Micro-Arc Oxidation Enhances the Blood Compatibility of Ultrafine-Grained Pure Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Coating
2.2. Surface Characterization
2.3. Hemolysis Test
2.4. Test of Dynamic Coagulation Time
2.5. Platelet Adhesion Test
2.6. Prothrombin Time (PT) and Activated Partial Thromboplastin Time (APTT) Tests
3. Results and Discussion
3.1. Microscopic Topography and Elemental Compositions of Oxidation Coating
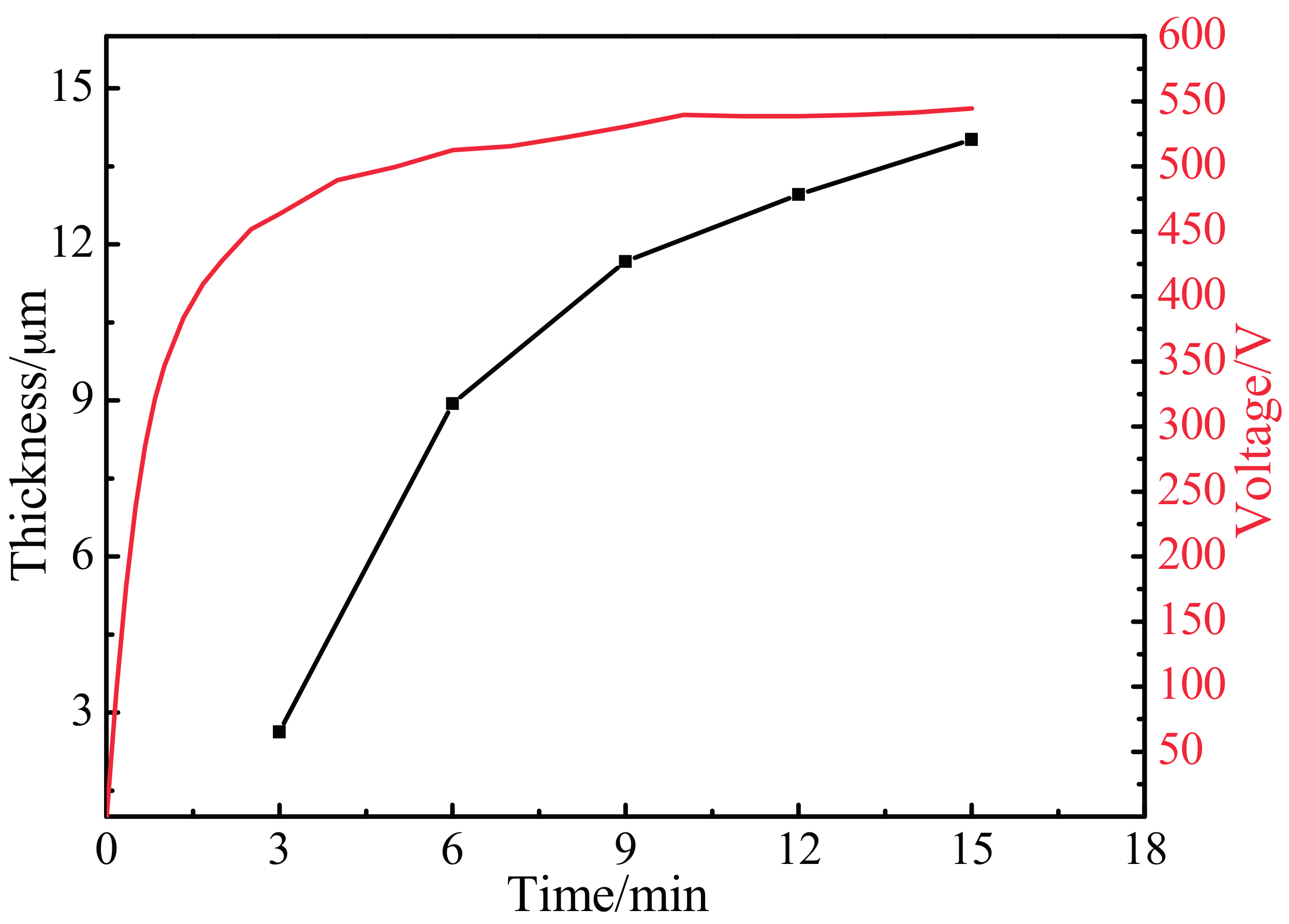
3.2. Variation of Oxidation Coating Thickness and Voltage
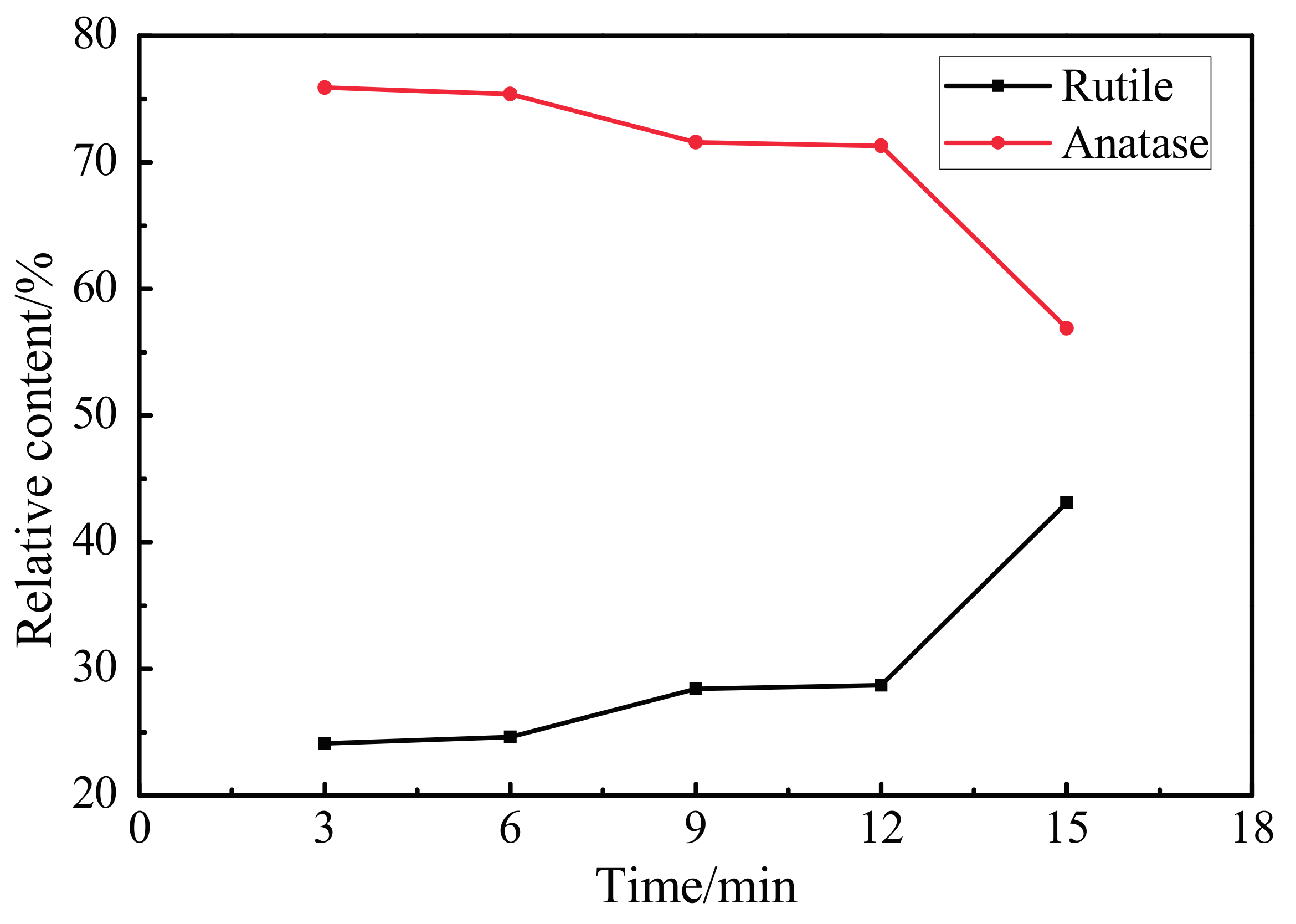
3.3. XRD Pattern of the Oxidation Coating
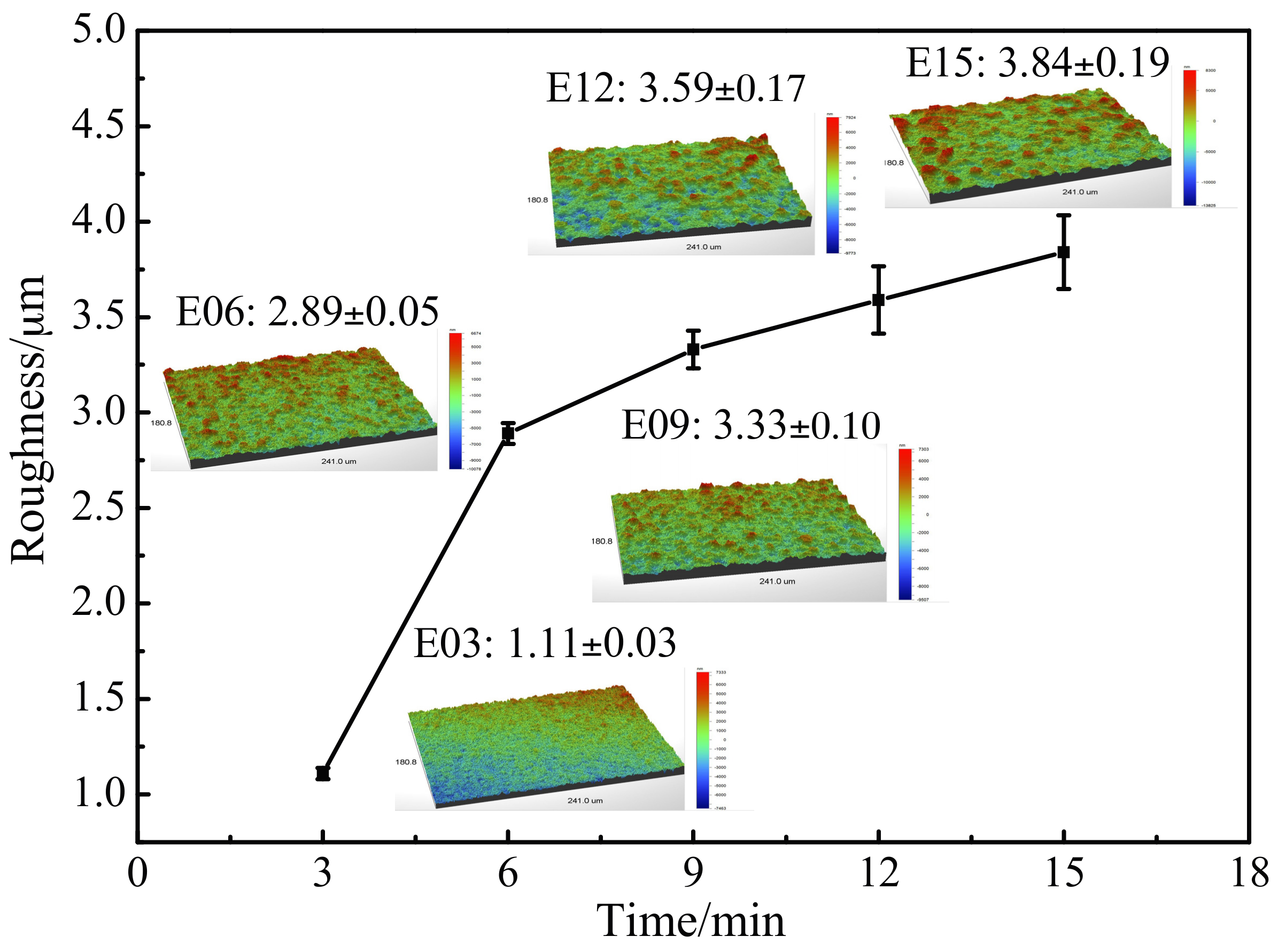
3.4. Oxidation Coating Roughness
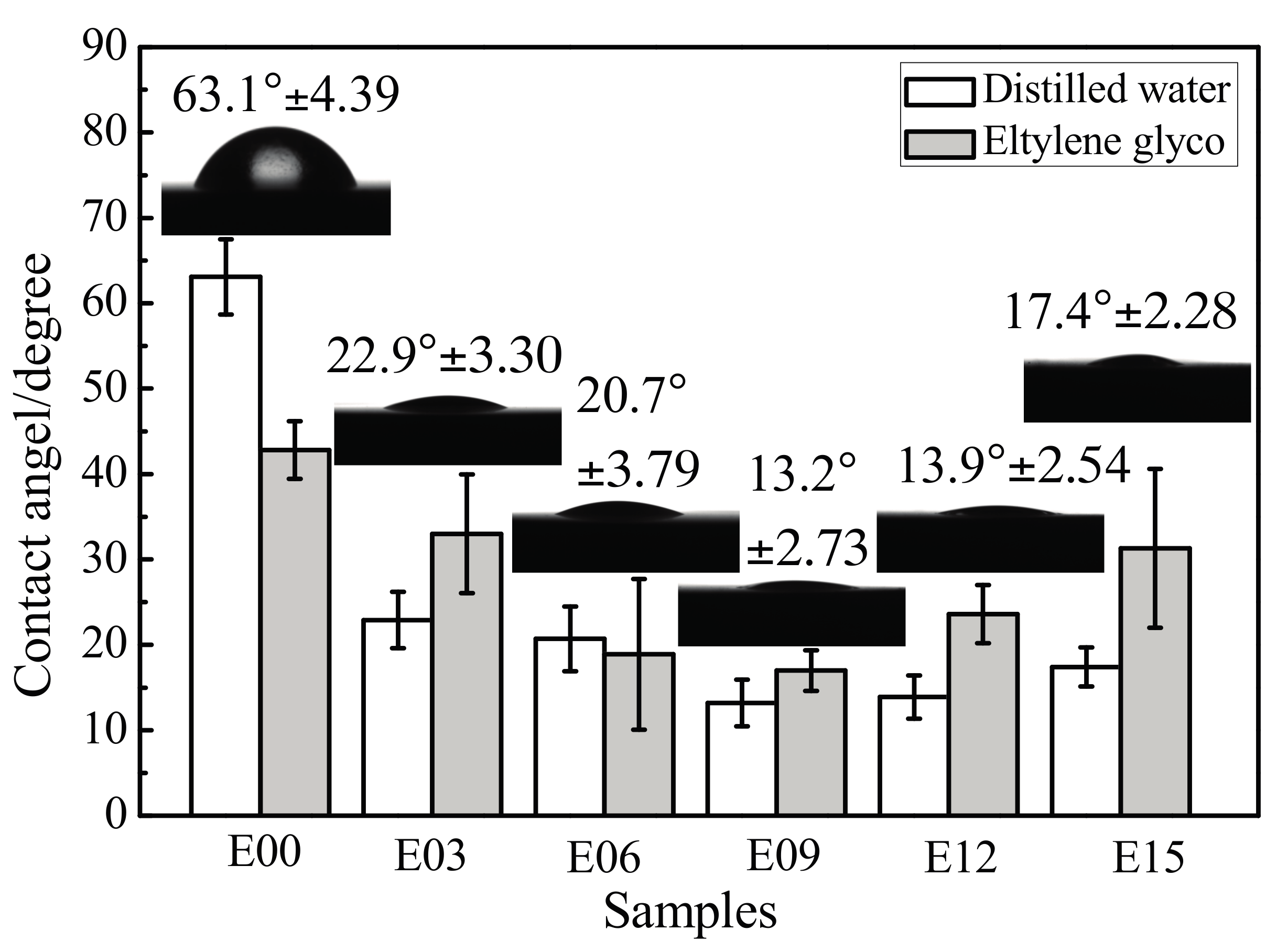
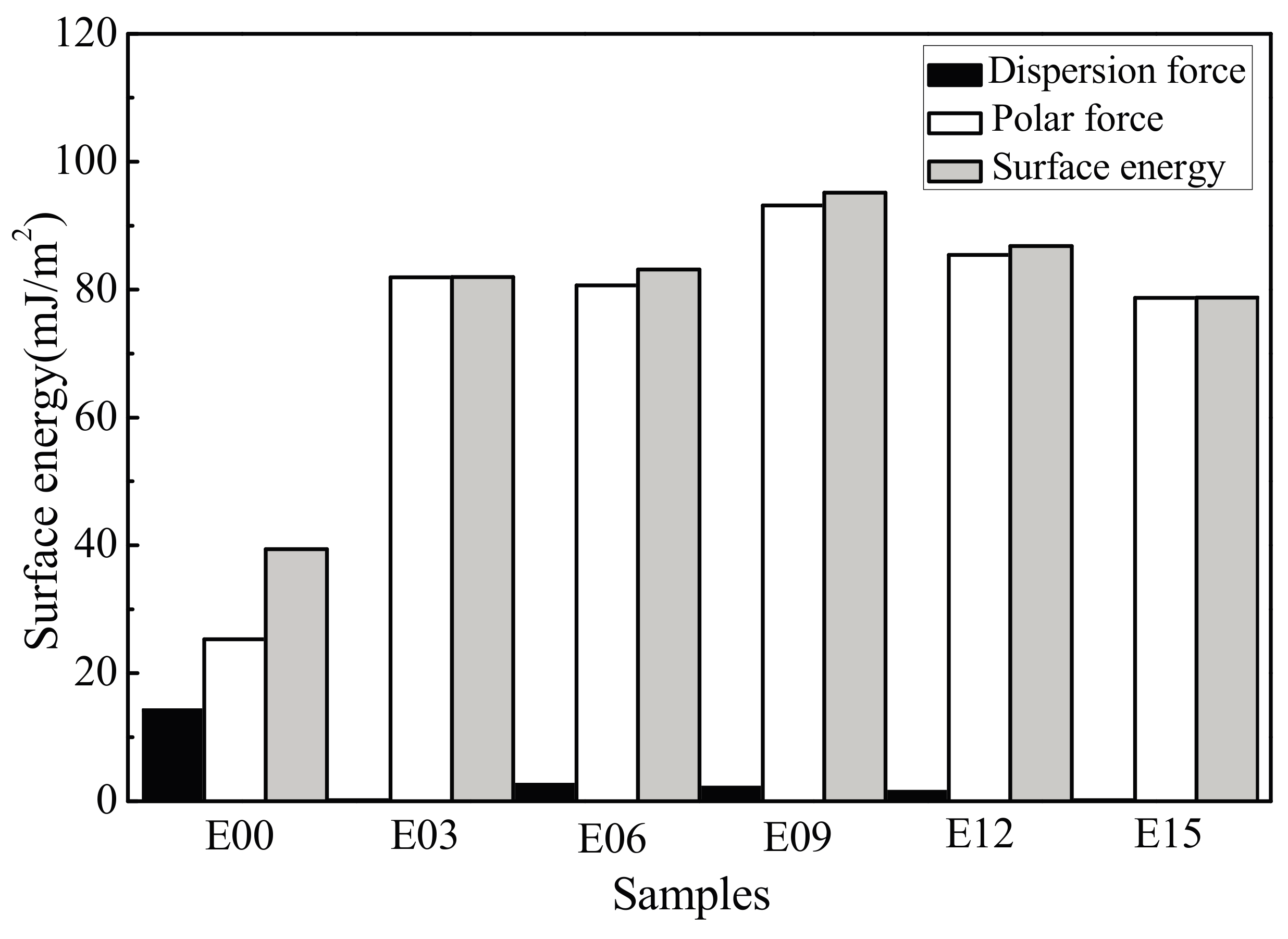
3.5. Coating Surface Wettability
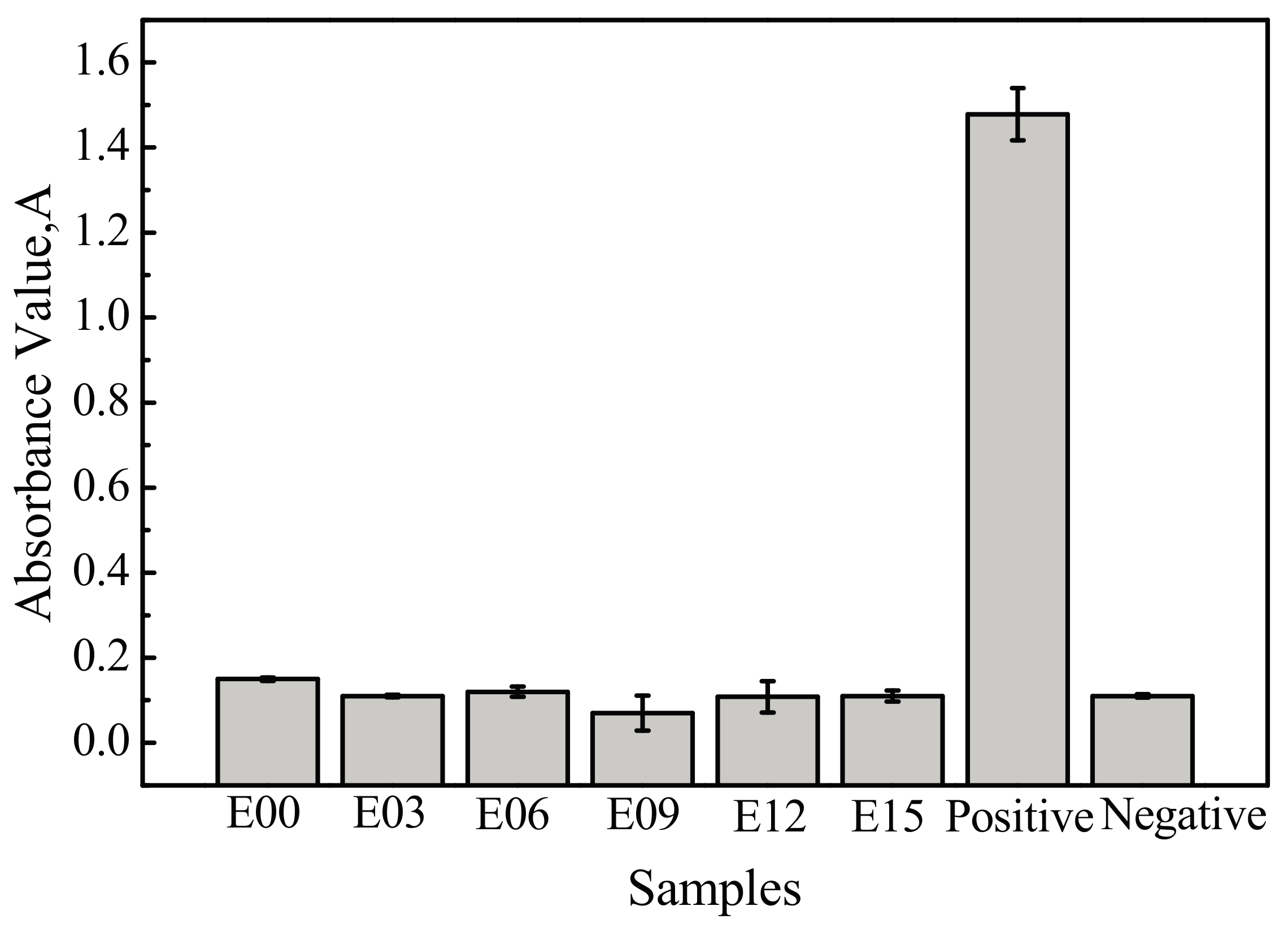
3.6. Hemolysis Rate
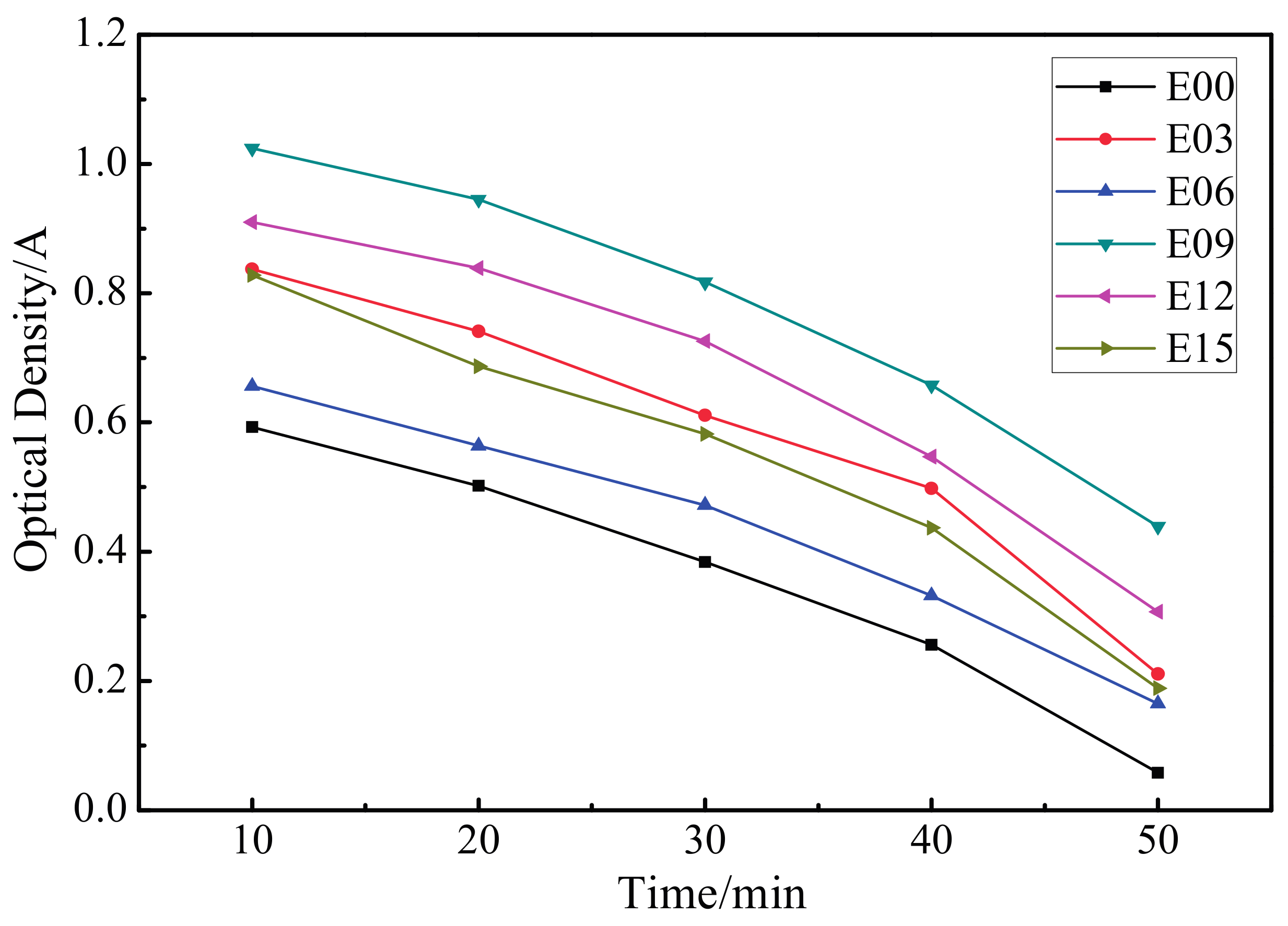
3.7. Dynamic Coagulation Time
3.8. Prothrombin Time and Activated Partial Thromboplastin Time
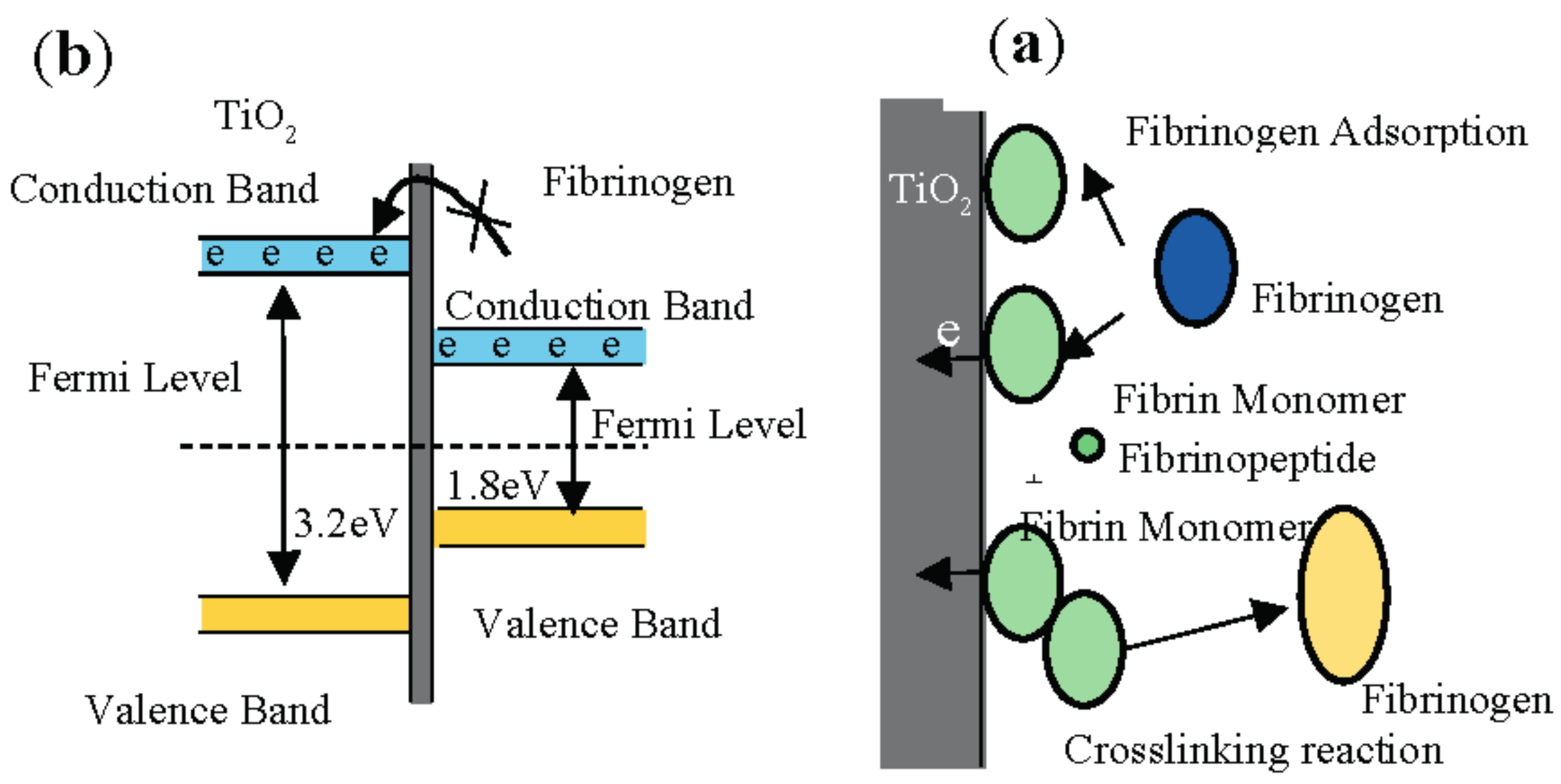
3.9. Platelet Adhesion Behavior
4. Conclusions
- (1)
- MAO technology can cause in situ oxidation of the ultrafine-grained pure titanium surface to generate a porous coating with mixed crystal structures of anatase- and rutile-phase TiO2.
- (2)
- After MAO modification, the samples with rough surface possessed smaller contact angles, higher surface energy, and more favorable wettability. The change in surface energy was primarily due to the increase of its polar force component. This was caused by how, after modification, the specific surface area of the MAO coating of the ultrafine-grained pure titanium increased, roughness increased, and the −OH− and −O2− oxygen-containing groups formed on the coating surface effectively introduced active hydrophilic groups.
