Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications
Abstract
:1. Historical Perspective
2. The Structure, Chemistry and Mechanical Properties of Bone
3. Transient Precursor Phases
4. Dissolution and Reprecipitation as Bone
5. Requirements from Calcium Phosphates for Medical Applications
6. Individual Calcium Phosphate Phases and Their Properties
6.1. MCPM
6.2. DCPA
6.3. DCPD
6.4. OCP
6.5. α-TCP
6.6. β-TCP
6.7. ACP
6.8. CDHA
6.9. TTCP
6.10. HAp
7. Nano-CaP
8. Biphasic and Triphasic CaP Formulations
9. Composite Calcium Phosphates
10. Functionally Graded Calcium Phosphates
11. Antibacterial Calcium Phosphates
12. The Effect of Sterilization
13. In Vitro and In Vivo Tests
14. CaP Coating Technologies
US FDA and International Standards Requirements
15. Clinical and Industrial Applications
16. The Future of Calcium Phosphates
- (1)
- Dental community mistrust. Interfacial failure of past dental implants coated with PS CaP has left a mark on CaP coatings. When first bursting into the dental industry as coatings, some PS CaP coatings failed within several months or years [850,851]. The cause has been attributed to the extensive dissolution of the coating as well as to its delamination [850,852]. These problems have since been eliminated; yet, many in the dental community have lost their faith in these coatings. A major campaign or sponsored Health Maintenance Organization (HMO) implants may reinstate CaP coatings to dental implants.
- (2)
- Short-term infection. As described earlier, most infections occur due to bacteria adhering to the implant’s surface during implantation, causing a biofilm to form on it prior to implantation [587,853]. In general, infections can be classified based on their time of onset. Most of the infections develop from an early contamination that occurs during the operation or in the first few days after surgery. Events such as these, which become symptomatic or anyway manifest shortly following surgery, within three months of implantation, have been referred to as “early” infections [589]. These infections can be prevented by CaP incorporated with either a drug release system or anti-fouling agents. Anti-fouling or on-demand drug release systems, if designed well, can also be used to prevent long-term infections. This is a goal marked by many companies today.
- (3)
- Long recovery time. CaPs are very good osteoconductive agents. Yet, the recovery period of implants integrated with CaPs is not immediate, unlike other techniques. For example, PMMA fixation of hip implants is immediate, and the patient may apply weight on the implantation site almost immediately after the operation. CaP coatings, and as such so are the scaffolds and cements, need time to allow good osseointegration, and thus extend the recovery period of patients. Encouraging faster integration, or somehow allowing for a bridge to such, may increase the use of CaP products.
- (4)
- Long-term issues with implants. Both long-term infection and resorption of the surrounding bone (e.g., due to stress shielding) introduce serious problems. Sensors allowing the doctor, or even the patient, to monitor the environment of the implant’s surface may allow earlier intervention, especially if such intervention can activate dormant agents within the coating.
- (5)
- Mechanical strength of scaffolds/cements for tissue engineering. Biodegradable scaffolds/cements are very limited in use because of poor mechanical stability, and limited promotion of vascularization. Composites of CaPs with biodegradable metals, e.g., magnesium, and incorporation of GFs could solve this intricate problem and increase the use of CaP products in reconstructive surgery. However, as described in Section 9, the inclusion of GFs is not in favour nowadays due to health safety issues.
- (6)
- Bone/cartilage, bone mineral/collagen and bone/tendon interfaces. While many efforts are focused on the issues described above, not enough attention is focused on bone interfaces. A better understanding of the biological systems is needed. For example, the bonding mechanism between the bone mineral and collagen remains unclear [854]. It is also unclear whether a rapid repair that is elicited by the new generation of bioceramics results from the enhancement of mineralization per se or whether there is a more complex signalling process involving proteins in collagen. If we were able to understand the fundamentals of bone response to specific ions and the signals they activate, then we could design better bioceramics for the future [854]. From application standpoint, CaP-based FGMs may very well address these issues and become the golden standard in CaP implants.
- (7)
- Transient precursor phases. As described in Section 3, ACP, DCPD and OCP have been suggested as transient precursor phases. However, there is yet no consensus in the scientific community regarding the prevalence of these phases and the exact mechanisms of biomineralization relevant to human bone remodelling. High-resolution, in situ structural and chemical studies of human bone formation and remodelling may be possible one day and clarify this old scientific debate. The outcomes of such studies may aid in developing better bone substitutes and CaP-based coatings.
17. Conclusions
Conflicts of Interest
References
- Dorozhkin, S.V. A detailed history of calcium orthophosphates from 1770s till 1950. Mater. Sci. Eng. C 2013, 33, 3085–3110. [Google Scholar] [CrossRef] [PubMed]
- Driskell, T.D. Early history of calcium phosphate materials and coatings. In Characterization and Performance of Calcium Phosphate Coatings for Implants; Horowitz, E., Parr, J.E., Eds.; American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 1994; pp. 1–9. [Google Scholar]
- Shackelford, J.F. Bioceramics—An historical perspective. Mater. Sci. Forum 1999, 293, 1–4. [Google Scholar] [CrossRef]
- Shepperd, J. The early biological history of calcium phosphates. In Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty; Epinette, J.A., Manley, M.T., Eds.; Springer: Paris, France, 2004; pp. 3–8. [Google Scholar]
- Leeuwenhoek, A. Microscopical observations concerning blood, milk, bone, the brain, spittle, and cuticula, etc. Philos. Trans. 1674, 9, 121–128. [Google Scholar] [CrossRef]
- Leeuwenhoek, A. Part of a letter of Mr. Anthony van Leeuwenhoeck, dated Delst, Sept. 10. 1697. Concerning the eggs of snails, roots of vegetables, teeth, and young Oysters. Philos. Trans. 1695, 19, 790–799. [Google Scholar] [CrossRef]
- Roscoe, H.E.; Schorlemmer, C. A Treatise on Chemistry. Volume I: The Non-Metallic Elements; Macmillan and Co.: London, UK, 1881; p. 751. [Google Scholar]
- Aikin, A.; Aikin, C.R. A Dictionary of Chemistry and Mineralogy, Vol. II; Printed for John and Arthur Arch, Corninll; William Phillips, George Yard, Lombard Street: London, UK, 1807; p. 176. [Google Scholar]
- Nicholson, W. A Dictionary of Practical and Theoretical Chemistry, with Its Application to the Arts and Manufactures, and to the Explanation of the Phenomena of Nature; Printed for Richard Phillips: London, UK, 1808. [Google Scholar]
- Parr, B. The London Medical Dictionary, Vol. I; Wentworth Press: London, UK, 1809; p. 786. [Google Scholar]
- Davy, H. Conversations on Chemistry; Didnep’s Press: London, UK, 1814; p. 383. [Google Scholar]
- Muhlenberg, W.F. Address in hygiene. In Transactions of the Medical Society of the State of Pennsylvania, at Its Thirty-Third Annual Session; Times Printing House: Philadelphia, PA, USA, 1832; Volume 14, p. 90. [Google Scholar]
- Percy, J. Notice of a new hydrated phosphate of lime. Mem. Proc. Chem. Soc. 1843, 2, 222–223. [Google Scholar] [CrossRef]
- Dana, J.D. On the occurrence of fluor spar, apatite and chondtodite in limestone. Philos. Mag. Ser. 3 1846, 29, 245–246. [Google Scholar]
- Lassaigne, M. Solubility of carbonate of lime in water containing carbonic acid. Philos. Mag. Ser. 3 1847, 30, 297–298. [Google Scholar] [CrossRef]
- Jenkins, E.E. Phosphate of Lime. Master’s Thesis, Medical College of the State of South Carolina, Charleston, SC, USA, 1853. [Google Scholar]
- Kramer, B.; Shear, M.J. Composition of bone. IV. Primary calcification. J. Biol. Chem. 1928, 79, 147–160. [Google Scholar] [CrossRef]
- Von Walter, P. Wiedereinheilung der bei der trapanation ausgebohrten knochenscheibe. J. Chir. Augen Heilkd. 1821, 2, 571. [Google Scholar]
- Macewen, W. Observations concerning transplantation of bone. Illustrated by a case of inter-human osseous transplantation, whereby over two-thirds of the shaft of a humerus was restored. Proc. R. Soc. Lond. 1881, 32, 232–247. [Google Scholar] [CrossRef]
- Cravens, J.E. Lacto-phosphate of lime; pathology and treatment of exposed dental pulps and sensitive dentine. Dent. Cosmos. 1876, 18, 463–469. [Google Scholar]
- Wells, H.G. Pathological calcification. J. Med. Res. 1906, 14, 491–525. [Google Scholar] [PubMed]
- Albee, F.H.; Morrison, H.F. Studies in bone growth triple calcium phosphate as a stimulus to osteogenesis. Ann. Surg. 1920, 71, 32–39. [Google Scholar] [PubMed]
- Albee, F.H. Bone-Graft Surgery; W.B. Saunders Company: Philadelphia, PA, USA; London, UK, 1915. [Google Scholar]
- Mehmel, M. On the structure of apatite. Z. Kristallogr. 1930, 75, 323–331. [Google Scholar]
- Náray-Szabó, S. The structure of apatite (CaF)Ca4(PO4)3. Z. Kristallogr. 1930, 75, 387–398. [Google Scholar]
- Hendricks, S.B.; Hill, W.L.; Jacob, K.D.; Jefferson, M.E. Structural characteristics of apatite-like substances and composition of rock and bone as determined from microscopical and X-ray diffraction analysis. Ind. Eng. Chem. 1931, 23, 1413–1418. [Google Scholar] [CrossRef]
- Möller, H.; Trömel, G. Röntgenographische untersuchung über den aufbau der anorganischen zahnsubstanz. Naturwissenschaften 1933, 21, 346–348. [Google Scholar] [CrossRef]
- Möller, H.; Trömel, G. Über die kristallorientierung im zahnschmelz. Naturwissenschaften 1936, 24, 377–378. [Google Scholar] [CrossRef]
- Bale, W.F.; Hodge, H.C.; Warren, S.L. Roentgen-ray diffraction studies of enamel and dentin. Am. J. Roentgenol. Radiat. Ther. 1934, 32, 369–376. [Google Scholar]
- Bredig, M.A.; Franck, H.H.; Fülnder, H. Beiträge zur kenntnis der kalk-phosphorsäure-verbindungen. Z. Elktrochem. 1932, 38, 158–164. [Google Scholar]
- Trömel, G. Beiträge zur kenntnis des systems kalziumoxyd-phosphorpentoxyd. Mitt. Kaiser-Wilhelm-Inst. Eisenforsch. Düsseldorf 1932, 14, 25–34. [Google Scholar]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10 (Suppl. 2), S96–S101. [Google Scholar] [PubMed]
- Huggins, C.B. The formation of bone under the influence of epithelium of the urinary tract. Arch. Surg. 1931, 22, 377–408. [Google Scholar] [CrossRef]
- Elmore, K.L.; Farr, T.D. Equilibrium in the system calcium oxide-phosphorous pentoxide-water. Ind. Eng. Chem. 1940, 32, 580–586. [Google Scholar] [CrossRef]
- Schram, W.R.; Fosdick, L.S. Stimulation of healing in long bones by use of artificial material. J. Oral Surg. 1948, 6, 209–217. [Google Scholar] [PubMed]
- Arnold, P.W. The nature of precipitated calcium phosphates. Trans. Faraday Soc. 1950, 46, 1061–1072. [Google Scholar] [CrossRef]
- Kingery, W.D., II. Cold-setting properties. J. Am. Ceram. Soc. 1950, 33, 242–246. [Google Scholar] [CrossRef]
- Köster, K.; Karbe, E.; Kramer, H.; Heide, H.; König, R. Experimental bone replacement with resorbable calcium phosphate ceramic. Langenbecks Arch. Chir. 1976, 341, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Brånemark, P.I. Osseointegration and its experimental background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.A.; Posner, A.S. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050–1052. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Betts, F. Synthetic amorphous calcium phosphate and its relation to bone mineral structure. Acc. Chem. Res. 1975, 8, 273–281. [Google Scholar] [CrossRef]
- Boskey, A.L. Amorphous calcium phosphate: The contention of bone. J. Dent. Res. 1997, 76, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Levitt, S.R.; Crayton, P.H.; Monroe, E.A.; Condrate, R.A. Forming methods for apatite prosthesis. J. Biomed. Mater. Res. 1969, 3, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, S.N.; Brady, J.M.; Getter, L.; Grower, M.F.; Driskell, T. Biodegradable ceramic implants in bone. Electron and light microscopic analysis. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 336–346. [Google Scholar] [CrossRef]
- Driskell, T.D.; Hassler, C.R.; Tennery, V.J.; McCoy, L.R.; Clarke, W.J. Calcium phosphate resorbable ceramics: A potential alternative to bone grafting. J. Dent. Res. 1973, 52, 123. [Google Scholar]
- Nery, E.B.; Lynch, K.L.; Hirthe, W.M.; Mueller, K.H. Bioceramic implants in surgically produced infrabony defects. J. Periodontol. 1975, 46, 328–347. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C.; Brilliant, J.D. Tricalcium phosphate as an adjunct to apical closure in pulpless permanent teeth. J. Endod. 1975, 1, 263–269. [Google Scholar] [CrossRef]
- Denissen, H.W.; de Groot, K. Immediate dental root implants from synthetic dense calcium hydroxylapatite. J. Prosthet. Dent. 1979, 42, 551–556. [Google Scholar] [CrossRef]
- León, B.; Jansen, J.A. (Eds.) Thin Calcium Phosphate Coatings for Medical Implants; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sudo, S.Z.; Schotzko, N.K.; Folke, L.E.A. Use of hydroxyapatite coated glass beads for preclinical testing of potential antiplaque agents. Appl. Environ. Microbiol. 1976, 32, 428–437. [Google Scholar] [PubMed]
- Bonfield, W.; Grynpas, M.D.; Tully, A.E.; Bowman, J.; Abram, J. Hydroxyapatite reinforced polyethylene—A mechanically compatible implant material for bone replacement. Biomaterials 1981, 2, 185–186. [Google Scholar] [CrossRef]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- De Groot, K. (Ed.) Bioceramics of Calcium Phosphate; CRC Press: Boca Raton, FL, USA, 1983. [Google Scholar]
- Aoki, H.; Kato, K.M.; Ogiso, M.; Tabata, T. Studies on the application of apatite to dental materials. J. Dent. Eng. 1977, 18, 86–89. [Google Scholar]
- Furlong, R.J.; Osborn, J.F. Fixation of hip prostheses by hydroxyapatite ceramic coatings. J. Bone Jt. Surg. Br. 1991, 73, 741–745. [Google Scholar]
- Randzio, J.; Thoma, K.; Alex, R.; Rhomberg, B. Healing and pharmacokinetics of a beta-tricalcium phosphate-gentamycin combination in animal studies (preliminary report). Dtsch. Zahnarztl. Z. 1985, 40, 668–671. [Google Scholar] [PubMed]
- Dorozhkin, S.V. Nanodimensional and nanocrystalline calcium orthophosphates. Am. J. Biomed. Eng. 2012, 2, 48–97. [Google Scholar] [CrossRef]
- Layrolle, P.; Lebugle, A. Characterization and reactivity of nanosized calcium phosphates prepared in anhydrous ethanol. Chem. Mater. 1994, 6, 1996–2004. [Google Scholar] [CrossRef]
- Li, Y.B.; de Wijn, J.; Klein, C.P.A.T.; de Meer, S.V.; de Groot, K. Preparation and characterization of nanograde osteoapatite-like rod crystals. J. Mater. Sci. Mater. Med. 1994, 5, 252–255. [Google Scholar]
- Shirkhanzadeh, M. X-ray diffraction and Fourier transform infrared analysis of nanophase apatite coatings prepared by electrocrystallization. Nanostruct. Mater. 1994, 4, 677–684. [Google Scholar] [CrossRef]
- Norman, M.E.; Elgendy, H.M.; Shors, E.C.; El-Amin, S.F.; Laurencin, C.T. An in-vitro evaluation of coralline porous hydroxyapatite as a scaffold for osteoblast growth. Clin. Mater. 1994, 17, 85–91. [Google Scholar] [CrossRef]
- Dekker, R.J.; de Bruijn, J.D.; van den Brink, I.; Bovell, Y.P.; Layrolle, P.; van Blitterswijk, C.A. Bone tissue engineering on calcium phosphate-coated titanium plates utilizing cultured rat bone marrow cells: A preliminary study. J. Mater. Sci. Mater. Med. 1998, 9, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.D.; Costantino, P.D.; Takagi, S.; Chow, L.C. BoneSource hydroxyapatite cement: A novel biomaterial for craniofacial skeletal tissue engineering and reconstruction. J. Biomed. Mater. Res. 1998, 43, 428–432. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium phosphates in oral biology and medicine. Monogr. Oral Sci. 1991, 15, 1–201. [Google Scholar] [PubMed]
- Aoki, H. Science and Medical Applications of Hydroxyapatite; JAAS: Tokyo, Japan, 1991. [Google Scholar]
- Elliott, J.C. Structure and Chemistry of the Apatites and Other Calcium Orthophosphates; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Brown, P.W.; Constantz, B. (Eds.) Hydroxyapatite and Related Materials; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- Horowitz, E.; Parr, J.E. Characterization and Performance of Calcium Phosphate Coatings for Implants; ASTM STP 1196; American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 1994. [Google Scholar]
- Epinette, J.A.; Manley, M.T. (Eds.) Fifteen Years of Clinical Experience with Hydroxyapatite Coatings in Joint Arthroplasty; Springer: Paris, France, 2004. [Google Scholar]
- Dorozhkin, S.V. Calcium Orthophosphates: Applications in Nature, Biology, and Medicine; Pan Stanford Publishing Pte.: Singapore, 2012. [Google Scholar]
- Heimann, R.B. (Ed.) Calcium Phosphate: Structure, Synthesis, Properties, and Applications; Nova Science Publishers: Hauppauge, NY, USA, 2014. [Google Scholar]
- Ben-Nissan, B. Advances in Calcium Phosphate Biomaterials; Springer: Berlin, Germany, 2014. [Google Scholar]
- Dey, A.; Mukhopadhyay, A.K. Microplasma Sprayed Hydroxyapatite Coatings; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Ann. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Rho, J.Y.; Kuhn-Spearing, L.; Zioupos, P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998, 20, 92–102. [Google Scholar] [CrossRef]
- Currey, J.D. Bones: Structure and Mechanics; Princeton University Press: Princeton, NJ, USA, 2002. [Google Scholar]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Fratzl, P.; Gupta, H.S.; Paschalis, E.P.; Roschger, P. Structure and mechanical quality of the collagen-mineral nano-composite in bone. J. Mater. Chem. 2004, 14, 2115–2123. [Google Scholar] [CrossRef]
- Weiner, S. Transient precursor strategy in mineral formation of bone. Bone 2006, 39, 431–433. [Google Scholar] [CrossRef] [PubMed]
- Grynpas, M.D.; Omelon, S. Transient precursor strategy or very small biological apatite crystals? Bone 2007, 41, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Fratzl, P.; Weinkamer, R. Nature’s hierarchical materials. Prog. Mater. Sci. 2007, 52, 1263–1334. [Google Scholar] [CrossRef]
- Olszta, M.J.; Cheng, X.; Jee, S.S.; Kumar, R.; Kim, Y.Y.; Kaufman, M.J.; Douglas, E.P.; Gower, L.B. Bone structure and formation: A new perspective. Mater. Sci. Eng. R 2007, 58, 77–116. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. A hierarchical structure for apatite crystals. J. Mater. Sci. Mater. Med. 2007, 18, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral—Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, Y.W.; Gao, H. On optimal hierarchy of load-bearing biological materials. Proc. R. Soc. B 2011, 278, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Sun, W.; Yang, X. First detection, characterization, and application of amorphous calcium phosphate in dentistry. J. Dent. Sci. 2012, 7, 316–323. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Matinlinna, J.P.; Chen, Z.; Pan, H. Insight into biological apatite: Physiochemical properties and preparation approaches. BioMed Res. Int. 2013, 2013, 929748. [Google Scholar] [CrossRef] [PubMed]
- Wegst, U.G.K.; Bai, H.; Saiz, E.; Tomsia, A.P.; Ritchie, R.O. Bioinspired structural materials. Nat. Mater. 2015, 14, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Wolff, J. The Law of Bone Remodeling; Springer: Berlin, Germany, 1986. [Google Scholar]
- Frost, H.M. Wolff’s Law and bone’s structural adaptations to mechanical usage: An overview for clinicians. Angle Orthod. 1994, 64, 175–188. [Google Scholar] [PubMed]
- Tortora, G.J.; Derrickson, B. Principles of Anatomy and Physiology, 11th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1993; p. 173. [Google Scholar]
- Lakstein, D.; Kopelovitch, W.; Barkay, Z.; Bahaa, M.; Hendel, D.; Eliaz, N. Enhanced osseointegration of grit-blasted, NaOH-treated and electrochemically hydroxyapatite-coated Ti–6Al–4V implants in rabbits. Acta Biomater. 2009, 5, 2258–2269. [Google Scholar] [CrossRef] [PubMed]
- Hassenkam, T.; Fantner, G.E.; Cutroni, J.A.; Weaver, J.C.; Morse, D.E.; Hansma, P.K. High-resolution AFM imaging of intact and fractured trabecular bone. Bone 2004, 35, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Ke, P.; Jiao, X.N.; Ge, X.H.; Xiao, W.M.; Yu, B. From macro to micro: Structural biomimetic materials by electrospinning. RSC Adv. 2014, 4, 39704–39724. [Google Scholar] [CrossRef]
- Koutsoukos, P.G.; Nancollas, G.H. Crystal growth of calcium phosphates—Epitaxial considerations. J. Cryst. Growth 1981, 53, 10–19. [Google Scholar] [CrossRef]
- Panda, R.N.; Hsieh, M.F.; Chung, R.J.; Chin, T.S. X-ray diffractometry and X-ray photoelectron spectroscopy investigations of nanocrytalline hydroxyapatite synthesized by a hydroxide gel technique: Structure and mechanical and thermal properties of condensed matter. Jpn. J. Appl. Phys. Part 1 2001, 40, 5030–5035. [Google Scholar] [CrossRef]
- Betts, F.; Blumenthal, N.C.; Posner, A.S. Bone mineralization. J. Cryst. Growth 1981, 53, 63–73. [Google Scholar] [CrossRef]
- Benezra, V.; Spector, M.; Hobbs, L.W. Characterization of mineral deposits on plasma-sprayed HA-coated Ti–6Al–4V. Mater. Res. Soc. 1995, 414, 165–170. [Google Scholar] [CrossRef]
- Danilchenko, S.N.; Kukharenko, O.G.; Moseke, C.; Protsenko, I.Y.; Sukhodub, L.F.; Sulkio-Cleff, B. Determination of the bone mineral crystallite size and lattice strain from diffraction line broadening. Cryst. Res. Technol. 2002, 37, 1234–1240. [Google Scholar] [CrossRef]
- Nyman, J.S.; Reyes, M.; Wang, X. Effect of ultrastructural changes on the toughness of bone. Micron 2005, 36, 566–582. [Google Scholar] [CrossRef] [PubMed]
- Kuhn-Spearing, L.; Rey, C.; Kim, H.M.; Glimcher, M.J. Carbonated apatite nanocrystals of bone. In Synthesis and Processing of Nanocrystalline Powder; The Minerals, Metals and Materials Society: Warrendale, PA, USA, 1996. [Google Scholar]
- Moreno, E.C.; Varughese, K. Crystal growth of calcium apatites from dilute solutions. J. Cryst. Growth 1981, 53, 20–30. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Khalil, K.A.; Sheikh, F.A.; Omran, A.N.; Gaihre, B.; Khil, S.M.; Kim, H.Y. Physiochemical characterizations of hydroxyapatite extracted from bovine bones by three different methods: Extraction of biologically desirable HAp. Mater. Sci. Eng. C 2008, 28, 1381–1387. [Google Scholar] [CrossRef]
- Arsenault, A.L. Crystal-collagen relationships in calcified turkey leg tendons visualized by selected-area dark field electron microscopy. Calcif. Tissue Int. 1988, 43, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Danilchenko, S.N.; Koropov, A.V.; Protsenko, I.Y.; Sulkio-Cleff, B.; Sukhodub, L.F. Thermal behavior of biogenic apatite crystals in bone: An X-ray diffraction study. Cryst. Res. Technol. 2006, 41, 268–275. [Google Scholar] [CrossRef]
- Janus, A.M.; Faryna, M.; Haberko, K.; Rakowska, A.; Panz, T. Chemical and microstructural characterization of natural hydroxyapatite derived from pig bones. Microchim. Acta 2008, 161, 349–353. [Google Scholar] [CrossRef]
- Kim, H.M.; Rey, C.; Glimcher, M.J. Isolation of calcium phosphate crystals of bone by non-aqueous methods at low temperature. J. Bone Miner. Res. 1995, 10, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Rey, C.; Glimcher, M.J. X-ray diffraction, electron microscopy, and Fourier transform infrared spectroscopy of apatite crystals isolated from chicken and bovine calcified cartilage. Calcif. Tissue Int. 1996, 59, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Rubin, M.A.; Jasiuk, I.; Taylor, J.; Rubin, J.; Ganey, T.; Apkarian, R.P. TEM analysis of the nanostructure of normal and osteoporotic human trabecular bone. Bone 2003, 33, 270–282. [Google Scholar] [CrossRef]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
- Suvorova, E.I.; Buffat, P.A. Electron diffraction from micro- and nanoparticles of hydroxyapatite. J. Microsc. 1999, 196, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Perloff, A.; Diorio, A.F. Refinement of the hydroxyapatite structure. Acta Cryst. 1958, 11, 308–309. [Google Scholar] [CrossRef]
- Elliott, J.C.; Mackie, P.E.; Young, R.A. Monoclinic hydroxyapatite. Science 1973, 180, 1055–1057. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.B.; Liu, X.Y. Hydroxyapatite: Hexagonal or monoclinic? Cryst. Growth Des. 2009, 9, 2991–2994. [Google Scholar] [CrossRef]
- De Leeuw, N.H. Local ordering of hydroxy groups in hydroxyapatite. Chem. Commun. 2001, 17, 1646–1647. [Google Scholar] [CrossRef]
- Vallet-Regi, M. Biomimetic Nanoceramics in Clinical Use: From Materials to Applications; Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Bonar, L.C.; Lees, S.; Mook, H.A. Neutron diffraction studies of collagen in fully mineralized bone. J. Mol. Biol. 1985, 181, 265–270. [Google Scholar] [CrossRef]
- Kuhn, L.T.; Grynpas, M.D.; Rey, C.C.; Wu, Y.; Ackerman, J.L.; Glimcher, M.J. A comparison of the physical and chemical differences between cancellous and cortical bovine bone mineral at two ages. Calcif. Tissue Int. 2008, 83, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Joschek, S.; Nies, B.; Krotz, R.; Göpferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Pasteris, J.D.; Wopenka, B.; Freeman, J.J.; Rogers, K.; Valsami-Jones, E.; van der Houwen, J.A.M.; Silva, M.J. Lack of OH in nanocrystalline apatite as a function of degree of atomic order: Implications for bone and biomaterials. Biomaterials 2004, 25, 229–238. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Development of nanocomposites for bone grafting. Compos. Sci. Technol. 2005, 65, 2385–2406. [Google Scholar] [CrossRef]
- Gordon, L.M.; Tran, L.; Joester, D. Atom probe tomography of apatites and bone-type mineralized tissues. ACS Nano 2012, 6, 10667–10675. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; da Silva Sasso, G.R.; Sasso-Cerri, E.; Simões, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F.; Gibson, L.J.; Wegst, U.; Olive, R. The mechanical properties of natural materials, I: Material property charts. Proc. R. Soc. Lond. Ser. A 1995, 450, 123–140. [Google Scholar] [CrossRef]
- Wegst, U.G.K.; Ashby, M.F. The mechanical efficiency of natural materials. Philos. Mag. 2004, 84, 2167–2186. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Buehler, M.J. Nanomechanics of functional and pathological amyloid materials. Nat. Nanotechnol. 2011, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Bonfield, W.; Wang, M.; Tanner, K.E. Interfaces in analogue biomaterials. Acta Mater. 1998, 46, 2509–2518. [Google Scholar] [CrossRef]
- Yamada, H. Strength of Biological Materials; Williams & Wilkins: Baltimore, MD, USA, 1970. [Google Scholar]
- Beniash, E.; Aizenberg, J.; Addadi, L.; Weiner, S. Amorphous calcium carbonate transforms into calcite during sea-urchin larval spicule growth. Proc. R. Soc. Lond. Ser. B 1997, 264, 461–465. [Google Scholar] [CrossRef]
- Beniash, E.; Metzler, R.A.; Lam, R.S.; Gilbert, P.U. Transient amorphous calcium phosphate in forming enamel. J. Struct. Biol. 2009, 166, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Towe, K.M.; Lowenstam, H.A. Ultrastructure and development of iron mineralization in the radular teeth of Cryptochiton stelleri (Mollusca). J. Ultrastruct. Res. 1967, 17, 1–13. [Google Scholar] [CrossRef]
- Politi, Y.; Arad, T.; Klein, E.; Weiner, S.; Addadi, L. Sea urchin spine calcite forms via a transient amorphous calcium carbonate phase. Science 2004, 306, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Mahamid, J.; Sharir, A.; Addadi, L.; Weiner, S. Amorphous calcium phosphate is a major component of the forming fin bones of zebrafish: Indications for an amorphous precursor phase. Proc. Natl. Acad. Sci. USA 2008, 105, 12748–12753. [Google Scholar] [CrossRef] [PubMed]
- Mahamid, J.; Aichmayer, B.; Shimoni, E.; Ziblat, R.; Li, C.; Siegel, S.; Paris, O.; Fratzl, P.; Weiner, S.; Addadi, L. Mapping amorphous calcium phosphate transformation into crystalline mineral from the cell to the bone in zebrafish fin rays. Proc. Natl. Acad. Sci. USA 2010, 107, 6316–6321. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Dynamics of biomineralization and biodemineralization. Met. Ions Life Sci. 2010, 4, 413–456. [Google Scholar] [PubMed]
