Phase Equilibria and Magnetic Phases in the Ce-Fe-Co-B System
Abstract
:1. Introduction
2. Literature Review
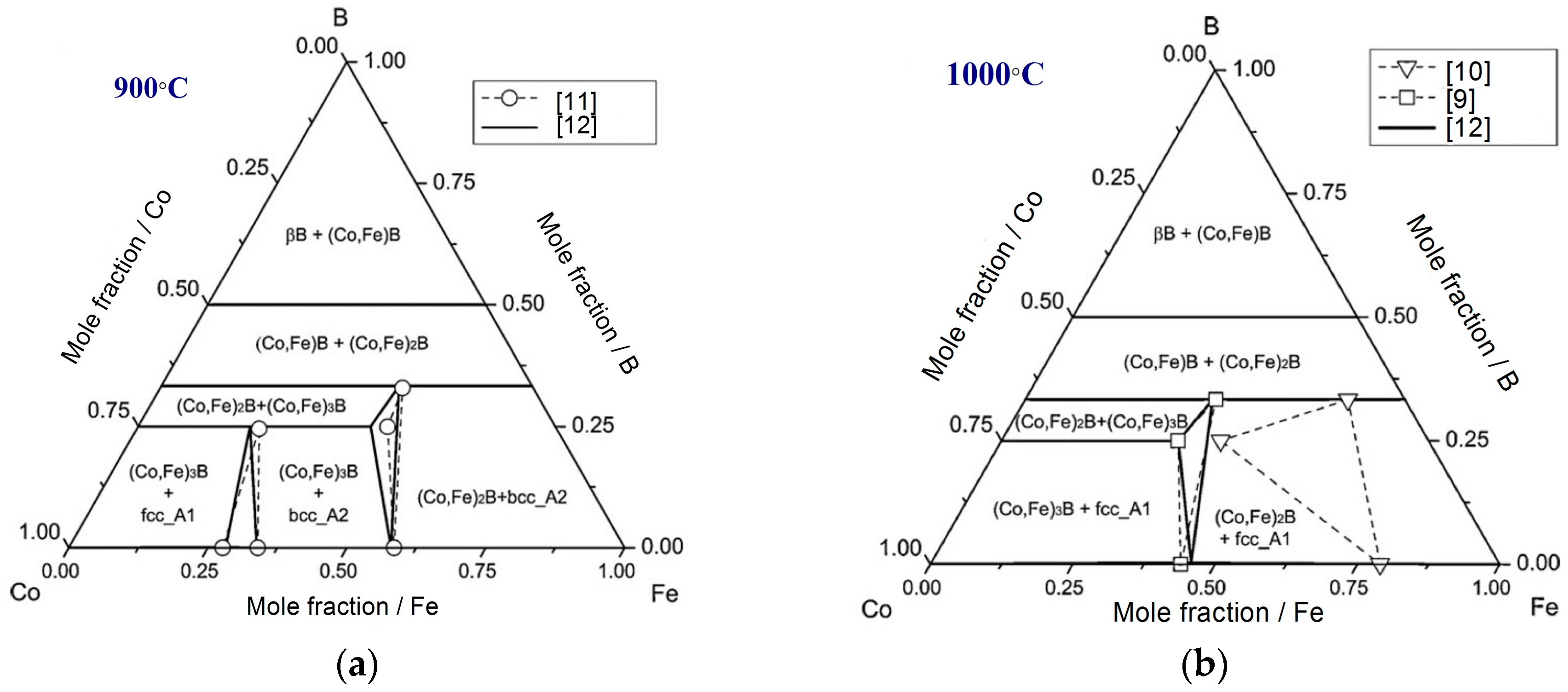
2.1. Ternary Systems
2.2. Quaternary System
3. Materials and Methods
4. Results and Discussion
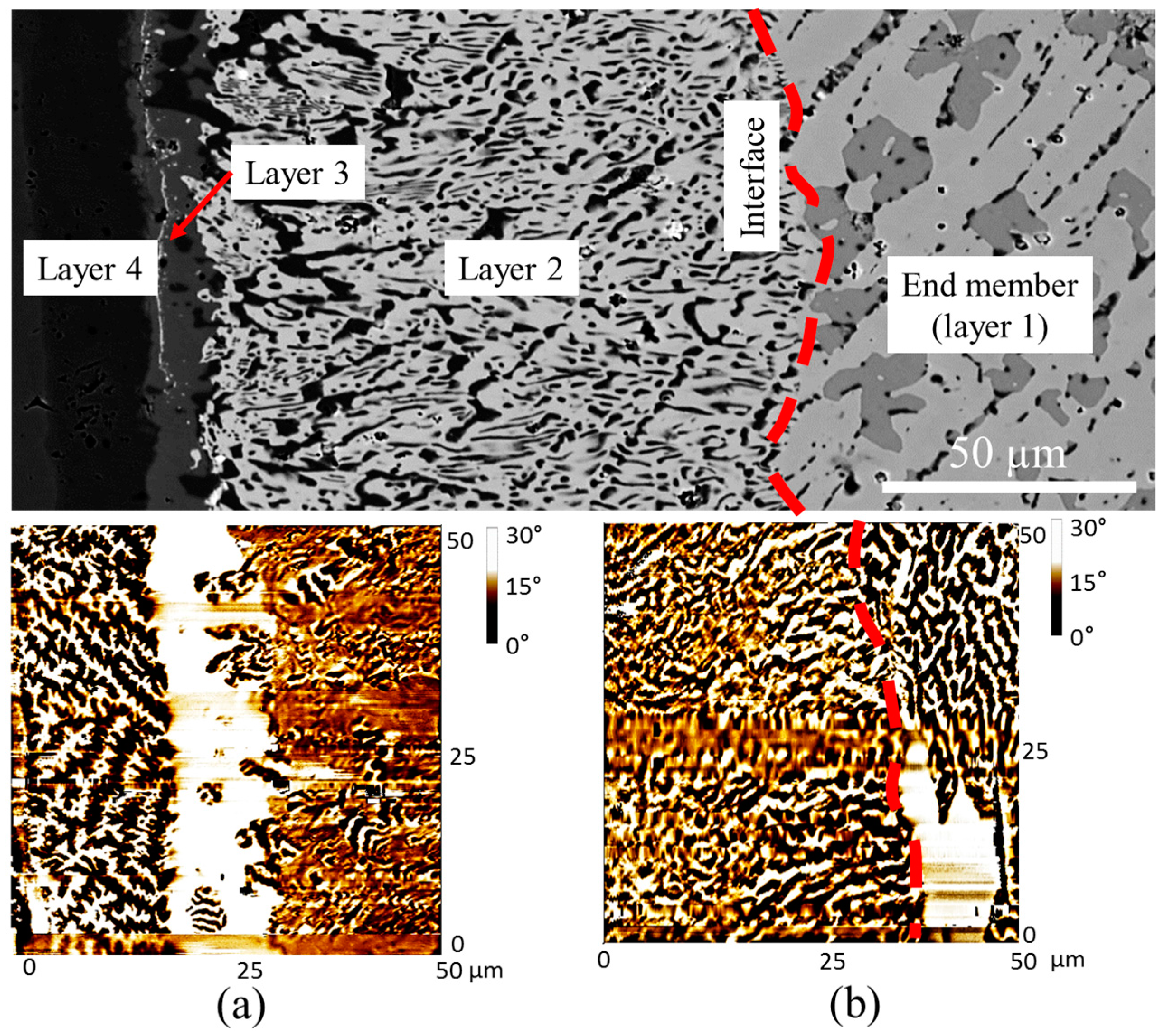
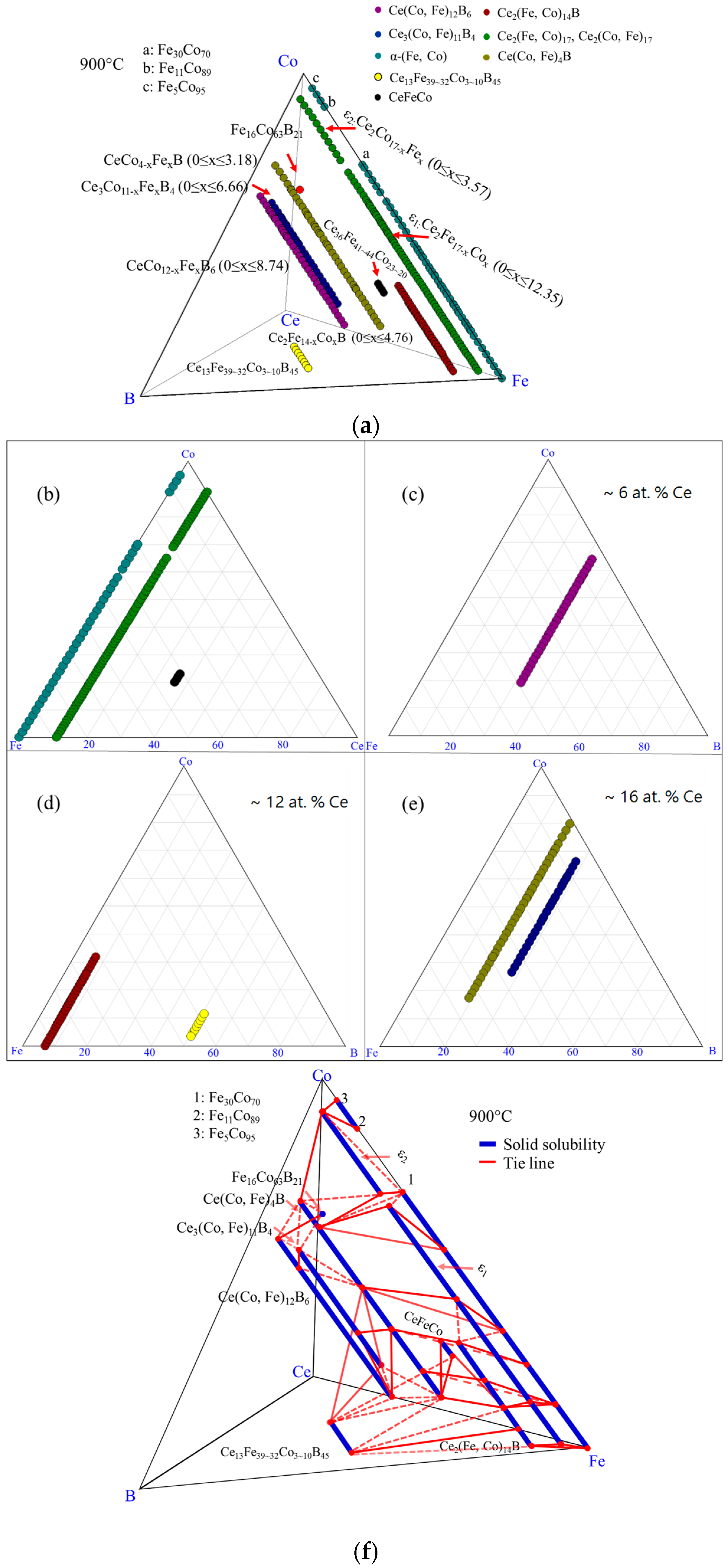
4.1. Diffusion Couples Results
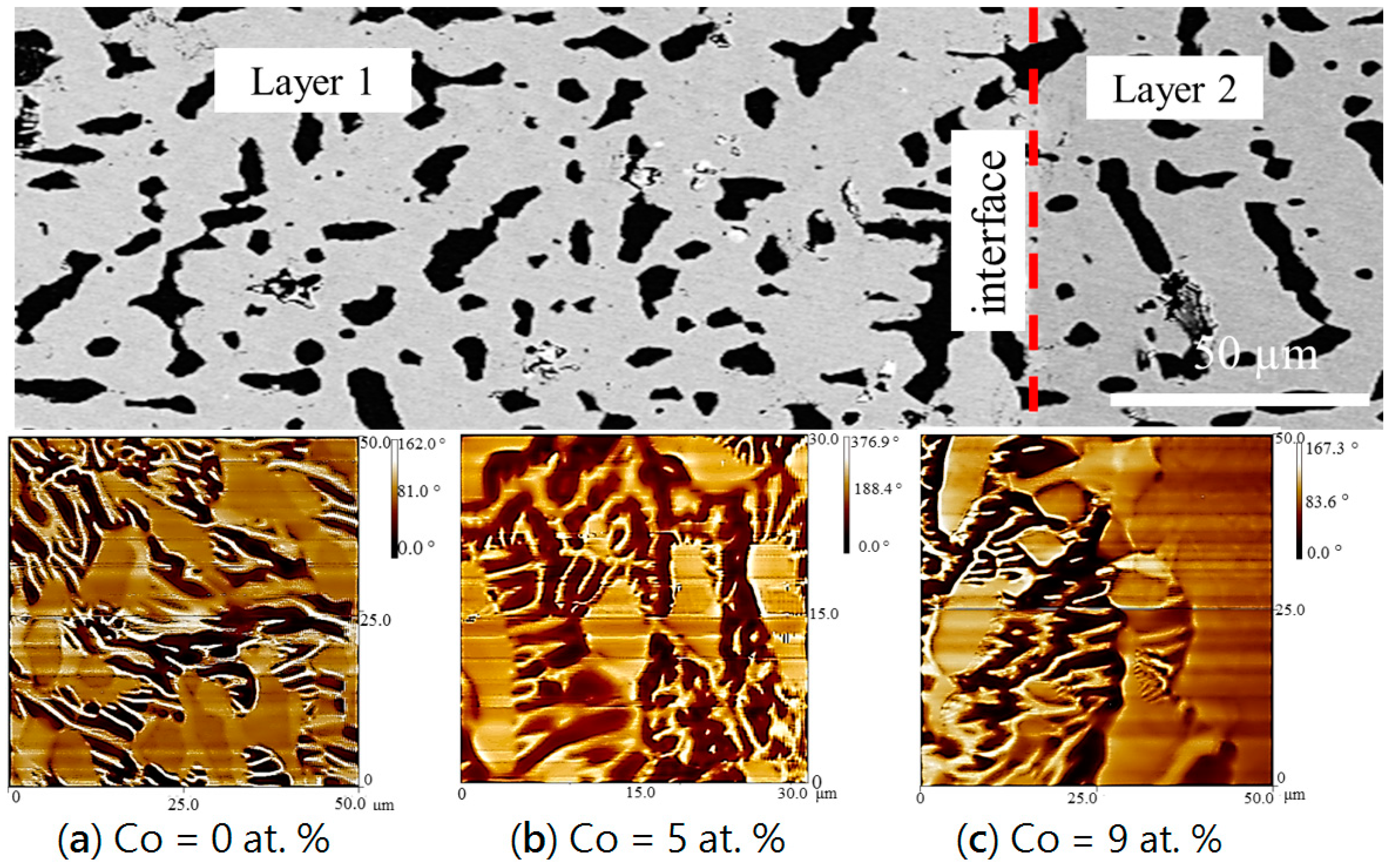
4.2. MFM Study on Diffusion Couples
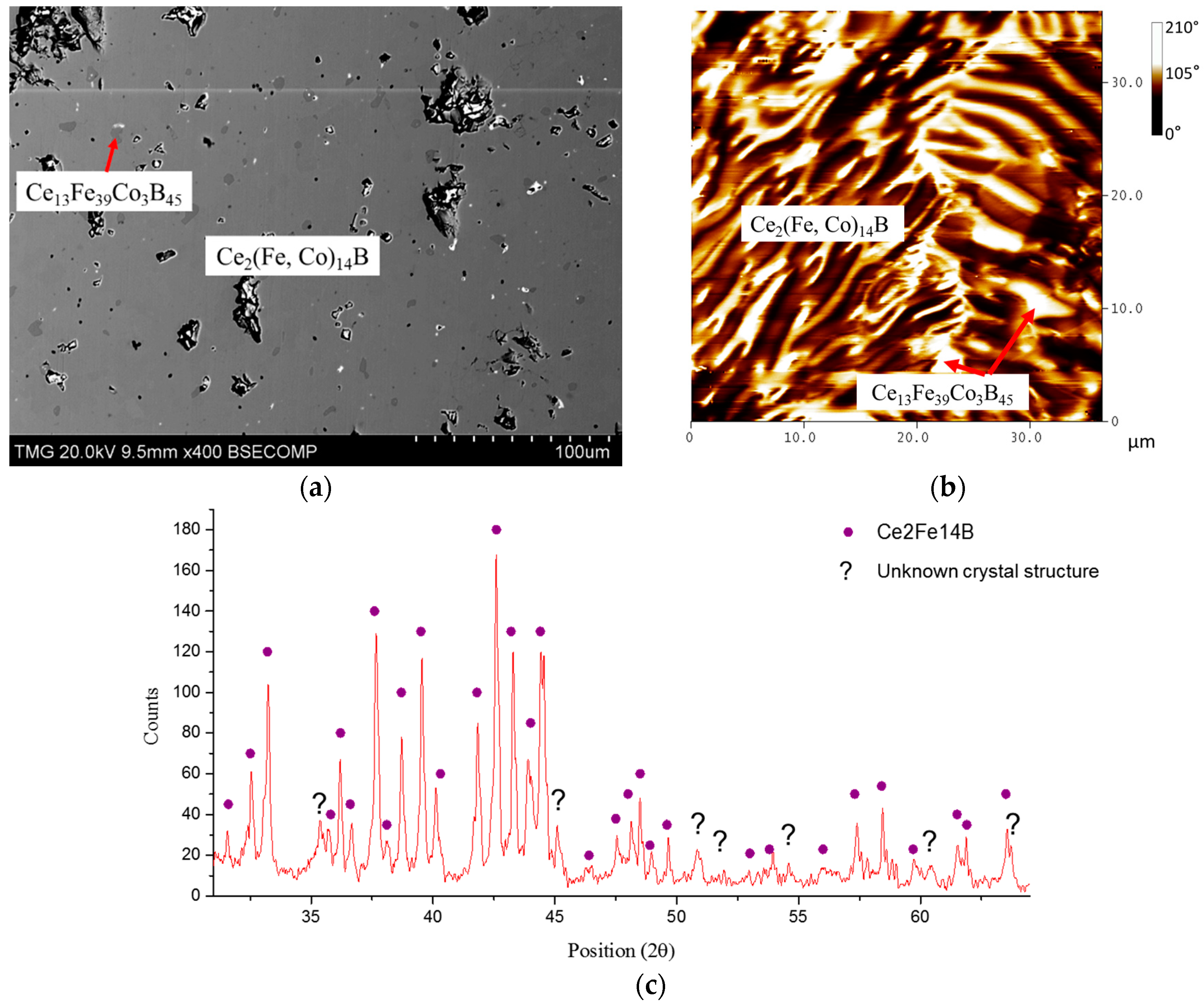
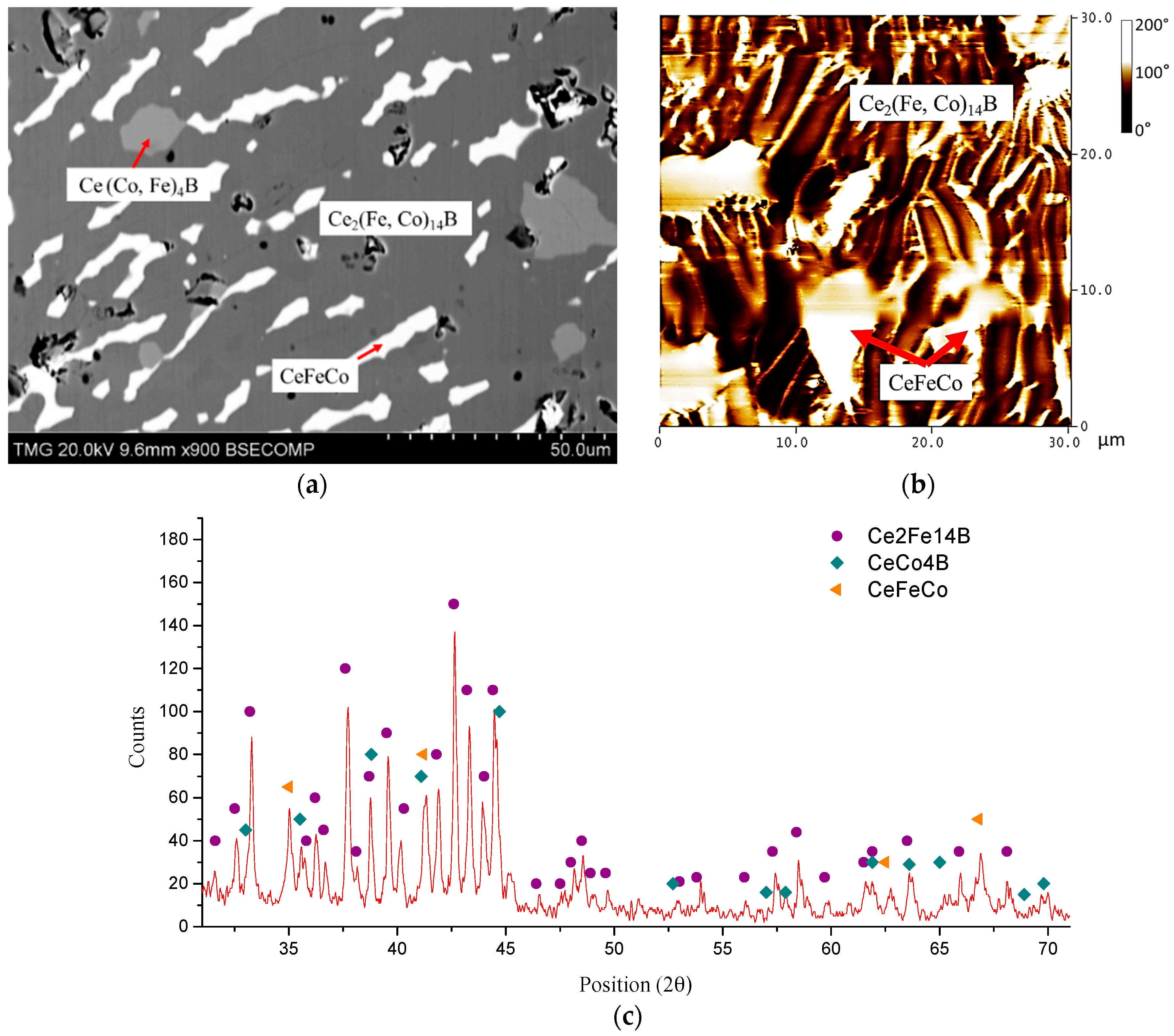
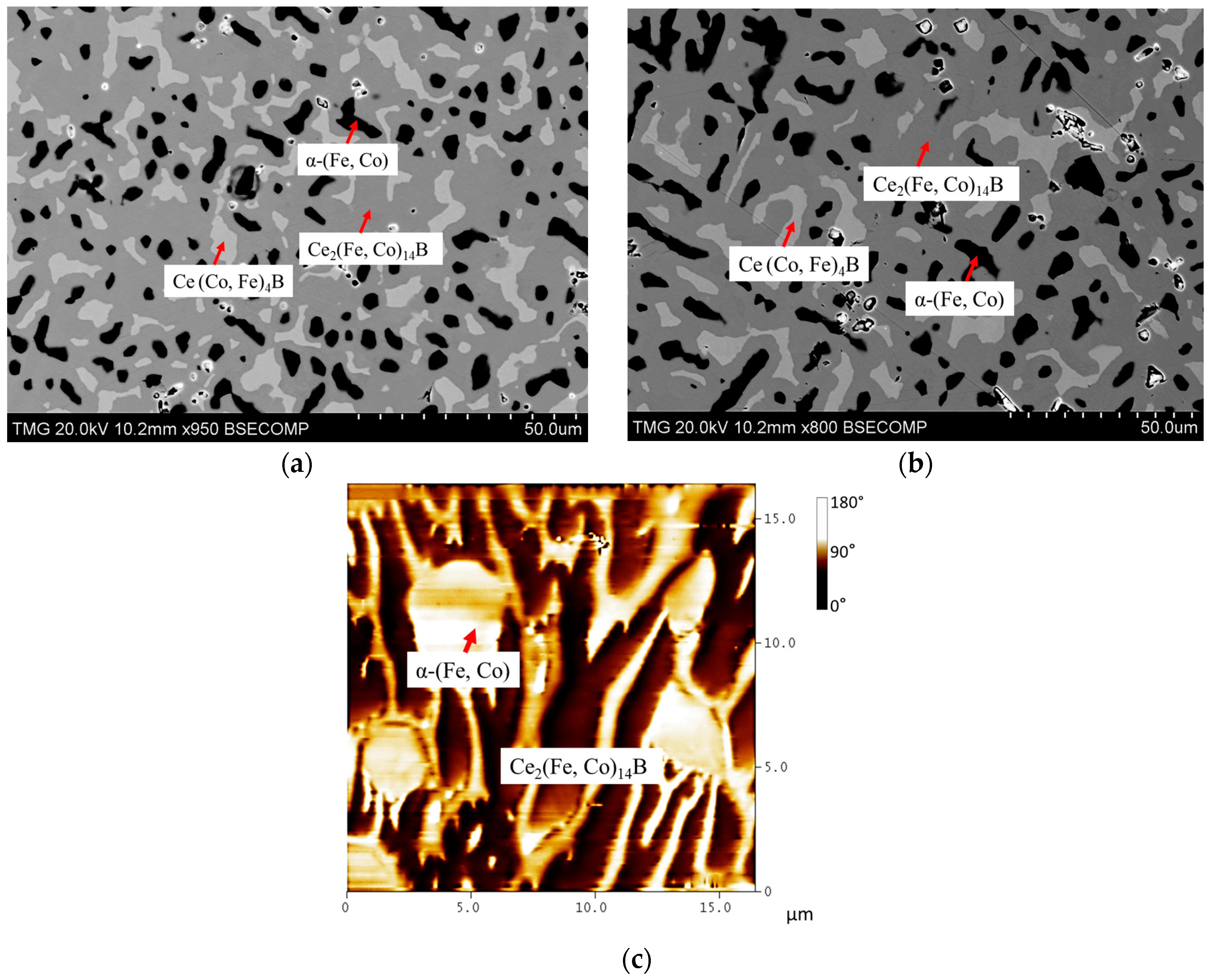
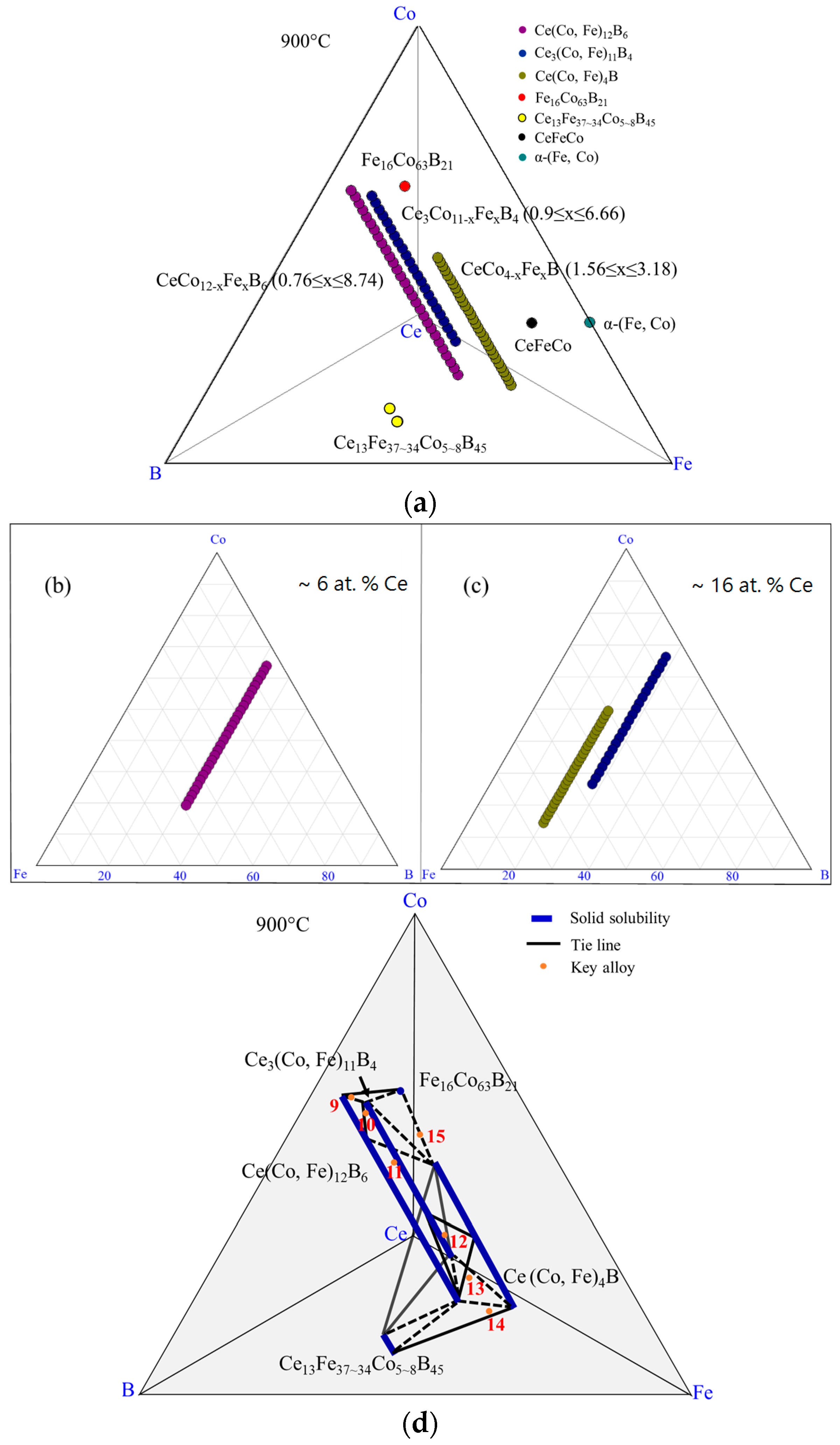
4.3. Key Alloys Study
4.3.1. Homogeneity ranges of Ce2(Fe, Co)14B and Ce(Co, Fe)4B
4.3.2. Homogeneity ranges of Ce(Co, Fe)12B6 and Ce3(Co, Fe)11B4
