Strategies to Achieve High-Performance White Organic Light-Emitting Diodes
Abstract
:1. Introduction
2. Parameters to Characterize WOLEDs
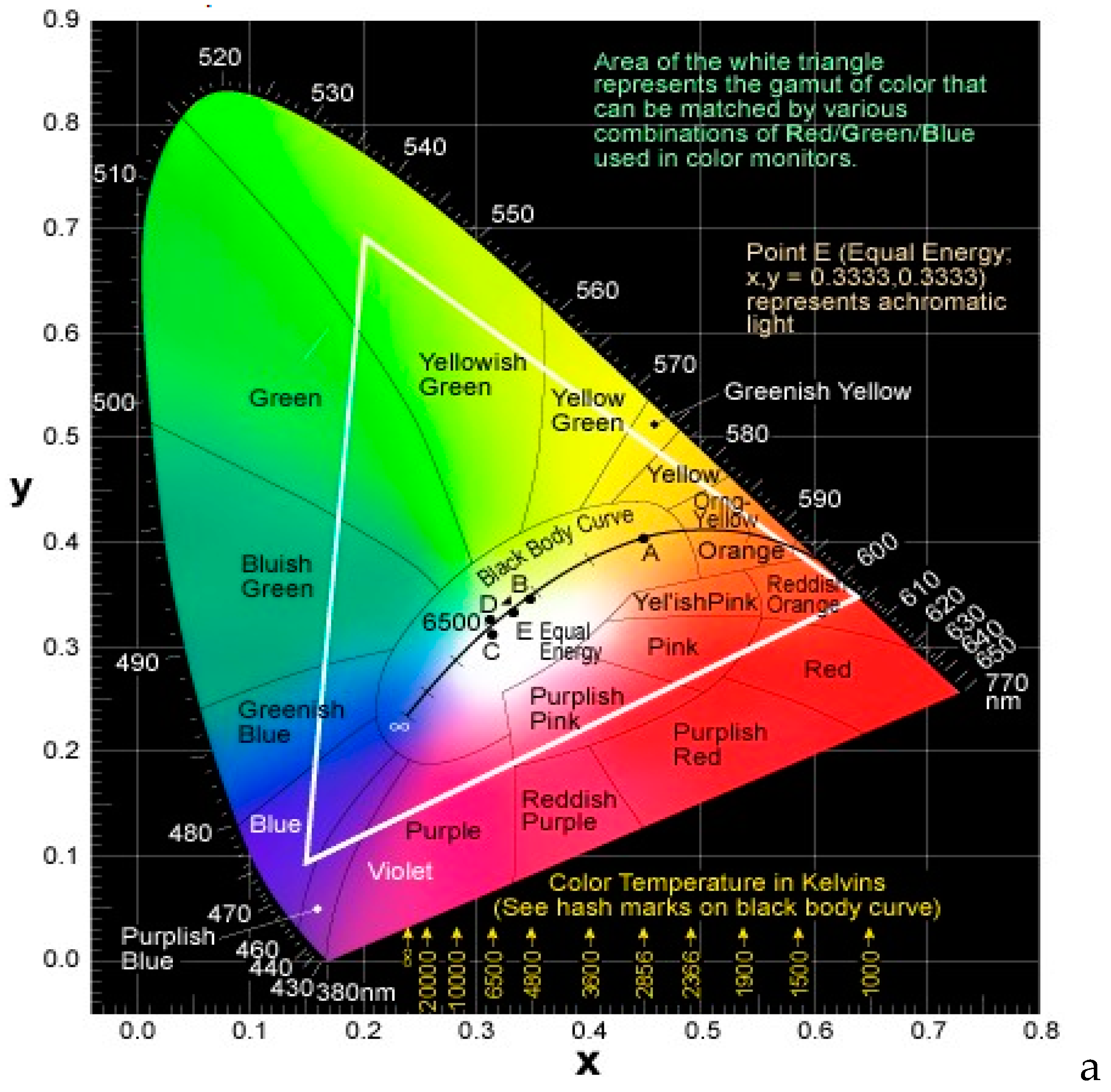
2.1. Emission Colors
2.2. Emission Efficiency
2.3. Lifetime
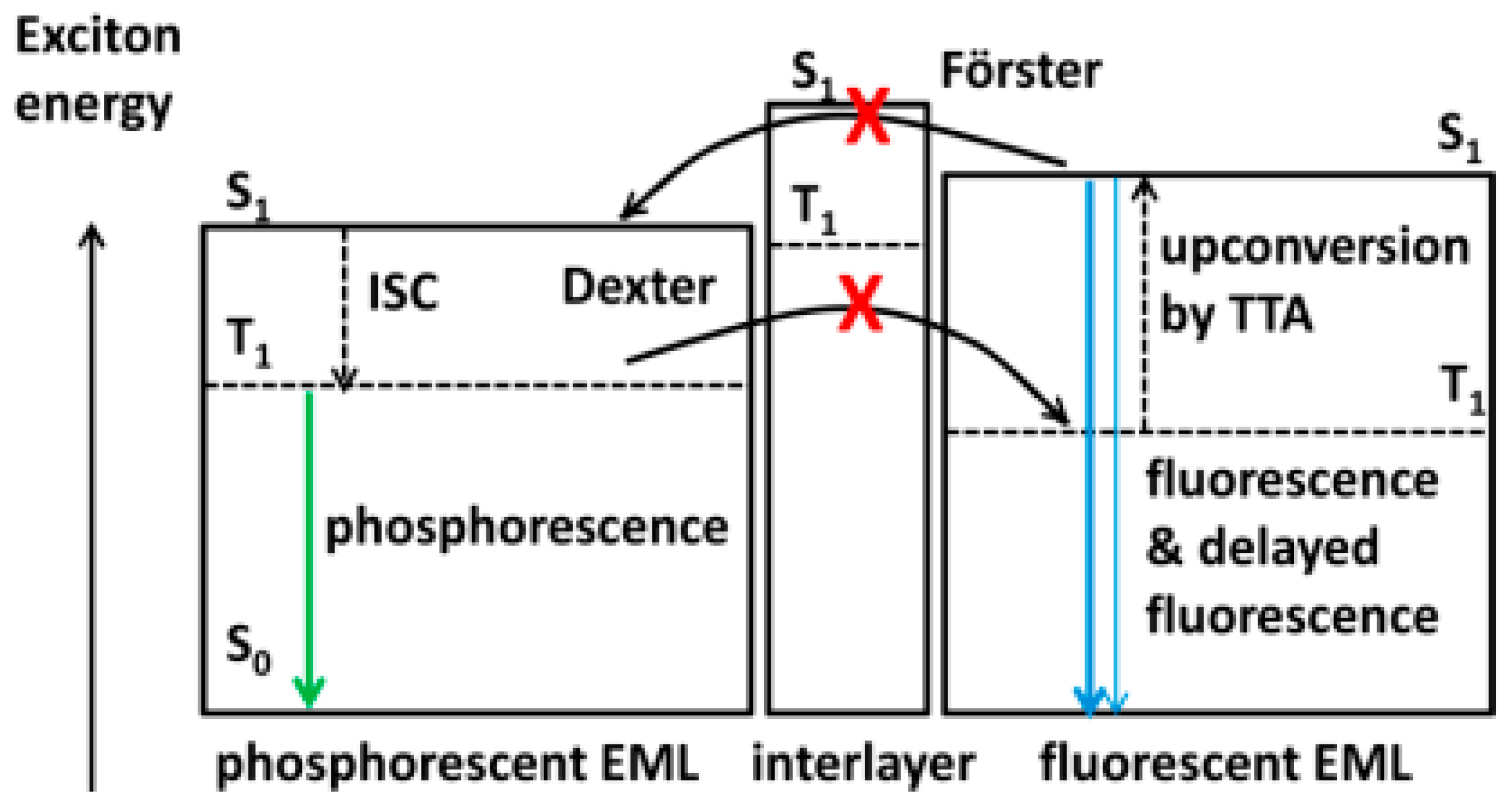
3. Fluorescent WOLEDs
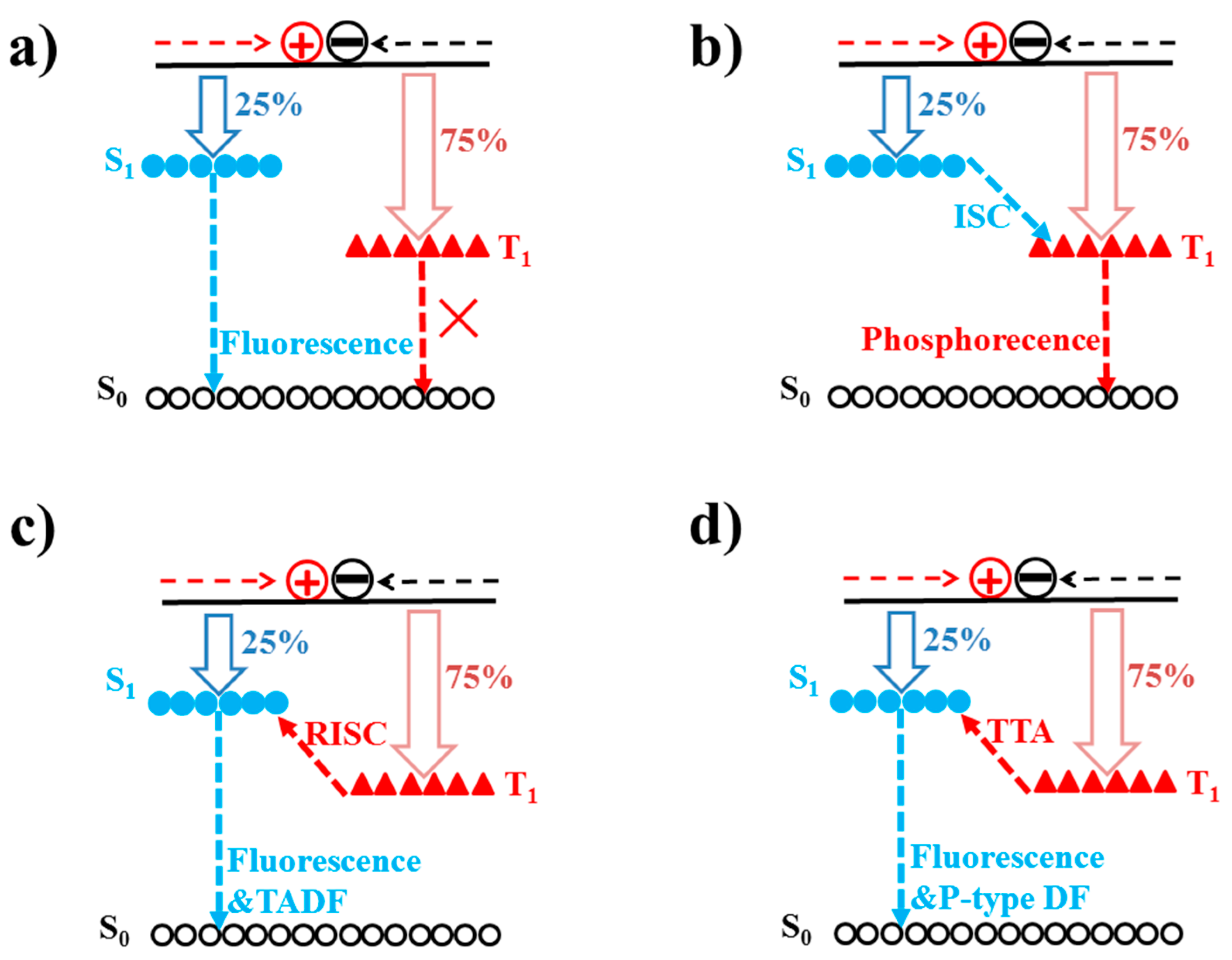
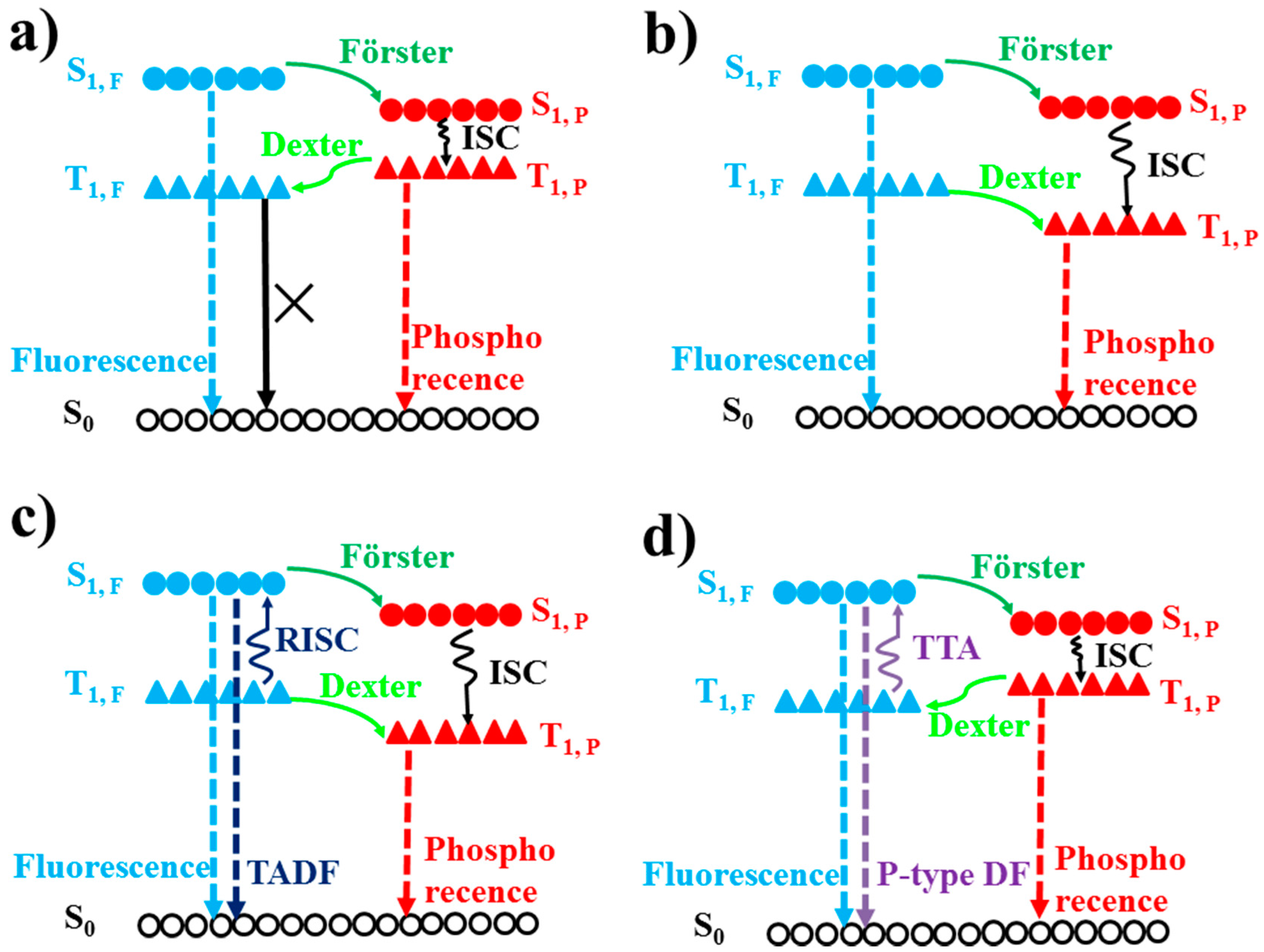
3.1. Fluorescence and Phosphorescence
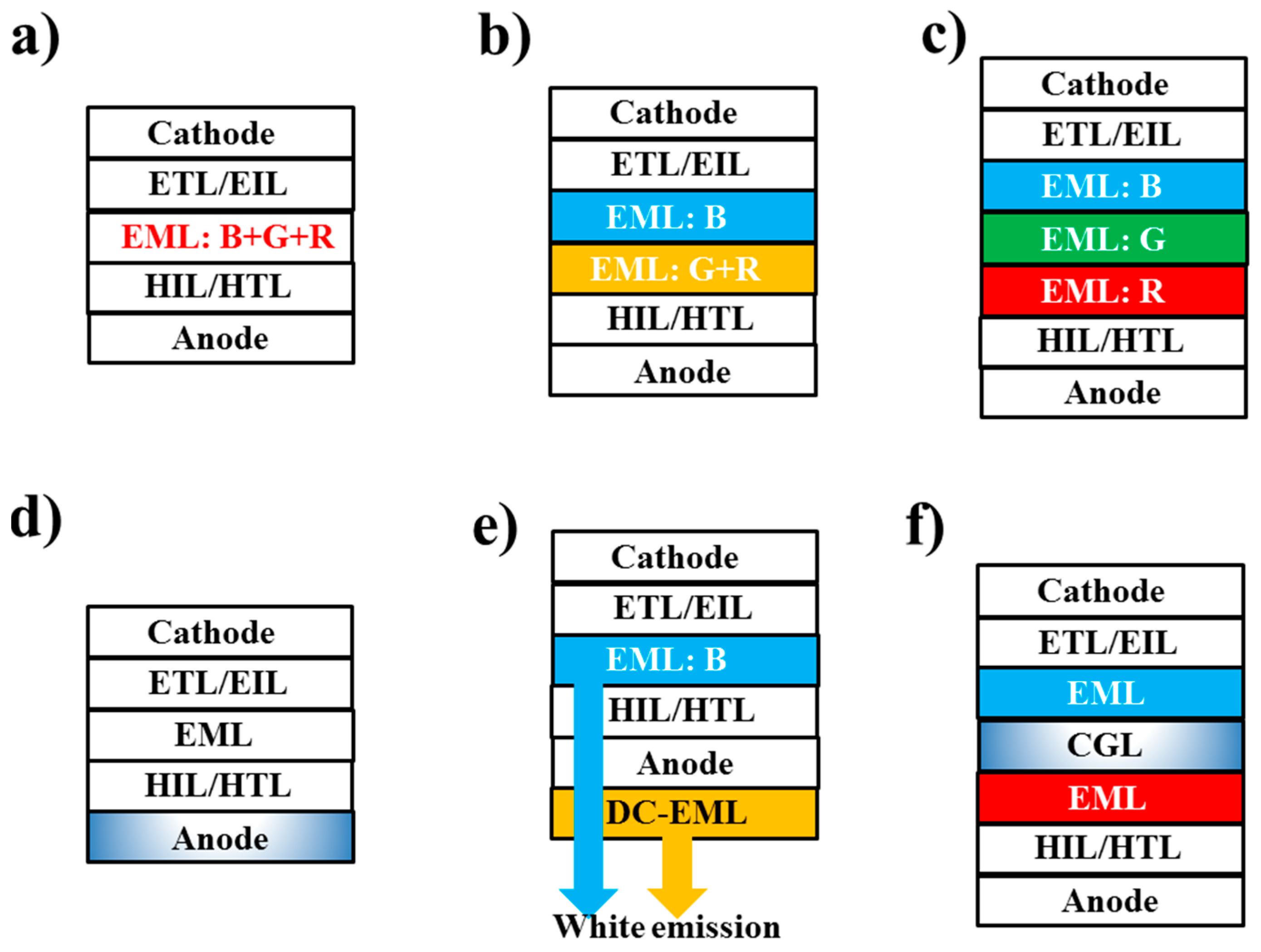
3.2. Device Architectures
3.3. High-Efficiency Fluorescent WOLEDs
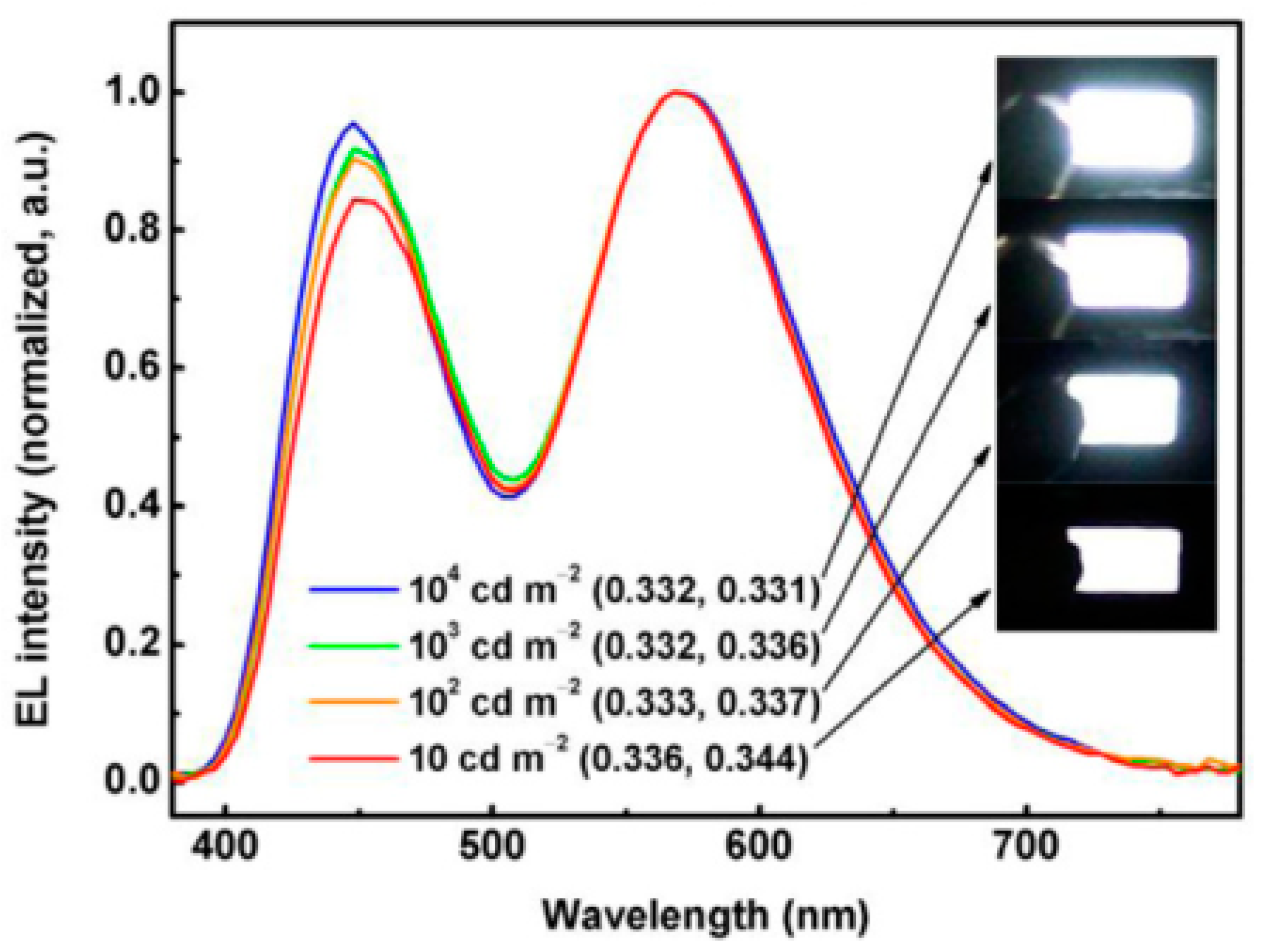
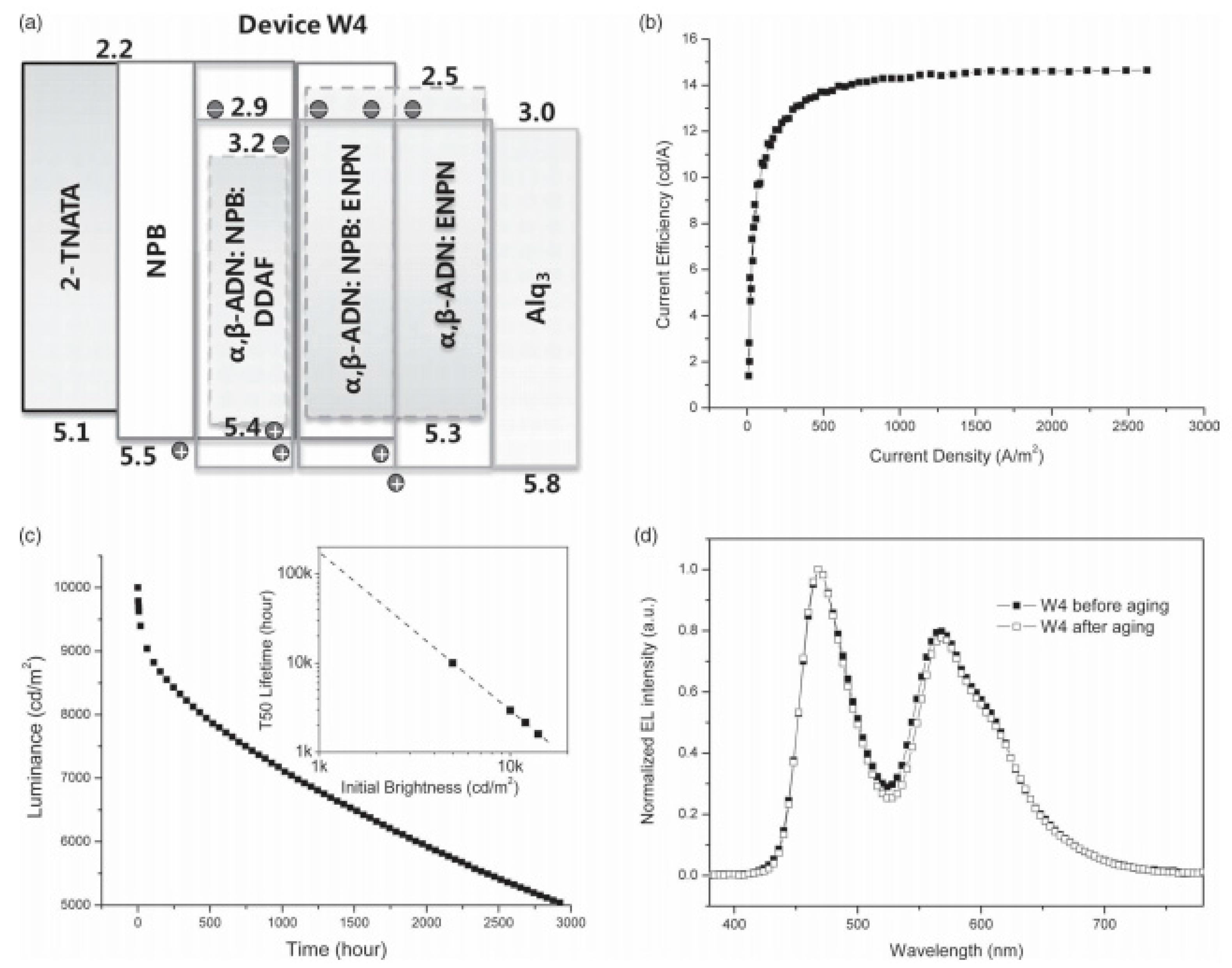
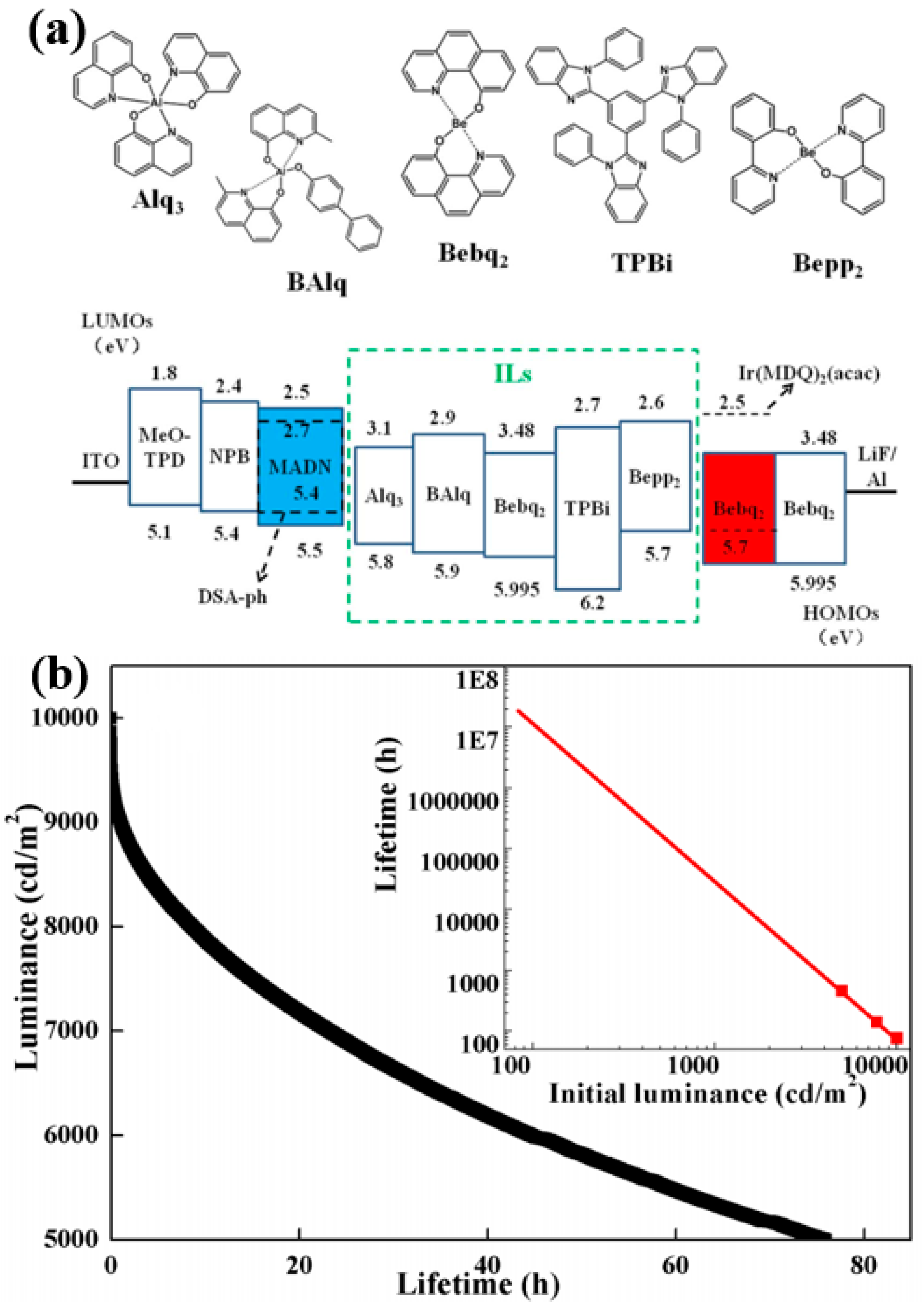
3.4. Long-Lifetime Fluorescent WOLEDs
4. Phosphorescent WOLEDs
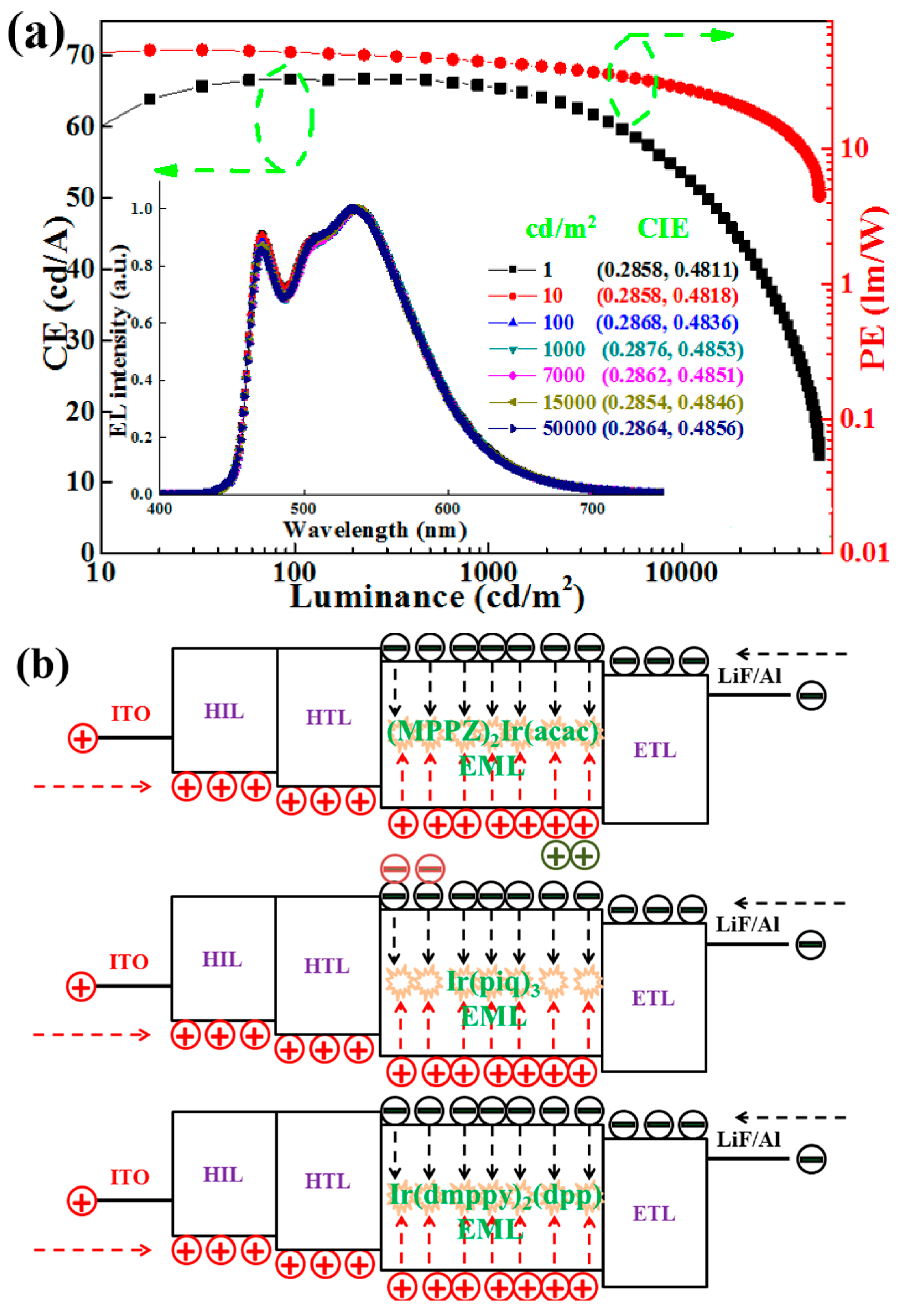
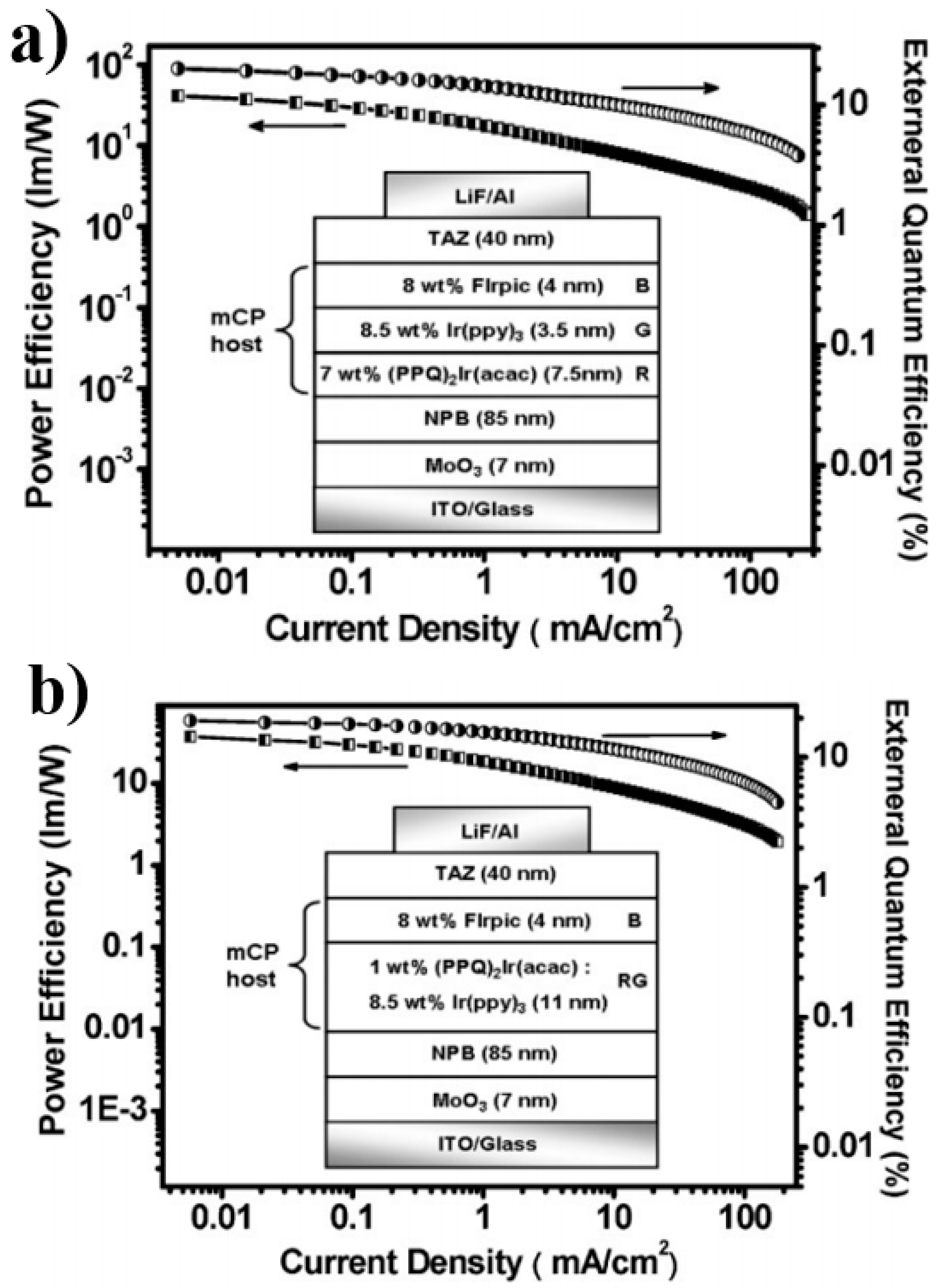
4.1. Single-EML Phosphorescent WOLEDs
4.1.1. Single-EML Phosphorescent WOLEDs with Unipolar Hosts
4.1.2. Single-EML Phosphorescent WOLEDs with Bipolar Hosts
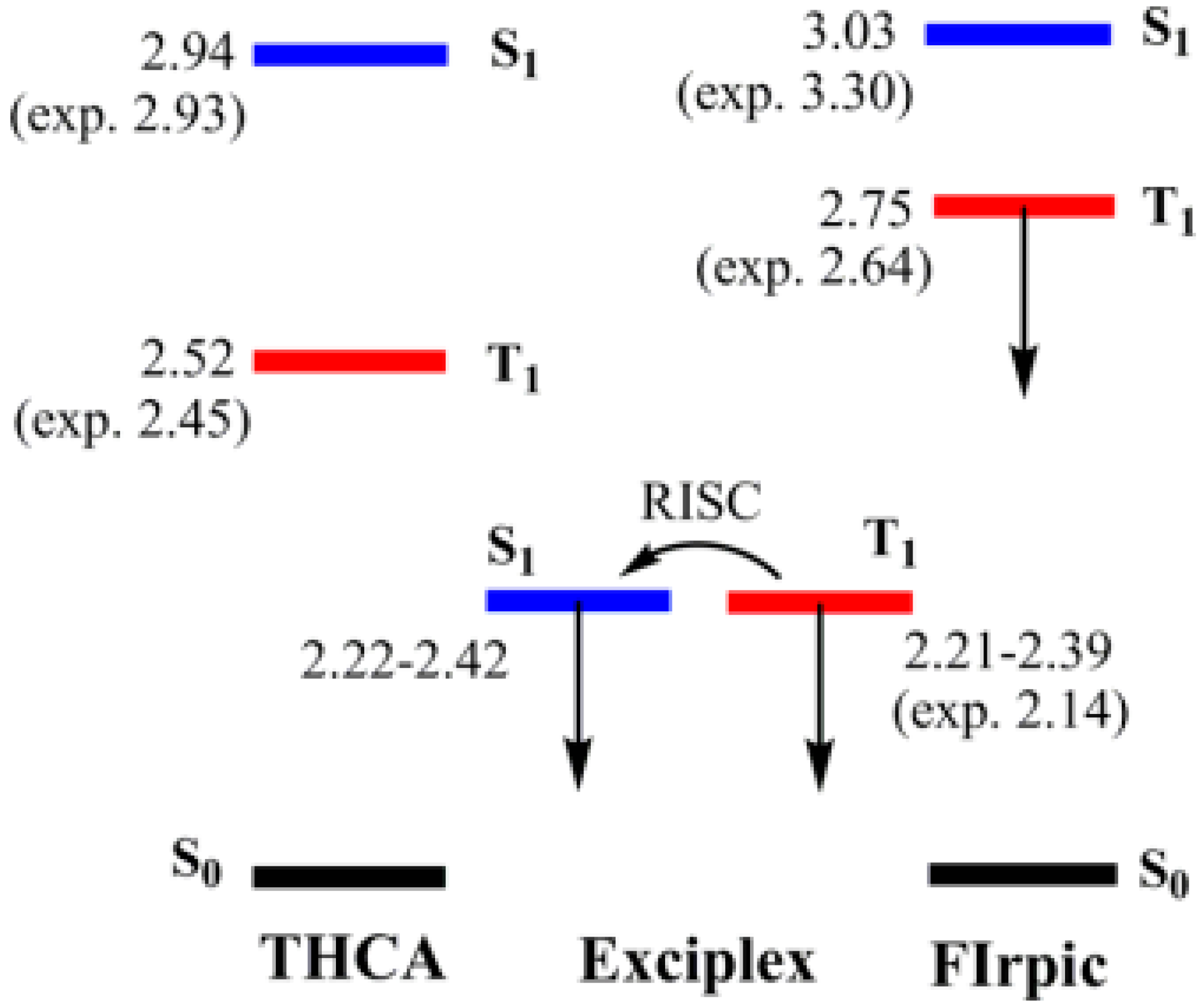
4.1.3. Single-EML Phosphorescent WOLEDs with Solely Exciplex Hosts
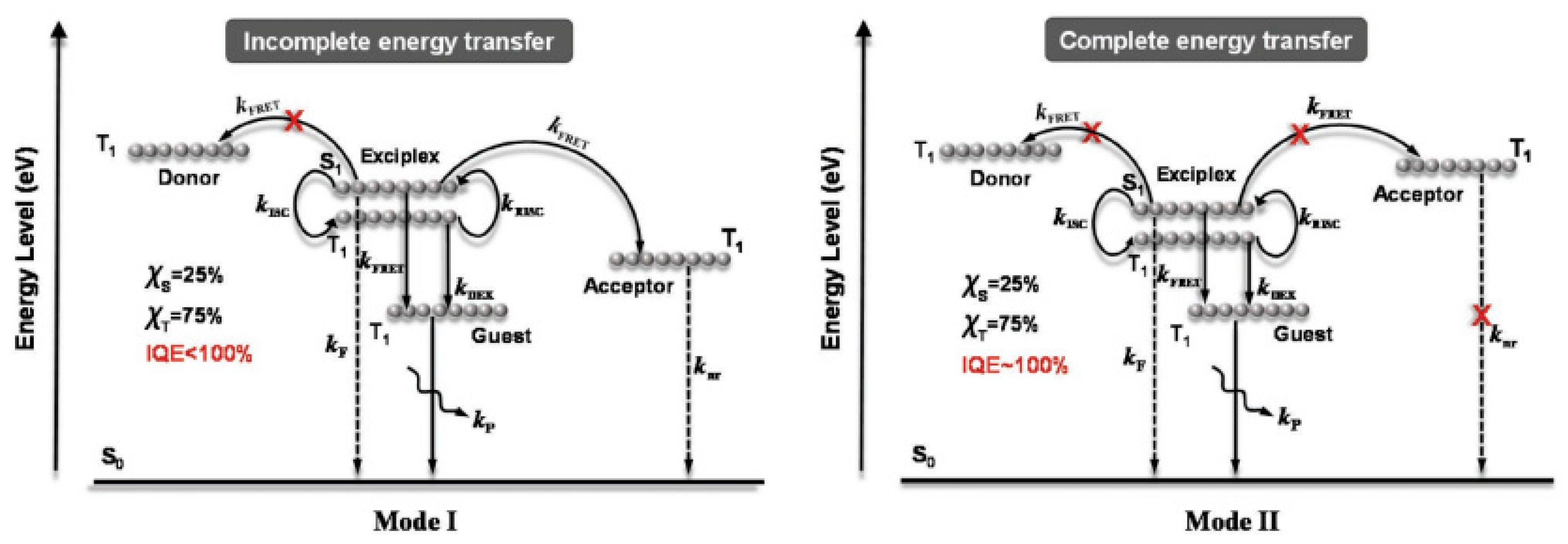
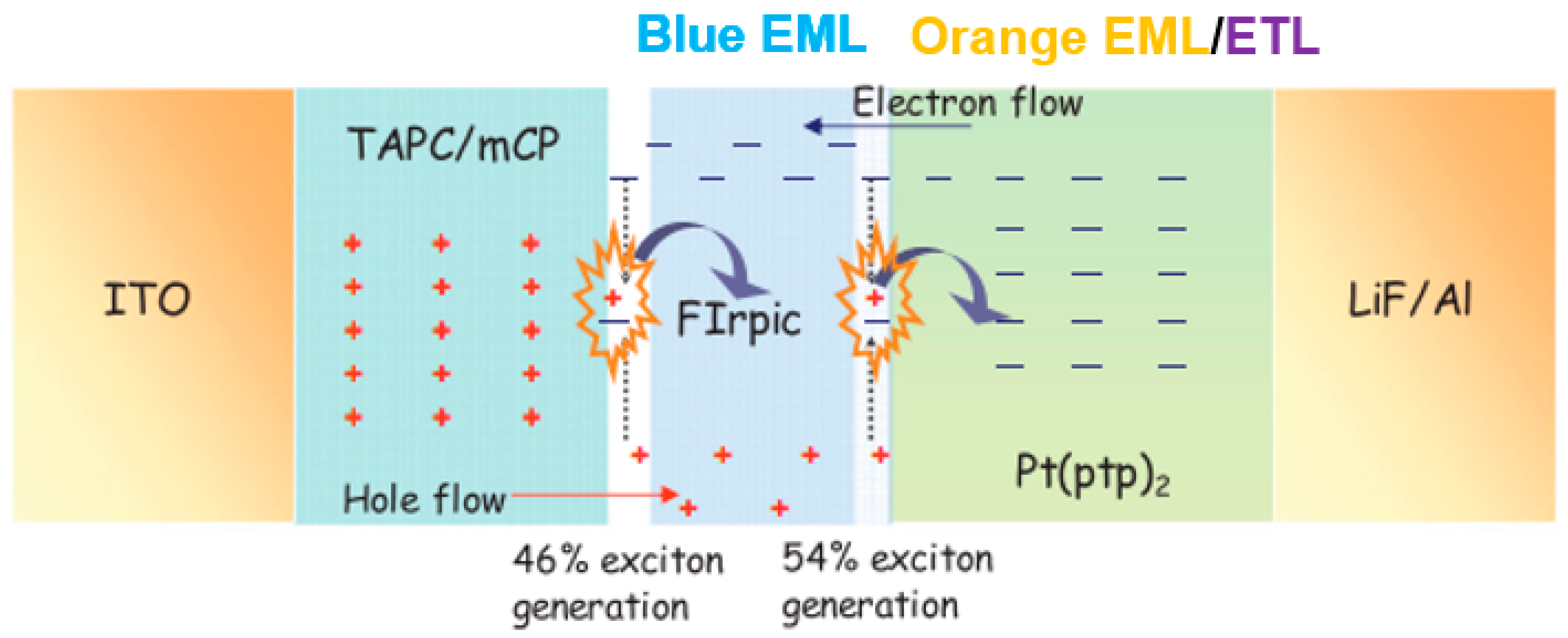
4.2. Multi-EML Phosphorescent WOLEDs
4.2.1. Carrier- and Exciton-Confining Structure
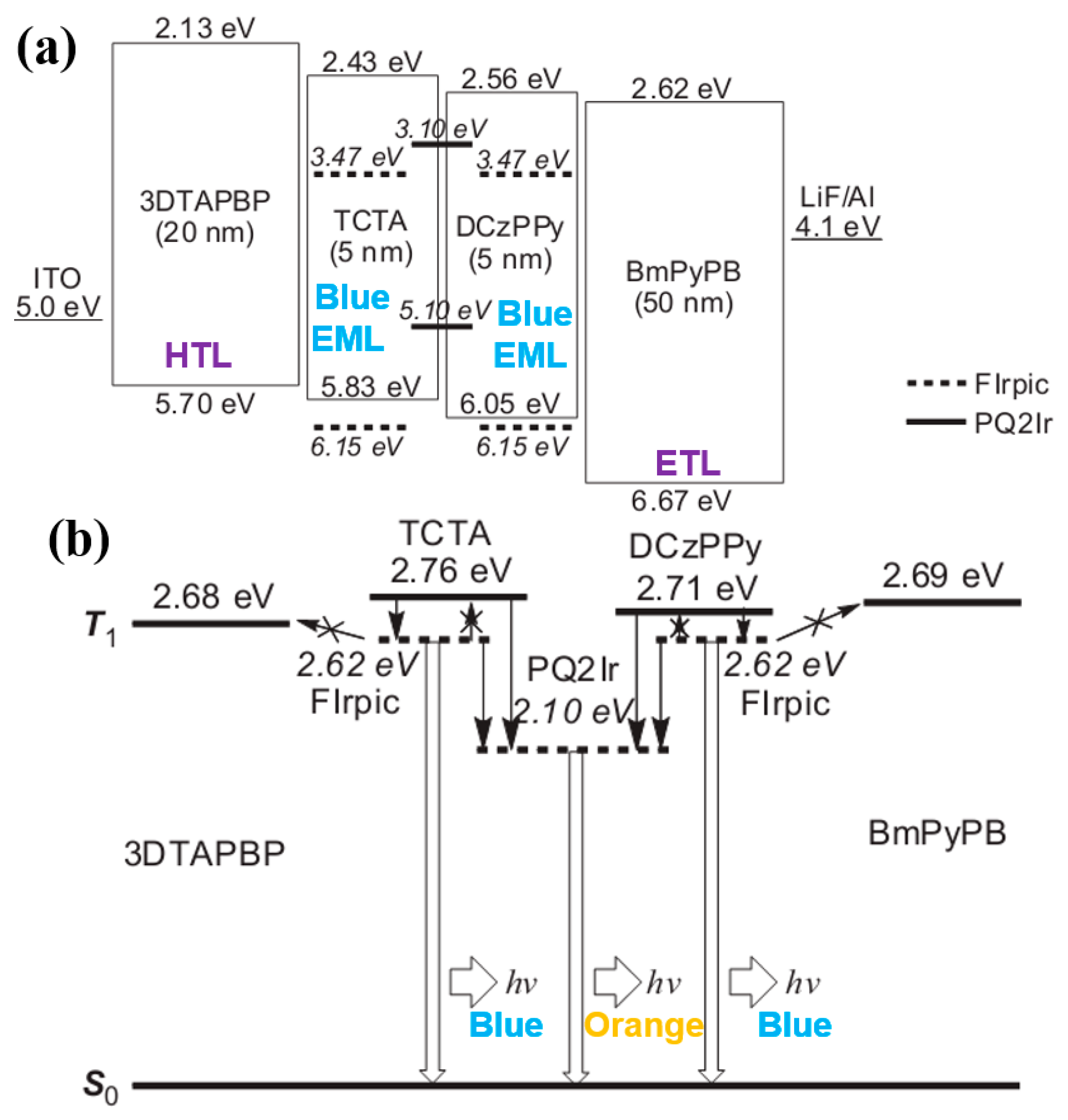
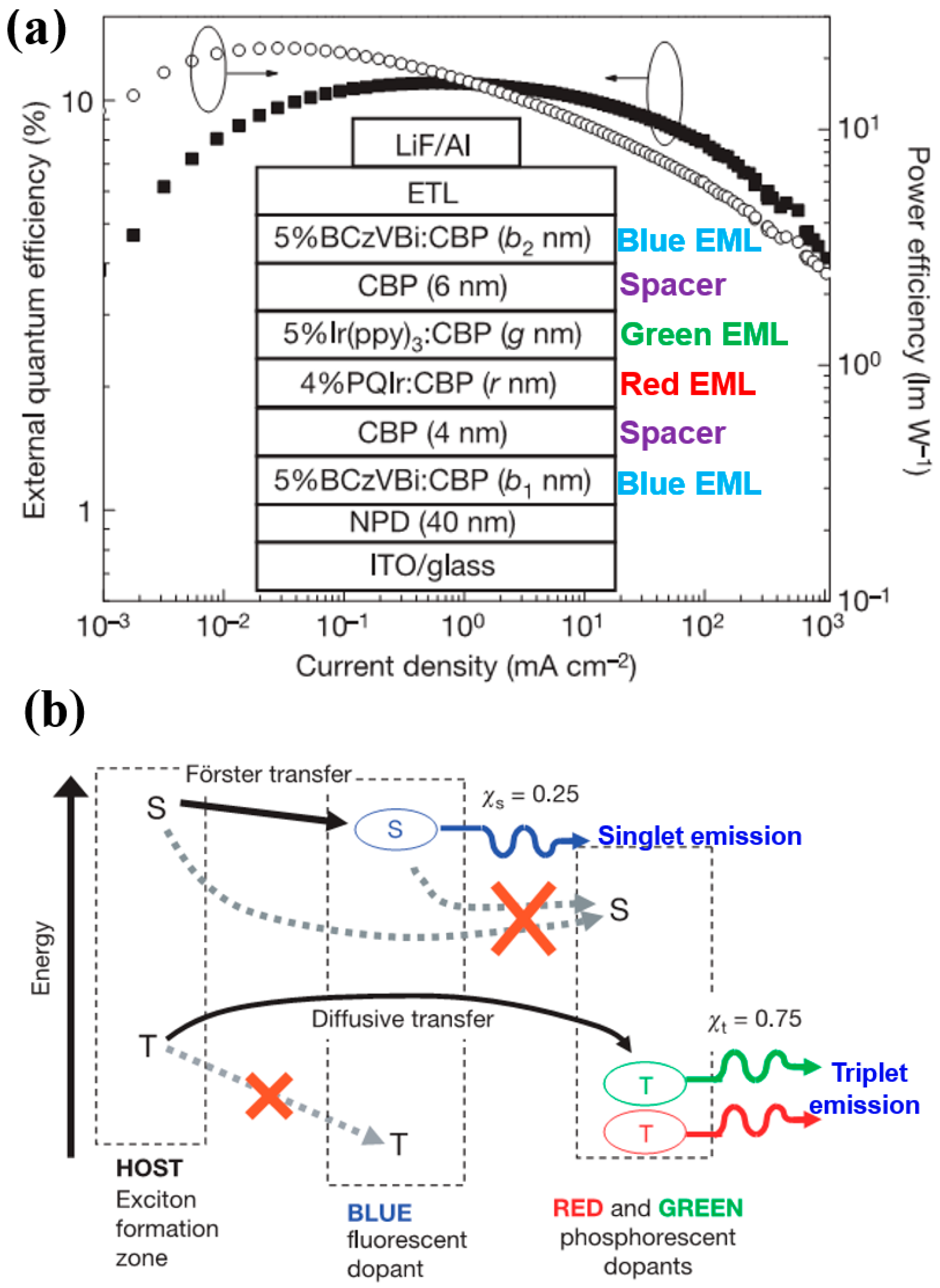
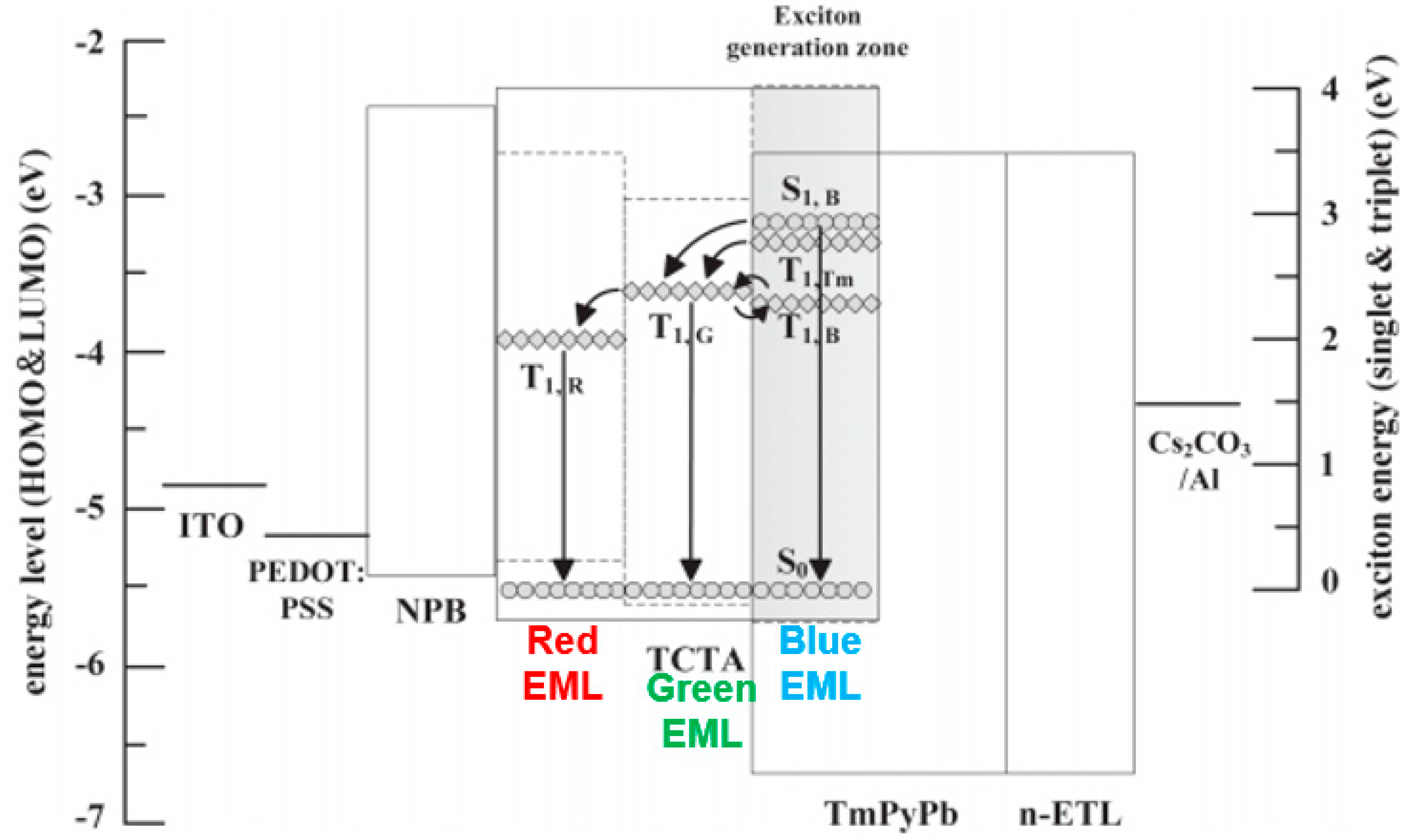
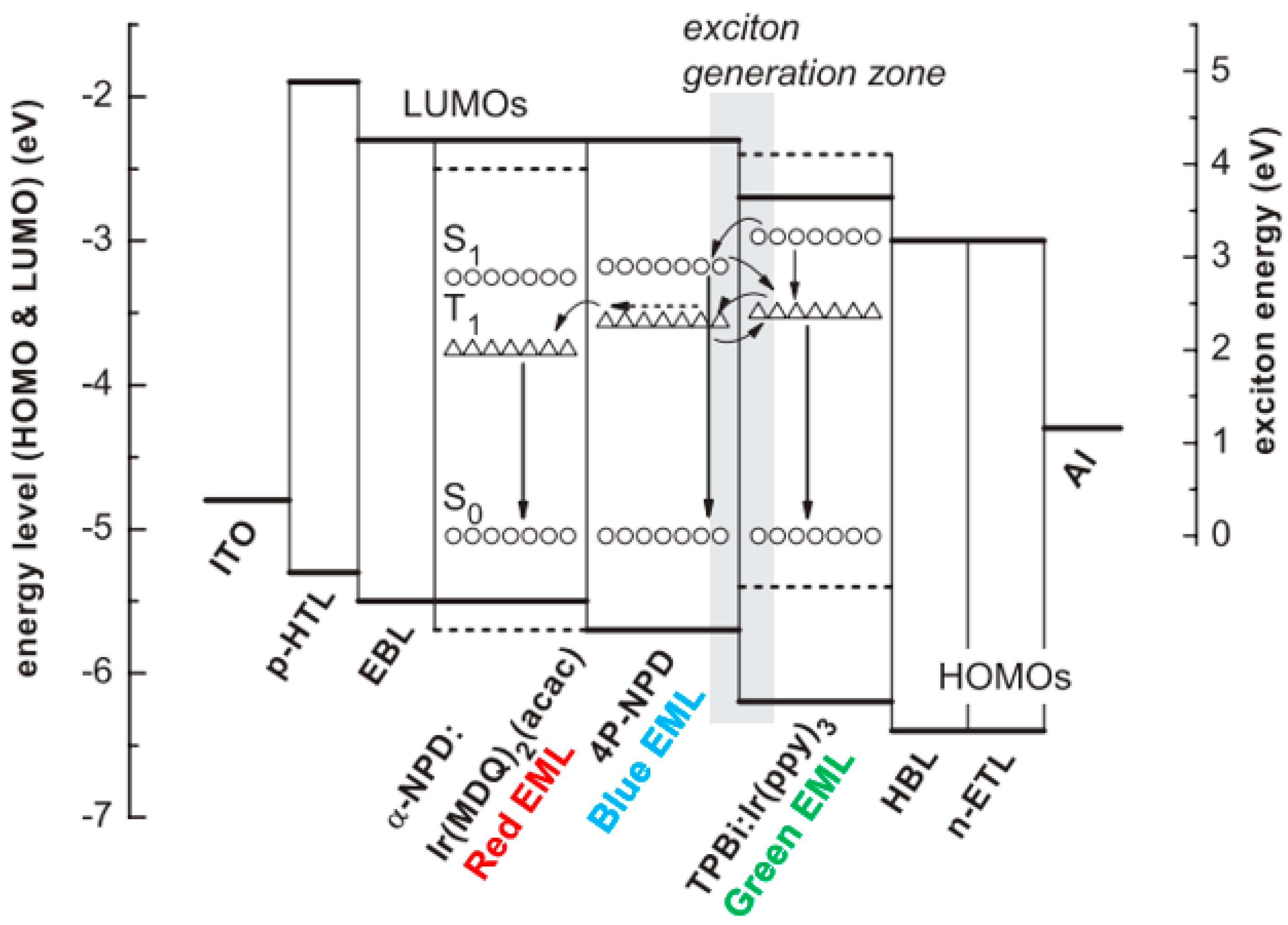
4.2.2. Combining Blue EML Surrounded by Green/Red EML Structure and Outcoupling Technology
4.2.3. Single-Host System Structure
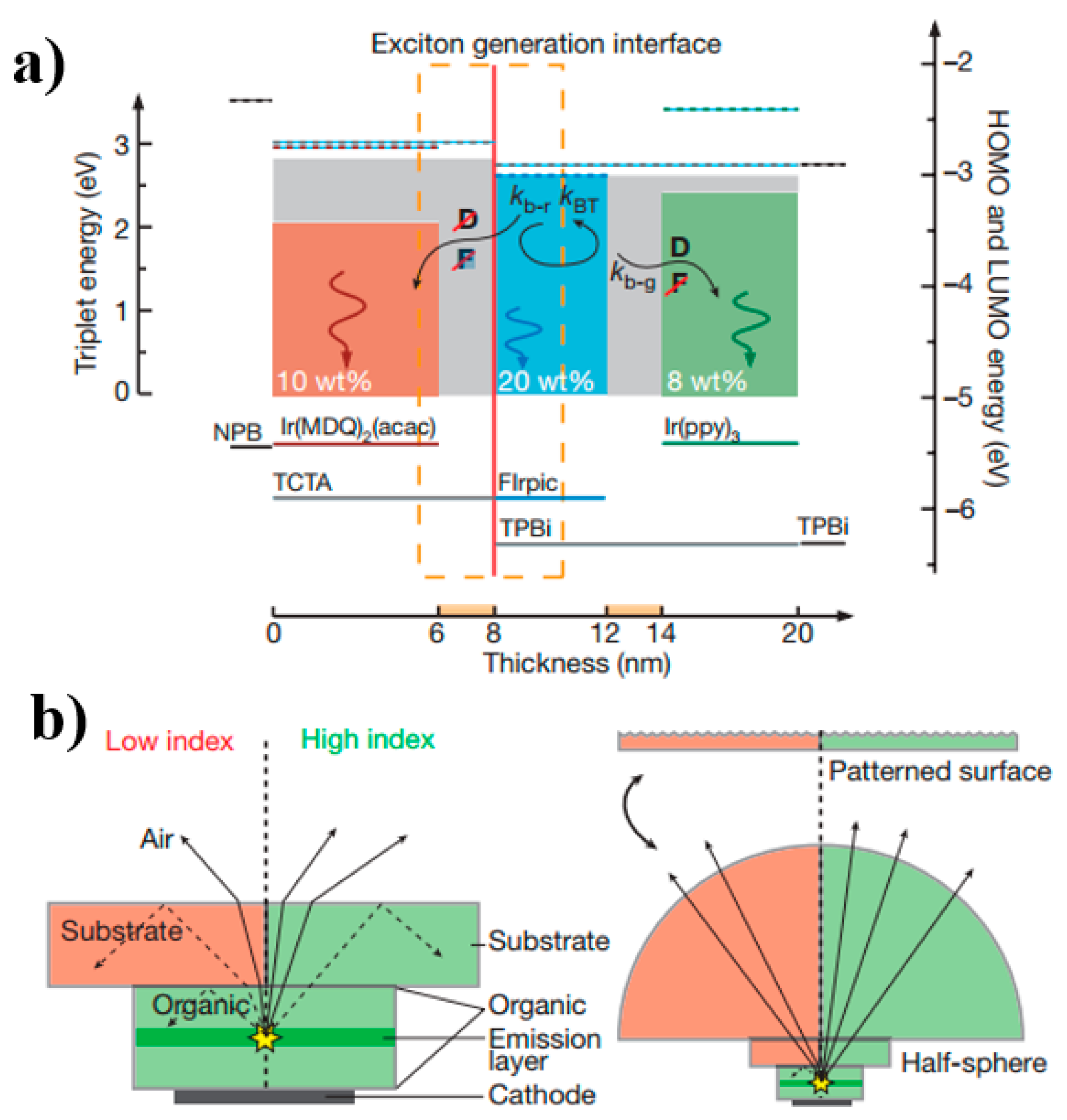
4.2.4. Triplet Exciton Conversion Structure
4.2.5. Incorporating Outcoupling Technology with Energy Cascade Structure
5. TADF WOLEDs
5.1. WOLEDs with All TADF Emitters
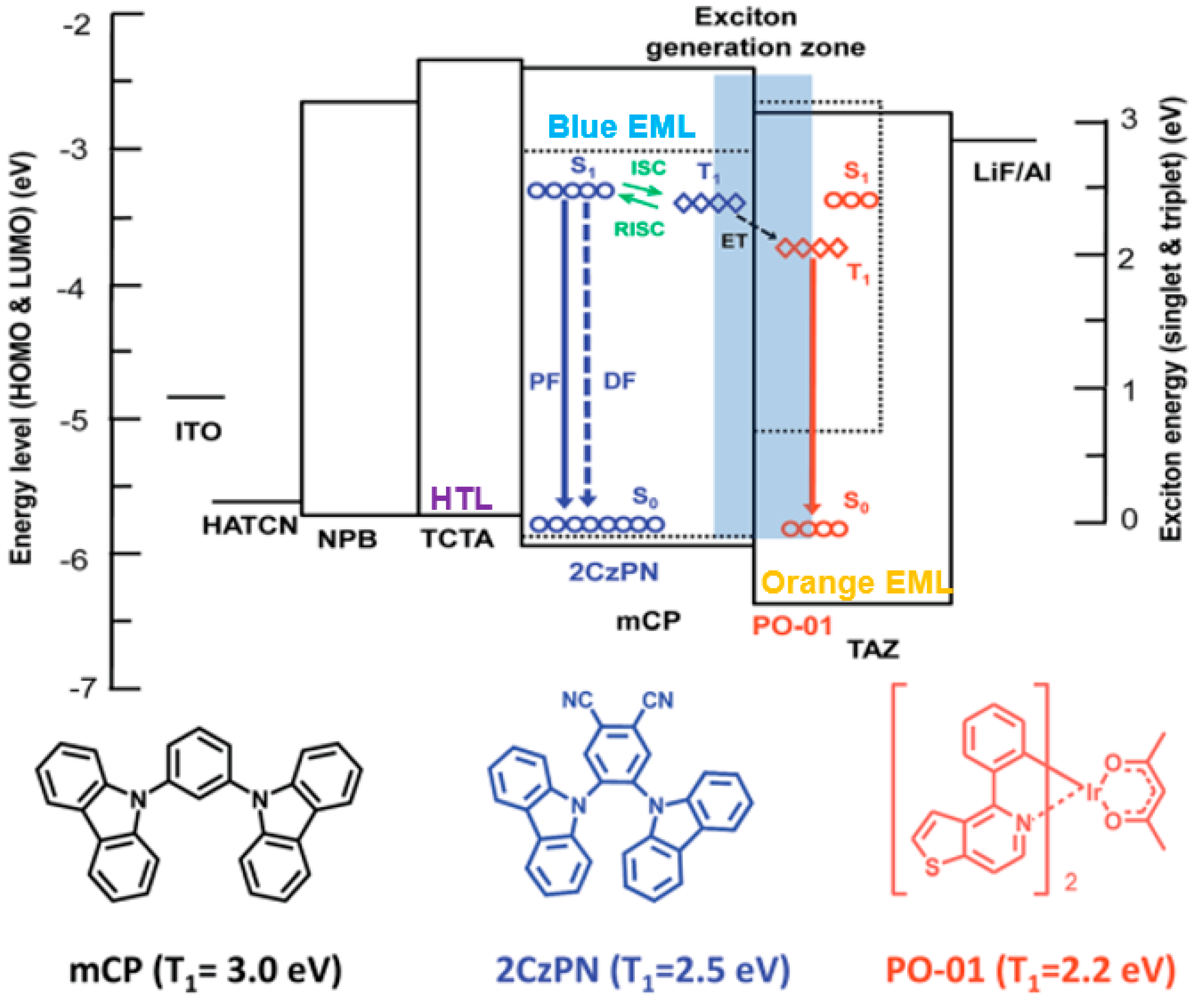
5.2. WOLEDs with TADF and Conventional Fluorescent Emitters
6. Hybrid WOLEDs
6.1. Types of Hybrid WOLEDs
6.2. Low-T1 Fluorophore-Based Hybrid WOLEDs
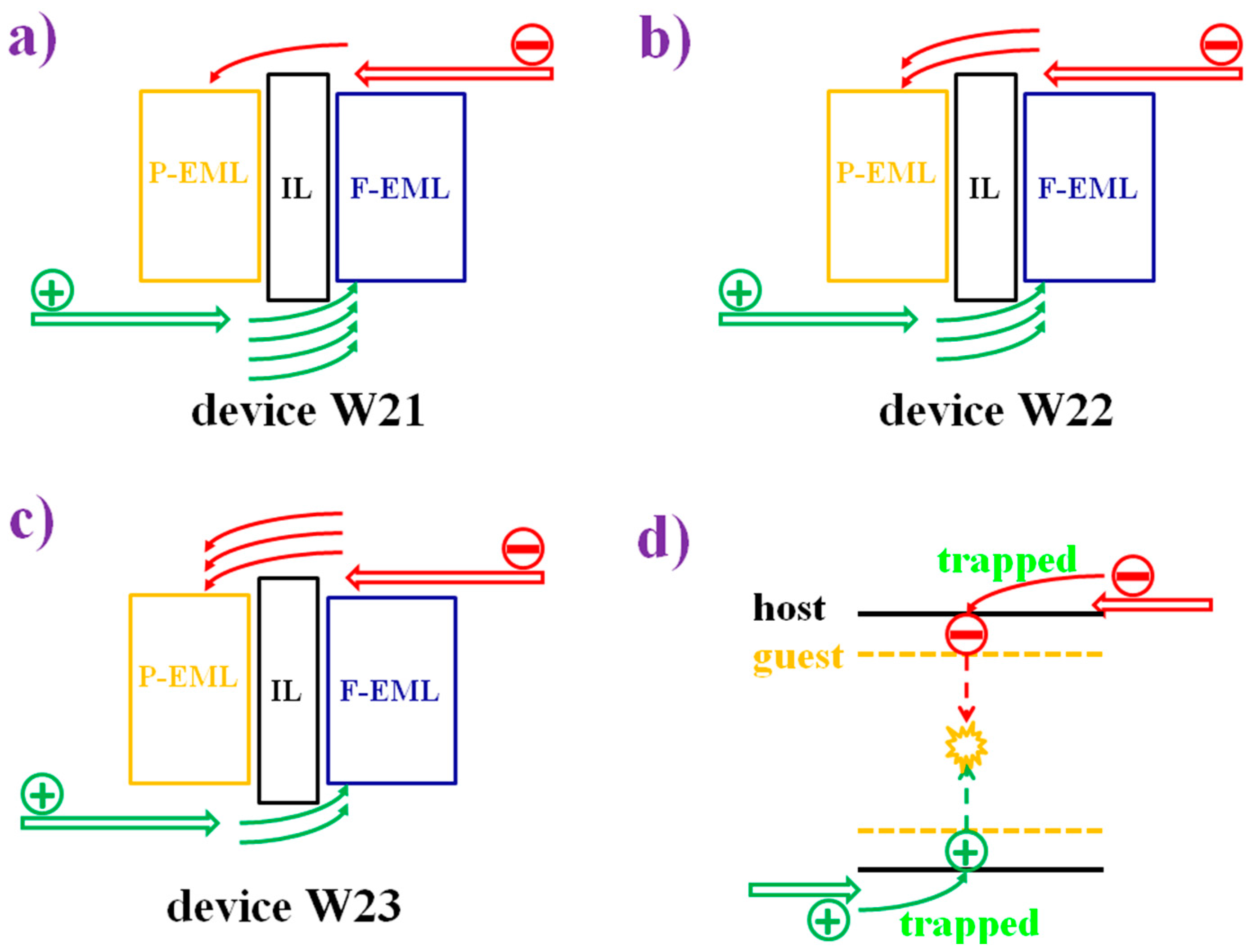
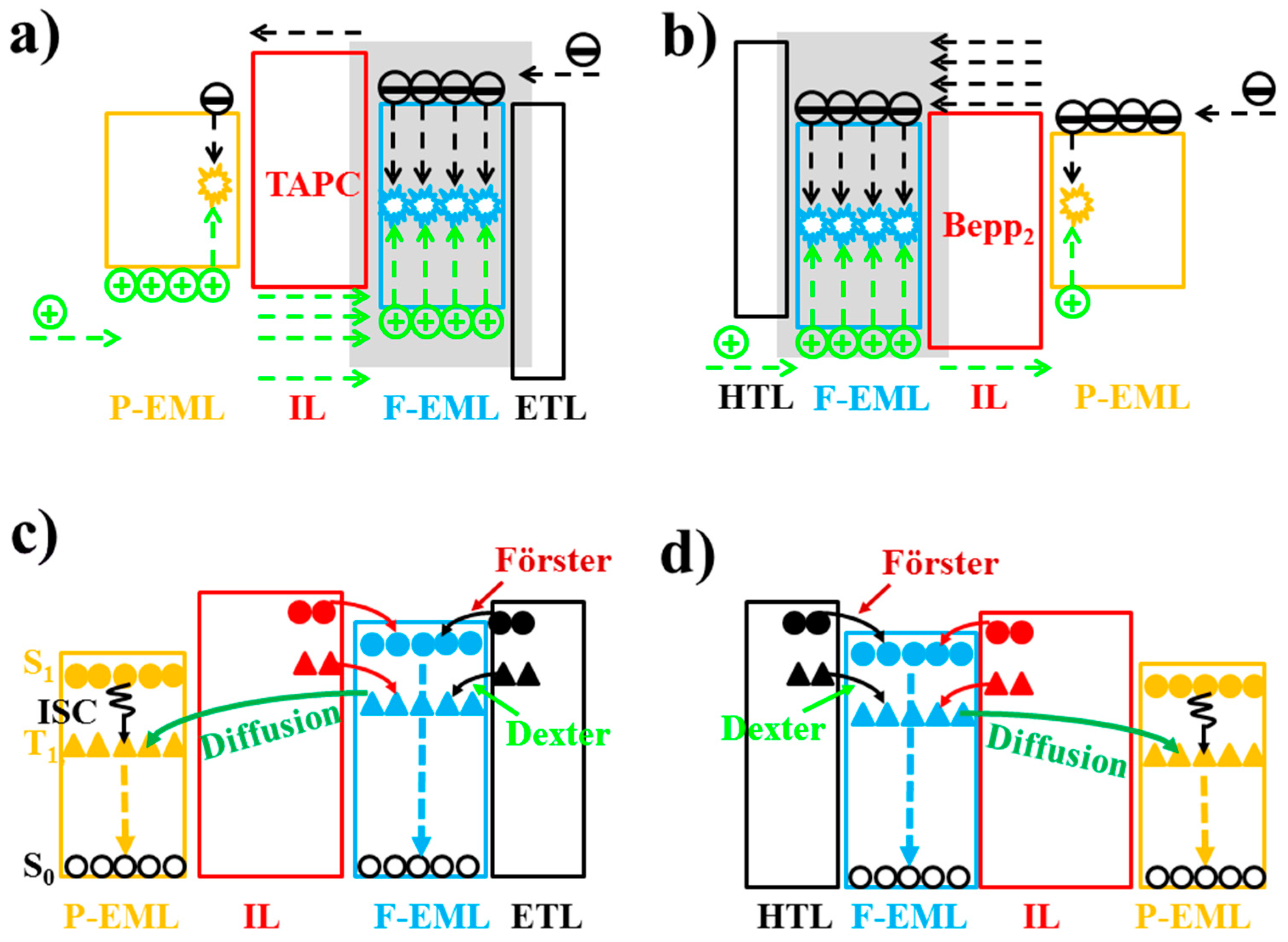
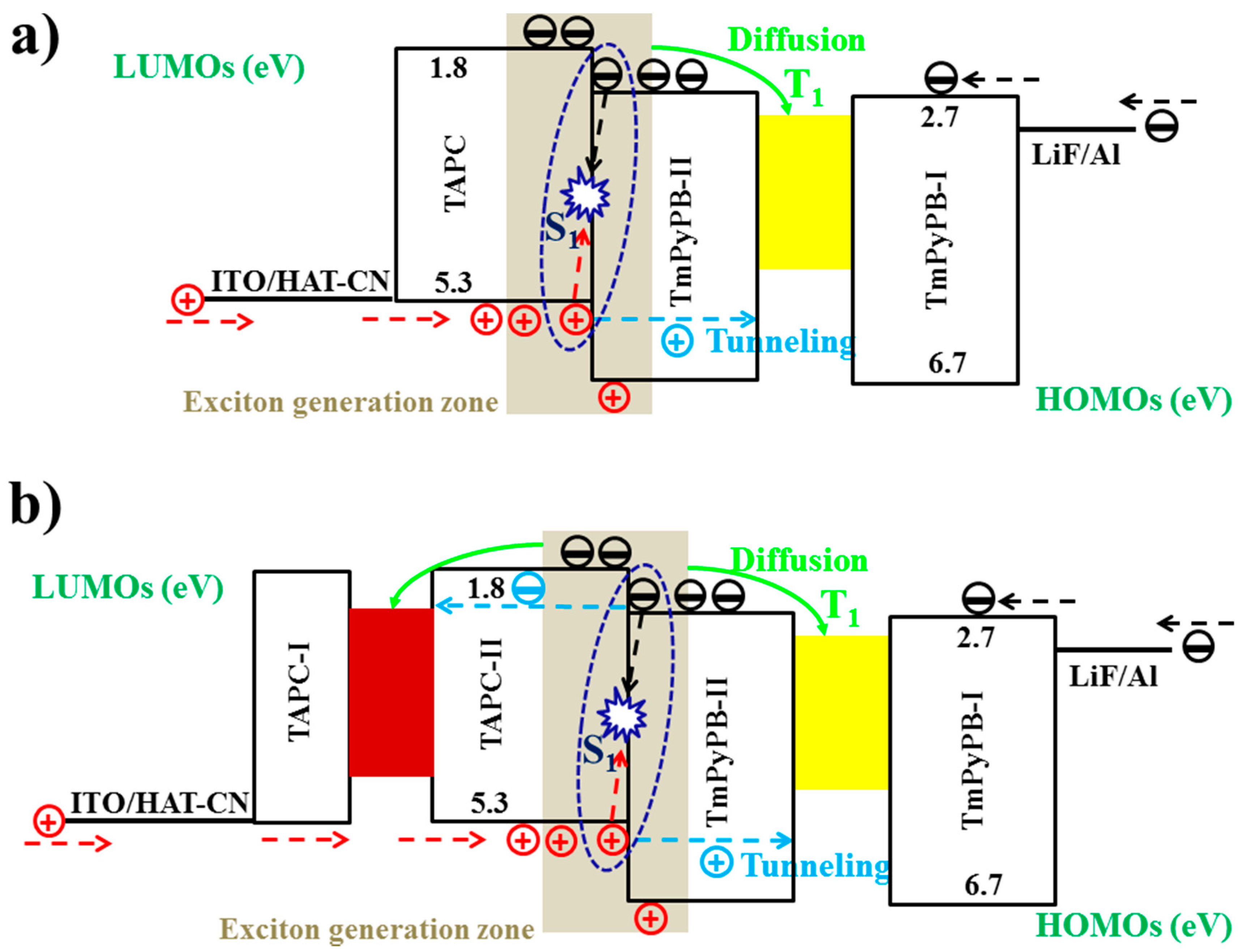
6.2.1. Bipolar Interlayer-Based Hybrid WOLEDs
6.2.2. Unipolar Interlayer-Based Hybrid WOLEDs
6.2.3. Interlayer-Free Hybrid WOLEDs
6.3. High-T1 Fluorophores Based Hybrid WOLEDs
6.3.1. Basic Concept of High-T1 Blue Fluorophore-Based Hybrid WOLEDs
6.3.2. Triplet-Harvesting Structure
6.3.3. Double Blue EMLs Structure
6.3.4. Single-EML Hybrid WOLEDs
6.3.5. TADF Hybrid WOLEDs
6.3.6. p-Type Delayed Fluorescence Hybrid WOLEDs
7. Doping-Free WOLEDs
8. Summary and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Names | Chemical Structures | Functions |
|---|---|---|
| 2-TNATA |  | HIL |
| 3CzTRZ | 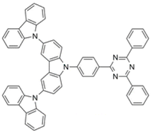 | Host |