- Glimcher, M.J. Recent studies of the mineral phase in bone and its possible linkage to the organic matrix by protein-bound phosphate bonds. Philos. Trans. R. Soc. Lond. Ser. B 1984, 304, 479–508. [Google Scholar] [CrossRef]
- Harper, R.; Posner, A. Measurement of non-crystalline calcium phosphate in bone mineral. Exp. Biol. Med. 1966, 122, 137–142. [Google Scholar] [CrossRef]
- Termine, J.D.; Posner, A.S. Infra-red determination of percentage of crystallinity in apatitic calcium phosphates. Nature 1966, 211, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Termine, J.D.; Posner, A.S. Infrared analysis of rat bone: Age dependency of amorphous and crystalline mineral fractions. Science 1966, 153, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Termine, J.D.; Posner, A.S. Amorphous/crystalline interrelationships in bone mineral. Calcif. Tissue Res. 1967, 1, 8–23. [Google Scholar] [CrossRef] [PubMed]
- Posner, A.S.; Blumenthal, N.C.; Boskey, A.L.; Betts, F. Synthetic analogue of bone-mineral formation. J. Dent. Res. 1975, 54, B88–B93. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.G.; Featherstone, J.D.; Duncan, J.F.; Cutress, T.W. Paracrystalline disorder of biological and synthetic carbonate-substituted apatites. J. Dent. Res. 1982, 61, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Brecevic, L.J.; Furedi-Milhofer, H. Precipitation of calcium phosphates from electrolyte solutions. II. The formation and transformation of precipitates. Calcif. Tissue Res. 1972, 10, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Pathways to biomineralization and biodemineralization of calcium phosphates: The thermodynamic and kinetic controls. Dalton Trans. 2009, 21, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Feenstra, T.P.; de Bruyn, P.L. Formation of calcium phosphates in moderately supersaturated solutions. J. Phys. Chem. 1979, 83, 475–479. [Google Scholar] [CrossRef]
- Eanes, E.D. Amorphous calcium phosphate: Thermodynamic and kinetic considerations. In Calcium Phosphates in Biological and Industrial Systems; Dordrecht, A.Z., Ed.; Kluwer Academic: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Tadic, D.; Peters, F.; Epple, M. Continuous synthesis of amorphous carbonated apatites. Biomaterials 2002, 23, 2553–2559. [Google Scholar] [CrossRef]
- Nagano, M.; Nakamura, T.; Kokubo, T.; Tanahashi, M.; Ogawa, M. Differences of bone bonding ability and degradation behaviour in vivo between amorphous calcium phosphate and highly crystalline hydroxyapatite coating. Biomaterials 1996, 17, 1771–1777. [Google Scholar] [CrossRef]
- Christoffersen, J.; Christoffersen, M.R.; Kibalczyc, W.; Andersen, F.A. A contribution to the understanding of the formation of calcium phosphates. J. Cryst. Growth 1989, 94, 767–777. [Google Scholar] [CrossRef]
- Feenstra, T.P.; de Bruyn, P.L. The Ostwald rule of stages in precipitation from highly supersaturated solutions: A model and its application to the formation of the nonstoichiometric amorphous calcium phosphate precursor phase. J. Colloid Interface Sci. 1981, 84, 66–72. [Google Scholar] [CrossRef]
- Tung, M.S.; Brown, W.E. An intermediate state in hydrolysis of amorphous calcium phosphate. Calcif. Tissue Int. 1983, 3, 783–790. [Google Scholar] [CrossRef]
- Watson, M.L.; Robinson, R.A. Collagen-crystal relationships in bone. II. Electron microscope study of basic calcium phosphate crystals. Am. J. Anat. 1953, 93, 25–59. [Google Scholar] [CrossRef] [PubMed]
- Eanes, E.D.; Termine, J.D.; Nylen, M.U. An electron microscopic study of the formation of amorphous calcium phosphate and its transformation to crystalline apatite. Calcif. Tissue Res. 1973, 12, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Zyman, Z.Z.; Rokhmistrov, D.V.; Glushko, V.I. Structural and compositional features of amorphous calcium phosphate at the early stage of precipitation. J. Mater. Sci. Mater. Med. 2010, 21, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Amorphous calcium (ortho)phosphates. Acta Biomater. 2010, 6, 4457–4475. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Conversion of amorphous calcium phosphate to microcrystalline hydroxyapatite. A pH-dependent, solution-mediated, solid-solid conversion. J. Phys. Chem. 1973, 77, 2313–2317. [Google Scholar] [CrossRef]
- Bar-Yosef, O.P.; Govrin-Lippman, R.; Garti, N.; Furedi-Milhofer, H. The influence of polyelectrolytes on the formation and phase transformation of amorphous calcium phosphate. Cryst. Growth Des. 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Tao, J.; Pan, H.; Zeng, Y.; Xu, R.; Tang, R. Roles of amorphous calcium phosphate and biological additives in the assembly of hydroxyapatite nanoparticles. J. Phys. Chem. B 2007, 111, 13410–13418. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liu, Y.; Sun, W.; Zhang, H. Amorphous calcium phosphate and its application in dentistry. Chem. Cent. J. 2011, 5, 40. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Vidavsky, N.; Weiner, S. Transient precursor amorphous phases in biomineralization. In the footsteps of Heinz A. Lowenstam. Z. Kristallogr. 2012, 227, 711–717. [Google Scholar] [CrossRef]
- He, G.; Dahl, T.; Veis, A.; George, A. Nucleation of apatite crystals in vitro by self-assembled dentin matrix protein 1. Nat. Mater. 2003, 2, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, T.; Onuma, K.; Yamamoto, A.; Iijima, M.; Shiba, K. Direct transformation from amorphous to crystalline calcium phosphate facilitated by motif programmed artificial proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 16866–16870. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Rey, C. Amorphous calcium phosphates: Synthesis, properties and uses in biomaterials. Acta Biomater. 2010, 6, 3362–3378. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.H.; Liu, X.Y.; Tang, R.K.; Xu, H.Y. Mystery of the transformation from amorphous calcium phosphate to hydroxyapatite. Chem. Commun. 2010, 46, 7415–7420. [Google Scholar] [CrossRef] [PubMed]
- Eanes, E.D.; Meyer, J.L. The maturation of crystalline calcium phosphates in aqueous suspensions at physiologic pH. Calcif. Tissue Res. 1977, 23, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Amjad, Z. Influence of polyelectrolytes on the precipitation of amorphous calcium phosphate. Colloids Surf. 1990, 48, 95–106. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Eanes, E.D. Effect of phospholipids on the transformation of amorphous calcium phosphate to hydroxyapatite in vitro. Calcif. Tissue Int. 1975, 19, 197–210. [Google Scholar] [CrossRef]
- Termine, J.D.; Eanes, E.D.; Conn, K.M. Phosphoprotein modulation of apatite crystallization. Calcif. Tissue Int. 1980, 31, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.M.; Wen, G.; Hirakawa, N.; Soloway, R.D.; Hong, N.K.; Crowther, R.S. Glycochenodeoxycholic acid inhibits calcium phosphate precipitation in vitro by preventing the transformation of amorphous calcium phosphate to calcium hydroxyapatite. J. Clin. Investig. 1991, 88, 1265–1271. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ryu, H.S.; Shin, H.; Jung, H.S.; Hong, K.S. In situ observation of hydroxyapatite nanocrystal formation from amorphous calcium phosphate in calcium-rich solutions. Mater. Chem. Phys. 2005, 91, 500–506. [Google Scholar] [CrossRef]
- Xin, R.; Leng, Y.; Wang, N. In situ TEM examinations of octacalcium phosphate to hydroxyapatite transformation. J. Cryst. Growth 2006, 289, 339–344. [Google Scholar] [CrossRef]
- Brown, W.E.; Smith, J.P.; Lehr, J.R.; Frazier, A.W. Octacalcium phosphate and hydroxyapatite: Crystallographic and chemical relations between octacalcium phosphate and hydroxyapatite. Nature 1962, 196, 1050–1055. [Google Scholar] [CrossRef]
- Mathew, M.; Takagi, S. Structures of biological minerals in dental research. J. Res. Natl. Inst. Stand. Technol. 2001, 106, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Tohda, H.; Yamada, M.; Yamaguchi, Y.; Yanagisawa, T. High-resolution electron microscopical observations of initial enamel crystals. J. Electron Microsc. 1997, 46, 97–101. [Google Scholar] [CrossRef]
- Crane, N.J.; Popescu, V.; Morris, M.D.; Steenhuis, P.; Ignelzi, M.A. Raman spectroscopic evidence for octacalcium phosphate and other mineral species deposited during intramembranous mineralization. Bone 2006, 39, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.; Chow, L. Chemical properties of bone mineral. Ann. Rev. Mater. Sci. 1976, 6, 213–236. [Google Scholar] [CrossRef]
- Glimcher, M.J.; Bonar, L.C.; Grynpas, M.D.; Landis, W.J.; Roufosse, A.H. Recent studies of bone mineral: Is the amorphous calcium phosphate theory valid? J. Cryst. Growth 1981, 53, 100–119. [Google Scholar] [CrossRef]
- Suvorova, E.I.; Buffat, P.A. Electron diffraction and high resolution transmission electron microscopy in the characterization of calcium phosphate precipitation from aqueous solutions under biomineralization conditions. Eur. Cells Mater. 2001, 1, 27–42. [Google Scholar] [CrossRef]
- Johnsson, M.S.A.; Nancollas, G.H. The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral Biol. Med. 1992, 3, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Brown, W.E.; Eidelman, N.; Tomazic, B. Octacalcium phosphates as a precursor in biomineral formation. Adv. Dent. Res. 1987, 1, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Kamakura, S.; Katagiri, T. Surface chemistry and biological responses to synthetic octacalcium phosphate. J. Biomed. Mater. Res. B 2006, 77, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Muragalelli, M.J.; Narusawa, H.; Shimada, T.; Iijima, M.; Aoba, T. Effects of fluoride on precipitation and hydrolysis of octacalcium phosphate in an experimental-model simulating enamel mineralization during amelogenesis. Cells Mater. 1992, 2, 221–230. [Google Scholar]
- Eliaz, N. Electrocrystallization of calcium phosphates. Isr. J. Chem. 2008, 48, 159–168. [Google Scholar] [CrossRef]
- Eliaz, N.; Sridhar, T.M. Electrocrystallization of hydroxyapatite and its dependence on solution conditions. Cryst. Growth Des. 2008, 8, 3965–3977. [Google Scholar] [CrossRef]
- Eliaz, N.; Kopelovitch, W.; Burstein, L.; Kobayashi, E.; Hanawa, T. Electrochemical processes of nucleation and growth of calcium phosphate on titanium supported by real-time quartz crystal microbalance measurements and X-ray photoelectron spectroscopy analysis. J. Biomed. Mater. Res. A 2009, 89, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Chusuei, C.C.; Goodman, D.W.; Van Stipdonk, M.J.; Justes, D.R.; Schweikert, E.A. Calcium phosphate phase identification using XPS and time-of-flight cluster SIMS. Anal. Chem. 1999, 71, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.B.; Campbell, C.T.; Graham, D.J.; Ratner, B.D. Surface characterization of hydroxyapatite and related calcium phosphates by XPS and TOF-SIMS. Anal. Chem. 2000, 72, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Ostwald, W. The formation and changes of solids. Z. Phys. Chem. 1897, 22, 289–302. (In German) [Google Scholar]
- Wang, H.; Eliaz, N.; Xiang, Z.; Hsu, H.P.; Spector, M.; Hobbs, L.W. Early bone apposition in vivo on plasma-sprayed and electrochemically deposited hydroxyapatite coatings on titanium alloy. Biomaterials 2006, 27, 4192–4203. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.-Y.; Wang, X.-X. Electrodeposition of hydroxyapatite coating on CoNiCrMo substrate in dilute solution. Surf. Coat. Technol. 2010, 204, 3205–3213. [Google Scholar] [CrossRef]
- Metoki, N.; Leifenberg-Kuznits, L.; Kopelovich, W.; Burstein, L.; Gozin, M.; Eliaz, N. Hydroxyapatite coatings electrodeposited at near-physiological conditions. Mater. Lett. 2014, 119, 24–27. [Google Scholar] [CrossRef]
- Metoki, N.; Rosa, C.M.R.; Zanin, H.; Marciano, F.R.; Eliaz, N.; Lobo, A.O. Electrodeposition and biomineralization of nano-β-tricalcium phosphate on graphenated carbon nanotubes. Surf. Coat. Technol. 2016, 297, 51–57. [Google Scholar] [CrossRef]
- Metoki, N.; Sadman, K.; Shull, K.; Mandler, D.; Eliaz, N. Electro-assisted deposition of calcium phosphate on self-assembled monolayers. Electrochim. Acta 2016, 206, 400–408. [Google Scholar] [CrossRef]
- Van der Houwen, J.A.M.; Valsami-Jones, E. The application of calcium phosphate precipitation chemistry to phosphorus recovery: The influence of organic ligands. Environ. Technol. 2001, 22, 1325–1335. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hahn, H.H.; Hoffmann, E. Effects of solution conditions on the precipitation of phosphate for recovery: A thermodynamic evaluation. Chemosphere 2002, 48, 1029–1034. [Google Scholar] [CrossRef]
- Plant, L.J.; House, W.A. Precipitation of calcite in the presence of inorganic phosphate. Colloids Surf. A Physicochem. Eng. Asp. 2002, 203, 143–153. [Google Scholar] [CrossRef]
- Song, Y.; Weidler, P.G.; Berg, U.; Nüesch, R.; Donnert, D. Calcite-seeded crystallization of calcium phosphate for phosphorus recovery. Chemosphere 2006, 63, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, X.; Chen, Y.; Xie, Q.; Lan, J.; Qian, M.; He, N. A comparative study on the dissolution and solubility of hydroxylapatite and fluorapatite at 25 °C and 45 °C. Chem. Geol. 2009, 268, 89–96. [Google Scholar] [CrossRef]
- Mañas, A.; Pocquet, M.; Biscans, B.; Sperandio, M. Parameters influencing calcium phosphate precipitation in granular sludge sequencing batch reactor. Chem. Eng. Sci. 2012, 77, 165–175. [Google Scholar] [CrossRef]
- Song, Y.; Qian, F.; Gao, Y.; Xiang, L.; He, M. Thermodynamic assessment of effects of solution conditions on precipitation and recovery of phosphorus from wastewater. Environ. Eng. Sci. 2015, 32, 574–581. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Biodegradation and bioresorption of calcium phosphate ceramics. Clin. Mater. 1993, 14, 65–88. [Google Scholar] [CrossRef]
- Habibovic, P.; Li, J.; van der Valk, C.M.; Meijer, G.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biological performance of uncoated and octacalcium phosphate-coated Ti6Al4V. Biomaterials 2005, 26, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Tfelt-Hansen, J.; Brown, E.M. The calcium-sensing receptor in normal physiology and pathophysiology: A review. Crit. Rev. Clin. Lab. Sci. 2005, 42, 35–70. [Google Scholar] [CrossRef] [PubMed]
- Hofer, A.M. Another dimension to calcium signaling: A look at extracellular calcium. J. Cell Sci. 2005, 118, 855–862. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.E.; Hobbs, L.W.; Rosen, V.B.; Spector, M. The ultrastructure of the plasma-sprayed hydroxyapatite-bone interface predisposing to bone bonding. Biomaterials 2002, 23, 725–733. [Google Scholar] [CrossRef]
- Daculsi, G.; LeGeros, R.Z.; Heughebaert, M.; Barbieux, I. Formation of carbonate-apatite crystals after implantation of calcium-phosphate ceramics. Calcif. Tissue Int. 1990, 46, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; LeGeros, R.Z.; Nery, E.; Lynch, K.; Kerebel, B. Transformation of biphasic calcium phosphate ceramics in vivo: Ultrastructural and physicochemical characterization. J. Biomed. Mater. Res. 1989, 23, 883–894. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; de Groot, K.; Chen, W.; Li, Y.; Zhang, X. Osseous substance formation induced in porous calcium phosphate ceramics in soft tissues. Biomaterials 1994, 15, 31–34. [Google Scholar] [CrossRef]
- Surmenev, R.A.; Surmeneva, M.A.; Ivanova, A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis—A review. Acta Biomater. 2014, 10, 557–579. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U. Osteoinduction in porous hydroxyapatite implanted in heterotopic sites of different animal models. Biomaterials 1996, 17, 31–35. [Google Scholar] [CrossRef]
- Yang, Z.; Yuan, H.; Tong, W.; Zou, P.; Chen, W.; Zhang, X. Osteogenesis in extraskeletally implanted porous calcium phosphate ceramics: Variability among different kinds of animals. Biomaterials 1996, 17, 2131–2137. [Google Scholar] [PubMed]
- Yuan, H.; Yang, Z.; Li, Y.; Zhang, X.; de Bruijn, J.D.; de Groot, K. Osteoinduction by calcium phosphate biomaterials. J. Mater. Sci. Mater. Med. 1998, 9, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Yang, Z.; de Bruijn, J.D.; de Groot, K.; Zhang, X. Material-dependent bone induction by calcium phosphate ceramics: A 2.5-year study in dog. Biomaterials 2001, 22, 2617–2623. [Google Scholar] [CrossRef]
- Yuan, H.; van den Doel, M.; Li, S.; van Blitterswijk, C.A.; de Groot, K.; de Bruijn, J.D. A comparison of the osteoinductive potential of two calcium phosphate ceramics implanted intramuscularly in goats. J. Mater. Sci. Mater. Med. 2002, 13, 1271–1275. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurent, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.M.; et al. Water-mediated structuring of bone apatite. Nat. Mater. 2013, 12, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Dissolution mechanism of calcium apatites in acids: A review of literature. World J. Methodol. 2012, 2, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ducheyne, P.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef]
- Schwartz, Z.; Boyan, B.D. Underlying mechanisms at the bone-biomaterial interface. J. Cell. Biochem. 1994, 56, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Puleo, D.A.; Nanci, A. Understanding and controlling the bone-implant interface. Biomaterials 1999, 20, 2311–2321. [Google Scholar] [CrossRef]
- Rahbek, O.; Overgaard, S.; Jensen, T.B.; Bendix, K.; Soballe, K. Sealing effect of hydroxyapatite coating: A 12-month study in canines. Acta Orthop. Scand. 2000, 71, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.W.; Hu, N.; Zorn, C.M.; McAfee, P.C. Bioactive titanium calcium phosphate coating for disc arthroplasty: Analysis of 58 vertebral end plates after 6- to 12-month implantation. Spine J. 2009, 9, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Surmenev, R.A. A review of plasma-assisted methods for calcium phosphate-based coatings fabrication. Surf. Coat. Technol. 2012, 206, 2035–2056. [Google Scholar] [CrossRef]
- Xu, S.; Long, J.; Sim, L.; Diong, C.H.; Ostrikov, K. RF plasma sputtering deposition of hydroxyapatite bioceramics: Synthesis, performance, and biocompatibility. Plasma Process. Polym. 2005, 2, 373–390. [Google Scholar] [CrossRef]
- Tomsia, A.P.; Launey, M.E.; Lee, J.S.; Mankani, M.H.; Wegst, U.G.K.; Saiz, E. Nanotechnology approaches for better dental implants. Int. J. Oral Maxillofac. Implants 2011, 26, 25–49. [Google Scholar]
- Mouriño, V.; Boccaccini, A.R. Bone tissue engineering therapeutics: Controlled drug delivery in three-dimensional scaffolds. J. R. Soc. Interface 2010, 7, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Ambard, A.J.; Mueninghoff, L. Calcium phosphate cement: Review of mechanical and biological properties. J. Prosthodont. 2006, 15, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Black, J. Biological Performance of Materials: Fundamentals of Biocompatibility; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Samavedi, S.; Whittington, A.R.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates. J. Mater. Sci. 2007, 42, 1061–1095. [Google Scholar] [CrossRef]
- Jimbo, R.; Ivarsson, M.; Koskela, A.; Sul, Y.T.; Johansson, C.B. Protein adsorption to surface chemistry and crystal structure modification of titanium surfaces. J. Oral Maxillofac. Res. 2010, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- Aronov, D.; Rosen, R.; Ron, E.Z.; Rosenman, G. Tunable hydroxyapatite wettability: Effect on adhesion of biological molecules. Proc. Biochem. 2006, 41, 2367–2372. [Google Scholar] [CrossRef]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Kilpadi, D.V.; Lemons, L.E. Surface energy characterization of unalloyed titanium implants. J. Biomed. Mater. Res. 1994, 28, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Nygren, H.; Ohlson, K. Implantation of hydrophilic and hydrophobic titanium discs in rat tibia: Cellular reactions on the surfaces during the first 3 weeks in bone. Biomaterials 2004, 25, 4759–4766. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Liu, X.M.; Vogler, E.A.; Donahue, H.J. Systematic variation in osteoblast adhesion and phenotype with substratum surface characteristics. J. Biomed. Mater. Res. A 2004, 68, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Eliaz, N.; Ritman-Hertz, O.; Aronov, D.; Weinberg, E.; Shenhar, Y.; Rosenman, G.; Weinreb, M.; Ron, E. The effect of surface treatments on the adhesion of electrochemically deposited hydroxyapatite coating to titanium and on its interaction with cells and bacteria. J. Mater. Sci. Mater. Med. 2011, 22, 1741–1752. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Shmueli, S.; Shur, I.; Benayahu, D.; Aronov, D.; Rosenman, G. The effect of surface treatment on the surface texture and contact angle of electrochemically deposited hydroxyapatite coating and on its interaction with bone-forming cells. Acta Biomater. 2009, 5, 3178–3191. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate cements and concretes. Materials 2009, 2, 221–291. [Google Scholar] [CrossRef]
- Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Vicat Needle; ASTM C191-13; ASTM International: West Conshohocken, PA, USA, 2013.
- Standard Test Method for Time of Setting of Hydraulic-Cement Paste by Gillmore Needles; ASTM C266-15; ASTM International: West Conshohocken, PA, USA, 2015.
- De Groot, K. Clinical applications of calcium phosphate biomaterials: A review. Ceram. Int. 1993, 19, 363–366. [Google Scholar] [CrossRef]
- Kreidler, E.R.; Hummel, F.A. Phase relations in the system SrO-P2O5 and the influence of water vapor on the formation of Sr4P2O9. Inorg. Chem. 1967, 6, 884–891. [Google Scholar] [CrossRef]
- De Groot, K. Effect of porosity and physicochemical properties on the stability, resorption, and strength of calcium phosphate ceramics, in bioceramics: Material characteristics versus in-vivo behavior. Ann. N. Y. Acad. Sci. 1988, 523, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Manjubala, I.; Sastry, T.P.; Suresh Kumar, R.V. Bone in-growth induced by biphasic calcium phosphate ceramic in femoral defect of dogs. J. Biomater. Appl. 2005, 19, 341–360. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.J.; Tadjiev, T.R.; Kim, J.W.; You, C.K.; Choi, S.K.; Park, K.B.; Ryoo, K.H.; Kim, S.Y. Comparative study of the degradation behavior of mechanically mixed and chemically precipitated biphasic calcium phosphates. Key Eng. Mater. 2006, 309–311, 227–230. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Z. Synthesis of biphasic ceramics of hydroxyapatite and β-tricalcium phosphate with controlled phase content and porosity. J. Mater. Chem. 1998, 8, 2237–2244. [Google Scholar] [CrossRef]
- Lin, F.; Liao, C.; Chen, K.; Sun, J.; Lin, C. Preparation of βTCP/HAP biphasic ceramics with natural bone structure by heating bovine cancellous bone with the addition of (NH4)2HPO4. J. Biomed. Mater. Res. A 2000, 51, 157–163. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Grossin, D. Bioactive ceramics: Physical chemistry. In Comprehensive Biomaterials; Ducheyne, P., Healy, K., Hutmacher, D., Grainger, D.E., Kirkpatrick, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 187–221. [Google Scholar]
- Bermúdez, O.; Boltong, M.G.; Driessens, F.C.M.; Planell, J.A. Optimization of a calcium orthophosphate cement formulation occurring in the combination of monocalcium phosphate monohydrate with calcium oxide. J. Mater. Sci. Mater. Med. 1994, 5, 67–71. [Google Scholar] [CrossRef]
- Huan, Z.; Chang, J. Novel bioactive composite bone cements based on the β-tricalcium phosphate-Monocalcium phosphate monohydrate composite cement system. Acta Biomater. 2009, 5, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013, 24, 1335–1363. [Google Scholar] [CrossRef] [PubMed]
- Van Wazer, J.R. Phosphorus and Its Compounds; Interscience: New York, NY, USA, 1958. [Google Scholar]
- Chow, L.C.; Takagi, S.; Shern, R.J.; Chow, T.H.; Takagi, K.K.; Sieck, B.A. Effects on whole saliva of chewing gums containing calcium phosphates. J. Dent. Res. 1994, 73, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphates in nature, biology and medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef]
- Van de Watering, F.C.J.; van den Beucken, J.J.J.P.; Felix Lanao, R.P.; Wolke, J.G.C.; Jansen, J.A. Biodegradation of calcium phosphate cement composites. In Degradation of Implant Materials; Eliaz, N., Ed.; Springer Science + Business Media: New York, NY, USA, 2012; Chapter 7; pp. 139–172. [Google Scholar]
- Constantz, B.; Barr, B.; Ison, I. Histological, chemical, and crystallographic analysis of four calcium phosphate cements in different rabbit osseous sites. J. Biomed. Mater. Res. 1998, 43, 451–461. [Google Scholar] [CrossRef]
- O’Neill, W.C. The fallacy of the calcium-phosphorus product. Kidney Int. 2007, 72, 792–796. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Formation and transformation of calcium phosphates: Relevance to vascular calcification. Z. Kardiol. 2001, 90 (Suppl. 3), III116–III125. [Google Scholar] [CrossRef]
- Bermúdez, O.; Boltong, M.G.; Driessens, F.C.M.; Planell, J.A. Development of some calcium phosphate cements from combinations of α-TCP, MCPM and CaO. J. Mater. Sci. Mater. Med. 1994, 5, 160–163. [Google Scholar] [CrossRef]
- Kurashina, K.; Kurita, H.; Hirano, M.; Kotani, A.; Klein, C.P.; de Groot, K. In vivo study of calcium phosphate cements: Implantation of an α-tricalcium phosphate/dicalcium phosphate dibasic/tetracalcium phosphate monoxide cement paste. Biomaterials 1997, 18, 539–543. [Google Scholar] [CrossRef]
- Driessens, F.C.M.; Planell, J.A.; Boltong, M.G.; Khairoun, I.; Ginebra, M.P. Osteotransductive bone cements. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Chow, L.C.; Ishikawa, K. Formation of hydroxyapatite in new calcium phosphate cements. Biomaterials 1998, 19, 1593–1599. [Google Scholar] [CrossRef]
- Yamamoto, H.; Niwa, S.; Hori, M.; Hattori, T.; Sawai, K.; Aoki, S.; Hirano, M.; Takeuchi, H. Mechanical strength of calcium phosphate cement in vivo and in vitro. Biomaterials 1998, 19, 1587–1591. [Google Scholar] [CrossRef]
- Crall, J.J.; Bjerga, J.M. Effects of DCPD/APF application and prolonged exposure to fluoride on caries-like lesion formation in vitro. J. Oral Pathol. Med. 1987, 16, 488–491. [Google Scholar] [CrossRef]
- Wefel, J.S.; Harless, J.D. The use of saturated DCPD in remineralization of artificial caries lesions in vitro. J. Dent. Res. 1987, 66, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Hoppenbrouwers, P.M.; Groenendijk, E.; Tewarie, N.R.; Driessens, F.C.M. Improvement of the caries resistance of human dental roots by a two-step conversion of the root mineral into fluoridated hydroxylapatite. J. Dent. Res. 1988, 67, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.J.; Charig, A.; Blake-Haskins, J.; Zhang, Y.P.; Miller, S.M.; Strannick, M.; Gaffar, A.; Margolis, H.C. In vivo detection of calcium from dicalcium phosphate dihydrate dentifrices in demineralized human enamel and plaque. Adv. Dent. Res. 1997, 11, 380–387. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Variations in the crystalline components of human dental calculus: I. crystallographic and spectroscopic methods of analysis. J. Dent. Res. 1974, 53, 45–50. [Google Scholar]
- Schroeder, H. Formation and inhibition of dental calculus. J. Periodontol. 1969, 40, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.C. Octacalcium Phosphate; Eanes, E.D., Ed.; Karger: Basel, Switzerland, 2001; p. 167. [Google Scholar]
- Brown, W.E. Octacalcium phosphate and hydroxyapatite: Crystal structure of octacalcium phosphate. Nature 1962, 196, 1048–1050. [Google Scholar] [CrossRef]
- Brown, W.E. Crystal growth of bone mineral. Clin. Orthop. Relat. Res. 1966, 44, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.G.A.; Wood, G.J.; Barry, J.C.; Featherstone, J.D.B. The structure of (100) defects in carbonated apatite crystallites: A high-resolution electron microscope study. Ultramicroscopy 1986, 19, 253–265. [Google Scholar] [CrossRef]
- Iijima, M.; Nelson, D.G.A.; Pan, Y.; Kreinbrink, A.T.; Adachi, M.; Goto, T.; Moriwaki, Y. Fluoride analysis of apatite crystals with a central planar OCP inclusion: Concerning the role of F- ions on apatite/OCP/apatite structure formation. Calcif. Tissue Int. 1996, 59, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Bodier-Houllé, P.; Steuer, P.; Voegel, J.C.; Cuisinier, F.J.G. First experimental evidence for human dentine crystal formation involving conversion of octacalcium phosphate to hydroxyapatite. Acta Crystallogr. Sect. D Biol. Crystallogr. 1998, 54, 1377–1381. [Google Scholar] [CrossRef]
