Structure-Dependent Spectroscopic Properties of Yb3+-Doped Phosphosilicate Glasses Modified by SiO2
Abstract
:1. Introduction
2. Experimental
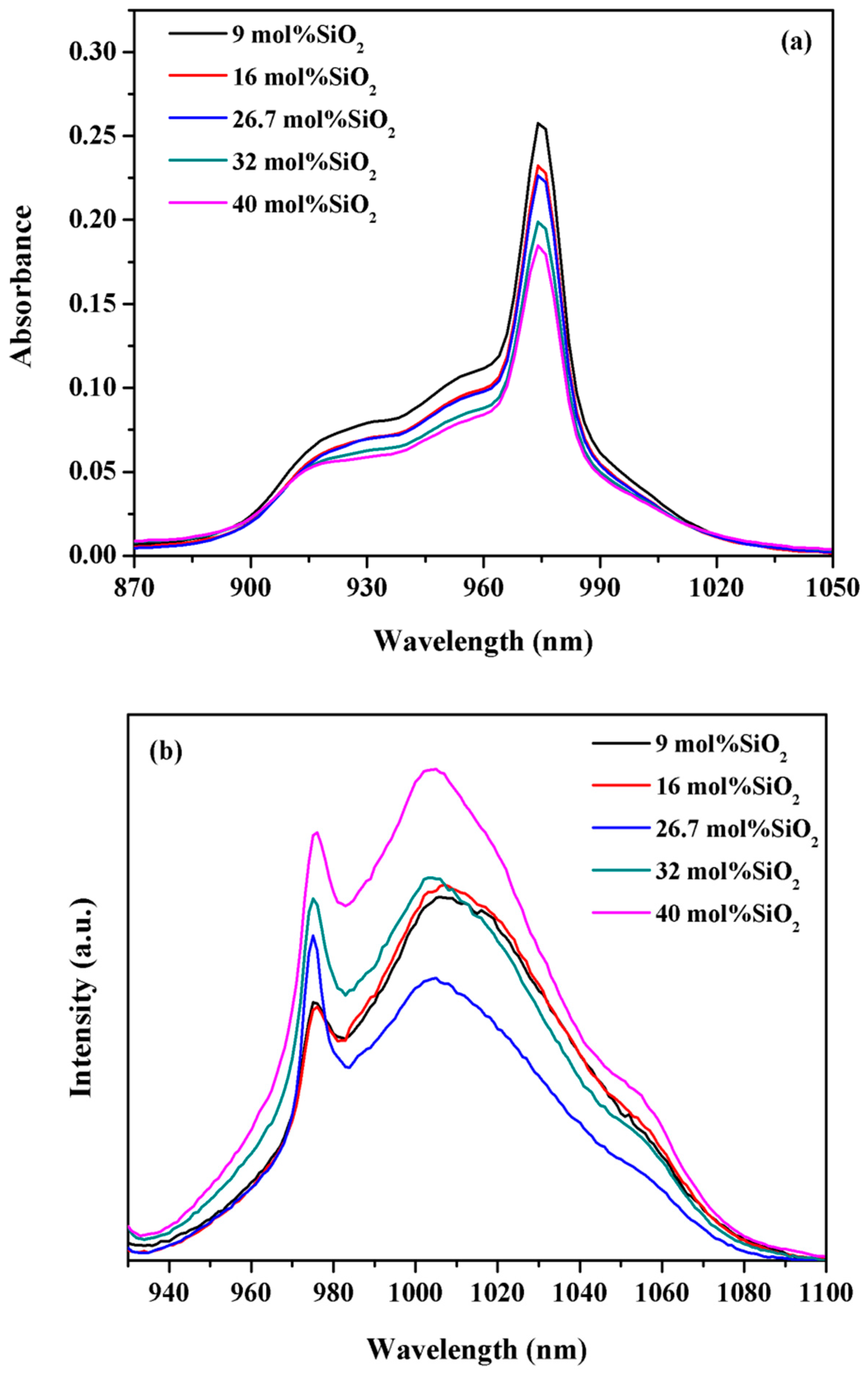
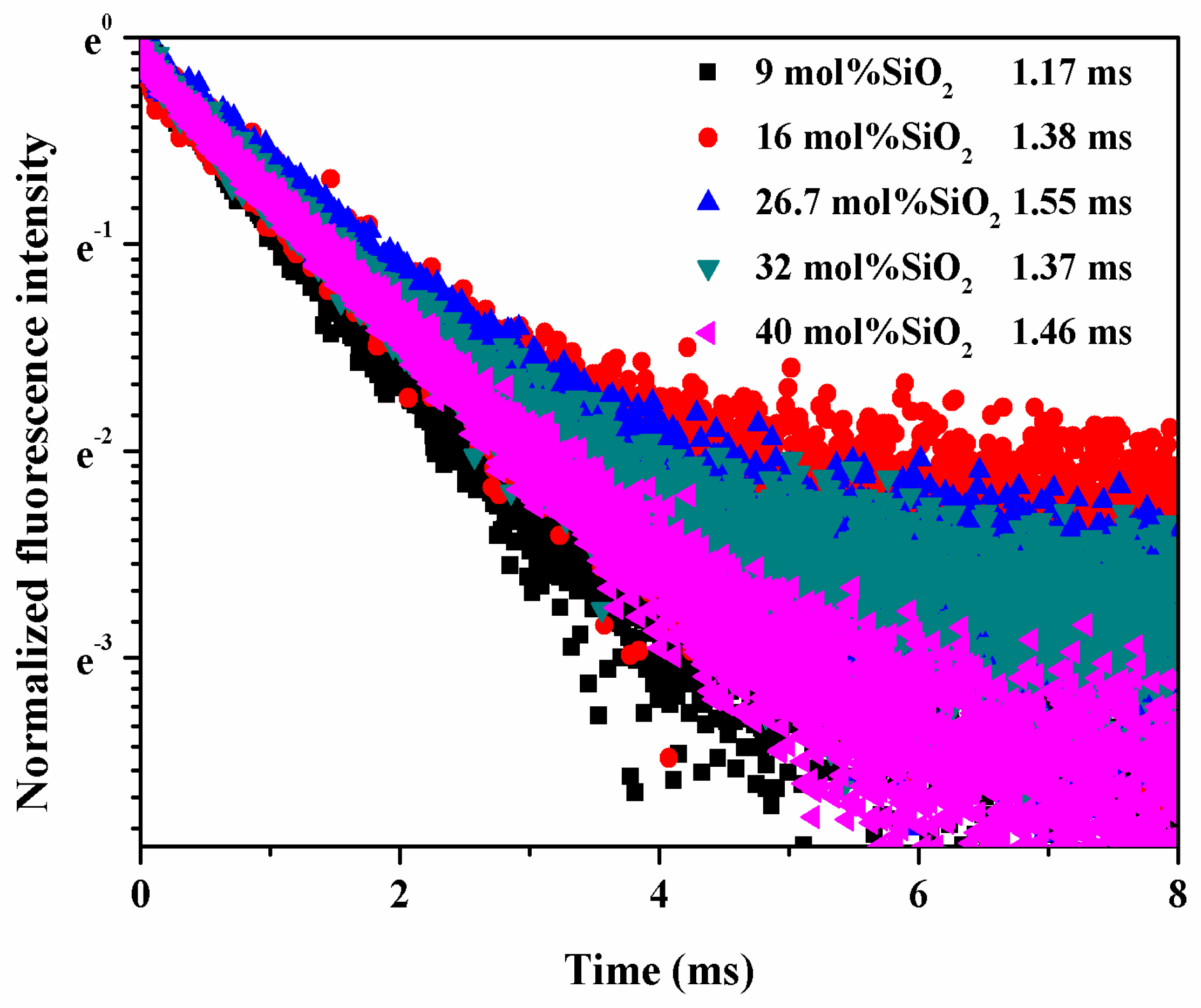
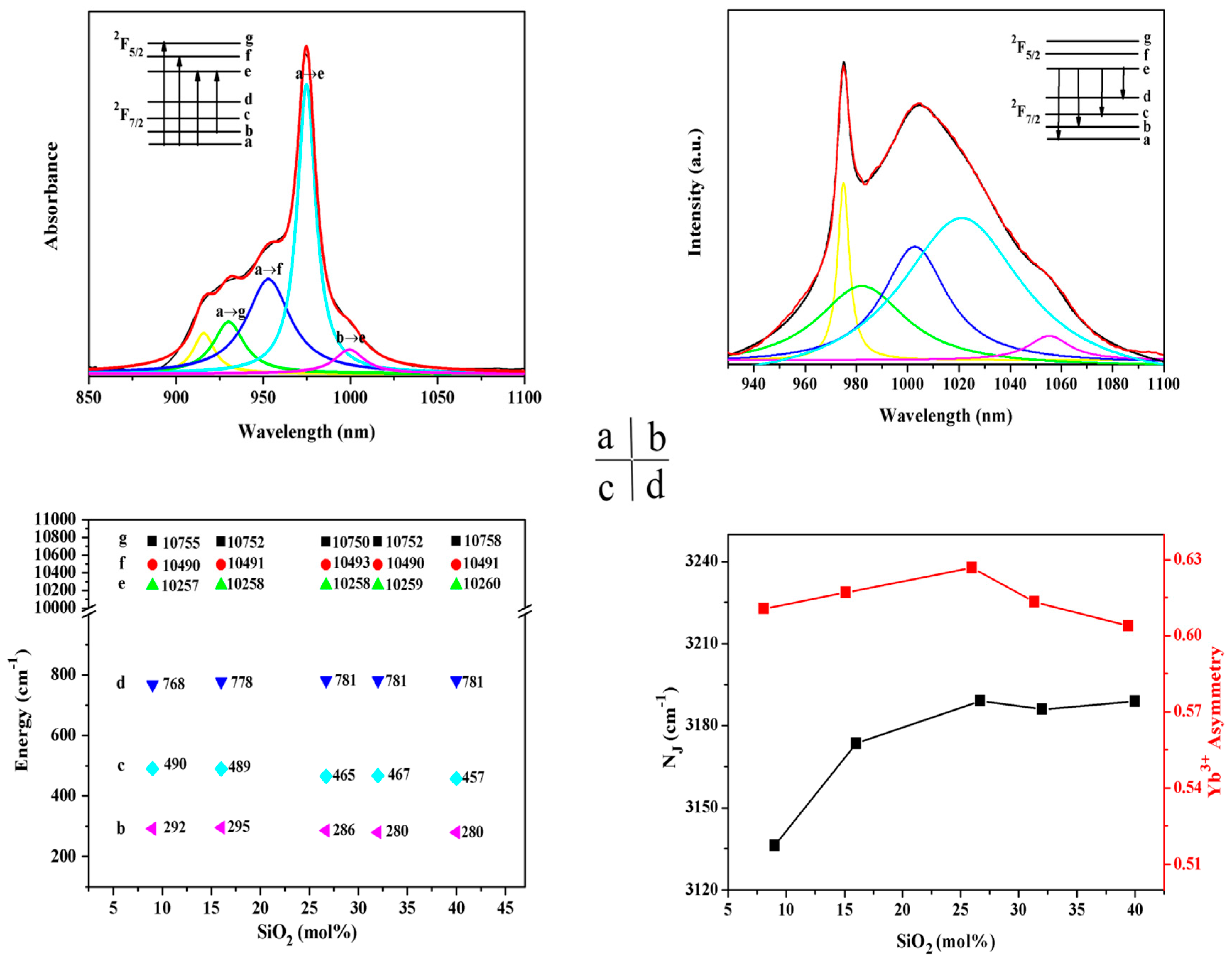
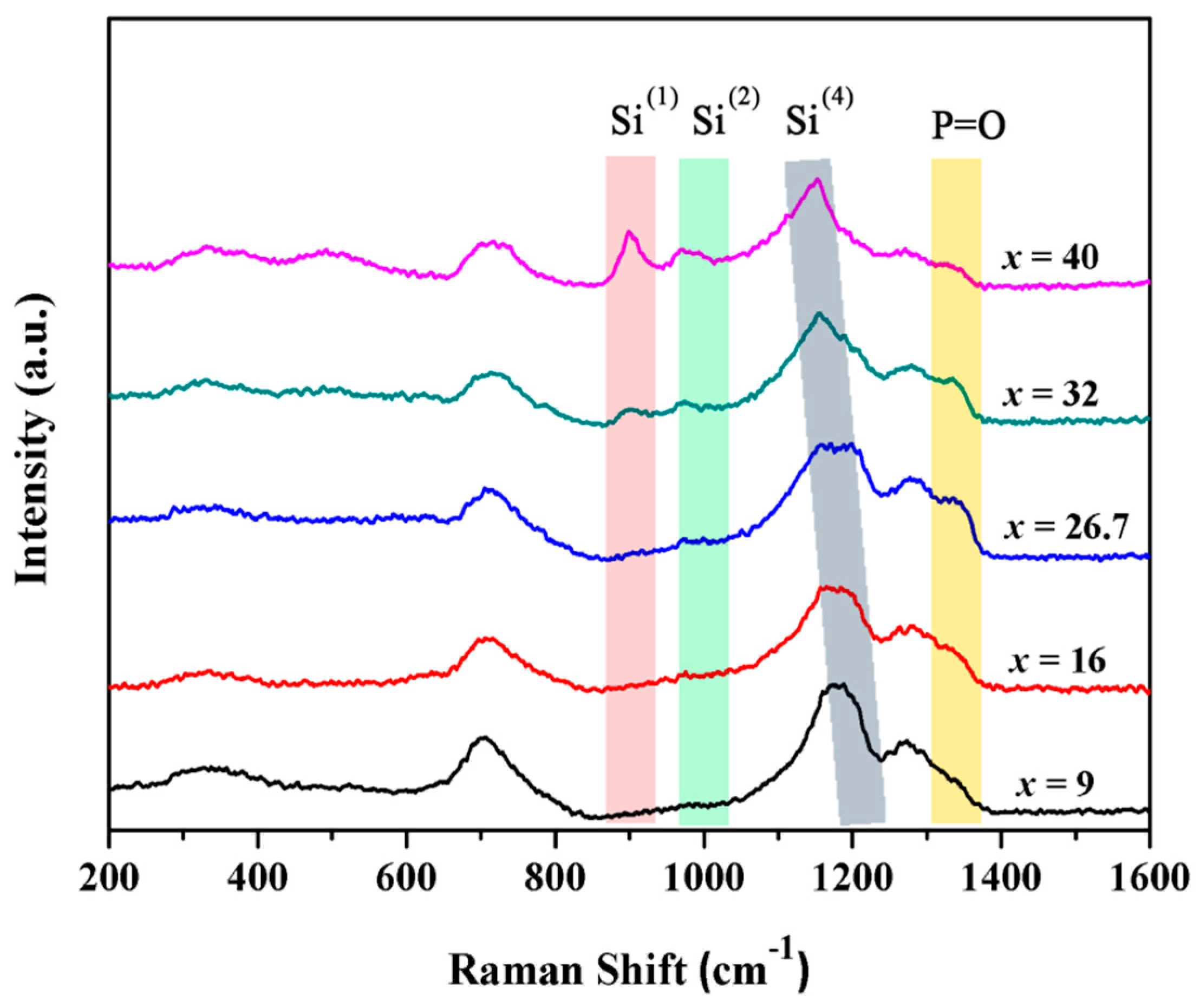
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zou, X.; Toratani, H. Evaluation of spectroscopic properties of Yb3+-doped glasses. Phys. Rev. B 1995, 52, 15889–15897. [Google Scholar] [CrossRef]
- Eger, D.; Atar, G.; Bruner, A.; Sfez, B.; Juwiler, I.; Kokki, T.; Kykkänen, P. Spectral characterization of Yb-doped silica layers for high power laser applications. Opt. Mater. 2011, 33, 438–443. [Google Scholar] [CrossRef]
- Zhang, L.; Pan, W.; Feng, J. Dependence of spectroscopic and thermal properties on concentration and temperature for Yb:Y2O3 transparent ceramics. J. Eur. Ceram. Soc. 2015, 35, 2547–2554. [Google Scholar] [CrossRef]
- Hönninger, C.; Paschotta, R.; Graf, M.; Morier-Genoud, F.; Zhang, G.; Moser, M.; Biswal, S.; Nees, J.; Braun, A.; Mourou, G.A.; et al. Ultrafast ytterbium-doped bulk lasers and laser amplifiers. Appl. Phys. B 1999, 69, 3–17. [Google Scholar] [CrossRef]