| 3DTAPBP | 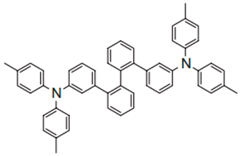 | HIL |
| 4CzPN | 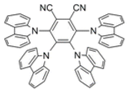 | Green TADF emitter |
| 4CzTPN-Ph | 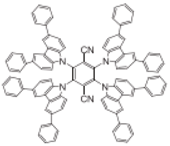 | Red TADF emitter |
| 4P-NPD |  | Host, HTL |
| α-NPD |  | Host, HTL |
| Alq3 | 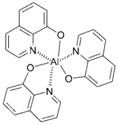 | Green fluorophore, host, ETL |
| ADN |  | Host |
| α,β-AND |  | Host |
| BAlq | 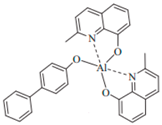 | ETL |
| B3PyMPM | 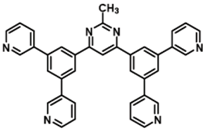 | ETL |
| B4PyMPM | 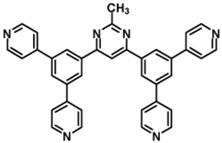 | ETL |
| BCzVBi |  | Blue fluorophore |
| BCP |  | Host, ETL |
| Bebq2 | 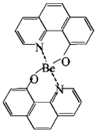 | ETL |
| Bepp2 | 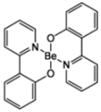 | Blue fluorophore, Host, ETL |
| BPBiPA | 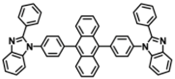 | ETL |
| Bphen | 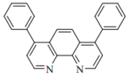 | Host, ETL |
| BTPEAn | 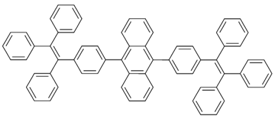 | Blue fluorophore |
| CBP | 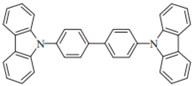 | Host |
| DACrs | 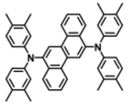 | Blue fluorophore |
| DADBT |  | Host |
| DBP | 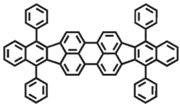 | Red fluorophore |
| DCM | 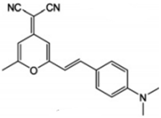 | Orange fluorophore |
| DCzPPy | 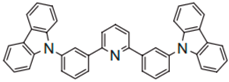 | Host |
| DDAF | 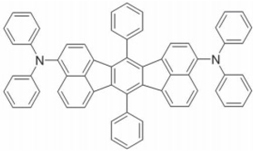 | Yellow fluorophore |
| DSA-ph |  | Blue fluorophore |
| ENPN | 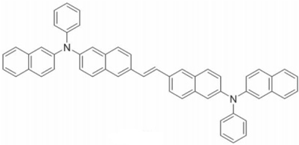 | Blue fluorophore |
| F4-TCNQ |  | HIL |
| (fbi)2Ir(acac) |  | Orange phosphor |
| FIr6 | 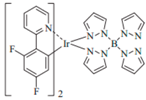 | Blue phosphor |
| FIrpic |  | Blue phosphor |
| HAT-CN | 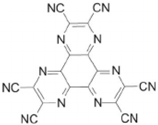 | HIL |
| Ir(2-phq)3 | 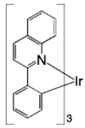 | Orange phosphor |
| Ir(dmppy)2(dpp) |  | Yellow phosphor |
| Ir(MDQ)2(acac) |  | Red phosphor |
| Ir(piq)3 | 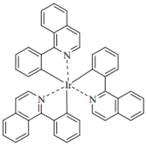 | Red phosphor |
| Ir(ppy)2(acac) | 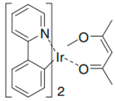 | Green phosphor |
| Ir(ppy)3 | 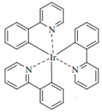 | Green phosphor |
| Liq |  | EIL |
| MADN | 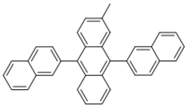 | Host |
| mCP | 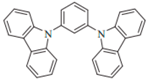 | Host, HTL |
| mCBP | 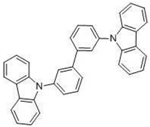 | Hosr |
| MeO-TPD | 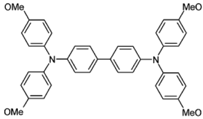 | HIL |
| m-MTDATA | 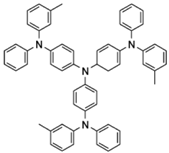 | HTL |
| (MPPZ)2Ir(acac) | 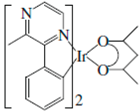 | Orange phosphor |
| N-1-PhTPA | 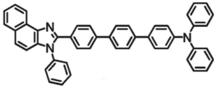 | Blue fluorophore |
| NPB | 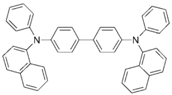 | Host, HTL |
| PEDOT: PSS | 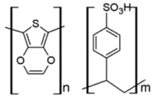 | HIL |
