Luminescent Lanthanoid Calixarene Complexes and Materials
Abstract
:1. Introduction
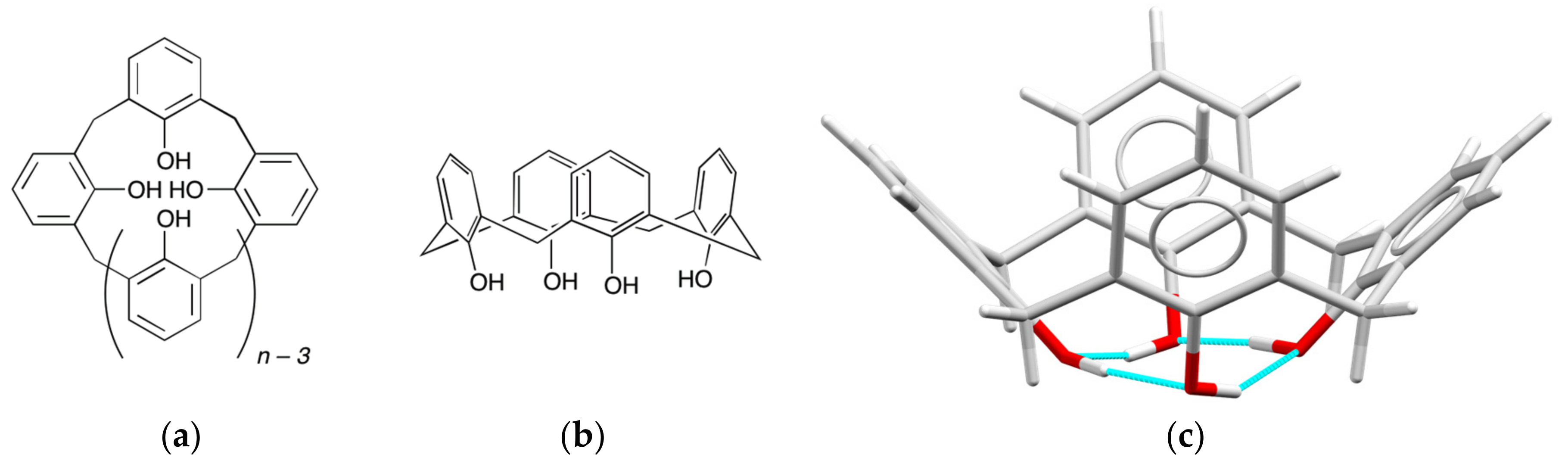
2. Background
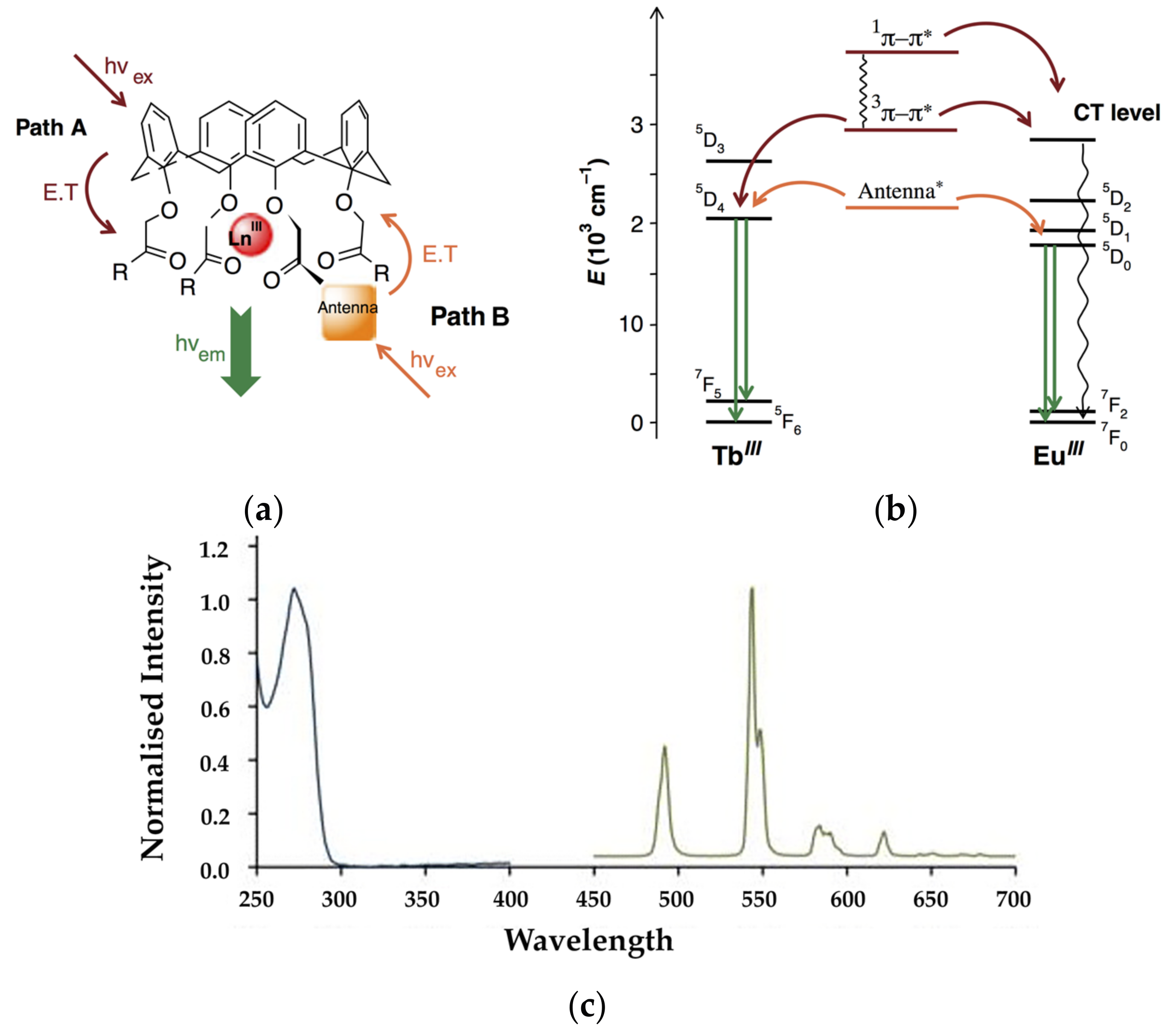
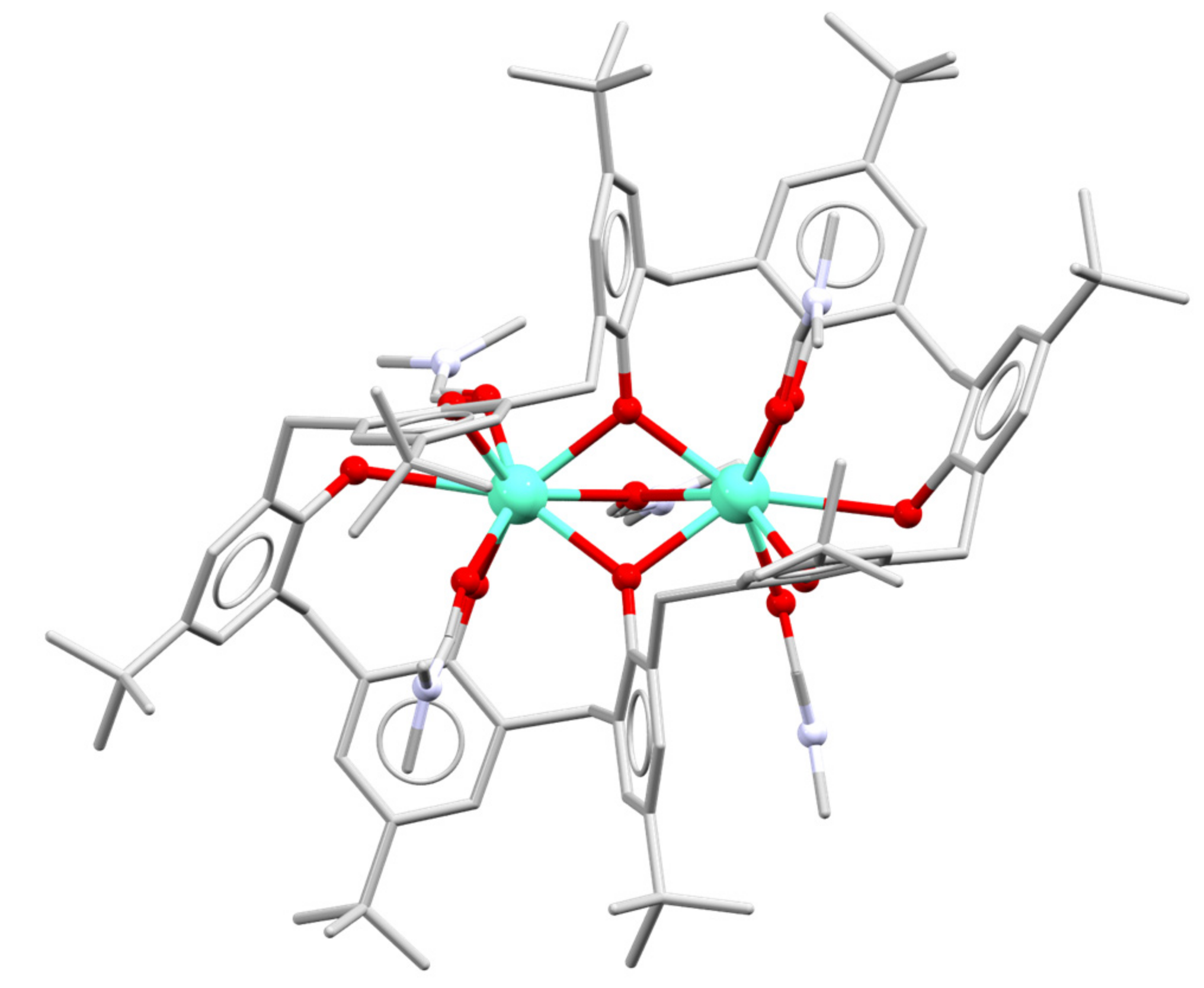
3. Early Work
4. Lanthanoid Calixarene Complexes in Functional Materials
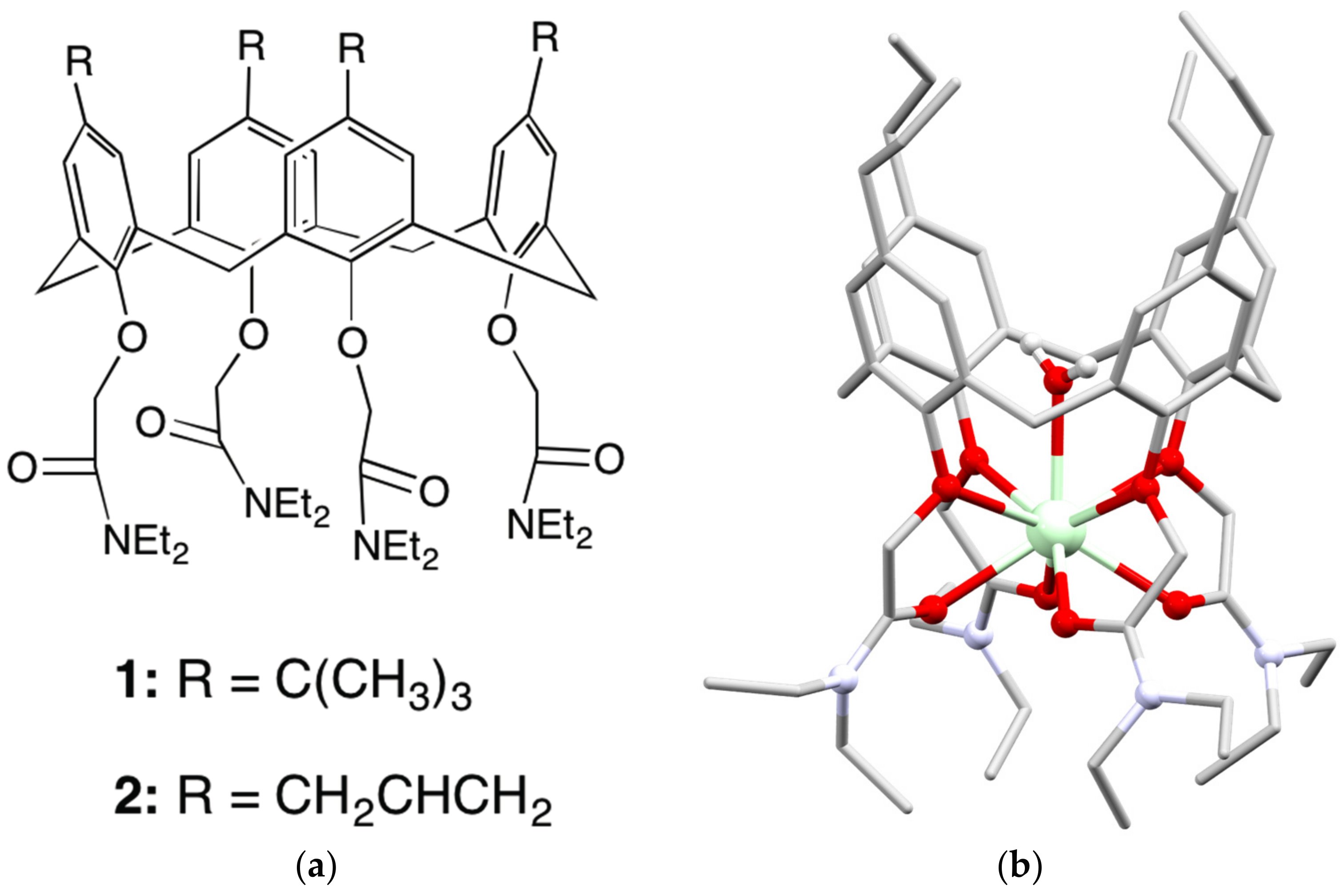
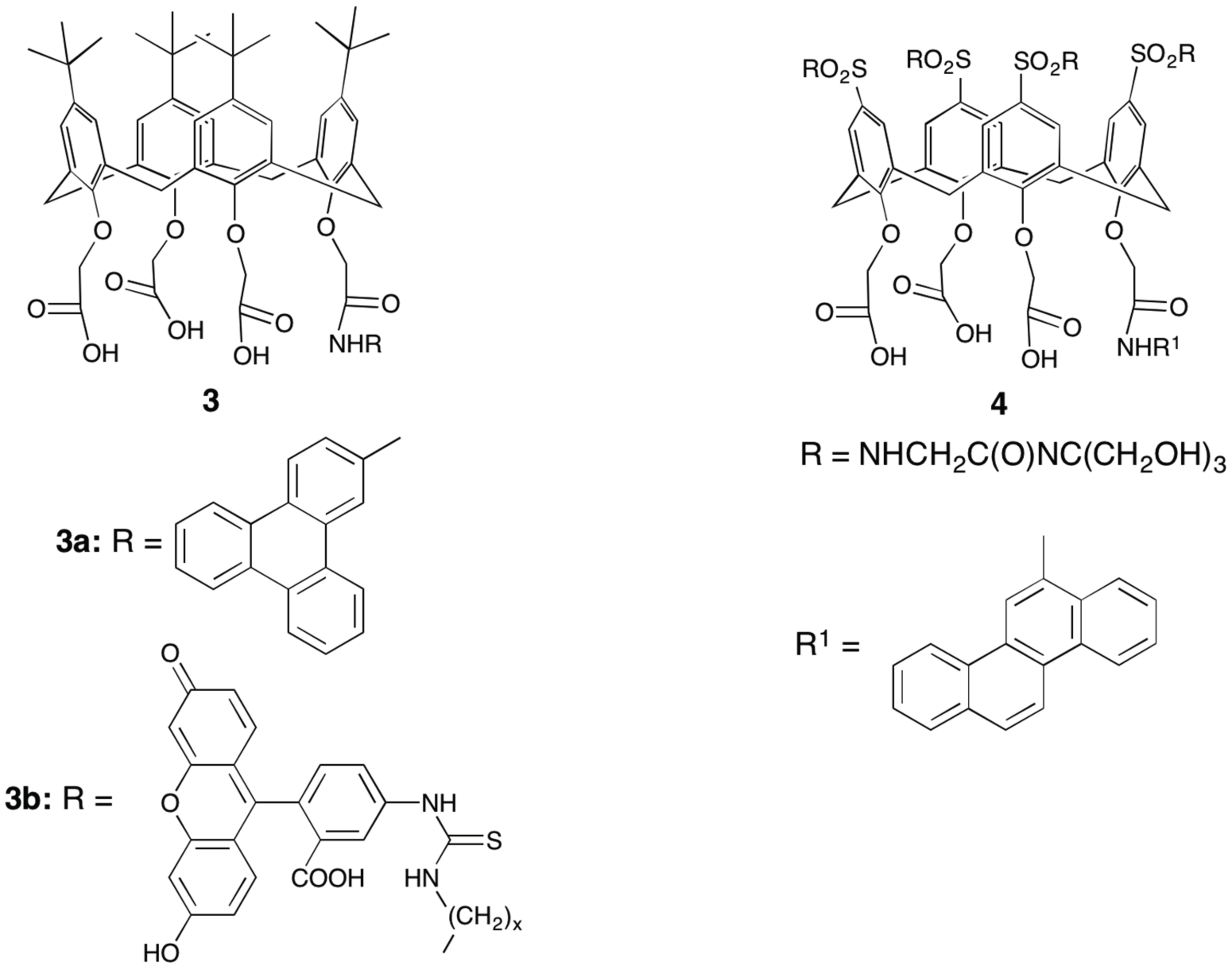
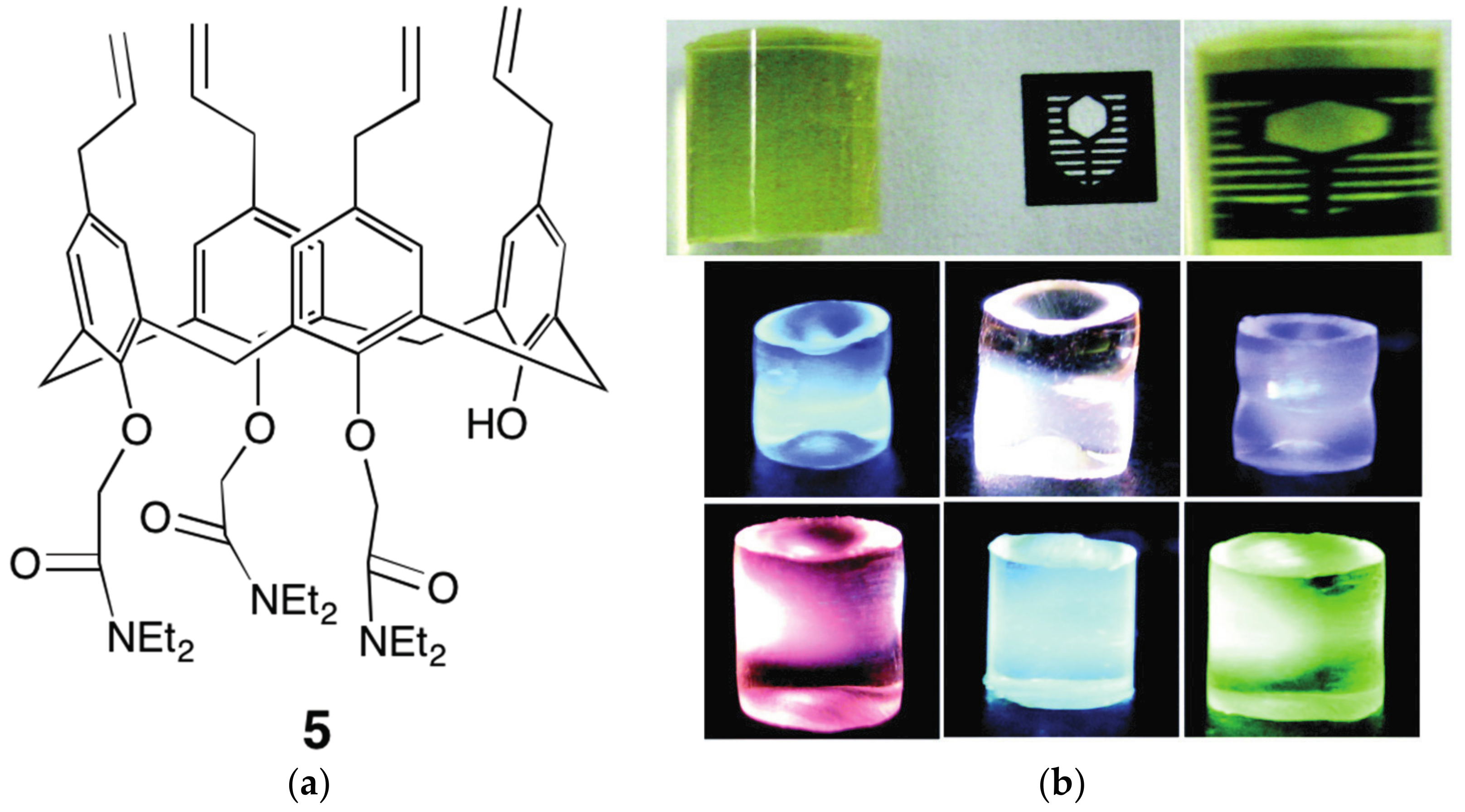
4.1. Polymeric Materials
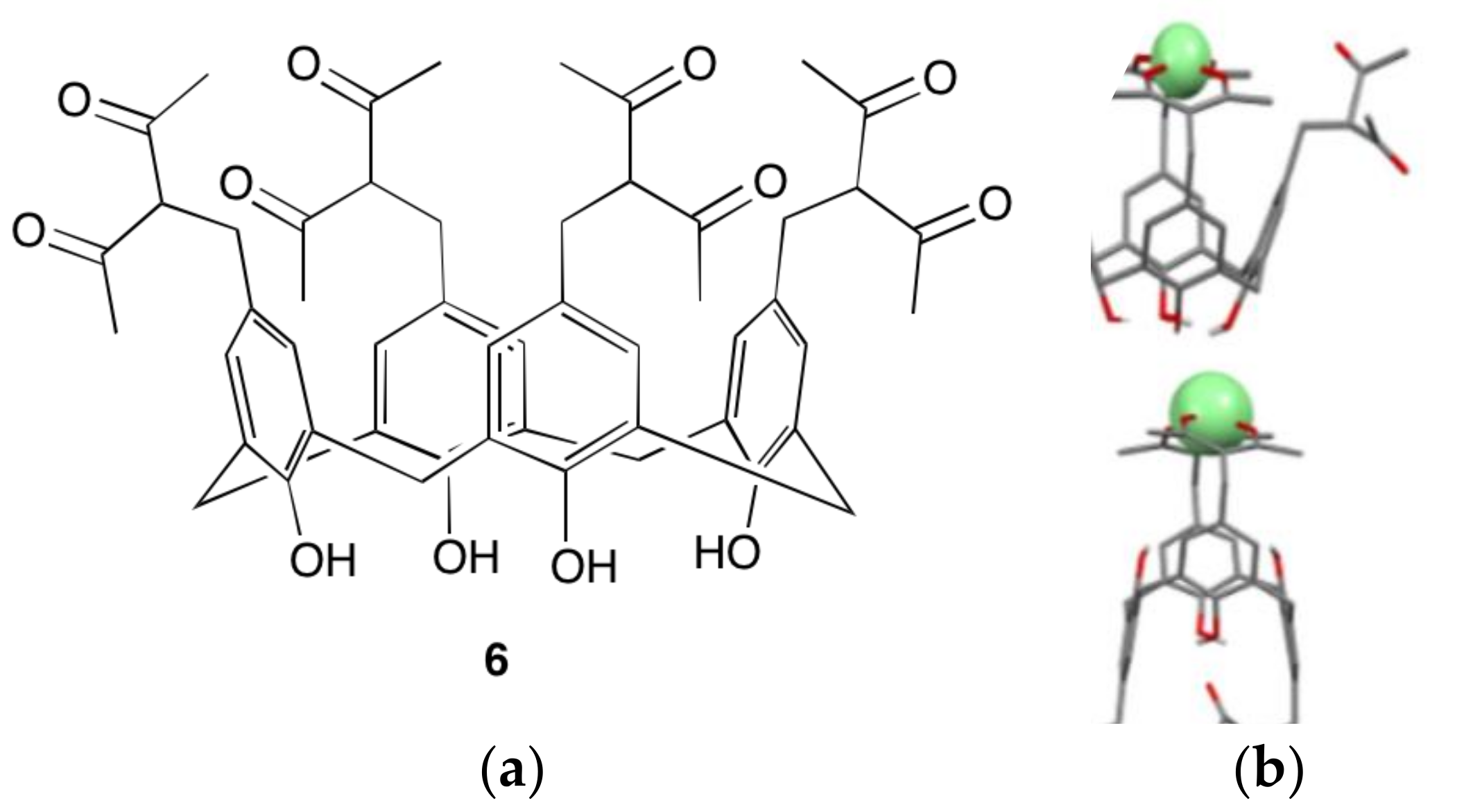
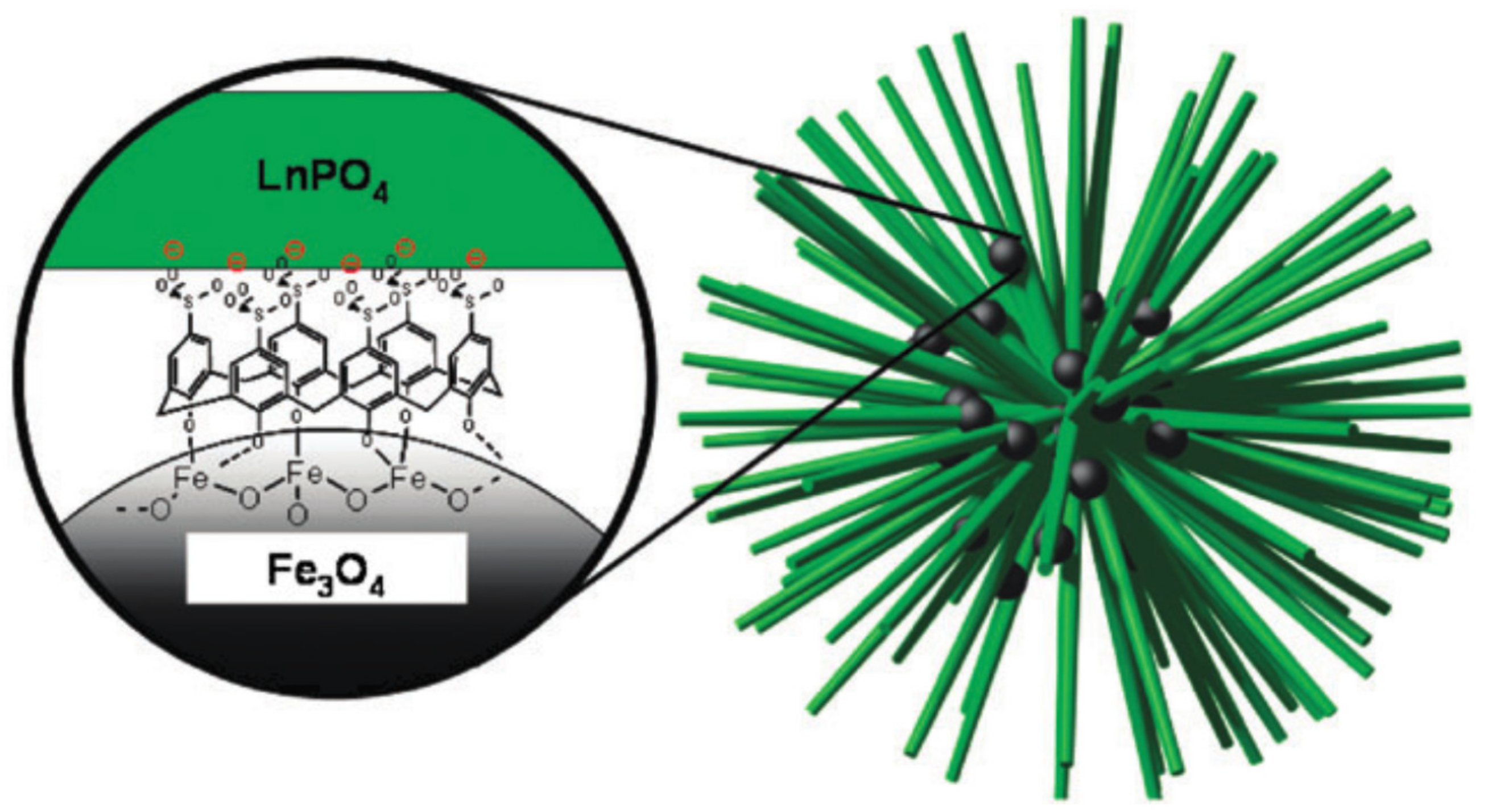
4.2. Nanoparticulate Materials
4.3. Lanthanoid Clusters
5. Conclusions
Conflicts of Interest
References
- Gutsche, C.D. Calixarenes: An Introduction, 2nd ed.; RSC Publishing: Cambridge, UK, 2008. [Google Scholar]
- Ogoshi, T.; Yamagishi, T.A.; Nakamoto, Y. Pillar-shaped macrocyclic hosts pillar[n]arenes: New key players for supramolecular chemistry. Chem. Rev. 2016, 116, 7937–8002. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Ko, K.C.; Lee, W.R.; Cho, J.; Moon, J.H.; Moon, D.; Sharma, A.; Lee, J.Y.; Kim, J.S.; Kim, S. Calix[n]triazoles and related conformational studies. Org. Lett. 2017, 19, 5509–5512. [Google Scholar] [CrossRef] [PubMed]
- Chinta, J.P.; Ramanujam, B.; Rao, C.P. Structural aspects of the metal ion complexes of the conjugates of calix[4]arene: Crystal structures and computational models. Coord. Chem. Rev. 2012, 256, 2762–2794. [Google Scholar] [CrossRef]
- Creaven, B.S.; Donlon, D.F.; McGinley, J. Coordination chemistry of calix[4]arene derivatives with lower rim functionalisation and their applications. Coord. Chem. Rev. 2009, 253, 893–962. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L. Application and luminescent properties of the complexes of lanthanide ions based on calixarene derivatives. Prog. Chem. 2010, 22, 427–432. [Google Scholar]