- DeLoach, L.D.; Payne, S.A.; Chase, L.L.; Smith, L.K.; Kway, W.L.; Krupke, W.F. Evaluation of absorption and emission properties of Yb3+-doped crystals for laser applications. IEEE J. Quantum Electron. 1993, 29, 1179–1191. [Google Scholar] [CrossRef]
- Snitzer, E. Optical maser action of Nd3+ in a barium crown glass. Phys. Rev. Lett. 1961, 7, 444–446. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, H.; Zeng, Q.J.; Tang, X.D.; Gan, F.X. Yb: Phosphate laser glass with high emission cross-section. J. Phys. Chem. Solids 2000, 61, 1217–1223. [Google Scholar] [CrossRef]
- Dai, S.X.; Sugiyama, A.; Hu, L.L.; Liu, Z.P.; Huang, G.S.; Jiang, Z.H. The spectrum and laser properties of ytterbium doped phosphate glass at low temperature. J. Non-Cryst. Solids 2002, 311, 138–144. [Google Scholar] [CrossRef]
- Krishnaiah, K.V.; Jayasankar, C.K.; Chaurasia, S.; Murali, C.G.; Dhareshwar, L.J. Preparation and characterization of Yb3+-doped metaphosphate glasses for high energy and high power laser applications. Sci. Adv. Mater. 2013, 5, 1–9. [Google Scholar] [CrossRef]
