229–1232. [Google Scholar] [CrossRef]
- Chaillout, C.; Cheong, S.W.; Fisk, Z.; Lehmann, M.S.; Marezio, M.; Morosin, B.; Schirber, J.E. The crystal structure of superconducting La2CuO4.032 by neutron diffraction. Physica C 1989, 158, 183–191. [Google Scholar] [CrossRef]
- Meyer, T.L.; Jiang, L.; Park, S.; Egami, T.; Lee, H.N. Strain-relaxation and critical thickness of epitaxial La1.85Sr0.15CuO4 films. APL Mater. 2015, 3, 126102. [Google Scholar] [CrossRef]
- Perry, J.K.; Tahir-Kheli, J.; Goddard, W.A. Antiferromagnetic band structure of La2CuO4: Becke-3-Lee-Yang-Parr calculations. Phys. Rev. B 2001, 63, 6. [Google Scholar] [CrossRef]
- Reehuis, M.; Ulrich, C.; Prokes, K.; Gozar, A.; Blumberg, G.; Komiya, S.; Ando, Y.; Pattison, P.; Keimer, B. Crystal structure and high-field magnetism of La2CuO4. Phys. Rev. B 2006, 73, 8. [Google Scholar] [CrossRef]
- Veith, G.M.; Chen, R.; Popov, G.; Croft, M.; Shokh, Y.; Nowik, I.; Greenblatt, M. Electronic, magnetic, and magnetoresistance properties of the n = 2 Ruddlesden–Popper phases Sr3Fe2−xCoxO7−δ (0.25 ≤ x ≤ 1.75). J. Solid State Chem. 2002, 166, 292–304. [Google Scholar] [CrossRef]
- Aguadero, A.; Escudero, M.J.; Perez, M.; Alonso, J.A.; Daza, L. Hyperstoichiometric La1.9Sr0.1NiO4+δ mixed conductor as novel cathode for intermediate temperature solid oxide fuel cells. J. Fuel Cell Sci. Technol. 2007, 4, 294–298. [Google Scholar] [CrossRef]
- Burriel, M.; Garcia, G.; Rossell, M.D.; Figueras, A.; Van Tendeloo, G.; Santiso, J. Enhanced high-temperature electronic transport properties in nanostructured epitaxial thin films of the Lan+1NinO3n+1 Ruddlesden-Popper series (n = 1, 2, 3, ∞). Chem. Mater. 2007, 19, 4056–4062. [Google Scholar] [CrossRef]
- Ferkhi, M.; Khelili, S.; Zerroual, L.; Ringuede, A.; Cassir, M. Synthesis, structural analysis and electrochemical performance of low-copper content La2Ni1−xCuxO4+δ materials as new cathodes for solid oxide fuel cells. Electrochim. Acta 2009, 54, 6341–6346. [Google Scholar] [CrossRef]
- Schroeder, M.; Dragan, M.A. Oxygen transport in La2−xSrxNiO4+δ: Membrane permeation and defect chemical modelling. J. Mater. Sci. 2007, 42, 1972–1983. [Google Scholar] [CrossRef]
- Vashook, V.V.; Ullmann, H.; Olshevskaya, O.P.; Kulik, V.P.; Lukashevich, V.E.; Kokhanovskij, L.V. Composition and electrical conductivity of some cobaltates of the type La2−xSrxCoO4.5−x/2±δ. Solid State Ion. 2000, 138, 99–104. [Google Scholar] [CrossRef]
- Ruddlesden, S.N.; Popper, P. The compound Sr3Ti2O7 and its structure. Acta Crystallogr. 1958, 11, 54–55. [Google Scholar] [CrossRef]
- Brown, I.D. Modeling the structures of La2NiO4. Z. Kristallogr. 1992, 199, 255–272. [Google Scholar] [CrossRef]
- Bassat, J.M.; Burriel, M.; Wahyudi, O.; Castaing, R.; Ceretti, M.; Veber, P.; Weill, I.; Villesuzanne, A.; Grenier, J.C.; Paulus, W.; et al. Anisotropic oxygen diffusion properties in Pr2NiO4+δ and Nd2NiO4+δ single crystals. J. Phys. Chem. C 2013, 117, 26466–26472. [Google Scholar] [CrossRef]