- Bradberry, S.J.; Savyasachi, A.J.; Martinez-Calvo, M.; Gunnlaugsson, T. Development of responsive visibly and nir luminescent and supramolecular coordination self-assemblies using lanthanide ion directed synthesis. Coord. Chem. Rev. 2014, 273, 226–241. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C.G. Lanthanide luminescence for biomedical analyses and imaging. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef] [PubMed]
- Atwood, J.L.; Barbour, L.J.; Jerga, A. Storage of methane and freon by interstitial van der waals confinement. Science 2002, 296, 2367–2369. [Google Scholar] [CrossRef] [PubMed]
- Bunzli, J.C.G. On the design of highly luminescent lanthanide complexes. Coord. Chem. Rev. 2015, 293, 19–47. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bunzli, J.C.G. Lanthanide luminescence for functional materials and bio-sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef] [PubMed]
- Eliseeva, S.V.; Bunzli, J.C.G. Rare earths: Jewels for functional materials of the future. New J. Chem. 2011, 35, 1165–1176. [Google Scholar] [CrossRef]
- Lincheneau, C.; Quinlan, E.; Kitchen, J.A.; McCabe, T.; Matthews, S.E.; Gunnlaugsson, T. Delayed lanthanide luminescent Tb(III) complexes formed from lower rim amide functionalised calix[4]arenes. Supramol. Chem. 2013, 25, 869–880. [Google Scholar] [CrossRef]
- Bunzli, J.C.G.; Froidevaux, P.; Harrowfield, J.M. Photophysical properties of lanthanide dinuclear complexes with p-tert-butylcalix[8]arene. Inorg. Chem. 1993, 32, 3306–3311. [Google Scholar] [CrossRef]
- Sato, N.; Shinkai, S. Energy-transfer luminescence of lanthanide ions encapsulated in sensitizer-modified calix[4]arenes. J. Chem. Soc. Perk. Trans. 2 1993, 621–624. [Google Scholar] [CrossRef]