- Hu, J.Y.; Yang, H.W.; Chen, Y.J.; Lin, J.S.; Lai, C.H.; Lee, Y.M.; Zhang, T. Properties and structure of Yb3+ doped zinc aluminum silicate phosphate glasses. J. Non-Cryst. Solids 2011, 357, 2246–2250. [Google Scholar] [CrossRef]
- Chen, Y.K.; Wen, L.; Hu, L.L.; Chen, W.; Guyot, Y.; Boulon, G. Raman and optical absorption spectroscopic investigation of Yb-Er codoped phosphate glasses containing SiO2. Chin. Opt. Lett. 2009, 7, 56–59. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C.; Hu, L.L.; Zhang, L.Y. Effect of SiO2 on the Stark splitting enlargement of Yb3+ in phosphate glass. Acta Phys. Sin. 2016, 5, 311–317. [Google Scholar]
- Li, X.; Zeng, H.D.; Ye, F.; Chen, J.D.; Chen, G.R.; Chen, D.P. Influence of SiO2 on the Luminous Properties of Nd3+-Doped Barium Silicophosphate. Nanosci. Nanotechnol. Lett. 2016, 8, 207–210. [Google Scholar] [CrossRef]
- Xu, W.B.; Ren, J.J.; Shao, C.Y.; Wang, X.; Wang, M.; Zhang, L.Y.; Chen, D.P.; Wang, S.K.; Yu, C.L.; Hu, L.L. Effect of P5+ on spectroscopy and structure of Yb3+/Al3+/P5+ co-doped silica glass. J. Lumin. 2015, 167, 8–15. [Google Scholar] [CrossRef]
- McCumber, D.E. Einstein Relations connecting broadband emission and absorption spectra. Phys. Rev. 1964, 136, A954–A957. [Google Scholar] [CrossRef]
