1. Introduction
Additive manufacturing of ceramics by stereolithography (SL) is a promising alternative to conventional manufacturing methods for the production of complex parts in small series where high resolution and surface quality is required. A ceramic green object is formed, layer by layer, by the selective photopolymerisation of a ceramic suspension containing photocurable monomer, photoinitiator, and ceramic powder [
1]. Curing is accomplished by either scanning the surface with a UV laser [
1,
2,
3] or by projecting an image with a dynamic light mask using Digital Light Processing (DLP) technology or an LCD [
4,
5]. After a three-dimensional ceramic green object has been shaped, the formed polymer matrix is removed through thermal debinding, and the resulting powder body sintered to form a dense ceramic part [
1]. Recently, alumina parts with high density and mechanical strength comparable to that of conventional manufacturing methods have been successfully manufactured using commercially available photocurable ceramic suspensions and a DLP-based stereolithography apparatus (SLA) from Lithoz GmbH, Vienna, Austria [
6].
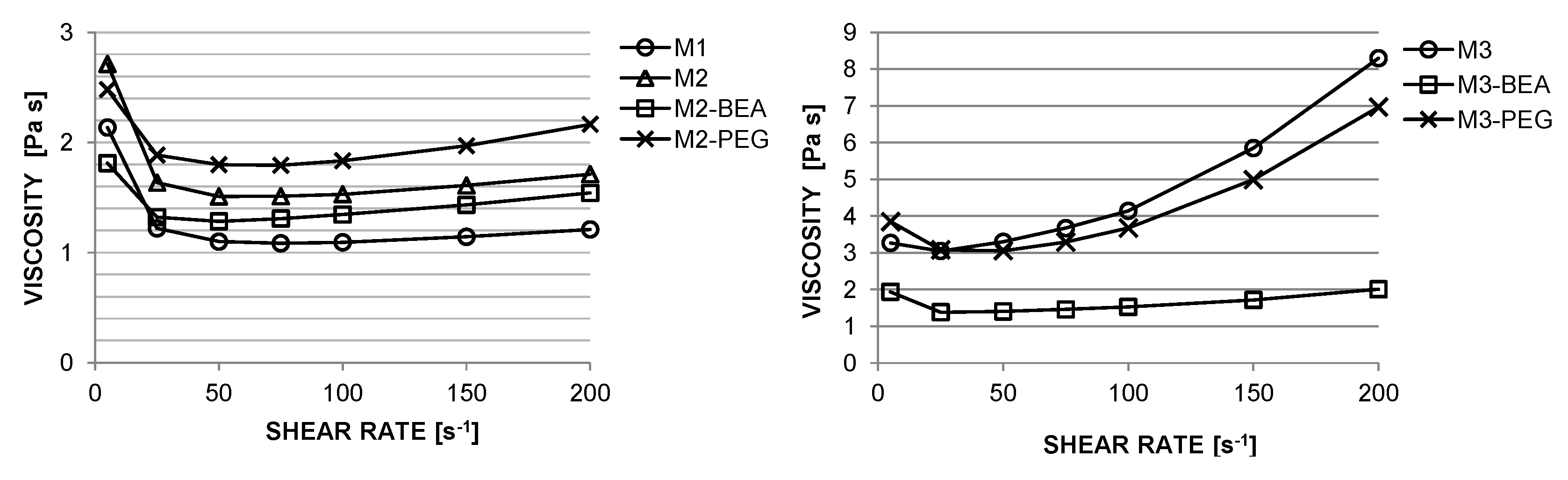
Photocurable suspensions must fulfil several requirements to be suitable for SL. A shear-thinning behaviour is desired to allow spreading of homogenous layers [
5]. Additionally, the suspensions must exhibit a sufficient cure depth to provide adequate integration between layers [
2,
7]. The cure depth (C
d) is related to the energy dose (E) by the Jacob’s equation, as C
d = D
pln(E/E
c), where D
p represents a sensitivity factor and E
c the critical energy required to initiate polymerisation [
8]. D
p and E
c of a photocurable resin depends on polymerization inhibitors and the absorption by the photoinitiator and inert dyes [
3]. When a ceramic powder is added to the resin, it induces light scattering, which depends on the powder loading and the refractive index contrast (Δn) between the resin and the ceramic powder [
3]. A higher Δn increases the light scattering effect. A high solids load of ceramic powder is required in order to manufacture dense ceramic parts, avoid the formation of cracks and defects, and achieve a uniform shrinkage during sintering [
5].