| PO-01 | 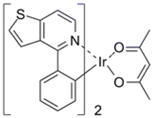 | Yellow phosphor |
| (PPQ)2Ir(acac) | 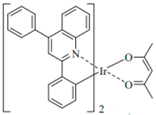 | Red phosphor |
| PQIr | 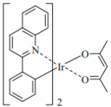 | Red phosphor |
| Pt(ptp)2 | 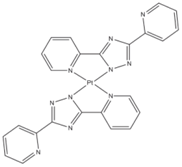 | Orange phosphor |
| PXZDSO2 | 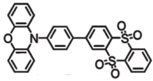 | Yellow TADF emitter |
| TAPC | 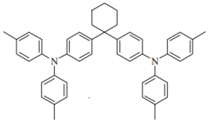 | HTL |
| TAZ |  | ETL |
| TCTA | 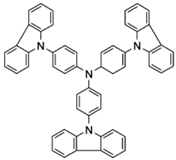 | Host, HTL |
| THCA | 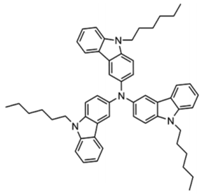 | HTL |
| TmPyPB | 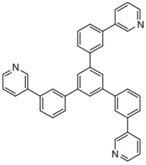 | ETL |
| TPBi |  | Host, ETL |
| TPD |  | HTL |
| Tris-PCz | 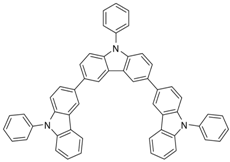 | HTL |
| UGH2 | 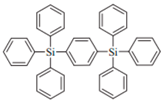 | Host |
References
- Tang, C.W.; VanSlyke, V.A. Organic Electroluminescent Diodes. Appl. Phys. Lett. 1987, 51, 913. [Google Scholar] [CrossRef]
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burns, P.L.; Homes, A.B. Light-Emitting Diodes Based on Conjugated Polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’brien, D.F.; You, Y.; Shoustikov, A.; Sibley, S.; Thompson, M.E.; Forrest, S.R. Highly Efficient Phosphorescent Emission From Organic Electroluminescent Devices. Nature 1998, 395, 151–154. [Google Scholar] [CrossRef]
- Helander, M.G.; Wang, Z.B.; Qiu, J.; Greiner, M.T.; Puzzo, D.P.; Liu, Z.W.; Lu, Z.H. Chlorinated Indium Tin Oxide Electrodes with High Work Function for Organic Device Compatibility. Science 2011, 332, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhou, G.; Wong, W.-Y. Functionalization of Phosphorescent Emitters and Their Host Materials by Main-Group Elements for Phosphorescent Organic Light-Emitting Devices. Chem. Soc. Rev. 2015, 44, 8484–8575. [Google Scholar] [CrossRef] [PubMed]
- Kido, J.; Hongawa, K.; Okuyama, K.; Nagai, K. White Light-Emitting Organic Electroluminescent Devices Using The Poly(N-vinylcarbazole) Emitter Layer Doped with Three Fluorescent Dyes. Appl. Phys. Lett. 1994, 64, 815. [Google Scholar] [CrossRef]
- Kido, J.; Kimura, M.; Nagai, K. Multilayer White Light-Emitting Organic Electroluminescent Device. Science 1995, 267, 1332–1334. [Google Scholar] [CrossRef] [PubMed]
- Yamae, K.; Kittichungchit, V.; Ide, N.; Ota, M.; Komoda, T. Invited Paper: Highly Efficient White OLEDs with Over 100 lm/W for General Lighting. SID Symp. Dig. Tech. Pap. 2014, 44, 916–919. [Google Scholar] [CrossRef]
