Structure and Mechanical Properties of Al-Cu-Fe-X Alloys with Excellent Thermal Stability
Abstract
:1. Introduction
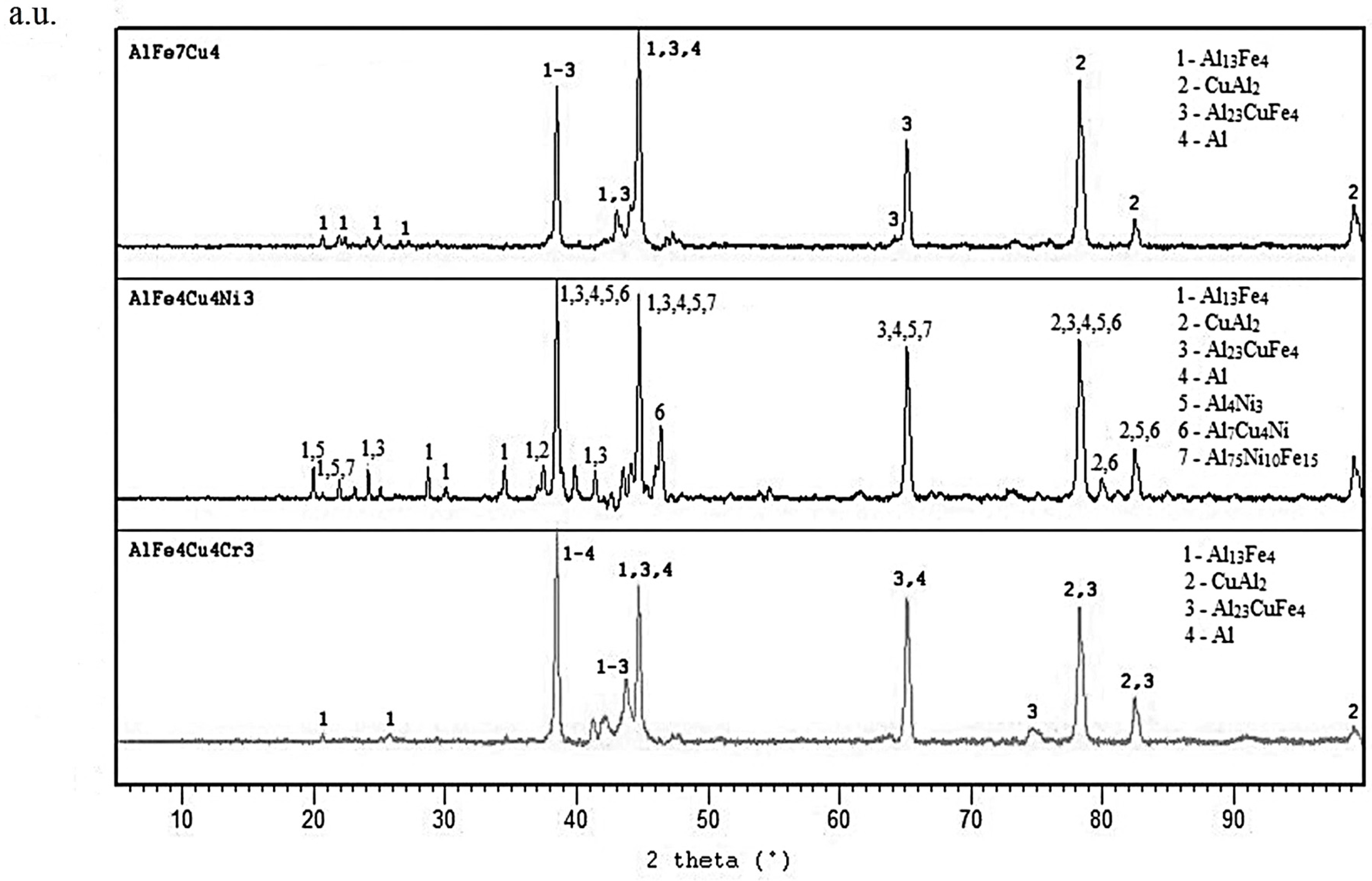
2. Results and Discussion
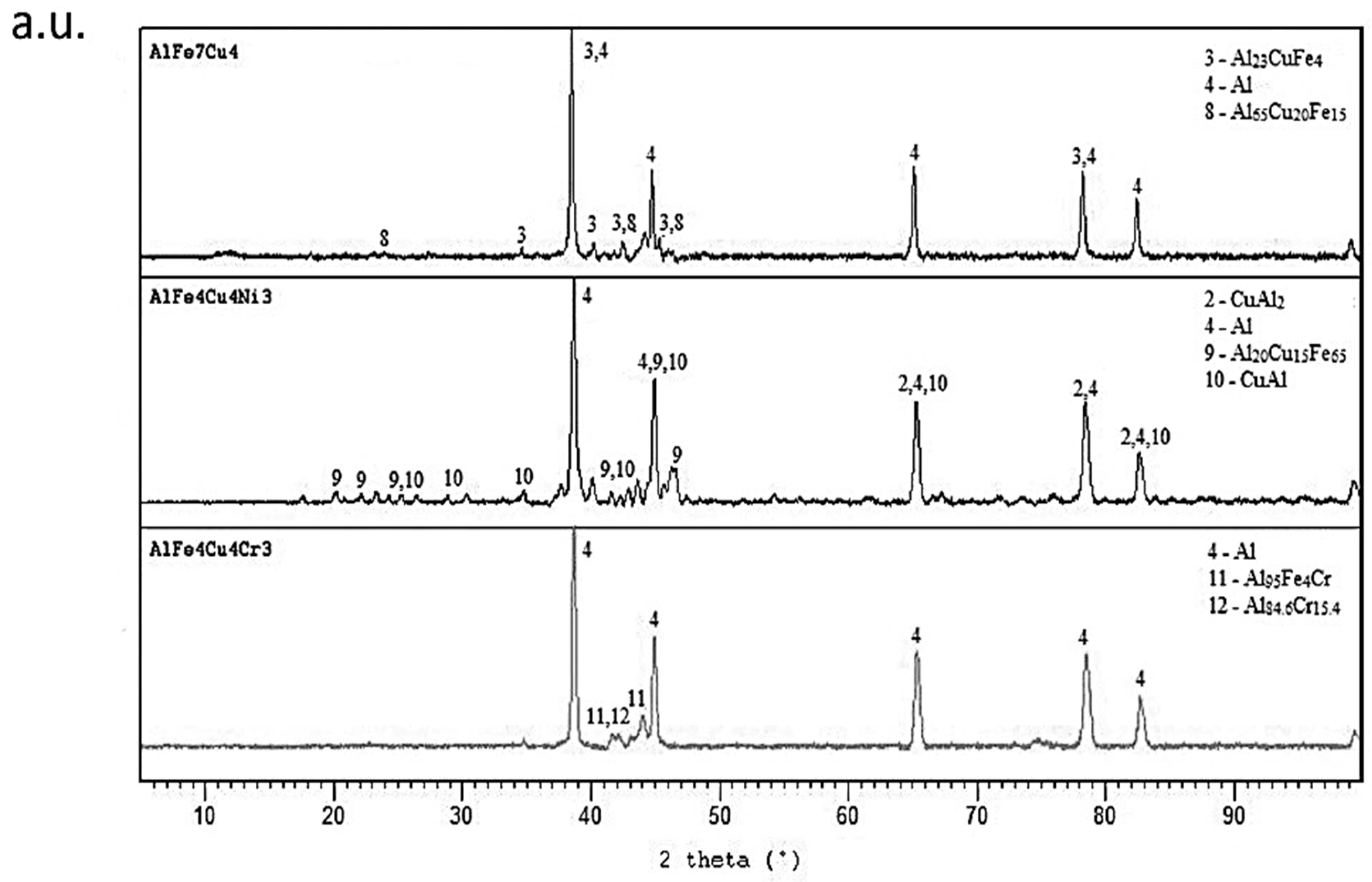
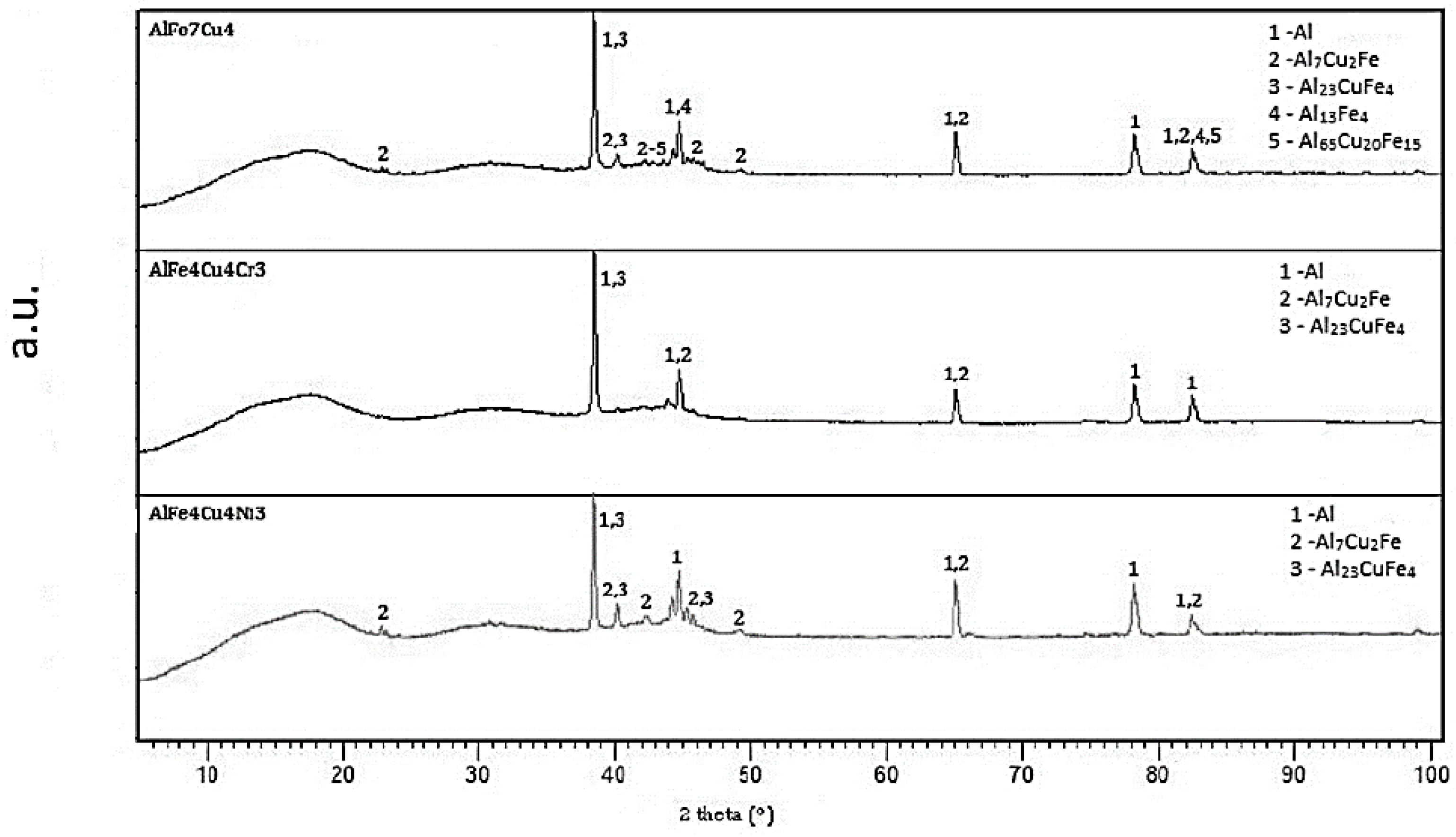
2.1. Microstructure of As-Cast Alloys
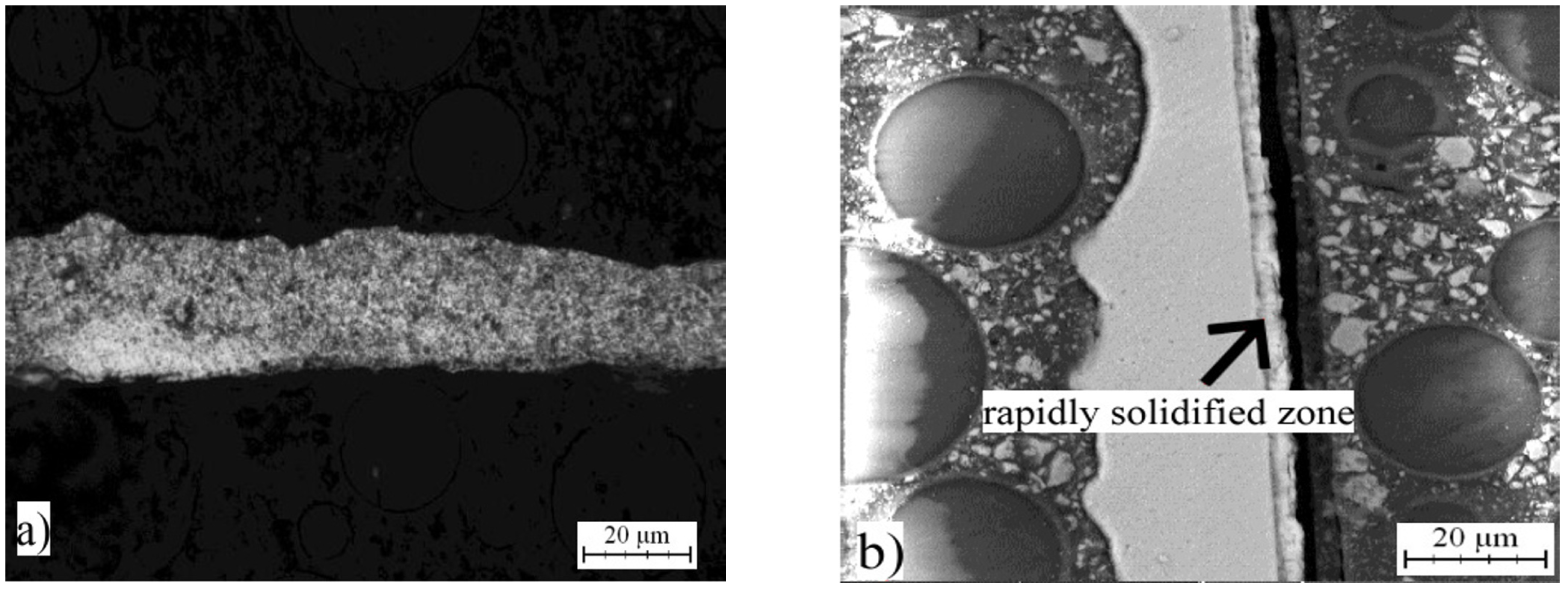
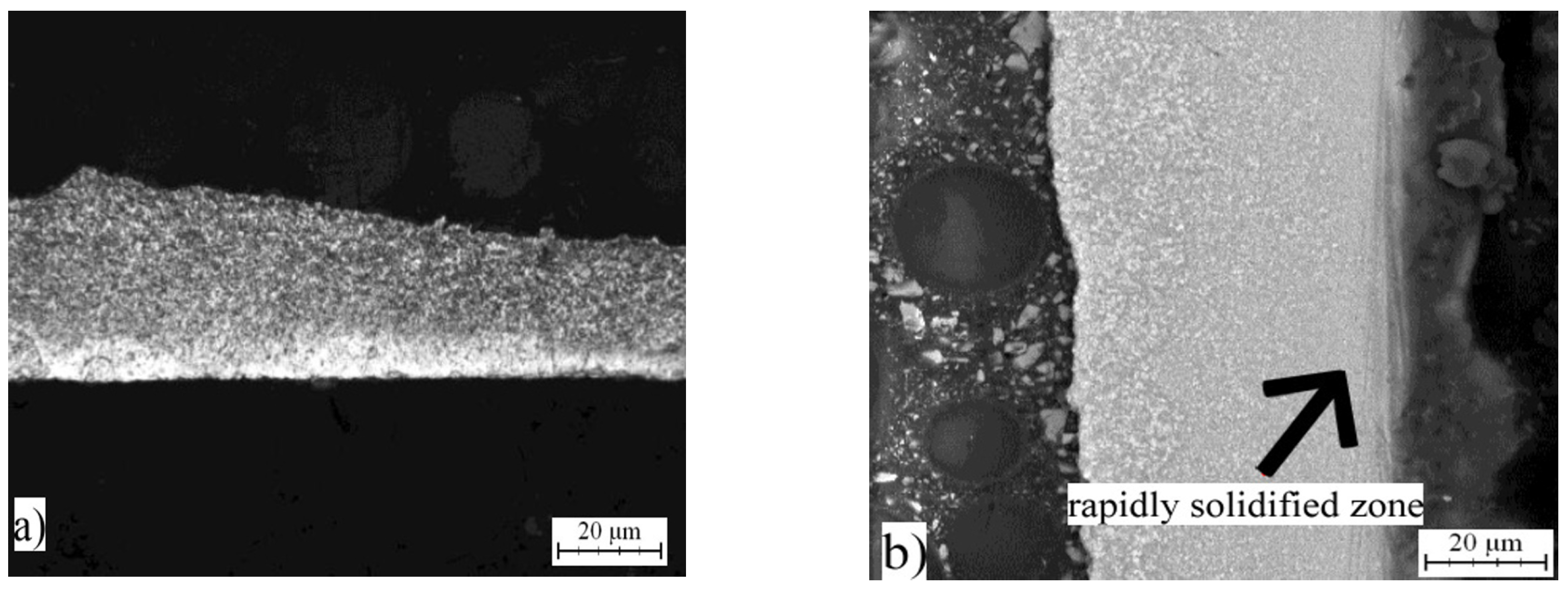
2.2. Microstructure of Rapidly Solidified Alloys
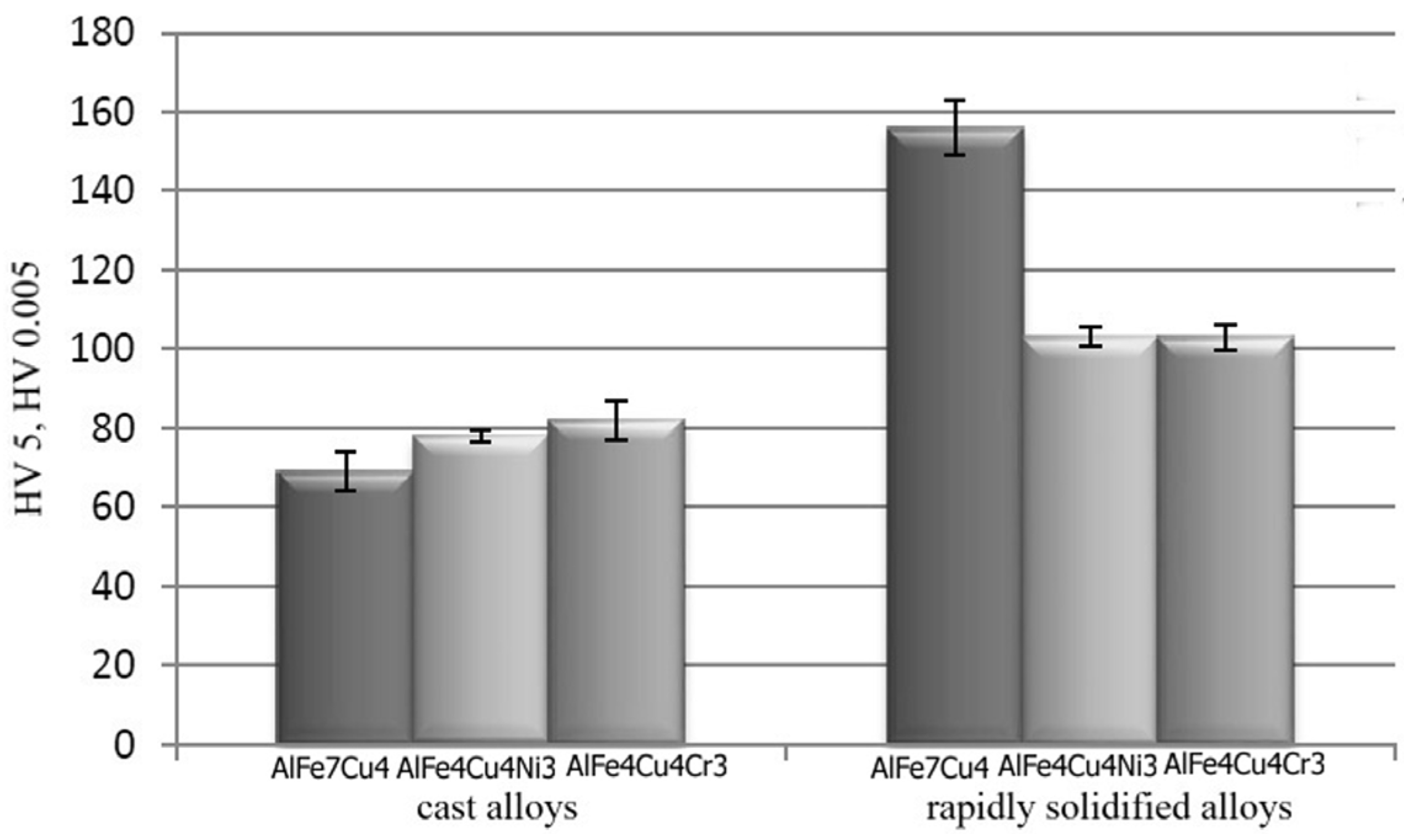
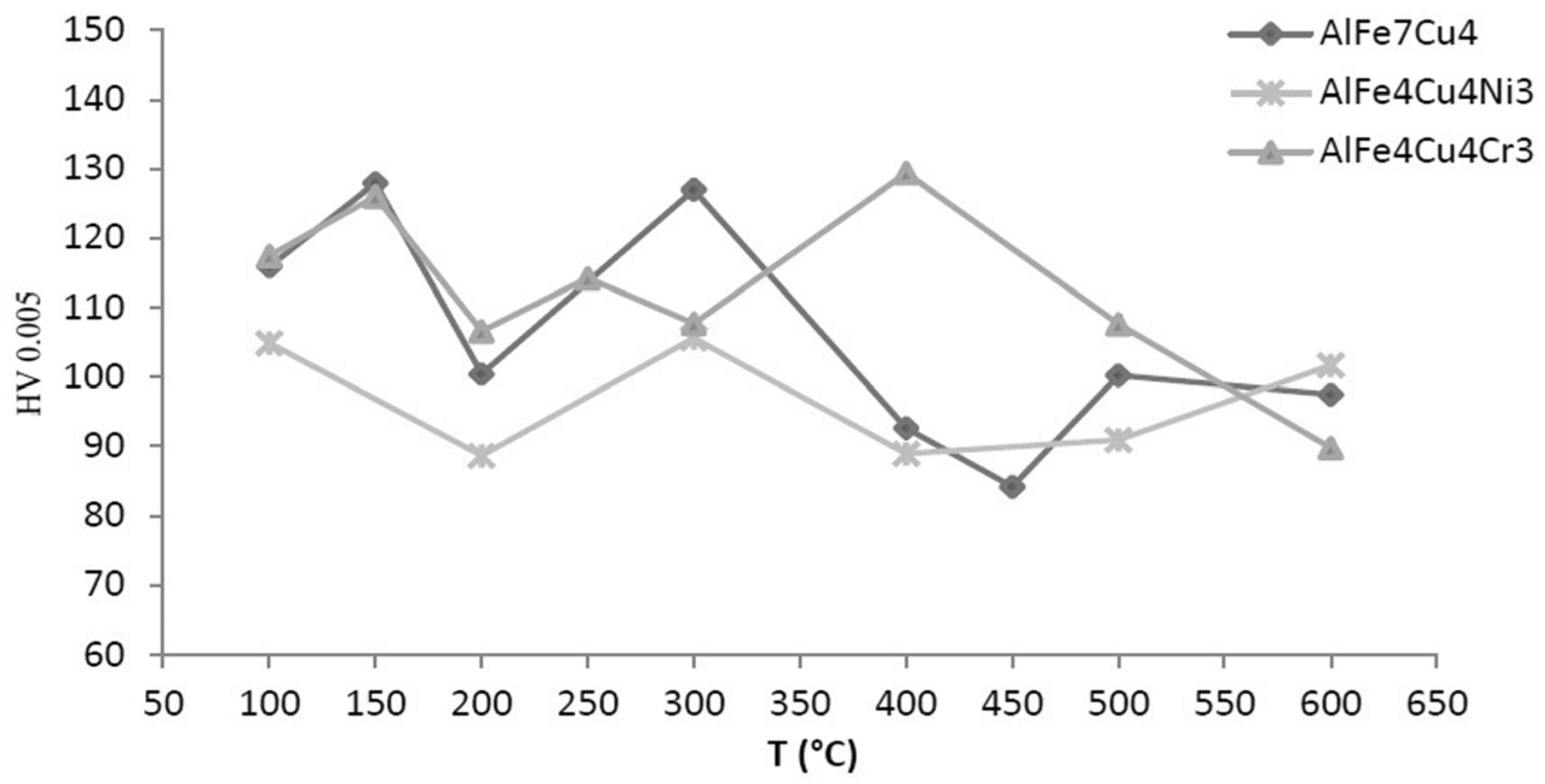
2.3. Hardness of Alloys
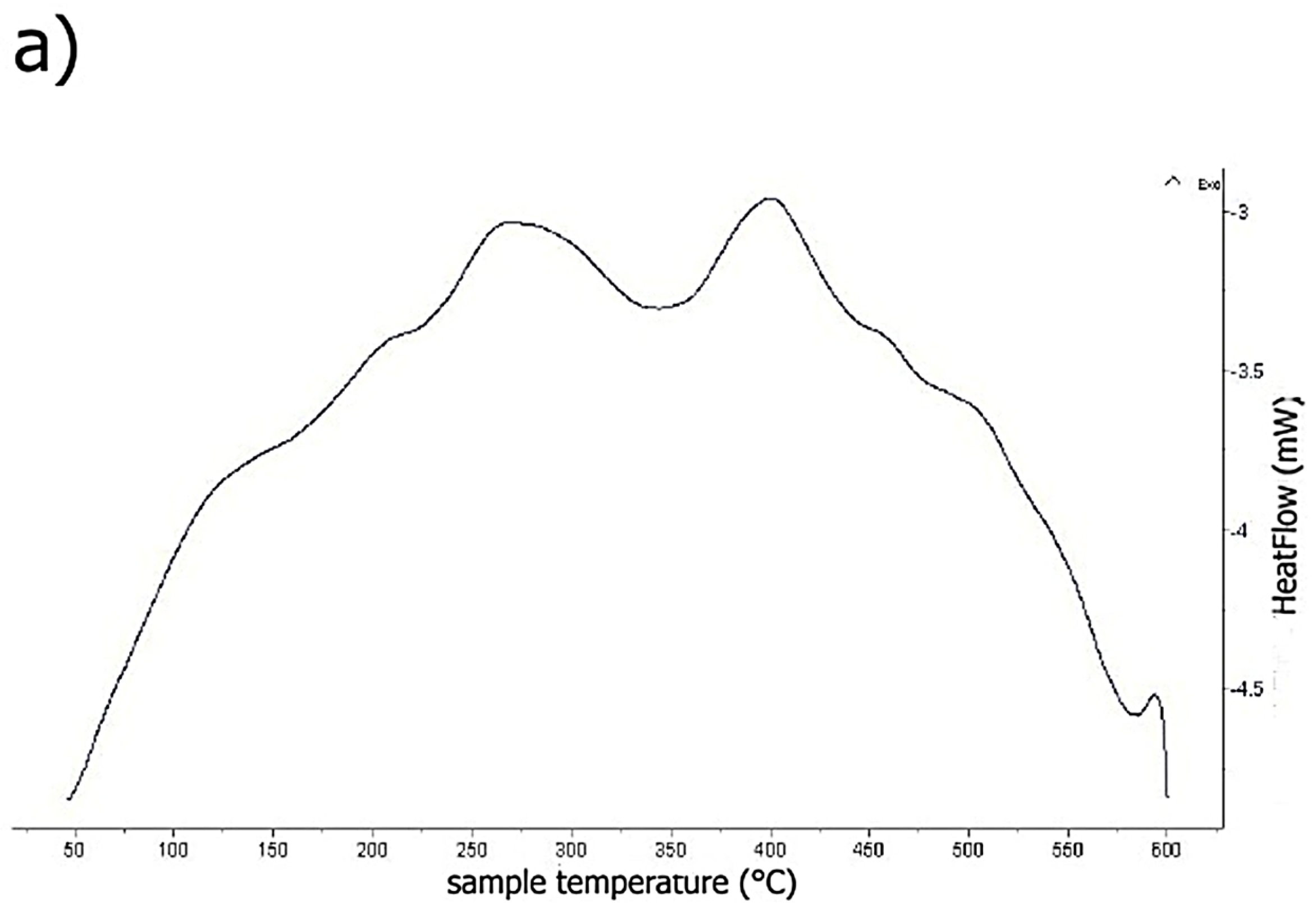
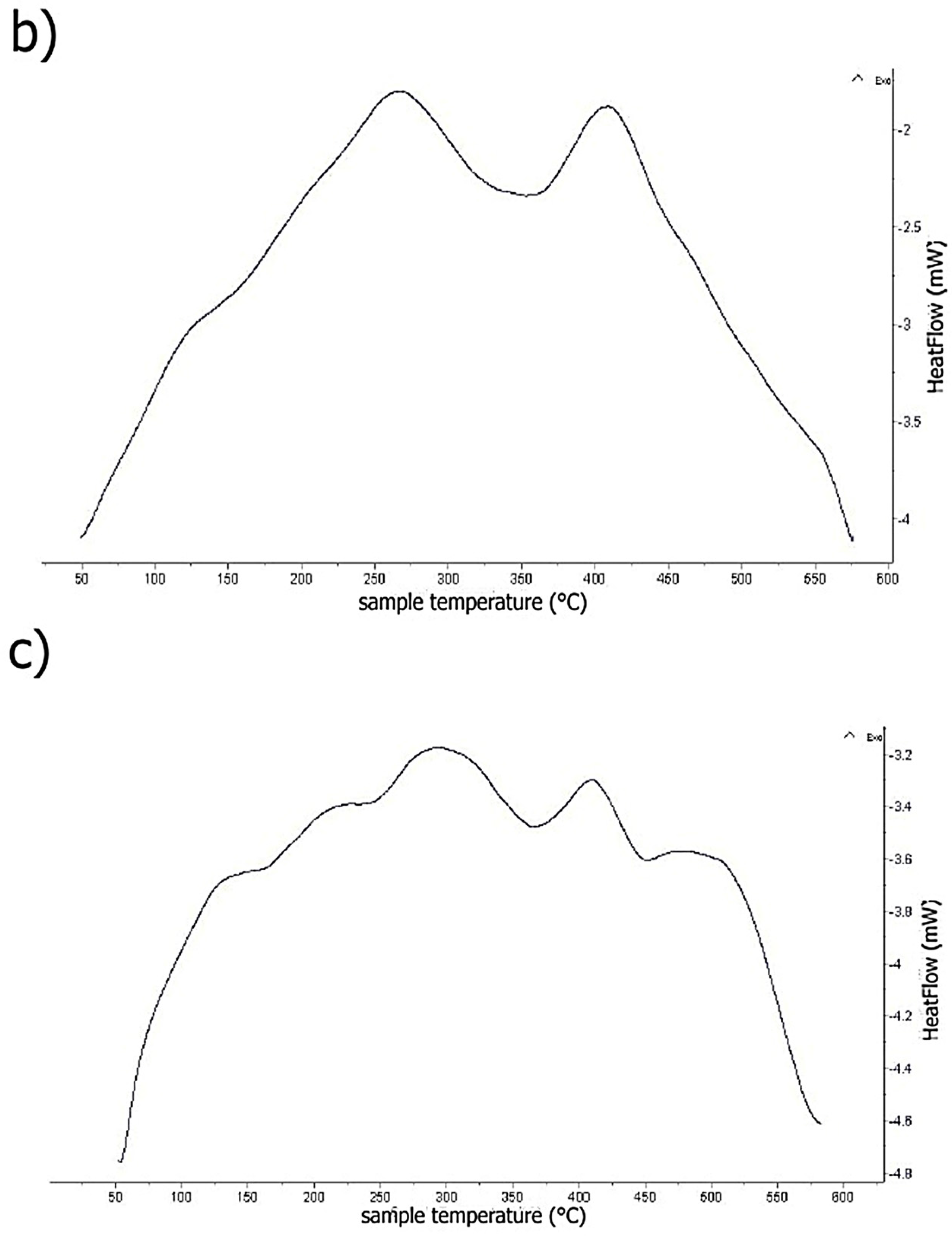
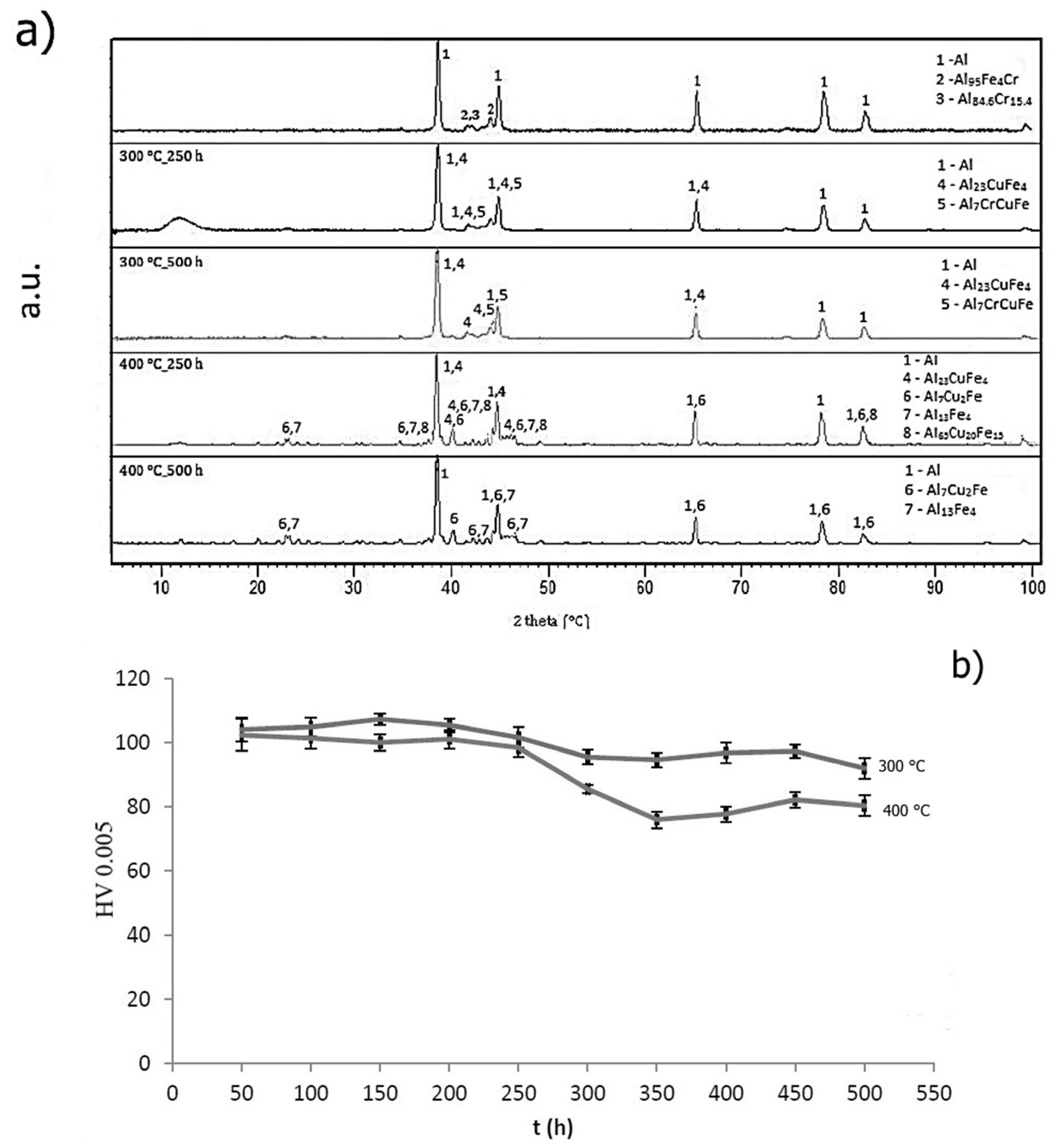
2.4. Thermal Stability of Rapidly Solidified Ribbons
2.4.1. Thermal Analysis
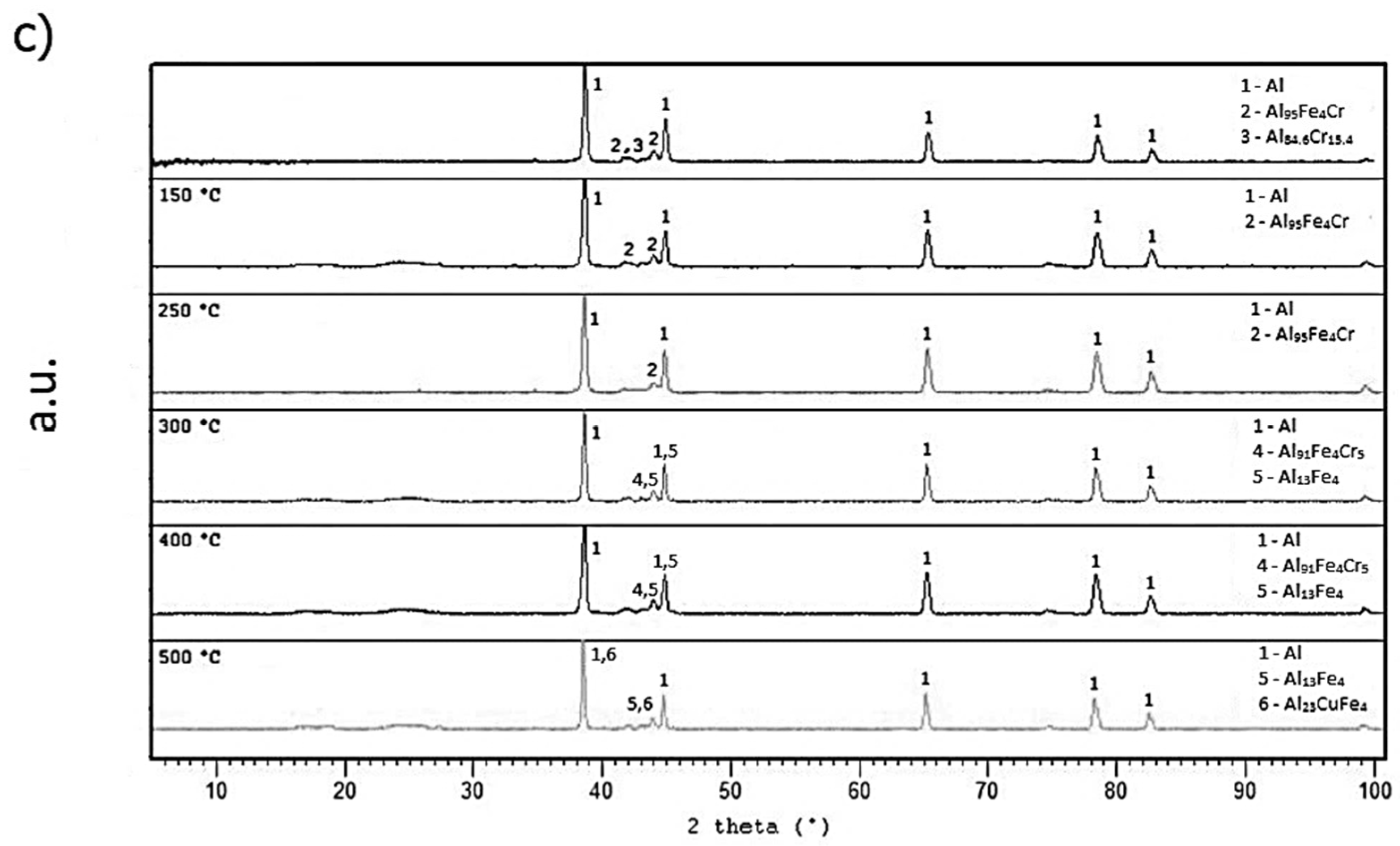
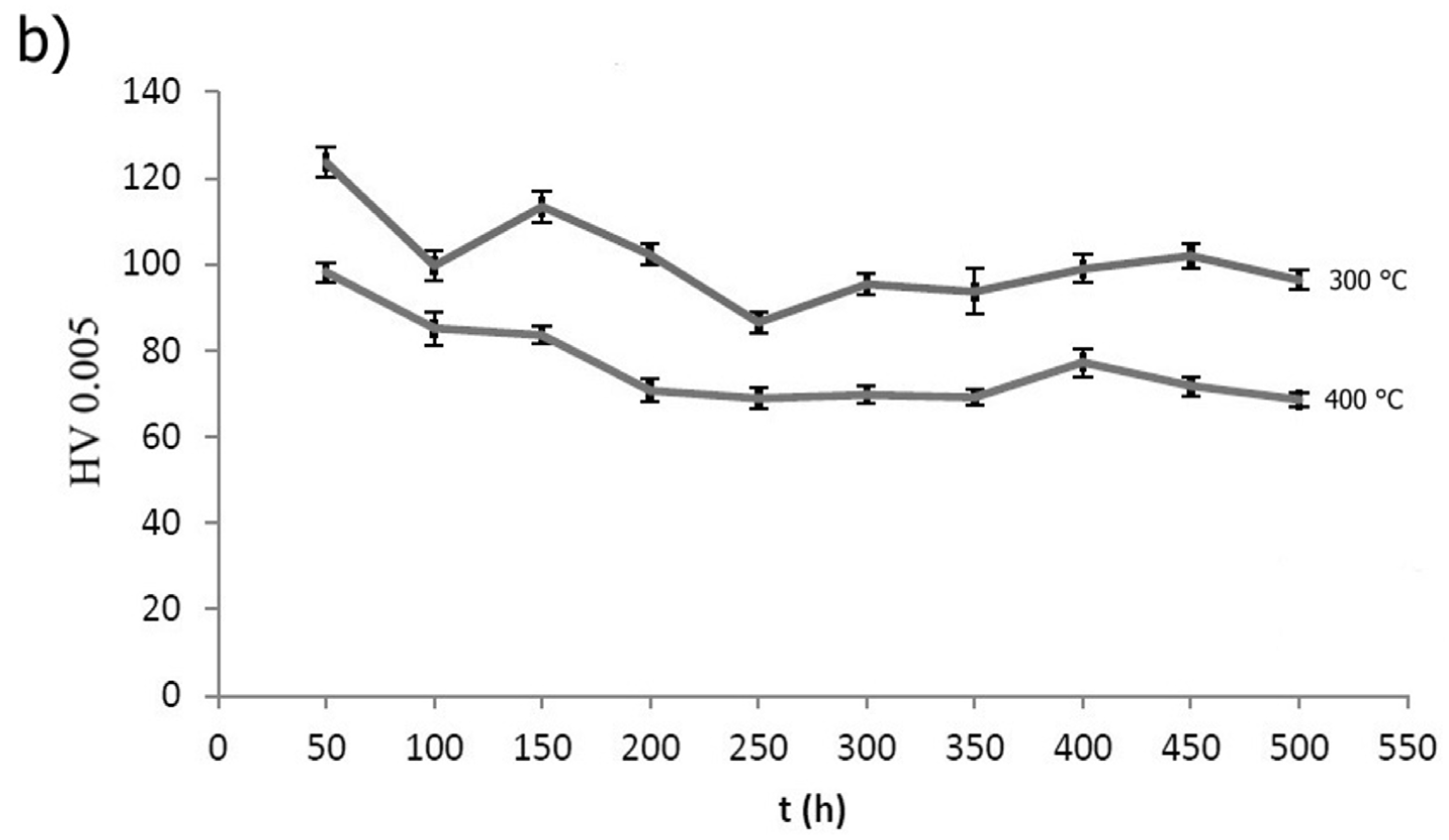
2.4.2. Short-Term Annealing
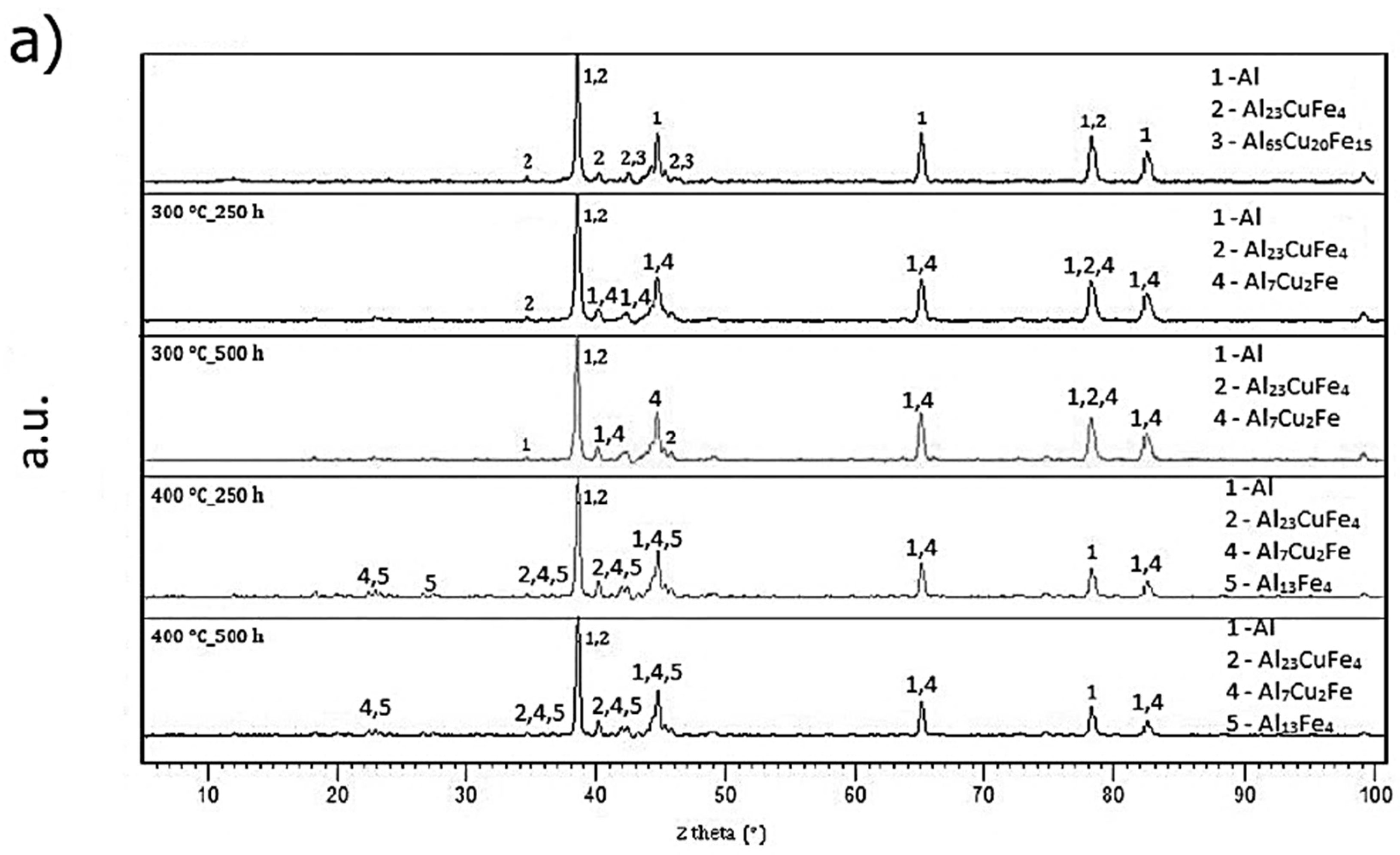
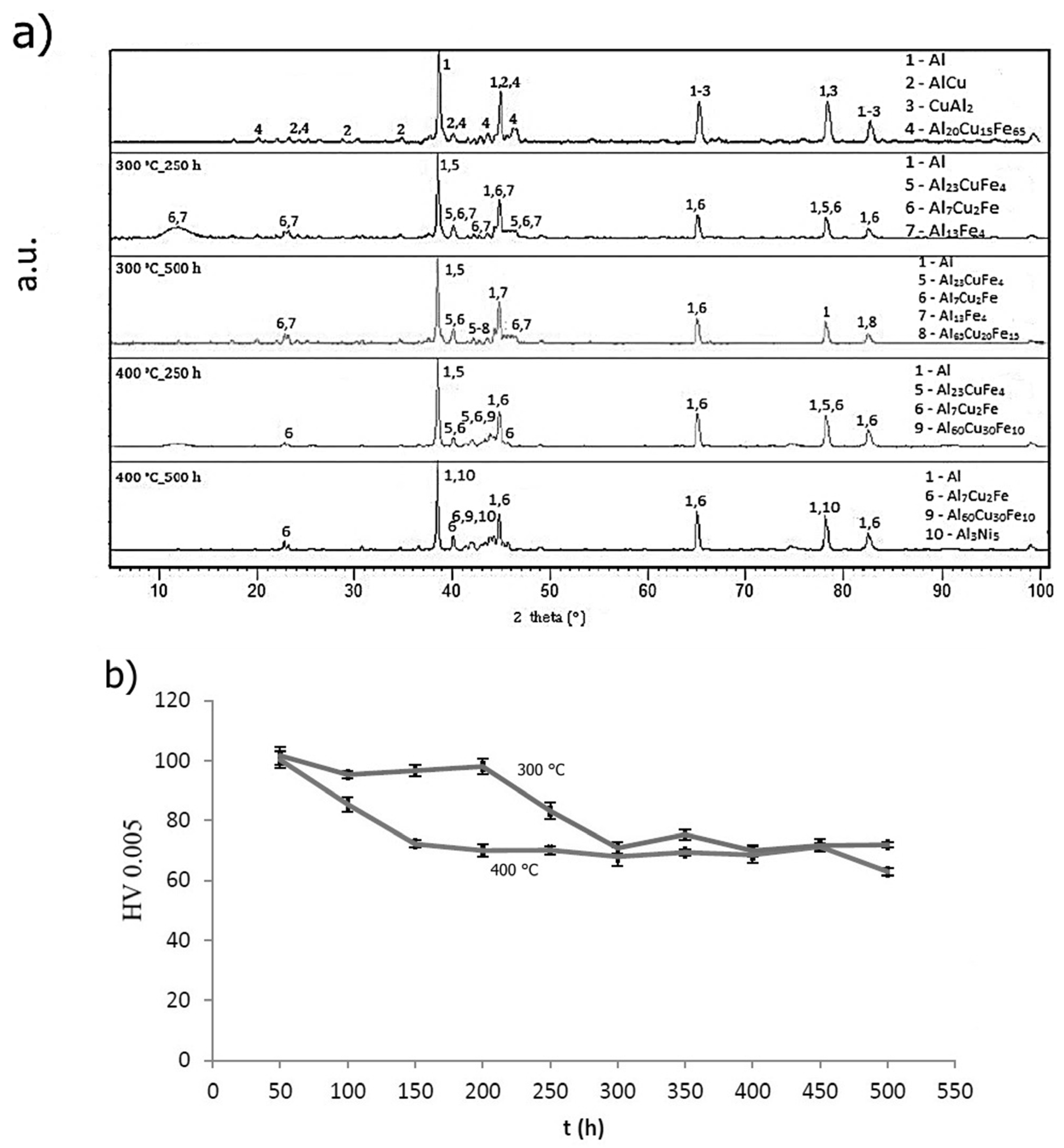
2.4.3. Long-Term Annealing
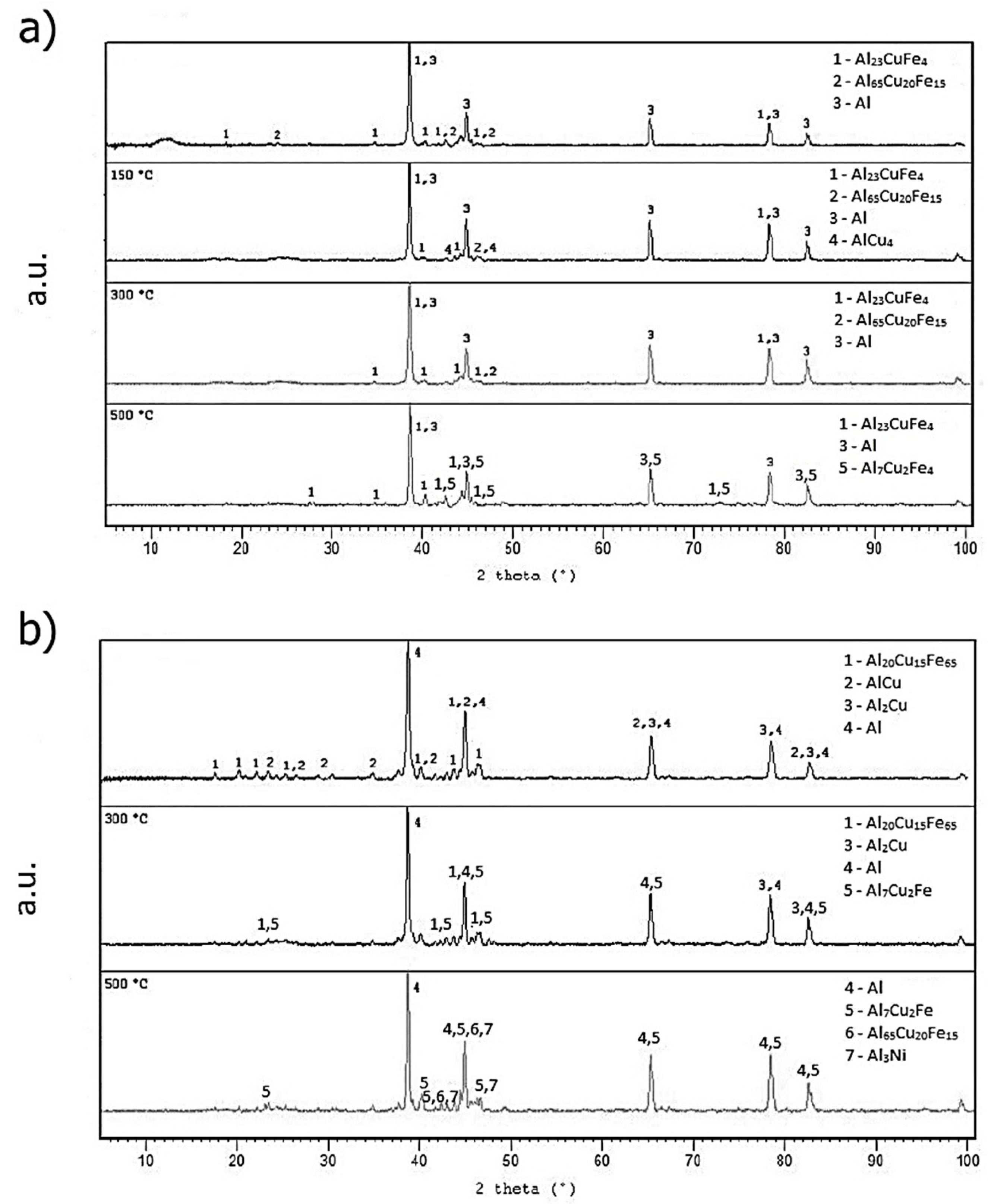
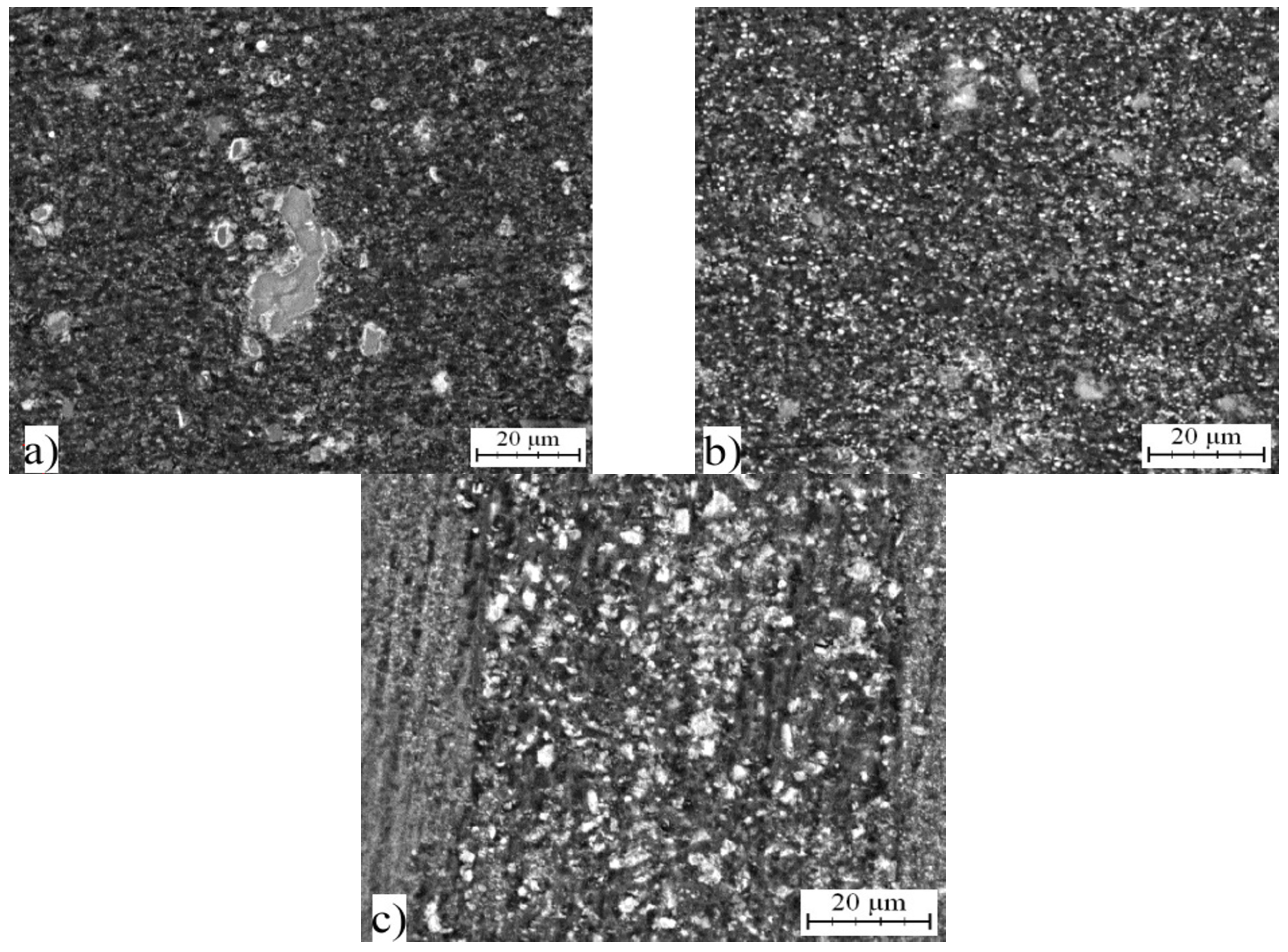
2.5. Microstructure and Phase Composition of Compact Alloys
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, T.S.; Suryanarayana, C.; Chun, B.S. Effect of alloying elements and degassing pressure on the structure and mechanical properties of rapidly solidified Al-20Si-5Fe-2X (X = Cr, Zr, or Ni) alloys. Mater. Sci. Eng. A 2000, 278, 113–120. [Google Scholar] [CrossRef]