- Yin, H.B.; Deng, P.Z.; Zhang, J.Z.; Gan, F.X. Determination of emission cross section of Yb3+ in glasses by the reciprocity method. Mater. Lett. 1997, 30, 29–33. [Google Scholar] [CrossRef]
- Dai, S.X.; Hu, L.L.; Sugiyama, A.; Izawa, Y.; Liu, Z.P.; Jiang, Z.H. Study of a new ytterbium doped phosphate laser glass. Chin. Sci. Bull. 2002, 47, 255–259. [Google Scholar] [CrossRef]
- Auzel, F. On the maximum splitting of the (2F7/2) ground state in Yb3+-doped solid state laser materials. J. Lumin. 2001, 93, 129–135. [Google Scholar] [CrossRef]
- Xu, W.B.; Yu, C.L.; Wang, S.K.; Lou, F.G.; Feng, S.Y.; Wang, M.; Zhou, Q.L.; Chen, D.P.; Hu, L.L.; Guzik, M.; et al. Effects of F− on the optical and spectroscopic properties of Yb3+/Al3+ co-doped silica glass. Opt. Mater. 2015, 42, 245–250. [Google Scholar] [CrossRef]
- Yang, B.H.; Liu, X.Q.; Wang, X.; Zhang, J.J.; Hu, L.L.; Zhang, L.Y. Compositional dependence of room-temperature Stark splitting of Yb3+ in several popular glass systems. Opt. Lett. 2014, 39, 1772–1774. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Dupree, R.; Holland, D.; Mortuza, M.G. Six-coordinated silicon in glasses. Nature 1987, 328, 416–417. [Google Scholar] [CrossRef]