- (3)
- In comparison to the ultrafine-grained pure titanium substrate, MAO coating reduced the hemolysis rate; extended the dynamic coagulation time, PT, and APTT; reduced the amount of platelet adhesion and degree of deformation; and increased the anticoagulant property. In particular, the sample with an oxidation time of 9 min had the lowest hemolysis rate; the longest dynamic coagulation time, PT, and APTT; the most favorable anticoagulant property; and the least platelet adhesion, with no aggregation generated and the lowest degree of deformation.
- (4)
- MAO is an ideal surface modification technique that can significantly enhance the blood compatibility of ultrafine-grained pure titanium. After MAO processing, the porous anatase- and rutile-phase TiO2 ceramic coating containing Si, Ca, and P possessed smaller contact angles for distilled water, smaller work function, and a more suitable surface energy (higher polar force components of the surface energy). This study showed that ultrafine-grained pure titanium modified by MAO possessed favorable blood compatibility, was in accordance with ISO requirements for medical materials, and has potential for use as a vascular stent material. However, further research is required on the short- and long-term effects of its implantation in the human body.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Civantos, A.; Martínezcampos, E.; Ramos, V.; Elvira, C.; Gallardo, A.; Abarrategi, A. Titanium coatings and surface modifications: Toward clinically useful bioactive implants. ACS Biomater. Sci. Eng. 2017, 3, 1245–1261. [Google Scholar] [CrossRef]