- Ou, Q.D.; Zhou, L.; Li, Y.Q.; Chen, S.; Chen, J.D.; Li, C.; Wang, Q.K.; Lee, S.T.; Tang, J.X. Light-Emitting Diodes: Extremely Efficient White Organic Light-Emitting Diodes for General Lighting. Adv. Funct. Mater. 2014, 24, 7249–7256. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Xu, M.; Tao, H.; Gao, D.; Zou, J.; Lan, L.; Ning, H.; Peng, J.; Cao, Y. Extremely Stable-color Flexible White Organic Light-emitting Diodes with Efficiency Exceeding 100 lm W−1. J. Mater. Chem. C 2014, 2, 9836–9841. [Google Scholar] [CrossRef]
- Liu, B.; Gao, D.; Wang, J.; Wang, X.; Wang, L.; Zou, J.; Ning, H.; Peng, J. Progress of White Organic Light-Emitting Diodes. Acta Phys. Chim. Sin. 2015, 31, 1823–1856. [Google Scholar]
- Wu, S.F.; Li, S.H.; Wang, Y.K.; Huang, C.C.; Sun, Q.; Liang, J.J.; Liao, L.S.; Fung, M.-K. White Organic LED with a Luminous Efficacy Exceeding 100 lm w−1 without Light Out-Coupling Enhancement Techniques. Adv. Funct. Mater. 2017, 27, 1701314. [Google Scholar] [CrossRef]
- Spindler, J.P.; Hatwar, T.K.; Miller, M.E.; Arnold, A.D.; Murdoch, M.J.; Kane, P.J.; Ludwicki, J.E.; Paula, J.; Alessi, P.J.; Slykey, S.A.V. System Considerations for RGBW OLED Displays. J. Soc. Inf. Display 2012, 14, 37–48. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lee, M.; Kang, K.; Kim, J.-O. Adaptive Color Saturation Control for Low Power RGBW OLED Displays. J. Disp. Technol. 2016, 12, 784–790. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, G.; Wong, W.-Y. Recent Design Tactics for High Performance White Polymer Light-Emitting Diodes. J. Mater. Chem. C 2014, 2, 1760–1778. [Google Scholar] [CrossRef]
- Jou, J.-H.; Kumar, S.; Agrawal, A.; Li, T.-H.; Sahoo, S. Approaches for Fabricating High Efficiency Organic Light Emitting Diodes. J. Mater. Chem. C 2015, 3, 2974–3002. [Google Scholar] [CrossRef]
- Liu, B.; Li, X.; Tao, H.; Zou, J.; Xu, M.; Wang, L.; Peng, J.; Cao, Y. Manipulation of Exciton Distribution for High-Performance Fluorescent/Phosphorescent Hybrid White Organic Light-Emitting Diodes. J. Mater. Chem. C 2017, 5, 7668–7683. [Google Scholar] [CrossRef]
- Jou, J.-H.; Hsieh, C.-Y.; Tseng, J.-R.; Peng, S.-H.; Jou, Y.-C.; Hong, J.H.; Shen, S.-M.; Tang, M.-C.; Chen, P.-C.; Lin, C.-H. Candle Light-Style Organic Light-Emitting Diodes. Adv. Funct. Mater. 2013, 23, 2750–2757. [Google Scholar] [CrossRef]
- Jou, J.-H.; Wu, R.-Z.; Yu, H.-H.; Li, C.-J.; Jou, Y.-C.; Peng, S.-H.; Chen, Y.-L.; Chen, C.-T.; Shen, S.-M.; Joers, P.; et al. Artificial Dusk-Light Based on Organic Light Emitting Diodes. ACS Photonics 2014, 1, 27–31. [Google Scholar] [CrossRef]
- Jou, J.H.; Wu, M.H.; Shen, S.M.; Wang, H.C.; Chen, S.Z.; Chen, S.H.; Lin, C.R.; Hsieh, Y.L. Sunlight-Style Color-Temperature Tunable Organic Light-Emitting Diode. Appl. Phys. Lett. 2009, 95, 013307. [Google Scholar] [CrossRef]
- OLED Lighting Companies. Available online: https://www.oled-info.com/companies/oled-lighting (accessed on 30 November 2017).