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Buschow, K.H.J. New permanent magnet materials. Mater. Sci. Rep. 1986, 1, 1–64. [Google Scholar] [CrossRef]
- Herbst, J.F.; Meyer, M.S.; Pinkerton, F.E. Magnetic hardening of Ce2Fe14B. J. Appl. Phys. 2012, 111, 1–3. [Google Scholar] [CrossRef]
- Skoug, E.J.; Meyer, M.S.; Pinkerton, F.E.; Tessema, M.M.; Haddad, D.; Herbst, J.F. Crystal structure and magnetic properties of Ce2Fe14−xCoxB alloys. J. Alloys Compd. 2013, 574, 552–555. [Google Scholar] [CrossRef]
- Bilonizhko, N.S.; Yu, B.; Kuz’ma, N.S. Ce-Fe-B system. Izv. Akad. Nauk SSSR Neorg. Mater. 1972, 8, 183–184. [Google Scholar]
- Dub, O.M.; Kuz’ma, Y.B. Ternary borides with the Nd2Fe14B structure. Soviet Powder Metall. Met. Ceram. 1986, 25, 572–575. [Google Scholar]
- Bezinge, A.; Braun, H.F.; Muller, J.; Yvon, K. Tetragonal rare earth (R) iron borides, R1+xFe4B4 (x ≈ 0.1) with incommensurate rare earth and iron substructures. Solid State Commun. 1985, 55, 131–135. [Google Scholar] [CrossRef]
- Dub, O.M.; Chaban, N.F.; Kuz’ma, Y.B. New borides of Pr5−xCo2+xB6-type structure. J. Less-Common Met. 1986, 117, 297–303. [Google Scholar] [CrossRef]
- Raghavan, V.; Raynor, G.V.; Rivlin, V.G. Phase Diagrams of Ternary Iron Alloys; ASM International: Materials Park, OH, USA, 1987; Part 3; pp. 297–300. [Google Scholar]
- Rogl, P.; Schuster, J.C.; Nowotny, H. Phase equilibrium and compound formation in Fe-M(metal)-B-X(non-metal) systems. In Boron in