- Bassat, J.M.; Odier, P.; Villesuzanne, A.; Marin, C.; Pouchard, M. Anisotropic ionic transport properties in La2NiO4+δ single crystals. Solid State Ion. 2004, 167, 341–347. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Lee, D.; Grimaud, A.; Crumlin, E.J.; Mezghani, K.; Habib, M.A.; Feng, Z.X.; Hong, W.T.; Biegalski, M.D.; Christen, H.M.; Shao-Horn, Y. Strain influence on the oxygen electrocatalysis of the (100)-oriented epitaxial La2NiO4+δ thin films at elevated temperatures. J. Phys. Chem. C 2013, 117, 18789–18795. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2−xSrxNiO4+δ. Solid State Ion. 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Gopalakrishnan, J.; Colsmann, G.; Reuter, B. Studies on La2−xSrxNiO4 (0 ≤ x ≤ 1) system. J. Solid State Chem. 1977, 22, 145–149. [Google Scholar] [CrossRef]
- Minervini, L.; Grimes, R.W.; Kilner, J.A.; Sickafus, K.E. Oxygen migration in La2NiO4+δ. J. Mater. Chem. 2000, 10, 2349–2354. [Google Scholar] [CrossRef]
- Opila, E.J.; Tuller, H.L.; Wuensch, B.J.; Maier, J. Oxygen tracer diffusion in La2−xSrxCuO4-δ single-crystals. J. Am. Ceram. Soc. 1993, 76, 2363–2369. [Google Scholar] [CrossRef]
- Mott, N.F.; Gurney, R.W. Electronic Processes in Ionic Crystals; Oxford University Press: London, UK, 1940. [Google Scholar]
- Burriel, M.; Garcia, G.; Santiso, J.; Kilner, J.A.; Richard, J.C.C.; Skinner, S.J. Anisotropic oxygen diffusion properties in epitaxial thin films of La2NiO4+δ. J. Mater. Chem. 2008, 18, 416–422. [Google Scholar] [CrossRef]
- Burriel, M.; Santiso, J.; Rossell, M.D.; Van Tendeloo, G.; Figueras, A.; Garcia, G. Enhancing total conductivity of La2NiO4+δ epitaxial thin films by reducing thickness. J. Phys. Chem. C 2008, 112, 10982–10987. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Chen, C.L. Measurement of oxygen transport kinetics in epitaxial La2NiO4+δ thin films by electrical conductivity relaxation. Solid State Ion. 2006, 177, 1461–1467. [Google Scholar] [CrossRef]
- Li, Z.A.; Haugsrud, R.; Norby, T. Oxygen bulk diffusion and surface exchange in Sr-substituted La2NiO4+δ. Solid State Ion. 2011, 184, 42–46. [Google Scholar] [CrossRef]
- Jorgensen, J.D.; Dabrowski, B.; Pei, S.; Richards, D.R.; Hinks, D.G. Structure of the interstitial oxygen defect in La2NiO4+δ. Phys. Rev. B 1989, 40, 2187–2199. [Google Scholar] [CrossRef]
- Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in tetragonal La2NiO4+δ: Molecular dynamics calculations. J. Mater. Chem. 2010, 20, 266–270. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Kovalevsky, A.V.; Naumovich, E.N.; Marques, F.M.B. Ionic transport in oxygen-hyperstoichiometric phases with K2NiF4-type structure. Solid State Ion. 2001, 143, 337–353. [Google Scholar] [CrossRef]
- Sayers, R.; Skinner, S.J. Evidence for the catalytic oxidation of La2NiO4+δ. J. Mater. Chem. 2010, 21, 414–419. [Google Scholar] [CrossRef]
- Cleave, A.R.; Kilner, J.A.; Skinner, S.J.; Murphy, S.T.; Grimes, R.W. Atomistic computer simulation of oxygen ion conduction mechanisms in La2NiO4. Solid State Ion. 2008, 179, 823–826. [Google Scholar] [CrossRef]
- Frayret, C.; Villesuzanne, A.; Pouchard, M. Application of density functional theory to the modeling of the mixed ionic and electronic conductor La2NiO4+δ: Lattice relaxation, oxygen mobility, and energetics of frenkel defects. Chem. Mater. 2005, 17, 6538–6544. [Google Scholar] [CrossRef]
