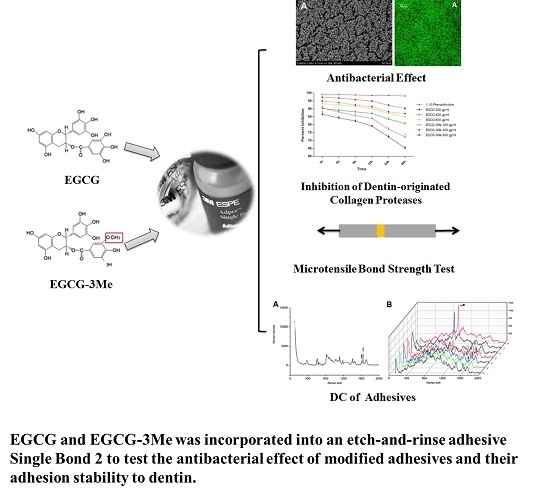
Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Adhesives
- (1)
- Adper SB 2 as the control (SB 2);
- (2)
- SB 2 + 200 μg/mL EGCG (EGCG 200);
- (3)
- SB 2 + 400 μg/mL EGCG (EGCG 400);
- (4)
- SB 2 + 600 μg/mL EGCG (EGCG 600);
- (5)
- SB 2 + 200 μg/mL EGCG-3Me (EGCG-3Me 200);
- (6)
- SB 2 + 400 μg/mL EGCG-3Me (EGCG-3Me 400);