- Aoba, T.; Komatsu, H.; Shimazu, Y.; Yagishita, H.; Taya, Y. Enamel mineralization and an initial crystalline phase. Connect. Tissue Res. 1998, 38, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Tomazic, B.B.; Brown, W.E.; Shoen, F.J. Physicochemical properties of calcific deposits isolated from porcine bioprosthetic heart valves removed from patients following 2–13 years function. J. Biomed. Mater. Res. 1994, 28, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Nancollas, G.H.; Wu, W. Biomineralization mechanisms: A kinetics and interfacial energy approach. J. Cryst. Growth 2000, 211, 137–142. [Google Scholar] [CrossRef]
- Kamakura, S.; Sasano, Y.; Homma, H.; Suzuki, O.; Kagayama, M.; Motegi, K. Implantation of octacalcium phosphate (OCP) in rat skull defects enhances bone repair. J. Dent. Res. 1999, 78, 1682–1687. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaei-Aval, F.; Sobhani, A.; Arab, M.R.; Sarani, S.A.; Heydari, M.H. The efficacy of implant of octacalcium phosphate in combination with bone matrix gelatin (BMG) on bone regeneration in skull defects in rat. Iran. J. Med. Sci. 2004, 29, 124–129. [Google Scholar]
- Suzuki, O.; Kamakura, S.; Katagiri, T.; Nakamura, M.; Zhao, B.; Honda, Y.; Kamijo, R. Bone formation enhanced by implanted octacalcium phosphate involving conversion into Ca-deficient hydroxyapatite. Biomaterials 2006, 27, 2671–2681. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, O.; Imaizumi, H.; Kamakura, S.; Katagiri, T. Bone regeneration by synthetic octacalcium phosphate and its role in biological mineralization. Curr. Med. Chem. 2008, 15, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Kikawa, T.; Kashimoto, O.; Imaizumi, H.; Kokubun, S.; Suzuki, O. Intramembranous bone tissue response to biodegradable octacalcium phosphate implant. Acta Biomater. 2009, 5, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Stefanic, M.; Krnel, K.; Pribosic, I.; Kosmac, T. Rapid biomimetic deposition of octacalcium phosphate coatings on zirconia ceramics (Y-TZP) for dental implant applications. Appl. Surf. Sci. 2012, 258, 4649–4656. [Google Scholar] [CrossRef]
- Habibovic, P.; van der Valk, C.M.; van Blitterswijk, C.A.; de Groot, K.; Meijer, G. Influence of octacalcium phosphate coating on osteoinductive properties of biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Matsui, K.; Kawai, T.; Kato, Y.; Matsui, A.; Suzuki, O.; Kamakura, S.; Echigo, S. Octacalcium phosphate collagen composites with titanium mesh facilitate alveolar augmentation in canine mandibular bone defects. Int. J. Oral Maxillofac. Surg. 2012, 41, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.; Yamashita, Y.; Nakano, Y.; Ohgaki, M.; Nakamura, S.; Yamashita, K.; Takagi, Y. Octacalcium phosphate-based cement as a pulp-capping agent in rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 749–755. [Google Scholar] [CrossRef] [PubMed]
- Kamakura, S.; Sasano, Y.; Nakamura, M.; Suzuki, O.; Ohki, H.; Kagayama, M.; Motegi, K. Initiation of alveolar ridge augmentation in the rat mandible by subperiosteal implantation of octacalcium phosphate. Arch. Oral Biol. 1996, 41, 1029–1038. [Google Scholar] [CrossRef]
- Kamakura, S.; Sasano, Y.; Homma, H.; Suzuki, O.; Kagayama, M.; Motegi, K. Experimental oral pathology: Implantation of octacalcium phosphate nucleates isolated bone formation in rat skull defects. Oral Dis. 2001, 7, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Nancollas, G.H. Calcium orthophosphates: Crystallization and dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Jiménez, M.J.; García-García, R.; Reyes-Gasga, J. Synthesis and hydrolysis of octacalcium phosphate and its characterization by electron microscopy and X-ray diffraction. J. Phys. Chem. Solids 2009, 70, 390–395. [Google Scholar] [CrossRef]
- Suzuki, O. Octacalcium phosphate: Osteoconductivity and crystal chemistry. Acta Biomater. 2010, 6, 3379–3387. [Google Scholar] [CrossRef] [PubMed]
- Heughebaert, J.C.; Zawacki, S.; Nancollas, G. The growth of octacalcium phosphate on beta tricalcium phosphate. J. Cryst. Growth 1983, 63, 83–90. [Google Scholar] [CrossRef]
- Yin, X.; Stott, M.J.; Rubio, A. α- and β-tricalcium phosphate: A density functional study. Phys. Rev. B 2003, 68, 205205. [Google Scholar] [CrossRef]
- Ten Huisen, K.S.; Brown, P.W. Formation of calcium-deficient hydroxyapatite from alpha-tricalcium phosphate. Biomaterials 1998, 19, 2209–2217. [Google Scholar] [CrossRef]
- Durucan, C.; Brown, P.W. alpha-Tricalcium phosphate hydrolysis to hydroxyapatite at and near physiological temperature. J. Mater. Sci. Mater. Med. 2000, 11, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Schroeder, L.W.; Dickens, B.; Brown, W.E. The crystal structure of α-Ca3(PO4)2. Acta Crystallogr. B 1977, 33, 1325–1333. [Google Scholar] [CrossRef]
- Yin, X.; Stott, M.J. Surface and adsorption properties of alpha-tricalcium phosphate. J. Chem. Phys. 2006, 124, 124701. [Google Scholar] [CrossRef] [PubMed]
- Dickens, B.; Schroeder, L.W.; Brown, W.E. Crystallographic studies of the role of Mg as a stabilizing impurity in β-Ca3(PO4)2, The crystal structure of pure β-Ca3(PO4)2. J. Solid State Chem. 1974, 10, 232–248. [Google Scholar] [CrossRef]
- De Aza, P.N.; Guitian, F.; Santos, C.; de Aza, S.; Cusco, R.; Artus, L. Vibrational properties of calcium phosphate compounds. 2. Comparison between hydroxyapatite and β-tricalcium phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Cheng, P.T.; Grabher, J.; LeGeros, R.Z. Effects of magnesium on calcium phosphate formation. Magnesium 1988, 7, 123–132. [Google Scholar] [PubMed]
- Tao, J.; Pan, H.; Zhai, H.; Wang, J.; Li, L.; Wu, J.; Jiang, W.; Xu, X.; Tang, R. Controls of tricalcium phosphate single-crystal formation from its amorphous precursor by interfacial energy. Cryst. Growth Des. 2009, 9, 3154–3160. [Google Scholar] [CrossRef]
- Hou, X.J.; Mao, K.Y.; Chen, D.F. Bone formation performance of beta-tricalcium phosphate sintered bone. J. Clin. Rehabil. Tissue. Eng. Res. 2008, 12, 9627–9630. [Google Scholar]
- Ohura, K.; Bohner, M.; Hardouin, P.; Lemaître, J.; Pasquier, G.; Flautre, B. Resorption of, and bone formation from, new beta-tricalcium phosphate-monocalcium phosphate cements: An in vivo study. J. Biomed. Mater. Res. 1996, 30, 193–200. [Google Scholar] [CrossRef]
- Mirtchi, A.A.; Lemaître, J.; Munting, E. Calcium phosphate cements: Study of the beta-tricalcium phosphate–dicalcium phosphate–calcite cements. Biomaterials 1990, 11, 83–88. [Google Scholar] [CrossRef]
- Metsger, D.S.; Driskell, T.D.; Paulsrud, J.R. Tricalcium phosphate ceramic—A resorbable bone implant: Review and current status. J. Am. Dent. Assoc. 1982, 105, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Epstein, N.E. Beta tricalcium phosphate: Observation of use in 100 posterolateral lumbar instrumented fusions. Spine J. 2009, 9, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Shayegan, A.; Petein, M.; Vanden Abbeele, A. The use of beta-tricalcium phosphate, white MTA, white Portland cement and calcium hydroxide for direct pulp capping of primary pig teeth. Dent. Traumatol. 2009, 25, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Weiner, M.L.; Salminen, W.F.; Larson, P.R.; Barter, R.A.; Kranetz, J.L.; Simon, G.S. Toxicological review of inorganic phosphates. Food Chem. Toxicol. 2001, 39, 759–786. [Google Scholar] [CrossRef]
- Jungbauer, A.; Hahn, R.; Deinhofer, K.; Luo, P. Performance and characterization of a nanophased porous hydroxyapatite for protein chromatography. Biotechnol. Bioeng. 2004, 87, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Van Velthuizen, J. Giant fluorapatite crystals: A question of locality. Miner. Rec. 1992, 23, 459–463. [Google Scholar]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Improved properties of amorphous calcium phosphate fillers in remineralizing resin composites. Dent. Mater. 1996, 12, 295–301. [Google Scholar] [CrossRef]
- Skrtic, D.; Hailer, A.W.; Takagi, S.; Antonucci, J.M.; Eanes, E.D. Quantitative assessment of the efficacy of amorphous calcium phosphate/methacrylate composites in remineralizing caries-like lesions artificially produced in bovine enamel. J. Dent. Res. 1996, 75, 1679–1686. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Effect of the monomer and filler system on the remineralizing potential of bioactive dental composites based on amorphous calcium phosphate. Polym. Adv. Technol. 2001, 12, 369–379. [Google Scholar] [CrossRef]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D. Amorphous calcium phosphate-based bioactive polymeric composites for mineralized tissue regeneration. J. Res. Natl. Inst. Stand. Technol. 2003, 108, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, D.; Antonucci, J.M.; Eanes, E.D.; Eidelman, N. Dental composites based on hybrid and surface-modified amorphous calcium phosphates. Biomaterials 2004, 25, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Skrtic, D.; Antonucci, J.M. Matrix resin effects on selected physicochemical properties of amorphous calcium phosphate composites. J. Bioact. Compat. Polym. 2005, 20, 29–49. [Google Scholar]
- Skrtic, D.; Antonucci, J.M. Dental composites based on amorphous calcium phosphate—Resin composition/physicochemical properties study. J. Biomater. Appl. 2007, 21, 375–393. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.N.R.; Schumacher, G.E.; Antonucci, J.M.; Skrtic, D. Adhesion of amorphous calcium phosphate composites bonded to dentin: A study in failure modality. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; O’Donnell, J.N.R.; Schumacher, G.E.; Skrtic, D. Amorphous calcium phosphate composites and their effect on composite–adhesive–dentin bonding. J. Adhes. Sci. Technol. 2009, 23, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Epple, M. Amorphous calcium phosphates as bone substitution materials. Eur. J. Trauma 2002, 28, 136–137. [Google Scholar]
- Yates, R.; Owens, J.; Jackson, R.; Newcombe, R.G.; Addy, M. A splitmouth placebo-controlled study to determine the effect of amorphous calcium phosphate in the treatment of dentine hypersensitivity. J. Clin. Periodontol. 1998, 25, 687–692. [Google Scholar] [CrossRef] [PubMed]
- Tung, M.S.; Eichmiller, F.C. Dental applications of amorphous calcium phosphates. J. Clin. Dent. 1999, 10, 1–6. [Google Scholar] [PubMed]
- Ambrosio, A.M.A.; Sahota, J.S.; Khan, Y.; Laurencin, CT. A novel amorphous calcium phosphate polymer ceramic for bone repair: I. Synthesis and characterization. J. Biomed. Mater. Res. 2001, 58, 295–301. [Google Scholar] [CrossRef]
- Dunn, W.J. Shear bond strength of an amorphous calcium-phosphate-containing orthodontic resin cement. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Berzins, D.W.; Bradley, T.G. Bond strength of an amorphous calcium phosphate-containing orthodontic adhesive. Angle Orthod. 2008, 78, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zhang, F.; Guo, J.; Wu, J.; Wu, W. Effects of amorphous calcium phosphate on periodontal ligament cell adhesion and proliferation in vitro. J. Med. Biol. Eng. 2008, 28, 107–112. [Google Scholar]
- Uysal, T.; Amasyali, M.; Koyuturk, A.E.; Sagdic, D. Efficiency of amorphous calcium phosphate-containing orthodontic composite and resin modified glass ionomer on demineralization evaluated by a new laser fluorescence device. Eur. J. Dent. 2009, 3, 127–134. [Google Scholar] [PubMed]
- Uysal, T.; Ulker, M.; Baysal, A.; Usumez, S. Microleakage between composite-wire and composite-enamel interfaces of flexible spiral wire retainers. Part 2: Comparison of amorphous calcium phosphate-containing adhesive with conventional lingual retainer composite. Eur. J. Orthod. 2009, 31, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Ulker, M.; Akdogan, G.; Ramoglu, S.I.; Yilmaz, E. Bond strength of amorphous calcium phosphate-containing orthodontic composite used as a lingual retainer adhesive. Angle Orthod. 2009, 79, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Ustdal, A.; Nur, M.; Catalbas, B. Bond strength of ceramic brackets bonded to enamel with amorphous calcium phosphate containing orthodontic composite. Eur. J. Orthod. 2010, 32, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Amasyali, M.; Ozcan, S.; Koyuturk, A.E.; Akyol, M.; Sagdic, D. In vivo effects of amorphous calcium phosphate-containing orthodontic composite on enamel demineralization around orthodontic brackets. Aust. Dent. J. 2010, 55, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Uysal, T.; Amasyali, M.; Koyuturk, A.E.; Ozcan, S.; Sagdic, D. Amorphous calcium phosphate-containing orthodontic composites. Do they prevent demineralisation around orthodontic brackets? Aust. Orthod. J. 2010, 26, 10–15. [Google Scholar] [PubMed]
- Xu, H.H.K.; Moreau, J.L.; Sun, L.; Chow, L.C. Nanocomposite containing amorphous calcium phosphate nanoparticles for caries inhibition. Dent. Mater. 2011, 27, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.; Walsh, D.; Fowler, C.E.; Mann, S. Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. CrystEngComm 2011, 13, 3692–3697. [Google Scholar] [CrossRef]
- Hamba, H.; Nikaido, T.; Inoue, G.; Sadr, A.; Tagami, J. Effects of CPP–ACP with sodium fluoride on inhibition of bovine enamel demineralization: A quantitative assessment using micro-computed tomography. J. Dent. 2011, 39, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Bar-Hillel, R.; Feuerstein, O.; Tickotsky, N.; Shapira, J.; Moskovitz, M. Effects of amorphous calcium phosphate stabilized by casein phosphopeptides on enamel de- and remineralization in primary teeth: An in vitro study. J. Dent. Child. 2012, 79, 9–14. [Google Scholar]
- Weir, M.D.; Chow, L.C.; Xu, H.H.K. Remineralization of demineralized enamel via calcium phosphate nanocomposite. J. Dent. Res. 2012, 91, 979–984. [Google Scholar] [CrossRef] [PubMed]
- Llena, C.; Forner, L.; Baca, P. Anticariogenicity of casein phosphopeptide–amorphous calcium phosphate: A review of the literature. J. Contemp. Dent. Pract. 2009, 10, 1–9. [Google Scholar] [PubMed]
- Giniger, M.; MacDonald, J.; Spaid, M.; Felix, H. A 180-day clinical investigation of the tooth whitening efficacy of a bleaching gel with added amorphous calcium phosphate. J. Clin. Dent. 2005, 16, 11–16. [Google Scholar] [PubMed]
- Reynolds, E.C.; Cai, F.; Cochrane, N.J.; Shen, P.; Walker, G.D.; Morgan, M.V.; Reynolds, C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J. Dent. Res. 2008, 87, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Yengopal, V.; Mickenautsch, S. Caries preventive effect of casein phosphopeptide-amorphous calcium phosphate (CPP-ACP): A meta-analysis. Acta Odontol. Scand. 2009, 67, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.L.; Chen, L.Y.; Zhang, D.; Zhang, P.L.; Liu, B.; Zhao, W.; Qi, H.Y. Effects of casein phosphopeptide-stabilized amorphous calcium phosphate solution on enamel remineralization. J. Clin. Rehabil. Tissue Eng. Res. 2009, 13, 4825–4828. [Google Scholar]
- Oreffo, R.O.C.; Driessens, F.C.M.; Planell, J.A.; Triffitt, J.T. Effects of novel calcium phosphate cements on human bone marrow fibroblastic cells. Tissue Eng. 1998, 4, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Hidaka, S. Studies on calcium-phosphate precipitation—Effects of metal-ions used in dental materials. J. Biomed. Mater. Res. 1994, 28, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G. Biphasic calcium phosphate concept applied to artificial bone, implant coating and injectable bone substitute. Biomaterials 1998, 19, 1473–1478. [Google Scholar] [CrossRef]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Lorenzo, L. Studies on calcium deficient apatites structure by means of MAS-NMR spectroscopy. J. Mater. Sci. Mater. Med. 2005, 16, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Rey, C.; Combes, C.; Drouet, C.; Sfihi, H. Chemical diversity of apatites. Adv. Sci. Technol. 2006, 49, 27–36. [Google Scholar] [CrossRef]
- Young, R.A.; Holcomb, D.W. Variability of hydroxyapatite preparations. Calcif. Tissue Int. 1981, 34, S17–S32. [Google Scholar]
- Blumenthal, N.C.; Betts, F.; Posner, A.S. Formation and structure of Ca-deficient hydroxyapatite. Calcif. Tissue Int. 1981, 33, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.; Ducheyne, P.; Radin, S. Determination of the Ca/P ratio in calcium-deficient hydroxyapatite using X-ray diffraction analysis. J. Mater. Sci. Mater. Med. 1993, 4, 105–168. [Google Scholar] [CrossRef]
- Ivanova, T.I.; Frank-Kamenetskaya, O.V.; Kol’tsov, A.B.; Ugolkov, V.L. Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal Decomposition. J. Solid State Chem. 2001, 160, 340–349. [Google Scholar] [CrossRef]
- Zhou, H.H.; Li, H.; Guo, L.H. Molecular and crystal structure characterization of calcium-deficient apatite. Key Eng. Mater. 2007, 330–332, 119–122. [Google Scholar] [CrossRef]
- Domashevskaya, E.P.; Al-Zubadi, A.A.; Goloshchapov, D.L.; Rumyantseva, N.A.; Seredin, P.V. Structure and composition of metal substituted calcium deficient hydroxyapatite. World Appl. Sci. J. 2014, 31, 2093–2100. [Google Scholar] [CrossRef]
- Bhat, S.S.; Waghmare, U.V.; Ramamurty, U. First-principles study of structure, vibrational, and elastic properties of stoichiometric and calcium-deficient hydroxyapatite. Cryst. Growth Des. 2014, 14, 3131–3141. [Google Scholar] [CrossRef]
- Brown, P.W.; Martin, R.I. An analysis of hydroxyapatite surface layer formation. J. Phys. Chem. B 1999, 103, 1671–1675. [Google Scholar] [CrossRef]
- Honghui, Z.; Hui, L.; Linghong, G. Molecular and crystal structure characterization of calcium deficient apatite. Key Eng. Mater. 2007, 330–332, 119–122. [Google Scholar]
- Moseke, C.; Gbureck, U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef] [PubMed]
- Dickens-Venz, S.H.; Takagi, S.; Chow, L.C.; Bowen, R.L.; Johnston, A.D.; Dickens, B. Physical and chemical properties of resin-reinforced calcium phosphate cements. Dent Mater. 1994, 10, 100–106. [Google Scholar] [CrossRef]
- Lee, Y.K.; Lim, B.S.; Kim, C.W. Mechanical properties of calcium phosphate based dental filling and regeneration materials. J. Oral Rehabil. 2003, 30, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Dickens, S.H.; Flaim, G.M.; Takagi, S. Mechanical properties and biochemical activity of remineralizing resin-based Ca–PO4 cements. Dent. Mater. 2003, 19, 558–566. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Takagi, S.; Sun, L.; Hussain, L.; Chow, L.C.; Guthrie, W.F.; Yen, J.H. Development of a non-rigid, durable calcium phosphate cement for use in periodontal bone repair. J. Am. Dent. Assoc. 2006, 137, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, A.; Fujikawa, K.; Takagi, S.; Chow, L.C. Histological analysis of calcium phosphate bone grafts for surgically created periodontal bone defects in dogs. Dent. Mater. J. 2008, 27, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Wang, J.; Shan, W.; Liu, X.; Ma, J.; Liu, C.; Fang, J.; Wei, S. Development of fluorapatite cement for dental enamel defects repair. J. Mater. Sci. Mater. Med. 2011, 22, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Thein-Han, W.; Liu, J.; Xu, H.H.K. Calcium phosphate cement with biofunctional agents and stem cell seeding for dental and craniofacial bone repair. Dent Mater. 2012, 28, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.K.; Weir, M.D.; Sun, L. Calcium and phosphate ion releasing composite: Effect of pH on release and mechanical properties. Dent Mater. 2009, 25, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Hayami, S.; Tsuji, I.; Toda, T. Histopathological study of a newly developed root canal sealer containing tetracalcium-dicalcium phosphates and 1.0% chondroitin sulfate. J. Endod. 1997, 23, 162–166. [Google Scholar] [CrossRef]
- White, T.J.; ZhiLi, D. Structural derivation and crystal chemistry of apatites. Acta Crystallogr. B 2003, 59, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.M.; Rakovan, J. The crystal structure of apatite, Ca5(PO4)3(F,OH,Cl). Rev. Mineral. Geochem. 2002, 48, 1–12. [Google Scholar] [CrossRef]
- Rulis, P.; Ouyang, L.; Ching, W.Y. Electronic structure and bonding in calcium apatite crystals: Hydroxyapatite, fluorapatite, chlorapatite, and bromapatite. Phys. Rev. B 2004, 70, 155104. [Google Scholar] [CrossRef]
- Calderín, L.; Stott, M.J.; Rubio, A. Electronic and crystallographic structure of apatites. Phys. Rev. B 2003, 67, 134106. [Google Scholar] [CrossRef]
- Hydroxyapatite Ca5(OH)(PO4)3. Available online: http://www.chemtube3d.com/solidstate/SShydroxyapatite.htm (accessed on 26 December 2016).
- Venkatesan, J.; Kim, S.K. Effect of temperature on isolation and characterization of hydroxyapatite from Tuna (Thunnus obesus) bone. Materials 2010, 3, 4761–4772. [Google Scholar] [CrossRef]
- Pramanik, N.; Mishra, D.; Banerjee, I.; Kumar Maiti, T.; Bhargava, P.; Pramanik, P. Chemical synthesis, characterization, and biocompatibility study of hydroxyapatite/chitosan phosphate nanocomposite for bone tissue engineering applications. Int. J. Biomater. 2009, 2009, 512417. [Google Scholar] [CrossRef] [PubMed]
- Gergely, G.; Wéber, F.; Lukács, I.; Tóth, A.L.; Horváth, Z.E.; Mihály, J.; Balázsi, C. Preparation and characterization of hydroxyapatite from eggshell. Ceram. Int. 2010, 36, 803–806. [Google Scholar] [CrossRef]
- Espanol, M.; Portillo, J.; Manero, J.M.; Ginebra, M.P. Investigation of the hydroxyapatite obtained as hydrolysis product of alpha-tricalcium phosphate by transmission electron microscopy. CrystEngComm 2010, 12, 3318–3326. [Google Scholar] [CrossRef]
- Neira, M.I.S. An Efficient Approach to the Synthesis of a Calcium Phosphate Bone-Cement and Its Reinforcement by Hydroxyapatite Crystals of Various Particle Morphologies; Universidade de Santiago de Compostela: Santiago, Spain, 2008. [Google Scholar]
- Jongprateep, O.; Nueangjumnong, C. Effects of synthesis techniques and initial reagents on compositions and particle morphology of hydroxyapatite. In Proceedings of the International Conference on Advanced Materials, Structures and Mechanical Engineering, Incheon, Korea, 29–31 May 2015; Kaloop, M., Ed.; CRC Press: Boca Raton, FL, USA, 2016. Chapter 53. pp. 251–254. [Google Scholar]
- Ahmed, S.; Ahsan, M. Synthesis of Ca-hydroxyapatite bioceramic from egg shell and its characterization. Bangladesh J. Sci. Ind. Res. 2008, 43, 501–512. [Google Scholar] [CrossRef]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Ramesh, S.; Tan, C.Y.; Sopyan, I.; Hamdi, M.; Teng, W.D. Consolidation of nanocrystalline hydroxyapatite powder. Sci. Technol. Adv. Mater. 2007, 8, 124–130. [Google Scholar] [CrossRef]
- Suzuki, S.; Sakamura, M.; Ichiyanagi, M.; Ozawa, M. Internal friction of hydroxyapatite and fluorapatite. Ceram. Int. 2004, 30, 625–627. [Google Scholar] [CrossRef]
- Ching, W.Y.; Rulis, P.; Misra, A. Ab initio elastic properties and tensile strength of crystalline hydroxyapatite. Acta Biomater. 2009, 5, 3067–3075. [Google Scholar] [CrossRef] [PubMed]
- Markovic, M.; Fowler, B.O.; Tung, M.S. Preparation and comprehensive characterization of a calcium hydroxyapatite reference material. J. Res. Natl. Inst. Stand. Technol. 2004, 109, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Willmann, G. Coating of implants with hydroxyapatite material connections between bone and metal. Adv. Eng. Mater. 1999, 1, 95–105. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Review: Material fundamentals and clinical performance of plasma sprayed hydroxyapatite coatings. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef] [PubMed]
- Ong, J.L.; Chan, D.C.N. Hydroxyapatites and their use as coatings in dental implants: A review. Crit. Rev. Biomed. Eng. 1999, 28, 667–707. [Google Scholar] [CrossRef]
- Geesink, R.G. Osteoconductive coatings for total joint arthroplasty. Clin. Orthop. Relat. Res. 2002, 395, 53–65. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Hench, L.L. Bioceramics. J. Am. Ceram. Soc. 1998, 81, 1705–1728. [Google Scholar] [CrossRef]
- Dey, A.; Mukhopadhyay, A.K.; Gangadharan, S.; Sinha, M.K.; Basu, D. Characterization of microplasma sprayed hydroxyapatite coating. J. Therm. Spray Technol. 2009, 18, 578–592. [Google Scholar] [CrossRef]
- Mangano, C.; Piattelli, A.; Perrotti, V.; Iezzi, G. Dense hydroxyapatite inserted into postextraction sockets: A histologic and histomorphometric 20-year case report. J. Periodontol. 2008, 79, 929–933. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, B.; Sidoti, M.C.; Roveri, N.; Tampieri, A.; Sandri, M.; Bertolazzi, L.; Galbusera, F.; Dubini, G.; Vena, P.; Contro, R. Controlled drug delivery from porous hydroxyapatite grafts: An experimental and theoretical approach. Mater. Sci. Eng. C 2005, 25, 207–213. [Google Scholar] [CrossRef]
- Palazzo, B.; Iafisco, M.; Laforgia, M.; Margiotta, N.; Natile, G.; Bianchi, C.L.; Walsh, D.; Mann, S.; Roveri, N. Biomimetic hydroxyapatite-drug nanocrystals as potential bone substitutes with antitumor drug delivery properties. Adv. Funct. Mater. 2007, 17, 2180–2188. [Google Scholar] [CrossRef]
- Tang, S.H.; Jin, A.M.; Lü, R.F.; Wang, X.D.; Wang, Y.F.; Yu, B.; Zhang, B.P. Preparation and in vivo release experiment of nanohydroxyapatite/gentamicin drug delivery system. J. Clin. Rehabil. Tissue Eng. Res. 2007, 11, 3573–3576. [Google Scholar]
- Ye, F.; Guo, H.; Zhang, H.; He, X. Polymeric micelle template synthesis of hydroxyapatite hollow nanoparticles for a drug delivery system. Acta Biomater. 2010, 6, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Niwa, M.; Sato, T.; Li, W.; Aoki, H.; Aoki, H.; Daisaku, T. Polishing and whitening properties of toothpaste containing hydroxyapatite. J. Mater. Sci. Mater. Med. 2001, 12, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.I.; Jeong, S.H.; Jang, S.O.; Kim, K.N.; Kwon, H.K.; Park, Y.D. Tooth whitening effect of toothpastes containing nano-hydroxyapatite. Key Eng. Mater. 2006, 309–311, 541–544. [Google Scholar] [CrossRef]
- Besinis, A.; van Noort, R.; Martin, N. Infiltration of demineralized dentin with silica and hydroxyapatite nanoparticles. Dent. Mater. 2012, 28, 1012–1023. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Clarkson, B.H.; Sun, K.; Mansfield, J.F. Self-assembly of synthetic hydroxyapatite nanorods into an enamel prism-like structure. J. Colloid Interface Sci. 2005, 288, 97–103. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Wolke, J.G.C.; Jansen, J.A. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 1998, 212, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.A.; Berndt, C.C. Biomedical application of apatites. In Phosphates: Geochemical, Geobiological and Materials Importance; Hughes, J.M., Kohn, M., Rakovan, J., Eds.; Mineralogical Society of America: Washington, DC, USA, 2002; pp. 631–672. [Google Scholar]
- Ferraz, M.P.; Monteiro, F.J.; Manuel, C.M. Hydroxyapatite nanoparticles: A review of preparation methodologies. J. Appl. Biomater. Biomech. 2004, 2, 74–80. [Google Scholar] [PubMed]
- Damien, E.; Revell, P.A. Coralline hydroxyapatite bone graft substitute: A review of experimental studies and biomedical applications. J. Appl. Biomater. Biomech. 2004, 2, 65–73. [Google Scholar] [PubMed]
- Ehrenfest, D.M.D.; Coelho, P.G.; Kang, B.S.; Sul, Y.T.; Albrektsson, T. Classification of osseointegrated implant surfaces: Materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Bral, A.; Mommaerts, M.Y. In vivo biofunctionalization of titanium patient-specific implants with nano hydroxyapatite and other nano calcium phosphate coatings: A systematic review. J. Cranio-Maxillofac. Surg. 2016, 44, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.; Eijofor, J.U. Increased osteoblast adhesion on nanophase metals: Ti, Ti6Al4V and CoCrMo. Biomaterials 2004, 25, 4731–4739. [Google Scholar] [CrossRef] [PubMed]
- Basu, B.; Dhirenda, K.; Ashok, K. Advanced Biomaterials: Fundamentals, Processing, and Applications; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]
- Catledge, S.A.; Fries, M.D.; Vohra, Y.K.; Lacefield, W.R.; Lemons, J.E.; Woodard, S.; Venugopalan, R. Nanostructured ceramics for biomedical implants. J. Nanosci. Nanotechnol. 2002, 2, 293–312. [Google Scholar] [CrossRef] [PubMed]
- Webster, T.J.; Siegel, R.W.; Bizios, R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials 2000, 21, 1803–1810. [Google Scholar] [CrossRef]
- Jimbo, R.; Xue, Y.; Hayashi, M.; Schwartz-Filho, H.O.; Andersson, M.; Mustafa, K.; Wennerberg, A. Genetic responses to nanostructured calcium phosphate-coated implants. J. Dent. Res. 2011, 90, 1422–1427. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Dittrich, R.; Lode, A.; Despang, F.; Gelinsky, M. Nanocrystalline spherical hydroxyapatite granules for bone repair: In vitro evaluation with osteoblast-like cells and osteoclasts. J. Mater. Sci. Mater. Med. 2013, 24, 1755–1766. [Google Scholar] [CrossRef] [PubMed]