- Yamashita, H.; Yoshino, H.; Nagata, K.; Yamaguchi, I.; Ookawa, M.; Maekawa, T. NMR and Raman Studies of Na2O-P2O5-SiO2 Glasses. J. Ceram. Soc. Jpn. 1998, 106, 539–544. [Google Scholar] [CrossRef]
- Zeng, H.D.; Jiang, Q.; Liu, Z.; Li, X.; Ren, J.; Chen, G.R.; Liu, F.D.; Peng, S. Unique sodium phosphosilicate glasses designed through extended topological constraint theory. J. Phys. Chem. B 2014, 118, 5177–5183. [Google Scholar] [CrossRef] [PubMed]
- Hudgens, J.J.; Brow, R.K.; Tallant, D.R.; Martin, S.W. Raman spectroscopy study of the structure of lithium and sodium ultraphosphate glasses. J. Non-Cryst. Solids 1998, 223, 21–31. [Google Scholar] [CrossRef]
- Plotnichenko, V.G.; Sokolov, V.O.; Koltashev, V.V.; Dianov, E.M. On the structure of phosphosilicate glasses. J. Non-Cryst. Solids 2002, 306, 209–226. [Google Scholar] [CrossRef]
- Ji, X.M.; Zeng, H.D.; Li, X.; Ye, F.; Chen, J.D.; Chen, G.R. High glass transition temperature barium silicophosphate glasses designed with topological constraint theory. J. Am. Ceram. Soc. 2016, 99, 1255–1258. [Google Scholar] [CrossRef]