- (7)
- SB 2 + 600 μg/mL EGCG-3Me (EGCG-3Me 600).
2.2. Antibacterial Test
2.2.1. Specimens Preparation
2.2.2. Bacteria Culture and Inoculation
2.2.3. OD 600 Evaluation
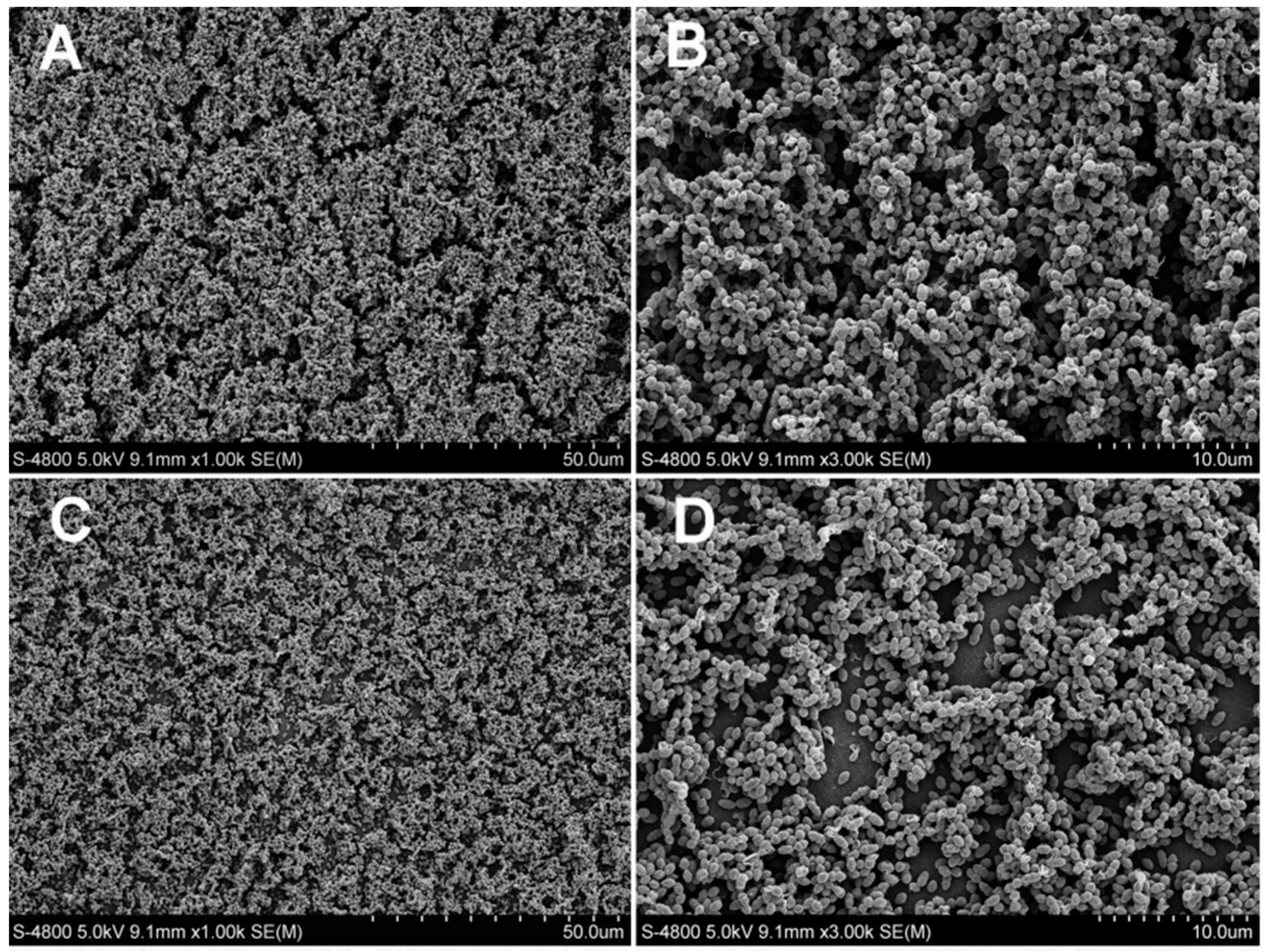
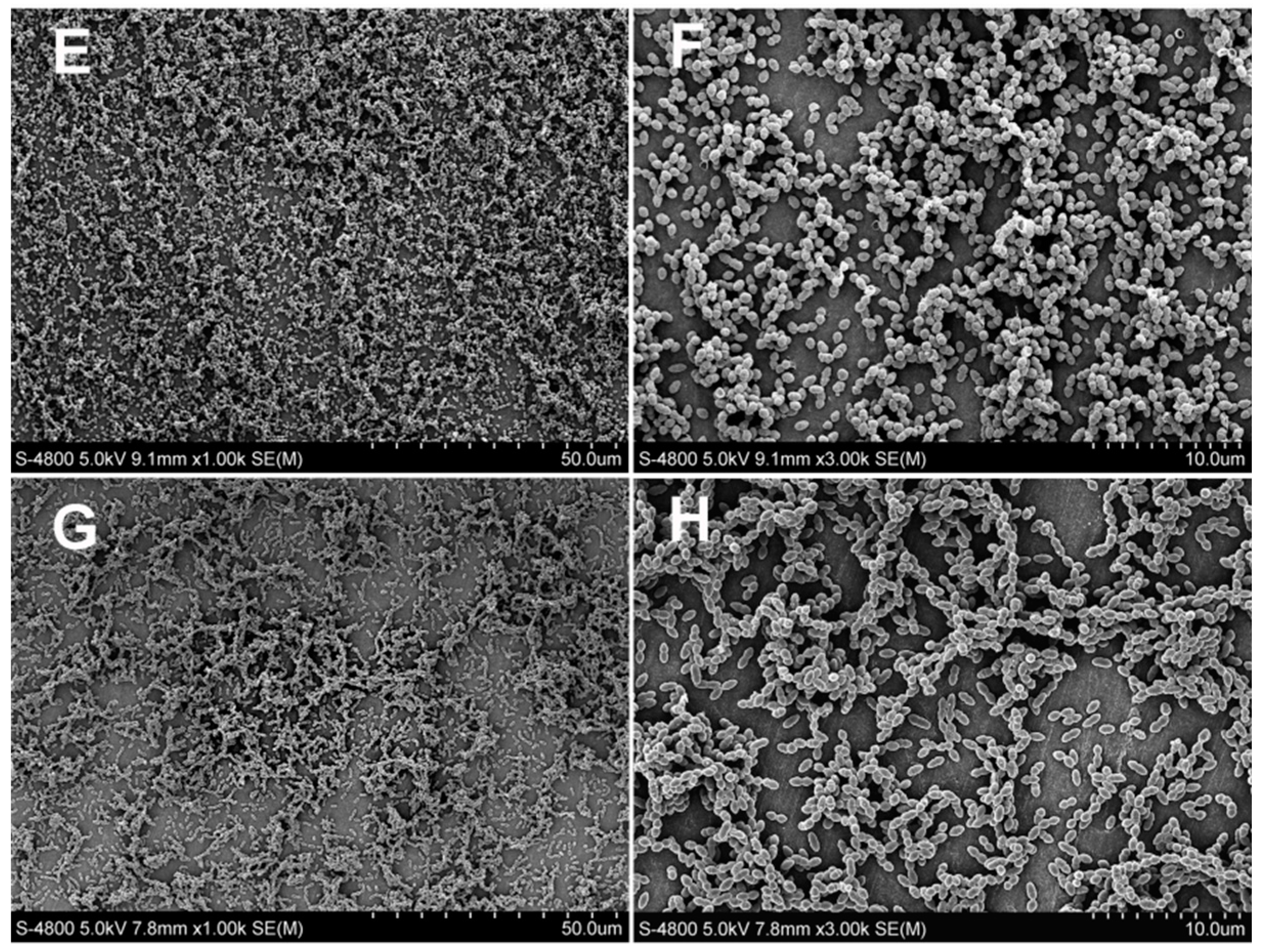
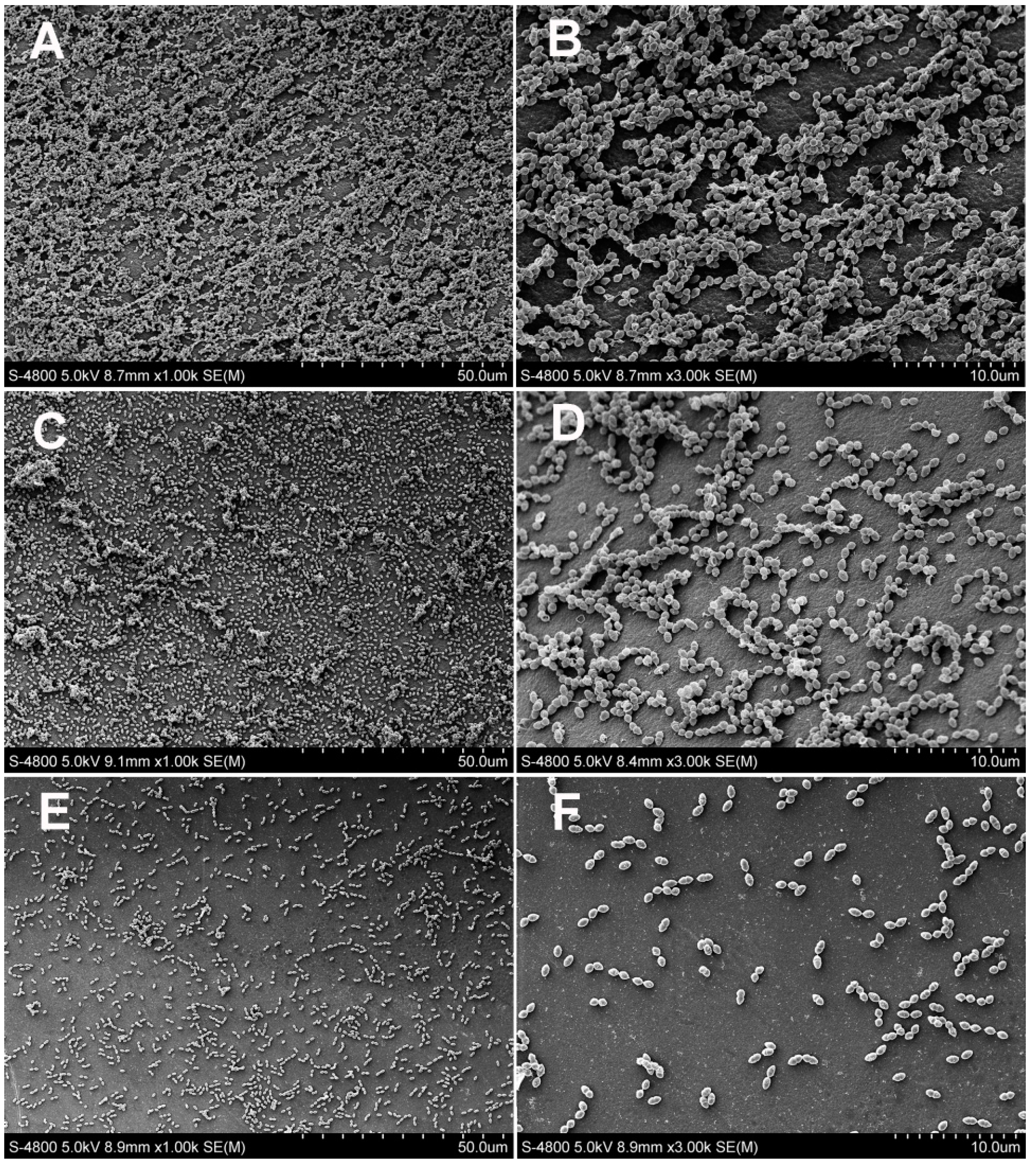
2.2.4. Scanning Electron Microscopy Observation