- Průša, F.; Vojtěch, D. Mechanical properties and thermal stability of Al-23Si-8Fe-1Cr and Al-23Si-8Fe-5Mn alloys prepared by powder metallurgy. Mater. Sci. Eng. A 2013, 565, 13–20. [Google Scholar] [CrossRef]
- Rajabi, M.; Simchi, A.; Vahidi, M.; Davami, P. Effect of particle size on the microstructure of rapidly solidified Al-20Si-5Fe-2X (X = Cu, Ni, Cr) powder. J. Alloy. Compd. 2008, 466, 111–118. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E.; Vuorinen, J. Preparation and characterisation of melt-spun Al-Cu-Fe quasicrystals. Intermetallics 2005, 13, 885–895. [Google Scholar] [CrossRef]
- Shechtman, D.; Blech, I.; Gratias, D.; Cahn, J.W. Metallic phase with long-range orientational order and no translational symmetry. Phys. Rev. Lett. 1984, 53, 1951. [Google Scholar] [CrossRef]
- Samavat, F.; Tavakoli, M.H.; Habibi, S.; Jaleh, B.; Ahmad, P.T. Quasicrystals. Open J. Phys. Chem. 2012, 2, 7–14. [Google Scholar] [CrossRef]
- Lityńska-Dobrzyńska, L.; Dutkiewicz, J.; Stan-Głowińska, K.; Wajda, W.; Dembinski, L.; Langlade, C.; Coddet, C. Characterization of aluminium matrix composites reinforced by Al-Cu-Fe quasicrystalline particles. J. Alloy. Compd. 2015, 643, 114–118. [Google Scholar] [CrossRef]