- Liu, B.; Shi, X.M.; Xiao, G.Y.; Lu, Y.P. In-situ preparation of scholzite conversion coatings on titanium and Ti-6Al-4V for biomedical applications. Colloids Surf. B 2017, 153, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Sekiguchi, T.; Ameyama, K. Mechanical properties of pure titanium and Ti-6Al-4V alloys with a new tailored nano/meso hybrid microstructure. Int. J. Mater. Res. 2013, 100, 796–799. [Google Scholar] [CrossRef]
- Wang, R.; He, X.; Gao, Y.; Zhang, X.; Yao, X.; Tang, B. Antimicrobial property, cytocompatibility and corrosion resistance of Zn-doped ZrO2/TiO2 coatings on Ti6Al4V implants. Mater. Sci. Eng. C 2017, 75, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Reshadi, F.; Faraji, G.; Baniassadi, M.; Tajeddini, M. Surface modification of severe plastically deformed ultrafine grained pure titanium by plasma electrolytic oxidation. Surf. Coat. Technol. 2017, 316, 113–121. [Google Scholar] [CrossRef]
- Luo, P.; McDonald, D.T.; Palanisamy, S.; Dargusch, M.S.; Xia, K. Ultrafine-grained pure Ti recycled by equal channel angular pressing with high strength and good ductility. J. Mater. Process. Technol. 2013, 213, 469–476. [Google Scholar] [CrossRef]
- Qarni, M.J.; Sivaswamy, G.; Rosochowski, A.; Boczkal, S. Effect of incremental equal channel angular pressing (I-ECAP) on the microstructural characteristics and mechanical behaviour of commercially pure titanium. Mater. Des. 2017, 122, 385–402. [Google Scholar] [CrossRef]