- Furphy, B.M.; Harrowfield, J.M.; Kepert, D.L.; Skelton, B.W.; White, A.H.; Wilner, F.R. Bimetallic lanthanide complexes of the calixarenes—Europium(III) and tert-butylcalix[8]arene. Inorg. Chem. 1987, 26, 4231–4236. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Ogden, M.I.; White, A.H. Lanthanide ion complexes of calixarenes. VIII. Bimetallic lanthanide complexes of p-t-Butylcalix[8]arene from dimethylformamide solutions. Aust. J. Chem. 1991, 44, 1249–1262. [Google Scholar] [CrossRef]
- Harrowfield, J.M.; Ogden, M.I.; White, A.H. Lanthanide ion complexes of calixarenes. VII. Bimetallic lanthanide complexes of para-tert-butylcalix[8]arene from Dimethylsulfoxide solutions. Aust. J. Chem. 1991, 44, 1237–1247. [Google Scholar] [CrossRef]
- Froidevaux, P.; Bunzli, J.C.G. Complexes of lanthanoid salts with macrocyclic ligands. 42. Energy-transfer processes in lanthanide dinuclear complexes with p-tert-butylcalix[8]arene—An example of dipole-dipolar mechanism. J. Phys. Chem. 1994, 98, 532–536. [Google Scholar] [CrossRef]
- Latva, M.; Takalo, H.; Mukkala, V.M.; Matachescu, C.; RodriguezUbis, J.C.; Kankare, J. Correlation between the lowest triplet state energy level of the ligand and lanthanide(III) luminescence quantum yield. J. Lumin. 1997, 75, 149–169. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Mecati, A.; Balzani, V.; Ungaro, R.; Ghidini, E.; Casnati, A.; Pochini, A. Encapsulation of lanthanide ions in calixarene receptors. A strongly luminescent terbium(3+) complex. J. Chem. Soc. Chem. Commun. 1990, 878–879. [Google Scholar] [CrossRef]
- Horrocks, W.D.; Sudnick, D.R. Lanthanide ion luminescence probes of the structure of biological macromolecules. Acc. Chem. Res. 1981, 14, 384–392. [Google Scholar] [CrossRef]