- Tabaković, A.; Kester, M.; Adair, J.H. Calcium phosphate-based composite nanoparticles in bioimaging and therapeutic delivery applications. WIREs Nanomed. Nanobiotechnol. 2012, 4, 96–112. [Google Scholar] [CrossRef] [PubMed]
- Adair, J.H.; Parette, M.P.; Altınoğlu, E.İ.; Kester, M. Nanoparticulate alternatives for drug delivery. ACS Nano 2010, 4, 4967–4970. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.H.; Huang, B.Y.; Zhou, K.C.; Huang, S.P.; Liu, F.; Li, Y.M.; Xue, Z.G.; Long, Z.G. Hydroxyapatite nanoparticles as a novel gene carrier. J. Nanopart. Res. 2004, 6, 307–311. [Google Scholar] [CrossRef]
- Ong, H.T.; Loo, J.S.C.; Boey, F.Y.C.; Russell, S.J.; Ma, J.; Peng, K.W. Exploiting the high-affinity phosphonate-hydroxyapatite nanoparticle interaction for delivery of radiation and drugs. J. Nanopart. Res. 2008, 10, 141–150. [Google Scholar] [CrossRef]
- Okada, M.; Furukawa, K.; Serizawa, T.; Yanagisawa, Y.; Tanaka, H.; Kawai, T.; Furuzono, T. Interfacial interactions between calcined hydroxyapatite nanocrystals and substrates. Langmuir 2009, 25, 6300–6306. [Google Scholar] [CrossRef] [PubMed]
- Dumbleton, J.; Manley, M.T. Hydroxyapatite-coated prostheses in total hip and knee arthroplasty. J. Bone Jt. Surg. Am. A 2004, 86, 2526–2540. [Google Scholar] [CrossRef]
- Van Blitterswijk, C.A.; Grote, J.J.; Kuypers, W.; Daems, W.T.H.; de Groot, K. Macropore tissue ingrowth: A quantitative and qualitative study on hydroxyapatite ceramic. Biomaterials 1986, 7, 137–143. [Google Scholar] [CrossRef]
- Uota, M.; Arakawa, H.; Kitamura, N.; Yoshimura, T.; Tanaka, J.; Kijima, T. Synthesis of high surface area hydroxyapatite nanoparticles by mixed surfactant-mediated approach. Langmuir 2005, 21, 4724–4728. [Google Scholar] [CrossRef] [PubMed]
- Mandler, D.; Eliaz, N.; Geuli, O.; Metoki, N. Electrochemically Driven Hydroxyapatite Nanoparticles Coating of Medical Implants. U.S. Provisional Patent Application 62/380,810, 29 August 2016. [Google Scholar]
- Geuli, O.; Metoki, N.; Eliaz, N.; Mandler, D. Electrochemically driven hydroxyapatite nanoparticles coating of medical implants. Adv. Funct. Mater. 2016, 26, 8003–8010. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Handbook of Nanostructured Biomaterials and their Applications in Nanobiotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2005; pp. 141–168. [Google Scholar]
- Murugan, R.; Ramakrishna, S. Nanostructured biomaterials. In Encyclopedia of Nanoscience and Nanotechnology; Nalwa, H.S., Ed.; American Scientific Publishers: Valencia, CA, USA, 2004; Volume 7, pp. 595–613. [Google Scholar]
- Ota, Y.; Iwashita, T. Novel preparation method of hydroxyapatite fibers. J. Am. Ceram. Soc. 1998, 81, 1665–1668. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Aqueous mediated synthesis of bioresorbable nanocrystalline hydroxyapatite. J. Cryst. Growth 2005, 274, 209–213. [Google Scholar] [CrossRef]
- Murugan, R.; Ramakrishna, S. Bioresorbable composite bone paste using polysaccharide based nano-hydroxyapatite. Biomaterials 2004, 25, 3829–3835. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Eibl, O.; Berthold, C.; Scheideler, L.; Geis-Gerstorfer, J. Structural characterization of nanocrystalline hydroxyapatite and adhesion of pre-osteoblast cells. Nanotechnology 2006, 17, 2711–2721. [Google Scholar] [CrossRef]
- Sun, W.; Chu, C.; Wang, J.; Zhao, H. Comparison of periodontal ligament cells responses to dense and nanophase hydroxyapatite. J. Mater. Sci. Mater. Med. 2007, 18, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Chai, C.; Ben-Nissan, B. Bioactive nanocrystalline sol-gel hydroxyapatite coatings. J. Mater. Sci. Mater. Med. 1999, 10, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Rusu, V.M.; Ng, C.H.; Wilke, M.; Tiersch, B.; Fratzl, P.; Peter, M.G. Size-controlled hydroxyapatite nanoparticles as self-organized organic-inorganic composite materials. Biomaterials 2005, 26, 5414–5426. [Google Scholar] [CrossRef] [PubMed]
- López-Macipe, A.; Gómez-Morales, J.; Rodríguez-Clemente, R. Nanosized hydroxyapatite precipitation from homogeneous calcium/citrate/phosphate solutions using microwave and conventional heating. Adv. Mater. 1998, 10, 49–53. [Google Scholar] [CrossRef]
- Zhang, S.; Consalves, K.E. Preparation and characterization of thermally stable nanohydroxyapatite. J. Mater. Sci. Mater. Med. 1997, 8, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Ioku, K.; Yamauchi, S.; Fujimori, H.; Goto, S.; Yoshimura, M. Hydrothermal preparation of fibrous apatite and apatite sheet. Solid State Ion. 2002, 151, 147–150. [Google Scholar] [CrossRef]
- Nakamura, S.; Tsobe, T.; Senna, M. Hydroxyapatite nano sol prepared via a mechanochemical route. J. Nanopart. Res. 2001, 3, 57–61. [Google Scholar] [CrossRef]
- Koumoulidis, G.C.; Vaimakis, T.C.; Sdoukos, A.T.; Boukos, N.K.; Trapalis, C.C. Preparation of hydroxyapatite lath-like particles using high-speed dispersing equipment. J. Am. Ceram. Soc. 2001, 84, 1203–1208. [Google Scholar] [CrossRef]
- Yang, Y.; Ong, J.L. Rapid sintering of hydroxyapatite by microwave processing. J. Mater. Sci. Lett. 2002, 21, 67–69. [Google Scholar] [CrossRef]
- Wang, J.; Shaw, L.L. Morphology-enhanced low-temperature sintering of nanocrystalline hydroxyapatite. Adv. Mater. 2007, 19, 2364–2369. [Google Scholar] [CrossRef]
- Fomin, A.S.; Barinov, S.M.; Ievlev, V.M.; Smirnov, V.V.; Mikhailov, B.P.; Belonogov, E.K.; Drozdova, N.A. Nanocrystalline hydroxyapatite ceramics produced by low-temperature sintering after high-pressure treatment. Doklady Akademii Nauk 2008, 418, 352–355. [Google Scholar] [CrossRef]
- Drouet, C.; Bosc, F.; Banu, M.; Largeot, C.; Combes, C.; Dechambre, G.; Estournès, C.; Raimbeaux, G.; Rey, C. Nanocrystalline apatites: From powders to biomaterials. Powder Technol. 2009, 190, 118–122. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Khorasani, M.T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Chang, J.; Liu, X.; Chen, L.; Zhou, Y. Synthesis of element-substituted hydroxyapatite with controllable morphology and chemical composition using calcium silicate as precursor. CrystEngComm 2011, 13, 4850–4855. [Google Scholar] [CrossRef]
- Lin, K.; Liu, X.; Chang, J.; Zhu, Y. Facile synthesis of hydroxyapatite nanoparticles, nanowires and hollow nano-structured microspheres using similar structured hard-precursors. Nanoscale 2011, 3, 3052–3055. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, C.; Wei, J.; Sun, J. Effects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblasts. J. Appl. Toxicol. 2012, 32, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Huang, X.; Cai, Y.; Tang, R.; Yang, D. Size effect of hydroxyapatite nanoparticles on proliferation and apoptosis of osteoblast-like cells. Acta Biomater. 2009, 5, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Khor, K.A.; Sui, J.J.; Zhang, J.H.; Chen, W.N. Protein expression profiles in osteoblasts in response to differentially shaped hydroxyapatite nanoparticles. Biomaterials 2009, 30, 5385–5391. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, Y.; Ikoma, T.; Tanaka, J.; Hoshi, K.; Ishihara, T.; Ogawa, Y.; Ueno, A. Injectable porous hydroxyapatite microparticles as a new carrier for protein and lipophilic drugs. J. Control. Release 2006, 110, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Walboomers, X.F.; van den Dolder, J.; Yang, F.; Bian, Z.; Fan, M.; Jansen, J.A. Non-viral bone morphogenetic protein 2 transfection of rat dental pulp stem cells using calcium phosphate nanoparticles as carriers. Tissue Eng. Part A 2008, 14, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Iafisco, M.; Palazzo, B.; Marchetti, M.; Margiotta, N.; Ostuni, R.; Natile, G.; Morpurgo, M.; Gandin, V.; Marzano, C.; Roveri, N. Smart delivery of antitumoral platinum complexes from biomimetic hydroxyapatite nanocrystals. J. Mater. Chem. 2009, 19, 8385–8392. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Biphasic, triphasic and multiphasic calcium orthophosphates. Acta Biomater. 2012, 8, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Ellinger, R.F.; Nery, E.B.; Lynch, K.L. Histological assessment of periodontal osseous defects following implantation of hydroxyapatite and biphasic calcium phosphate ceramics: A case report. Int. J. Periodontics Restor. Dent. 1986, 3, 22–33. [Google Scholar]
- Lobo, S.E.; Arinzeh, L.T. Biphasic calcium phosphate ceramics for bone regeneration and tissue engineering applications. Materials 2010, 3, 815–826. [Google Scholar] [CrossRef]
- Daculsi, G.; Baroth, S.; LeGeros, R.Z. 20 years of biphasic calcium phosphate bioceramics development and applications. In Advance in Bioceramics and Porous Ceramics II; Narayan, R., Colombo, P., Singh, D., Salem, J., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 45–58. [Google Scholar]
- Arinzeh, T.L.; Tran, T.; Mcalary, J.; Daculsi, G. A comparative study of biphasic calcium phosphate ceramics for human mesenchymal stem-cell-induced bone formation. Biomaterials 2005, 26, 3631–3638. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.N.; Pereira, M.M.; Goes, A.M.; Leite, M.F. Effect of biphasic calcium phosphate on human macrophage functions in vitro. J. Biomed. Mater. Res. A 2003, 65, 475–481. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ren, W.; Tian, X.; Liu, W.; Wu, S.; Chen, X. Comparative study on in vivo response of porous calcium carbonate composite ceramic and biphasic calcium phosphate ceramic. Mater. Sci. Eng. C 2016, 64, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Amirian, J.; Linh, N.T.B.; Min, Y.K.; Lee, B.T. Bone formation of a porous Gelatin-Pectin-biphasic calcium phosphate composite in presence of BMP-2 and VEGF. Int. J. Biol. Macromol. 2015, 76, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Sadiasa, A.; Kim, M.S.; Lee, B.T. Poly(lactide-co-glycolide acid)/biphasic calcium phosphate composite coating on a porous scaffold to deliver simvastatin for bone tissue engineering. J. Drug Target. 2013, 21, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium orthophosphate-containing biocomposites and hybrid biomaterials for biomedical applications. J. Funct. Biomater. 2015, 6, 708–832. [Google Scholar] [CrossRef] [PubMed]
- Allo, B.A.; Costa, D.O.; Dixon, S.J.; Mequanint, K.; Rizkalla, A.S. Bioactive and biodegradable nanocomposites and hybrid biomaterials for bone regeneration. J. Funct. Biomater. 2012, 3, 432–463. [Google Scholar] [CrossRef] [PubMed]
- Gremillard, L.; Meille, S.; Chevalier, J.; Zhao, J.; Fridrici, V.; Kapsa, P.; Geringer, J.; Uribe, J. Degradation of bioceramics. In Degradation of Implant Materials; Eliaz, N., Ed.; Springer Science + Business Media: New York, NY, USA, 2012; Chapter 9; pp. 195–252. [Google Scholar]
- Perez, R.A.; Kim, H.W.; Ginebra, M.P. Polymeric additives to enhance the functional properties of calcium phosphate cements. J. Tissue Eng. 2012, 3, 2041731412439555. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Ye, J. Construction and properties of poly(lactic-co-glycolic acid)/calcium phosphate cement composite pellets with microspheres-in-pellet structure for bone repair. Ceram. Int. 2016, 42, 5587–5592. [Google Scholar] [CrossRef]
- Ishikawa, K.; Miyamoto, Y.; Kon, M.; Nagayama, M.; Asaoka, K. Non-decay type fast-setting calcium phosphate cement: Composite with sodium alginate. Biomaterials 1995, 16, 527–532. [Google Scholar] [CrossRef]
- Cao, C.; Li, H.; Li, J.; Liu, C.; Yang, H.; Li, B. Mechanical reinforcement of injectable calcium phosphate cement/silk fibroin (SF) composite by mineralized SF. Ceram. Int. 2014, 40, 13987–13993. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Burguera, E.F.; Carey, L.E. Strong, macroporous, and in situ-setting calcium phosphate cement-layered structures. Biomaterials 2007, 28, 3786–3796. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef] [PubMed]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Panchagnula, R. Skeletal drug delivery systems. Int. J. Pharm. 2000, 206, 1–12. [Google Scholar] [CrossRef]
- Roy, A.; Jhunjhunwala, S.; Bayer, E.; Fedorchak, M.; Little, S.R.; Kumta, P.N. Porous calcium phosphate-poly(lactic-co-glycolic) acid composite bone cement: A viable tunable drug delivery system. Mater. Sci. Eng. C 2016, 59, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Xiao, G.Y.; Wang, X.; Dong, Z.G.; Ma, Z.Y.; Li, L.; Li, Y.H.; Pan, X.; Nie, L. Biocompatibility and osteogenesis of calcium phosphate composite scaffolds containing simvastatin-loaded PLGA microspheres for bone tissue engineering. J. Biomed. Mater. Res. Part A 2015, 103, 3250–3258. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kwon, J.; Khang, G.; Lee, D. Reduction of inflammatory responses and enhancement of extracellular matrix formation by vanillin-incorporated poly(lactic-co-glycolic acid) scaffolds. Tissue Eng. Part A 2012, 18, 1967–1978. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.T.; Ling, L.S.; Hussain, R. Injectable magnesium-doped brushite cement for controlled drug release application. J. Mater. Sci. 2016, 51, 7427–7439. [Google Scholar] [CrossRef]
- Boroujeni, N.M.; Zhou, H.; Luchini, T.J.; Bhaduri, S.B. Development of multi-walled carbon nanotubes reinforced monetite bionanocomposite cements for orthopedic applications. Mater. Sci. Eng. C 2013, 33, 4323–4330. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Zhou, H.; Leaman, D.W.; Goel, V.K.; Agarwal, A.K.; Bhaduri, S.B. Sustained release of small molecules from carbon nanotube-reinforced monetite calcium phosphate cement. Mater. Sci. Eng. C 2014, 43, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Sadollah, A.; Bahreininejad, A.; Hamdi, M.; Purbolaksono, J. Optimum mechanical behavior of calcium phosphate cement/hydroxyl group functionalized multi-walled carbon nanotubes/bovine serum albumin composite using metaheuristic algorithms. Neural Comput. Appl. 2014, 24, 193–200. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, D.; Huan, Z.; Wu, C.; Chang, J. Novel tricalcium silicate/magnesium phosphate composite bone cement having high compressive strength, in vitro bioactivity and cytocompatibility. Acta Biomater. 2015, 21, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Sopcak, T.; Medvecky, L.; Giretova, M.; Stulajterova, R.; Durisin, J.; Girman, V.; Faberova, M. Effect of phase composition of calcium silicate phosphate component on properties of brushite based composite cements. Mater. Charact. 2016, 117, 17–29. [Google Scholar] [CrossRef]
- Geffers, M.; Barralet, J.E.; Groll, J.; Gbureck, U. Dual-setting brushite–silica gel cements. Acta Biomater. 2015, 11, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, C.; Zhang, X.; Gu, X.; Zhang, J.; Yuan, Y.; Liu, C.; Shi, J.; Wang, J.; Li, Y. Preparation of an rhBMP-2 loaded mesoporous bioactive glass-calcium phosphate cement porous composite scaffold for rapid bone tissue regeneration. J. Mater. Chem. B 2015, 3, 8558–8566. [Google Scholar] [CrossRef]
- Verron, E.; Bouler, J.M.; Guicheux, J. Controlling the biological function of calcium phosphate bone substitutes with drugs. Acta Biomater. 2012, 8, 3541–3551. [Google Scholar] [CrossRef] [PubMed]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Blom, E.J.; Klein-Nulend, J.; Wolke, J.G.C.; van Waas, M.A.J.; Driessens, F.C.M.; Burger, E.H. Transforming growth factor-beta1 incorporation in a calcium phosphate bone cement—Material properties and release characteristics. J. Biomed. Mater. Res. 2002, 59, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.R.; Kim, H.K.; Kwon, J.Y.; Kim, T.K.; Choi, Y.M.; Kim, K.H. The incorporation of platelet-rich plasma into calcium phosphate cement enhances bone regeneration in osteoporosis. Pain Phys. 2014, 17, E737–E745. [Google Scholar]
- Hustedt, J.W.; Blizzard, D.J. The controversy surrounding bone morphogenetic proteins in the spine: A review of current research. Yale J. Biol. Med. 2014, 87, 549–561. [Google Scholar] [PubMed]
- Epstein, N.E. Complications due to the use of BMP/INFUSE in spine surgery: The evidence continues to mount. Surg. Neurol. Int. 2013, 4, S343–S352. [Google Scholar] [CrossRef] [PubMed]
- Qiao, P.; Wang, J.; Xie, Q.; Li, F.; Dong, L.; Xu, T. Injectable calcium phosphate–alginate–chitosan microencapsulated MC3T3-E1 cell paste for bone tissue engineering in vivo. Mater. Sci. Eng. C 2013, 33, 4633–4639. [Google Scholar] [CrossRef] [PubMed]
- Romeo, H.E.; Fanovich, M.A. Functionalized bridged silsesquioxane-based nanostructured microspheres: Performance as novel drug-delivery devices in bone tissue-related applications. J. Biomater. Appl. 2012, 26, 987–1012. [Google Scholar] [CrossRef] [PubMed]
- Nouri-Felekori, M.; Mesgar, A.S.M.; Mohammadi, Z. Development of composite scaffolds in the system of gelatin-calcium phosphate whiskers/fibrous spherulites for bone tissue engineering. Ceram. Int. 2015, 41, 6013–6019. [Google Scholar] [CrossRef]
- Sarikaya, B.; Aydin, H.M. Collagen/beta-tricalcium phosphate based synthetic bone grafts via dehydrothermal processing. BioMed Res. Int. 2015, 2015, 576532. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.S.; Seto, J.; Wagermaier, W.; Zaslansky, P.; Boesecke, P.; Fratzl, P. Cooperative deformation of mineral and collagen in bone at the nanoscale. Proc. Natl. Acad. Sci. USA 2006, 103, 17741–17746. [Google Scholar] [CrossRef] [PubMed]
- Fantner, G.E.; Hassenkam, T.; Kindt, J.H.; Weaver, J.C.; Birkedal, H.; Pechenik, L.; Cutroni, J.A.; Cidade, G.A.; Stucky, G.D.; Morse, D.E.; et al. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat. Mater. 2005, 4, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, T.; Zhang, G.F.; Yu, X.; Zhang, J.; Zhou, G.; Tang, Z.H. A comparative evaluation of the mechanical properties of two calcium phosphate/collagen composite materials and their osteogenic effects on adipose-derived stem cells. Stem Cells Int. 2016, 2016, 6409546. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.; Ku, S.H.; Lee, M.; Park, C.B. Bone-like peptide/hydroxyapatite nanocomposites assembled with multi-level hierarchical structures. Soft Matter 2011, 7, 7201–7206. [Google Scholar] [CrossRef]
- Li, C.; Born, A.K.; Schweizer, T.; Zenobi-Wong, M.; Cerruti, M.; Mezzenga, R. Amyloid-hydroxyapatite bone biomimetic composites. Adv. Mater. 2014, 26, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Hadisi, Z.; Nourmohammadin, J.; Mohammadi, J. Composite of porous starch-silk fibroin nanofiber-calcium phosphate for bone regeneration. Ceram. Int. 2015, 41, 10745–10754. [Google Scholar] [CrossRef]
- Zhang, Y.; Ni, M.; Zhang, M.; Ratner, B. Calcium phosphate-chitosan composite scaffolds for bone tissue engineering. Tissue Eng. 2003, 9, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M. Synthesis and characterization of macroporous chitosan/calcium phosphate composite scaffolds for tissue engineering. J. Biomed. Mater. Res. 2001, 55, 304–312. [Google Scholar] [CrossRef]
- Kozłowska, J.; Sionkowska, A. Effects of different crosslinking methods on the properties of collagen-calcium phosphate composite materials. Int. J. Biol. Macromol. 2015, 74, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xie, J.; Lipner, J.; Yuan, X.; Thomopoulos, S.; Xia, Y. Nanofiber scaffolds with gradations in mineral content for mimicking the tendon-to-bone insertion site. Nano Lett. 2009, 9, 2763–2768. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.G.; Antonucci, J.M.; Liu, D.W.; Skrtic, D. In vitro cytotoxicity of amorphous calcium phosphate composites. J. Bioact. Compat. Polym. 2005, 20, 279–295. [Google Scholar] [CrossRef]
- Maeda, Y.; Hojo, H.; Shimohata, N.; Choi, S.; Yamamoto, K.; Takato, T.; Chung, U.; Ohba, S. Bone healing by sterilizable calcium phosphate tetrapods eluting osteogenic molecules. Biomaterials 2013, 34, 5530–5537. [Google Scholar] [CrossRef] [PubMed]
- Okumura, M.; Ohgushi, H.; Tamai, S. Bonding osteogenesis in coralline hydroxyapatite combined with bone marrow cells. Biomaterials 1991, 12, 411–416. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Zhang, X.P.; Xiao, G.Y.; Hou, Y.; Cheng, L.; Si, M.; Wang, S.S.; Li, Y.H.; Nie, L. In vitro and in vivo evaluation of calcium phosphate composite scaffolds containing BMP-VEGF loaded PLGA microspheres for the treatment of avascular necrosis of the femoral head. Mater. Sci. Eng. C 2016, 60, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Yaylaoǧlu, M.; Korkusuz, P.; Örs, Ü.; Korkusuz, F.; Hasirci, V. Development of a calcium phosphate-gelatin composite as a bone substitute and its use in drug release. Biomaterials 1999, 20, 711–719. [Google Scholar] [CrossRef]
- Trajano, V.C.C.; Costa, K.J.R.; Lanza, C.R.M.; Sinisterra, R.D.; Cortés, M.E. Osteogenic activity of cyclodextrin-encapsulated doxycycline in a calcium phosphate PCL and PLGA composite. Mater. Sci. Eng. C 2016, 64, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M. Calcium phosphate/chitosan composite scaffolds for controlled in vitro antibiotic drug release. J. Biomed. Mater. Res. 2002, 62, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kataoka, K. Nano-structured composites based on calcium phosphate for cellular delivery of therapeutic and diagnostic agents. Nano Today 2009, 4, 508–517. [Google Scholar] [CrossRef]
- Lin, X.; de Groot, K.; Wang, D.; Hu, Q.; Wismeijer, D.; Liu, Y. A review paper on biomimetic calcium phosphate coatings. Open Biomed. Eng. J. 2015, 9 (Suppl. 1-M4), 56–64. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Cai, S.; Liu, T.; Wu, X. Calcium phosphate glass/MgF2 double layered composite coating for improving the corrosion resistance of magnesium alloy. J. Alloys Compd. 2014, 591, 34–40. [Google Scholar] [CrossRef]
- Thonglem, S.; Rujijanagul, G.; Eitssayeam, S.; Pengpat, K. Fabrication of P2O5–CaO–Na2O glasses doped with magnesium oxide for artificial bone applications. Ceram. Int. 2013, 39, S537–S540. [Google Scholar] [CrossRef]
- Huang, Y.; Yana, Y.; Pang, X.; Ding, Q.; Han, S. Bioactivity and corrosion properties of gelatin-containing and strontium-doped calcium phosphate composite coating. Appl. Surf. Sci. 2013, 282, 583–589. [Google Scholar] [CrossRef]
- Wang, M.J.; Chao, S.C.; Yen, S.K. Electrolytic calcium phosphate/zirconia composite coating on AZ91D magnesium alloy for enhancing corrosion resistance and bioactivity. Corros. Sci. 2016, 104, 47–60. [Google Scholar] [CrossRef]
- Su, Y.; Li, D.; Su, Y.; Lu, C.; Niu, L.; Lian, J.; Li, G. Improvement of the biodegradation property and biomineralization ability of magnesium-hydroxyapatite composites with dicalcium phosphate dihydrate and hydroxyapatite coatings. ACS Biomater. Sci. Eng. 2016, 2, 818–828. [Google Scholar] [CrossRef]
- Kim, H.W.; Lee, S.Y.; Bae, C.J.; Noh, Y.J.; Kim, H.E.; Kim, H.M.; Ko, J.S. Porous ZrO2 bone scaffold coated with hydroxyapatite with fluorapatite intermediate layer. Biomaterials 2003, 24, 3277–3284. [Google Scholar] [CrossRef]
- Arce, J.E.; Arce, A.E.; Aguilar, Y.; Yate, L.; Moya, S.; Rincόn, C.; Gutiérrez, O. Calcium phosphate-calcium titanate composite coatings for orthopedic applications. Ceram. Int. 2016, 42, 10322–10331. [Google Scholar] [CrossRef]
- Fan, Y.; Duan, K.; Wang, R. A composite coating by electrolysis-induced collagen self-assembly and calcium phosphate mineralization. Biomaterials 2005, 26, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Hunziker, E.B.; Randall, N.X.; de Groot, K.; Layrolle, P. Proteins incorporated into biomimetically prepared calcium phosphate coatings modulate their mechanical strength and dissolution rate. Biomaterials 2003, 24, 65–70. [Google Scholar] [CrossRef]
- Leonor, I.B.; Alves, C.M.; Azevedo, H.S.; Reis, R.L. Effects of protein incorporation on calcium phosphate coating. Mater. Sci. Eng. C 2009, 29, 913–918. [Google Scholar] [CrossRef]
- De Jonge, L.T.; van den Beucken, J.J.J.P.; Leeuwenburgh, S.C.G.; Hamers, A.A.J.; Wolke, J.G.C.; Jansen, J.A. In vitro responses to electrosprayed alkaline phosphatase/calcium phosphate composite coatings. Acta Biomater. 2009, 5, 2773–2782. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Yokoyama, Y.; Uchida, M.; Ito, A. The formation of an antibacterial agent-apatite composite coating on a polymer surface using a metastable calcium phosphate solution. Biomaterials 2006, 27, 3295–3303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Xu, W.; Chen, F.; Qi, C.; Lu, B.Q.; Zhang, H.; Wu, J.; Qian, Q.R.; Zhu, Y.J. Amorphous calcium phosphate nanospheres/polylactide composite coated tantalum scaffold: Facile preparation, fast biomineralization and subchondral bone defect repair application. Colloids Surf. B Biointerfaces 2014, 123, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Q.; Jia, Z.; Xu, X.; Cheng, Y.; Zheng, Y.; Xi, T.; Wei, S. Graphene oxide/hydroxyapatite composite coatings fabricated by electrophoretic nanotechnology for biological applications. Carbon 2014, 67, 185–197. [Google Scholar] [CrossRef]
- Janković, A.; Eraković, S.; Mitrić, M.; Matić, I.Z.; Juranić, Z.D.; Tsui, G.C.P.; Tang, C.; Mišković-Stanković, V.; Rhee, K.Y.; Park, S.J. Bioactive hydroxyapatite/graphene composite coating and its corrosion stability in simulated body fluid. J. Alloys Compd. 2015, 624, 148–157. [Google Scholar] [CrossRef]
- Santos, C.; Piedade, C.; Uggowitzer, P.J.; Montemor, M.F.; Carmezim, M.J. Parallel nano-assembling of a multifunctional GO/HapNP coating on ultrahigh-purity magnesium for biodegradable implants. Appl. Surf. Sci. 2015, 345, 387–393. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Li, H. Nanostructural characteristics of vacuum cold-sprayed hydroxyapatite/graphene-nanosheet coatings for biomedical applications. J. Therm. Spray Technol. 2014, 23, 1149–1156. [Google Scholar] [CrossRef]
- Gao, F.; Xu, C.; Hu, H.; Wang, Q.; Gao, Y.; Chen, H.; Guo, Q.; Chen, D.; Eder, D. Biomimetic synthesis and characterization of hydroxyapatite/graphene oxide hybrid coating on Mg alloy with enhanced corrosion resistance. Mater. Lett. 2015, 138, 25–28. [Google Scholar] [CrossRef]
- Zanin, H.; Saito, E.; Marciano, F.R.; Ceragioli, H.J.; Granato, A.E.C.; Porcionatto, M.; Lobo, A.O. Fast preparation of nano-hydroxyapatite/superhydrophilic reduced graphene oxide composites for bioactive applications. J. Mater. Chem. B 2013, 1, 4947–4955. [Google Scholar] [CrossRef]
- Zanin, H.; Rosa, C.; Eliaz, N.; May, P.; Marciano, F.; Lobo, A.P. Assisted deposition of nano-hydroxyapatite onto exfoliated carbon nanotube oxide scaffolds. Nanoscale 2015, 7, 10218–10232. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-González, C.; Cid-Luna, H.E.; Salas, P.; Castaño, V.M. Hydroxyapatite-functionalized graphene: A new hybrid nanomaterial. J. Nanomater. 2014, 2014, 940903. [Google Scholar] [CrossRef]
- Núñez, J.D.; Benito, A.M.; González, R.; Aragón, J.; Arenal, R.; Maser, W.K. Integration and bioactivity of hydroxyapatite grown on carbon nanotubes and graphene oxide. Carbon 2014, 79, 590–604. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Zhang, Y.; Li, H.; Tan, Y.; Luo, L.; Duan, J.; Lia, K.; Banks, C.E. Facile and controllable synthesis of hydroxyapatite/graphene hybrid materials with enhanced sensing performance towards ammonia. Analyst 2015, 140, 5235–5242. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ouyang, Z.; Ren, Z.; Li, J.; Zhang, P.; Wei, G.; Su, Z. Self-assembled peptide nanofibers on graphene oxide as a novel nanohybrid for biomimetic mineralization of hydroxyapatite. Carbon 2015, 89, 20–30. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. In-situ deposition of hydroxyapatite on graphene nanosheets. Mater. Res. Bull. 2013, 48, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Hu, K.; Fang, D.; Shang, L.; Tran, S.D.; Cerruti, M. Graphene and hydroxyapatite self-assemble into homogeneous, free standing nanocomposite hydrogels for bone tissue engineering. Nanoscale 2015, 7, 7992–8002. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, C.; Zhai, H.; Zhu, G.; Pan, H.; Xuab, X.; Tang, R. Biomimetic graphene oxide-hydroxyapatite composites via in situ mineralization and hierarchical assembly. RSC Adv. 2014, 4, 25398. [Google Scholar] [CrossRef]
- Depan, D.; Pesacretab, T.C.; Misra, R.D.K. The synergistic effect of a hybrid graphene oxide-chitosan system and biomimetic mineralization on osteoblast functions. Biomater. Sci. 2014, 2, 264–274. [Google Scholar] [CrossRef]