- Dubois, J.C.; Kang, S.S.; Perrot, A. Towards applications of quasicrystals. Mater. Sci. Eng. A 1994, 179, 122–126. [Google Scholar] [CrossRef]
- Huttunen-Saarivirta, E. Microstructure, fabrication and properties of quasicrystalline Al–Cu–Fe alloys: A review. J. Alloy. Compd. 2004, 363, 154–178. [Google Scholar] [CrossRef]
- Laplanche, G.; Bonneville, J.; Joulain, A.; Gauthier-Brunet, V.; Dubois, S. Mechanical properties of Al-Cu-Fe quasicrystalline and crystalline phases: An analogy. Intermetallics 2014, 50, 54–58. [Google Scholar] [CrossRef]
- Ali, F.; Scudino, S.; Liu, G.; Srivastava, V.C.; Mukhopadhyay, N.K.; Samadi Khoshkhoo, M.; Prashanth, K.G.; Uhlenwinkel, V.; Calin, M.; Eckert, J. Modeling the strengthening effect of Al-Cu-Fe quasicrystalline particles in Al-based metal matrix composites. J. Alloy. Compd. 2012, 536, 130–133. [Google Scholar] [CrossRef]
- Singh, A.; Ranganathan, S. A transmission icosahedral electron microscopic study of twins-II. A rapidly solidified Al-Cu-Fe alloy. Acta. Metall. 1995, 43, 3553–3562. [Google Scholar] [CrossRef]
- Guo, J.Q.; Kazama, N.S. Mechanical properties of rapidly solidified Al-Ti-Fe, Al-Cu-Fe and Al-Fe-Cu-Ti based alloys extruded from their atomized powders. Mater. Sci. Eng. A 1997, 232, 177–182. [Google Scholar] [CrossRef]