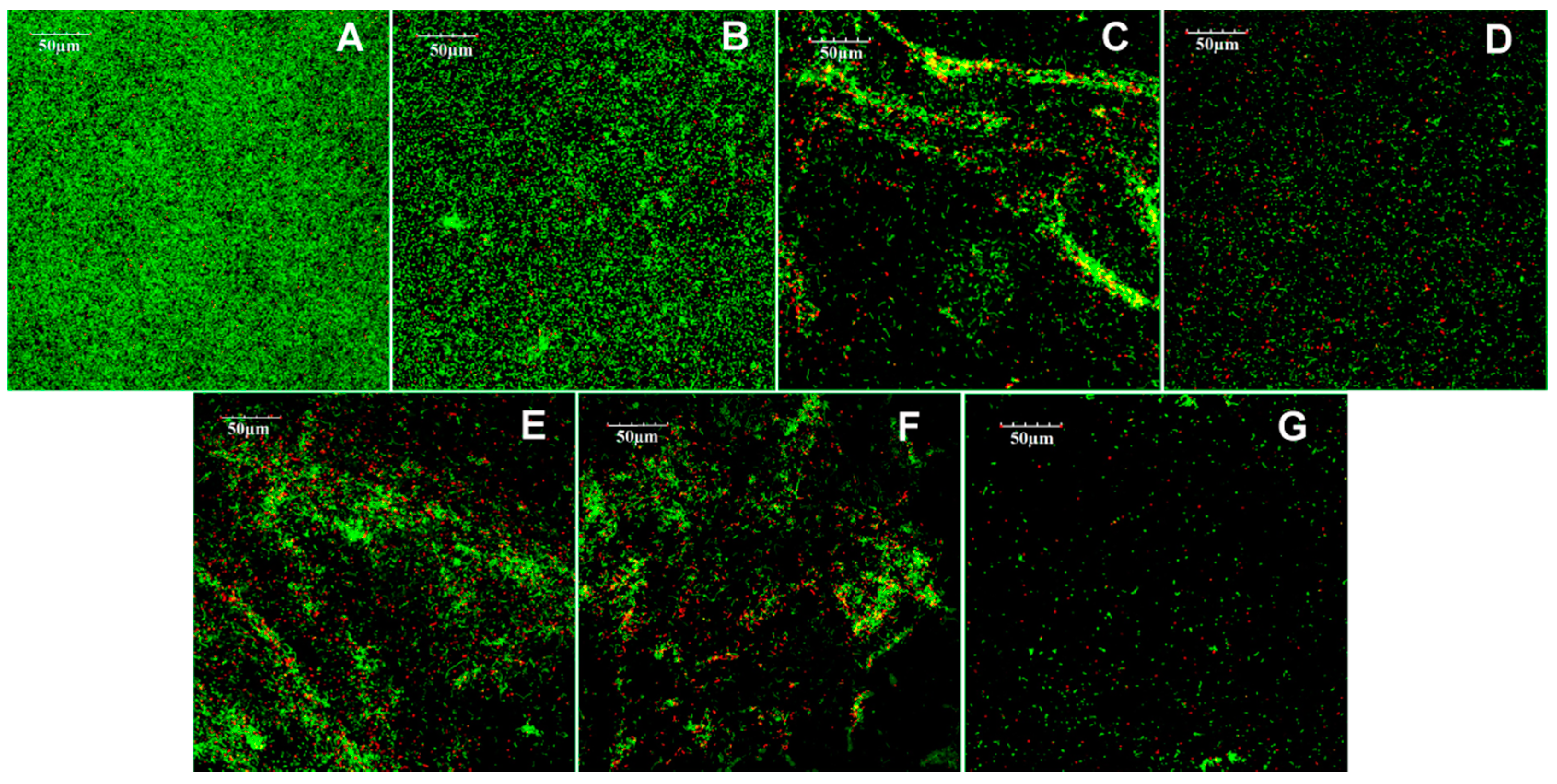
2.2.5. Confocal Laser Scanning Microscope Observation
2.3. Microtensile Bond Strength Test
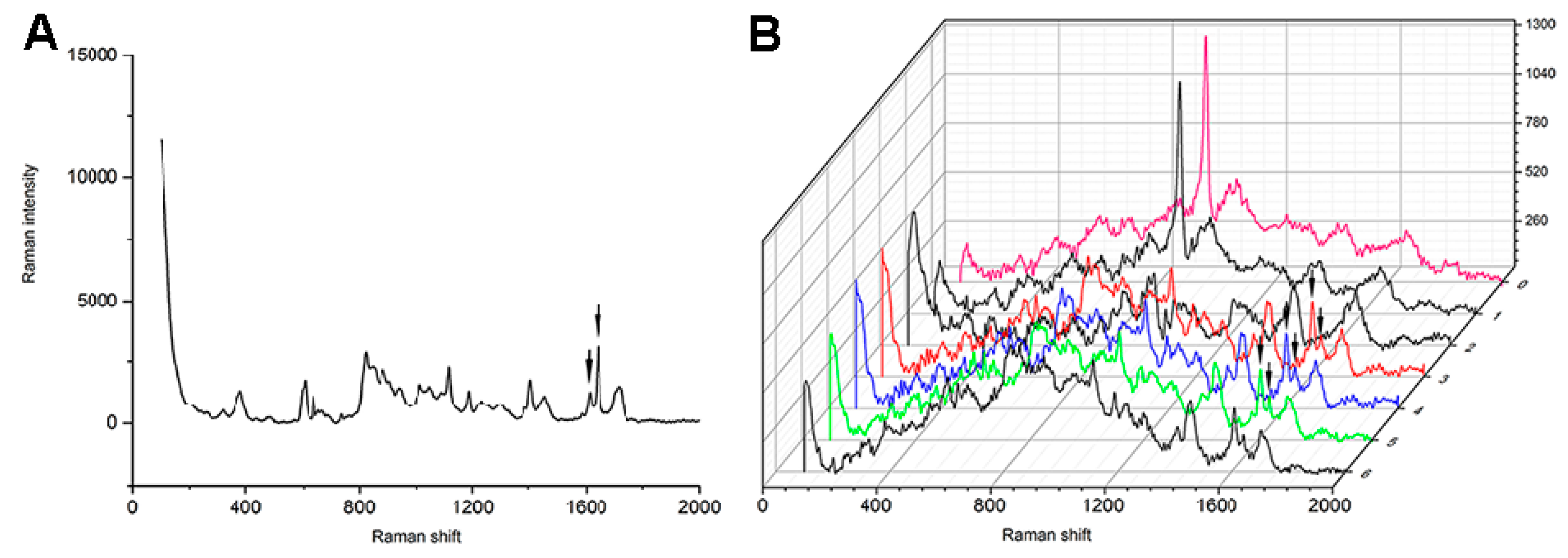
2.4. Micro-Raman Analysis for the Degrees of Conversion Measurements
2.5. Inhibition of Dentin-Originated Collagen Proteases Activities
2.5.1. Preparation of Dentin Powder
2.5.2. Preparation of the Experimental Solutions
- (1)
- Distilled water (negative control);
- (2)
- 1,10-phenanthroline (positive control);
- (3)
- 200 μg/mL EGCG (EGCG 200);
- (4)
- 400 μg/mL EGCG (EGCG 400);
- (5)
- 600 μg/mL EGCG (EGCG 600);
- (6)
- 200 μg/mL EGCG-3Me (EGCG-3Me 200);
- (7)
- 400 μg/mL EGCG-3Me (EGCG-3Me 400);
- (8)
- 600 μg/mL EGCG-3Me (EGCG-3Me 600).