- Kim, S.S.; Park, M.S.; Jeon, O.; Choi, C.Y.; Kim, B.S. Poly(lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Webster, T.J. Biological response to and toxicity of nanoscale implant materials. In Degradation of Implant Materials; Eliaz, N., Ed.; Springer Science + Business Media: New York, NY, USA, 2012; Chapter 18; pp. 481–508. [Google Scholar]
- Narayan, R.J.; Hobbs, L.W.; Jin, C.; Rabiei, A. The use of functionally gradient materials in medicine. J. Oncol. Manag. 2006, 58, 52–56. [Google Scholar] [CrossRef]
- Wang, M.; Yang, X.Y.; Khor, K.A.; Wang, Y. Preparation and characterization of bioactive monolayer and functionally graded coatings. J. Mater. Sci. Mater. Med. 1999, 10, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Vaz, L.; Lopes, A.; Almeida, M. Porosity control of hydroxyapatite implants. J. Mater. Sci. Mater. Med. 1999, 10, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Pompe, W.; Worch, H.; Epple, M.; Friess, W.; Gelinsky, M.; Greil, P.; Hempele, U.; Scharnweber, D.; Schulte, K. Functionally graded materials for biomedical applications. Mater. Sci. Eng. A 2003, 362, 40–60. [Google Scholar] [CrossRef]
- Werner, J.; Linner-Krčmar, B.; Friess, W.; Greil, P. Mechanical properties and in vitro cell compatibility of hydroxyapatite ceramics with graded pore structure. Biomaterials 2002, 23, 4285–4294. [Google Scholar] [CrossRef]
- Schiller, C.; Rasche, C.; Wehmöller, M.; Beckmann, F.; Eufinger, H.; Epple, M.; Weihe, S. Geometrically structured implants for cranial reconstruction made of biodegradable polyesters and calcium phosphate/calcium carbonate. Biomaterials 2004, 25, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Wehmöller, M.; Neuking, K.; Epple, M.; Annen, T.; Eufinger, H. Mechanical characteristics of functionally graded biodegradable implants for skull bone reconstruction. Materialwissenschaft und Werkstofftechnik 2006, 37, 413–415. [Google Scholar] [CrossRef]
- Eufinger, H.; Rasche, C.; Lehmbrock, J.; Wehmöller, M.; Weihe, S.; Schmitz, I.; Schiller, C.; Epple, M. Performance of functionally graded implants of polylactides and calcium phosphate-calcium carbonate in an ovine model for computer assisted craniectomy and cranioplasty. Biomaterials 2007, 28, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Lindner, M.; Bergmann, C.; Telle, R.; Fischer, H. Calcium phosphate scaffolds mimicking the gradient architecture of native long bones. J. Biomed. Mater. Res. A 2014, 102, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A.; Lin, C.Y.; Navaratnam, M.; Rawlings, R.D.; McShane, H.B. A functionally gradient material produced by a powder metallurgical process. J. Mater. Sci. Lett. 1993, 12, 1516–1518. [Google Scholar]
- Takahashi, H. Mechanical properties of functional gradient materials of titanium-apatite and titanium zirconia for dental use. J. Jpn. Dent. Mater. 1993, 12, 595–612. [Google Scholar]
- Wang, Y.; Khor, K.; Cheang, P. Thermal spraying of functionally graded calcium phosphate coatings for biomedical implants. J. Therm. Spray Technol. 1998, 7, 50–57. [Google Scholar] [CrossRef]
- Wang, C.X.; Chen, Z.Q.; Wang, M.; Liu, Z.Y.; Wang, P.L.; Zheng, S.X. Functionally graded calcium phosphate coatings produced by ion beam sputtering/mixing deposition. Biomaterials 2001, 22, 1619–1626. [Google Scholar] [CrossRef]
- Xu, S.; Long, J.D.; Ostrikov, K.N.; Lu, J.H.; Diong, C.H. RF magnetron sputtering deposition of bioactive Ca-P-based coatings on Ti–6Al–4V alloy. IEEE Trans. Plasma Sci. 2002, 30, 118–119. [Google Scholar] [CrossRef]
- Rabiei, A.; Thomas, B.; Jin, C.; Narayan, R.; Cuomo, J.; Yang, Y.; Ong, J.L. A study on functionally graded HA coatings processed using ion beam assisted deposition with in situ heat treatment. Surf. Coat. Technol. 2006, 200, 6111–6116. [Google Scholar] [CrossRef]
- Rabiei, A.; Thomas, B.; Neville, B.; Lee, J.W.; Cuomo, J. A novel technique for processing functionally graded HA coatings. Mater. Sci. Eng. C 2007, 27, 523–528. [Google Scholar] [CrossRef]
- Bai, X.; Sandukas, S.; Appleford, M.R.; Ong, J.L.; Rabiei, A. Deposition and investigation of functionally graded calcium phosphate coatings on titanium. Acta Biomater. 2009, 5, 3563–3572. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, A.; Blalock, T.; Thomas, B.; Cuomo, J.; Yang, Y.; Ong, J. Microstructure, mechanical properties, and biological response to functionally graded HA coatings. Mater. Sci. Eng. C 2007, 27, 529–533. [Google Scholar] [CrossRef]
- Manjubala, I.; Kumar, T.S. Effect of TiO2–Ag2O additives on the formation of calcium phosphate based functionally graded bioceramics. Biomaterials 2000, 21, 1995–2002. [Google Scholar] [CrossRef]
- Roy, M.; Krishna Balla, V.; Bandyopadhyay, A.; Bose, S. Compositionally graded hydroxyapatite/tricalcium phosphate coating on Ti by laser and induction plasma. Acta Biomater. 2011, 7, 866–873. [Google Scholar] [CrossRef] [PubMed]
- Marković, S.; Lukić, M.J.; Škapin, S.D.; Stojanović, B.; Uskoković, D. Designing, fabrication and characterization of nanostructured functionally graded HAp/BCP ceramics. Ceram. Int. 2015, 41, 2654–2667. [Google Scholar] [CrossRef]
- Farnoush, H.; Aldıç, G.; Çimenoğlu, H. Functionally graded HA–TiO2 nanostructured composite coating on Ti–6Al–4V substrate via electrophoretic deposition. Surf. Coat. Technol. 2015, 265, 7–15. [Google Scholar] [CrossRef]
- Kumar, R.R.; Wang, M. Modulus and hardness evaluations of sintered bioceramic powders and functionally graded bioactive composites by nano-indentation technique. Mater. Sci. Eng. A 2002, 338, 230–236. [Google Scholar] [CrossRef]
- Cattini, A.; Bellucci, D.; Sola, A.; Pawłowski, L.; Cannillo, V. Microstructural design of functionally graded coatings composed of suspension plasma sprayed hydroxyapatite and bioactive glass. J. Biomed. Mater. Res. B Appl. Biomater. 2014, 102, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Manjubala, I.; Sivakumar, M.; Sampath Kumar, T.S.; Panduranga Rao, K. Synthesis and characterization of functional gradient materials using Indian corals. J. Mater. Sci. Mater. Med. 2000, 11, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; More, K.; Rouleau, C.M.; Rabiei, A. Functionally graded hydroxyapatite coatings doped with antibacterial components. Acta Biomater. 2010, 6, 2264–2273. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Olsen Horton, C.; Guelcher, S.A.; Goldstein, A.S.; Whittington, A.R. Fabrication of a model continuously graded co-electrospun mesh for regeneration of the ligament-bone interface. Acta Biomater. 2011, 7, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Samavedi, S.; Guelcher, S.A.; Goldsteina, A.S.; Whittington, A.R. Response of bone marrow stromal cells to graded co-electrospun scaffolds and its implications for engineering the ligament-bone interface. Biomaterials 2012, 33, 7727–7735. [Google Scholar] [CrossRef] [PubMed]
- Erisken, C.; Kalyon, D.M.; Wang, H. Functionally graded electrospun polycaprolactone and β-tricalcium phosphate nanocomposites for tissue engineering applications. Biomaterials 2008, 29, 4065–4073. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yang, D.; Yan, W.; Shi, Z.; Feng, J.; Gao, Y.; Weng, W.; Yan, S. Osteochondral repair using the combination of fibroblast growth factor and amorphous calcium phosphate/poly(l-lactic acid) hybrid materials. Biomaterials 2007, 28, 3091–3100. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Chen, K.C.; Chan, Y.J.; Tsai, C.C.; Chen, H.H.; Shih, C.C. Dental implant failure associated with bacterial infection and long-term bisphosphonate usage: A case report. Implant Dent. 2012, 21, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Virk, A.; Osmon, D.R. Prosthetic joint infection. Curr. Treat. Opt. Infect. Dis. 2001, 3, 287–300. [Google Scholar]
- Mombelli, A. Microbiology and antimicrobial therapy of peri-implantitis. Periodontol 2000, 28, 177–189. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. Implant infections and infection-resistant materials. In Degradation of Implant Materials; Eliaz, N., Ed.; Springer Science + Business Media: New York, NY, USA, 2012; Chapter 13; pp. 347–357. [Google Scholar]
- Kienapfel, H.; Kühn, K.D. (Eds.) The Infected Implant; Springer: Berlin, Germany, 2009. [Google Scholar]
- Khosravi, A.D.; Ahmadi, F.; Salmanzadeh, S.; Dashtbozorg, A.; Montazeri, E.A. Study of bacteria isolated from orthopedic implant infections and their antimicrobial susceptibility pattern. Res. J. Microbiol. 2009, 4, 158–163. [Google Scholar] [CrossRef]
- Kühn, K.D.; Vogt, S. Local Antibiotics in Arthroplasty: State of the Art from an Interdisciplinary Point of View; Ghim, W., Ed.; Georg Thieme: Stuttgart, Germany, 2007; pp. 23–30. [Google Scholar]
- Trampuz, A.; Zimmerli, W. Antimicrobial agents in orthopaedic surgery: Prophylaxis and treatment. Drugs 2006, 66, 1089–1105. [Google Scholar] [CrossRef] [PubMed]
- Toms, A.D.; Davidson, D.; Masri, B.A.; Duncan, C.P. The management of peri-prosthetic infection in total joint arthroplasty. J. Bone Jt. Surg. Br. 2006, 88, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Darouiche, R.O.; Mansouri, M.D.; Zakarevicz, D.; Alsharif, A.; Landon, G.C. In vivo efficacy of antimicrobial-coated devices. J. Bone Jt. Surg. Am. 2007, 89, 792–797. [Google Scholar] [CrossRef]
- Moskowitz, J.S.; Blaisse, M.R.; Samuel, R.E.; Hsu, H.P.; Harris, M.B.; Martin, S.D.; Lee, J.C.; Spector, M.; Hammond, P.T. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials 2010, 31, 6019–6030. [Google Scholar] [CrossRef] [PubMed]
- Moojen, D.J.F. Implant-Related Infections: Application of PCR-Based Diagnostics and New Antimicrobial Strategies in Prevention and Treatment. Ph.D. Thesis, University Medical Center Utrecht, Utrecht, The Netherlands, 2010. [Google Scholar]
- Sudo, A.; Hasegawa, M.; Fukuda, A.; Uchida, A. Treatment of infected hip arthroplasty with antibiotic-impregnated calcium hydroxyapatite. J. Arthroplast. 2008, 23, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.; Curry, A.; Gambhir, A.K.; Panigrahi, H.; Walker, C.R.; Wilkins, E.G.; Worsley, M.A.; Kay, P.R. Intraoperative bacterial contamination in operations for joint replacement. J. Bone Jt. Surg. Br. 1999, 81, 886–889. [Google Scholar] [CrossRef]
- Isiklar, Z.U.; Darouiche, R.O.; Landon, G.C.; Beck, T. Efficacy of antibiotics alone for orthopaedic device related infections. Clin. Orthop. Relat. Res. 1996, 332, 184–189. [Google Scholar] [CrossRef]
- Trampuz, A.; Zimmerli, W. Prosthetic joint infections: Update in diagnosis and treatment. Swiss Med. Wkly. 2005, 135, 243–251. [Google Scholar] [PubMed]
- Bose, S.; Tarafder, S.; Edgington, J.; Bandyopadhyay, A. Calcium phosphate ceramics in drug delivery. JOM 2011, 63, 93–98. [Google Scholar] [CrossRef]
- Govindan, R.; Girija, E. Drug loaded phosphate glass/hydroxyapatite nanocomposite for orthopedic applications. J. Mater. Chem. B 2014, 2, 5468–5477. [Google Scholar] [CrossRef]
- Laurent, F.; Bignon, A.; Goldnadel, J.; Chevalier, J.; Fantozzi, G.; Viguier, E.; Roger, T.; Boivin, G.; Hartmann, D. A new concept of gentamicin loaded HAP/TCP bone substitute for prophylactic action: In vitro release validation. J. Mater. Sci. Mater. Med. 2008, 19, 947–951. [Google Scholar] [CrossRef] [PubMed]
- Brohede, U.; Forsgren, J.; Roos, S.; Mihranyan, A.; Engqvist, H.; Strømme, M. Multifunctional implant coatings providing possibilities for fast antibiotics loading with subsequent slow release. J. Mater. Sci. Mater. Med. 2009, 20, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Mohan, N.; Yokogawa, Y.; Varma, H. Pulsed laser deposition of hydroxyapatite on nanostructured titanium towards drug eluting implants. Mater. Sci. Eng. C 2013, 33, 2899–2904. [Google Scholar] [CrossRef]
- Baro, M.; Sánchez, E.; Delgado, A.; Perera, A.; Évora, C. In vitro–in vivo characterization of gentamicin bone implants. J. Control. Release 2002, 83, 353–364. [Google Scholar] [CrossRef]
- Altomare, L.; Visai, L.; Bloise, N.; Arciola, C.R.; Ulivi, L.; Canadiani, G.; Cigada, A.; Chiesa, R.; De Nardo, L. Electrochemically deposited gentamicin-loaded calcium phosphate coating for bone tissue integration. Int. J. Artif. Organs 2012, 35, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, M.; Mystiridou, E.; Mouzakis, D.; Zaoutsos, S.; Fatouros, D.G.; Bouropoulos, N. Preparation, characterization and in vitro assessment of ibuprofen loaded calcium phosphate/gypsum bone cements. Cryst. Res. Technol. 2016, 51, 41–48. [Google Scholar] [CrossRef]
- Shao, F.; Liu, L.; Fan, K.; Cai, Y.; Yao, J. Ibuprofen loaded porous calcium phosphate nanospheres for skeletal drug delivery system. J. Mater. Sci. 2012, 47, 1054–1058. [Google Scholar] [CrossRef]
- Peter, B.; Pioletti, D.P.; Laïb, S.; Bujoli, B.; Pilet, P.; Janvier, P.; Guicheux, J.; Zambelli, P.Y.; Bouler, J.M.; Gauthier, O. Calcium phosphate drug delivery system: Influence of local zoledronate release on bone implant osteointegration. Bone 2005, 36, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, M.; Matsuda, Y.; Suwa, Y.; Fox, J.L.; Higuchi, W.I. A novel skeletal drug-delivery system using self-setting calcium phosphate cement. 4. Effects of the mixing solution volume on the drug-release rate of heterogeneous aspirin-loaded cement. J. Pharm. Sci. 1994, 83, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Takechi, M.; Miyamoto, Y.; Ishikawa, K.; Nagayama, M.; Kon, M.; Asaoka, K.; Suzuki, K. Effects of added antibiotics on the basic properties of anti-washout-type fast-setting calcium phosphate cement. J. Biomed. Mater. Res. 1998, 39, 308–316. [Google Scholar] [CrossRef]
- Ratier, A.; Gibson, I.R.; Best, S.M.; Freche, M.; Lacout, J.L.; Rodriguez, F. Setting characteristics and mechanical behaviour of a calcium phosphate bone cement containing tetracycline. Biomaterials 2001, 22, 897–901. [Google Scholar] [CrossRef]
- Luginbuehl, V.; Ruffieux, K.; Hess, C.; Reichardt, D.; von Rechenberg, B.; Nuss, K. Controlled release of tetracycline from biodegradable β-tricalcium phosphate composites. J. Biomed. Mater. Res. Part B Appl. Biomater. 2010, 92, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Radin, S.; Campbell, J.T.; Ducheyne, P.; Cuckler, J.M. Calcium phosphate ceramic coatings as carriers of vancomycin. Biomaterials 1997, 18, 777–782. [Google Scholar] [CrossRef]
- Gautier, H.; Daculsi, G.; Merle, C. Association of vancomycin and calcium phosphate by dynamic compaction: In vitro characterization and microbiological activity. Biomaterials 2001, 22, 2481–2487. [Google Scholar] [CrossRef]
- Fu, C.; Song, B.; Wan, C.; Savino, K.; Wang, Y.; Zhang, X.; Yates, M.Z. Electrochemical growth of composite hydroxyapatite coatings for controlled release. Surf. Coat. Technol. 2015, 276, 618–625. [Google Scholar] [CrossRef]
- Hayes, G.; Gibson, T. A review of local antibiotic implants and applications to veterinary orthopedic surgery. Vet. Comp. Orthop. Traumatol. 2013, 26, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Van de Belt, H.; Neut, D.; Schenk, W.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J. Infection of orthopedic implants and the use of antibiotic-loaded bone cements. Acta Orthop. Scand. 2001, 72, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, M.; Manjubala, I.; Rao, K.P. Preparation, characterization and in-vitro release of gentamicin from coralline hydroxyapatite-chitosan composite microspheres. Carbohydr. Polym. 2002, 49, 281–288. [Google Scholar] [CrossRef]
- Belcarz, A.; Ginalska, G.; Zalewska, J.; Rzeski, W.; Slósarczyk, A.; Kowalczuk, D.; Godlewski, P.; Niedźwiadek, J. Covalent coating of hydroxyapatite by keratin stabilizes gentamicin release. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 89, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Tadic, D.; Welzel, T.; Seidel, P.; Wüst, E.; Dingeldein, E.; Epple, M. Controlled release of gentamicin from biomimetic calcium phosphate in vitro. Comparison of four different incorporation methods. Materialwissenschaft und Werkstofftechnik 2004, 35, 1001–1005. [Google Scholar] [CrossRef]
- Sivakumar, M.; Rao, K.P. Preparation, characterization and in vitro release of gentamicin from coralline hydroxyapatite-gelatin composite microspheres. Biomaterials 2002, 23, 3175–3181. [Google Scholar] [CrossRef]
- Goodman, S.B.; Yao, Z.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [PubMed]
- Stigter, M.; Bezemer, J.; de Groot, K.; Layrolle, P. Incorporation of different antibiotics into carbonated hydroxyapatite coatings on titanium implants, release and antibiotic efficacy. J. Control. Release 2004, 99, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Stigter, M.; de Groot, K.; Layrolle, P. Incorporation of tobramycin into biomimetic hydroxyapatite coating on titanium. Biomaterials 2002, 23, 4143–4153. [Google Scholar] [CrossRef]
- Thomas, M.B.; Metoki, N.; Mandler, D.; Eliaz, N. In situ potentiostatic deposition of calcium phosphate with gentamicin-loaded chitosan nanoparticles on titanium alloy surfaces. Electrochim. Acta 2016, 222, 355–360. [Google Scholar] [CrossRef]
- Zilberman, M.; Elsner, J.J. Antibiotic-eluting medical devices for various applications. J. Control. Release 2008, 130, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Metoki, N.; Geuli, O.; Sharabani-Yosef, O.; Zada, T.; Reches, M.; Mandler, D.; Eliaz, N. Quickly manufactured, drug eluting, calcium phosphate composite coating. ChemistrySelect 2017, 2, 753–758. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. A pharmacological and toxicological profile of silver as an antimicrobial agent in medical devices. Adv. Pharmacol. Sci. 2010, 2010, 910686. [Google Scholar] [CrossRef] [PubMed]
- Lansdown, A.B. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [PubMed]
- Xie, C.; Lu, X.; Wang, K. Pulse electrochemical synthesis of spherical hydroxyapatite and silver nanoparticles mediated by the polymerization of polypyrrole on metallic implants for biomedical applications. Part. Part. Syst. Charact. 2015, 32, 630–635. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Li, C.; Huang, Y.; Ding, Q.; Pang, X. Preparation and characterization of chitosan-silver/hydroxyapatite composite coatings on TiO2 nanotube for biomedical applications. Appl. Surf. Sci. 2015, 332, 62–69. [Google Scholar] [CrossRef]
- Orozco Carmona, V.; Martinez Pérez, C.; de Lima, R.; Fraceto, L.F.; Romero García, J.; Ledezma Pérez, E.; Marke, S.; Rodríguez González, C.; Macías, A.H.; Martínez-Villafa, A. Effect of silver nanoparticles in a hydroxyapatite coating applied by atmospheric plasma spray. Int. J. Electrochem. Sci. 2014, 9, 7471–7494. [Google Scholar]
- Yan, Y.; Zhang, X.; Huang, Y.; Ding, Q.; Pang, X. Antibacterial and bioactivity of silver substituted hydroxyapatite/TiO2 nanotube composite coatings on titanium. Appl. Surf. Sci. 2014, 314, 348–357. [Google Scholar] [CrossRef]
- Chimutengwende-Gordon, M.; Pendegrass, C.; Bayston, R.; Blunn, G. Preventing infection of osseointegrated transcutaneous implants: Incorporation of silver into preconditioned fibronectin-functionalized hydroxyapatite coatings suppresses Staphylococcus aureus colonization while promoting viable fibroblast growth in vitro. Biointerphases 2014, 9, 031010. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.M.; Lu, X.; Wang, K.F.; Meng, F.Z.; Jiang, O.; Zhang, H.P.; Zhi, W.; Fang, L.M. Silver nanoparticles and growth factors incorporated hydroxyapatite coatings on metallic implant surfaces for enhancement of osteoinductivity and antibacterial properties. ACS Appl. Mater. Interfaces 2014, 6, 8580–8589. [Google Scholar] [CrossRef] [PubMed]
- Massa, M.A.; Covarrubias, C.; Bittner, M.; Fuentevilla, I.A.; Capetillo, P.; von Marttens, A.; Carvajal, J.C. Synthesis of new antibacterial composite coating for titanium based on highly ordered nanoporous silica and silver nanoparticles. Mater. Sci. Eng. C 2014, 45, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Eraković, S.; Janković, A.; Matic, I.Z.; Juranić, Z.D.; Vukasinovic-Sekulic, M.; Stevanović, T.; Mišković-Stanković, V. Investigation of silver impact on hydroxyapatite/lignin coatings electrodeposited on titanium. Mater. Chem. Phys. 2013, 142, 521–530. [Google Scholar] [CrossRef]
- Ionita, D.; Ungureanu, C.; Demetrescu, I. Electrochemical and antibacterial performance of CoCrMo alloy coated with hydroxyapatite or silver nanoparticles. J. Mater. Eng. Perform. 2013, 22, 3584–3591. [Google Scholar] [CrossRef]
- Pishbin, F.; Mourino, V.; Gilchrist, J.B.; McComb, D.W.; Kreppel, S.; Salih, V.; Ryan, M.P.; Boccaccini, A.R. Single-step electrochemical deposition of antimicrobial orthopaedic coatings based on a bioactive glass/chitosan/nano-silver composite system. Acta Biomater. 2013, 9, 7469–7479. [Google Scholar] [CrossRef] [PubMed]
- Eraković, S.; Janković, A.; Veljović, D.; Palcevskis, E.; Mitrić, M.; Stevanović, T.; Janaćković, D.; Mišković-Stanković, V. Corrosion stability and bioactivity in simulated body fluid of silver/hydroxyapatite and silver/hydroxyapatite/lignin coatings on titanium obtained by electrophoretic deposition. J. Phys. Chem. B 2013, 117, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Bir, F.; Khireddine, H.; Touati, A.; Sidane, D.; Yala, S.; Oudadesse, H. Electrochemical depositions of fluorohydroxyapatite doped by Cu2+, Zn2+, Ag+ on stainless steel substrates. Appl. Surf. Sci. 2012, 258, 7021–7030. [Google Scholar] [CrossRef]
- Zhang, F.; Lin, L.X.; Wang, G.W. A high-throughput electrochemical impedance spectroscopy evaluation of bioresponsibility of the titanium microelectrode array integrated with hydroxyapatite and silver. Electrochim. Acta 2012, 85, 152–161. [Google Scholar] [CrossRef]
- Ionita, D.; Dilea, M.; Titorencu, I.; Demetrescu, I. Merit and demerit effects of silver nanoparticles in the bioperformance of an electrodeposited hydroxyapatite: Nanosilver composite coating. J. Nanopart. Res. 2012, 14, 1152. [Google Scholar] [CrossRef]
- Ghani, Y.; Coathup, M.J.; Hing, K.A.; Blunn, G.W. Development of a hydroxyapatite coating containing silver for the prevention of peri-prosthetic infection. J. Orthop. Res. 2012, 30, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, B.; Wang, Y.; Zhou, X.; Weng, J.; Qu, S.; Feng, B.; Watari, F.; Ding, Y.; Leng, Y. Nano-Ag-loaded hydroxyapatite coatings on titanium surfaces by electrochemical deposition. J. R. Soc. Interface 2011, 8, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, Y.; Lu, X.; Zhou, X.; Qu, S.; Feng, B.; Weng, J. Preparation of HA/Ag composite coatings by pulse electrochemical deposition on titanium substrate. Rare Met. Mater. Eng. 2010, 39, 1835–1839. [Google Scholar]
- Pang, X.; Zhitomirsky, I. Electrodeposition of hydroxyapatite-silver-chitosan nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 3815–3821. [Google Scholar] [CrossRef]
- Simon, V.; Albon, C.; Simon, S. Silver release from hydroxyapatite self-assembling calcium-phosphate glasses. J. Non-Cryst. Solids 2008, 354, 1751–1755. [Google Scholar] [CrossRef]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Dingeldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticulate silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, X.; Mao, H.; Li, T.; Zhao, R.; Yan, Y.; Pang, X. Osteoblastic cell responses and antibacterial efficacy of Cu/Zn co-substituted hydroxyapatite coatings on pure titanium using electrodeposition method. RSC Adv. 2015, 5, 17076–17086. [Google Scholar] [CrossRef]
- Grenho, L.; Salgado, C.L.; Fernandes, M.H.; Monteiro, F.J.; Ferraz, M.P. Antibacterial activity and biocompatibility of three-dimensional nanostructured porous granules of hydroxyapatite and zinc oxide nanoparticles—An in vitro and in vivo study. Nanotechnology 2015, 26, 315101. [Google Scholar] [CrossRef] [PubMed]
- Morejón-Alonso, L.; García Carrodeguas, R.; García-Menocal, J.A.D.; Pérez, J.A.A.; Manent, S.M. Effect of sterilization on the properties of CDHA-OCP-β-TCP biomaterial. Mater. Res. 2007, 10, 15–20. [Google Scholar] [CrossRef]
- Goldman, M.; Pruitt, L. Comparison of the effects of gamma radiation and low temperature hydrogen peroxide gas plasma sterilization on the molecular structure, fatigue resistance, and wear behavior of UHMWPE. J. Biomed. Mater. Res. 1998, 40, 378–384. [Google Scholar] [CrossRef]
- Noah, E.M.; Chen, J.; Jiao, X.; Heschel, I.; Pallua, N. Impact of sterilization on the porous design and cell behavior in collagen sponges prepared for tissue engineering. Biomaterials 2002, 23, 2855–2861. [Google Scholar] [CrossRef]
- Takechi, M.; Miyamoto, Y.; Momota, Y.; Yuasa, T.; Tatehara, S.; Nagayama, M.; Ishikawa, K. Effects of various sterilization methods on the setting and mechanical properties of apatite cement. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Suwanprateeb, J.; Tanner, K.E.; Turner, S.; Bonfield, W. Influence of sterilization by gamma irradiation and of thermal annealing on creep of hydroxyapatite-reinforced polyethylene composites. J. Biomed. Mater. Res. Part A 1998, 39, 16–22. [Google Scholar] [CrossRef]
- Smith, D.; Pilliar, R.; Chernecky, R. Dental implant materials. I. Some effects of preparative procedures on surface topography. J. Biomed. Mater. Res. 1991, 25, 1045–1068. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Schmitt, M.; Bouler, J.M.; Daculsi, G. Chemical transformation of some biologically relevant calcium phosphates in aqueous media during a steam sterilization. J. Mater. Sci. Mater. Med. 2000, 11, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Jarry, C.; Chaput, C.; Chenite, A.; Renaud, M.A.; Buschmann, M.; Leroux, J.C. Effects of steam sterilization on thermogelling chitosan-based gels. J. Biomed. Mater. Res. 2001, 58, 127–135. [Google Scholar] [CrossRef]
- Whyte, M.; Brockhurst, P. The effect of steam sterilization on the properties of set dental gypsum models. Aust. Dent. J. 1996, 41, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Barbieri, D.; Davison, N.; Yan, Y.; de Bruijn, J.D.; Yuan, H. Zinc in calcium phosphate mediates bone induction: In vitro and in vivo model. Acta Biomater. 2014, 10, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Komath, M.; Varma, H. Development of a fully injectable calcium phosphate cement for orthopedic and dental applications. Bull. Mater. Sci. 2003, 26, 415–422. [Google Scholar] [CrossRef]
- Li, X.; Guo, B.; Xiao, Y.; Yuan, T.; Fan, Y.; Zhang, X. Influences of the steam sterilization on the properties of calcium phosphate porous bioceramics. J. Mater. Sci. Mater. Med. 2016, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Gomes, P.S.; Duarte, J.A.; Franke, R.P.; Almeida, M.M.; Costa, M.E.; Fernandes, M.H. Relevance of the sterilization-induced effects on the properties of different hydroxyapatite nanoparticles and assessment of the osteoblastic cell response. J. R. Soc. Interface 2012, 9, 3397–3410. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.C.; Ju, C.P.; Lin, J.H.C. Gamma-radiation effect on morphology and properties of TTCP/DCPA-derived calcium phosphate cement. Mater. Trans. 2005, 46, 1701–1705. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Ishikawa, K.; Takechi, M.; Toh, T.; Yoshida, Y.; Nagayama, M.; Kon, M.; Asaoka, K. Tissue response to fast-setting calcium phosphate cement in bone. J. Biomed. Mater. Res. 1997, 37, 457–464. [Google Scholar] [CrossRef]
- Lebugle, A.; Rodrigues, A.; Bonnevialle, P.; Voigt, J.J.; Canal, P.; Rodriguez, F. Study of implantable calcium phosphate systems for the slow release of methotrexate. Biomaterials 2002, 23, 3517–3522. [Google Scholar] [CrossRef]
- Zahraoui, C.; Sharrock, P. Influence of sterilization on injectable bone biomaterials. Bone 1999, 25 (Suppl. 2), 63S–65S. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Helebrant, A.; Jonasova, L.; Sanda, L. The influence of simulated body fluid composition on carbonated hydroxyapatite formation. Ceramics 2002, 46, 9–14. [Google Scholar]
- Oyane, A.; Kim, H.M.; Furuya, T.; Kokubo, T.; Miyazaki, T.; Nakamura, T. Preparation and assessment of revised simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 65, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Filgueiras, M.R.; La Torre, G.; Hench, L.L. Solution effects on the surface reactions of a bioactive glass. J. Biomed. Mater. Res. 1993, 27, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Takadama, H.; Hashimoto, M.; Mizuno, M.; Kokubo, T. Round-robin test of SBF for in vitro measurement of apatite-forming ability of synthetic materials. Phosphorus Res. Bull. 2004, 17, 119–125. [Google Scholar] [CrossRef]
- Standard Test Method for Conducting Cyclic Potentiodynamic Polarization Measurements to Determine the Corrosion Susceptibility of Small Implant Devices; ASTM F2129-15; ASTM International: West Conshohocken, PA, USA, 2015.