The most critical and time-consuming step during the manufacturing process is the debinding of the green part. The crosslinked polymer network is removed thermally by slowly heating the part to 600 °C in air. If the debinding rate is too high, volatile products from the decomposition will not have time to diffuse out from the structure, leading to pressure buildup and the formation of cracks or layer delamination [
9,
10]. The mass transport becomes increasingly diffusion-limited as the features of the component increase in size, leading to long debinding times. One proposed solution to reduce delamination during debinding is by adding a plasticizing agent to reduce internal stresses generated in the part owing to polymerization shrinkage [
11]. Defects can also be avoided by introducing open spaces into the structure by adding components or solvents designed to evaporate or decompose at a lower temperature than the thermal decomposition temperature of the polymer matrix [
12]. Bae and Halloran [
13] found that residual unpolymerized monomer in the green object caused cracks during debinding, possibly owing to internal stresses caused by thermally-initiated polymerization during the debinding.
Most publications regarding SL of ceramics do not fully describe resin compositions and/or processing conditions and they rarely reveal crucial difficulties or solutions for success. In this work, the aim was to document the entire SL processing of alumina utilizing specific resin compositions by exploring processing properties and impact on delamination and defects in shaped and sintered materials. This included the characterization of rheological, curing, and debinding properties, and micro-structural evaluation of sintered specimens manufactured by using the commercially available DLP-based SLA Cerafab 7500 from Lithoz GmbH, Vienna, Austria.
2. Materials and Methods
2.1. Starting Materials
The alumina powder used in the study was CT 3000SG (Almatis), a commercial α-alumina powder with d50 = 0.5 μm and a surface area of 7.5 m2/g. Hypermer KD-1 (Croda)—a polyester/polyamine condensation polymer with a cationic head group—was used as a dispersant in order to obtain a low viscosity and high homogeneity of the photocurable suspensions. Three radically polymerisable monomers with low skin irritation were used: ethoxylated(2) 1,6-hexanediol diacrylate (HDEODA), di(trimethylolpropane) tetraacrylate (DiTMPTA), and dipentaerythritol penta-/hexa-acrylate (DPHA) (Sigma Aldrich, Schnelldorf, Germany).
HDEODA was used as the main constituent, owing to its low viscosity, whereas DiTMPTA and DPHA were added in order to increase the crosslinking of the resins. Camphorquinone (CQ)—an
α-diketone with an absorption peak at 470 nm which is commonly used for visible light photopolymerization of dental composite resins—was selected as photoinitiator. The efficiency of CQ can be significantly increased by the addition of an amine with an abstractable
α-hydrogen as a co-initiator [
14]. The tertiary amine 2-(dimethylamino) ethyl methacrylate (DMAEMA) was selected for this purpose. Butoxy ethyl acetate (BEA) and poly(ethylene glycol) 200 (PEG-200) were evaluated as non-reactive diluents. Properties of the evaluated monomers and diluents can be found in
Table 1.
The selection of a suitable cleaning liquid to remove un-polymerized material from the additively-manufactured parts is important in order to ensure an easy cleaning process without swelling and/or layer delamination. Acetone, ethanol, 1-octanol, iso-propanol, PEG-200, and a mixture of dibasic esters (DBE, Sigma Aldrich) were evaluated as cleaning medium.
2.2. Preparation of Ceramic Suspensions
A special pre-preparation route for the alumina powder was utilized where it was ball milled for 24 h in tert-amyl alcohol together with 2.5 wt % of the dispersant based on the powder weight. In order to facilitate time-efficient preparation of homogenous low-viscosity resin suspensions, the ball milled suspension was freeze granulated. Freeze granulation allows the preparation of homogenous granules that are easy to disintegrate by spraying the suspension into liquid nitrogen and subsequently removing the solvent by freeze-drying [
15]. Resins were prepared by dissolving photoinitiator (0.33 wt % based on the resin weight) in the acrylate monomer and diluent mixtures according to
Table 2, and finally, the co-initiator was added. The CQ–amine molar ratio was 1:2. The freeze granulated powder was added in portions during continuous impeller mixing until a solids load of 50 vol % was achieved. Prior to loading the suspensions into the SLA, they were de-aired by vacuum treatment.