2.5.3. Treatment of the Dentin Powder
2.6. Statistical Analysis
3. Results
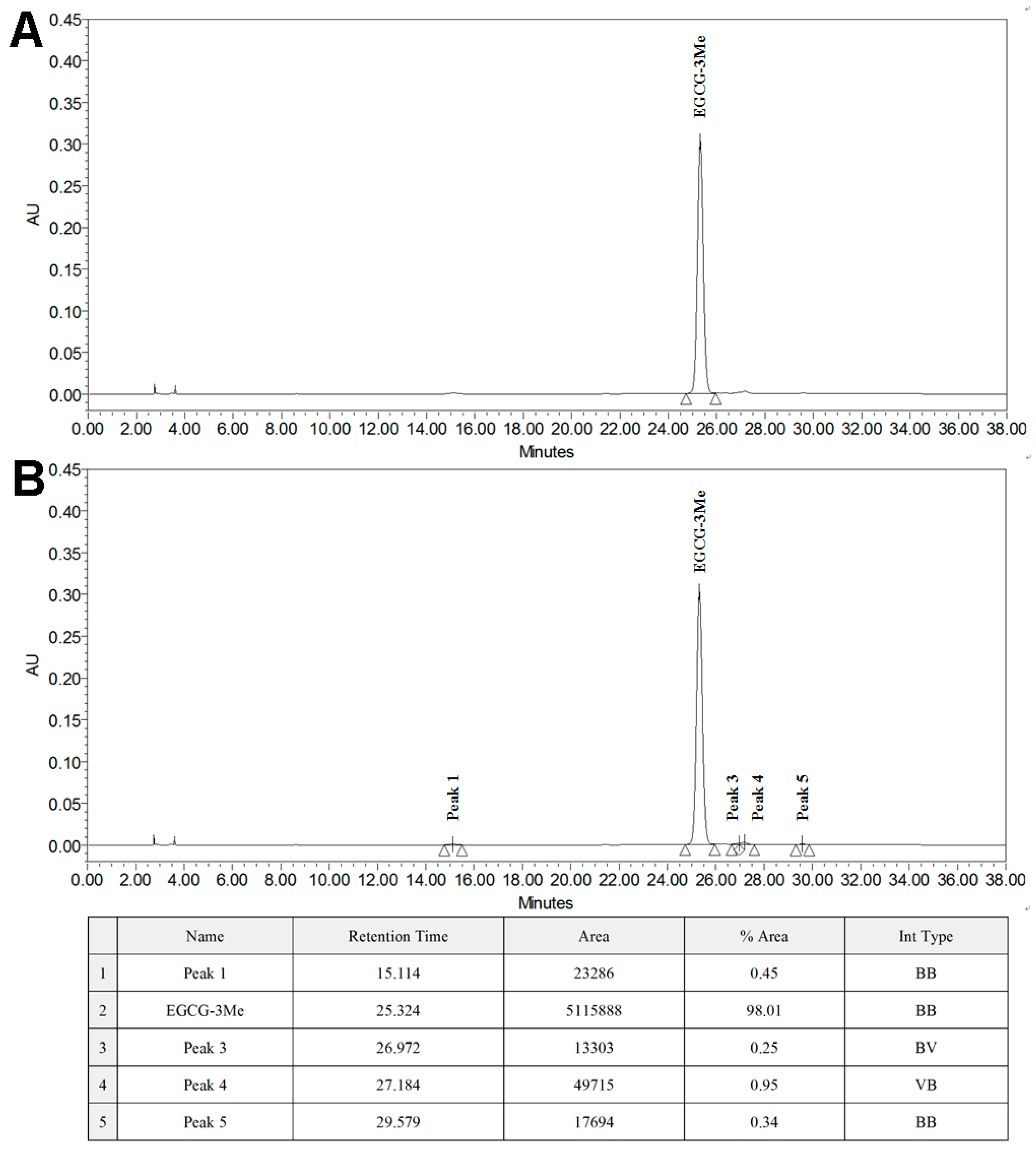
3.1. Isolation of EGCG-3Me
3.2. Antibacterial Test
3.3. Microtensile Bond Strength
3.4. Inhibition of Dentin-Originated Collagen Proteases Activities
3.5. Degree of Conversion
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of interest
References
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J.L. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.C.; Marouf, A.S.; Al-Hindi, A.M. Photo-polymerization shrinkage-stress kinetics in resin-composites: Methods development. Dent. Mater. 2003, 19, 1–11. [Google Scholar] [CrossRef]
- Garcia-Godoy, F.; Kramer, N.; Feilzer, A.J.; Frankenberger, R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. In vivo. Dent. Mater. 2010, 26, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; de Munck, J.; Yoshida, Y.; Inoue, S.; Vargas, M.; Vijay, P.; van Landuyt, K.; Lambrechts, P.; Vanherle, G. Buonocore memorial lecture. Adhesion to enamel and dentin: Current status and future challenges. Oper. Dent. 2003, 28, 215–235. [Google Scholar] [PubMed]
- Frost, P.M. An audit on the placement and replacement of restorations in a general dental practice. Prim. Dent. Care 2002, 9, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Deligeorgi, V.; Mjor, I.A.; Wilson, N.H. An overview of reasons for the placement and replacement of restorations. Prim. Dent. Care 2001, 8, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Brackett, M.G.; Li, N.; Brackett, W.W.; Sword, R.J.; Qi, Y.P.; Niu, L.N.; Pucci, C.R.; Dib, A.; Pashley, D.H.; et al. The critical barrier to progress in dentine bonding with the etch-and-rinse technique. J. Dent. 2011, 39, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tjaderhane, L.; Breschi, L.; Mazzoni, A.; Li, N.; Mao, J.; Pashley, D.H.; Tay, F.R. Limitations in bonding to dentin and experimental strategies to prevent bond degradation. J. Dent. Res. 2011, 90, 953–968. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Domb, A.J.; Weiss, E.I. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J. Dent. 2007, 35, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Zalkind, M.M.; Keisar, O.; Ever-Hadani, P.; Grinberg, R.; Sela, M.N. Accumulation of streptococcus mutans on light-cured composites and amalgam: An in vitro study. J. Esthet. Dent. 1998, 10, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Torii, M.; Tsuchitani, Y.; McCabe, J.F.; Russell, R.R. Incorporation of bacterial inhibitor into resin composite. J. Dent. Res. 1994, 73, 1437–1443. [Google Scholar] [PubMed]
- Sakaguchi, R.L. Review of the current status and challenges for dental posterior restorative composites: Clinical, chemistry, and physical behavior considerations. Summary of discussion from the portland composites symposium (POCOS) June 17–19, 2004, Oregon Health and Science University, Portland, Oregon. Dent. Mater. 2005, 21, 3–6. [Google Scholar] [PubMed]
- Jokstad, A.; Bayne, S.; Blunck, U.; Tyas, M.; Wilson, N. Quality of dental restorations. FDI commission project 2–95. Int. Dent. J. 2001, 51, 117–158. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.M.; Pereira, J.C.; Yoshiyama, M.; Pashley, D.H. A review of polymerization contraction: The influence of stress development versus stress relief. Oper. Dent. 1996, 21, 17–24. [Google Scholar] [PubMed]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In vitro antibacterial effects of the dentin primer of clearfil protect bond. Dent. Mater. 2006, 22, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Esteves, C.M.; Ota-Tsuzuki, C.; Reis, A.F.; Rodrigues, J.A. Antibacterial activity of various self-etching adhesive systems against oral streptococci. Oper. Dent. 2010, 35, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S. Bio-active restorative materials with antibacterial effects: New dimension of innovation in restorative dentistry. Dent. Mater. J. 2009, 28, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.; Chai, Z.; Zhang, L.; Xiao, Y.