- Eliaz, N. Biomaterials and corrosion. In Corrosion Science and Technology; Kamachi Mudali, U., Raj, B., Eds.; Alpha Science International: Oxford, UK, 2008; Chapter 12; pp. 356–397. [Google Scholar]
- Ghanaati, S.; Barbeck, M.; Orth, C.; Willershausen, I.; Thimm, B.W.; Hoffmann, C.; Rasic, A.; Sader, R.A.; Unger, R.E.; Peters, F.; et al. Influence of β-tricalcium phosphate granule size and morphology on tissue reaction in vivo. Acta Biomater. 2010, 6, 4476–4487. [Google Scholar] [CrossRef] [PubMed]
- Okuda, T.; Ioku, K.; Yonezawa, I.; Minagi, H.; Kawachi, G.; Gonda, Y.; Murayama, H.; Shibata, Y.; Minami, S.; Kamihira, S.; et al. The effect of the microstructure of β-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials 2007, 28, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Cancedda, R.; Giannoni, P.; Mastrogiacomo, M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials 2007, 28, 4240–4250. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Zaazou, M.H.; Chow, L.C.; Mahmoud, A.A.; Zaki, D.Y.; Basha, M.; Abdel Hamid, M.A.; Khallaf, M.E.; Sharaf, N.F.; Hamdy, T.M. Injectable nanoamorphous calcium phosphate based in situ gel systems for the treatment of periapical lesions. Biomed. Mater. 2015, 10, 065006. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.P.; Patka, P.; Wolke, J.G.; de Blieck-Hogervorst, J.M.; de Groot, K. Long-term in vivo study of plasma sprayed coatings on titanium alloys of tetracalcium phosphate, hydroxyapatite and alpha-tricalcium phosphate. Biomaterials 1994, 15, 146–150. [Google Scholar] [CrossRef]
- Dupraz, A.; Rohanizadeh, R.; Delecrin, J.; Pilet, P.; Passuti, N.; Daculsi, G. Ultrastructural study of long term implanted Ca-P particulate materials into rabbit bones. In Bioceramics; Sedel, L., Rey, C., Eds.; Elsevier: Oxford, UK, 1997; Volume 10, pp. 191–194. [Google Scholar]
- Hashemi, A.; Bednar, D.; Ziada, S. Pullout strength of pedicle screws augmented with particulate calcium phosphate: An experimental study. Spine J. 2009, 9, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lord, M.S.; Foss, M.; Besenbacher, F. Influence of nanoscale surface topography on protein adsorption and cellular response. Nano Today 2010, 5, 66–78. [Google Scholar] [CrossRef]
- Martínez, E. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann. Anat. Anat. Anz. 2009, 191, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Appleford, M.R.; Oh, S.; Cole, J.A.; Carnes, D.L.; Lee, M.; Bumgardner, J.D.; Haggard, W.O.; Ong, J.L. Effects of trabecular calcium phosphate scaffolds on stress signaling in osteoblast precursor cells. Biomaterials 2007, 28, 2747–2753. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Ohashi, R.; Yokogawa, Y.; Nishizawa, K.; Nagata, F.; Kawamoto, Y.; Kameyama, T.; Toriyama, M. Initial anchoring and proliferation of fibroblast L-929 cells on unstable surface of calcium phosphate ceramics. J. Biosci. Bioeng. 1999, 87, 320–327. [Google Scholar] [CrossRef]
- Hesaraki, S.; Nezafati, N. In vitro biocompatibility of chitosan/hyaluronic acid-containing calcium phosphate bone cements. Bioprocess. Biosyst. Eng. 2014, 37, 1507–1516. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Pan, F.; Yu, G.; Yang, L.; Zhang, E.; Yang, K. In vitro and in vivo evaluation of the surface bioactivity of a calcium phosphate coated magnesium alloy. Biomaterials 2009, 30, 1512–1523. [Google Scholar] [CrossRef] [PubMed]
- Shu, R.; McMullen, R.; Baumann, M.J.; McCabe, L.R. Hydroxyapatite accelerates differentiation and suppresses growth of MC3T3-E1 osteoblasts. J. Biomed. Mater. Res. A 2003, 67, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.V.; Story, B.J.; La, D.; Wagner, W.R.; LeGeros, J.P. Highly crystalline MP-1 hydroxylapatite coating. Part I: In vitro characterization and comparison to other plasma-sprayed hydroxylapatite coatings. Clin. Oral Implants Res. 1999, 10, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Gomi, K.; Lowenberg, B.; Shapiro, G.; Davies, J.E. Resorption of sintered synthetic hydroxyapatite by osteoclasts in vitro. Biomaterials 1993, 14, 91–96. [Google Scholar] [CrossRef]
- Malik, M.A.; Puleo, D.A.; Bizios, R.; Doremus, R.H. Osteoblasts on hydroxyapatite, alumina and bone surfaces in vitro: Morphology during the first 2 h of attachment. Biomaterials 1992, 13, 123–128. [Google Scholar] [CrossRef]
- Jalota, S.; Bhaduri, S.B.; Tas, A.C. In vitro testing of calcium phosphate (HA, TCP, and biphasic HA-TCP) whiskers. J. Biomed. Mater. Res. A 2006, 78, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Le Nihouannen, D.; Daculsi, G.; Saffarzadeh, A.; Gauthier, O.; Delplace, S.; Pilet, P.; Layrolle, P. Ectopic bone formation by microporous calcium phosphate ceramic particles in sheep muscles. Bone 2005, 36, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Burgess, A.V.; Story, B.J.; Wagner, W.R.; Trisi, P.; Pikos, M.A.; Guttenberg, S.A. Highly crystalline MP-1 hydroxylapatite coating. Part II: In vivo performance on endosseous root implants in dogs. Clin. Oral Implants Res. 1999, 10, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Kon, E.; Muraglia, A.; Corsi, A.; Bianco, P.; Marcacci, M.; Martin, I.; Boyde, A.; Ruspantini, I.; Chistolini, P.; Rocca, M.; et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J. Biomed. Mater. Res. 2000, 49, 328–337. [Google Scholar] [CrossRef]
- Meenaghan, M.A.; Natiella, J.R.; Moresi, J.L.; Flynn, H.E.; Wirth, J.E.; Baier, R.E. Tissue response to surface-treated tantalum implants: Preliminary observations in primates. J. Biomed. Mater. Res. 1979, 13, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Baier, R.E.; Meyer, A.E.; Natiella, J.R.; Natiella, R.R.; Carter, J.M. Surface properties determine bioadhesive outcomes: Methods and results. J. Biomed. Mater. Res. 1984, 18, 337–355. [Google Scholar] [CrossRef] [PubMed]
- Baier, R.E. Applied chemistry at protein interfaces. Adv. Chem. Ser. 1975, 145, 1–25. [Google Scholar]
- Bormashenko, E.; Pogreb, R.; Whyman, G.; Bormashenko, Y.; Erlich, M. Vibration-induced Cassie-Wenzel wetting transition on rough surfaces. Appl. Phys. Lett. 2007, 90, 201917. [Google Scholar] [CrossRef]
- Lafuma, A.; Quere, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M.; Noel, B.; Dufresne, E.; Judas, D.; Iost, A.; Hardouin, P. Qualitative and quantitative study of human osteoblast adhesion on materials with various surface roughnesses. J. Biomed. Mater. Res. 2000, 49, 155–166. [Google Scholar] [CrossRef]
- Matsuzaka, K.; Walboomers, X.F.; Yoshinari, M.; Inoue, T.; Jansen, J.A. The attachment and growth behavior of osteoblast-like cells on microtextured surfaces. Biomaterials 2003, 24, 2711–2719. [Google Scholar] [CrossRef]
- Ball, M.D.; Downes, S.; Scotchford, C.A.; Antonov, E.N.; Bagratashvili, V.N.; Popov, V.K.; Lo, W.J.; Grant, D.M.; Howdle, S.M. Osteoblast growth on titanium foils coated with hydroxyapatite by pulsed laser ablation. Biomaterials 2001, 22, 337–347. [Google Scholar] [CrossRef]
- Narayanan, R.; Kim, S.Y.; Kwon, T.Y.; Kim, K.H. Nanocrystalline hydroxyapatite coatings from ultrasonated electrolyte: Preparation, characterization, and osteoblast responses. J. Biomed. Mater. Res. A 2008, 87, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Benayahu, D.; Fried, A.; Efraty, M.; Robey, P.G.; Wientroub, S. Bone marrow interface: Preferential attachment of an osteoblastic marrow stromal cell line. Cell Biochem. 1995, 59, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Weng, J.; Yang, B.C.; Qu, S.X.; Zhang, X.D. Characterization of titanium surfaces with calcium and phosphate and osteoblast adhesion. Biomaterials 2004, 25, 3421–3428. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Son, D.W.; Ong, J.L.; Kim, K.H. Characterization of hydrothermally treated anodic oxides containing Ca and P on titanium. J. Mater. Sci. Mater. Med. 2003, 14, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Cheung, H.S.; Story, M.T.; McCarty, D.J. Mitogenic effects of HA and calcium pyrophosphate dihydrate crystals on cultured mammalian cells. Arthritis Rheum. 1984, 27, 665–674. [Google Scholar] [CrossRef]
- Cheung, H.S.; McCarty, D.J. Mitogenesis induced by calcium containing crystal: Role of intracellular dissolution. Exp. Cell Res. 1985, 157, 63–70. [Google Scholar] [CrossRef]
- Knabe, C.; Gildenhaar, R.; Berger, G.; Ostapowicwz, W.; Fitzer, R.; Radlanski, R.J.; Gross, U. Morphological evaluation of osteoblasts cultured on different calcium phosphate ceramics. Biomaterials 1997, 18, 1339–1347. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Matsudaira, P. Molecular Cell Biology; Freeman & Co.: New York, NY, USA, 2004; pp. 821–823. [Google Scholar]
- Zhu, X.; Chen, J.; Scheideler, L.; Altebaeumer, T.; Geis-Gerstorfer, J.; Kern, D. Cellular reactions of osteoblasts to micron- and submicron-scale porous structures of titanium surfaces. Cells Tissues Organs 2004, 178, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Shur, I.; Zilberman, M.; Benayahu, D.; Einav, S. Adhesion molecule expression by osteogenic cells cultured on various biodegradable scaffolds. J. Biomed. Mater. Res. A 2005, 75, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Olmo, N.; Martín, A.I.; Salinas, A.J.; Turnay, J.; Vallet-Regí, M.; Lizarbe, M.A. Bioactive sol-gel glasses with and without a hydroxycarbonate apatite layer as substrates for osteoblast cell adhesion and proliferation. Biomaterials 2003, 24, 3383–3393. [Google Scholar] [CrossRef]
- Takebe, J.; Itoh, S.; Okada, J.; Ishibashi, K. Anodic oxidation and hydrothermal treatment of titanium results in a surface that causes increased attachment and altered cytoskeletal morphology of rat bone marrow stromal cells in vitro. J. Biomed. Mater. Res. 2000, 51, 398–407. [Google Scholar] [CrossRef]
- Su, W.T.; Chu, I.M.; Yang, J.Y.; Lin, C.D. The geometric pattern of a pillared substrate influences the cell-process distribution and shapes of fibroblasts. Micron 2006, 37, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Wilkinson, C. Topographical control of cells. Biomaterials 1997, 18, 1573–1583. [Google Scholar] [CrossRef]
- Clark, P.; Connolly, P.; Curtis, A.S.G.; Dow, J.A.T.; Wilkinson, C.D.W. Topographical control of cell behaviour: II. Multiple grooved substrata. Development 1990, 108, 635–644. [Google Scholar] [PubMed]
- Zinger, O.; Anselme, K.; Denzer, A.; Habersetzer, P.; Wieland, M.; Jeanfils, J.; Hardouin, P.; Landolt, D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.J.; Sullivan, P.J.; Dong, W.P.; Mainsah, E.; Luo, N.; Mathia, T.; Zahouani, H. The Development of Methods for Characterization of Roughness in Three Dimensions; EUR 15178 EN of Commission of the European Communities; University of Birmingham: Birmingham, UK, 1993. [Google Scholar]
- Boyan, B.D.; Lohmann, C.H.; Dean, D.D.; Sylvia, V.L.; Cochran, D.L.; Schwartz, Z. Mechanisms involved in osteoblast response to implant surface morphology. Ann. Rev. Mater. Res. 2001, 31, 357–371. [Google Scholar] [CrossRef]
- Webster, T.J.; Ergun, C.; Doremus, R.H.; Lanford, W.A. Increased osteoblast adhesion on titanium-coated hydroxylapatite that forms CaTiO3. J. Biomed. Mater. Res. A 2003, 67, 975–980. [Google Scholar] [CrossRef] [PubMed]
- Brentel, A.S.; de Vasconcellos, L.M.; Oliveira, M.V.; Graça, M.L.; de Vasconcellos, L.G.; Cairo, C.A.; Carvalho, Y.R. Histomophometric analysis of pure titanium implants with porous surface vs. rough surface. J. Appl. Oral Sci. 2006, 14, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Peraire, C.; Arias, J.L.; Bernal, D.; Pou, J.; León, B.; Arañó, A.; Roth, W. Biological stability and osteoconductivity in rabbit tibia of pulsed laser deposited hydroxylapatite coatings. J. Biomed. Mater. Res. A 2006, 77, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Hacking, S.A.; Bobyn, J.D.; Tanzer, M.; Krygier, J.J. The osseous response to corundum blasted implant surfaces in a canine hip model. Clin. Orthop. 1999, 364, 240–253. [Google Scholar] [CrossRef]
- Yang, C.Y.; Wang, B.C.; Lee, T.M.; Chang, E.; Chang, G.L. Intramedullary implant of plasma sprayed hydroxyapatite coating: An interface study. J. Biomed. Mater. Res. 1997, 36, 39–48. [Google Scholar] [CrossRef]
- Summer, D.R.; Bryan, J.M.; Urban, R.M.; Kuszak, J.R. Measuring the volume of bone ingrowth: A comparison of three techniques. J. Orthop. Res. 1990, 8, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Feighan, J.E.; Goldberg, V.M.; Davy, D.; Parr, J.A.; Stevenson, S. The influence of surface-blasting on the incorporation of titanium-alloy implants in a rabbit intramedullary model. J. Bone Jt. Surg. Am. 1995, 77, 1380–1395. [Google Scholar] [CrossRef]
- Stewart, M.; Welter, J.F.; Goldberg, V.M. Effect of hydroxyapatite/tricalcium-phosphate coating on osseointegration of plasma-sprayed titanium alloy implants. J. Biomed. Mater. Res. A 2004, 69, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hacking, S.A.; Tanzer, M.; Harvey, E.J.; Krygier, J.J.; Bobyn, J.D. Relative contributions of chemistry and topography to the osseointegration of hydroxyapatite coatings. Clin. Orthop. 2002, 405, 24–38. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Schwabe, P.; Stange, R.; Hoffmann, J.; Südkamp, N.P.; Haas, N.P.; Raschke, M. A new electrochemically graded hydroxyapatite coating for osteosynthetic implants promotes implant osteointegration in a rat model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2002, 63, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Jinno, T.; Goldberg, V.M.; Davy, D.; Stevenson, S. Osseointegration of surface-blasted implants made of titanium alloy and cobalt-chromium alloy in a rabbit intramedullary model. J. Biomed. Mater. Res. 1998, 42, 20–29. [Google Scholar] [CrossRef]
- Oosterbos, C.J.M.; Vogely, H.Ch.; Nijhof, M.W.; Fleer, A.; Verbout, A.J.; Tonino, A.J.; Dhert, W.J. Osseointegration of hydroxyapatite-coated and noncoated Ti6Al4V implants in the presence of local infection: A comparative histomorphometrical study in rabbits. J. Biomed. Mater. Res. 2002, 60, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Jinno, T.; Davy, D.T.; Goldberg, V.M. Comparison of hydroxyapatite and hydroxyapatite tricalcium-phosphate coatings. J. Arthroplast. 2002, 17, 902–909. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of osteoconductive biomaterials: Calcium phosphate. Clin. Orthop. 2002, 395, 725–733. [Google Scholar] [CrossRef]
- Kilpadi, K.L.; Chang, P.L.; Bellis, S.L. Hydroxyapatite binds more serum proteins, purified integrins, and osteoblast precursor cells than titanium or steel. J. Biomed. Mater. Res. 2001, 57, 258–267. [Google Scholar] [CrossRef]
- Overgaard, S.; Bromose, U.; Lind, M.; Bunger, C.; Soballe, K. The influence of crystallinity of the hydroxyapatite coatings on the fixation of implants. Mechanical and histomorphometric result. J. Bone Jt. Surg. Br. 1999, 81, 725–731. [Google Scholar] [CrossRef]
- Wong, M.; Eulenberger, J.; Schenk, R.; Hunziker, E. Effect of surface topology on the osseointegration of implant materials in trabecular bone. J. Biomed. Mater. Res. 1995, 29, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Svehia, M.; Morberg, P.; Zicat, B.; Bruce, W.; Sonnabend, D.; Walsh, W.R. Morphometric and mechanical evaluation of titanium implant integration: Comparison of five surface structures. J. Biomed. Mater. Res. 2000, 51, 15–22. [Google Scholar] [CrossRef]
- Campbell, A.A. Bioceramics for implant coatings. Mater. Today 2003, 6, 26–30. [Google Scholar] [CrossRef]
- Jaffe, W.L.; Scott, D.F. Total hip arthroplasty with hydroxyapatite-coated prostheses. J. Bone Jt. Surg. Am. 1996, 78, 1918–1934. [Google Scholar] [CrossRef]
- Soballe, K.; Overgaard, S. The current status of hydroxyapatite coating of prostheses. J. Bone Jt. Surg. Br. 1996, 78, 689–691. [Google Scholar]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate deposits: Preparation, properties and biomedical applications. Mater. Sci. Eng. C 2015, 55, 272–326. [Google Scholar] [CrossRef] [PubMed]
- Gross, K.; Berndt, C. Thermal processing of hydroxyapatite for coating production. J. Biomed. Mater. Res. 1998, 39, 580–587. [Google Scholar] [CrossRef]
- De Groot, K.; Geesink, R.G.T.; Klein, C.P.A.T.; Serekian, P. Plasma sprayed coatings of hydroxyapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.C.; Chang, E. Influence of residual stress on bonding strength and fracture of plasma-sprayed hydroxyapatite coatings on Ti–6Al–4V substrate. Biomaterials 2001, 22, 1827–1836. [Google Scholar] [CrossRef]
- Takeuchi, S.; Ito, M.; Takeda, K. Modelling of residual stress in plasma-sprayed coatings: Effect of substrate temperature. Surf. Coat. Technol. 1990, 43, 426–435. [Google Scholar] [CrossRef]
- Roşu, R.A.; Şerban, V.A.; Uţu, D.; Locovei, C.; Sfirloaga, P. Characterisation of the titanium-hydroxyapatite biocompatible composite layers deposited by HVOF thermal spraying method. In Proceedings of the Metal 2011, 20th Anniversary International Conference on Metallurgy and Materials, Brno, Czech Republic, 18–20 May 2011; pp. 711–716. [Google Scholar]
- Davison, N.L.; Su, J.; Yuan, H.; van den Beucken, J.J.; de Bruijn, J.D.; de Groot, B.F. Influence of surface microstructure and chemistry on osteoinduction and osteoclastogenesis by biphasic calcium phosphate discs. Eur. Cells Mater. 2015, 29, 314–329. [Google Scholar] [CrossRef]
- Jansen, J.; Wolke, J.G.; Swann, S.; van der Waerden, J.P.; de Groot, K. Application of magnetron sputtering for producing ceramic coatings on implant materials. Clin. Oral Implant Res. 1993, 4, 28–34. [Google Scholar] [CrossRef]
- Arias, J.L.; Mayor, M.B.; Pou, J.; Leng, Y.; León, B.; Pérez-Amor, M. Micro- and nano-testing of calcium phosphate coatings produced by pulsed laser deposition. Biomaterials 2003, 24, 3403–3408. [Google Scholar] [CrossRef]
- Tucker, B.E.; Cottell, C.M.; Auyeung, R.C.; Spector, M.; Nancollas, G.H. Pre-conditioning and dual constant composition dissolution kinetics of pulsed laser deposited hydroxyapatite thin films on silicon substrates. Biomaterials 1996, 17, 631–637. [Google Scholar] [CrossRef]
- Yoshinari, M.; Ohtsuka, Y.; Dérand, T. Thin hydroxyapatite coating produced by the ion beam dynamic mixing method. Biomaterials 1994, 15, 529–535. [Google Scholar] [CrossRef]
- Luo, Z.S.; Cui, F.Z.; Li, W.Z. Low-temperature crystallization of calcium phosphate coatings synthesized by ion-beam-assisted deposition. J. Biomed. Mater. Res. 1999, 46, 80–86. [Google Scholar] [CrossRef]
- Lacefield, W. Hydroxyapatite coatings. Ann. N. Y. Acad. Sci. 1988, 523, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Spoto, G.; Ciliberto, E.; Allen, G.C. A new synthetic route to hydroxyapatite coatings. J. Mater. Chem. 1994, 4, 1849–1850. [Google Scholar] [CrossRef]
- Russell, S.W.; Luptak, K.A.; Suchicital, C.T.A.; Alford, T.L.; Pizziconi, V.B. Chemical and structural evolution of sol–gel-derived hydroxyapatite thin films under rapid thermal processing. J. Am. Ceram. Soc. 1996, 79, 837–842. [Google Scholar] [CrossRef]
- Liu, D.M.; Troczynski, T.; Tseng, W.J. Water-based sol-gel synthesis of hydroxyapatite: Process development. Biomaterials 2001, 22, 1721–1730. [Google Scholar] [CrossRef]
- Ducheyne, P.; Radin, S.; Heughebaert, M.; Heughebaert, J.C. Calcium-phosphate ceramic coatings on porous titanium—Effect of structure and composition on electrophoretic deposition, vacuum sintering and in vitro dissolution. Biomaterials 1990, 11, 244–254. [Google Scholar] [CrossRef]
- Wei, M.; Ruys, A.J.; Milthorpe, B.K.; Sorrell, C.C. Precipitation of hydroxyapatite nanoparticles: Effects of precipitation method on electrophoretic deposition. J. Mater. Sci. Mater. Med. 2005, 16, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, T.M.; Eliaz, N.; Kamachi Mudali, U.; Baldev, R. Electrophoretic deposition of hydroxyapatite coatings and corrosion aspects of metallic implants. Corros. Rev. 2002, 20, 255–293. [Google Scholar] [CrossRef]
- Eliaz, N.; Sridhar, T.M.; Kamachi Mudali, U.; Baldev, R. Electrochemical and electrophoretic deposition of hydroxyapatite for orthopaedic applications. Surf. Eng. 2005, 21, 238–242. [Google Scholar] [CrossRef]
- Wen, H.B.; de Wijn, J.R.; Cui, F.Z.; de Groot, K. Preparation of calcium phosphate coatings on titanium implant materials by simple chemistry. J. Biomed. Mater. Res. 1998, 41, 227–236. [Google Scholar] [CrossRef]
- Tas, A.C. Synthesis of biomimetic Ca-hydroxyapatite powders at 37 degrees C in synthetic body fluids. Biomaterials 2000, 21, 1429–1438. [Google Scholar] [PubMed]
- Redepenning, J.; McIsaac, J.P. Electrocrystallization of brushite coatings on prosthetic alloys. Chem. Mater. 1990, 2, 625–627. [Google Scholar] [CrossRef]
- Royer, P.; Rey, C. Calcium-phosphate coatings for orthopedic prosthesis. Surf. Coat. Technol. 1991, 45, 171–177. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M. Electrochemical preparation of bioactive calcium phosphate coatings on porous substrates by the periodic pulse technique. J. Mater. Sci. Lett. 1993, 12, 16–19. [Google Scholar]
- Vijayaraghavan, T.V.; Bensalem, A. Electrodeposition of apatite coating on pure titanium and titanium-alloys. J. Mater. Sci. Lett. 1994, 13, 1782–1785. [Google Scholar] [CrossRef]
- Shirkhanzadeh, M. Direct formation of nanophase hydroxyapatite on cathodically polarized electrodes. J. Mater. Sci. Mater. Med. 1998, 9, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Eliyahu, M. Electrochemical processes of nucleation and growth of hydroxyapatite on titanium supported by real-time electrochemical atomic force microscopy. J. Biomed. Mater. Res. Part A 2007, 80, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Metoki, N.; Mandler, D.; Eliaz, N. Effect of decorating titanium with different self-assembled monolayers on the electrodeposition of calcium phosphate. Cryst. Growth Des. 2016, 16, 2756–2764. [Google Scholar] [CrossRef]
- Munting, E. The contributions and limitations of hydroxyapatite coatings to implant fixation—A histomorphometric study of load bearing implants in dogs. Int. Orthop. 1996, 20, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Layrolle, P.; de Bruijn, J.; van Blitterswijk, C.A.; de Groot, K. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J. Biomed. Mater. Res. 2001, 57, 327–335. [Google Scholar] [CrossRef]
- Barrere, F.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biomimetic coatings on titanium: A crystal growth study of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2001, 12, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.J.E.M.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovic, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Commun. 2013, 4, 1507. [Google Scholar] [CrossRef] [PubMed]
- Waterman, J.; Pietak, A.; Birbilis, N.; Woodfield, T.; Dias, G.; Staiger, M.P. Corrosion resistance of biomimetic calcium phosphate coatings on magnesium due to varying pretreatment time. Mater. Sci. Eng. B 2011, 176, 1756–1760. [Google Scholar] [CrossRef]
- Sarkar, P.; Nicholson, P.S. Electrophoretic deposition (EPD): Mechanisms, kinetics, and application to ceramics. J. Am. Ceram. Soc. 1996, 79, 1987–2002. [Google Scholar] [CrossRef]
- Wei, M.; Ruys, A.; Milthorpe, B.; Sorrell, C.; Evans, J. Electrophoretic deposition of hydroxyapatite coatings on metal substrates: A nanoparticulate dual-coating approach. J. Sol-Gel Sci. Technol. 2001, 21, 39–48. [Google Scholar] [CrossRef]
- Ma, J.; Liang, C.; Kong, L.; Wang, C. Colloidal characterization and electrophoretic deposition of hydroxyapatite on titanium substrate. J. Mater. Sci. Mater. Med. 2003, 14, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, J.; Cheng, W.; Zhang, R. Thick hydroxyapatite coatings by electrophoretic deposition. Mater. Lett. 2002, 57, 99–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Dunn, M.F.; Xiao, T.; Tomsia, A.P.; Saiz, E. Nanostructured Hydroxyapatite Coatings for Improved Adhesion and Corrosion Resistance for Medical Implants; Cambridge University Press: Cambridge, UK, 2001; Volume 703. [Google Scholar]
- Cui, F.; Luo, Z. Biomaterials modification by ion-beam processing. Surf. Coat. Technol. 1999, 112, 278–285. [Google Scholar] [CrossRef]
- Ohtsuka, Y.; Matsuura, M.; Chida, N.; Yoshinari, M.; Sumii, T.; Dérand, T. Formation of hydroxyapatite coating on pure titanium substrates by ion beam dynamic mixing. Surf. Coat. Technol. 1994, 65, 224–230. [Google Scholar] [CrossRef]
- Cui, F.; Luo, Z.; Feng, Q. Highly adhesive hydroxyapatite coatings on titanium alloy formed by ion beam assisted deposition. J. Mater. Sci. Mater. Med. 1997, 8, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.F.; Johnson, S.; Kumar, D.; Jelinek, M.; Chrisey, D.B.; Doraiswamy, A.; Jin, C.; Narayan, R.J.; Mihailescu, I.N. Pulsed laser deposition of hydroxyapatite thin films. Mater. Sci. Eng. C 2007, 27, 484–494. [Google Scholar] [CrossRef]
- Fernández-Pradas, J.; Sardin, G.; Clèries, L.; Serra, P.; Ferrater, C.; Morenza, J. Deposition of hydroxyapatite thin films by excimer laser ablation. Thin Solid Films 1998, 317, 393–396. [Google Scholar] [CrossRef]
- Lo, W.J.; Grant, D.M.; Ball, M.D.; Welsh, B.S.; Howdle, S.M.; Antonov, E.N.; Bagratashvili, V.N.; Popov, V.K. Physical, chemical, and biological characterization of pulsed laser deposited and plasma sputtered hydroxyapatite thin films on titanium alloy. J. Biomed. Mater. Res. 2000, 50, 536–545. [Google Scholar] [CrossRef]
- Zeng, H.; Lacefield, W.R. XPS, EDX and FTIR analysis of pulsed laser deposited calcium phosphate bioceramic coatings: The effects of various process parameters. Biomaterials 2000, 21, 23–30. [Google Scholar] [CrossRef]
- Cheung, J.; Horwitz, J. Pulsed laser deposition history and laser-target interactions. MRS Bull. 1992, 17, 30–36. [Google Scholar] [CrossRef]
- Uhlmann, D.; Suratwala, T.; Davidson, K.; Boulton, J.; Teowee, G. Sol-gel derived coatings on glass. J. Non-Cryst. Solids 1997, 218, 113–122. [Google Scholar] [CrossRef]
- Guglielmi, M. Sol-gel coatings on metals. J. Sol-Gel Sci. Technol. 1997, 8, 443–449. [Google Scholar] [CrossRef]
- Hu, L.; Yoko, T.; Kozuka, H.; Sakka, S. Effects of solvent on properties of sol-gel-derived TiO2 coating films. Thin Solid Films 1992, 219, 18–23. [Google Scholar] [CrossRef]
- Brinker, C.J.; Scherer, G.W. Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing; Academic Press: Boston, MA, USA, 2013. [Google Scholar]
- Brinker, C.J.; Ashley, C.S.; Cairncross, R.A.; Chen, K.S.; Hurd, A.J.; Reed, S.T.; Samuel, J.; Schunk, P.R.; Schwartz, R.W.; Scotto, C.S. Sol-gel derived ceramic films—Fundamentals and applications. In Metallurgical and Ceramic Protective Coatings; Stern, K.H., Ed.; Chapman & Hall: London, UK, 1996; Chapter 6; pp. 112–151. [Google Scholar]
- Olding, T.; Sayer, M.; Barrow, D. Ceramic sol-gel composite coatings for electrical insulation. Thin Solid Films 2001, 398, 581–586. [Google Scholar] [CrossRef]
- Varma, H.; Kalkura, S.; Sivakumar, R. Polymeric precursor route for the preparation of calcium phosphate compounds. Ceram. Int. 1998, 24, 467–470. [Google Scholar] [CrossRef]
- Gross, K.; Chai, C.; Kannangara, G.; Ben-Nissan, B.; Hanley, L. Thin hydroxyapatite coatings via sol-gel synthesis. J. Mater. Sci. Mater. Med. 1998, 9, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Layrolle, P.; Ito, A.; Tateishi, T. Sol-gel synthesis of amorphous calcium phosphate and sintering into microporous hydroxyapatite bioceramics. J. Am. Ceram. Soc. 1998, 81, 1421–1428. [Google Scholar] [CrossRef]
- Hsieh, M.F.; Perng, L.H.; Chin, T.S.; Perng, H.G. Phase purity of sol-gel-derived hydroxyapatite ceramic. Biomaterials 2001, 22, 2601–2607. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, Y.K.; Kim, K.M.; Kim, K.N. Bioactive calcium phosphate coating prepared on H2O2-treated titanium substrate by electrodeposition. Surf. Coat. Technol. 2005, 195, 252–257. [Google Scholar] [CrossRef]
- Park, J.H.; Lee, D.Y.; Oh, K.T.; Lee, Y.K.; Kim, K.N. Bioactive calcium phosphate coating on sodium hydroxide-pretreated titanium substrate by electrodeposition. J. Am. Ceram. Soc. 2004, 87, 1792–1794. [Google Scholar] [CrossRef]
- Rončević, I.S.; Grubač, Z.; Metikoš-Huković, M. Electrodeposition of hydroxyapatite coating on AZ91D alloy for biodegradable implant application. Int. J. Electrochem. Sci. 2014, 9, 5907–5923. [Google Scholar]
- Stoica, E.D.; Nicolae, M.; Gebert, A. Electrodeposited calcium phosphate coatings on Ti6Al4V and Ti6Al7Nb anodized surfaces with intermediate nanotubes layer. Metal. Int. 2012, 17, 13–16. [Google Scholar]
- Wang, Y.Q.; Tao, J.; Wang, L.; He, P.T.; Wang, T. HA coating on titanium with nanotubular anodized TiO2 intermediate layer via electrochemical deposition. Trans. Nanoferr. Met. Soc. China 2008, 18, 631–635. [Google Scholar] [CrossRef]
- Hanawa, T. Electrochemical techniques to obtain biofunctional materials. In Applications of Electrochemistry and Nanotechnology in Biology and Medicine I; Modern Aspects of Electrochemistry; Eliaz, N., Ed.; Springer Science + Business Media: Berlin, Germany, 2011; Volume 52, pp. 343–376. [Google Scholar]
- Katić, J.; Metikoš-Hukovíca, M.; Škapin, S.D.; Petravić, M.; Varašanec, M. The potential-assisted deposition as valuable tool for producing functional apatite coatings on metallic materials. Electrochim. Acta 2014, 127, 173–179. [Google Scholar] [CrossRef]
- Wang, H.; Eliaz, N.; Hobbs, L.W. The nanostructure of an electrochemically deposited hydroxyapatite coating. Mater. Lett. 2011, 65, 2455–2457. [Google Scholar] [CrossRef]
- Lin, D.Y.; Wang, X.X. A novel method to synthesize hydroxyapatite coating with hierarchical structure. Colloids Surf. B Biointerfaces 2011, 82, 637–640. [Google Scholar] [CrossRef] [PubMed]
- Han, H.M.; Mikhalovsky, S.V.; Phillips, G.J.; Lloyd, A.W. Calcium phosphate sonoelectrodeposition on carbon fabrics and its effect on osteoblast cell viability in vitro. New Carbon Mater. 2007, 22, 121–125. [Google Scholar] [CrossRef]
- Zhang, R.; Metoki, N.; Sharabani-Yosef, O.; Zhu, H.; Eliaz, N. Hydroxyapatite/mesoporous graphene/single-walled carbon nanotubes freestanding flexible hybrid membranes for regenerative medicine. Adv. Funct. Mater. 2016, 26, 7965–7974. [Google Scholar] [CrossRef]
- Callahan, T.J.; Gantenberg, J.B.; Sands, B.E. Calcium phosphate (Ca-P) coating draft guidance for preparation of Food and Drug Administration (FDA) submissions for orthopedic and dental endosseous implants. In ASTM STP 1196: Characterization and Performance of Calcium Phosphate Coatings for Implants; Horowitz, E., Parr, J.E., Eds.; ASTM International: West Conshohocken, PA, USA, 1994; pp. 185–197. [Google Scholar]
- Guidance for Industry and FDA Staff—Class II Special Controls Guidance Document: Root-Form Endosseous Dental Implants and Endosseous Dental Abutments. Document Issued on 12 May 2004. Available online: http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072424.htm (accessed on 4 February 2017).
- Standard Specification for Calcium Phosphate Coatings for Implantable Materials; ASTM F1609-08(2014); ASTM International: West Conshohocken, PA, USA, 2014.
- Implants for Surgery—Hydroxyapatite—Part 2: Coatings of Hydroxyapatite; BS ISO 13779-2:2008; British Standards Institution: London, UK, 2008.
- Standard Test Method for Shear Testing of Calcium Phosphate Coatings and Metallic Coatings; ASTM F1044-05(2011)e1; ASTM International: West Conshohocken, PA, USA, 2011.
- Standard Test Method for Tension Testing of Calcium Phosphate and Metallic Coatings; ASTM F1147-05(2011); ASTM International: West Conshohocken, PA, USA, 2005.
- Implants for Surgery—Hydroxyapatite—Part 4: Determination of Coating Adhesion Strength; BS ISO 13779-4:2002; British Standards Institution: London, UK, 2002.
- Implants for Surgery—Hydroxyapatite—Part 3: Chemical Analysis and Characterization of Crystallinity and Phase Purity Coatings of Hydroxyapatite; BS ISO 13779-3:2008; British Standards Institution: London, UK, 2008.
- Standard Specification for Composition of Hydroxylapatite for Surgical Implants; ASTM F1185-03(2014); ASTM International: West Conshohocken, PA, USA, 2014.