2.3. Additive Manufacturing of Ceramic Parts
Test specimens with the dimensions 2 mm × 5 mm × 10 mm were manufactured using a Cerafab 7500 from Lithoz GmbH, Vienna, Austria, designed to allow additive manufacturing of ceramic green parts by stereolithography. The DLP-based system uses a Digital Micromirror Device (DMD) with a resolution of 1920 × 1080 pixels, resulting in a lateral resolution of 40 × 40 μm in the build plane. The light source is a series of LEDs emitting 460 nm blue light. The fabrication of each layer starts by the application of a thin layer of ceramic suspension across the bottom of a circular transparent vat by a wiper blade. The building platform descends into the vat until it is positioned at a distance equal to the desired layer thickness from the bottom of the vat. An image corresponding to the shape of the layer is projected onto the transparent vat from below using a DMD, selectively curing the material. The vat is tilted down to detach the cured layer, and the building platform ascends to allow recoating of the suspension layer at the bottom of the vat. The layer thickness was set to 25 μm, and the cure depth was varied between 50–150 μm by adjusting the exposure time. Manufactured parts were carefully removed from the building platform with a razor blade. Un-polymerized suspension was removed from the test specimens using cleaning liquid and compressed air. Thermal debinding was performed by rate-controlled extraction in a custom-built furnace with an integrated high-precision balance. The weight loss was monitored and used to control the temperature increase using custom-built computer software. A weight loss rate of 0.05 wt %/min was used up to 600 °C, resulting in a debinding cycle of approximately 50 h, and directly followed by a pre-sintering for 2 h at 900 °C. Finally, the test specimens were sintered at 1600 °C for 2 h. All thermal treatments were conducted in air.
2.4. Characterization
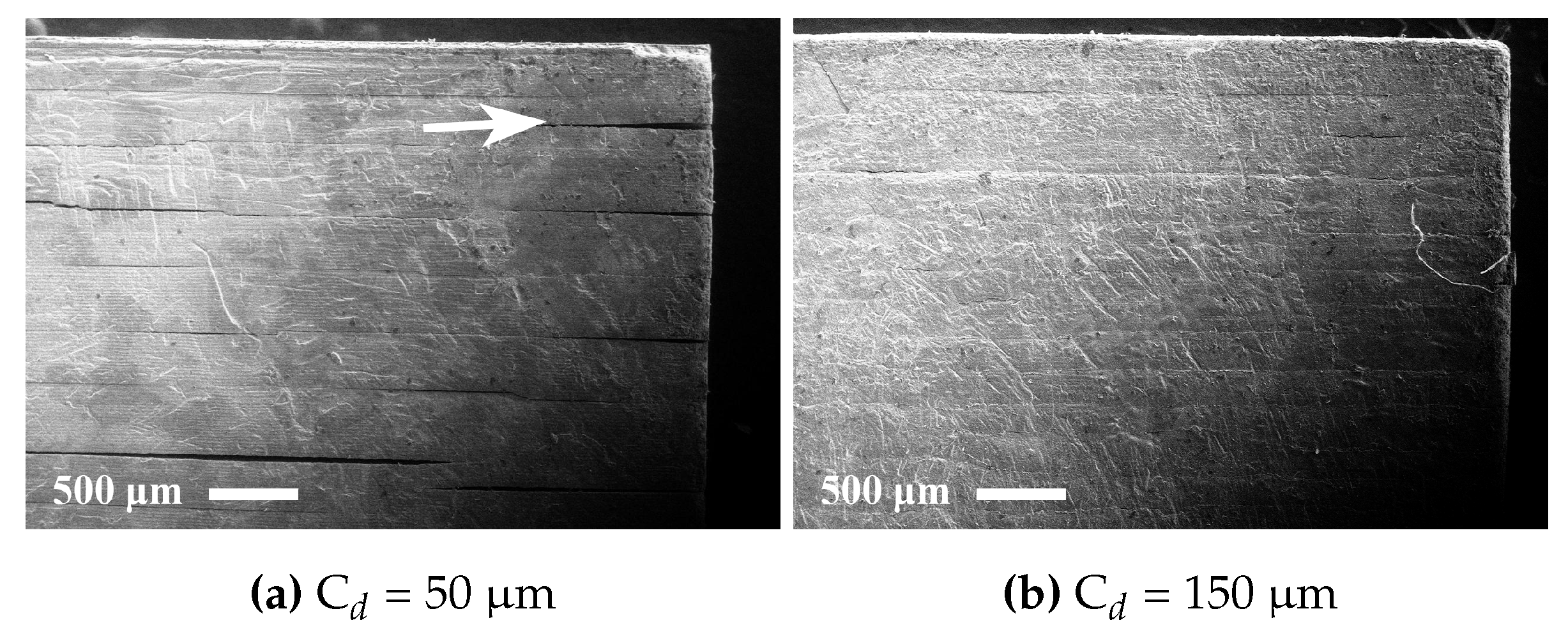