- Gunderov, D.V.; Polyakov, A.V.; Semenova, I.P.; Raab, G.I.; Churakova, A.A.; Gimaltdinova, E.I.; Sabirov, I.; Segurado, J.; Sitdikov, V.D.; Alexandrov, I.V.; et al. Evolution of microstructure, macrotexture and mechanical properties of commercially pure Ti during ECAP-conform processing and drawing. Mater. Sci. Eng. A 2013, 562, 128–136. [Google Scholar] [CrossRef]
- Shi, J.Z.; Chen, C.Z.; Zhang, S.; Wu, Y. Application of surface modification in biomedical materials Research. Surf. Rev. Lett. 2000, 14, 361–369. [Google Scholar] [CrossRef]
- Mitamura, Y.; Hosooka, K.; Matsumoto, T.; Otaki, K.; Sakai, K.; Tanabe, T.; Yuta, T.; Mikami, T. Development of a ceramic heart valve. J. Biomater. Appl. 1989, 4, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Nandakumar, D.; Bendavid, A.; Martin, P.J.; Harris, K.D.; Ruys, A.J.; Lord, M.S. Fabrication of Semiordered Nanopatterned Diamond-like Carbon and Titania Films for Blood Contacting Applications. ACS Appl. Mater. Interfaces 2016, 8, 6802–6810. [Google Scholar] [CrossRef] [PubMed]
- Schvezov, C.E.; Alterach, M.A.; Vera, M.L.; Rosenberger, M.R.; Ares, A.E. Characteristics of hemocompatible TiO2, nano-films produced by the sol-gel and anodic oxidation techniques. JOM 2010, 62, 84–87. [Google Scholar] [CrossRef]
- Chen, J.; Yang, P.; Liao, Y.; Wang, J.; Chen, H.; Sun, H.; Huang, N. Effect of the duration of UV irradiation on the anticoagulant properties of titanium dioxide films. ACS Appl. Mater. Interfaces 2015, 7, 4423–4432. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, F.; Li, C.; Zheng, Z.; Wang, X.; Liu, X.; Chen, A.; Jiang, Z. Improvement of blood compatibility of artificial heart valves via titanium oxide film coated on low temperature isotropic carbon. Surf. Coat. Technol. 2000, 128, 36–42. [Google Scholar] [CrossRef]