- De Sena, L.A.; de Andrade, M.C.; Rossi, A.M.; de Almeida Soares, G. Hydroxyapatite deposition by electrophoresis on titanium sheets with different surface finishing. J. Biomed. Mater. Res. 2002, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Piveteau, L.D.; Gasser, B.; Schlapbach, L. Evaluating mechanical adhesion of sol-gel titanium dioxide coatings containing calcium phosphate for metal implant application. Biomaterials 2000, 21, 2193–2201. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Bioactive metals prepared by surface modification: Preparation and properties. In Applications of Electrochemistry and Nanotechnology in Biology and Medicine I; Modern Aspects of Electrochemistry; Eliaz, N., Ed.; Springer Science + Business Media: Berlin, Germany, 2011; Volume 52, pp. 377–421. [Google Scholar]
- Nishiguchi, S.; Fujibayashi, S.; Kim, H.M.; Kokubo, T.; Takashi Nakamura, T. Biology of alkali- and heat-treated titanium implants. J. Biomed. Mater. Res. A. 2003, 67, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Miyaji, F.; Kim, H.M. Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J. Am. Ceram. Soc. 1996, 79, 1127–1129. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Nakamura, T. Effect of heat treatment on apatite-forming ability of Ti induced by alkali treatment. J. Mater. Sci. Mater. Med. 1997, 8, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T. Apatite formation on surfaces of ceramics, metals and polymers in body environment. Acta Mater. 1998, 46, 2519–2527. [Google Scholar] [CrossRef]
- Chosa, N.; Taira, M.; Saitoh, S.; Sato, N.; Araki, Y. Characterization of apatite formed on alkaline-heat-treated Ti. J. Dent. Res. 2004, 83, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Bearinger, J.P.; Orme, C.A.; Gilbert, J.L. Effect of hydrogen peroxide on titanium surfaces: In situ imaging and step-polarization impedance spectroscopy of commercially pure titanium and titanium, 6-aluminum, 4-vanadium. J. Biomed. Mater. Res. A 2003, 67, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Rohanizadeh, R.; Al-Sadeq, M.; LeGeros, R.Z. Preparation of different forms of titanium oxide on titanium surface: Effects on apatite deposition. J. Biomed. Mater. Res. A 2004, 71, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Tanahashi, M.; Matsuda, T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 1997, 34, 305–315. [Google Scholar] [CrossRef]
- Metoki, N.; Liu, L.; Beilis, E.; Eliaz, N.; Mandler, D. Preparation and characterization of alkylphosphonic acid self-assembled monolayers on titanium alloy by chemisorption and electrochemical deposition. Langmuir 2014, 30, 6791–6799. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Mandal, T.; Prakash, P.; Garg, A.; Balani, K. Electrophoretic deposition of nanocrystalline hydroxyapatite on Ti6Al4V/TiO2 substrate. J. Coat. Technol. Res. 2013, 10, 263–275. [Google Scholar] [CrossRef]
- Bhawanjali, S.; Revathi, A.; Popat, K.C.; Geetha, M. Surface modification of Ti–13Nb–13Zr and Ti–6Al–4V using electrophoretic deposition (EPD) for enhanced cellular interaction. Mater. Technol. 2014, 29 (Suppl. 1), B54–B58. [Google Scholar] [CrossRef]
- Wen, C.; Guan, S.; Peng, L.; Ren, C.; Wang, X.; Hu, Z. Characterization and degradation behavior of AZ31 alloy surface modified by bone-like hydroxyapatite for implant applications. Appl. Surf. Sci. 2009, 255, 6433–6438. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Li, J.; Zhao, C.; Zhang, X. Electrodeposition of Ca–P coatings on biodegradable Mg alloy: In vitro biomineralization behavior. Acta Biomater. 2010, 6, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Bakhsheshi-Rad, H.; Idris, M.; Abdul-Kadir, M. Synthesis and in vitro degradation evaluation of the nano-HA/MgF2 and DCPD/MgF2 composite coating on biodegradable Mg–Ca–Zn alloy. Surf. Coat. Technol. 2013, 222, 79–89. [Google Scholar] [CrossRef]
- Shirdar, M.R.; Izman, S.; Taheri, M.M.; Assadian, M.; Abdul Kadir, M.R. Effect of post-treatment techniques on corrosion and wettability of hydroxyapatite-coated Co–Cr–Mo alloy. Arab. J. Sci. Eng. 2015, 40, 1197–1203. [Google Scholar] [CrossRef]
- Lee, Y.P.; Wang, C.K.; Huang, T.H.; Chen, C.C.; Kao, C.T.; Ding, S.J. In vitro characterization of postheat-treated plasma-sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2005, 197, 367–374. [Google Scholar] [CrossRef]
- Caulier, H.; van der Waerden, J.P.; Paquay, Y.C.; Wolke, J.G.; Kalk, W.; Naert, I.; Jansen, J.A. Effect of calcium phosphate (Ca-P) coatings on trabecular bone response: A histological study. J. Biomed. Mater. Res. 1995, 29, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Lu, Y.; Su, Y.; Hu, J.; Lian, J.; Li, G. Enhancing the corrosion resistance and surface bioactivity of a calcium-phosphate coating on a biodegradable AZ60 magnesium alloy via a simple fluorine post-treatment method. RSC Adv. 2015, 5, 56001–56010. [Google Scholar] [CrossRef]
- Suge, T.; Ishikawa, K.; Kawasaki, A.; Yoshiyama, M.; Asaoka, K. Effects of fluoride on the calcium phosphate precipitation method for dentinal tubule occlusion. J. Dent. Res. 1995, 74, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Coe, S.; Grant, D.M.; Grime, G.W.; Jeynes, C. Accurate determination of the Ca:P ratio in rough hydroxyapatite samples by SEM-EDS, PIXE and RBS—A comparative study. X-ray Spectrom. 2009, 38, 343–347. [Google Scholar] [CrossRef]
- Lin, K.; Wu, C.; Chang, J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014, 10, 4071–4102. [Google Scholar] [CrossRef] [PubMed]
- Pryor, L.S.; Gage, E.; Langevin, C.J.; Herrera, F.; Breithaupt, A.D.; Gordon, C.R.; Afifi, A.M.; Zins, J.E.; Meltzer, H.; Gosman, A.; et al. Review of bone substitutes. Craniomaxillofac. Trauma Reconstr. 2009, 2, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Blitch, E.L.; Ricotta, P.J. Introduction to bone grafting. J. Foot Ankle Surg. 1996, 35, 458–462. [Google Scholar] [CrossRef]
- Sokolova, V.; Epple, M. Inorganic nanoparticles as carriers of nucleic acids into cells. Angew. Chem. Int. Ed. 2008, 47, 1382–1395. [Google Scholar] [CrossRef] [PubMed]
- SGS Dental Implant System. Available online: http://sgs-dental.com/ (accessed on 14 March 2017).
- Augmentos® 3D Scaffold. Available online: http://www.innotere.de/cms/products/augmentos.html (accessed on 10 February 2017).
- Calcibon® granules. Available online: http://www.dijagfarm.com/repo/2012/09/calcibon-granules.jpg (accessed on 10 February 2017).
- Megasonex® Nano-Hydroxyapatite Toothpaste. Available online: http://megasonex.com/products/toothpaste/ (accessed on 14 March 2017).
- Biorepair Oral Care. Available online: http://www.biorepair.it/Total-Protection-Plus (accessed on 14 March 2017).
- Osteovit® Xenograft Bone Substitute. Available online: http://www.medicalexpo.com/prod/aesculap/product-70641-662983.html (accessed on 10 February 2017).
- DePuy Synthes CORAIL® Cementless Hip Prosthesis. Available online: http://www.medicalexpo.com/prod/depuy-synthes-79814.html#product-item_631053 (accessed on 10 February 2017).
- DePuy Synthes DBX™ Material. Available online: https://emea.depuysynthes.com/hcp/biomaterials/products/qs/dbx-material (accessed on 10 February 2017).
- Baltag, I.; Watanabe, K.; Kusakari, H.; Taguchi, N.; Miyakawa, O.; Kobayashi, M.; Ito, N. Long-term changes of hydroxyapatite-coated dental implants. J. Biomed. Mater. Res. 2000, 53, 76–85. [Google Scholar] [CrossRef]
- Gineste, L.; Gineste, M.; Ranz, X.; Ellefterion, A.; Guilhem, A.; Rouquet, N.; Frayssinet, P. Degradation of hydroxylapatite, fluorapatite, and fluorhydroxyapatite coatings of dental implants in dogs. J. Biomed. Mater. Res. 1999, 48, 224–234. [Google Scholar] [CrossRef]
- Overgaard, S.; Lind, M.; Josephsen, K.; Maunsbach, A.B.; Bünger, C.; Søballe, K. Resorption of hydroxyapatite and fluorapatite ceramic coatings on weight-bearing implants: A quantitative and morphological study in dogs. J. Biomed. Mater. Res. 1998, 39, 141–152. [Google Scholar] [CrossRef]
- Lidwell, O.M.; Lowbury, E.J.; Whyte, W.; Blowers, R.; Stanley, S.J.; Lowe, D. Airborne contamination of wounds in joint replacement operations: The relationship to sepsis rates. J. Hosp. Infect. 1983, 4, 111–131. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphates as bioceramics: State of the art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. The future of biomedical materials. J. Mater. Sci. Mater. Med. 2006, 17, 1025–1028. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.J.; Pirzada, D.; Cai, M.; Mohanty, P.; Bandyopadhyay, A. Bioceramic coating of hydroxyapatite on titanium substrate with Nd-YAG laser. Mater. Sci. Eng. C 2005, 25, 541–547. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Ye, C.; Erasquin, U.J.; Huynh, T.; Cai, C.; Cheng, G.J. Laser engineered multilayer coating of biphasic calcium phosphate/titanium nanocomposite on metal substrates. ACS Appl. Mater. Interfaces 2011, 3, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Krishna Balla, V.; Das, M.; Bose, S.; Ram, G.D.J.; Manna, I. Laser surface modification of 316L stainless steel with bioactive hydroxyapatite. Mater. Sci. Eng. C 2013, 33, 4594–4598. [Google Scholar] [CrossRef] [PubMed]
- Nag, S.; Paital, S.R.; Nandawana, P.; Mahdak, K.; Ho, Y.H.; Vora, H.D.; Banerjee, R.; Dahotre, N.B. Laser deposited biocompatible Ca-P coatings on Ti–6Al–4V: Microstructural evolution and thermal modeling. Mater. Sci. Eng. C 2013, 33, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Jakus, A.E.; Rutz, A.L.; Jordan, S.W.; Kannan, A.; Mitchell, S.M.; Yun, C.; Koube, K.D.; Yoo, S.C.; Whiteley, H.E.; Richter, C.P.; et al. Hyperelastic “bone”: A highly versatile, growth factor-free, osteoregenerative, scalable, and surgically friendly biomaterial. Sci. Transl. Med. 2016, 8, 358ra127. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Butscher, A.; Bohner, M.; Doebelin, N.; Hofmann, S.; Müller, R. New depowdering-friendly designs for three-dimensional printing of calcium phosphate bone substitutes. Acta Biomater. 2013, 9, 9149–9158. [Google Scholar] [CrossRef] [PubMed]
- Miranda, P.; Saiz, E.; Gryn, K.; Tomsia, A.P. Sintering and robocasting of β-tricalcium phosphate scaffolds for orthopaedic applications. Acta Biomater. 2006, 2, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Carrel, J.P.; Wiskott, A.; Moussa, M.; Rieder, P.; Scherrer, S.; Durual, S. A 3D printed TCP/HA structure as a new osteoconductive scaffold for vertical bone augmentation. Clin. Oral Implants Res. 2016, 27, 55–62. [Google Scholar] [CrossRef] [PubMed]
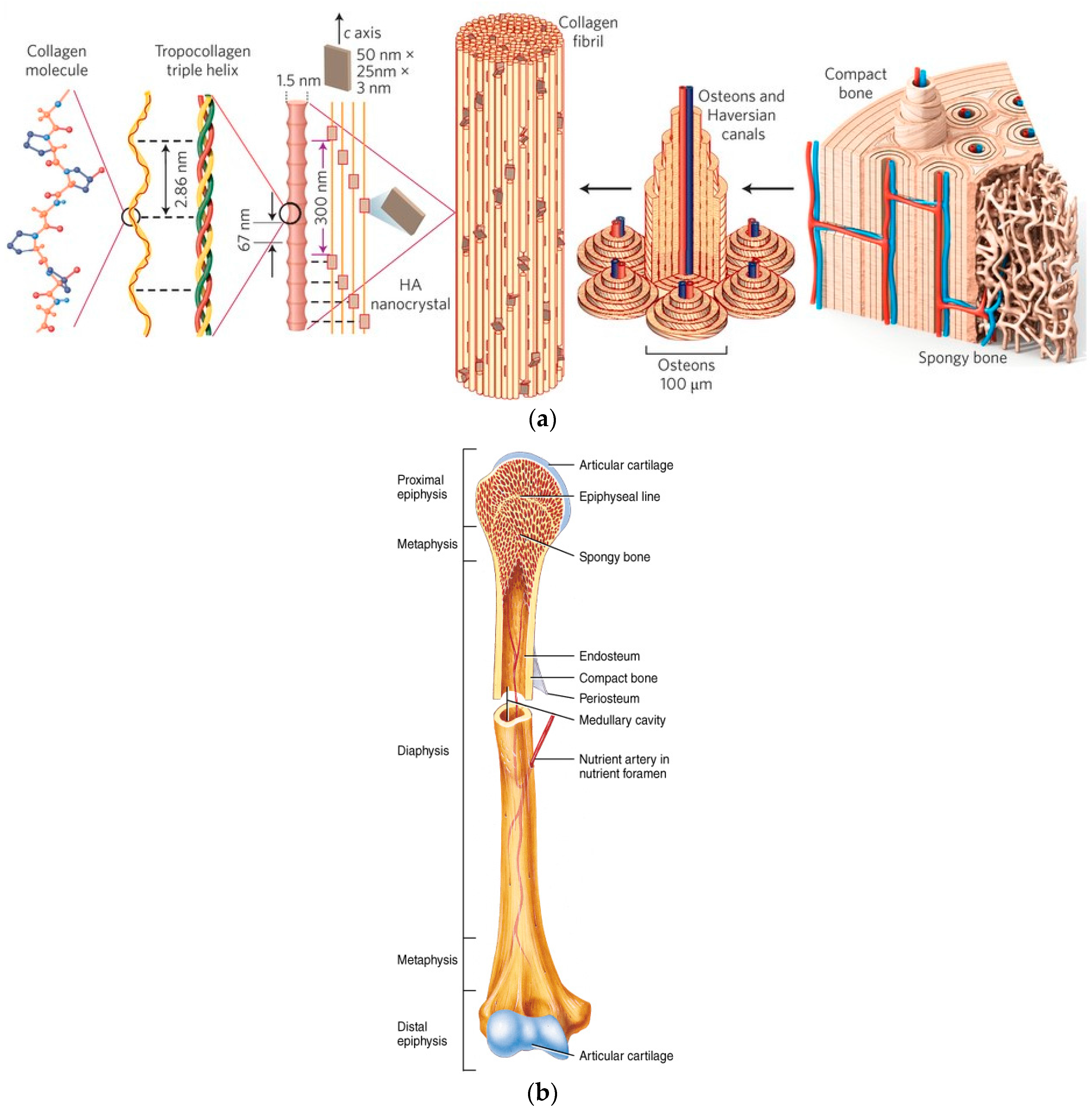
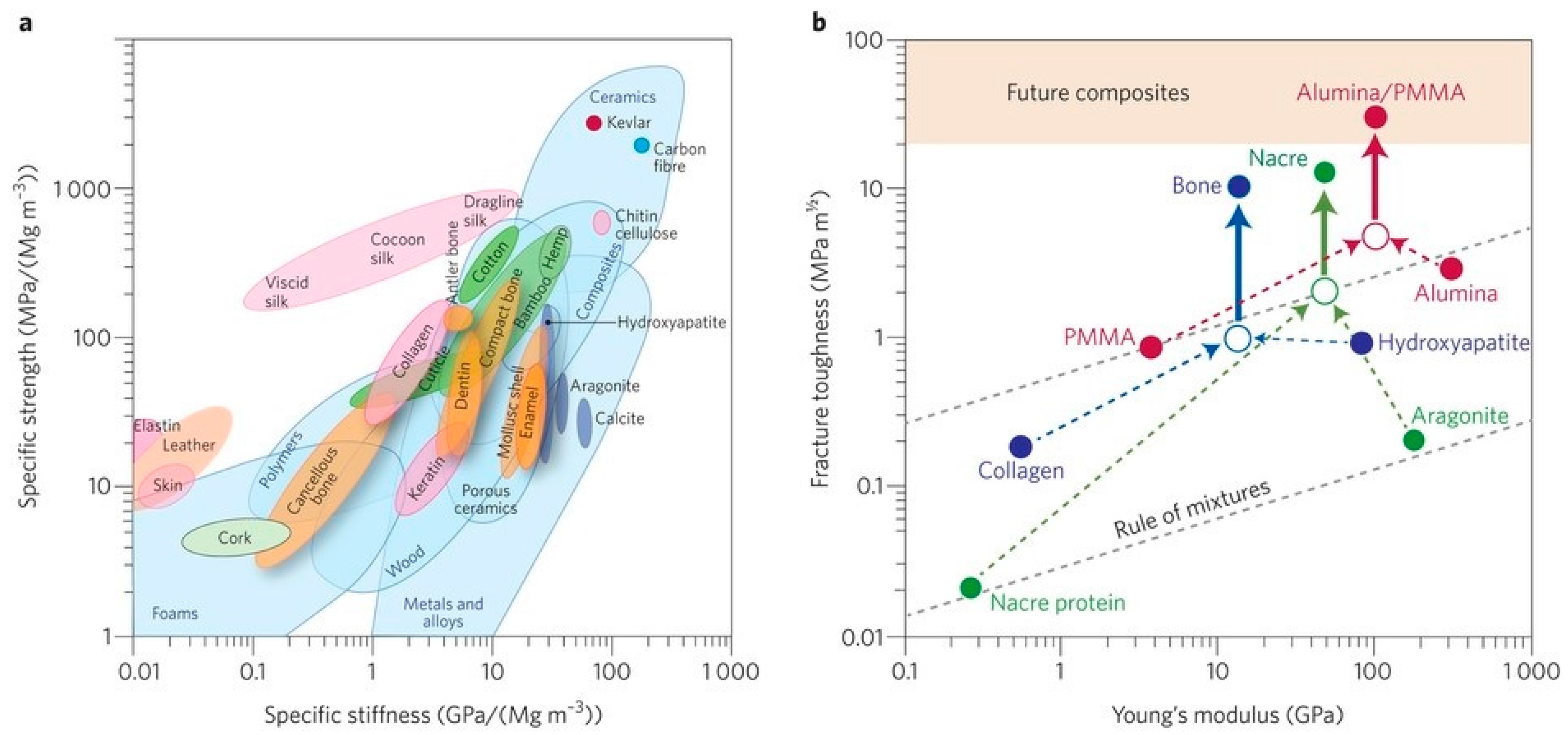
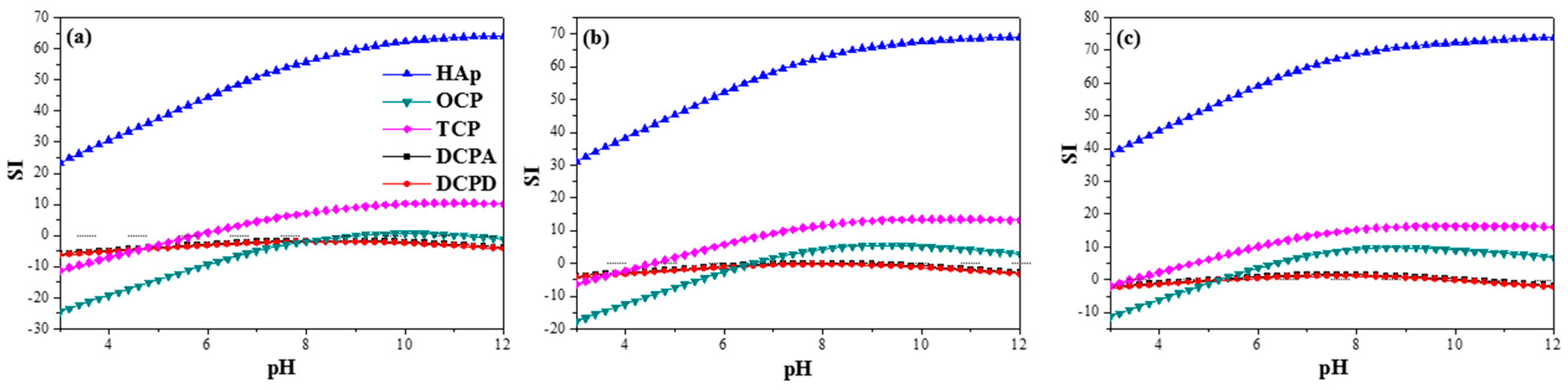
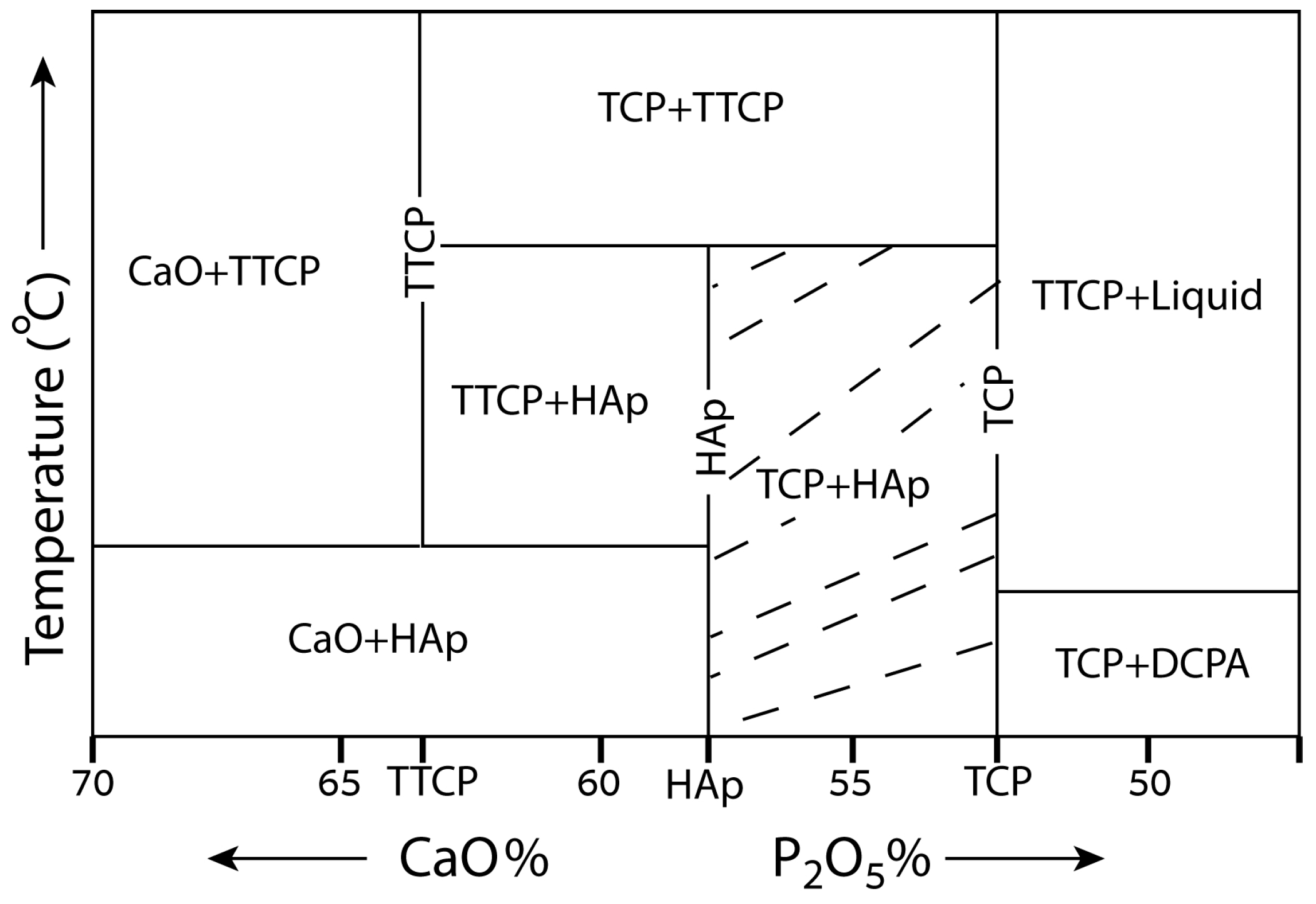

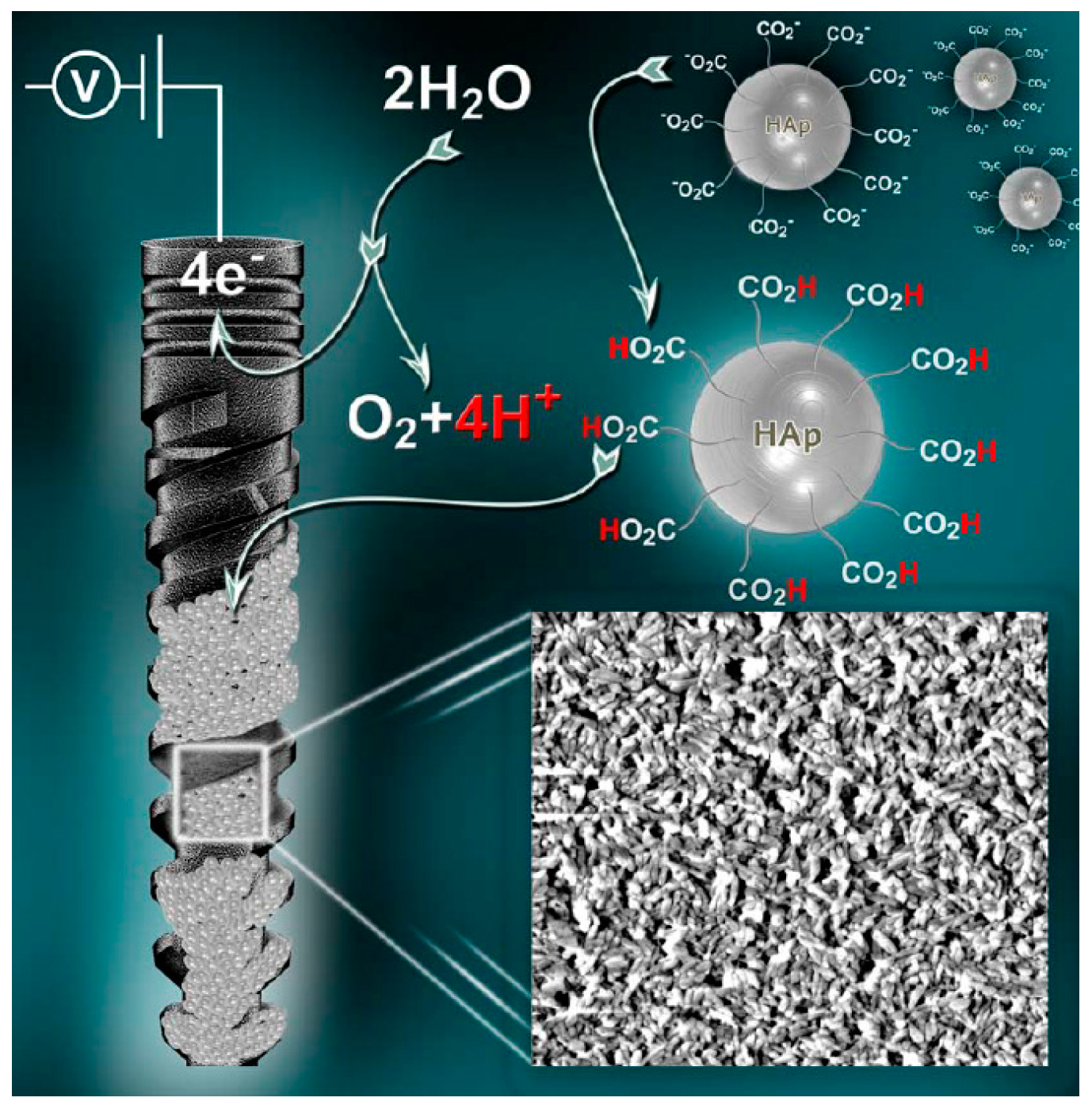
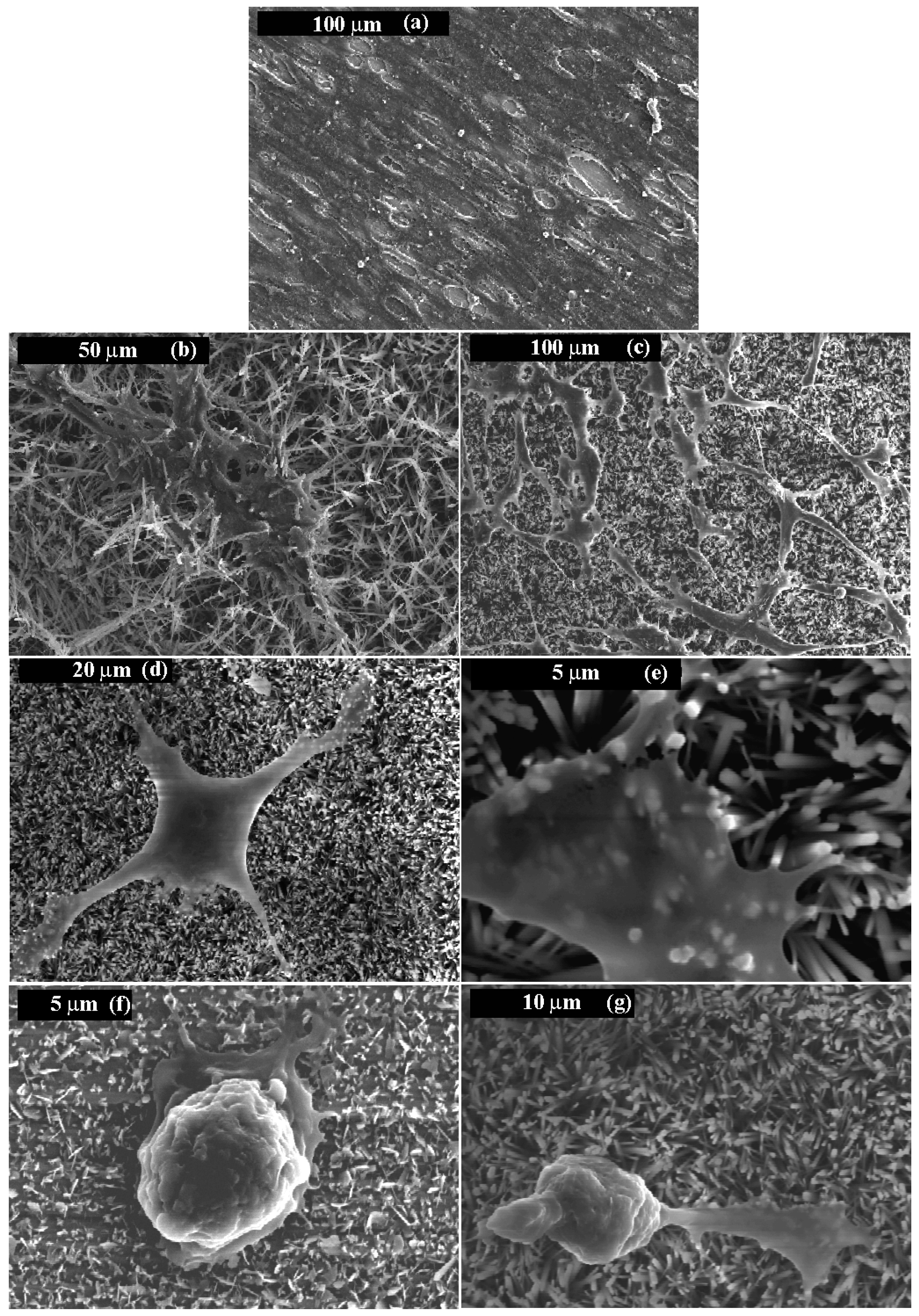
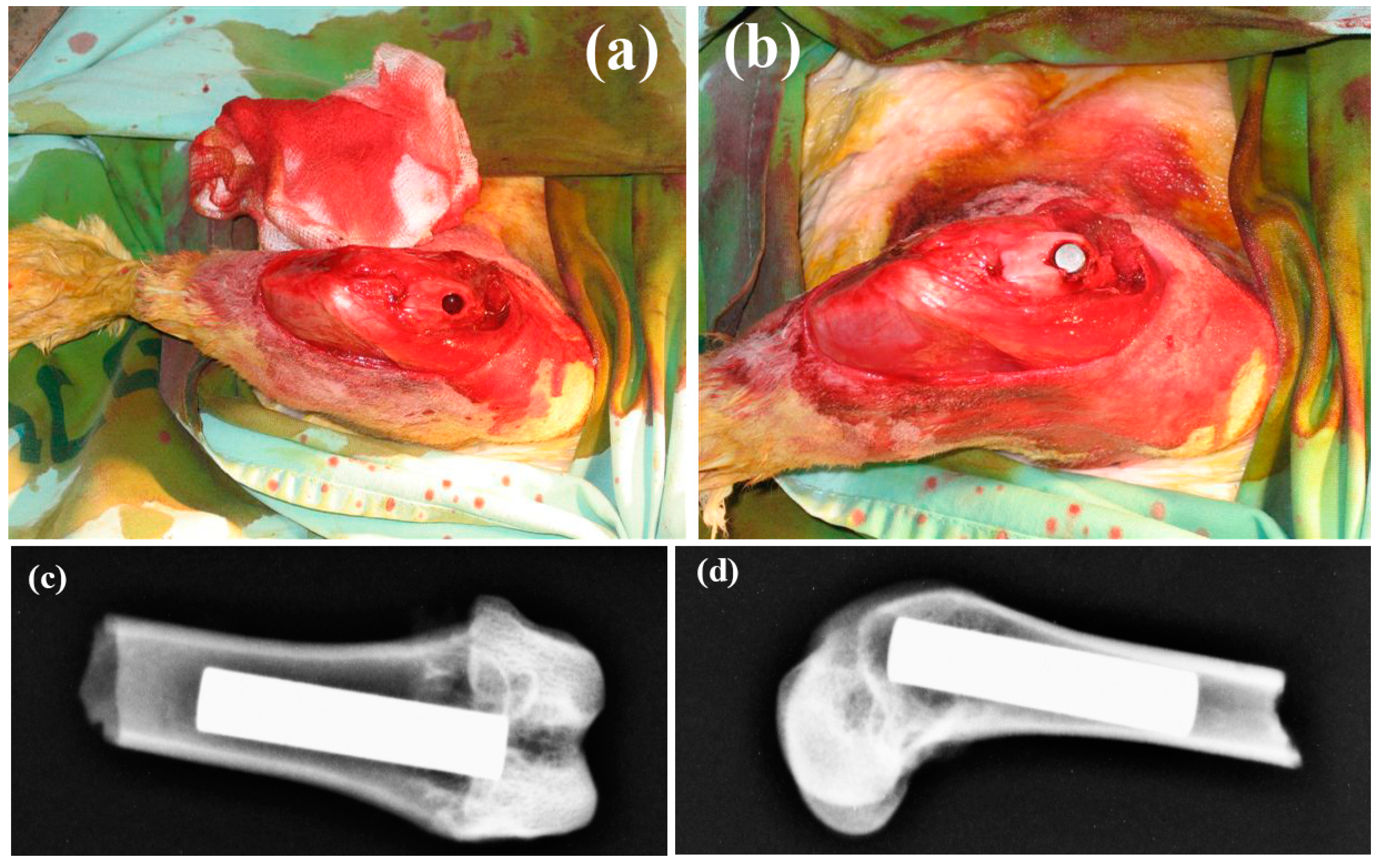

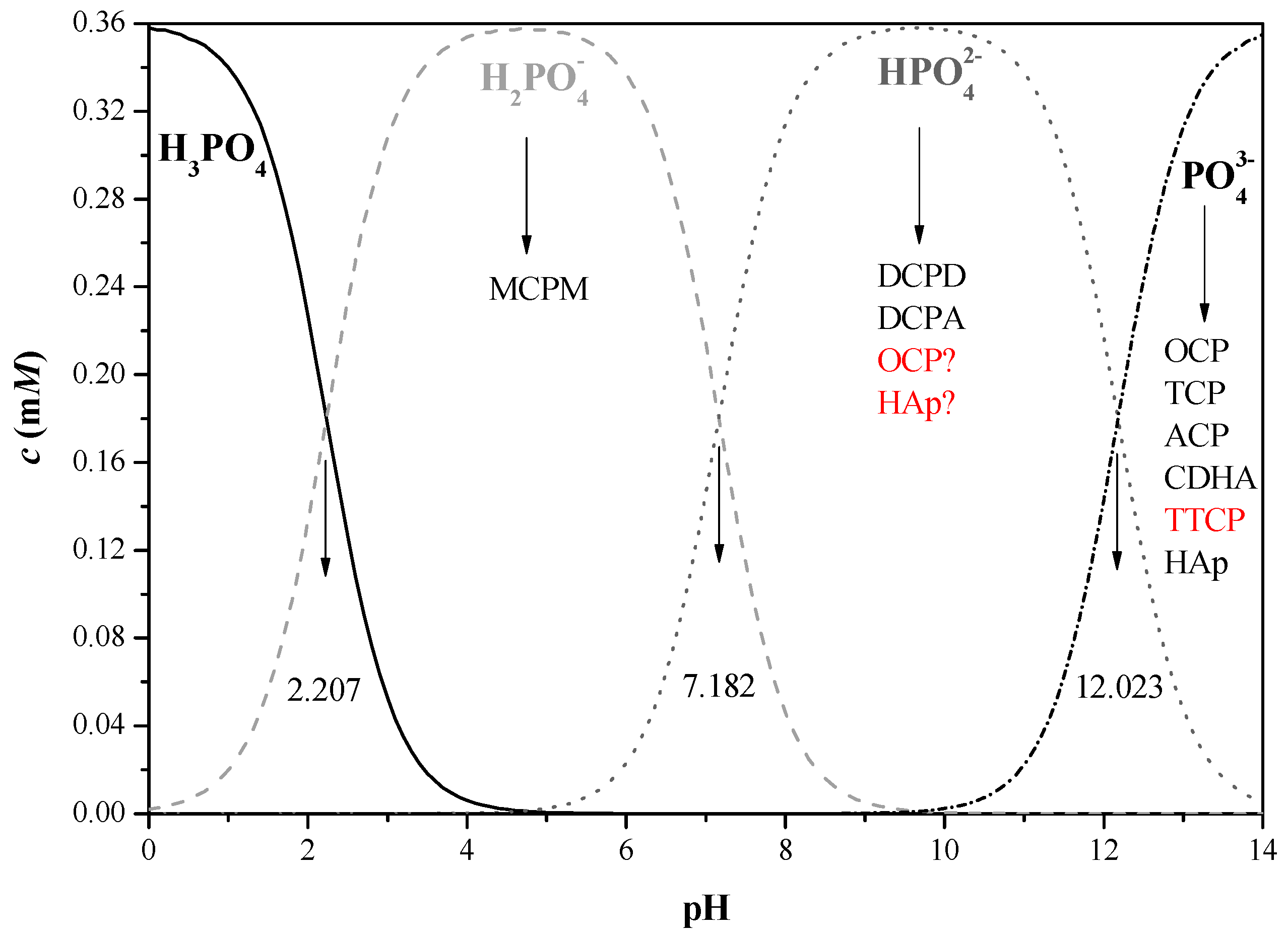
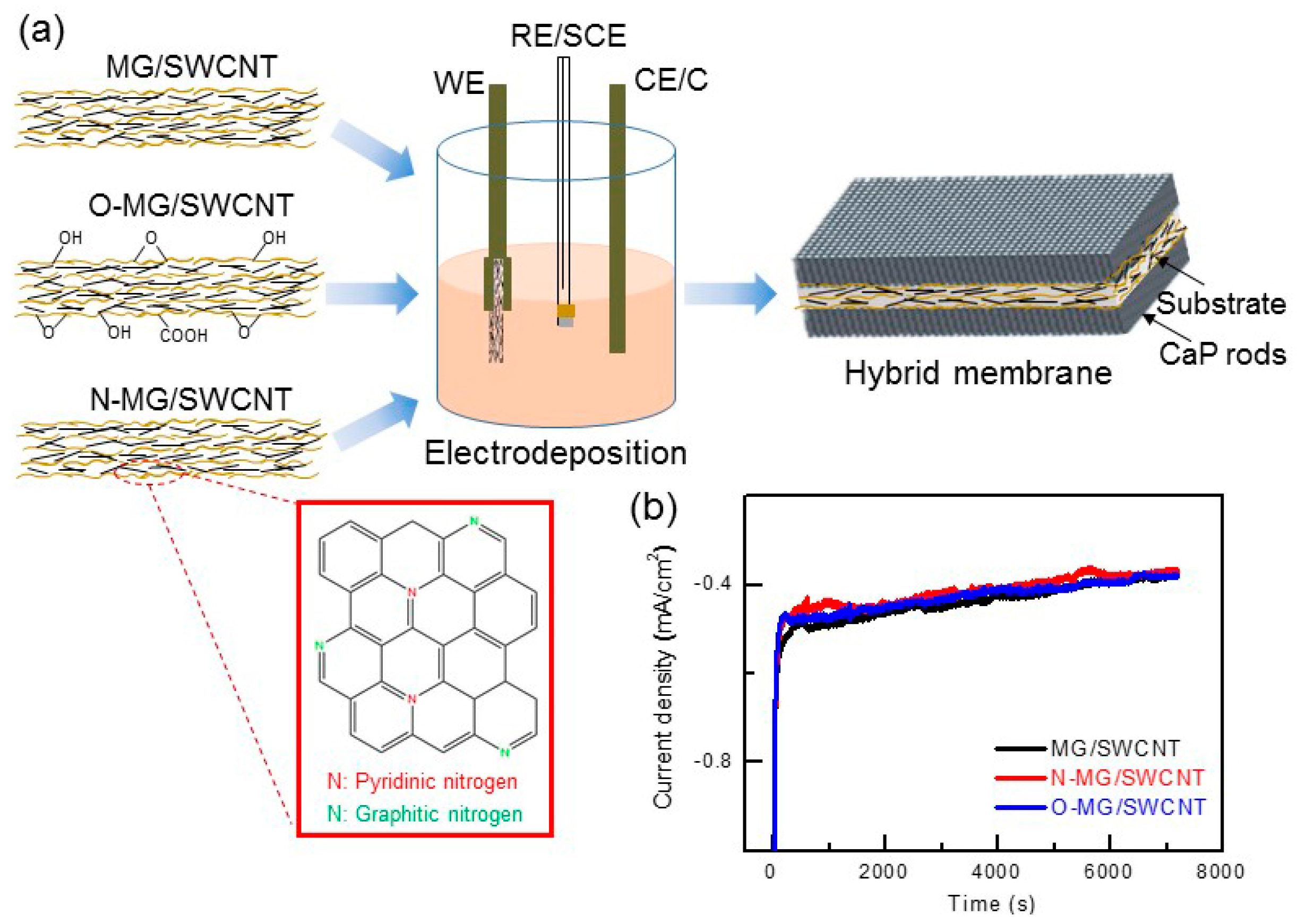
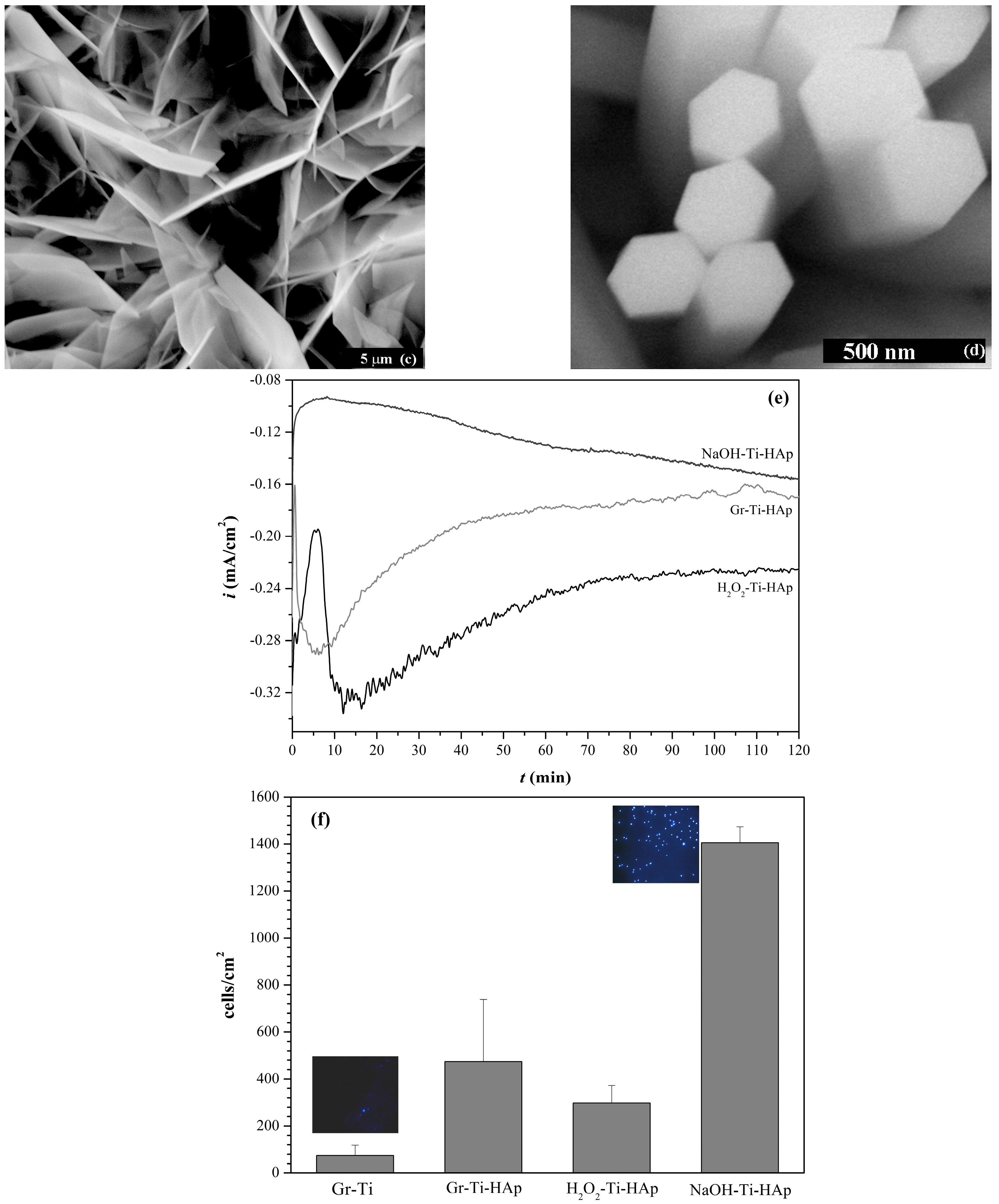

| Inorganic Phase | Organic Phase |
|---|---|
| HAp ≈ 60 | Collagen ≈ 20 |
| H2O ≈ 9 | Non-collagenous proteins (osteocalcin, osteonectin, osteopontin, thrombospondin, morphogenetic proteins, sialoprotein, serum proteins) ≈ 3 |
| Carbonate ≈ 4 | Traces: Polysaccharides, lipids, cytokines |
| Citrate ≈ 0.9 | Primary bone cells: osteoblasts, osteocytes, osteoclasts |
| Na+ ≈ 0.7 | - |
| Mg2+ ≈ 0.5 | |
| Cl− ≈ 0.13 | |
| Others: K+, F−, Zn2+, Fe2+, Cu2+, Sr2+, Pb2+ |
| Property | Definition/Function |
|---|---|
| Bioactivity | The inherent ability of a material to participate in specific biological reactions or have an effect on living tissues |
| Biocompatibility | The ability of a material to perform with an appropriate host response in a specific application |
| Bioactive fixation | Reactive surfaces form chemical bonding with bone, thus minimizing the fibrous capsule formation |
| Biostability | The ability of a material to maintain its properties in vivo |
| Crystallinity | Higher level of crystallinity prevents fast resorption (dissolution) of the bioceramic in body fluids |
| Interfacial stability and good adhesion | Prevent mechanical failures under load-bearing conditions |
| Osseointegration | Direct anchorage of an implant by the formation of bony tissue around it without growth of fibrous tissue at the bone/implant interface |
| Osteoconduction | Ability to provide a scaffold for the formation of new bone |
| Osteoinduction | The process by which osteogenesis is induced. This term means that primitive, undifferentiated and pluripotent cells are somehow stimulated to develop into the bone-forming cell lineage |
| Resorption | Gradual degradation over time to replace the biomaterial with the natural host tissue |
| Therapeutic capabilities | Templates for the in situ delivery of drugs and growth factors at required times |
| Wettability | The property that indicates a material’s ability to attract/repel water molecules |
| Ca/P Molar Ratio | Name | Formula | pH Stability Range | Density (g/cm3) |
|---|---|---|---|---|
| 0.5 | MCPM (monobasic calcium phosphate monohydrate) | Ca(H2PO4)2·H2O | 0.0–2.0 | 2.22 |
| 1.0 | DCPA (dicalcium phosphate anhydrous, Monetite) | CaHPO4 | 2.0–5.5 (>80 °C) | 2.929 |
| 1.0 | DCPD (dibasic calcium phosphate dehydrate, Brushite) | CaHPO4·2H2O | 2.0–6.0 | 2.319 |
| 1.33 | OCP (octacalcium phosphate) | Ca8(HPO4)2(PO4)4·5H2O | 5.5–7.0 | 2.673 |
| 1.5 | α-TCP (α-tricalcium phosphate) | α-Ca3(PO4)2 | Precipitated from aqueous solutions only at T > 1125 °C | 2.814 3 |