- Parfitt, D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Molecular dynamics study of oxygen diffusion in Pr2NiO4+δ. Phys. Chem. Chem. Phys. 2010, 12, 6834–6836. [Google Scholar] [CrossRef] [PubMed]
- Allan, N.L.; Mackrodt, W.C. Oxygen ion migration in La2CuO4. Philos. Mag. A 1991, 64, 1129–1132. [Google Scholar] [CrossRef]
- Kushima, A.; Parfitt, D.; Chroneos, A.; Yildiz, B.; Kilner, J.A.; Grimes, R.W. Interstitialcy diffusion of oxygen in tetragonal La2CoO4+δ. Phys. Chem. Chem. Phys. 2011, 13, 2242–2249. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Yashiro, K.; Sato, K.; Mizusaki, J. Oxygen nonstoichiometry and defect equilibrium in La2−xSrxNiO4+δ. Solid State Ion. 2009, 180, 368–376. [Google Scholar] [CrossRef]
- Vashook, V.V.; Trofimenko, N.E.; Ullmann, H.; Makhnach, L.V. Oxygen nonstoichiometry and some transport properties of LaSrNiO4−δ nickelate. Solid State Ion. 2000, 131, 329–336. [Google Scholar] [CrossRef]
- Mazo, G.N.; Savvin, S.N. The molecular dynamics study of oxygen mobility in La2−xSrxCuO4−δ. Solid State Ion. 2004, 175, 371–374. [Google Scholar] [CrossRef]
- Savvin, S.N.; Mazo, G.N.; Ivanov-Schitz, A.K. Simulation of ion transport in layered cuprates La2−xSrxCuO4−δ. Crystallogr. Rep. 2008, 53, 291–301. [Google Scholar] [CrossRef]
- Kanai, H.; Mizusaki, J.; Tagawa, H.; Hoshiyama, S.; Hirano, K.; Fujita, K.; Tezuka, M.; Hashimoto, T. Defect chemistry of La2−xSrxCuO4−δ: Oxygen nonstoichiometry and thermodynamic stability. J. Solid State Chem. 1997, 131, 150–159. [Google Scholar] [CrossRef]
- Teske, K.; Ullmann, H.; Trofimenko, N. Thermal analysis of transition metal and rare earth oxide system-gas interactions by a solid electrolyte-based coulometric technique. J. Therm. Anal. 1997, 49, 1211–1220. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G.; Whitfield, P.S.; Davidson, I.J. Oxygen transport in the La2Ni1−xCoxO4+δ system. Solid State Ion. 2005, 176, 1895–1901. [Google Scholar] [CrossRef]
- Alyoshin, V.A.; Romanova, I.P.; Mikhailova, D.; Oswald, S.; Senyshyn, A.; Ehrenberg, H. Oxygen nonstoichiometry of tetragonal La2−xSrxCuO4−δ (x = 0.15–1.2) and in situ XPS studies at elevated temperatures. J. Phys. Chem. A 2010, 114, 13362–13369. [Google Scholar] [CrossRef] [PubMed]
- Tealdi, C.; Ferrara, C.; Mustarelli, P.; Islam, M.S. Vacancy and interstitial oxide ion migration in heavily doped La2−xSrxCoO4±δ. J. Mater. Chem. 2012, 22, 8969–8975. [Google Scholar] [CrossRef]
- De Souza, R.A.; Ramadan, A.; Horner, S. Modifying the barriers for oxygen-vacancy migration in fluorite-structured CeO2 electrolytes through strain: A computer simulation study. Energy Environ. Sci. 2012, 5, 5445–5453. [Google Scholar] [CrossRef]
- Jiang, J.; Hu, X.C.; Shen, W.D.; Ni, C.Y.; Hertz, J.L. Improved ionic conductivity in strained yttria-stabilized zirconia thin films. Appl. Phys. Lett. 2013, 102, 143901. [Google Scholar] [CrossRef]
- Kushima, A.; Yildiz, B. Oxygen ion diffusivity in strained yttria stabilized zirconia: Where is the fastest strain? J. Mater. Chem. 2010, 20, 4809–4819. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Chroneos, A. Impact of uniaxial strain and doping on oxygen diffusion in CeO2. Sci. Rep. 2014, 4, 6068. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, B. “Stretching” the energy landscape of oxides-effects on electrocatalysis and diffusion. MRS Bull. 2014, 39, 147–156. [Google Scholar] [CrossRef]