- Yu, S.; Yu, Z.; Wang, G.; Matthew, S.D.; Zhang, M. Evaluation of Haemocompatibility of TLM Titanium Alloy with Surface Heparinization. Rare Met. Mater. Eng. 2009, 38, 384–388. [Google Scholar] [CrossRef]
- Sunny, M.C.; Sharma, C.P. Titanium-protein interaction: Changes with oxide layer thickness. J. Biomater. Appl. 1991, 6, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Chen, Y.R.; Luo, J.M.; Yi, J.; Lu, R.; Xiao, J.; Xue, Z.N.; Liu, X.H. In vitro investigation of blood compatibility of Ti with oxide layers of rutile structure. J. Biomater. Appl. 1994, 8, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.R.; Park, K. Effect of surface hydrophobicity on the conformational changes of adsorbed fibrinogen. J. Colloid Interface Sci. 1991, 144, 271–281. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.C.; Chen, J.Y.; Zhao, J.Z.; He, L.; Qi, S.K.; Zhang, X.D. Surface characterization of titanium and adsorption of bovine serum albumin. Mater. Charact. 2002, 49, 129–137. [Google Scholar] [CrossRef]
- Yang, G.L.; He, F.M.; Hu, J.A.; Wang, X.X.; Zhao, S.F. Biomechanical comparison of biomimetically and electrochemically deposited hydroxyapatite-coated porous titanium implants. J. Oral Maxil. Surg. 2010, 68, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.; Shen, Y.G.; Alajmi, Z.; Yang, S.Y.; Sun, J.M.; Zhang, H.M. Sol-gel preparation and properties of Ag-TiO2 films on surface roughened Ti-6Al-4V alloy. Mater. Sci. Technol. 2014, 31, 501–505. [Google Scholar] [CrossRef]