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of streptococcus mutans. J. Dent. 2009, 37, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Stanislawczuk, R.; Pereira, F.; Munoz, M.A.; Luque, I.; Farago, P.V.; Reis, A.; Loguercio, A.D. Effects of chlorhexidine-containing adhesives on the durability of resin-dentine interfaces. J. Dent. 2014, 42, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, J.; Chen, L.; Li, D.; Tan, Y. The incorporation of chlorhexidine in a two-step self-etching adhesive preserves dentin bond in vitro. J. Dent. 2009, 37, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer mdpb. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Cheng, L.; Weir, M.D.; Xu, H.H.; Antonucci, J.M.; Kraigsley, A.M.; Lin, N.J.; Lin-Gibson, S.; Zhou, X. Antibacterial amorphous calcium phosphate nanocomposites with a quaternary ammonium dimethacrylate and silver nanoparticles. Dent. Mater. 2012, 28, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Strategies to prevent hydrolytic degradation of the hybrid layer—A review. Dent. Mater. 2013, 29, 999–1011. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.C.; Kern, M. The role of host-derived dentinal matrix metalloproteinases in reducing dentin bonding of resin adhesives. Int. J. Oral Sci. 2009, 1, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Tjaderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.R.; Carvalho, R.M.; Tay, F.R.; et al. Optimizing dentin bond durability: Control of collagen degradation by matrix metalloproteinases and cysteine cathepsins. Dent. Mater. 2013, 29, 116–135. [Google Scholar] [CrossRef] [PubMed]
- Komori, P.C.; Pashley, D.H.; Tjaderhane, L.; Breschi, L.; Mazzoni, A.; de Goes, M.F.; Wang, L.; Carrilho, M.R. Effect of 2% chlorhexidine digluconate on the bond strength to normal versus caries-affected dentin. Oper. Dent. 2009, 34, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Majd, H.; Weir, M.D.; Arola, D.D.; Xu, H.H. Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dent. Mater. 2015, 31, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Ferrazzano, G.F.; Amato, I.; Ingenito, A.; Zarrelli, A.; Pinto, G.; Pollio, A. Plant polyphenols and their anti-cariogenic properties: A review. Molecules 2011, 16, 1486–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.T.; Leite, A.L.; Hannas, A.R.; Buzalaf, M.A. Gels containing mmp inhibitors prevent dental erosion in situ. J. Dent. Res. 2010, 89, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, J.; Buer, J.; Pietschmann, T.; Steinmann, E. Anti-infective properties of epigallocatechin-3-gallate (EGCG), a component of green tea. Br. J. Pharmacol. 2013, 168, 1059–1073. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Huang, X.; Huang, C.; Wang, Y.; Zhang, Y. Epigallocatechin-3-gallate (EGCG) enhances the therapeutic activity of a dental adhesive. J. Dent. 2012, 40, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Guo, J.; Deng, D.; Chen, Z.; Huang, C. Effect of adjunctive application of epigallocatechin-3-gallate and ethanol-wet bonding on adhesive-dentin bonds. J. Dent. 2016, 44, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Neri, J.R.; Yamauti, M.; da Silveira, F.D.; Mendonca, J.S.; de Carvalho, R.M.; Santiago, S.L. Influence of dentin biomodification with epigallocatechin-3-gallate on the bond strength of self-etch adhesive: Twelve-month results. Int. J. Adhes. Adhes. 2016, 71, 81–86. [Google Scholar] [CrossRef]