- Wong, W.-Y.; Ho, C.L. Heavy Metal Organometallic Electrophosphors Derived From Multi-Component Chromophores. Coord. Chem. Rev. 2009, 253, 1709–1758. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, D. Management of Charges and Excitons for High-performance White Organic Light-emitting Diodes. Chem. Soc. Rev. 2010, 39, 2387–2398. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Lin, C.-C.; Yeh, C.-W.; Liu, R.-S. Light Converting Inorganic Phosphors for White Light-Emitting Diodes. Materials 2010, 3, 2172–2195. [Google Scholar] [CrossRef]
- Kamtekar, K.T.; Monkman, A.P.; Bryce, M.R. Recent Advances in White Organic Light-Emitting Materials and Devices (WOLEDs). Adv. Mater. 2010, 22, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Kohenen, A.; Meerholz, K. White Organic Light-Emitting Diodes. Adv. Mater. 2011, 23, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Tyan, Y.S. Organic Light-Emitting-Diode Lighting Overview. J. Photonics Energy 2011, 1, 011009. [Google Scholar] [CrossRef]
- Sasabe, H.; Kido, J. Development of High Performance OLEDs for General Lighting. J. Mater. Chem. C 2013, 1, 1699–1707. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Lu, Z.-H. White Organic Light-Emitting Diodes for Solid-State Lighting. J. Disp. Technol. 2013, 9, 459–468. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, F.; Ma, D. Hybrid White OLEDs with Fluorophors and Phosphors. Mater. Today 2014, 17, 175–183. [Google Scholar] [CrossRef]
- Gohri, V.; Hofmann, S.; Reineke, S.; Rosenow, T.; Thomschke, M.; Levichkova, M.; Lüssem, B.; Leo, K. White Top-Emitting Organic Light-Emitting Diodes Employing a Heterostructure of Down-Conversion Layers. Org. Electron. 2011, 12, 2126–2130. [Google Scholar] [CrossRef]
- Mondal, E.; Hung, W.Y.; Dai, H.C.; Wong, K.T. Fluorene-Based Asymmetric Bipolar Universal Hosts for White Organic Light Emitting Devices. Adv. Funct. Mater. 2013, 23, 3096–3105. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Wu, Q.; Shi, H.; Mei, Y.; Zhang, R.; Wang, L.; Huang, W. Efficient, Color-Stable Flexible White Top-Emitting Organic Light-Emitting Diodes. Org. Electron. 2013, 14, 3037–3045. [Google Scholar] [CrossRef]
- Kim, B.S.; Yook, K.S.; Lee, J.Y. Above 20% External Quantum Efficiency in Novel Hybrid White Organic Light-Emitting Diodes Having Green Thermally Activated Delayed Fluorescent Emitter. Sci. Rep. 2014, 4, 6019. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shin, H.; Kim, J.J. High-Efficiency Orange and Tandem White Organic Light-Emitting Diodes Using Phosphorescent Dyes with Horizontally Oriented Emitting Dipoles. Adv. Mater. 2014, 26, 5864–5868. [Google Scholar] [CrossRef] [PubMed]
- Kuei, C.Y.; Tsai, W.L.; Tong, B.; Jiao, M.; Lee, W.K.; Chi, Y.; Wu, C.-C.; Liu, S.-H.; Lee, G.-H.; Chou, P.-T. Bis-Tridentate Ir(Iii) Complexes with Nearly Unitary RGB Phosphorescence and Organic Light-Emitting Diodes with External Quantum Efficiency Exceeding 31%. Adv. Mater. 2016, 28, 2795–2800. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Alle, R.; Becker, H.; Meerholz, K. On the Origin of the Color Shift in White-Emitting OLEDs. Adv. Mater. 2007, 19, 4460–4465. [Google Scholar] [CrossRef]
- Chen, S.; Qu, Q.; Kong, M.; Zhao, X.; Yu, Z.; Jia, P.; Huang, W. On the Origin of the Shift in Color in White Organic Light-Emitting Diodes. J. Mater. Chem. C 2013, 1, 3508–3524. [Google Scholar] [CrossRef]
- Shao, Y.; Yang, Y. White Organic Light-Emitting Diodes Prepared by a Fused Organic Solid Solution Method. Appl. Phys. Lett. 2005, 86, 073510. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Wang, L.; Yan, X.; Tao, H.; Su, Y.; Gao, D.; Lan, L.; Zou, J.; Peng, J. Investigation and Optimization of Each Organic Layer: A Simple But Effective Approach Towards Achieving High-Efficiency Hybrid White Organic Light-Emitting Diodes. Org. Electron. 2014, 15, 926–936. [Google Scholar] [CrossRef]
- Liu, B.; Wang, L.; Xu, M.; Tao, H.; Xia, X.; Zou, J.; Su, Y.; Gao, D.; Lan, L.; Peng, J. Simultaneous Achievement of Low Efficiency Roll-Off and Stable Color in Highly Efficient Single-Emitting-Layer Phosphorescent White Organic Light-Emitting Diodes. J. Mater. Chem. C 2014, 2, 5870–5877. [Google Scholar] [CrossRef]
- Pan, B.; Wang, B.; Wang, Y.; Xu, P.; Wang, L.; Chen, J.; Ma, D. A Simple Carbazole-N-Benzimidazole Bipolar Host Material for Highly Efficient Blue and Single Layer White Phosphorescent Organic Light-Emitting Diodes. J. Mater. Chem. C 2014, 2, 2466–2469. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Wang, L.; Tao, H.; Su, Y.; Gao, D.; Lan, L.; Zou, J.; Peng, J. Very-High Color Rendering Index Hybrid White Organic Light-Emitting Diodes with Double Emitting Nanolayers. Nano Micro Lett. 2014, 6, 335–339. [Google Scholar] [CrossRef]
- Min, S.P.; Park, H.J.; Kim, O.Y.; Lee, J.Y. High Color Rendering Index in Phosphorescent White Organic Light-Emitting Diodes Using a Yellowish-Green Dopant with Broad Light Emission. Org. Electron. Phys. Mater. Appl. 2013, 14, 1504–1509. [Google Scholar]
- Chang, C.-H.; Chen, C.-C.; Wu, C.-C.; Chang, S.-Y.; Hung, J.-Y.; Chi, Y. High-Color-Rendering Pure-White Phosphorescent Organic Light-Emitting Devices Employing Only Two Complementary Colors. Org. Electron. 2010, 11, 266–272. [Google Scholar] [CrossRef]
- Sasabe, H.; Takamatsu, J.; Motoyama, T.; Watanabe, S.; Wagenblast, G.; Langer, N.; Molt, O.; Fuchs, E.; Lennartz, C.; Kido, J. High-Efficiency Blue and White Organic Light-Emitting Devices Incorporating a Blue Iridium Carbene Complex. Adv. Mater. 2010, 22, 5003–5007. [Google Scholar] [CrossRef] [PubMed]
- D’Andrade, B.W.; Thompson, M.E.; Forrest, S.R. Controlling Exciton Diffusion in Multilayer White Phosphorescent Organic Light Emitting Devices. Adv. Mater. 2002, 14, 147–151. [Google Scholar] [CrossRef]
- Jou, J.H.; Shen, S.M.; Lin, C.R.; Wang, Y.S.; Chou, Y.C.; Chen, S.Z.; Jou, Y.C. Efficient Very-High Color Rendering Index Organic Light-Emitting Diode. Org. Electron. 2011, 12, 865–868. [Google Scholar] [CrossRef]
- Wang, D.; Li, W.L.; Su, Z.S.; Li, T.L.; Chu, B.; Bi, D.F.; Chen, L.L.; Su, W.M.; He, H. Broad Wavelength Modulating and Design of Organic White Diode Based on Lighting by Using Exciplex Emission From Mixed Acceptors. Appl. Phys. Lett. 2006, 89, 233511. [Google Scholar] [CrossRef]
- Yang, H.; Shi, Y.; Zhao, Y.; Meng, Y.; Hu, W.; Hou, J.; Liu, S. High Colour Rendering Index White Organic Light-Emitting Devices with Three Emitting Layers. Displays 2008, 29, 327–332. [Google Scholar] [CrossRef]
- Chang, C.H.; Tien, K.C.; Chen, C.C.; Lin, M.S.; Cheng, H.C.; Liu, S.H.; Wu, C.C.; Hung, J.Y.; Chiu, Y.C.; Chi, Y. Efficient Phosphorescent White Oleds with High Color Rendering Capability. Org. Electron. 2010, 11, 412–418. [Google Scholar] [CrossRef]
- Chen, S.; Tan, G.; Wong, W.Y.; Kwok, H.S. White Organic Light-Emitting Diodes with Evenly Separated Red, Green, and Blue Colors for Efficiency/Color-Rendition Trade-Off Optimization. Adv. Funct. Mater. 2011, 21, 3785–3793. [Google Scholar] [CrossRef]
- Hao, Y.; Meng, W.; Xu, H.; Wang, H.; Liu, X.; Xu, B. White Organic Light-Emitting Diodes Based on a Novel Zn Complex with High CRI Combining Emission From Excitons and Interface-Formed Electroplex. Org. Electron. 2011, 12, 136–142. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zhang, L.; Wen, X.; Yin, Y.; Liu, S.; Xie, W.; Zhao, H.; Tao, S. Ultra-High General and Special Color Rendering Index White Organic Light-Emitting Device Based on a Deep Red Phosphorescent Dye. Org. Electron. 2013, 14, 3201–3205. [Google Scholar] [CrossRef]
- Yu, L.; Liu, J.; Hu, S.; He, R.; Yang, W.; Wu, H.; Peng, J. Red, Green, and Blue Light-Emitting Polyfluorenes Containing a Dibenzothiophene-S, S-Dioxide Unit and Efficient High-Color-Rendering-Index White-Light-Emitting Diodes Made Therefrom. Adv. Funct. Mater. 2013, 23, 4366–4376. [Google Scholar] [CrossRef]
- Chang, Y.C.; Tang, K.C.; Pan, H.A.; Liu, S.H.; Koshevoy, I.O.; Karttunen, A.J.; Hung, W.-Y.; Cheng, M.-H.; Chou, P.-T. Harnessing Fluorescence Versus Phosphorescence Branching Ratio in (Phenyl)n-Bridged (n = 0–5) Bimetallic Au(I) Complexes. J. Phys. Chem. C 2013, 117, 9623–9632. [Google Scholar] [CrossRef]
- You, H.; Ma, D. Efficient White Organic Light-Emitting Diodes Using Europium Complex as the Red Unit. J. Phys. D Appl. Phys. 2018, 41, 155113. [Google Scholar] [CrossRef]
- Yong, J.C.; Yook, K.S.; Lee, J.Y. Cool and Warm Hybrid White Organic Light-Emitting Diode with Blue Delayed Fluorescent Emitter Both as Blue Emitter and Triplet Host. Sci. Rep. 2015, 5, 7859. [Google Scholar]
- Fröbel, M.; Schwab, T.; Kliem, M.; Hofmann, S.; Leo, K.; Gather, M.C. Get It White: Color-Tunable Ac/Dc OLEDs. Light Sci. Appl. 2015, 4, e247. [Google Scholar] [CrossRef] [Green Version]
- Krotkus, S.; Kasemann, D.; Lenk, S.; Leo, K.; Reineke, S. Adjustable White-Light Emission From a Photo-Structured Micro-OLED Array. Light Sci. Appl. 2016, 5, e16121. [Google Scholar] [CrossRef]
- Guan, N.; Dai, X.; Messanvi, A.; Zhang, H.; Yan, J.; Gautier, E.; Bougerol, C.; Julien, F.H.; Durand, C.; Eyery, J.; et al. Flexible White Light Emitting Diodes Based on Nitride Nanowires and Nanophosphors. ACS Photonics 2016, 3, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Xu, M.; Wang, L.; Tao, H.; Su, Y.; Gao, D.; Zou, J.; Lan, L.; Peng, J. Comprehensive Study on the Electron Transport Layer in Blue Flourescent Organic Light-Emitting Diodes. ECS J. Solid State Sci. Technol. 2015, 2, R258–R261. [Google Scholar] [CrossRef]
- Li, X.; Xie, F.; Zhang, S.; Hou, J.; Choy, W.C. MoOx and V2Ox as Hole and Electron Transport Layers Through Functionalized Intercalation in Normal and Inverted Organic Optoelectronic Devices. Light Sci. Appl. 2015, 4, e273. [Google Scholar] [CrossRef] [Green Version]
- Gomez, E.F.; Steckl, A.J. Improved Performance of OLEDs on Cellulose/Epoxy Substrate Using Adenine as a Hole Injection Layer. ACS Photonics 2015, 2, 439–445. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Ma, D.-G.; Sun, H.-D.; Chen, J.-S.; Guo, Q.-X.; Wang, Q.; Zhao, Y.-B. Organic Semiconductor Heterojunctions: Electrode-Independent Charge Injectors for High-Performance Organic Light-Emitting Diodes. Light Sci. Appl. 2016, 5, e16042. [Google Scholar] [CrossRef]
- Marchal, W.; Verboven, I.; Kesters, J.; Moeremans, B.; De, D.C.; Bonneux, G.; Elen, K.; Conings, B.; Maes, W.; Boyen, H.G.; et al. Steering the Properties of MoOx Hole Transporting Layers In OPVs And OLEDs: Interface Morphology Vs. Electronic Structure. Materials 2017, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- VanSlyke, S.A.; Chen, C.H.; Tang, C.W. Organic Electroluminescent Devices with Improved Stability. Appl. Phys. Lett. 1996, 69, 2160–2162. [Google Scholar] [CrossRef]
- Fery, C.; Racine, B.; Vaufrey, D.; Doyeux, H.; Cina, S. Physical Mechanism Responsible for the Stretched Exponential Decay Behavior of Aging Organic Light-emitting Diodes. Appl. Phys. Lett. 2005, 87, 213502. [Google Scholar] [CrossRef]
- Chu, T.Y.; Chen, J.F.; Chen, S.Y.; Chen, C.H. Comparative Study of Single and Multiemissive Layers in Inverted White Organic Light-Emitting Devices. Appl. Phys. Lett. 2006, 89, 113502. [Google Scholar] [CrossRef]
- Yu, J.N.; Zhang, M.Y.; Li, C.; Shang, Y.Z.; Lv, Y.F.; Wei, B.; Huang, W. Fine-Tuning the Thicknesses of Organic Layers to Realize High-Efficiency and Long-Lifetime Blue Organic Light-Emitting Diodes. Chin. Phys. B 2012, 21, 083303. [Google Scholar] [CrossRef]
- Chua, T.-Y.; Chen, J.-F.; Chen, S.-Y.; Chen, C.-J.; Chen, C.H. Highly Efficient and Stable Inverted Bottom-Emission Organic Light Emitting Devices. Appl. Phys. Lett. 2006, 89, 053503. [Google Scholar] [CrossRef]
- Meerheim, R.; Walzer, K.; Pfeiffer, M.; Leo, K. Ultrastable and Efficient Red Organic Light Emitting Diodes with Doped Transport Layers. Appl. Phys. Lett. 2006, 89, 061111. [Google Scholar] [CrossRef]
- Lindla, F.; Boesing, M.; Gemmern, P.V.; Bertram, D.; Keiper, D.; Heuken, M.; Kalisch, H.; Jansen, R.H. Employing Exciton Transfer Molecules to Increase the Lifetime of Phosphorescent Red Organic Light Emitting Diodes. Appl. Phys. Lett. 2011, 98, 173304. [Google Scholar] [CrossRef]
- So, F.; Kondakov, D. Degradation Mechanisms in Small-Molecule and Polymer Organic Light-Emitting Diodes. Adv. Mater. 2010, 22, 3762–3777. [Google Scholar]
- Seifert, R.; Moraes, I.R.D.; Scholz, S.; Gather, M.C.; Lüssem, B.; Leo, K. Chemical Degradation Mechanisms of Highly Efficient Blue Phosphorescent Emitters Used for Organic Light Emitting Diodes. Org. Electron. 2013, 14, 115–123. [Google Scholar] [CrossRef]
- Baldo, M.A.; O’Brien, D.F.; Thompson, M.E.; Forrest, S.R. Excitonic Singlet-Triplet Ratio in a Semiconducting Organic Thin Film. Phys. Rev. B 1999, 60, 14422–14428. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Thompson, M.E.; Forrest, S.R. Nearly 100% Internal Phosphorescence Efficiency in an Organic Light-Emitting Device. J. Appl. Phys. 2001, 90, 5048–5051. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, L.; Chen, J.; Ma, D. Improving Color Stability of Blue/Orange Complementary White OLEDs by Using Single-Host Double-Emissive Layer Structure: Comprehensive Experimental Investigation into the Device Working Mechanism. Org. Electron. 2012, 13, 1340–1348. [Google Scholar] [CrossRef]
- Liu, B.; Xu, M.; Tao, H.; Ying, L.; Zou, J.; Wu, H.; Peng, J. Highly Efficient Red Phosphorescent Organic Light-Emitting Diodes Based on Solution Processed Emissive Layer. J. Lumin. 2013, 142, 35–39. [Google Scholar] [CrossRef]
- Xiang, C.; Koo, W.; So, F.; Sasabe, H.; Kido, J. A Systematic Study on Efficiency Enhancements in Phosphorescent Green, Red and Blue Microcavity Organic Light Emitting Devices. Light Sci. Appl. 2013, 2, e74. [Google Scholar] [CrossRef]
- Fleetham, T.; Huang, L.; Li, J. Tetradentate Platinum Complexes for Efficient and Stable Excimer-Based White OLEDs. Adv. Funct. Mater. 2015, 24, 6066–6073. [Google Scholar] [CrossRef]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly Efficient Organic Light-Emitting Diodes From Delayed Fluorescence. Nature 2012, 492, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, T.; Yasuda, T.; Lee, S.Y.; Kondo, R.; Adachi, C. A six-carbazole-decorated Cyclophosphazene as A Host with High Triplet Energy to Realize Efficient Delayed-Fluorescence OLEDs. Mater. Horiz. 2014, 1, 264–269. [Google Scholar] [CrossRef]
- Zhang, Q.; Tsang, D.; Kuwabara, H.; Hatae, Y.; Li, B.; Takahashi, T.; Lee, S.Y.; Yasuda, T.; Adachi, C. Nearly 100% Internal Quantum Efficiency in Undoped Electroluminescent Devices Employing Pure Organic Emitters. Adv. Mater. 2015, 27, 2096–2100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Cai, M.H.; Zhang, Y.G.; Zhang, D.Q.; Duan, L. Sterically Shielded Blue Thermally Activated Delayed Fluorescence Emitters with Improved Efficiency and Stability. Mater. Horiz. 2016, 3, 145–151. [Google Scholar] [CrossRef]
- Liang, Y.; Bing, Y.; Ma, Y.G. Progress in Next-Generation Organic Electroluminescent Materials: Material Design Beyond Exciton Statistics. Sci. Sin. Chim. 2014, 57, 335–345. [Google Scholar]
- Kondakov, D.Y. Triplet-triplet Annihilation in Highly Efficient Fluorescent Organic Light-emitting Diodes: Current State and Future Outlook. Philos. Trans. R. Soc. A 2015, 373, 20140321. [Google Scholar] [CrossRef] [PubMed]
- Gray, V.; Dreos, A.; Erhart, P.; Albinsson, B.; Mothpoulsen, K.; Abrahamsson, M. Loss Channels in Triplet-Triplet Annihilation Photon Upconversion: Importance of Annihilator Singlet and Triplet Surface Shapes. Phys. Chem. Chem. Phys. 2017, 19, 10931. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Gao, Z.; Tang, X.; He, X.; Gao, Y.; Li, J.; Sun, X.; Liu, Y.; Liu, H.; Yang, B.; et al. Highly Efficient and Stable Pure Blue Nondoped Organic Light-Emitting Diodes at High Luminance Based on Phenanthroimidazole-Pyrene Derivative Enabled by Triplei-Triplet Annihilation. Dyes Pigments 2017, 142, 189–197. [Google Scholar] [CrossRef]