- Yang, Y.; Lai, Y.; Zhang, Q.; Wu, K.; Zhang, L.; Lin, C.; Tang, P. A novel electrochemical strategy for improving blood compatibility of titanium-based biomaterials. Colloids Surf. B 2010, 79, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wei, D.; Feng, W.; Cheng, S.; Yang, H.; Li, B.; Wang, Y.; Jia, D.; Zhou, Y. Bioactive coating with hierarchical double porous structure on titanium surface formed by two-step microarc oxidation treatment. Surf. Coat. Technol. 2014, 252, 148–156. [Google Scholar] [CrossRef]
- Zhao, Q.M.; Cheng, L.; Liu, Z.T.; Zhao, J.J. Surface characteristics of Zinc-TiO2 coatings prepared via micro-arc oxidation. Compos. Interfaces 2014, 21, 585–593. [Google Scholar] [CrossRef]
- Wang, M.S.; Lee, F.P.; Shen, Y.D.; Chen, C.H.; Ou, K.L.; Ou, S.F. Surface, Biocompatible and Hemocompatible Properties of Meta-Amorphous Titanium Oxide Film. Int. J. Appl. Ceram. Technol. 2015, 12, 341–350. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Jayaraman, M.; Meyer, U.; Bühner, M.; Joos, U.; Wiesmann, H.P. Influence of titanium surfaces on attachment of osteoblast-like cells in vitro. Biomaterials 2004, 25, 625–631. [Google Scholar] [CrossRef]
- Spurr, P.A.; Myers, H. Quantitative analysis of anatase-rutile mixtures with an X-ray diffractometer. Anal. Chem. 1957, 29, 760–762. [Google Scholar] [CrossRef]
- Fan, H.; Chen, P.; Qi, R.; Zhai, J.; Wang, J.; Chen, L.; Chen, L.; Sun, Q.; Song, Y.; Han, D.; et al. Greatly improved blood compatibility by microscopic multiscale design of surface architectures. Small 2009, 5, 2144–2148. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.L.; Liu, F.; Luo, J.M.; Zhong, Z.C. In vitro blood compatibility of alumina coatings prepared by micro-arc oxidation on biomedical NiTi alloy. Appl. Mech. Mater. 2012, 184–185, 1021–1024. [Google Scholar] [CrossRef]