- Driscoll, C.R.; Skelton, B.W.; Massi, M.; Ogden, M.I. Structural characterisation and photophysical properties of lanthanoid complexes of a tetra-amide functionalised calix[4]arene. Supramol. Chem. 2016, 28, 567–574. [Google Scholar] [CrossRef]
- Sabbatini, N.; Guardigli, M.; Manet, I.; Ungaro, R.; Casnati, A.; Fischer, C.; Ziessel, R.; Ulrich, G. Synthesis and luminescence of Eu3+ and Tb3+ complexes with novel calix[4]arene ligands carrying 2,2’-bipyridine subunits. New J. Chem. 1995, 19, 137–140. [Google Scholar]
- Casnati, A.; Fischer, C.; Guardigli, M.; Isernia, A.; Manet, I.; Sabbatini, N.; Ungaro, R. Synthesis of calix[4]arene receptors incorporating (2,2′-bipyridin-6-yl)methyl and (9-methyl-1,10-phenanthrolin-2-yl)methyl chromophores and luminescence of their Eu3+ and Tb3+ complexes. J. Chem. Soc., Perk. Trans. 2 1996, 395–399. [Google Scholar] [CrossRef]
- Ulrich, G.; Ziessel, R.; Manet, I.; Guardigli, M.; Sabbatini, N.; Fraternali, F.; Wipff, G. Calix[4]arene podands and barrelands incorporating 2,2′-bipyridine moieties and their lanthanide complexes: Luminescence properties. Chem. Eur. J. 1997, 3, 1815–1822. [Google Scholar] [CrossRef]
- Rudkevich, D.M.; Verboom, W.; Vandertol, E.; Vanstaveren, C.J.; Kaspersen, F.M.; Verhoeven, J.W.; Reinhoudt, D.N. Calix[4]aarene-triacids as receptors for lanthanides—Synthesis and luminescence of neutral Eu3+ and Tb3+ complexes. J. Chem. Soc. Perk. Trans. 2 1995, 131–134. [Google Scholar] [CrossRef]
- Steemers, F.J.; Verboom, W.; Reinhoudt, D.N.; Vandertol, E.B.; Verhoeven, J.W. New sensitizer-modified calix[4]arenes enabling near-uv excitation of complexed luminescent lanthanide ions. J. Am. Chem. Soc. 1995, 117, 9408–9414. [Google Scholar] [CrossRef]