| 1.5 | β-TCP (β-ticalcium phosphate) | β-Ca3(PO4)2 | Precipitated from aqueous solutions only at T > 800 °C | 3.067 3 |
| 1.2–2.2 | ACP (amorphous calcium phosphate) | CaxHy(PO4)z·nH2O, n = 3–4.5, 15%–20% H2O | ~5–12 1 | - |
| 1.50–1.67 | CDHA (calcium deficient hydroxyapatite, CDHAp; precipitated HAp, pHA, pHAp) | Ca10−x(HPO4)x(PO4)6−x(OH)2−x (0 < x < 2) 2 | 6.5–9.5 | - |
| 1.67 | HAp, or OHAp (Hydroxyapatite) | Ca10(PO4)6(OH)2 | 9.5–12.0 | 3.155 |
| 2.0 | TTCP, or TetCP (tetracalcium phosphate, Hilgenstockite) | Ca4(PO4)2O | Precipitated from aqueous solutions only at T > 1300 °C | 3.056 3 |
| Name | Solubility at 37 °C, −log(Ks) | Solubility at 25 °C, −log(Ks) | Solubility at 25 °C, g/L |
|---|---|---|---|
| MCPM | -- | 1.14 | ~18 |
| DCPA | 7.02 | 6.90 | ~0.048 |
| DCPD | 6.63 | 6.59 | ~0.088 |
| OCP | 95.9 | 96.6 | ~0.0081 |
| α-TCP | 25.5 | 25.5 | ~0.0025 |
| β-TCP | 29.5 | 28.9 | ~0.0005 |
| ACP | 1 | 1 | - |
| CDHA | ~85.1 | 85.1 | ~0.0094 |
| HAp | 117.2 | 116.8 | ~0.0003 |
| TTCP | 37–42 | 38–44 | ~0.0007 |
| Name | Space Group | Unit Cell Parameters | JCPDS 1,2 File |
|---|---|---|---|
| MCPM | Triclinic | a = 6.250, b = 11.892, c = 5.629 Å α = 96.67°, β = 114.20°, γ = 92.95° | 00-009-0347 |
| DCPA | Triclinic | a = 6.910, b = 6.627, c = 6.998 Å α = 96.34°, β = 103.82°, γ = 88.33° | 00-003-0398, 00-004-0513, 00-009-0080, 01-075-1520, 04-009-3755, 04-009-6216, 04-011-3070 |
| DCPD | Monoclinic Ia | a = 5.812, b = 15.180, c = 6.239 Å β = 116.42° | 00-009-0077, 00-011-0293, 04-008-2081, 04-013-3344 |
| OCP | Triclinic | a = 9.529, b = 18.994, c = 6.855 Å α = 92.33°, β = 90.13°, γ = 79.93° | 00-026-1056, 00-044-0778, 04-013-3883, 04-016-3473 |
| α-TCP | Orthorhombic (Monoclinic P21/a?) | a = 15.220, b = 20.710, c = 9.109 Å α = β = γ = 90° | 00-009-0348, 00-029-0359 |
| β-TCP | Rhombohedral R3c | a = b = 10.439, c = 37.375 Å α = β = 90.00°, γ = 120.00° | 00-009-0169, 04-002-4776, 04-008-8714 |
| CDHA | Hexagonal P63/m | a = b = 9.4157–9.4490, c = 6.8777–6.8865 Å α = β = 90°, γ = 120° | 00-046-0905 |
| HAp | Monoclinic P21/b or Hexagonal P63/m 3 | a = 9.84214, b = 2a, c = 6.8814 Å, γ = 120° (monoclinic). a = b = 9.418, c = 6.884 Å α = β = 90.00°, γ = 120.00° (hexagonal) | 00-009-0432, 00-024-0033, 01-074-0565, 01-074-0566, 01-084-1998, 01-089-4405, 04-007-2837, 04-007-5086, 04-016-1185 |
| TTCP | Monoclinic P21 | a = 7.018, b = 11.980, c = 9.469 Å, α = γ = 90.00°, β = 90.88° | 00-011-0232, 00-025-1137 |
| Property | Value | Comments |
|---|---|---|
| Binding energy | −280.6 eV | Reference [373] |
| Kohn-Sham gap | −5.4 eV | Reference [373] |
| Dielectric constant | 7.40–10.47 | - |
| Thermal conductivity | 0.013 W/(cm·K) | - |
| Relative density | 95%–99.5% | - |
| Decomposition temperature | >1000 °C | - |
| Melting point | 1614 °C | - |
| Tensile strength | 38–300 MPa ~3 MPa | For dense HAp For porous HAp |
| Compressive strength | 120–900 MPa 2–100 MPa | For dense HAp For porous HAp |
| Bending strength | 38–250 MPa 2–11 MPa | For dense HAp For porous HAp |
| Young’s (elastic) modulus | 35–120 GPa | For dense HAp |
| Fracture toughness | 0.7–1.2 MPa·m1/2 | Decreases almost linearly with porosity [383] |
| Fracture energy | 2.3–20 J/m2 | Behaves like a typical brittle ceramic |
| Vickers hardness | 3–7 GPa | For dense HAp |
| Poisson’s ratio | 0.27 | For synthetic HAp (bones ~0.3) |
| Biocompatibility | High | - |
| Bioactivity | High | - |
| Biodegradation | Low | - |
| Cellular compatibility | High | - |
| Osteoinduction | Nil | - |
| Osteoconduction | High | - |
| Medium | Na+ | K+ | Ca2+ | Mg2+ | Cl− | Organic Acids (mg/L) | Proteins (mg/L) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Human blood plasma | 142.0 | 3.6–5.5 | 2.1–2.6 | 1.5 | 95.0–107.0 | 27.0 | 0.65–1.45 | 0.5 | 210 | 66,300 |
| Synovial fluid | 136.0 | 4.0 | 1.5 | - | 107.5 | 30.8 | 1.0 | 0.5 | - | 15,000 |
| Original SBF | 142.0 | 5.0 | 2.5 | 1.5 | 148.8 | 4.2 | 1.0 | - | - | - |
| Corrected SBF (c-SBF) | 142.0 | 5.0 | 2.5 | 1.5 | 147.8 | 4.2 | 1.0 | 0.5 | - | - |
| Revised SBF (r-SBF) | 142.0 | 5.0 | 2.5 | 1.5 | 103.0 | 27.0 | 1.0 | 0.5 | - | - |
| Newly improved SBF (n-SBF) | 142.0 | 5.0 | 2.5 | 1.5 | 103.0 | 4.2 | 1.0 | 0.5 | - | - |
| Phosphate-buffered saline (PBS) | 157.0 | 4.5 | - | - | 139.7 | - | 10.0 + 1.8 | - | - | - |
| Ringer’s | 291.3 | 10.8 | 6.3 | - | 212.0 | 3.3 | - | - | - | - |
| Hanks’ balanced salts solution (HBSS) | 141.7 | 5.7 | 1.7 | 0.8 | 145.6 | 4.2 | 0.7 | 0.8 | - | - |
| Property | Requirement | Testing Method | Standard | Comment |
|---|---|---|---|---|
| Chemical composition | Ca/P = 1.67–1.76 | Inductively coupled plasma/mass spectroscopy (ICP/MS) or atomic absorption (AAS) | ISO 13779-3 ASTM F1088 ASTM F1185 | Atomic ratio. Analyse enough samples to produce a meaningful mean and variance (i.e., 95% confidence interval) |
| Trace elements | As: max 3 ppm, Cd: max 5 ppm, Hg: max 5 ppm, Pb: max 30 ppm, Total heavy metals: 50 ppm | Inductively coupled plasma/mass spectroscopy (ICP/MS) or atomic absorption (AAS) | ISO 13779-3 ISO 10993-17 ASTM F1185 | - |
| Phase content | Only HAp and OCP | XRD + FTIR to identify functional groups | ASTM F2024 ISO 13779-3 | XPS analysis: [185]. Superimpose relevant JCPDS/ICDD lines. Provide a table identifying all peaks by intensity, d-spacing and 2θ. Specify scan range and scan rate. Report preferred orientations, effect of strain, etc. Characteristic absorption bands for HAp: 570, 962 and 1050 cm−1 for ; 630 and 3540 cm−1 for |
| Percentage of crystallinity | min 45% crystalline HAp, max 5% other crystalline phases, Balance: amorphous | - | ISO 13779-3 | - |
| Adhesion strength | min 15 MPa (under tension) | - | ISO 13779-4 ASTM F1147 in tension. ASTM F1044 shear adhesive strength | Provide SEM images at 100× of the epoxy/coating/substrate prior to testing to demonstrate any potential penetration of the epoxy. At least 10 samples. Report STDEV |
| Microporosity and macroporosity | - | - | ASTM F1854 | Report average porosity size and overall pore volume |
| Surface coverage | - | Microscopic examination at 10× magnification. SEM images at 100× of the coated implant surfaces and of a cross-sectioned area of the implant showing the coating interface | - | Report “bare” areas, “pinholes,” cracking, foreign debris, unmelts, chips, delamination, the appearance at the coating/substrate interface, etc. Provide photomicrographs at 100× |
| Coating thickness | - | Cross-sections | ASTM F1854 ASTM E376 | Report distinct layers, if exist, and tolerance |
| Colour | - | Macroscopic examination | - | Ensure a uniform and consistent appearance |
| Surface roughness | - | - | ANSI/ASME B46.1 | Ra and the tolerances of the substrate and coating should be reported |
| Abrasion resistance | - | - | None available for CaP. ASTM F1978 may be used | Need to determine if the coating will spall. |
| Fatigue strength | - | Three-point bending, rotating beam, or modified static test methods for testing of dental implants | ASTM F1160. ISO 7206 for hip prostheses | Both the coating/substrate interface and the effect on the substrate should be evaluated. The effect of the coating on the resulting fatigue strength of an actual implant should also be considered. Provide SEM images of failure regions. Provide S/N curve. Test for the worst-case scenario. For femoral stems, the S/N curve may be substituted with testing of the stem at a load of 3–4 times body weight and R = 0.1 for 10 M cycles. A sample size of 5 is required |
| Solubility products and dissolution rate | - | In a physiologically similar solution such as tris-HCl buffered solution at 37 °C and pH = 3.0 and 7.3 | ASTM F1926 | Measurement should include dissolved Ca and P. Weight loss should also be measured. If compound contains other elements such as F, these should be measured too. Monitor pH changes. Calculate Ksp |
| Density of the coating | 2.98 g/cm3 | Helium pycnometer | - | - |
| Animal studies | - | - | - | Check Guidance for the Arrangement and Content of a Premarket Approval (PMA) Application for an Endosseous Implant for Prosthetic Attachment |
| Clinical studies | - | - | - | Check Guidance for the Arrangement and Content of a Premarket Approval (PMA) Application for an Endosseous Implant for Prosthetic Attachment |
| Composition | Product Name | Producer |
|---|---|---|
| β-TCP | adbone®TCP | Medbone, Portugal |
| Bioresorb | Sybron Implant Solutions, Germany | |
| Biosorb | SBM S.A., France | |
| Calciresorb | Ceraver, France | |
| Cerasorb | Curasan, Germany | |
| Ceros | Thommen Medical, Switzerland | |
| Conduit | DePuy Spine, USA | |
| JAX | Smith and Nephew Orthopaedics, USA | |
| Osferion | Olympus Terumo Biomaterials, Japan | |
| OsSatura TCP | Integra Orthobiologics, CA, USA | |
| SynthoGraft | Synthograft, MA, USA | |
| Triha+ | Teknimed, France | |
| Vitoss | Orthovita, PA, USA | |
| CDHA | Osteogen | Impladent, NY, USA |
| HAp | Actifuse | ApaTech, UK |
| Apaceram | Pentax, Japan ApaTech, UK | |
| ApaPore | ApaTech, UK | |
| Bioroc | Depuy-Bioland, France | |
| Bonefil | Pentax, Japan | |
| Bonetite | Pentax, Japan | |
| Boneceram | Sumitomo Osaka Cement, Japan | |
| Bone Source | Stryker Orthopaedics, NJ, USA | |
| Calcitite | Zimmer, IN, USA | |
| Cerapatite | Ceraver, France | |
| Neobone | Toshiba Ceramics, Japan | |
| Ostegraf | Ceramed, CO, USA | |
| Ostim | Heraeus Kulzer, Germany | |
| Synatite | SBM, France | |
| Coralline HAp | Interpore | Interpore, CA, USA |
| ProOsteon | Interpore, CA, USA | |
| Algae-derived HAp | Algipore | Dentsply Friadent, Germany |
| Bovine bone apatite (unsintered) | BioOss | Geitslich, Switzerland |
| Laddec | Ost-Developpement, France | |
| Lubboc | Ost-Developpement, France | |
| Oxbone | Bioland biomateriaux, France | |
| Tutoplast | IOP, CA, USA | |
| Bovine bone apatite (sintered) | Cerabone | aap Implantate, Germany |
| Endobon | Biomet Deutschland GmbH, Germany | |
| Osteograf | Ceramed, CO, USA | |
| PepGen P-15 | Dentsply Friadent, Germany | |
| XenoGraft | Staumann, Switzerland | |
| HAp + collagen | Bioimplant | Connectbiopharm, Russia |
| Bonject | Koken, Japan | |
| CollapAn | Intermedapatite, Russia | |
| HAPCOL | Polystom, Russia | |
| Healos Fx | DePuy Spine, USA | |
| LitAr | LitAr, Russia | |
| HAp + sodium alginate | Bialgin | Biomed, Russia |
| HAp + PLLA | SuperFIXSORB30 | Takiron, Japan |
| HAp + Polyethylene | HAPEX | Gyrus, TN, USA |
| HAp + CaSO4 | Hapset | LifeCore, MIN, USA |
| BCP (HAp + β-TCP) | 4Bone | MIS, Israel |
| BCP | Medtronic, MN, USA | |
| Biosel | Depuy Bioland, France | |
| BoneSave | Stryker Orthopaedics, NJ, USA | |
| Calciresorb | Ceraver, France | |
| CellCeram | Scaffdex, Finland | |
| Ceraform | Teknimed, France | |
| Ceratite | NGK Spark Plug, Japan | |
| Eurocer | FH Orthopedics, France | |
| Graftys BCP | Graftys, France | |
| Hatric | Arthrex, Naples, FL, USA | |
| Indost | Polystom, Russia | |
| MBCP Gel In’Oss (contains also hydrogel) | Biomatlante, France | |
| Kainos | Signus, Germany | |
| Mastergraft | Medtronic, IN, USA | |
| Maxresorb | Staumann, Switzerland | |
| MBCP | Biomatlante, France | |
| OptiMX | Exactech, FL, USA | |
| OsSatura BCP | Integra Orthobiologics, CA, USA | |
| Osteosynt | Einco, Brazil | |
| Repros | JRI Orthopaedics, UK | |
| SBS | Expanscience, France | |
| TCH | Kasios, France | |
| Triosite | Zimmer, IN, USA | |
| Tribone | Stryker, Europe | |
| Valeos | Innov’spine, France | |
| BCP (HAp + α-TCP) | Skelite | Millennium Biologix, ON, Canada |
| BCP (DCPD/HAp) | BONIT | DOT Medical Implants Solutions, Germany |
| BONITex | DOT Medical Implants Solutions, Germany | |
| BCP + collagen | Allograft | Zimmer, IN, USA |
| BCP + fibrin | TricOS | Baxter BioScience, France |
| BCP + silicon | FlexHA | Xomed, FL, USA |
| FA + BCP (HAp + β-TCP) | FtAP | Polystom, Russia |
| β-TCP + PMMA | Cal-CEMEX | Tecres Spa, Italy |
| rhBMP-2 on the surface of HAp/β-TCP | CowellBMP | Cowellmedi Co (CWM), Korea |
| TTCP + DCPA + saline | BoneSource HAC | Stryker Instruments, MI, USA |
| α-TCP + TTCP + sodium glycerophosphate + (lime + phosphoric acid) | Cementek | Teknimed LC, France |
| CaP within lyophilized type I bovine collagen sponges | CopiOS Sponge | Zimmer Biomet Spine, CO, USA |
| ACP + DCPD | Biobon (α-BSM) | Etex, MA, USA |
| BCP (HAp + β-TCP) granules, bovine collagen and bone marrow aspirate | Collagraft | Zimmer, IN, USA |
| β-TCP granules and polymer | Therigraft Putty | Therics, OH, USA |
| β-TCP granules and an aqueous solution of glycerol and carboxymethylcellulose (CMC) | JAXTCP | Smith and Nephew, USA |
| HAp, P-15 peptide and aqueous Na-hyaluronate solution | Pepgen P-15 flow | Dentsply, PA, USA |
| α-TCP + TTCP + CaHPO4 + HAp | BIOPEX | Taisho Pharmaceutical, Japan |
| BCP (DCPD + β-TCP) | ChronOS | DePuy Synthes, PA, USA |
| Carbonate apatite | Healos | Orquest, CA, USA |
| Norian SRS | Synthes, PA, USA |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliaz, N.; Metoki, N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials 2017, 10, 334. https://doi.org/10.3390/ma10040334
Eliaz N, Metoki N. Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications. Materials. 2017; 10(4):334. https://doi.org/10.3390/ma10040334
Chicago/Turabian StyleEliaz, Noam, and Noah Metoki. 2017. "Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications" Materials 10, no. 4: 334. https://doi.org/10.3390/ma10040334