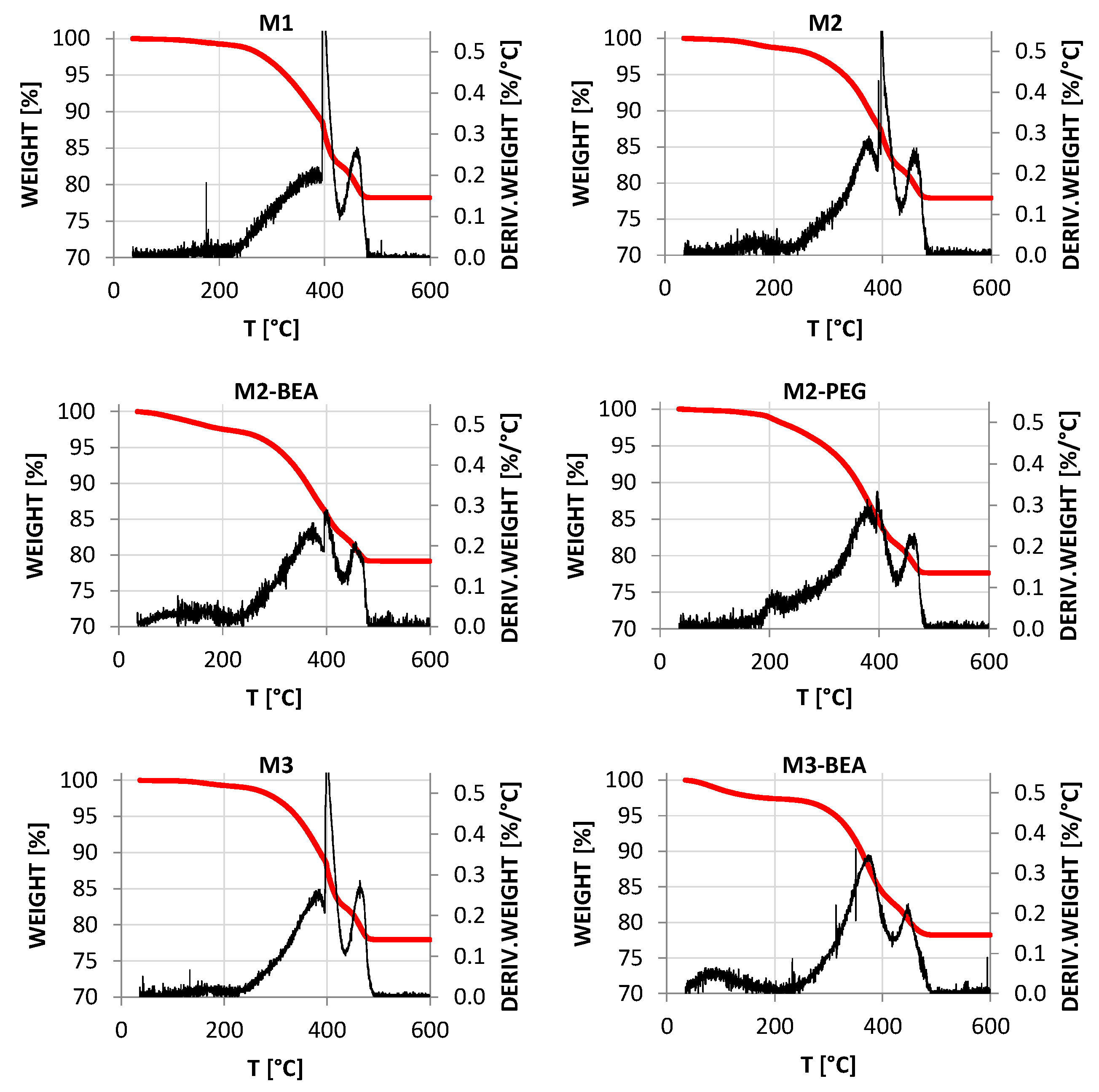
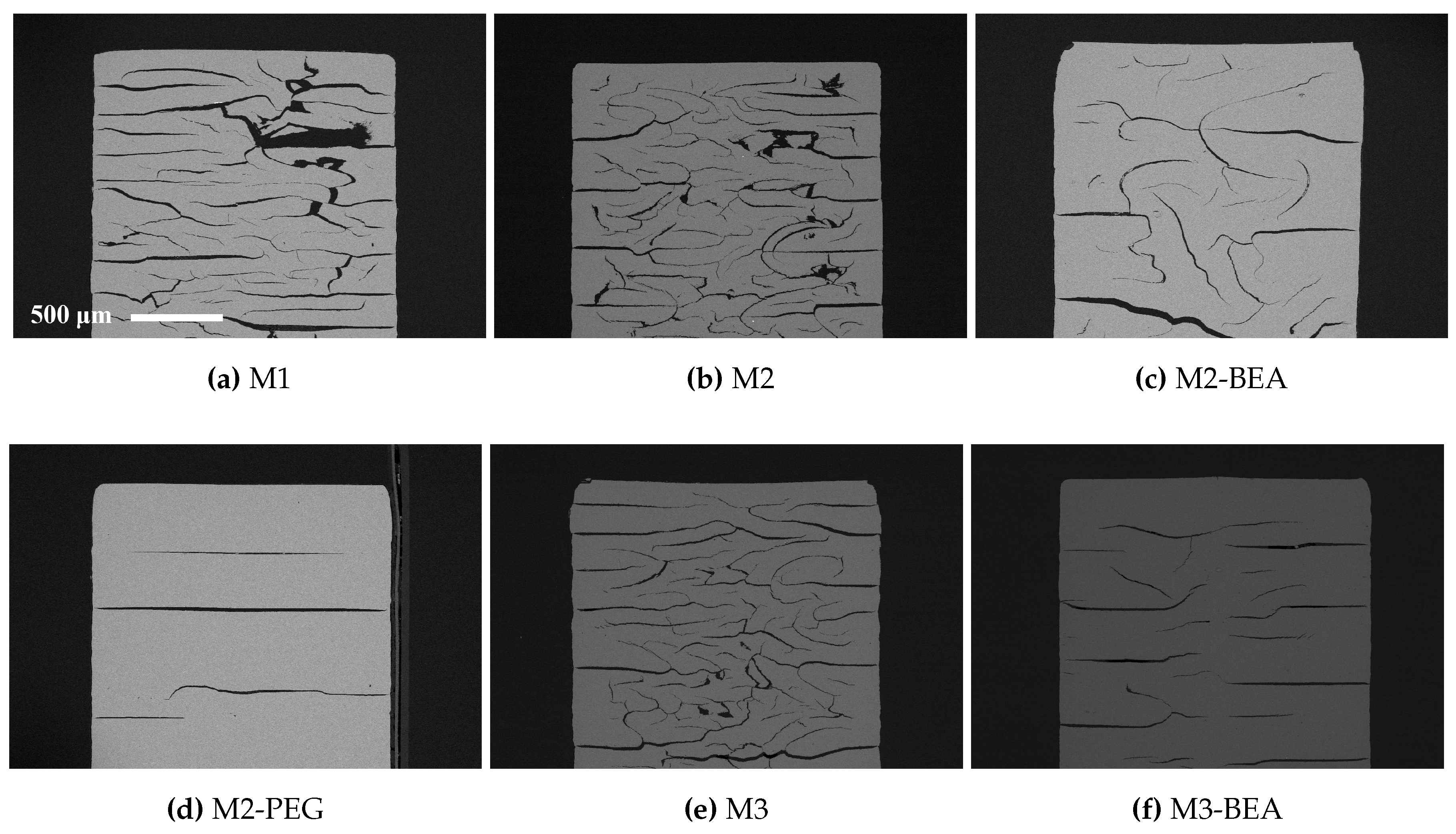
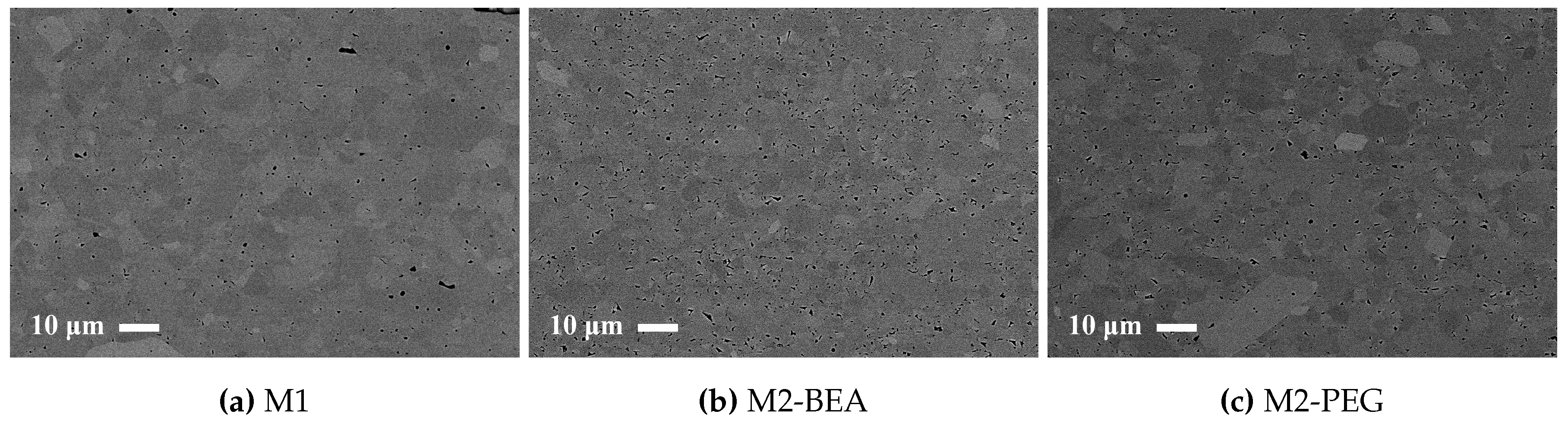
The rheology in terms of steady state equilibrium viscosity of the ceramic suspensions was determined with a Nova Melts rheometer (Reologica Instruments AB, Lund, Sweden) using a 15/2 cone plate configuration in rotational mode. Measurements were performed at 25 °C for shear rates in the range of 1–200 s−1. The polymerization shrinkage of the unfilled resins was determined by helium pycnometry using an AccuPyc 1330 (Micromeritics, Norcross, GA, USA). A known volume of liquid resin was polymerized by irradiating it with 460 nm blue light, having a light intensity of 48 mW/cm2 for 60 s, and the volume of the resulting cross-linked polymer was measured. The cure depth of the ceramic suspensions was determined by polymerising suspensions from below on a glass plate, after which the thickness of the cured film was measured using a digital micrometer. The light intensity of the light source was determined using a light intensity meter from Ophir Photonics, Israel, with a 3A-P-FS-12 sensor and the incident energy calculated from the irradiated time. Surface delamination was evaluated by SEM for sintered specimens manufactured using varying cure depth. The thermal decomposition of the suspensions and ceramic green parts was analysed by thermogravimetric analysis (TGA) in air (50 mL/min) between 25 °C and 600 °C using a temperature ramp of 2 K/min. SEM analysis of additively-manufactured test specimens was performed using a JEOL JSM-6610 LV low vacuum SEM (JEOL Ltd., Akishima, Tokyo, Japan). Surfaces of test specimens manufactured using varying cure depths were evaluated by secondary electron SEM at 14 kV. Cross-sections of test specimens manufactured from different resin compositions were evaluated using backscattered electron SEM at 10 kV in high vacuum in order to determine the influence of resin composition on the defect formation.