- Wolbers, M.P.O.; van Veggel, F.; Peters, F.G.A.; van Beelen, E.S.E.; Hofstraat, J.W.; Geurts, F.A.J.; Reinhoudt, D.N. Sensitised near-infrared emission from Nd3+ and Er3+ complexes of fluorescein-bearing calix[4]arene cages. Chem. Eur. J. 1998, 4, 772–780. [Google Scholar] [CrossRef]
- Steemers, F.J.; Meuris, H.G.; Verboom, W.; Reinhoudt, D.N.; vanderTol, E.B.; Verhoeven, J.W. Water-soluble neutral calix[4]arene-lanthanide complexes: Synthesis and luminescence properties. J. Org. Chem. 1997, 62, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhang, H.J. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.M.; Holliday, B.J. Luminescent lanthanide-containing metallopolymers. Coord. Chem. Rev. 2012, 256, 1520–1530. [Google Scholar] [CrossRef]
- Driscoll, C.R.; Reid, B.L.; McIldowie, M.J.; Muzzioli, S.; Nealon, G.L.; Skelton, B.W.; Stagni, S.; Brown, D.H.; Massi, M.; Ogden, M.I. A “plug-and-play” approach to the preparation of transparent luminescent hybrid materials based on poly(methyl methacrylate), a calix[4]arene cross-linking agent, and terbium ions. Chem. Commun. 2011, 47, 3876–3878. [Google Scholar] [CrossRef] [PubMed]
- Ennis, B.W.; Muzzioli, S.; Reid, B.L.; D’Alessio, D.M.; Stagni, S.; Brown, D.H.; Ogden, M.I.; Massi, M. Recyclable calix[4]arene-lanthanoid luminescent hybrid materials with color-tuning and color-switching properties. Dalton Trans. 2013, 42, 6894–6901. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.F.; Zhang, H.Y.; Yan, B. Photoactive binary and ternary lanthanide (Eu3+, Tb3+, Nd3+) hybrids with p-tert-butylcalix[4]arene derived Si–O linkages and polymers. Dalton Trans. 2010, 39, 8882–8892. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikov, A.; Solovieva, S.; Antipin, I.; Ferlay, S. Coordination polymers based on calixarene derivatives: Structures and properties. Coord. Chem. Rev. 2017, 352, 151–186. [Google Scholar] [CrossRef]
