UV-Induced Photocatalytic Cashmere Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of TiO2 Colloid
2.2. Preparation of TiO2-Coated Cashmere Fabrics
2.3. Characterization
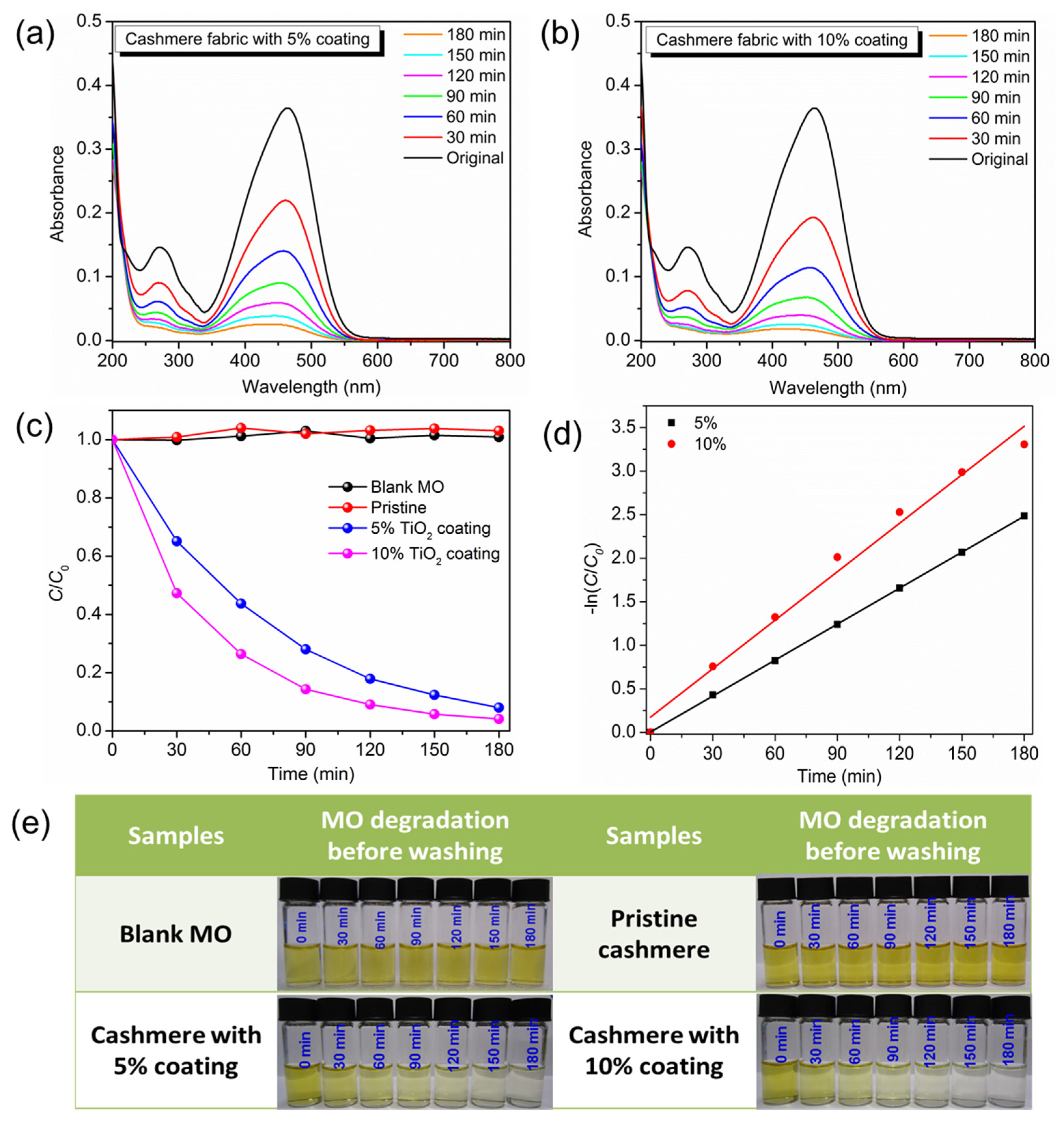
2.4. Photocatalytic Studies
2.5. Stability Study
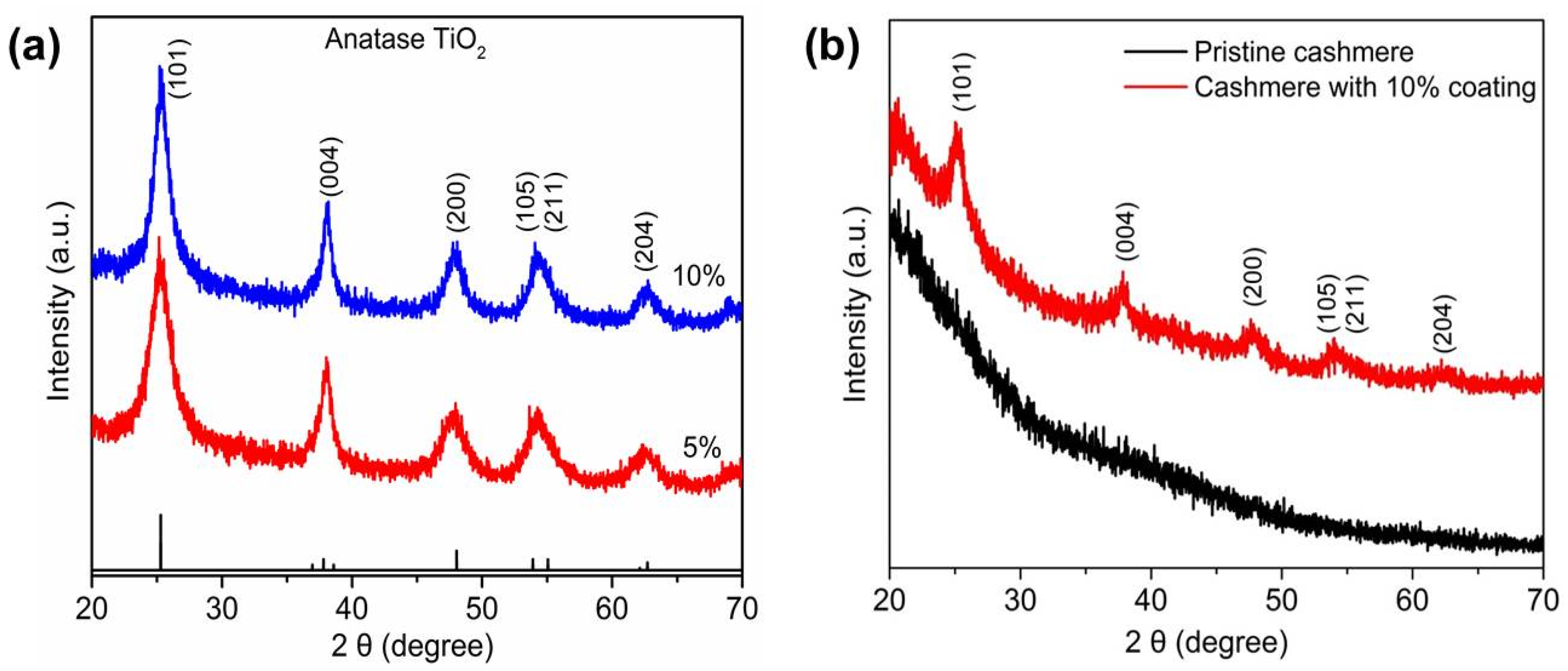
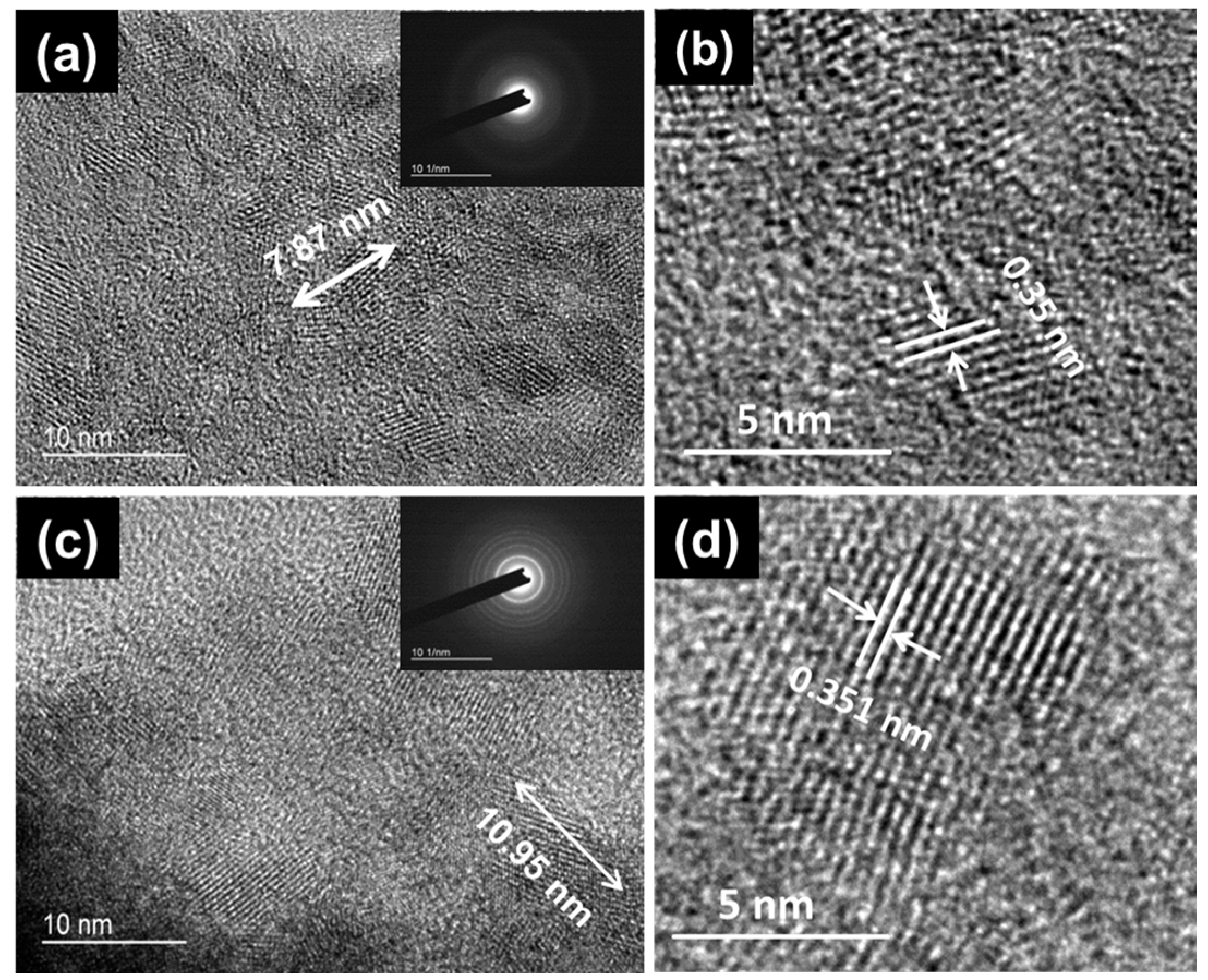
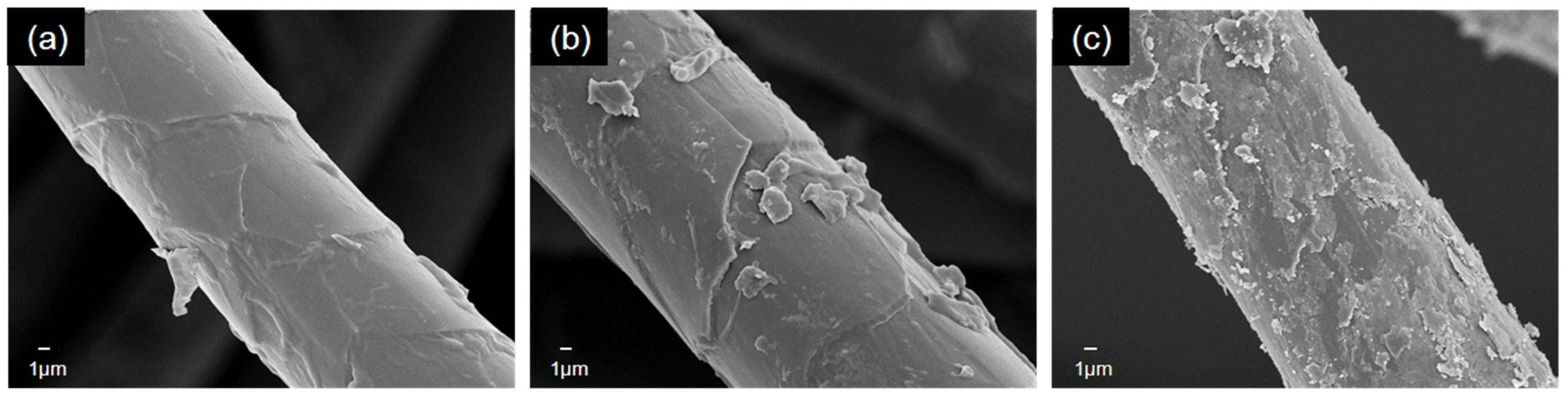
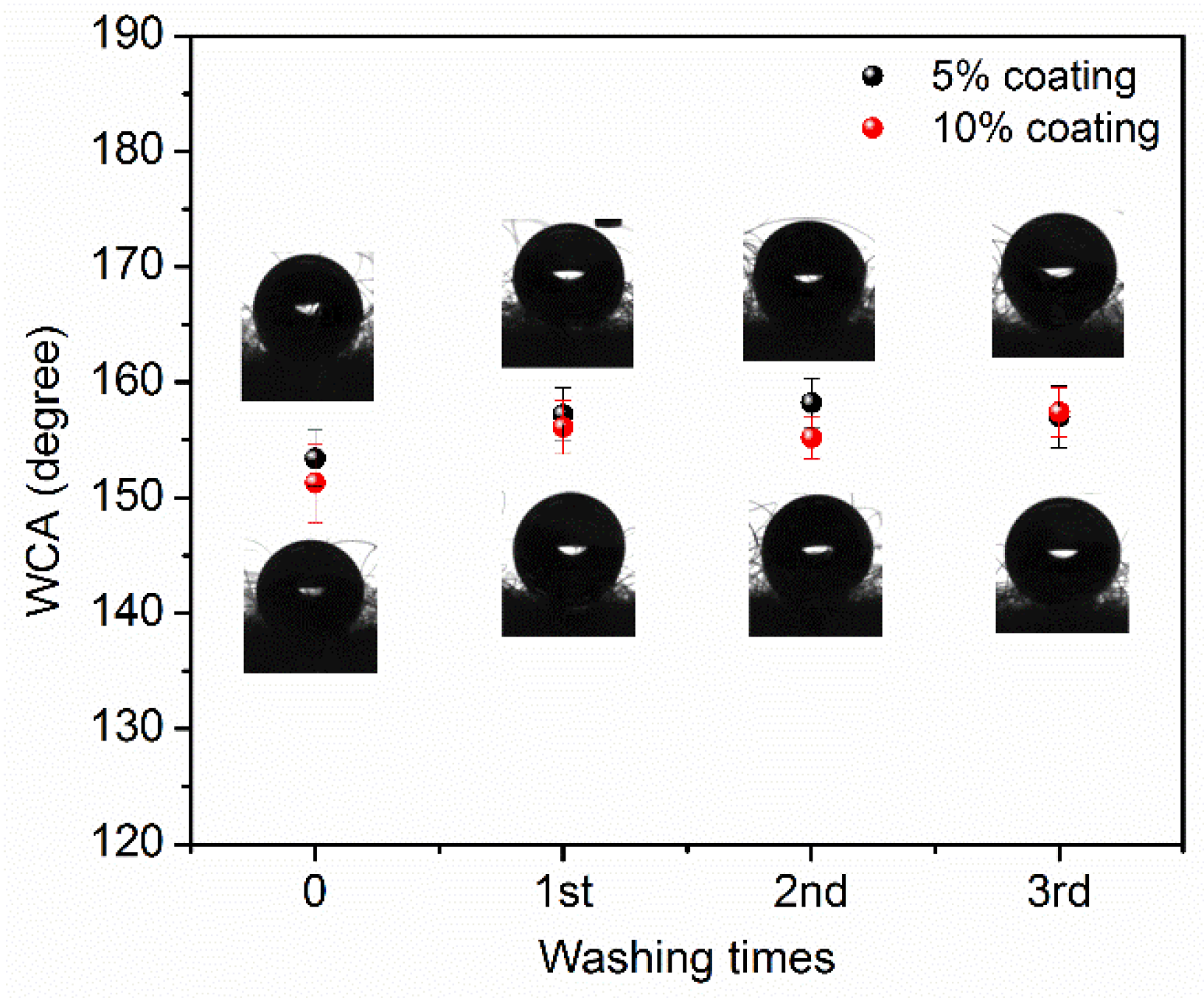
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ragesh, P.; Ganesh, V.A.; Naira, S.V.; Nair, A.S. A review on ‘self-cleaning and multifunctional materials’. J. Mater. Chem. A 2014, 2, 14773–14797. [Google Scholar] [CrossRef]
- Fresno, F.; Portela, R.; Suarez, S.; Coronado, J.M. Photocatalytic materials: Recent achievements and near future trends. J. Mater. Chem. A 2014, 2, 2863–2884. [Google Scholar] [CrossRef]
- Manjumol, K.A.; Mini, L.; Mohamed, A.P.; Hareesh, U.S.; Warrier, K.G.K. A hybrid sol-gel approach for novel photoactive and hydrophobic titania coatings on aluminium metal surfaces. RSC Adv. 2013, 3, 18062–18070. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A. Self-cleaning fibers via nanotechnology: A virtual reality. J. Mater. Chem. 2011, 21, 7858–7869. [Google Scholar] [CrossRef]