Steel Proceeding of the International Symposium; Metallurgical Society of AIME: Milwaukee, WI, USA, 1979; pp. 33–43. [Google Scholar]
- Pradelli, G.; Gianoglio, C.; Quadrini, E. The Co-Fe-B system in the presence of liquid. Met. Ital. 1981, 73, 351–355. [Google Scholar]
- Van Loo, F.J.J.; van Beek, J.A. Reactions and phase relations in the Fe-Ni-B and Fe-Co-B dystems. Z. Metallkd. 1989, 80, 245–250. [Google Scholar]
- Liu, Y.Q.; Zhao, X.S.; Yang, J.; Shen, J.Y. Thermodynamic optimization of the Boron-Cobalt-Iron system. J. Alloys Compd. 2011, 509, 4805–4810. [Google Scholar] [CrossRef]
- Bilonizhko, H.C.; Kuz’ma, N.S.; Yu, B. Cerium-cobalt-boron system. Izv. Akad. Nauk SSSR Neorg. Mater. 1974, 10, 265–269. [Google Scholar]
- Kuz’ma, Y.B.; Bilonizhko, N.S.; Mykhalenko, S.I.; Stepanchikova, G.F.; Chaban, N.F. The interaction of transition and rare earth metals with boron. J. Less-Common Met. 1979, 67, 51–57. [Google Scholar] [CrossRef]
- Jurczyk, M. Crystallographic and magnetic characteristics of the Ce3Co20B compound. Phys. Status Solidi A 1987, 100, K173–K176. [Google Scholar] [CrossRef]
- Kuz’ma, Y.B.; Chernyak, G.V.; Chaban, N.F. New borides of rare-earth metals with the structure of SrNi12B6 type. Dopov. Akad. Nauk Ukr. RSR Ser. A 1981, 12–80. [Google Scholar]
- Dub, O.M.; Skolozdra, R.V.; Kuz’ma, Y.B.; Dubenko, N.S. Magnetic and electrical parameters of ternary rare-earth borides containing cobalt and nickel. Inorg. Mater. 1990, 26, 1034–1037. [Google Scholar]