- Huo, C.; Wan, S.B.; Lam, W.H.; Li, L.; Wang, Z.; Landis-Piwowar, K.R.; Chen, D.; Dou, Q.P.; Chan, T.H. The challenge of developing green tea polyphenols as therapeutic agents. Inflammopharmacology 2008, 16, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Maeda-Yamamoto, M.; Ema, K.; Monobe, M.; Tokuda, Y.; Tachibana, H. Epicatechin-3-O-(3″-O-methyl)-gallate content in various tea cultivars (Camellia sinensis L.) and its in vitro inhibitory effect on histamine release. J. Agric. Food Chem. 2012, 60, 2165–2170. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Landis-Piwowar, K.; Chan, T.H.; Dou, Q.P. Green tea polyphenols as proteasome inhibitors: Implication in chemoprevention. Curr. Cancer Drug Targets 2011, 11, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Kurita, I.; Maeda-Yamamoto, M.; Tachibana, H.; Kamei, M. Antihypertensive effect of benifuuki tea containing O-methylated EGCG. J. Agric. Food Chem. 2010, 58, 1903–1908. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Suzuki, M.; Miyase, T.; Yoshino, K.; Maeda-Yamamoto, M. Novel antiallergic catechin derivatives isolated from oolong tea. J. Agric. Food Chem. 1999, 47, 1906–1910. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm effect of dental adhesive with cationic monomer. J. Dent. Res. 2009, 88, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ehara, A.; Torii, M.; Ebisu, S. Antibacterial activity of dentine primer containing mdpb after curing. J. Dent. 1998, 26, 267–271. [Google Scholar] [CrossRef]
- Li, F.; Weir, M.D.; Chen, J.; Xu, H.H. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Acquaviva, P.A.; Cerutti, F.; Adami, G.; Gagliani, M.; Ferrari, M.; Gherlone, E.; Cerutti, A. Degree of conversion of three composite materials employed in the adhesive cementation of indirect restorations: A micro-raman analysis. J. Dent. 2009, 37, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L.; Cooper, P.R.; Smith, A.J. Dentin matrix component solubilization by solutions of PH relevant to self-etching dental adhesives. J. Adhes. Dent. 2013, 15, 407–412. [Google Scholar] [PubMed]
- Tezvergil-Mutluay, A.; Mutluay, M.M.; Gu, L.S.; Zhang, K.; Agee, K.A.; Carvalho, R.M.; Manso, A.; Carrilho, M.; Tay, F.R.; Breschi, L.; et al. The anti-mmp activity of benzalkonium chloride. J. Dent. 2011, 39, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, S.; Seseogullari-Dirihan, R.; Uctasli, M.; Tay, F.R.; Pashley, D.H.; Tezvergil-Mutluay, A. Effect of polyacrylic acid on dentin protease activities. Dent. Mater. 2015, 31, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Shin, D.H. Antibacterial effect of self-etching adhesive systems on streptococcus mutans. Restor. Dent. Endod. 2014, 39, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.M.; Goncalves, R.B.; Pimenta, L.A.; Bedran-Russo, A.K.; Pereira, P.N. In vitro evaluation of caries inhibition promoted by self-etching adhesive systems containing antibacterial agents. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 75, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, J.; Yang, X.; Xu, X.; Li, D.; Chen, L. MMP-inhibitory effect of chlorhexidine applied in a self-etching adhesive. J. Adhes. Dent. 2011, 13, 111–115. [Google Scholar] [PubMed]
- Cadenaro, M.; Pashley, D.H.; Marchesi, G.; Carrilho, M.; Antoniolli, F.; Mazzoni, A.; Tay, F.R.; Di Lenarda, R.; Breschi, L. Influence of chlorhexidine on the degree of conversion and e-modulus of experimental adhesive blends. Dent. Mater. 2009, 25, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Anusavice, K.J.; Zhang, N.Z.; Shen, C. Controlled release of chlorhexidine from udma-tegdma resin. J. Dent. Res. 2006, 85, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Amaral, F.L.; Colucci, V.; Palma-Dibb, R.G.; Corona, S.A. Assessment of in vitro methods used to promote adhesive interface degradation: A critical review. J. Esthet. Restor. Dent. 2007, 19, 340–353. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.S.; Darvell, B.W. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Miyazaki, M.; Sato, M.; Onose, H.; Moore, B.K. Influence of thermal cycling on dentin bond strength of two-step bonding systems. Am. J. Dent. 1998, 11, 118–122. [Google Scholar] [PubMed]