- Andersen, S.J.; Guo, X.Y.; Høier, R.; Waterloo, G. Microstructure of rapidly solidified A1-7.5Cu-2.5Fe. Mater. Sci. Eng. A 1994, 179–180, 665–668. [Google Scholar] [CrossRef]
- Novák, P.; Kubatík, T.; Vystrčil, J.; Hendrych, R.; Kříž, J.; Mlynár, J.; Vojtěch, D. Powder metallurgy preparation of Al-Cu-Fe quasicrystals using mechanical alloying and spark plasma sintering. Intermetallics 2014, 52, 131–137. [Google Scholar] [CrossRef]
- Lee, S.M.; Jung, J.H.; Fleury, E.; Kim, W.T.; Kim, D.H. Metal matrix composites reinforced by gas-atomised Al-Cu-Fe powders. Mater. Sci. Eng. A 2000, 294–296, 99–103. [Google Scholar] [CrossRef]
- Tsai, A.P.; Inoue, A.; Masumoto, T. New quasicrystals in AI65Cu20Mn15 (M = Cr, Mn or Fe) systems prepared by rapid solidification. J. Mater. Sci. Lett. 1988, 7, 322–326. [Google Scholar] [CrossRef]
- Inoue, A. Amorphous, nanoquasicrystalline and nanocrystalline alloys in Al-based systems. Prog. Mater. Sci. 1998, 43, 365–520. [Google Scholar] [CrossRef]
- Ustinov, A.I.; Movchan, B.A.; Polishchuk, S.S. Formation of nanoquasicrystalline Al-Cu-Fe coatings at electron beam physical vapour deposition. Scripta. Mat. 2004, 50, 533–537. [Google Scholar] [CrossRef]
- Rosas, G.; Perez, R. On the transformations of the ψ-AlCuFe icosahedral phase. Mater. Lett. 2001, 47, 225–230. [Google Scholar] [CrossRef]