- Mayeshiba, T.; Morgan, D. Strain effects on oxygen migration in perovskites. Phys. Chem. Chem. Phys. 2015, 17, 2715–2721. [Google Scholar] [CrossRef] [PubMed]
- Petrie, J.R.; Mitra, C.; Jeen, H.; Choi, W.S.; Meyer, T.L.; Reboredo, F.A.; Freeland, J.W.; Eres, G.; Lee, H.N. Strain control of oxygen vacancies in epitaxial strontium cobaltite films. Adv. Funct. Mater. 2016, 26, 1564–1570. [Google Scholar] [CrossRef]
- Islam, M.S.; Cherry, M.; Catlow, C.R.A. Oxygen diffusion in LaMnO3 and LaCoO3 perovskite-type oxides: A molecular dynamics study. J. Solid State Chem. 1996, 124, 230–237. [Google Scholar] [CrossRef]
- Cherry, M.; Islam, M.S.; Catlow, C.R.A. Oxygen ion migration in perovskite-type oxides. J. Solid State Chem. 1995, 118, 125–132. [Google Scholar] [CrossRef]
- De Souza, R.A.; Kilner, J.A. Oxygen transport in La1−xSrxMn1−yCoyO3±δ. Part I. Oxygen tracer diffusion. Solid State Ion. 1998, 106, 175–187. [Google Scholar] [CrossRef]
- Ishigaki, T.; Yamauchi, S.; Kishio, K.; Mizusaki, J.; Fueki, K. Diffusion of oxide ion vacancies in perovskite-type oxides. J. Solid State Chem. 1988, 73, 179–187. [Google Scholar] [CrossRef]
- Ishigaki, T.; Yamauchi, S.; Mizusaki, J.; Fueki, K.; Tamura, H. Tracer diffusion-coefficient of oxide ions in LaCoO3 single-crystal. J. Solid State Chem. 1984, 54, 100–107. [Google Scholar] [CrossRef]
- Routbort, J.L.; Doshi, R.; Krumpelt, M. Oxygen tracer diffusion in La1−xSrxCoO3. Solid State Ion. 1996, 90, 21–27. [Google Scholar] [CrossRef]
- Van Doorn, R.H.E.; Fullarton, I.C.; De Souza, R.A.; Kilner, J.A.; Bouwmeester, H.J.M.; Burggraaf, A.J. Surface oxygen exchange of La0.3Sr0.7CoO3−δ. Solid State Ion. 1997, 96, 1–7. [Google Scholar] [CrossRef]
- Kilner, J.A.; Shaw, C.K.M. Mass transport in La2Ni1−xCoxO4+δ oxides with the K2NiF4 structure. Solid State Ion. 2002, 154, 523–527. [Google Scholar] [CrossRef]
- Li, Z.A.; Haugsrud, R.; Smith, J.B.; Norby, T. Transport properties and defect analysis of La1.9Sr0.1NiO4+δ. Solid State Ion. 2009, 180, 1433–1441. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. A comparison of the transport properties of La2−xSrxNi1−yFeyO4+δ where 0 < x < 0.2 and 0 < y < 0.2. Ionics 1999, 5, 171–174. [Google Scholar]
- Burriel, M.; Téllez, H.; Chater, R.J.; Castaing, R.; Veber, P.; Zaghrioui, M.; Ishihara, T.; Kilner, J.A.; Bassat, J.-M. Influence of crystal orientation and annealing on the oxygen diffusion and surface exchange of La2NiO4+δ. J. Phys. Chem. C 2016, 120, 17927–17938. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Steil, M.C.; Dordor, P.; Mauvy, F.; Grenier, J.C. Oxygen transport properties of La2Ni1−xCuxO4+δ mixed conducting oxides. Solid State Sci. 2003, 5, 973–981. [Google Scholar] [CrossRef]
- Claus, J.; Borchardt, G.; Weber, S.; Hiver, J.M.; Scherrer, S. Combination of EBSP measurements and sims to study crystallographic orientation dependence of diffusivities in a polycrystalline material: Oxygen tracer diffusion in La2−xSrxCuO4±δ. Mater. Sci. Eng. B 1996, 38, 251–257. [Google Scholar] [CrossRef]