- Gándara, F.; Gutiérrez-Puebla, E.; Iglesias, M.; Snejko, N.; Monge, M.Á. Isolated hexanuclear hydroxo lanthanide secondary building units in a rare-earth polymeric framework based on p-sulfonatocalix[4]arene. Cryst. Growth Des. 2010, 10, 128–134. [Google Scholar] [CrossRef]
- Barros, B.S.; de Oliveira, C.A.F.; Kulesza, J.; Alves, S.; Bochenska, M. Synthesis, characterization and luminescent properties of new coordination polymers based on p-tert-butylcalix[4]arene-tetracarboxylic acid and lanthanide cations. In Adaptive, Active and Multifunctional Smart Materials Systems; Vincenzini, P., Hahn, Y.B., Iannotta, S., Lendlein, A., Palermo, V., Paul, S., Sibilia, C., Silva, S.R.P., Srinivasan, G., Eds.; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; Volume 77, pp. 132–137. [Google Scholar]
- Lee, J.Y.; Kim, H.J.; Jung, J.H.; Sim, W.; Lee, S.S. Networking of calixcrowns: From heteronuclear endo/exocyclic coordination polymers to a photoluminescence switch. J. Am. Chem. Soc. 2008, 130, 13838–13839. [Google Scholar] [CrossRef] [PubMed]
- Zairov, R.; Mustafina, A.; Shamsutdinova, N.; Nizameev, I.; Moreira, B.; Sudakova, S.; Podyachev, S.; Fattakhova, A.; Safina, G.; Lundstrom, I.; et al. High performance magneto-fluorescent nanoparticles assembled from terbium and gadolinium 1,3-diketones. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed]
- Zairov, R.; Shamsutdinova, N.; Podyachev, S.; Sudakova, S.; Gimazetdinova, G.; Rizvanov, I.; Syakaev, V.; Babaev, V.; Amirov, R.; Mustafina, A. Structure impact in antenna effect of novel upper rim substituted tetra-1,3-diketone calix[4]arenes on Tb(III) green and Yb(III) NIR-luminescence. Tetrahedron 2016, 72, 2447–2455. [Google Scholar] [CrossRef]