- Tcherdyntsev, V.V.; Kaloshkin, S.D.; Shelekhov, E.V.; Salimon, A.I.; Sartori, S.; Principi, G. Quasicrystalline phase formation in the mechanically alloyed Al-Cu-Fe system. Intermetallics 2005, 13, 841–847. [Google Scholar] [CrossRef]
- Tsai, A.; Inoue, A.; Masumoto, T. Preparation of a new AI-Cu-Fe quasicrystal with large grain sizes by rapid solidification. J. Mater. Sci. Lett. 1987, 6, 1403–1405. [Google Scholar] [CrossRef]
- Li, L.; Bi, Q.; Yang, J.; Fu, L.; Wang, L.; Wang, S.; Liu, W. Large-scale synthesis of Al-Cu-Fe submicron quasicrystals. Scripta. Mat. 2008, 59, 587–590. [Google Scholar] [CrossRef]
- Soltani, N.; Jafari Nodooshan, H.R.; Bahrami, A.; Pech-Canul, M.I.; Liu, W.; Wu, G. Effect of hot extrusion on wear properties of Al-15 wt % Mg2Si in situ metal matrix composites. Mater. Design 2014, 53, 774–781. [Google Scholar] [CrossRef]
- Deaquino-Lara, R.; Soltani, N.; Bahrami, A.; Gutiérrez-Castañeda, E.; García-Sánchez, E.; Hernandez-Rodriguez, M.A.L. Tribological characterization of Al7075-graphite composites fabricated by mechanical alloying and hot extrusion. Mater. Design 2015, 67, 224–231. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.I. The effect of Ti on mechanical properties of extruded in-situ Al-15 pct Mg2Si composite. Metall. Mater. Trans. A 2013, 44, 4366–4373. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Moghimi, F.M.; Pech-Canul, M.I.; Hajaghasi, A. The simultaneous effect of extrusion and T6 treatment on the mechanical properties of Al-15 wt % Mg2Si composite. J. Heat Treat. Mater. 2012, 67, 378–385. [Google Scholar] [CrossRef]
- Rosas, G.; Reyes-Gasga, J.; Pérez, R. Morphological characteristics of the rapidly and conventionally solidified alloys of the AlCuFe system. Mater. Charact. 2007, 58, 765–770. [Google Scholar] [CrossRef]
- Wang, E.R.; Hui, X.D.; Wang, S.S.; Zhao, Y.F.; Chen, G.L. Improved mechanical properties in cast Al-Si alloys by combined alloying of Fe and Cu. Mater. Sci. Eng. A 2010, 527, 7878–7884. [Google Scholar] [CrossRef]
- Zhang, L.; Lück, R. Phase equilibria of the icosahedral Al-Cu-Fe phase. J. Alloy. Compd 2002, 342, 53–56. [Google Scholar] [CrossRef]
- Ohashi, T.; Dai, L.; Fukatsu, N. Decomposition characteristics of Al-Mn-Zr alloys rapidly-quenched from melt. Metall. Trans. A 1986, 17, 799–806. [Google Scholar] [CrossRef]
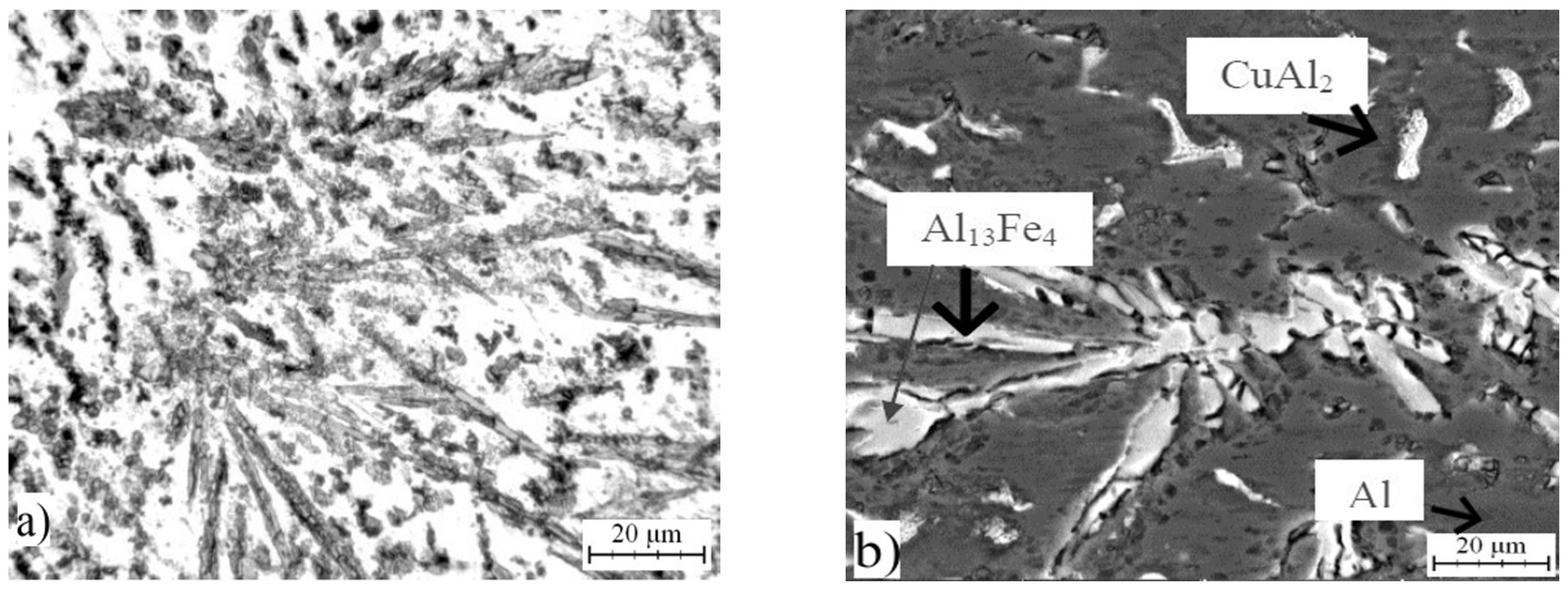
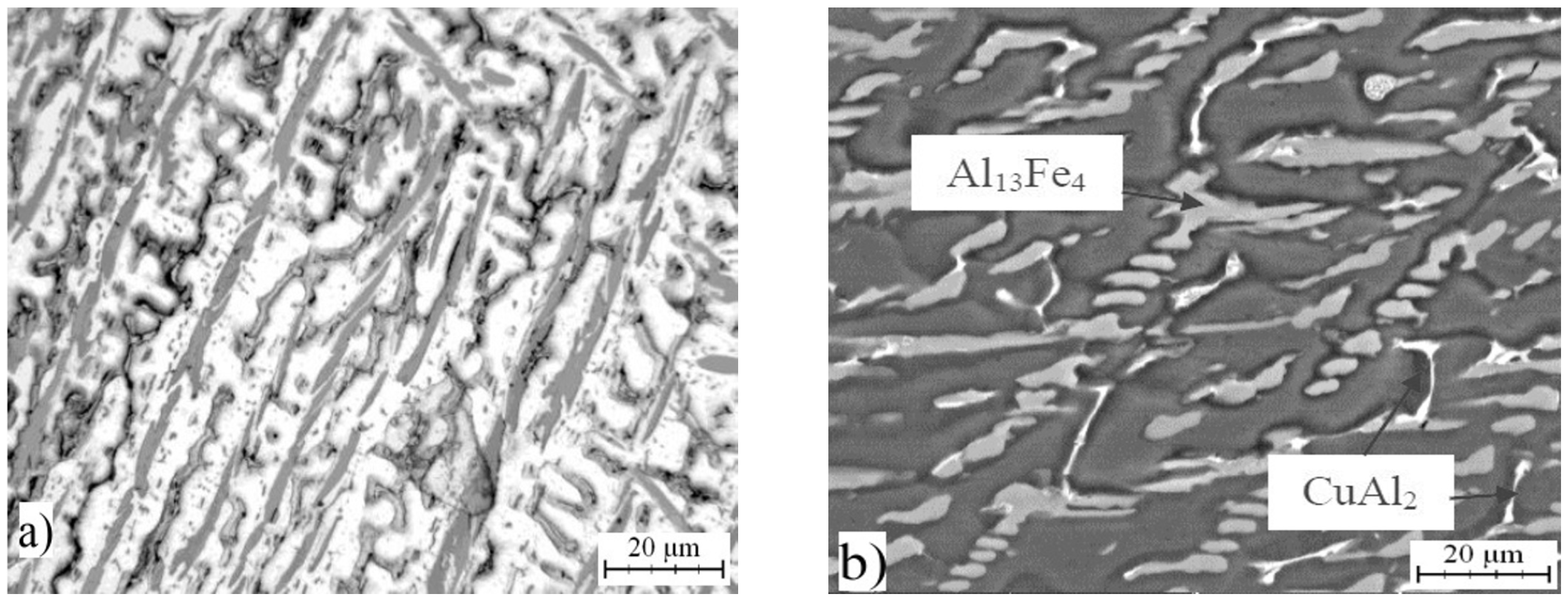
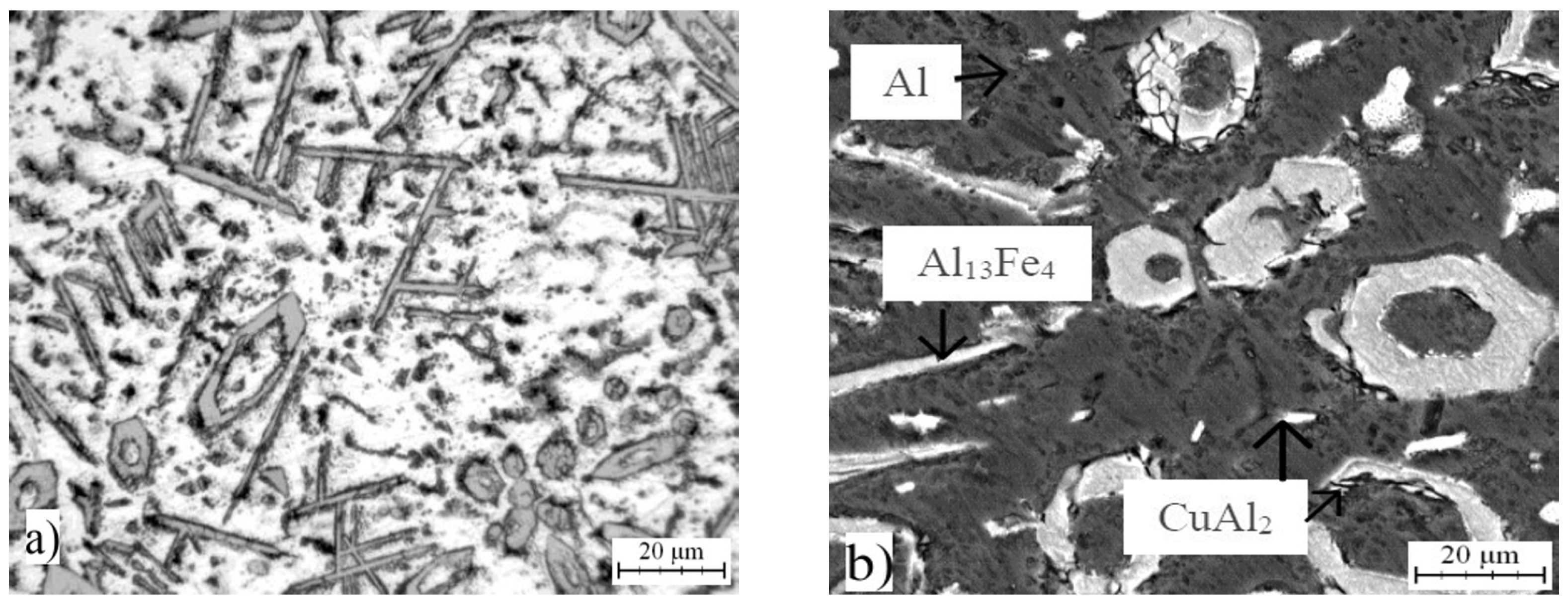

| Phase | Chemical Composition in wt % | ||
|---|---|---|---|
| Al | Fe | Cu | |
| Al | 98 ± 7.86 | 1 ± 1.74 | 1 ± 0.37 |