- Lisman, T.; Bakhtiari, K.; Pereboom, I.T.; Hendriks, H.G.; Meijers, J.C.; Porte, R.J. Normal to increased thrombin generation in patients undergoing liver transplantation despite prolonged conventional coagulation tests. J. Hepatol. 2010, 52, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Elbert, D.L.; Hubbell, J.A. Surface treatments of polymers for biocompatibility. Annu. Rev. Mater. Sci. 1996, 26, 365–394. [Google Scholar] [CrossRef]
- Sawyer, P.N.; Schwann, G.; Stanczewski, B. Effects of a New Hemostatic Agent on Blood Coagulation. Biomater. Med. Devices Artif. Organs 1983, 11, 135. [Google Scholar] [CrossRef] [PubMed]
- Dash, B.C.; Réthoré, G.; Monaghan, M.; Fitzgerald, K.; Gallagher, W.; Pandit, A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials 2010, 31, 8188–8197. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Ding, J. N.; Xu, X. J.; He, Y. Q.; Lei, X. C. Blood Compatibility of TiO2-HA Bioceramic Coating on Titanium. Rare Met. Mater. Eng. 2017, 46, 1299–1304. [Google Scholar]
- Baurschmidt, P.; Schaldach, M. Alloplastic materials for heart-valve prostheses. Med. Biol. Eng. Comput. 1980, 18, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Kaelble, D.H.; Moacanin, J. A surface energy analysis of bioadhesion. Polymer 1977, 18, 475–482. [Google Scholar] [CrossRef]
- Michiardi, A.; Aparicio, C.; Ratner, B.D.; Planell, J.A.; Gil, J. The influence of surface energy on competitive protein adsorption on oxidized NiTi surfaces. Biomaterials 2007, 28, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Kuo, W.H.; Wang, M.J.; Chien, H.W.; Wei, T.C.; Lee, C.; Tsai, W.B. Surface modification with poly (sulfobetaine methacrylate-co-acrylic acid) to reduce fibrinogen adsorption, platelet adhesion, and plasma coagulation. Biomacromolecules 2011, 12, 4348–4356. [Google Scholar] [CrossRef] [PubMed]