- Shamsutdinova, N.A.; Podyachev, S.N.; Sudakova, S.N.; Mustafina, A.R.; Zairov, R.R.; Burilov, V.A.; Nizameev, I.R.; Rizvanov, I.K.; Syakaev, V.V.; Gabidullin, B.M.; et al. A facile synthetic route to convert Tb(III) complexes of novel tetra-1,3-diketone calix[4]resorcinarene into hydrophilic luminescent colloids. New J. Chem. 2014, 38, 4130–4140. [Google Scholar] [CrossRef]
- Fang, J.; Saunders, M.; Guo, Y.L.; Lu, G.Z.; Raston, C.L.; Iyer, K.S. Green light-emitting Lapo4: Ce3+:Tb3+ koosh nanoballs assembled by p-sulfonato-calix[6]arene coated superparamagnetic Fe3O4. Chem. Commun. 2010, 46, 3074–3076. [Google Scholar] [CrossRef] [PubMed]
- Harrowfield, J.; Koutsantonis, G. Calixarenes as cluster keepers. In Calixarenes in the Nanoworld; Vicens, J., Harrowfield, J., Baklouti, L., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 197–212. [Google Scholar]
- Furphy, B.M.; Harrowfield, J.M.; Ogden, M.I.; Skelton, B.W.; White, A.H.; Wilner, F.R. Lanthanide ion complexes of the calixarenes. Part 4. Double inclusion by p-t-butylcalix[4]arene (H4L). Crystal structures of [Eu2(HL)2(dmf)4].7dmf (dmf = dimethylformamide) and H4L.dmso (dmso = dimethyl sulphoxide). J. Chem. Soc. Dalton Trans. 1989, 2217–2221. [Google Scholar] [CrossRef]
- Sanz, S.; McIntosh, R.D.; Beavers, C.M.; Teat, S.J.; Evangelisti, M.; Brechin, E.K.; Dalgarno, S.J. Calix[4]arene-supported rare earth octahedra. Chem. Commun. 2012, 48, 1449–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Bilyk, A.; Hall, A.K.; Harrowfield, J.M.; Hosseini, M.W.; Skelton, B.W.; White, A.H. A unique rare-earth cluster within a calixarene sandwich: Parallels in the chemistry of cyclosiloxanes and calixarenes. Aust. J. Chem. 2001, 53, 895–898. [Google Scholar] [CrossRef]