| Al13Fe4 | 63 ± 0.35 | 35 ± 0.36 | 2 ± 0.69 |
| CuAl2 | 66 ± 0.95 | 1 ± 0.04 | 33 ± 0.99 |
| Phase | Chemical Composition in wt % | |||
|---|---|---|---|---|
| Al | Fe | Cu | Ni | |
| Al | 95 ± 2.26 | 1 ± 0.56 | 3 ± 1.10 | 1 ± 0.63 |
| Al13Fe4 | 72 ± 2.98 | 16 ± 1.39 | 1 ± 0.51 | 11 ± 1.10 |
| CuAl2 | 75 ± 8.63 | 1 ± 0.07 | 22 ± 8.16 | 2 ± 0.54 |
| Phase | Chemical Composition in wt % | |||
|---|---|---|---|---|
| Al | Fe | Cu | Cr | |
| Al | 97 ± 1.56 | 0.5 ± 0.53 | 2 ± 0.64 | 0.5 ± 0.44 |
| Al13Fe4 | 76 ± 3.38 | 11 ± 3.04 | 3 ± 0.47 | 10 ± 2.87 |
| CuAl2 | 71 ± 10.77 | 0.5 ± 0.21 | 28 ± 10.56 | 0.5 ± 0 |
| Alloy | Crystallite Size (Å) |
|---|---|
| AlFe7Cu4 | 1558 |
| AlFe4Cu4Ni3 | 1279 |
| AlFe4Cu4Cr3 | 989 |
| Alloy | d(111) (Å) | |
|---|---|---|
| As-Cast Alloys | Rapidly Solidified Alloys | |
| AlFe7Cu4 | 2.33794 | 2.33796 |
| AlFe4Cu4Ni3 | 2.33770 | 2.32526 |
| AlFe4Cu4Cr3 | 2.33728 | 2.33131 |
| Alloy | Crystallite Size (Å) | ||||
|---|---|---|---|---|---|
| Not-Annealed | 150 °C | 250 °C | 300 °C | 500 °C | |
| AlCu7Fe4 | 486 | 1975 | - | 878 | 1625 |
| AlCu4Fe4Ni3 | 660 | - | - | 1591 | 2922 |
| AlCu4Fe4Cr3 | 434 | 445 | 808 | 1225 | 2186 |
| Alloy | Crystallite Size (Å) | |||
|---|---|---|---|---|
| 300 °C, 250 h | 300 °C, 500 h | 400 °C, 250 h | 400 °C, 500 h | |