- Zhang, B.; Tan, G.; Lam, C.S.; Yao, B.; Ho, C.L.; Liu, L.; Xie, Z.; Wong, W.Y.; Ding, J.; Wang, L. High-Efficiency Single Emissive Layer White Organic Light-Emitting Diodes Based on Solution-Processed Dendritic Host and New Orange-Emitting Iridium Complex. Adv. Mater. 2012, 24, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wu, H.; Lam, C.S.; Wang, C.; Zhu, J.; Zhong, C.; Hu, S.; Ho, C.L.; Zhou, G.J.; Wu, H.B.; et al. Simultaneous Optimization of Charge-Carrier Balance and Luminous Efficacy in Highly Efficient White Polymer Light-Emitting Devices. Adv. Mater. 2011, 23, 2976–2980. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Liu, J.; Wu, H.; Yang, W.; Peng, J.; Cao, Y. High-Efficiency and Good Color Quality White Light-Emitting Devices Based on Polymer Blend. Org. Electron. 2009, 10, 843–848. [Google Scholar] [CrossRef]
- Wu, H.; Zou, J.; Liu, F.; Wang, L.; Mikhailovsky, A.; Bazan, G.C.; Yang, W.; Cao, Y. Efficient Single Active Layer Electrophosphorescent White Polymer Light-Emitting Diodes. Adv. Mater. 2008, 20, 696–702. [Google Scholar] [CrossRef]
- Aizawa, N.; Pu, Y.J.; Watanabe, M.; Chiba, T.; Ideta, K.; Toyota, N.; Igarashi, M.; Suzuri, Y.; Sasabe, H.; Kido, J. Solution-Processed Multilayer Small-Molecule Light-Emitting Devices with High-Efficiency White-Light Emission. Nat. Commun. 2014, 5, 5756. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhong, Z.; Xue, S.; Zhou, Y.; Meng, Y.; Hu, Z.; Ai, N.; Wang, J.; Wang, L.; Peng, J.; et al. Highly Efficient, Solution Processed Electrofluorescent Small Molecule White Organic Light-Emitting Diodes with a Hybrid Electron Injection Layer. ACS Appl. Mater. Interfaces 2014, 6, 8345–8352. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Duan, L.; Qiao, J.; Zhang, D.; Dong, G.; Wang, L.; Qiu, Y. Efficient Solution-Processed Small-Molecule Single Emitting Layer Electrophosphorescent White Light-Emitting Diodes. Org. Electron. 2010, 11, 1344–1350. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, J.; Ma, D.; Cheng, Y. Highly Efficient Single-Emitting-Layer White Organic Light-Emitting Diodes with Reduced Efficiency Roll-Off. Appl. Phys. Lett. 2009, 94, 103503. [Google Scholar] [CrossRef]
- Yin, Y.; Piao, X.; Li, Y.; Wang, Y. High-Efficiency and Low-Efficiency-Roll-Off Single-Layer White Organic Light-Emitting Devices with a Bipolar Transport Host. Appl. Phys. Lett. 2012, 101, 063306. [Google Scholar] [CrossRef]
- Jou, J.H.; Lin, C.C.; Li, T.H.; Li, C.J.; Peng, S.H.; Yang, F.C.; Thomas, K.R.J.; Kumar, D.; Chi, Y.; Hsu, B.-D. Plant Growth Absorption Spectrum Mimicking Light Sources. Materials 2015, 8, 5265–5275. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Liu, X.; Zhang, X.; Wei, F.; Zhu, W.; Jiang, X.; Zhang, Z.; Xu, S. Top-Emitting Organic Light-Emitting Devices With Cavity Effect. Acta Phys. Sin. 2007, 56, 1088–1092. [Google Scholar]
- Cao, J.; Jiang, X.; Zhang, Z. Research of Tricolor Microcavity Top-Emitting Organic Light-Emitting Devices with White Emitting Layer. Acta Phys. Sin. 2007, 56, 3493–3498. [Google Scholar]
- Dodabalapur, A.; Rothberg, L.J.; Miller, T.M. Color Variation with Electroluminescent Organic Semiconductors in Multimode Resonant Cavities. Appl. Phys. Lett. 1994, 65, 2308–2310. [Google Scholar] [CrossRef]
- Hsu, S.F.; Lee, C.C.; Hwang, S.W.; Chen, C.H. Highly Efficient Top-Emitting White Organic Electroluminescent Devices. Appl. Phys. Lett. 2005, 86, 253508. [Google Scholar] [CrossRef]
- Liu, C.; Liu, S.; Tien, K.; Hsu, M.; Chang, H.; Chang, C.; Yang, C.; Wu, C. Microcavity Top-Emitting Organic Light-Emitting Devices Integrated with Diffusers for Simultaneous Enhancement of Efficiencies and Viewing Characteristics. Appl. Phys. Lett. 2009, 94, 103302. [Google Scholar] [CrossRef]
- Thomschke, M.; Reineke, S.; Lussem, B.; Leo, K. Highly Efficient White Top-Emitting Organic Light-Emitting Diodes Comprising Laminated Microlens Films. Nano Lett. 2012, 12, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Duggal, A.R.; Shiang, J.J.; Heller, C.M.; Foust, D.F. Organic Light-Emitting Devices for Illumination Quality White Light. Appl. Phys. Lett. 2002, 80, 3470–3478. [Google Scholar] [CrossRef]
- Krummacher, B.C.; Choong, V.; Mathai, M.K.; Choulis, S.A.; So, F.; Jermann, F.; Fiedler, T.; Zachau, M. Highly Efficient White Organic Light-Emitting Diode. Appl. Phys. Lett. 2006, 88, 113506. [Google Scholar] [CrossRef]
- Ji, W.; Zhang, L.; Gao, R.; Zhang, L.; Xie, W.; Zhang, H.; Li, B. Top-Emitting White Organic Light-Emitting Devices with Down-Conversion Phosphors: Theory and Experiment. Opt. Express 2008, 16, 15489–15494. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chopra, N.; Bera, D.; Maslow, S.; Zheng, Y.; Holloway, P.; Xue, J.; So, F. Down-Conversion White Organic Light-Emitting Diodes Using Microcavity Structure. Adv. Energy Mater. 2011, 1, 174–178. [Google Scholar] [CrossRef]
- Pu, Y.J.; Chiba, T.; Ideta, K.; Takahashi, S.; Aizawa, N.; Hikichi, T.; Kido, J. Fabrication of Organic Light-Emitting Devices Comprising Stacked Light-Emitting Units by Solution-Based Processes. Adv. Mater. 2015, 27, 1327–1332. [Google Scholar] [CrossRef] [PubMed]
- Yook, K.S.; Jeon, S.O.; Min, S.Y.; Lee, J.Y.; Yang, H.J.; Noh, T.; Kang, S.K.; Lee, T.W. Highly Efficient p-i-n and Tandem Organic Light-Emitting Devices Using an Air-Stable and Low-Temperature-Evaporable Metal Azide as an n-Dopant. Adv. Funct. Mater. 2010, 20, 1797–1802. [Google Scholar] [CrossRef]
- Sun, H.D.; Guo, Q.X.; Yang, D.Z.; Chen, Y.H.; Chen, J.S.; Ma, D.G. High Efficiency Tandem Organic Light Emitting Diode Using an Organic Heterojunction as the Charge Generation Layer: An Investigation into the Charge Generation Model and Device Performance. ACS Photonics 2015, 2, 271–279. [Google Scholar] [CrossRef]
- Lei, D.; Tang, X.; Xu, M.F.; Shi, X.B.; Wang, Z.K.; Liao, L.S. Lithium Hydride Doped Intermediate Connector for High-Efficiency and Long-Term Stable Tandem Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2014, 6, 18228–18232. [Google Scholar]
- Kido, J.; Matsumoto, T.; Nakada, T.; Endo, J.; Mori, K.; Kawamura, N.; Yokoi, A. 27.1: Invited Paper: High Efficiency Organic EL Devices having Charge Generation Layers. Dig. Tech. Pap. Soc. Inf. Disp. Int. Symp. 2003, 34, 964–965. [Google Scholar] [CrossRef]
- Ding, L.; Sun, Y.Q.; Chen, H.; Zu, F.S.; Wang, Z.K.; Liao, L.S. A novel intermediate connector with improved charge generation and separation for large-area tandem white organic lighting devices. J. Mater. Chem. C 2014, 2, 10403–10408. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Huang, S.; Shi, X.; Wu, X.; He, G. A highly efficient, transparent and stable charge generation unit based on a p-doped monolayer. Org. Electron. 2013, 14, 1337–1343. [Google Scholar] [CrossRef]
- Guo, F.; Ma, D. White organic light-emitting diodes based on tandem structures. Appl. Phys. Lett. 2005, 87, 173510. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, J.F.; Hwang, S.W.; Chen, C.H. Highly efficient white organic electroluminescent devices based on tandem architecture. Appl. Phys. Lett. 2005, 87, 253501. [Google Scholar] [CrossRef]
- Son, Y.H.; Kim, Y.J.; Park, M.J.; Oh, H.Y.; Park, J.S.; Yang, J.H.; Suh, M.C.; Kwon, J.H. Small single–triplet energy gap bipolar host materials for phosphorescent blue and white organic light emitting diodes. J. Mater. Chem. C 2013, 1, 5008–5014. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, T.; Ye, K.Q.; Wu, Y.; Liu, Y.; Wang, Y. High-efficiency and high-quality white organic light-emitting diode employing fluorescent emitters. Org. Electron. 2011, 12, 29–33. [Google Scholar] [CrossRef]
- Chuen, C.H.; Tao, Y.T. Highly-bright white organic light-emitting diodes based on a single emission layer. Appl. Phys. Lett. 2002, 81, 4499–4501. [Google Scholar] [CrossRef]
- Kim, N.H.; Kim, Y.-H.; Yoon, J.-A.; Lee, S.Y.; Ryu, D.H.; Wood, R.; Moon, C.-B.; Kim, W.Y. Color optimization of single emissive white OLEDs via energy transfer between RGB fluorescent dopants. J. Lumin. 2013, 143, 723–728. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, D.Q.; Wu, K.W.; Huang, X.Q.; Wang, L.D.; Qiu, Y. Controlling the Recombination Zone of White Organic Light-Emitting Diodes with Extremely Long Lifetimes. Adv. Funct. Mater. 2011, 21, 3540–3545. [Google Scholar] [CrossRef]
- D’Andrade, B.W.; Holmes, R.J.; Forrest, S.R. Efficient Organic Electrophosphorescent White-Light-Emitting Device with a Triple Doped Emissive Layer. Adv. Mater. 2004, 16, 624–628. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, J.; Ma, D.; Cheng, Y.; Wang, L.; Jing, X.; Wang, F. Harvesting Excitons Via Two Parallel Channels for Efficient White Organic LEDs with Nearly 100% Internal Quantum Efficiency: Fabrication and EmissionMechanism Analysis. Adv. Funct. Mater. 2010, 19, 84–95. [Google Scholar] [CrossRef]
- Wang, Q.; Ding, J.Q.; Zhang, Z.Q.; Ma, D.G.; Cheng, Y.X.; Wang, L.X.; Wang, F.S. A high-performance tandem white organic light-emitting diode combining highly effective white-units and their interconnection layer. J. Appl. Phys. 2009, 105, 076101. [Google Scholar] [CrossRef]
- Huang, W.Y.; Chen, Z.W.; You, H.W.; Fan, F.C.; Chen, H.F.; Wong, K.T. Efficient carrier- and exciton-confining device structure that enhances blue PhOLED efficiency and reduces efficiency roll-off. Org. Electron. 2011, 12, 575–581. [Google Scholar] [CrossRef]
- Zhao, X.H.; Zhang, Z.S.; Qian, Y.; Yi, M.D.; Xie, L.H.; Hu, C.P.; Xie, G.H.; Xu, H.; Han, C.M.; Zhao, Y.; et al. A bulky pyridinylfluorene-fuctionalizing approach to synthesize diarylfluorene-based bipolar host materials for efficient red, green, blue and white electrophosphorescent devices. J. Mater. Chem. C 2013, 1, 3482–3490. [Google Scholar] [CrossRef]
- Gong, S.; Chen, Y.; Luo, J.; Yang, C.; Zhong, C.; Qin, J.; Ma, D. Bipolar Tetraarylsilanes as Universal Hosts for Blue, Green, Orange, and White Electrophosphorescence with High Efficiency and Low Efficiency Roll-Off. Adv. Funct. Mater. 2011, 21, 1168–1178. [Google Scholar] [CrossRef]
- Huang, H.; Yang, X.; Wang, Y.; Pan, B.; Wang, L.; Chen, J.; Ma, D.; Yang, C. Optimizing the conjugation between N,N′-dicarbazolyl-3,5-benzene and triphenylphosphine oxide as bipolar hybrids for highly efficient blue and single emissive layer white phosphorescent OLEDs. Org. Electron. 2013, 14, 2573–2581. [Google Scholar] [CrossRef]
- Liu, M.-S.; Yang, S.-J.; Chang, H.-W.; Huang, Y.-H.; Tsai, Y.-T.; Wu, C.-C.; Chou, S.-H.; Mondal, E.; Wong, K.-T. Incorporation of a CN group into mCP: A new bipolar host material for highly efficient blue and white electrophosphorescent devices. J. Mater. Chem. 2012, 22, 16114–16120. [Google Scholar]
- Liu, B.Q.; Wang, L.; Gao, D.Y.; Xu, M.; Zhu, X.H.; Zou, J.H.; Lan, L.F.; Ning, H.L.; Peng, J.B.; Cao, Y. Harnessing charge and exciton distribution towards extremely high performance: The critical role of guests in single-emitting-layer white OLEDs. Mater. Horiz. 2015, 2, 536–544. [Google Scholar] [CrossRef]
- Su, S.-J.; Gonmori, E.; Sasabe, H.; Kido, J. Highly efficient organic blue- and white-light-emitting devices having a carrier- and exciton-confining structure for reduced efficiency roll-off. Adv. Mater. 2008, 20, 4189–4194. [Google Scholar] [CrossRef]
- Reineke, S.; Lindner, F.; Schwartz, G.; Seidler, N.; Walzer, K.; Lüssem, B.; Leo, K. White organic light-emitting diodes with fluorescent tube efficiency. Nature 2009, 459, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, J.; Ma, D.; Cheng, Y.; Wang, L.; Wang, F. Manipulating Charges and Excitons within a Single-Host System to Accomplish Efficiency/CRI/Color-Stability Trade-off for High-Performance OWLEDs. Adv. Mater. 2009, 21, 2397–2401. [Google Scholar] [CrossRef]
- Chang, Y.L.; Song, Y.; Wang, Z.; Helander, M.G.; Qiu, J.; Chai, L.; Liu, Z.; Scholes, G.D.; Lu, Z. Highly Efficient Warm White Organic Light-Emitting Diodes by Triplet Exciton Conversion. Adv. Funct. Mater. 2013, 23, 705–712. [Google Scholar] [CrossRef]
- Bulovic, V.; Khalfin, V.B.; Gu, G.; Burrows, P.E.; Garbuzov, D.Z.; Forrest, S.R. Weak microcavity effects in organic light-emitting devices. Phys. Rev. B 1998, 58, 3730–3740. [Google Scholar] [CrossRef]
- Chutinan, A.; Ishihara, K.; Asano, T.; Fujita, M.; Noda, S. Theoretical analysis on light-extraction efficiency of organic light-emitting diodes using FDTD and mode-expansion methods. Org. Electron. 2005, 6, 3–9. [Google Scholar] [CrossRef]
- Kim, S.Y.; Jeong, W.I.; Mayr, C.; Park, Y.S.; Kim, K.H.; Lee, J.H.; Moon, C.K.; Brütting, W.; Kim, J.J. Organic Light-Emitting Diodes with 30% External Quantum Efficiency Based on a Horizontally Oriented Emitter. Adv. Funct. Mater. 2013, 23, 3896–3900. [Google Scholar] [CrossRef]
- Möller, S.; Forrest, S. Improved light out-coupling in organic light emitting diodes employing ordered microlens arrays. J. Appl. Phys. 2002, 91, 3324–3327. [Google Scholar] [CrossRef]
- Yang, J.P.; Bao, Q.Y.; Xu, Z.Q.; Li, Y.Q.; Tang, J.X.; Shen, S. Light out-coupling enhancement of organic light-emitting devices with microlens array. Appl. Phys. Lett. 2010, 97, 223303. [Google Scholar] [CrossRef]
- Lim, J.; Oh, S.S.; Kim, D.Y.; Cho, S.H.; Kim, I.T.; Han, S.H.; Takezoe, H.; Choi, E.H.; Cho, G.S.; Seo, Y.H.; et al. Enhanced out-coupling factor of microcavity organic light-emitting devices with irregularmicrolens array. Opt. Express 2006, 14, 6564–6571. [Google Scholar] [CrossRef] [PubMed]
- Do, Y.; Kim, Y.C.; Song, Y.W.; Cho, C.O.; Jeon, H.; Lee, Y.J.; Kim, S.H.; Lee, Y.H. Enhanced light extraction from organic light-emitting diodes with 2D SiO2/SiNx photonic crystals. Adv. Mater. 2003, 15, 1214–1218. [Google Scholar] [CrossRef]
- Matioli, E.; Brinkley, S.; Kelchner, K.M.; Hu, Y.-L.; Nakamura, S.; DenBaars, S.; Speck, J.; Weisbuch, C. High-brightness polarized light-emitting diodes. Light Sci. Appl. 2012, 1, 479–482. [Google Scholar] [CrossRef]
- Ziebarth, J.M.; Saafir, A.K.; Fan, S.; McGehee, M.D. Extracting Light from Polymer Light-Emitting Diodes Using Stamped Bragg Gratings. Adv. Funct. Mater. 2004, 14, 451–456. [Google Scholar] [CrossRef]
- Bi, Y.G.; Feng, J.; Li, Y.F.; Zhang, X.L.; Liu, Y.F.; Jin, Y.; Sun, H.B. Broadband light extraction from white organic light-emitting devices by employing corrugated metallic electrodes with dual periodicity. Adv. Mater. 2013, 25, 6969–6974. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.S.; Li, B.; Huang, S.P.; Nomura, H.; Tanaka1, H.; Adachi, C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photonics 2014, 8, 326–332. [Google Scholar] [CrossRef]
- Wang, H.; Meng, L.Q.; Shen, X.X.; Wei, X.F.; Zheng, X.L.; Lv, X.P.; Yi, Y.P.; Wang, Y.; Wang, P.F. Light-Emitting Diodes: Highly Efficient Orange and Red Phosphorescent Organic Light-Emitting Diodes with Low Roll-Off of Efficiency using a Novel Thermally Activated Delayed Fluorescence Material as Host. Adv. Mater. 2015, 27, 4041–4047. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Lee, J.Y. Engineering of Mixed Host for High External Quantum Efficiency above 25% in Green Thermally Activated Delayed Fluorescence Device. Adv. Funct. Mater. 2015, 24, 3970–3977. [Google Scholar] [CrossRef]
- Rajamalli, P.; Senthilkumar, N.; Gandeepan, P.; Huang, P.-Y.; Huang, M.-J.; Yang, C.-Y.; Chiu, M.-J.; Chu, L.-K.; Lin, H.-W.; Cheng, C.-H. A New Molecular Design Based on Thermally Activated Delayed Fluorescence for Highly Efficient Organic Light Emitting Diodes. J. Am. Chem. Soc. 2016, 138, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Lampande, R.; Im, J.B.; Lee, J.M.; Lee, J.Y.; Kwon, J.H. Controlling the exciton lifetime of blue thermally activated delayed fluorescence emitters using a heteroatom-containing pyridoindole donor moiety. Mater. Horiz. 2017, 4, 619–624. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Mao, Z.; Xie, Z.L.; Zhang, Y.; Liu, S.W.; Zhao, J.; Xu, J.R.; Chi, Z.G.; Aldred, M.P. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Thermally Activated Delayed Fluorescence Materials Towards the Breakthrough of Organoelectronics. Adv. Mater. 2014, 26, 7931–7958. [Google Scholar] [CrossRef] [PubMed]