- Routbort, J.L.; Rothman, S.J.; Flandermeyer, B.K.; Nowicki, L.J.; Baker, J.E. Oxygen diffusion in La2−xSrxCuO4−δ. J. Mater. Res. 1988, 3, 116–121. [Google Scholar] [CrossRef]
- Shannon, R.D.; Prewitt, C.T. Effective ionic radii in oxides and fluorides. Acta Cryst. 1969, B25, 925–946. [Google Scholar] [CrossRef]
- Lee, D.; Lee, Y.-L.; Grimaud, A.; Hong, W.T.; Biegalski, M.D.; Morgan, D.; Shao-Horn, Y. Strontium influence on the oxygen electrocatalysis of La2−xSrxNiO4±δ (0.0 ≤ xSr ≤ 1.0) thin films. J. Mater. Chem. A 2014, 2, 6480–6487. [Google Scholar] [CrossRef]
- Allan, N.L.; Lawton, J.M.; Mackrodt, W.C. A comparison of the calculated lattice and defect structures of La2CuO4, La2NiO4, Nd2CuO4, Pr2CuO4, Y2CuO4, Al2CuO4: Relationship to high-Tc superconductivity. Philos. Mag. B 1989, 59, 191–206. [Google Scholar] [CrossRef]
- Allan, N.L.; Mackrodt, W.C. Defect chemistry of La2CuO4, Nd2CuO4 and Pr2CuO4 doped by tetravalent cations: Relevance to high-Tc behavior. Philos. Mag. Lett. 1989, 60, 183–186. [Google Scholar] [CrossRef]
- Naumovich, E.N.; Patrakeev, M.V.; Kharton, V.V.; Yaremchenko, A.A.; Lopinovich, D.I.; Marques, F.M.B. Oxygen nonstoichiornetry in La2Ni(M)O4+δ (M = Cu, Co) under oxidizing conditions. Solid State Sci. 2005, 7, 1353–1362. [Google Scholar] [CrossRef]
- Lee, Y.-L.; Lee, D.; Wang, X.R.; Lee, H.N.; Morgan, D.; Shao-Horn, Y. Kinetics of oxygen surface exchange on epitaxial Ruddlesden–Popper phases and correlations to first-principles descriptors. J. Phys. Chem. Lett. 2016, 7, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Lee, Y.-L.; Shao-Horn, Y.; Morgan, D. Oxygen point defect chemistry in Ruddlesden–Popper oxides (La1−xSrx)2MO4±δ (M = Co, Ni, Cu). J. Phys. Chem. Lett. 2016, 7, 1939–1944. [Google Scholar] [CrossRef] [PubMed]
- Amow, G.; Skinner, S.J. Recent developments in Ruddlesden–Popper nickelate systems for solid oxide fuel cell cathodes. J. Solid State Electrochem. 2006, 10, 538–546. [Google Scholar] [CrossRef]
- Chen, Y.; Téllez, H.; Burriel, M.; Yang, F.; Tsvetkov, N.; Cai, Z.; McComb, D.W.; Kilner, J.A.; Yildiz, B. Segregated chemistry and structure on (001) and (100) surfaces of (La1−xSrx)2CoO4 override the crystal anisotropy in oxygen exchange kinetics. Chem. Mater. 2015, 27, 5436–5450. [Google Scholar] [CrossRef]
- Aidhy, D.S.; Liu, B.; Zhang, Y.W.; Weber, W.J. Strain-induced phase and oxygen-vacancy stability in ionic interfaces from first-principles calculations. J. Phys. Chem. C 2014, 118, 30139–30144. [Google Scholar] [CrossRef]
- Aidhy, D.S.; Zhang, Y.W.; Weber, W.J. (001) SrTiO3 vertical bar (001) MGO interface and oxygen-vacancy stability from first-principles calculations. ACS Appl. Mater. Interfaces 2014, 6, 15536–15541. [Google Scholar] [PubMed]
- Fronzi, M.; Cereda, S.; Tateyama, Y.; De Vita, A.; Traversa, E. Ab initio investigation of defect formation at ZrO2-CeO2 interfaces. Phys. Rev. B 2012, 86, 085407. [Google Scholar] [CrossRef]
- Shen, W.D.; Jiang, J.; Hertz, J.L. Beneficial lattice strain in heterogeneously doped ceria. J. Phys. Chem. C 2014, 118, 22904–22912. [Google Scholar] [CrossRef]
- Shen, W.D.; Jiang, J.; Ni, C.Y.; Voras, Z.; Beebe, T.P.; Hertz, J.L. Two-dimensional vacancy trapping in yttria doped ceria. Solid State Ion. 2014, 255, 13–20. [Google Scholar] [CrossRef]