| Glass | σabs (975 nm) (10−20 cm2) | σem(975 nm) (10−20 cm2) | σabs (1005 nm) (10−20 cm2) | σem (1005 nm) (10−20 cm2) | τexp (ms) | σem × τexp (10−20 cm2·ms) |
|---|---|---|---|---|---|---|
| BPS9 | 1.09 | 1.46 | 0.15 | 0.86 | 1.17 | 1.01 |
| BPS16 | 0.98 | 1.30 | 0.13 | 0.76 | 1.38 | 1.04 |
| BPS26.7 | 0.85 | 1.13 | 0.12 | 0.71 | 1.55 | 1.10 |
| BPS32 | 0.80 | 1.07 | 0.11 | 0.66 | 1.37 | 0.91 |
| BPS40 | 0.62 | 0.83 | 0.11 | 0.62 | 1.46 | 0.90 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zeng, H.; Yang, B.; Ye, F.; Chen, J.; Chen, G.; Smith, A.T.; Sun, L. Structure-Dependent Spectroscopic Properties of Yb3+-Doped Phosphosilicate Glasses Modified by SiO2. Materials 2017, 10, 241. https://doi.org/10.3390/ma10030241
Wang L, Zeng H, Yang B, Ye F, Chen J, Chen G, Smith AT, Sun L. Structure-Dependent Spectroscopic Properties of Yb3+-Doped Phosphosilicate Glasses Modified by SiO2. Materials. 2017; 10(3):241. https://doi.org/10.3390/ma10030241
Chicago/Turabian StyleWang, Ling, Huidan Zeng, Bin Yang, Feng Ye, Jianding Chen, Guorong Chen, Andew T. Smith, and Luyi Sun. 2017. "Structure-Dependent Spectroscopic Properties of Yb3+-Doped Phosphosilicate Glasses Modified by SiO2" Materials 10, no. 3: 241. https://doi.org/10.3390/ma10030241