| Elements | E03 | E06 | E09 | E12 | E15 |
|---|---|---|---|---|---|
| O | 75.42 | 76.05 | 77.44 | 77.50 | 76.37 |
| Si | 2.66 | 4.04 | 3.31 | 3.46 | 3.97 |
| P | 0.93 | 1.71 | 1.73 | 1.79 | 2.27 |
| Ca | 0.93 | 2.17 | 2.94 | 3.20 | 4.50 |
| Ti | 20.06 | 15.01 | 15.20 | 14.04 | 12.89 |
| Samples | |||
|---|---|---|---|
| Distilled water | 72.1 | 19.9 | 52.2 |
| Ethylene glycol | 48.0 | 29.0 | 19.0 |
| Samples | E00 | E03 | E06 | E09 | E12 | E15 | Positive |
|---|---|---|---|---|---|---|---|
| PT | 11.87 ± 0.15 | 11.93 ± 0.19 | 12.09 ± 0.16 | 13.72 ± 0.73 | 13.46 ± 0.0.59 | 12.60 ± 0.53 | 12.08 ± 0.23 |
| APTT | 26.5 ± 0.10 | 29.6 ± 0.32 | 31.8 ± 0.42 | 36.0 ± 0.21 | 30.9 ± 0.25 | 27.8 ± 0.38 | 28 ± 0.26 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Zhang, K.; Wu, C.; Lei, X.; Ding, J.; Shi, X.; Liu, C. Micro-Arc Oxidation Enhances the Blood Compatibility of Ultrafine-Grained Pure Titanium. Materials 2017, 10, 1446. https://doi.org/10.3390/ma10121446
Xu L, Zhang K, Wu C, Lei X, Ding J, Shi X, Liu C. Micro-Arc Oxidation Enhances the Blood Compatibility of Ultrafine-Grained Pure Titanium. Materials. 2017; 10(12):1446. https://doi.org/10.3390/ma10121446
Chicago/Turabian StyleXu, Lin, Kun Zhang, Cong Wu, Xiaochun Lei, Jianning Ding, Xingling Shi, and Chuncheng Liu. 2017. "Micro-Arc Oxidation Enhances the Blood Compatibility of Ultrafine-Grained Pure Titanium" Materials 10, no. 12: 1446. https://doi.org/10.3390/ma10121446