- Cho, S.Y.; Chung, Y.-C.; Ahn, K.; Lee, J.-H.; Kim, B.-K.; Kim, H. Oxygen transport in epitaxial La0.875Sr0.125CoO3−δ thin-film cathodes for solid oxide fuel cells: Roles of anisotropic strain. Scr. Mater. 2016, 115, 141–144. [Google Scholar] [CrossRef]
- Gan, L.-Y.; Akande, S.O.; Schwingenschlogl, U. Anisotropic O vacancy formation and diffusion in LaMnO3. J. Mater. Chem. A 2014, 2, 19733–19737. [Google Scholar] [CrossRef]
- Kushima, A.; Yildiz, B. Role of lattice strain and defect chemistry on the oxygen vacancy migration at the (8.3% Y2O3-ZrO2)/SrTiO3 hetero-interface: A first principles study. ECS Trans. 2009, 25, 1599–1609. [Google Scholar]
- Petrie, J.R.; Jeen, H.; Barron, S.C.; Meyer, T.L.; Lee, H.N. Enhancing perovskite electrocatalysis through strain tuning of the oxygen deficiency. J. Am. Chem. Soc. 2016, 138, 7252–7255. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Shimada, T.; Araki, Y.; Wang, J.; Kitamura, T. Defect-strain engineering for multiferroic and magnetoelectric properties in epitaxial (110) ferroelectric lead titanate. Phys. Rev. B 2015, 92, 104106. [Google Scholar] [CrossRef]
- Tsvetkov, N.; Lu, Q.; Chen, Y.; Yildiz, B. Accelerated oxygen exchange kinetics on Nd2NiO4+δ thin films with tensile strain along c-axis. ACS Nano 2015, 9, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Bishop, S.R.; Duncan, K.L.; Wachsman, E.D. Thermo-chemical expansion in strontium-doped lanthanum cobalt iron oxide. J. Am. Ceram. Soc. 2010, 93, 4115–4121. [Google Scholar] [CrossRef]
- Borovskikh, L.; Mazo, G.; Kemnitz, E. Reactivity of oxygen of complex cobaltates La1−xSrxCoO3−δ and LaSrCoO4. Solid State Sci. 2003, 5, 409–417. [Google Scholar] [CrossRef]
- Cai, Z.H.; Kubicek, M.; Fleig, J.; Yildiz, B. Chemical heterogeneities on La0.6Sr0.4CoO3-δ thin films-correlations to cathode surface activity and stability. Chem. Mater. 2012, 24, 1116–1127. [Google Scholar] [CrossRef]
- Hardy, J.S.; Templeton, J.W.; Edwards, D.J.; Lu, Z.G.; Stevenson, J.W. Lattice expansion of LSCF-6428 cathodes measured by in situ XRD during SOFC operation. J. Power Sources 2012, 198, 76–82. [Google Scholar] [CrossRef]
- Hashimoto, S.; Fukuda, Y.; Kuhn, M.; Sato, K.; Yashiro, K.; Mizusaki, J. Oxygen nonstoichiometry and thermo-chemical stability of La0.6Sr0.4Co1−yFeyO3−δ (y = 0.2, 0.4, 0.6, 0.8). Solid State Ion. 2010, 181, 1713–1719. [Google Scholar] [CrossRef]
- Kahoul, A.; Hammouche, A.; Poillerat, G.; De Doncker, R.W. Electrocatalytic activity and stability of La1−xCaxCoO3 perovskite-type oxides in alkaline medium. Catal. Today 2004, 89, 287–291. [Google Scholar] [CrossRef]
- Kharton, V.V.; Yaremchenko, A.A.; Patrakeev, M.V.; Naumovich, E.N.; Marques, F.M.B. Thermal and chemical induced expansion of La0.3Sr0.7(Fe,Ga)O3−δ ceramics. J. Eur. Ceram. Soc. 2003, 23, 1417–1426. [Google Scholar] [CrossRef]
- Lugovy, M.; Aman, A.; Chen, Y.; Orlovskaya, N.; Kuebler, J.; Graule, T.; Reece, M.J.; Ma, D.; Stoica, A.D.; An, K. In-situ neutron diffraction of LaCoO3 perovskite under uniaxial compression. II. Elastic properties. J. Appl. Phys. 2014, 116, 013504. [Google Scholar] [CrossRef]