| AlFe7Cu4 | 498 | 714 | 498 | 774 |
| AlFe4Cu4Ni3 | 687 | 793 | 694 | 1360 |
| AlFe4Cu4Cr3 | 745 | 805 | 749 | 858 |
| Alloy | () |
|---|---|
| AlFe7Cu4 | 698 |
| AlFe4Cu4Ni3 | 850 |
| AlFe4Cu4Cr3 | 1060 |
| Alloy | d(111) (Å) |
|---|---|
| AlFe7Cu4 | 2.33625 |
| AlFe4Cu4Ni3 | 2.3368 |
| AlFe4Cu4Cr3 | 2.3354 |
| Alloy | Weighed Portion (g) | ||||
|---|---|---|---|---|---|
| AlFe11 | AlCr11 | Al | Cu | Ni | |
| AlFe7Cu4 | 318 | - | 162 | 20 | - |
| AlFe4Cu4Ni3 | 182 | - | 283 | 20 | 15 |
| AlFe4Cu4Cr3 | 182 | 136 | 162 | 20 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Školáková, A.; Novák, P.; Mejzlíková, L.; Průša, F.; Salvetr, P.; Vojtěch, D. Structure and Mechanical Properties of Al-Cu-Fe-X Alloys with Excellent Thermal Stability. Materials 2017, 10, 1269. https://doi.org/10.3390/ma10111269
Školáková A, Novák P, Mejzlíková L, Průša F, Salvetr P, Vojtěch D. Structure and Mechanical Properties of Al-Cu-Fe-X Alloys with Excellent Thermal Stability. Materials. 2017; 10(11):1269. https://doi.org/10.3390/ma10111269
Chicago/Turabian StyleŠkoláková, Andrea, Pavel Novák, Lucie Mejzlíková, Filip Průša, Pavel Salvetr, and Dalibor Vojtěch. 2017. "Structure and Mechanical Properties of Al-Cu-Fe-X Alloys with Excellent Thermal Stability" Materials 10, no. 11: 1269. https://doi.org/10.3390/ma10111269