- Chen, S.B.; Mahmood, N.; Beiner, M.; Binder, W.H. Self-healing materials from v- and h-shaped supramolecular architectures. Angew. Chem. Int. Ed. 2015, 54, 10188–10192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; Lu, X.; Xin, Z.; Zhou, C.L. A self-cleaning polybenzoxazine/TiO2 surface with superhydrophobicity and superoleophilicity for oil/water separation. Nanoscale 2015, 7, 19476–19483. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.Y.; Wang, W.; Mao, L.H.; Wang, C.F.; Chen, S. Versatile superhydrophobic and photocatalytic films generated from TiO2-SiO2@PDMS and their applications on fabrics. J. Mater. Chem. A 2014, 2, 4178–4184. [Google Scholar] [CrossRef]
- Cao, C.Y.; Ge, M.Z.; Huang, J.Y.; Li, S.H.; Deng, S.; Zhang, S.N.; Chen, Z.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. Robust fluorine-free superhydrophobic PDMS-ormosil@fabrics for highly effective self-cleaning and efficient oil-water separation. J. Mater. Chem. A 2016, 4, 12179–12187. [Google Scholar] [CrossRef]
- Li, S.H.; Huang, J.Y.; Ge, M.Z.; Cao, C.Y.; Deng, S.; Zhang, S.N.; Chen, G.Q.; Zhang, K.Q.; Al-Deyab, S.S.; Lai, Y.K. Robust flower-like TiO2@cotton fabrics with special wettability for effective self-cleaning and versatile oil/water separation. Adv. Mater. Interfaces 2015, 2. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, X.; Boo, C.; Zhu, S.Y.; Lu, Y.; Fan, W.; Huo, M.X.; Elimelech, M.; Yang, X. Self-cleaning anti-fouling hybrid ultrafiltration membranes via side chain grafting of poly(aryl ether sulfone) and titanium dioxide. J. Membr. Sci. 2017, 529, 1–10. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.T.; Tryk, D.A. Tio2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. Tio2 photocatalysis: A historical overview and future prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285. [Google Scholar] [CrossRef]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Superhydrophobic and photocatalytic self-cleaning cotton. J. Mater. Chem. A 2014, 2, 18005–18011. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A.; Leung, S.K. Understanding photocatalytic behavior on biomaterials: Insights from TiO2 concentration. J. Colloid Interface Sci. 2009, 339, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Daoud, W.A.; Leung, S.K.; Tung, W.S.; Xin, J.H.; Cheuk, K.; Qi, K. Self-cleaning keratins. Chem. Mater. 2008, 20, 1242–1244. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A. Self-cleaning surface functionalisation of keratins: Effect of heat treatment and formulation preparation time on photocatalysis and fibres mechanical properties. Surf. Eng. 2010, 26, 525–531. [Google Scholar] [CrossRef]
- Moafi, H.F.; Shojaee, A.F.; Zanjanchi, M.A. Photocatalytic self-cleaning of wool fibers coated with synthesized nano-sized titanium dioxide. Int. J. Polym. Mater. 2011, 60, 591–602. [Google Scholar] [CrossRef]
- Montazer, M.; Pakdel, E. Self-cleaning and color reduction in wool fabric by nano titanium dioxide. J. Text. Inst. 2011, 102, 343–352. [Google Scholar] [CrossRef]
- Pakdel, E.; Daoud, W.A.; Wang, X.G. Self-cleaning and superhydrophilic wool by TiO2/SiO2 nanocomposite. Appl. Surf. Sci. 2013, 275, 397–402. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A.; Henrion, G. Enhancement of anatase functionalization and photocatalytic self-cleaning properties of keratins by microwave-generated plasma afterglow. Thin Solid Films 2013, 545, 310–319. [Google Scholar] [CrossRef]
- Tung, W.S.; Daoud, W.A. Photocatalytic self-cleaning keratins: A feasibility study. Acta Biomater. 2009, 5, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bosc, F.; Ayral, A.; Albouy, P.A.; Guizard, C. A simple route for low-temperature synthesis of mesoporous and nanocrystalline anatase thin films. Chem. Mater. 2003, 15, 2463–2468. [Google Scholar] [CrossRef]
- Deng, J.; Tao, J.; Li, X.L.; Wu, T. Lower-temperature preparation and photoelectrochemical properties of anatase TiO2 sol. Energy Environ. Biol. Mater. 2011, 685, 87–97. [Google Scholar]