- Samal, D.; Kumar, P.S.A. A critical re-examination and a revised phase diagram of La1−xSrxCoO3. J. Phys. Condens. Matter. 2011, 23, 9. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.S.; Fleig, J.; Cristiani, G.; Stuhlhofer, B.; Habermeier, H.U.; Maier, J. Quantitative comparison of mixed conducting SOFC cathode materials by means of thin film model electrodes. J. Electrochem. Soc. 2007, 154, B931–B941. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.W.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Petric, A.; Huang, P.; Tietz, F. Evaluation of La-Sr-Co-Fe-O perovskites for solid oxide fuel cells and gas separation membranes. Solid State Ion. 2000, 135, 719–725. [Google Scholar] [CrossRef]
- Skinner, S.J. Characterisation of La2NiO4+δ using in-situ high temperature neutron powder diffraction. Solid State Sci. 2003, 5, 419–426. [Google Scholar] [CrossRef]
- Huang, B.X.; Malzbender, J.; Steinbrech, R.W. Thermo-mechanical properties of La2NiO4+δ. J. Mater. Sci. 2011, 46, 4937–4941. [Google Scholar] [CrossRef]
- Makhnach, L.V.; Pankov, V.V.; Strobel, P. High-temperature oxygen non-stoichiometry, conductivity and structure in strontium-rich nickelates La2−xSrxNiO4−δ (x = 1 and 1.4). Mater. Chem. Phys. 2008, 111, 125–130. [Google Scholar] [CrossRef]
- Millburn, J.E.; Green, M.A.; Neumann, D.A.; Rosseinsky, M.J. Evolution of the structure of the K2NiF4 phases La2−xSrxNiO4+δ with oxidation state: Octahedral distortion and phase separation (0.2 ≤ x ≤ 1.0). J. Solid State Chem. 1999, 145, 401–420. [Google Scholar] [CrossRef]
- Nakamura, T.; Yashiro, K.; Sato, K.; Mizusaki, J. Structural analysis of La2−xSrxNiO4+δ by high temperature X-ray diffraction. Solid State Ion. 2010, 181, 292–299. [Google Scholar] [CrossRef]
- Kharton, V.V.; Kovalevsky, A.V.; Avdeev, M.; Tsipis, E.V.; Patrakeev, M.V.; Yaremchenko, A.A.; Naumovich, E.N.; Frade, J.R. Chemically induced expansion of La2NiO4+δ-based materials. Chem. Mater. 2007, 19, 2027–2033. [Google Scholar] [CrossRef]
- Kharton, V.V.; Yaremchenko, A.A.; Shaula, A.L.; Patrakeev, M.V.; Naumovich, E.N.; Loginovich, D.I.; Frade, J.R.; Marques, F.M.B. Transport properties and stability of Ni-containing mixed conductors with perovskite- and K2NiF4-type structure. J. Solid State Chem. 2004, 177, 26–37. [Google Scholar] [CrossRef]
- Tietz, F.; Raj, I.A.; Zahid, M.; Mai, A.; Stover, D. Survey of the quasi-ternary system La0.8Sr0.2MnO3-La0.8Sr0.2CoO3-La0.8Sr0.2FeO3. Prog. Solid State Chem. 2007, 35, 539–543. [Google Scholar] [CrossRef]
- Ullmann, H.; Trofimenko, N.; Tietz, F.; Stover, D.; Ahmad-Khanlou, A. Correlation between thermal expansion and oxide ion transport in mixed conducting perovskite-type oxides for SOFC cathodes. Solid State Ion. 2000, 138, 79–90. [Google Scholar] [CrossRef]
- Tai, L.W.; Nasrallah, M.M.; Anderson, H.U.; Sparlin, D.M.; Sehlin, S.R. Structure and electrical properties of La1−xSrxCo1−yFeyO3. Part 2. The system La1−xSrxCo0.2Fe0.8O3. Solid State Ion. 1995, 76, 273–283. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G.; Whitfield, P.S.; Davidson, L.J. Structure, stability and electrical properties of the La2−xSrxMnO4±δ solid solution series. Solid State Ion. 2006, 177, 1849–1853. [Google Scholar] [CrossRef]