- Zhang, L.; Wang, D.Y.; Fan, J.; Li, F.; Chen, Y.J.; Chen, J.H. Stability of bonds made to superficial vs. deep dentin, before and after thermocycling. Dent. Mater. 2014, 30, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Pang, E.K.; Kim, C.S.; Yoo, Y.J.; Cho, K.S.; Chai, J.K.; Kim, C.K.; Choi, S.H. Inhibitory effects of green tea polyphenol (–)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J. Periodontal Res. 2004, 39, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.M.; Aguiar, T.R.; Phansalkar, R.; McAlpine, J.B.; Napolitano, J.G.; Chen, S.N.; Araujo, L.S.; Pauli, G.F.; Bedran-Russo, A. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014, 10, 3288–3294. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial activity and bonding ability of an adhesive incorporating an antibacterial monomer DMAE-CB. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Asmussen, E. Restorative resins: Hardness and Strength vs. Quantity of remaining double bonds. Scand. J. Dent. Res. 1982, 90, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Epasinghe, D.J.; Yiu, C.K.; Burrow, M.F.; Tay, F.R.; King, N.M. Effect of proanthocyanidin incorporation into dental adhesive resin on resin-dentine bond strength. J. Dent. 2012, 40, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Kirita, M.; Honma, D.; Tanaka, Y.; Usui, S.; Shoji, T.; Sami, M.; Yokota, T.; Tagashira, M.; Muranaka, A.; Uchiyama, M.; et al. Cloning of a novel O-methyltransferase from camellia sinensis and synthesis of O-methylated EGCG and evaluation of their bioactivity. J. Agric. Food Chem. 2010, 58, 7196–7201. [Google Scholar] [CrossRef] [PubMed]
- Dickens, S.H.; Cho, B.H. Interpretation of bond failure through conversion and residual solvent measurements and weibull analyses of flexural and microtensile bond strengths of bonding agents. Dent. Mater. 2005, 21, 354–364. [Google Scholar] [CrossRef] [PubMed]


| Materials | Composition | Application Procedures |
|---|---|---|
| Adper Single Bond 2 (3 M ESPE, St. Paul, MN, USA) | bis-GMA; HEMA; Polyalkenoic; Acid copolymer; Photoinitiators; Ethanol; Water. | Phosphoric acid-etching for 15 s; rinse with water for 10 s; dry with paper points; apply two coats of adhesive; air-dry for 5 s; light cure for 10 s. |
| Adhesive | OD 600 Values |
|---|---|
| SB 2 | 0.438 ± 0.029 a, |
| EGCG 200 | 0.379 ± 0.039 a, |
| EGCG 400 | 0.292 ± 0.026 b |
| EGCG 600 | 0.219 ± 0.037 c |
| EGCG-3Me 200 | 0.241 ± 0.037 b,c |
| EGCG-3Me 400 | 0.201 ± 0.025 c |
| EGCG-3Me 600 | 0.136 ± 0.028 d |
| Time | Adhesive | ||||||
|---|---|---|---|---|---|---|---|
| SB 2 | EGCG 200 | EGCG 400 | EGCG 600 | EGCG-3Me 200 | EGCG-3Me 400 | EGCG-3Me 600 | |
| Immediate | 33.44 ± 6.43 a,1 | 32.64 ± 5.30 a,1 | 30.38 ± 3.64 a,1 | 30.20 ± 4.83 a,1 | 30.43 ± 5.20 a,1 | 31.20 ± 4.17 a,1 | 31.51 ± 3.08 a,1 |
| After Thermocycling | 20.80 ± 2.22 b,1 | 27.09 ± 2.96 a,2 | 27.61 ± 1.40 a,2 | 27.76 ± 1.83 a,2 | 27.82 ± 2.36 a,2 | 29.60 ± 2.04 a,2 | 29.99 ± 2.26 a,2 |
| Adhesive | Degree of Conversion (%) | ||
|---|---|---|---|
| Adhesive-Composite Interface | Middle of the Hybrid Layer | Bottom of the Hybrid Layer that Adjoined the Dentin | |
| SB 2 | 73.14 ± 0.85 a,1 | 72.23 ± 0.89 a,1 | 72.00 ± 1.84 a,1 |
| EGCG 200 | 71.05 ± 1.49 a,c,1 | 69.05 ± 0.93 a,c,1 | 68.90 ± 0.38 a,d,1 |
| EGCG 400 | 69.34 ± 2.02 a,c,1 | 65.53 ± 0.45 c,1,2 | 62.56 ± 1.85 c,2 |
| EGCG 600 | 62.49 ± 2.61 b,1 | 56.03 ± 1.11 b,2 | 53.83 ± 1.89 b,2 |
| EGCG-3Me 200 | 71.26 ± 1.14 a,c,1 | 69.11 ± 2.84 a,c,1 | 68.84 ± 2.66 a,d,1 |
| EGCG-3Me 400 | 69.55 ± 1.66 a,c,1 | 67.47 ± 1.16 c,1 | 65.91 ± 2.80 c,d,1 |
| EGCG-3Me 600 | 67.42 ± 1.70 c,1 | 65.23 ± 1.84 c,1,2 | 62.03 ± 1.95 c,2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, H.-H.; Zhang, L.; Yu, F.; Li, F.; Liu, Z.-Y.; Chen, J.-H. Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin. Materials 2017, 10, 183. https://doi.org/10.3390/ma10020183
Yu H-H, Zhang L, Yu F, Li F, Liu Z-Y, Chen J-H. Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin. Materials. 2017; 10(2):183. https://doi.org/10.3390/ma10020183
Chicago/Turabian StyleYu, Hao-Han, Ling Zhang, Fan Yu, Fang Li, Zheng-Ya Liu, and Ji-Hua Chen. 2017. "Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin" Materials 10, no. 2: 183. https://doi.org/10.3390/ma10020183