- Masui, K.; Nakanotani, H.; Adachi, C. Analysis of exciton annihilation in high-efficiency sky-blue organic light-emitting diodes with thermally activated delayed fluorescence. Org. Electron. 2013, 14, 2721–2726. [Google Scholar] [CrossRef]
- Guo, J.J.; Li, X.L.; Nie, H.; Luo, W.W.; Gan, S.F.; Hu, S.M.; Hu, R.R.; Qin, A.J.; Zhao, Z.J.; Su, S.J.; et al. Achieving High-Performance Nondoped OLEDs with Extremely Small Efficiency Roll-Off by Combining Aggregation-Induced Emission and Thermally Activated Delayed Fluorescence. Adv. Funct. Mater. 2017, 27, 1606458. [Google Scholar] [CrossRef]
- Nishide, J.-I.; Nakanotani, H.; Hiraga, Y.; Adachi, C. High-efficiency white organic light-emitting diodes using thermally activated delayed fluorescence. Appl. Phys. Lett. 2014, 104, 233304. [Google Scholar] [CrossRef]
- Zhang, D.; Duan, L.; Li, C.; Li, Y.; Li, H.; Zhang, D.; Qiu, Y. High-Efficiency Fluorescent Organic Light-Emitting Devices Using Sensitizing Hosts with a Small Singlet-Triplet Exchange Energy. Adv. Mater. 2014, 26, 5050–5055. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Nakanotani, H.; Adachi, C. High-Efficiency White Organic Light-Emitting Diodes Based on a Blue Thermally Activated Delayed Fluorescent Emitter Combined with Green and Red Fluorescent Emitters. Adv. Mater. 2015, 27, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Zhang, T.Y.; Li, W.L.; Su, Z.S.; Chu, B.; Yan, X.W.; Jin, F.M.; Gao, Y.; Wu, H. Organic Electronics Highly efficient and color stable single-emitting-layer fluorescent WOLEDs with delayed fluorescent host. Org. Electron. 2015, 23, 208–212. [Google Scholar] [CrossRef]
- Li, X.L.; Xie, G.Z.; Liu, M.; Chen, D.C.; Cai, X.Y.; Peng, J.B.; Cao, Y.; Su, S.J. High-Efficiency WOLEDs with High Color-Rendering Index based on a Chromaticity-Adjustable Yellow Thermally Activated Delayed Fluorescence Emitter. Adv. Mater. 2016, 28, 4614–4619. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Cheng, G.; Zhao, Y.; Feng, J.; Liu, S.Y.; Zhang, M.; Ma, Y.G.; Shen, J.C. White-electrophosphorescence devices based on rhenium complexes. Appl. Phys. Lett. 2003, 83, 4716–4718. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Q.; Zhao, Y.B.; Yang, D.Z.; Zhao, F.C.; Chen, J.S.; Ma, D.G. A hybrid white organic light-emitting diode with above 20% external quantum efficiency and extremely low efficiency roll-off. J. Mater. Chem. C 2014, 2, 7494–7504. [Google Scholar] [CrossRef]
- Sun, N.; Zhao, Y.B.; Zhao, F.C.; Chen, Y.H.; Yang, D.Z.; Chen, J.S.; Ma, D.G. A white organic light-emitting diode with ultra-high color rendering index, high efficiency, and extremely low efficiency roll-off. Appl. Phys. Lett. 2014, 105, 013303. [Google Scholar] [CrossRef]
- Li, X.L.; Ouyang, X.H.; Chen, D.C.; Cai, X.Y.; Liu, M.; Ge, Z.Y.; Cao, Y.; Su, S.J. Highly efficient blue and warm white organic light-emitting diodes with a simplified structure. Nanotechnology 2016, 27, 124001. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Ouyang, X.H.; Liu, M.; Ge, Z.Y.; Peng, J.B.; Cao, Y.; Su, S.J. Highly efficient single-and multi-emission-layer fluorescent/phosphorescent hybrid white organic light-emitting diodes with ∼20% external quantum efficiency. J. Mater. Chem. C 2015, 3, 9233–9239. [Google Scholar] [CrossRef]
- Ouyang, X.H.; Li, X.L.; Bai, Y.Q.; Mi, D.B.; Ge, Z.Y.; Su, S.J. Highly-efficient hybrid white organic light-emitting diodes based on a high radiative exciton ratio deepblue emitter with improved concentration of phosphorescent dopant. RSC Adv. 2015, 5, 32298–32306. [Google Scholar] [CrossRef]
- Liu, B.Q.; Tao, H.; Su, Y.J.; Gao, D.Y.; Lan, L.F.; Zou, J.H.; Peng, J.B. Color-stable, reduced efficiency roll-off hybrid white organic light emitting diodes with ultra high brightness. Chin. Phys. B 2013, 22, 077303. [Google Scholar] [CrossRef]
- Liu, B.Q.; Xu, M.; Wang, L.; Su, Y.J.; Gao, D.Y.; Tao, H.; Lan, L.F.; Zou, J.H.; Peng, J.B. High-Performance Hybrid White Organic Light-Emitting Diodes Comprising Ultrathin Blue and Orange Emissive Layers. Appl. Phys. Express 2013, 6, 122101. [Google Scholar] [CrossRef]
- Wang, L.; Lei, G.T.; Yi, X.H. White Organic Light Emitting Diodes Based on Combination of Fluorescence and Phosphorescence. Prog. Chem. 2008, 20, 1050–1056. [Google Scholar]
- Hofmann, S.; Furno, M.; Lussem, B.; Leo, K.; Gather, M.C. Investigation of triplet harvesting and outcoupling efficiency in highly efficient two-color hybrid white organic light-emitting diodes. Phys. Status Solidi A 2013, 210, 1467–1475. [Google Scholar] [CrossRef]
- Zhou, G.J.; Yang, X.L.; Wong, W.-Y.; Wang, Q.; Suo, S.; Ma, D.G.; Feng, J.K.; Wang, L.X. A Robust Yellow-Emitting Metallophosphor with Electron-Injection/-Transporting Traits for Highly Efficient White Organic Light-Emitting Diodes. ChemPhysChem 2011, 12, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Zou, J.H.; Zhou, Z.W.; Wang, L.; Xu, M.; Tao, H.; Gao, D.Y.; Lan, L.Y.; Ning, H.L.; Peng, J.B. Efficient single-emitting layer hybrid white organic light-emitting diodes with low efficiency roll-off, stable color and extremely high luminance. J. Ind. Eng. Chem. 2015, 30, 85–91. [Google Scholar] [CrossRef]
- Liu, B.Q.; Xu, M.; Tao, H.; Su, Y.J.; Gao, D.Y.; Zou, J.H.; Lan, L.F.; Peng, J.B. The effect of spacer in hybrid white organic light emitting diodes. Chin. Sci. Bull. 2014, 59, 3090–3097. [Google Scholar] [CrossRef]
- Liu, B.Q.; Xu, M.; Wang, L.; Tao, H.; Su, Y.J.; Gao, D.Y.; Lan, L.F.; Zou, J.H.; Peng, J.B. Simplified hybrid white organic light-emitting diodes with efficiency/efficiency roll-off/color rendering index/color-stability trade-off. Phys. Status Solidi RRL 2014, 8, 719–723. [Google Scholar] [CrossRef]
- Schwartz, G.; Reineke, S.; Rosenow, T.C.; Walzer, K.; Leo, K. Triplet Harvesting in Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2009, 19, 1319–1333. [Google Scholar] [CrossRef]
- Poloek, A.; Chen, C.-T.; Chen, C.-T. High performance hybrid white and multi-colour electroluminescence from a new host material for a heteroleptic naphthyridinolate platinum complex dopant. J. Mater. Chem. C 2014, 2, 1376–1380. [Google Scholar] [CrossRef]
- Poloek, A.; Lin, C.-W.; Chen, C.-T.; Chen, C.-T. High colour rendering index and colour stable hybrid white efficient OLEDs with a double emitting layer structure using a single phosphorescence dopant of heteroleptic platinum complexes. J. Mater. Chem. C 2014, 2, 10343–10356. [Google Scholar] [CrossRef]
- Liu, B.Q.; Xu, M.; Wang, L.; Zou, J.H.; Tao, H.; Su, Y.J.; Gao, D.Y.; Ning, H.L.; Lan, L.F.; Peng, J.B. Regulating charges and excitons in simplified hybrid white organic light-emitting diodes: The key role of concentration in single dopant host-guest systems. Org. Electron. 2014, 15, 2616–2623. [Google Scholar] [CrossRef]
- Chen, P.; Xie, W.F.; Li, K.; Guan, T.; Duan, Y.; Zhao, Y.; Liu, S.Y.; Ma, C.S.; Zhang, L.Y.; Li, B. White organic light-emitting devices with a bipolar transport layer between blue fluorescent and orange phosphorescent emitting layers. Appl. Phys. Lett. 2007, 91, 023505. [Google Scholar] [CrossRef]
- Zhang, L.J.; Hua, Y.L.; Wu, X.M.; Wang, Y.; Yin, S.G. White organic light-emitting device with both phosphorescent and fluorescent emissive layers. Chin. Phys. B 2008, 17, 3097–3102. [Google Scholar]
- Ho, C.L.; Wong, W.Y.; Wang, Q.; Ma, D.G.; Wang, L.X.; Lin, Z.Y. Multifunctional Iridium-Carbazolyl Orange Phosphor for HighPerformance Two-Element WOLED Exploiting Exciton-Managed Fluorescence/Phosphorescence. Adv. Funct. Mater. 2008, 18, 928–937. [Google Scholar] [CrossRef]
- Peng, Q.M.; Chen, P.; Sun, J.X.; Li, F. Magnetic field effects on electroluminescence emanated simultaneously from blue fluorescent and red phosphorescent emissive layers of an organic light-emitting diode. Org. Electron. 2012, 13, 3040–3044. [Google Scholar] [CrossRef]
- Liu, B.Q.; Zou, J.H.; Su, Y.J.; Gao, D.Y.; Lan, L.F.; Tao, H.; Peng, J.B. Hybrid white organic light emitting diodes with low efficiency roll-off, stable color and extreme brightness. J. Lumin. 2014, 151, 161–164. [Google Scholar] [CrossRef]
- Sun, Y.R.; Giebink, N.C.; Kanno, H.; Ma, B.W.; Thompson, M.E.; Forrest, S.R. Management of Singlet and Triplet Excitons for Efficient White Organic Light-emitting Devices. Nature 2006, 440, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.C.; Zhang, Z.Q.; Liu, Y.P.; Dai, Y.F.; Chen, J.S.; Ma, D.G. A hybrid white organic light-emitting diode with stable color and reduced efficiency roll-off by using a bipolar charge carrier switch. Org. Electron. 2012, 13, 1049–1055. [Google Scholar] [CrossRef]
- Zhao, F.C.; Chen, Y.H.; Wang, Q.; Ma, D.G. Studies of fluorescence/phosphorescence hybrid white organic light-emitting diodes. Sci. Sin. Chim. 2013, 43, 398–406. [Google Scholar] [CrossRef]
- Schwartz, G.; Ke, T.-H.; Wu, C.-C.; Walzer, K.; Leo, K. Balanced ambipolar charge carrier mobility in mixed layers for application in hybrid white organic light-emitting diodes. Appl. Phys. Lett. 2008, 93, 073304. [Google Scholar] [CrossRef]
- Schwartz, G.; Reineke, S.; Walzer, K.; Leo, K. Reduced efficiency roll-off in high-efficiency hybrid white organic light-emitting diodes. Appl. Phys. Lett. 2008, 92, 053311. [Google Scholar] [CrossRef]
- Wang, Y.; Hua, Y.L.; Wu, X.M.; Zhang, L.J.; Hou, Q.C.; Zhang, N.; Ma, L.; Cheng, X.M.; Yin, S.G. Application of mixed interface in white-electrophosphorescent devices: An efficient approach to adjust the distributions of carriers. Appl. Phys. Lett. 2008, 93, 113302. [Google Scholar] [CrossRef]
- Yook, K.S.; Jeon, S.O.; Joo, C.W.; Lee, J.Y. Color stability and suppressed efficiency roll-off in white organic light-emitting diodes through management of interlayer and host properties. J. Ind. Eng. Chem. 2009, 15, 420–422. [Google Scholar] [CrossRef]
- Liu, B.Q.; Wang, L.; Zou, J.H.; Tao, H.; Su, Y.J.; Gao, D.Y.; Xu, M.; Lan, L.F.; Peng, J.B. Investigation on spacers and structures: A simple but effective approach toward high-performance hybrid white organic light emitting diodes. Synth. Met. 2013, 184, 5–9. [Google Scholar] [CrossRef]
- Schwartz, G.; Fehse, K.; Pfeiffer, M.; Walzer, K.; Leo, K. Highly efficient white organic light emitting diodes comprising an interlayer to separate fluorescent and phosphorescent regions. Appl. Phys. Lett. 2006, 89, 083509. [Google Scholar] [CrossRef]
- Liu, B.Q.; Nie, H.; Zhou, X.B.; Hu, S.B.; Luo, D.X.; Gao, D.Y.; Zou, J.H.; Xu, M.; Wang, L.; Zhao, Z.J.; et al. Manipulation of Charge and Exciton Distribution Based on Blue Aggregation-Induced Emission Fluorophors: A Novel Concept to Achieve High-Performance Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 776–783. [Google Scholar] [CrossRef]
- Liu, B.Q.; Xu, Z.P.; Zou, J.H.; Tao, H.; Xu, M.; Gao, D.Y.; Lan, L.F.; Wang, L.; Ning, H.L.; Peng, J.B. High-performance hybrid white organic light-emitting diodes employing p-type interlayers. J. Ind. Eng. Chem. 2015, 27, 240–244. [Google Scholar] [CrossRef]
- Ho, C.L.; Lin, M.F.; Wong, W.Y.; Wong, W.-K.; Chen, C.H. High-efficiency and color-stable white organic light-emitting devices based on sky blue electrofluorescence and orange electrophosphorescence. Appl. Phys. Lett. 2008, 92, 083301. [Google Scholar] [CrossRef]
- Xia, Z.Y.; Su, J.H.; Chang, C.S.; Chen, C.H. Hybrid white organic light-emitting devices based on phosphorescent iridium–benzotriazole orange–red and fluorescent blue emitters. J. Lumin. 2013, 135, 323–326. [Google Scholar] [CrossRef]
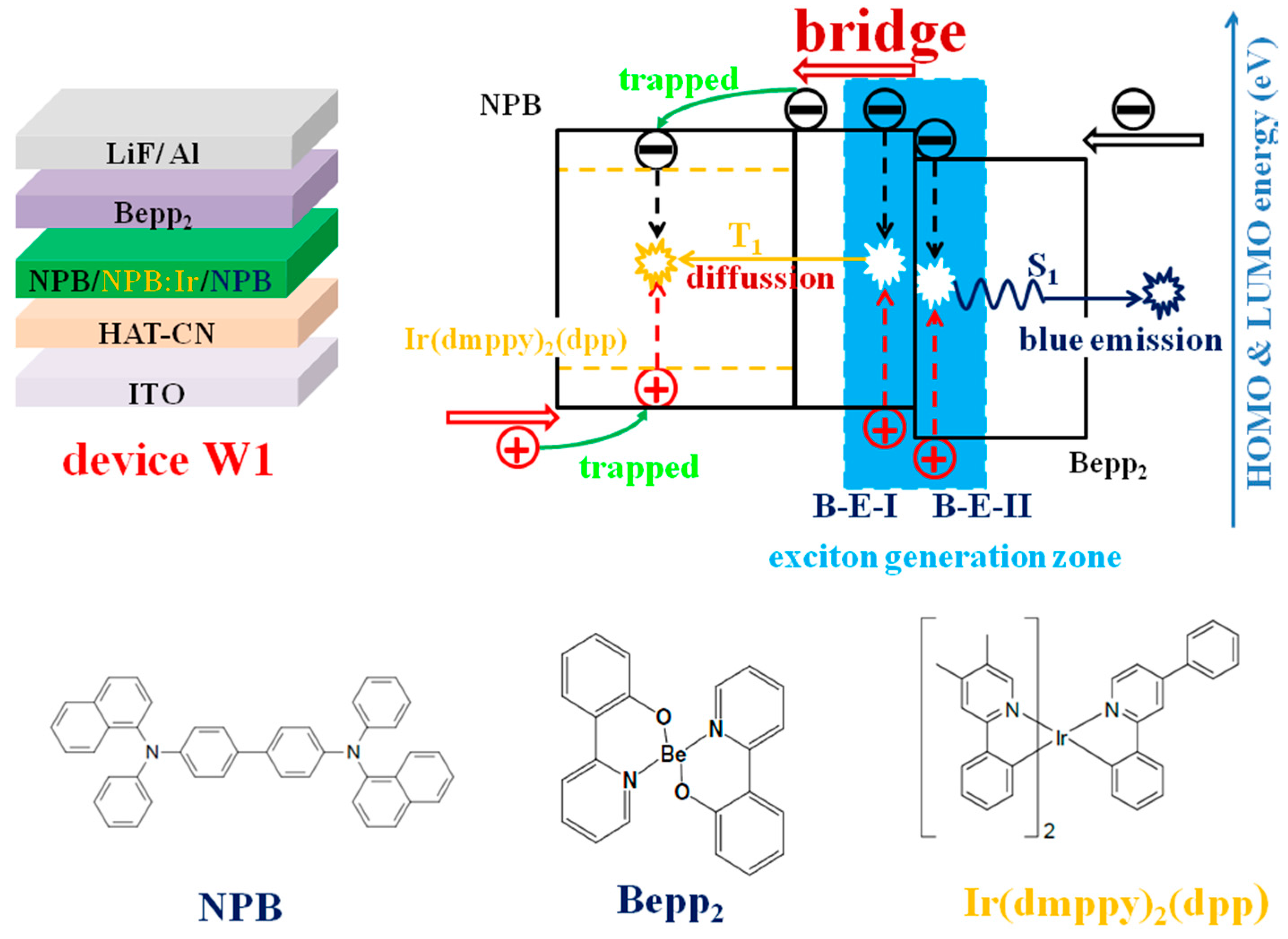
- Luo, D.X.; Yang, Y.F.; Xiao, Y.; Zhao, Y.; Yang, Y.B.; Liu, B.Q. Regulating Charge and Exciton Distribution in High-Performance Hybrid White Organic Light-Emitting Diodes with n-Type Interlayer Switch. Nano Micro Lett. 2017, 9, 37. [Google Scholar] [CrossRef]
- Liu, B.Q.; Wang, L.; Xu, M.; Tao, H.; Zou, J.H.; Gao, D.Y.; Lan, L.F.; Ning, H.L.; Peng, J.B.; Cao, Y. Efficient Hybrid White Organic Light-emitting Diodes with Extremely Long Lifetime: The Effect of n-type Interlayer. Sci. Rep. 2014, 4, 7198. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.V.; Su, Y.K.; Huang, T.S.; Chen, Y.-C. White Organic Light Emitting Devices Based on Multiple Emissive Nanolayers. Nano Micro Lett. 2010, 2, 242–246. [Google Scholar]
- Du, X.Y.; Tao, S.L.; Huang, Y.; Yang, X.X.; Ding, X.L.; Zhang, X.H. Efficient fluorescence/phosphorescence white organic light-emitting diodes with ultra high color stability and mild efficiency roll-off. Appl. Phys. Lett. 2015, 107, 183304. [Google Scholar] [CrossRef]
- Sun, N.; Wang, Q.; Zhao, Y.B.; Chen, Y.H.; Yang, D.Z.; Zhao, F.C.; Chen, J.S.; Ma, D.G. High-Performance Hybrid White Organic Light-Emitting Devices without Interlayer between Fluorescent and Phosphorescent Emissive Regions. Adv. Mater. 2014, 26, 1617–1621. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Tao, S.L.; Chen, M.M.; Mo, H.W.; Ng, T.W.; Liu, X.K.; Zhang, X.H.; Zhao, W.M.; Lee, C.-S. A new multifunctional fluorenyl carbazole hybrid for high performance deep blue fluorescence, orange phosphorescent host and fluorescence/phosphorescence white OLEDs. Dyes Pigments 2013, 97, 273–277. [Google Scholar] [CrossRef]
- Yu, J.; Yin, Y.M.; Liu, W.B.; Zhang, W.; Zhang, L.T.; Xie, W.F.; Zhao, H.Y. Effect of the greenish-yellow emission on the color rendering index of white organic light-emitting devices. Org. Electron. 2014, 15, 2817–2821. [Google Scholar] [CrossRef]
- Liu, W.; Chen, Z.; Zheng, C.J.; Liu, X.K.; Wang, K.; Li, F.; Dong, Y.P.; Ou, X.M.; Zhang, X.H. A novel nicotinonitrile derivative as an excellent multifunctional blue fluorophore for highly efficient hybrid white organic light-emitting devices. J. Mater. Chem. C 2015, 3, 8817–8823. [Google Scholar] [CrossRef]
- Schwartz, G.; Pfeiffer, M.; Reineke, S.; Walzer, K.; Leo, K. Harvesting Triplet Excitons from Fluorescent Blue Emitters in White Organic Light-Emitting Diodes. Adv. Mater. 2007, 19, 3672–3676. [Google Scholar] [CrossRef]
- Liu, B.Q.; Wang, L.; Gao, D.Y.; Zou, J.H.; Ning, H.L.; Peng, J.B.; Cao, Y. Extremely high-efficiency and ultrasimplified hybrid white organic light-emitting diodes exploiting double multifunctional blue emitting layers. Light Sci. Appl. 2016, 5, e16137. [Google Scholar] [CrossRef]
- Peng, T.; Yang, Y.; Bi, H.; Liu, Y.; Hou, Z.; Wang, Y. Highly efficient white organic electroluminescence device based on a phosphorescent orange material doped in a blue host emitter. J. Mater. Chem. 2011, 21, 3551–3553. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, F.; Zhao, Y.; Chen, J.; Ma, D. Ultra-simple hybrid white organic light-emitting diodes with high efficiency and CRI trade-off: Fabrication and emission-mechanism analysis. Org. Electron. 2012, 13, 2807–2815. [Google Scholar] [CrossRef]
- Zheng, C.-J.; Wang, J.; Ye, J.; Lo, M.-F.; Liu, X.-K.; Fung, M.-K.; Zhang, X.-H.; Lee, C.-S. Novel Efficient Blue Fluorophors with Small Singlet-Triplet Splitting: Hosts for Highly Efficient Fluorescence and Phosphorescence Hybrid WOLEDs with Simplified Structure. Adv. Mater. 2013, 25, 2205–2211. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Luo, D.X.; Zou, J.H.; Gao, D.Y.; Ning, H.L.; Wang, L.; Peng, J.B.; Cao, Y. A host–guest system comprising high guest concentration to achieve simplified and high-performance hybrid white organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 6359–6366. [Google Scholar] [CrossRef]
- Ye, J.; Zheng, C.-J.; Ou, X.-M.; Zhang, X.-H.; Fung, M.-K.; Lee, C.-S. Management of Singlet and Triplet Excitons in a Single Emission Layer: A Simple Approach for a High-Efficiency Fluorescence/Phosphorescence Hybrid White Organic Light-Emitting Device. Adv. Mater. 2012, 24, 3410–3414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.D.; Duan, L.; Zhang, Y.G.; Cai, M.H.; Zhang, D.Q.; Qiu, Y. Highly efficient hybrid warm white organic light-emitting diodes using a blue thermally activated delayed fluorescence emitter: Exploiting the external heavy-atom effect. Light Sci. Appl. 2015, 4, e232. [Google Scholar] [CrossRef]