- Riza, F.; Ftikos, C. Influence of A- and B-site doping on the properties of the system La2CoO4±δ. J. Eur. Ceram. Soc. 2007, 27, 571–573. [Google Scholar] [CrossRef]





| Material | Ea/eV | Methodology | Mechanism | Ref. |
|---|---|---|---|---|
| La2NiO4+δ | 0.29 | MD | Interstitialcy mechanism | [55] |
| La2NiO4+δ | 0.55 | Static atomistic simulation | Vacancy mechanism | [66] |
| La2NiO4+δ | 1.2 | DFT | Interstitial mechanism | [67] |
| La2NiO4+δ | 0.51 | MD | Interstitialcy mechanism | [63] |
| Pr2NiO4+δ | 0.49–0.64 | MD | Interstitialcy mechanism | [68] |
| La2CoO4+δ | 0.31 | MD | Interstitialcy mechanism | [70] |
| La2CoO4+δ | 0.73–0.80 | DFT | Interstitialcy mechanism | [70] |
| La2CoO4+δ | 1.27–1.39 | DFT | Interstitial mechanism | [70] |
| x (Sr) | La2−xSrxCuO4±δ | La2−xSrxNiO4±δ | La2−xSrxCoO4±δ |
|---|---|---|---|
| 4 ± δ [Ref.] | 4 ± δ [Ref.] | 4 ± δ [Ref.] | |
| 0.0 | 4.01 [75] | 4.14 [76] | 4.15 [77] |
| 0.1 | 4.00 [75] | 4.12 [76] | − |
| 0.2 | 3.99 [75] | 4.11 [76] | − |
| 0.3 | 3.98 [75] | 4.09 [76] | − |
| 0.5 | 3.90 [78] | 4.06 [76] | 4.07 [46] |
| 1.0 | 3.60 [78] | 3.99 [76] | 4.00 [46] |
| 1.4 | − | 3.95 [76] | 3.90 [46] |
| Material | Ea/eV | Temperature Range/°C | Comment | Ref. |
|---|---|---|---|---|
| Pr2NiO4+δ | 0.67 | 450–700 | Single crystal, a-b plane | [49] |
| Pr2NiO4+δ | 1.10 | 450–700 | Single crystal, c-direction | [49] |
| Pr2NiO4+δ | 0.76 | 550–850 | Polycrystalline | [51] |
| La2NiO4+δ | 0.81 | 525–600 | Single crystal, a-b plane | [97] |
| La2NiO4+δ | 0.75 | 450–600 | Single crystal, c-direction | [97] |
| La2NiO4+δ | 0.87 | 500–850 | Polycrystalline | [51] |
| Nd2NiO4+δ | 1.38 | 450–700 | Single crystal, a-b plane | [49] |
| Nd2NiO4+δ | 1.27 | 450–700 | Single crystal, c-direction | [49] |
| Nd2NiO4+δ | 1.05 | 550–850 | Polycrystalline | [51] |
| La2CuO4+δ | 0.81 | 390–600 | Single crystal | [56] |
| La2CuO4+δ | 1.18 | 527–727 | Polycrystalline | [98] |
| La2CoO4+δ | 0.13 | 450–700 | Polycrystalline | [77] |
| Material | TEC (×10−6 K−1) | Temperature/°C | Ref. |
|---|---|---|---|
| La0.8Sr0.2CoO3−δ | 19.1 | 30–1000 | [141] |
| La0.6Sr0.4CoO3−δ | 20.5 | 30–1000 | [142] |
| La0.8Sr0.2Co0.2Fe0.8O3−δ | 15.4 | 100– 800 | [143] |
| La0.6Sr0.4Co0.2Fe0.8O3−δ | 15.3 | 100–600 | [143] |
| La2NiO4+δ | 11.0 | 650–950 | [138] |
| La1.8Sr0.2NiO4+δ | 11.2 | 650–950 | [138] |
| La1.6Sr0.4NiO4+δ | 12.0 | 650–950 | [138] |
| La0.6Sr1.4MnO4±δ | 13.5 | 30–800 | [144] |
| La0.2Sr1.8MnO4±δ | 16.5 | 30–800 | [144] |
| La0.5Sr0.5Co0.5Fe0.5O4−δ | 13.5 | 30–700 | [145] |
| La2Ni0.9Co0.1O4+δ | 13.8 | 100–900 | [139] |
| La1.3Sr0.7CoO4−δ | 9.6 | 30–1000 | [27] |
| La1.4Sr0.6CoO4−δ | 10.1 | 30–700 | [145] |
| LaSrCoO4−δ | 14.3 | 30–1000 | [27] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, D.; Lee, H.N. Controlling Oxygen Mobility in Ruddlesden–Popper Oxides. Materials 2017, 10, 368. https://doi.org/10.3390/ma10040368
Lee D, Lee HN. Controlling Oxygen Mobility in Ruddlesden–Popper Oxides. Materials. 2017; 10(4):368. https://doi.org/10.3390/ma10040368
Chicago/Turabian StyleLee, Dongkyu, and Ho Nyung Lee. 2017. "Controlling Oxygen Mobility in Ruddlesden–Popper Oxides" Materials 10, no. 4: 368. https://doi.org/10.3390/ma10040368