- Iki, N.; Tanaka, T.; Hiro-oka, S.; Shinoda, K. Self-assembly of a trilanthanide(III) core sandwiched between two thiacalix[4]arene ligands. Eur. J. Inorg. Chem. 2016, 5020–5027. [Google Scholar] [CrossRef]
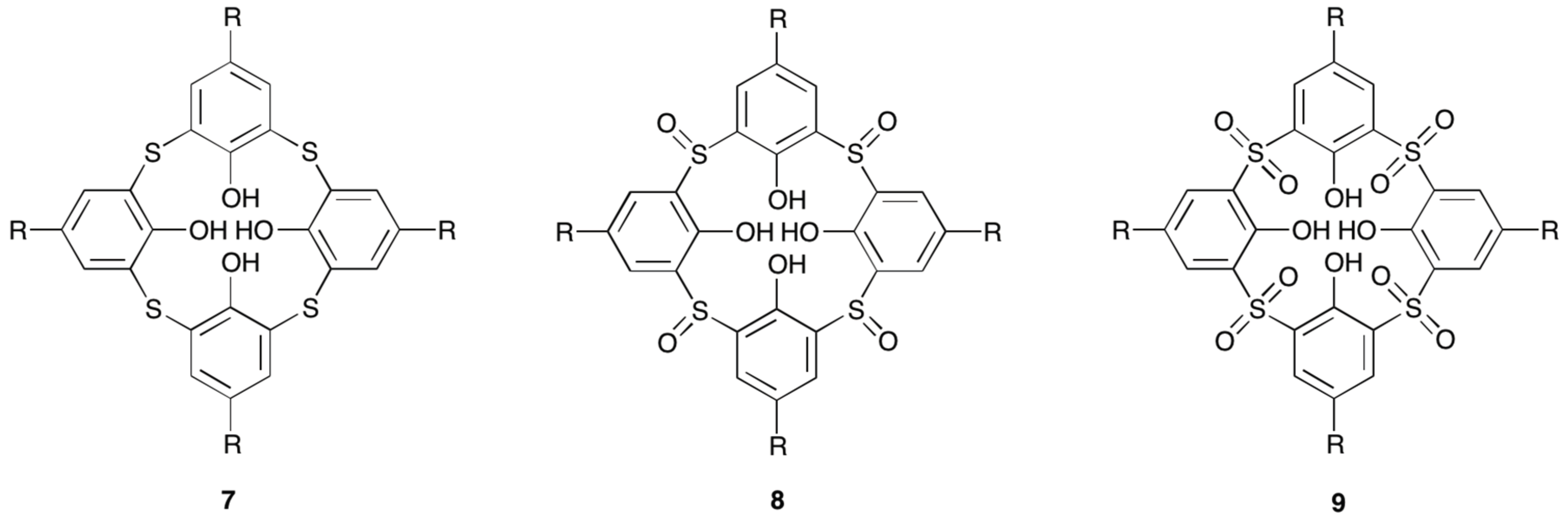
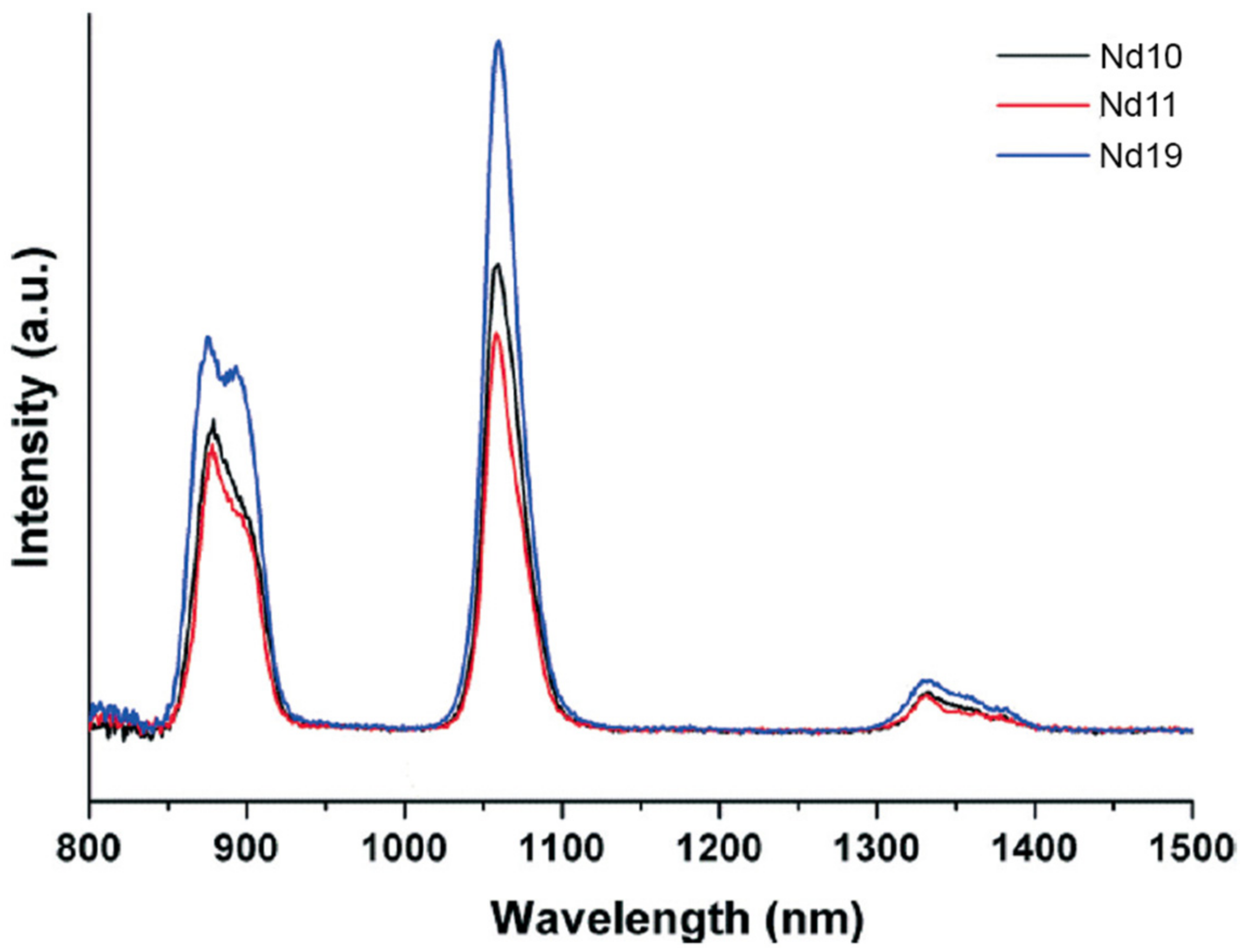
- Su, K.Z.; Jiang, F.L.; Wu, M.Y.; Qian, J.J.; Pang, J.D.; Yuan, D.Q.; Hong, M.C. Syntheses, structures, luminescence and magnetic properties of three high-nuclearity neodymium compounds based on mixed sulfonylcalix[4]arene-phosphonate ligands. CrystEngComm 2016, 18, 4921–4928. [Google Scholar] [CrossRef]
- Shangguan, L.Q.; Xing, H.; Mondal, J.H.; Shi, B.B. Novel rare earth fluorescent supramolecular polymeric assemblies constructed by orthogonal pillar[5]arene-based molecular recognition, Eu(III)-coordination and π–π donor–acceptor interactions. Chem. Commun. 2017, 53, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Shamsutdinova, N.; Zairov, R.; Mustafina, A.; Podyachev, S.; Sudakova, S.; Nizameev, I.; Kadirov, M.; Amirov, R. Interfacial interactions of hard polyelectrolyte-stabilized luminescent colloids with substrates. Colloids Surf. A 2015, 482, 231–240. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Massi, M.; Ogden, M.I. Luminescent Lanthanoid Calixarene Complexes and Materials. Materials 2017, 10, 1369. https://doi.org/10.3390/ma10121369
Massi M, Ogden MI. Luminescent Lanthanoid Calixarene Complexes and Materials. Materials. 2017; 10(12):1369. https://doi.org/10.3390/ma10121369
Chicago/Turabian StyleMassi, Massimiliano, and Mark I. Ogden. 2017. "Luminescent Lanthanoid Calixarene Complexes and Materials" Materials 10, no. 12: 1369. https://doi.org/10.3390/ma10121369