4. Conclusions
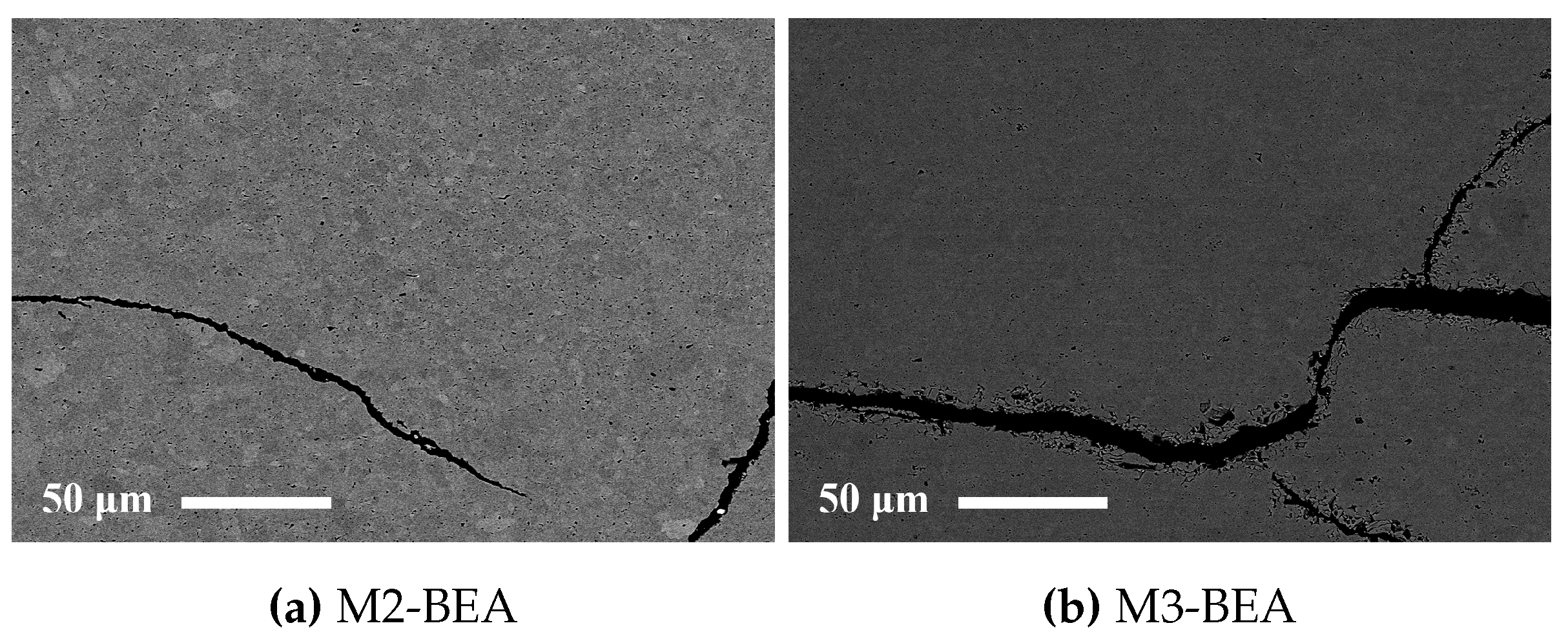
This study showed that it was possible to achieve visible light photocurable alumina suspensions with a high solids load and a viscosity level and cure depth suitable for the SL process. It was not possible to manufacture defect-free parts using the resin compositions in the study, but the influence of several parameters on defect formation could be determined. Delamination in additively-manufactured green objects could be minimized by increasing the cure depth and using a cleaning liquid compatible with the resin. The resin composition was found to greatly influence the formation of delamination and intra-laminar cracks in specimens during debinding and sintering. By the addition of a non-reactive component to the suspensions, the thermal debinding of the polymer matrix could be altered, and cracks minimized. The non-reactive component was found to decrease polymerization shrinkage, leading to reduced built-in stresses in the parts. Additionally, using a non-reactive component which decomposed rather than evaporated led to less residual porosity. The addition of a highly functional crosslinker to the resin gave no clear improvement in layer delamination.
With a relative density close to 99% and pores below 5 μm in size, there is potential to achieve a material with a mechanical strength in the same range as conventionally produced alumna if the process-related cracks can be eliminated. It is believed that this can be achieved by selecting monomers with more suitable thermal decomposition patterns and optimizing the amount and type of non-reactive component.