- Han, S.J.; Choi, S.H.; Kim, S.S.; Cho, M.; Jang, B.; Kim, D.Y.; Yoon, J.; Hyeon, T. Low-temperature synthesis of highly crystalline tio2 nanocrystals and their application to photocatalysis. Small 2005, 1, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.J.; Lee, J.W.; Yoon, H.S.; Jang, H.D.; Kim, J.G.; Yang, J.S.; Yoo, S.J. Photoelectric characteristics of nanocrystalline TiO2 film prepared from TiO2 colloid sol for dye-sensitized solar cell. Bull. Korean Chem. Soc. 2009, 30, 2365–2370. [Google Scholar]
- Katoch, A.; Kim, H.; Hwang, T.; Kim, S.S. Preparation of highly stable TiO2 sols and nanocrystalline TiO2 films via a low temperature sol-gel route. J. Sol-Gel Sci. Technol. 2012, 61, 77–82. [Google Scholar] [CrossRef]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Photostable self-cleaning cotton by a copper(II) porphyrin/TiO2 visible-light photocatalytic system. ACS Appl. Mat. Interfaces 2013, 5, 4753–4759. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Daoud, W.A.; Langford, S.J. Self-cleaning cotton by porphyrin-sensitized visible-light photocatalysis. J. Mater. Chem. 2012, 22, 4083–4088. [Google Scholar] [CrossRef]