- Wu, Z.B.; Luo, J.J.; Sun, N.; Zhu, L.P.; Sun, H.D.; Yu, L.; Yang, D.Z.; Qiao, X.F.; Chen, J.S.; Yang, C.L.; et al. HighPerformance Hybrid White Organic Light-Emitting Diodes with Superior Efficiency/Color Rendering Index/Color Stability and Low Efficiency Roll-Off Based on a Blue Thermally Activated Delayed Fluorescent Emitter. Adv. Funct. Mater. 2016, 26, 3306–3313. [Google Scholar] [CrossRef]
- Luo, D.X.; Yang, Y.B.; Huang, L.; Liu, B.Q.; Zhao, Y. High-performance hybrid white organic light-emitting diodes exploiting blue thermally activated delayed fluorescent dyes. Dyes Pigments 2017, 147, 83–89. [Google Scholar] [CrossRef]
- Zhang, D.D.; Duan, L.; Li, Y.L.; Zhang, D.Q.; Qiu, Y. Highly efficient and color-stable hybrid warm white organic light-emitting diodes using a blue material with thermally activated delayed fluorescence. J. Mater. Chem. C 2014, 2, 8191–8197. [Google Scholar] [CrossRef]
- Zhang, D.D.; Zhang, D.Q.; Duan, L. Exploiting p-Type Delayed Fluorescence in Hybrid White OLEDs: Breaking the Trade-off between High Device Efficiency and Long Lifetime. ACS Appl. Mater. Interfaces 2016, 8, 23197–23203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Oswald, I.W.H.; Yang, X.L.; Zhou, G.J.; Jia, H.P.; Qiao, Q.Q.; Chen, Y.H.; Jason, H.-H.; Gnade, B.E. A Non-Doped Phosphorescent Organic Light-Emitting Device with Above 31% External Quantum Efficiency. Adv. Mater. 2014, 26, 8107–8113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Oswald, I.W.H.; Perez, M.R.; Jia, H.P.; Gnade, B.E.; Omary, M.A. Exciton and Polaron Quenching in Doping-Free Phosphorescent Organic Light-Emitting Diodes from a Pt(II)-Based Fast Phosphor. Adv. Funct. Mater. 2013, 23, 5420–5428. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Chen, J.S.; Ma, D.G. Ultrathin Nondoped Emissive Layers for Efficient and Simple Monochrome and White Organic Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2013, 5, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.X.; Xiao, Y.; Hao, M.M.; Zhao, Y.; Yang, Y.B.; Gao, Y.; Liu, B.Q. Doping-free white organic light-emitting diodes without blue molecular emitter: An unexplored approach to achieve high performance via exciplex emission. Appl. Phys. Lett. 2017, 110, 061105. [Google Scholar] [CrossRef]
- Liu, B.; Nie, H.; Lin, G.; Hu, S.; Gao, D.; Zou, J.; Xu, M.; Wang, L.; Zhao, Z.; Ning, H.; et al. High-Performance Doping-Free Hybrid White OLEDs Based on Blue Aggregation-Induced Emission Luminogens. ACS Appl. Mater. Interfaces 2017, 9, 34162–34171. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, L.; Tao, H.; Xu, M.; Zou, J.; Ning, H.; Peng, J.; Cao, Y. Doping-Free Tandem White Organic Light-Emitting Diodes. Sci. Bull. 2017, 62, 1193–1200. [Google Scholar] [CrossRef]
- Xue, K.W.; Sheng, R.; Duan, Y.; Chen, P.; Chen, B.Y.; Wang, X.; Duan, Y.H.; Zhao, Y. Efficient non-doped monochrome and white phosphorescent organic light-emitting diodes based on ultrathin emissive layers. Org. Electron. 2015, 26, 451–457. [Google Scholar] [CrossRef]
- Xue, K.W.; Han, G.G.; Duan, Y.; Chen, P.; Yang, Y.Q.; Yang, D.; Duan, Y.H.; Wang, X.; Zhao, Y. Doping-free orange and white phosphorescent organic light-emitting diodes with ultra-simply structure and excellent color stability. Org. Electron. 2015, 18, 84–88. [Google Scholar] [CrossRef]
- Yin, Y.M.; Yu, J.; Cao, H.T.; Zhang, L.T.; Sun, H.Z.; Xie, W.F. Efficient non-doped phosphorescent orange, blue and white organic light-emitting devices. Sci. Rep. 2014, 4, 6754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Oswald, I.W.H.; Perez, M.R.; Jia, H.P.; Shahub, A.A.; Qiao, Q.Q.; Gnade, B.E.; Omary, M.A. Doping-Free Organic Light-Emitting Diodes with Very High Power Efficiency, Simple Device Structure, and Superior Spectral Performance. Adv. Funct. Mater. 2014, 24, 4746–4752. [Google Scholar] [CrossRef]
- Liu, B.Q.; Tao, H.; Wang, L.; Gao, D.Y.; Liu, W.C.; Zou, J.H.; Xu, M.; Ning, H.L.; Peng, J.B.; Cao, Y. High-performance doping-free hybrid white organic light-emitting diodes: The exploitation of ultrathin emitting nanolayers (<1 nm). Nano Energy 2016, 26, 26–36. [Google Scholar] [CrossRef]
- Luo, D.; Li, X.-L.; Zhao, Y.; Gao, Y.; Liu, B. High-Performance Blue Molecular Emitter-Free and Doping-Free Hybrid White Organic Light-Emitting Diodes: An Alternative Concept to Manipulate Charges and Excitons Based on Exciplex and Electroplex Emission. ACS Photonics 2017, 4, 1566–1575. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X.K.; Zheng, C.J.; Ye, J.; Liu, C.L.; Li, F.; Ou, X.M.; Lee, C.S.; Zhang, X.H. High Performance Exciplex-Based Fluorescence-Phosphorescence White Organic Light-Emitting Device with Highly Simplified Structure. Chem. Mater. 2015, 27, 5206–5211. [Google Scholar] [CrossRef]
- Hung, W.Y.; Fang, G.C.; Lin, S.W.; Cheng, S.H.; Wong, K.T.; Kuo, T.Y.; Chou, P.T. The First Tandem, All-exciplex-based WOLED. Sci. Rep. 2014, 4, 5161. [Google Scholar] [CrossRef] [PubMed]
- Chapran, M.; Angioni, E.; Findlay, N.J.; Breig, B.; Cherpak, V.; Stakhira, P.; Tuttle, T.; Volyniuk, D.; Grazulevicius, J.V.; Nastishin, Y.A.; et al. An ambipolar BODIPY derivative for a white exciplex OLED and cholesteric liquid crystal laser towards multi-functional devices. ACS Appl. Mater. Interfaces 2017, 9, 4750–4757. [Google Scholar] [CrossRef] [PubMed]
- Cherpak, V.; Stakhira, P.; Minaev, B.; Baryshnikov, G.; Stromylo, E.; Helzhynskyy, I.; Chapran, M.; Volyniuk, D.; Tomkute-Luksiene, D.; Malinauskas, T.; et al. Efficient “Warm-White” OLEDs Based on the Phosphorescent bis-Cyclometalated iridium(III) Complex. J. Phys. Chem. C 2014, 118, 11271–11278. [Google Scholar] [CrossRef]
- Cekaviciute, M.; Simokaitiene, J.; Volyniuk, D.; Sini, G.; Grazulevicius, J.V. Arylfluorenyl-substituted metoxytriphenylamines as deep blue exciplex forming bipolar semiconductors for white and blue organic light emitting diodes. Dyes Pigments 2017, 140, 187–202. [Google Scholar] [CrossRef]
- Michaleviciute, A.; Gurskyte, E.; Volyniuk, D.Y.; Cherpak, V.V.; Sini, G.; Stakhira, P.Y.; Grazulevicius, J.V. Star-Shaped Carbazole Derivatives for Bilayer White Organic Light-Emitting Diodes CombiningEmission from Both Excitons and Exciplexes. J. Phys. Chem. C 2012, 116, 20769–20778. [Google Scholar] [CrossRef]
- Huang, H.; Zhao, F.; Liu, L.; Zhang, F.; Wu, X.G.; Shi, L.; Zou, B.; Pei, Q.; Zhong, H. Emulsion Synthesis of Size-Tunable CH3NH3PbBr3 Quantum Dots: An Alternative Route toward Efficient Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2015, 7, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.J.; Wang, Y.A.; Li, L.S.; Wang, D.; Zhu, T.; Xu, J.; Yang, C.; Li, Y. Bright, Multicoloured Light-emitting Diodes Based on Quantum Dots. Nat. Photonics 2007, 1, 717–722. [Google Scholar] [CrossRef]
- Yang, Y.X.; Zheng, Y.; Cao, W.R.; Titov, A.; Hyvonen, J.; Manders, J.R.; Xue, J.G.; Holloway, P.H.; Qian, L. High-Efficiency Light-Emitting Devices Based on Quantum Dots with Tailored Nanostructures. Nat. Photonics 2015, 9, 259–266. [Google Scholar] [CrossRef]
- Dai, X.L.; Zhang, Z.X.; Jin, Y.Z.; Niu, Y.; Cao, H.J.; Liang, X.Y.; Chen, L.W.; Wang, J.P.; Peng, X.G. Solution-Processed, High Performance Light-Emitting Diodes Based on Quantum Dots. Nature 2014, 515, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Nadal, B.; Mahler, B.; Aubin, H.; Dubertret, B. Quasi-2D Colloidal Semiconductor Nanoplatelets for Narrow Electroluminescence. Adv. Funct. Mater. 2014, 24, 295–302. [Google Scholar] [CrossRef]
- Fan, F.; Kanjanaboos, P.; Saravanapavanantham, M.; Beauregard, E.; Ingram, G.; Yassitepe, E.; Adachi, M.M.; Voznyy, O.; Johnston, A.K.; Walters, G.; et al. Colloidal CdSe1-X SX Nanoplatelets with Narrow and Continuously-Tunable Electroluminescence. Nano Lett. 2015, 15, 4611–4615. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Yuan, Z.; Tian, Y.; Wang, X.; Wang, J.C.; Xin, Y.; Hanson, K.; Ma, B.; Gao, H. Bright Light-Emitting Diodes Based on Organometal Halide Perovskite Nanoplatelets. Adv. Mater. 2016, 28, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xu, L.; Wang, T.; Song, J.; Chen, J.; Xue, J.; Dong, Y.; Cai, B.; Shan, Q.; Han, B.; et al. 50-Fold EQE Improvement up to 6.27% of Solution-Processed All-Inorganic Perovskite CsPbBr3 QLEDs via Surface Ligand Density Control. Adv. Mater. 2017, 29, 1603885. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Liu, S.; Zhang, H.; Wang, R.; Xie, W.; Zhang, H. Ultrasonic Spray Processed, Highly Efficient All-Inorganic Quantum-Dot Light-Emitting Diodes. ACS Photonics 2017, 4, 1271–1278. [Google Scholar] [CrossRef]
- Jiang, C.; Zhong, Z.; Liu, B.; He, Z.; Zou, J.; Wang, L.; Wang, J.; Peng, J.B.; Cao, Y. Coffee-Ring-Free Quantum Dot Thin Film Using Inkjet Printing from a Mixed-Solvent System on Modified ZnO Transport Layer for Light-Emitting Devices. ACS Appl. Mater. Interfaces 2016, 8, 26162–26168. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, H.; Liu, B.; Zhong, Z.; Zou, J.; Wang, J.; Wang, L.; Peng, J.; Cao, Y. Improved performance of inverted quantum dots light emitting devices by introducing double hole transport layers. Org. Electron. 2016, 31, 82–89. [Google Scholar] [CrossRef]
- Xiao, Z.; Jia, X.; Ding, L. Ternary organic solar cells offer 14% power conversion efficiency. Sci. Bull. 2017. [Google Scholar] [CrossRef]
- Xiao, Z.; Liu, F.; Geng, X.; Zhang, J.; Ding, L. A carbon-oxygen-bridged ladder-type building block for efficient donor and acceptor materials used in organic solar cells. Sci. Bull. 2017, 62, 1331–1336. [Google Scholar] [CrossRef]
- Xiao, Z.; Jia, X.; Li, D.; Wang, S.; Ding, L. 26 mA cm−2 Jsc from organic solar cells with a low-bandgap nonfullerene acceptor. Sci. Bull. 2017. [Google Scholar] [CrossRef]
- Camara, C.G.; Escobar, J.V.; Hird, J.R.; Putterman, S.J. Correlation between nanosecond X-ray flashes and stick–slip friction in peeling tape. Nature 2008, 455, 1089–1092. [Google Scholar] [CrossRef]
- Ducrot, E.; Chen, Y.; Bulters, M.; Sijbesma, R.P.; Creton, C. Toughening elastomers with sacrificial bonds and watching them break. Science 2014, 344, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Boldyreva, E. Mechanochemistry of inorganic and organic systems: What is similar, what is different? Chem. Soc. Rev. 2013, 42, 7719–7738. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, C.-N.; Yamada, H.; Nishikubo, K.; Zheng, X.-G. Electro-Mechano-Optical Conversions in Pr3+-Doped BaTiO3-CaTiO3 Ceramics. Adv. Mater. 2005, 17, 1254–1258. [Google Scholar] [CrossRef]
- Babu, S.S.; Hollamby, M.J.; Aimi, J.; Ozawa, H.; Saeki, A.; Seki, S.; Kobayashi, K.; Hagiwara, K.; Yoshizawa, M.; Mohwald, H.; et al. Nonvolatile liquid anthracenes for facile full-colour luminescence tuning at single blue-light excitation. Nat. Commun. 2013, 4, 1969. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Kwon, S.-H.; Petrášek, Z.; Park, O.K.; Jun, S.W.; Shin, K.; Choi, M., II; Park, Y.; Park, K.; Na, H.B.; et al. High-resolution three-photon biomedical imaging using doped ZnS nanocrystals. Nat. Mater. 2013, 12, 359–366. [Google Scholar]
- An, Z.; Zheng, C.; Tao, Y.; Chen, R.; Shi, H.; Chen, T.; Wang, Z.; Li, H.; Deng, R.; Liu, X.; et al. Stabilizing triplet excited states for ultralong organic phosphorescence. Nat. Mater. 2015, 14, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Y.; Wang, X.; Kuang, S.Y.; Su, L.; Li, H.Y.; Wang, Y.; Pan, C.; Wang, Z.L.; Zhu, G. Dynamic Triboelectrification-Induced Electroluminescence and its Use in Visualized Sensing. Adv. Mater. 2016, 28, 6656–6664. [Google Scholar] [CrossRef] [PubMed]
- Yoshihiro, O. Color rendering and luminous efficacy of white LED spectra. Proc. SPIE 2004, 88, 5530. [Google Scholar]
- Liu, B.; Lan, L.; Zou, J.; Peng, J. A novel organic light-emitting diode by utilizing double hole injection layer. Acta Phys. Sin. 2013, 62, 087302. [Google Scholar]
- Hong, T.; Gao, D.; Liu, B.; Wang, L.; Zou, J.; Xu, M.; Peng, J. Enhancement of tandem organic light-emitting diode performance by inserting an ultra-thin Ag layer in charge generation layer. Acta Phys. Sin. 2017, 1, 017302. [Google Scholar]
- Kim, D.Y.; Park, J.H.; Lee, J.W.; Hwang, S.; Oh, S.J.; Kim, J.; Sone, C.; Schubert, E.F.; Kim, J.K. Overcoming the fundamental light-extraction efficiency limitations of deep ultraviolet light-emitting diodes by utilizing transverse-magnetic-dominant emission. Light Sci. Appl. 2015, 4, e263. [Google Scholar] [CrossRef]
- Preinfalk, J.B.; Eiselt, T.; Wehlus, T.; Rohnacher, V.; Hanemann, T.; Gomard, G.; Lemmer, U. Large-Area Screen-Printed Internal Extraction Layers for Organic Light-Emitting Diodes. ACS Photonics 2017, 4, 928–933. [Google Scholar] [CrossRef]
- Koh, T.-W.; Spechler, J.A.; Lee, K.M.; Arnold, C.B.; Rand, B.P. Enhanced Outcoupling in Organic Light-Emitting Diodes via a High-Index Contrast Scattering Layer. ACS Photonics 2015, 2, 1366–1372. [Google Scholar] [CrossRef]


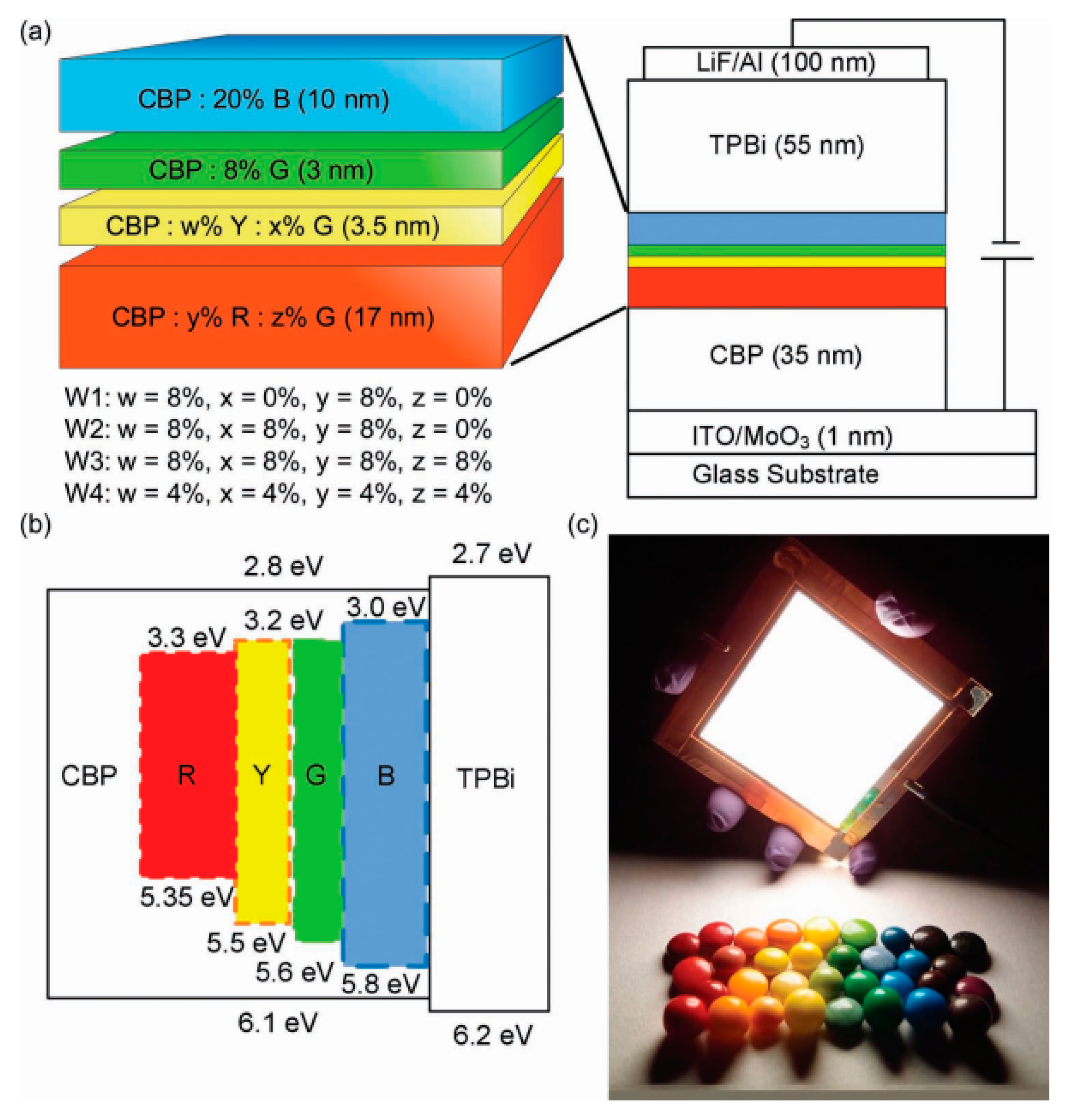
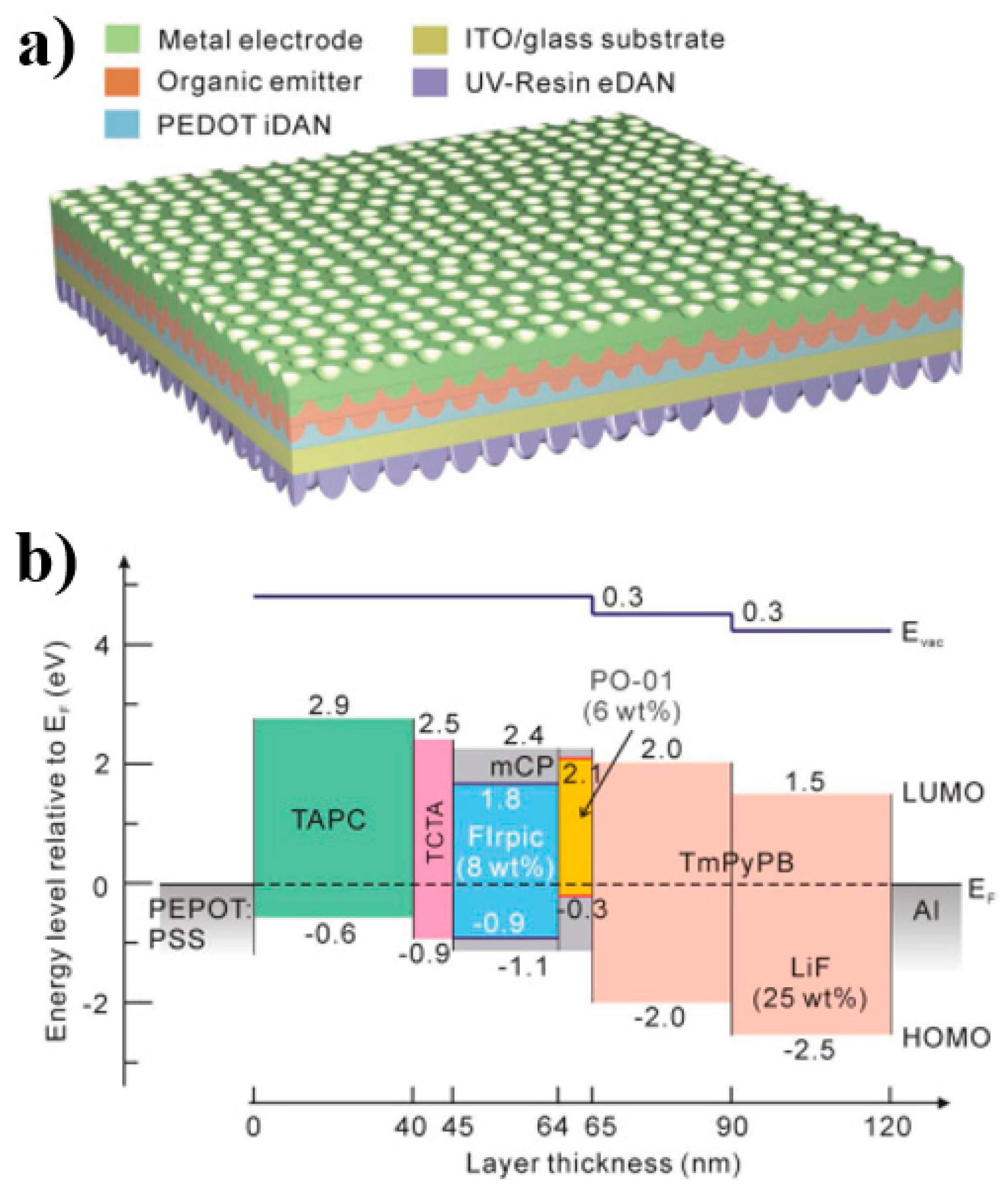
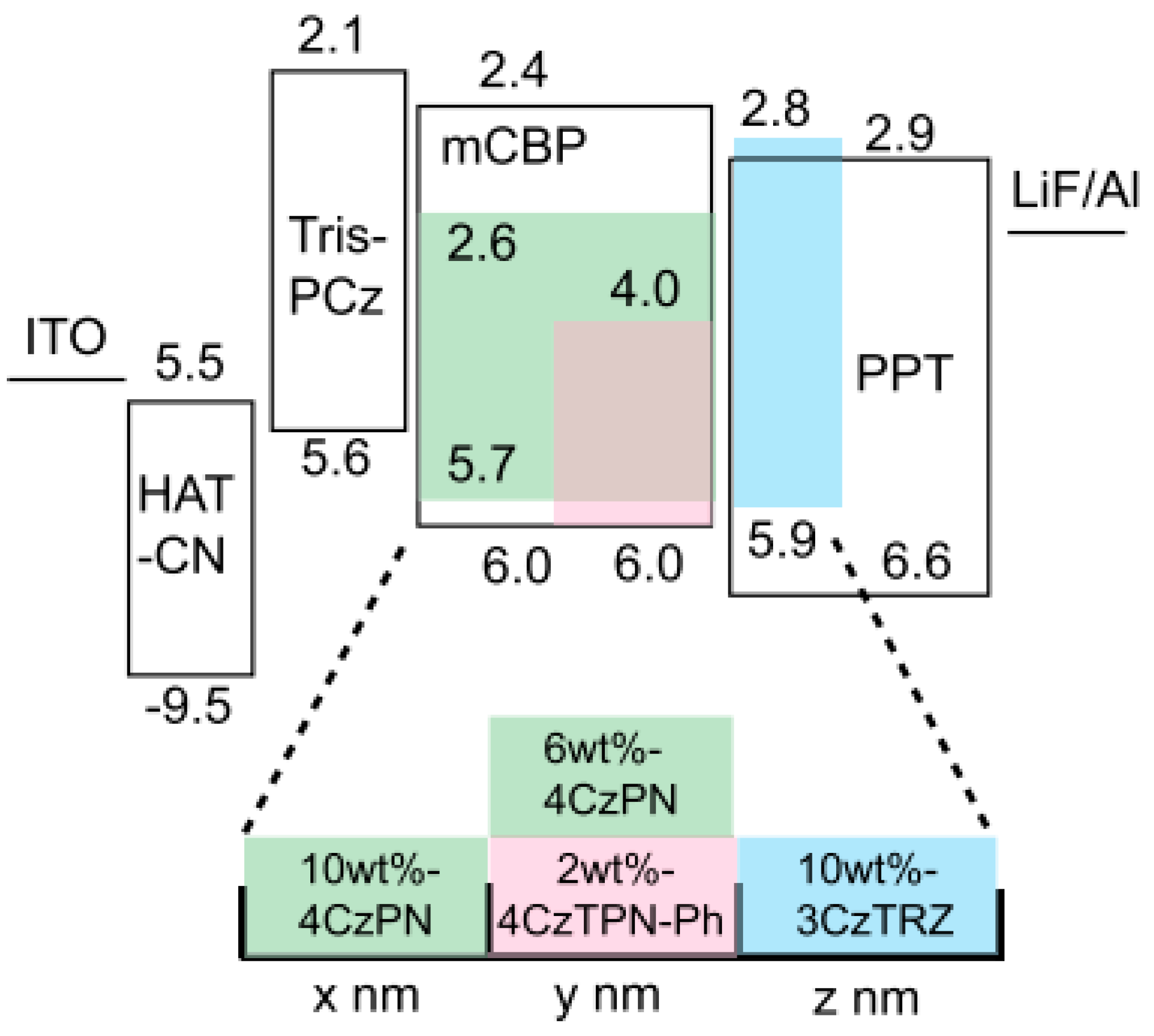
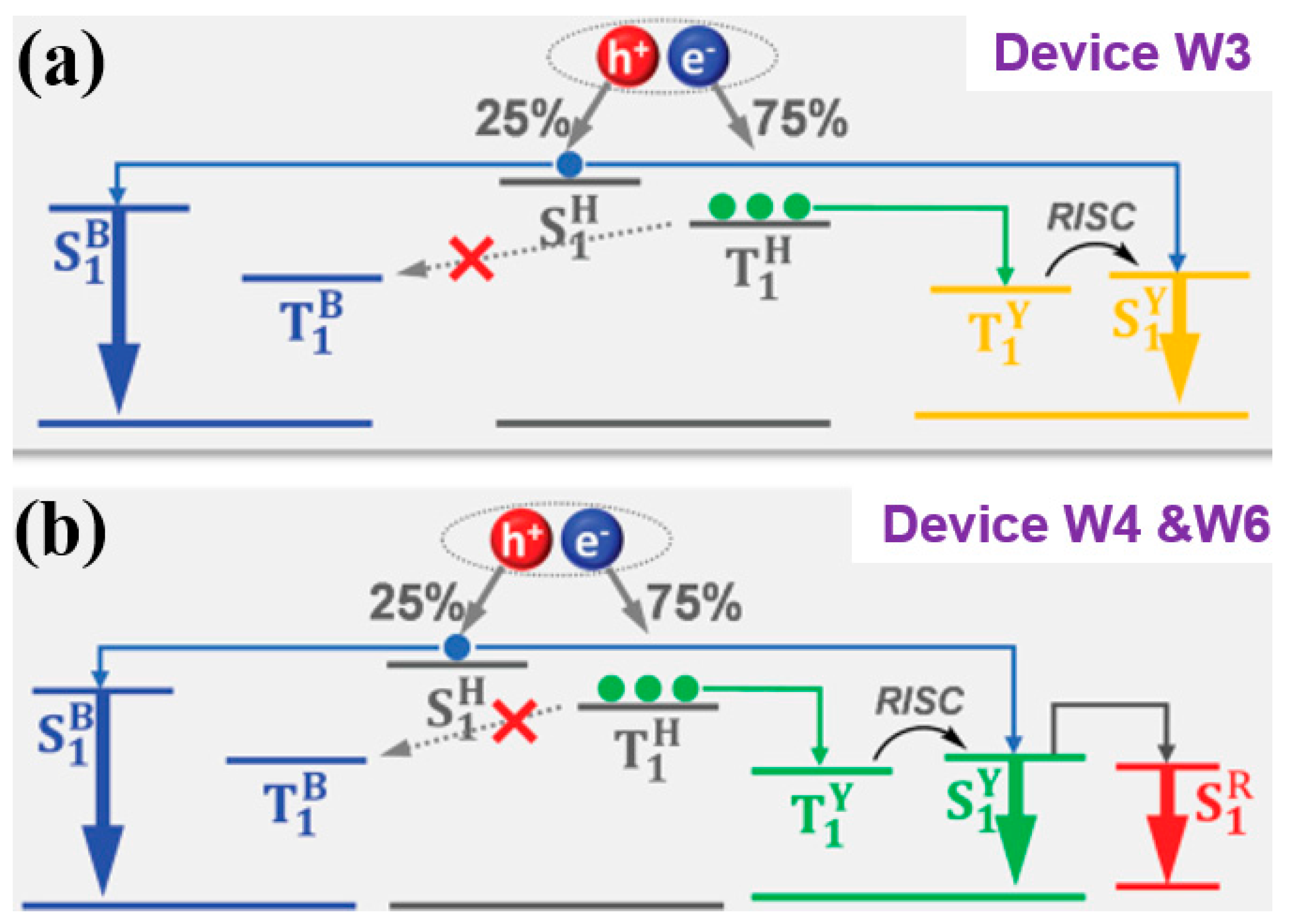

| Devices a | Von/V1000 b (v) | EQEmax/EQE1000 c (%) | CEmax/CE1000 d (cd A−1) | PEmax/PE1000 e (lm W−1) | CIE1000 f | CRI g |
|---|---|---|---|---|---|---|
| Ref. [120] h | 3.0/- | 5.6/- | 14.0/- | 9.2/- | (0.334, 0.337) | 81 |
| Ref. [123] h | -/- | 6.3/- | 14.7/- | -/- | (0.335, 0.355) | - |
| Ref. [124] i | 3.3/5 | 12/- | -/- | 42/- | (0.43, 0.45) | 80 |
| Ref. [125] i | -/- | 19.3/- | -/- | 72.2/- | (0.33, 0.39) | 71 |
| Ref. [132] i | -/- | -/- | 113.6/111.7 | 92.5/75.5 | (0.288, 0.485) | - |
| Ref. [12] i | 2.5/3.6 | 28.1/21.5 | 83.6/61.0 | 105/59.5 | (0.40, 0.48) | - |
| Ref. [133] i | 2.83/3.79 | -/25 | -/- | /44 | (0.335, 0.396) | 68 |
| Ref. [134] i | -/3 | -/34 | -/- | -/90 | (0.41, 0.49) | 69 |
| Ref. [135] i | -/- | 20.1/- | -/- | 41.3/- | (0.38, 0.45) | 85 |
| Ref. [136] i | -/- | -/24.5 | -/57.7 | -/33.8 | (0.44, 0.46) | 81 |
| Ref. [9] i | -/<4 | 56.1/54.6 | -/142 | 132.8/123.4 | (0.339, 0.458) | - |
| Ref. [156] j | 3.6/7.8 | 17/8.5 | 45.6/20.6 | 34.1/8.3 | (0.33, 0.41) | - |
| Ref. [160] j | 3.4/5.4 | 19.2/14.2 | 51.4/37.3 | 47.5/21.8 | (0.348, 0.457) | 69 |
| Ref. [184] k | -/- | 18.7/- | -/- | 37.6/- | (0.40, 0.41) | 85 |
| Ref. [193] k | -/- | -/- | 70.2/- | 43.4/- | (0.44, 0.50) | 46 |
| Ref. [198] k | 2.8/- | -/- | 20.6/19.0 | 20.9/11.9 | (0.277, 0.354) | 73 |
| Ref. [201] k | 3.1/- | 32.3/28.9 | 76.8/68.9 | 70.9/58.3 | (0.43, 0.43) | 83 |
| Ref. [205] k | ~2.5/3.46 | -/16.1 | -/- | -/37.5 | (0.44, 0.47) | 86 |
| Ref. [206] k | 2.4/3.1 | -/- | 84.5/- | 106.3/65.1 | (0.43, 0.48) | 47 |
| Ref. [211] k | 2.4/- | 26.6/21.2 | 53.5/42.6 | 67.2/33.5 | (0.46, 0.44) | - |
| Ref. [215] k | -/- | 38.4/26.2 | -/- | 80.1/37.5 | (0.45, 0.48) | - |
| Ref. [216] k | -/- | 19.1/17.6 | 49.6/- | 49.3/- | (0.43, 0.46) | 80 |
| Ref. [226] l | 2.4/- | -/- | -/- | 49.5/30.0 | (0.44, 0.47) | 65 |
| Ref. [227] l | 2.5/- | 11.6/- | 32.5/- | 39.3/18.2 | (0.42, 0.48) | - |
| Ref. [228] l | -/- | 16.8/- | 49.6/- | 56.4/40.0 | (0.42, 0.51) | 56 |
| Ref. [235] l | 2.2/- | 5/- | 15/- | -/- | - | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, X.-L.; Luo, D.; Xiao, P.; Xiao, W.; Song, Y.; Ang, Q.; Liu, B. Strategies to Achieve High-Performance White Organic Light-Emitting Diodes. Materials 2017, 10, 1378. https://doi.org/10.3390/ma10121378
Zhang L, Li X-L, Luo D, Xiao P, Xiao W, Song Y, Ang Q, Liu B. Strategies to Achieve High-Performance White Organic Light-Emitting Diodes. Materials. 2017; 10(12):1378. https://doi.org/10.3390/ma10121378
Chicago/Turabian StyleZhang, Lirong, Xiang-Long Li, Dongxiang Luo, Peng Xiao, Wenping Xiao, Yuhong Song, Qinshu Ang, and Baiquan Liu. 2017. "Strategies to Achieve High-Performance White Organic Light-Emitting Diodes" Materials 10, no. 12: 1378. https://doi.org/10.3390/ma10121378