- Wong, A.; Daoud, W.A.; Liang, H.; Szeto, Y.S. The effect of aging and precursor concentration on room-temperature synthesis of nanocrystalline anatase TiO2. Mater. Lett. 2014, 117, 82–85. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.X.; Liu, L.Q.; Reunchan, P.; Umezawa, N.; Ye, J.H. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Zoccola, M.; Lu, N.; Mossotti, R.; Innocenti, R.; Montarsolo, A. Identification of wool, cashmere, yak, and angora rabbit fibers and quantitative determination of wool and cashmere in blend: A near infrared spectroscopy study. Fibers Polym. 2013, 14, 1283–1289. [Google Scholar] [CrossRef]
- Tester, D.H. Fine structure of cashmere and superfine Merino wool fibers. Text. Res. J. 1987, 57, 213–219. [Google Scholar] [CrossRef]
- Banerjee, S.; Dionysiou, D.D.; Pillai, S.C. Self-cleaning applications of TiO2 by photo-induced hydrophilicity and photocatalysis. Appl. Catal. B Environ. 2015, 176, 396–428. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Daoud, W.A. UV-Induced Photocatalytic Cashmere Fibers. Materials 2017, 10, 1414. https://doi.org/10.3390/ma10121414
Wang L, Daoud WA. UV-Induced Photocatalytic Cashmere Fibers. Materials. 2017; 10(12):1414. https://doi.org/10.3390/ma10121414
Chicago/Turabian StyleWang, Lingyun, and Walid A. Daoud. 2017. "UV-Induced Photocatalytic Cashmere Fibers" Materials 10, no. 12: 1414. https://doi.org/10.3390/ma10121414