- Critchley, J.K. Low melting point alloys of cerium with iron, cobalt and plutonium. U.K. At. Energy Auth. Harwell Lab. Memo AERE-M 1959, 488, 1–7. [Google Scholar]
- ASM Alloy Phased Diagram Database. Available online: http://www1.asminternational.org/AsmEnterprise/APD/BrowseAPD.aspx (accessed on 3 November 2015).
- Su, X.; Zhang, W.J.; Du, Z.M. A thermodynamic modelling of the Co-Ce system. J. Alloys Compd. 1998, 267, 121–127. [Google Scholar] [CrossRef]
- Mansey, R.C.; Raynor, G.V.; Harris, I.R. Rare-earth intermediate phases VI. Pseudo-binary systems between cubic laves phases formed by rare-earth metals with iron, cobalt, nickel, aluminum and rhodium. J. Less-Common Met. 1968, 14, 337–347. [Google Scholar] [CrossRef]
- Harris, I.R.; Longworth, G. X-ray and Mössbauer studies of the pseudo-binary system Ce(Fe1−xNix)2. J. Less-Common Met. 1976, 45, 63–77. [Google Scholar] [CrossRef]
- Longworth, G.; Harris, I.R. Mössbauer effect study of the pseudo-binary system Ce(Fe1−xCox)2. J. Less-Common Met. 1975, 41, 175–185. [Google Scholar] [CrossRef]
- Putz, H.; Brandenburg, K. Pearson’s Crystal Data, Crystal Structure Database for Inorganic Compounds, CD-ROM Software Version 1.3; ASM International: Materials Park, OH, USA, 2007.
- Wang, T.; Kevorkov, D.; Mostafa, A.; Medraj, M. Experimental investigation of the phase equilibria in the Al-Mn-Zn system at 400 °C. J. Mater. 2014, 2014, 451587. [Google Scholar] [CrossRef]
- X’Pert HighScore Plus, version 2.2b (2.2.2); PANalytical: Almelo, The Netherlands, 2006.
- Hartmann, U. Magnetic Force Microscopy. Annu. Rev. Mater. Sci. 1999, 29, 53–87. [Google Scholar] [CrossRef]
- Fujii, H.; Satyanarayana, M.V.; Wallace, W.E. Magnetic and crystallographic properties of substituted Ce2Co17−xTx compounds (T = Ti, V, Cr, Mn, Fe, Cu, Zr, and Hf). J. Appl. Phys. 1982, 53, 2371–2373. [Google Scholar] [CrossRef]
- Yang, L.; Dayal, K. Effect of lattice orientation, surface modulation, and applied fields on free-surface domain microstructure in ferroelectrics. Acta Mater. 2011, 59, 6594–6603. [Google Scholar] [CrossRef]
- Denton, A.R.; Ashcroft, N.W. Vegard’s Law. Phys. Rev. A 1991, 43, 3161–3164. [Google Scholar] [CrossRef] [PubMed]
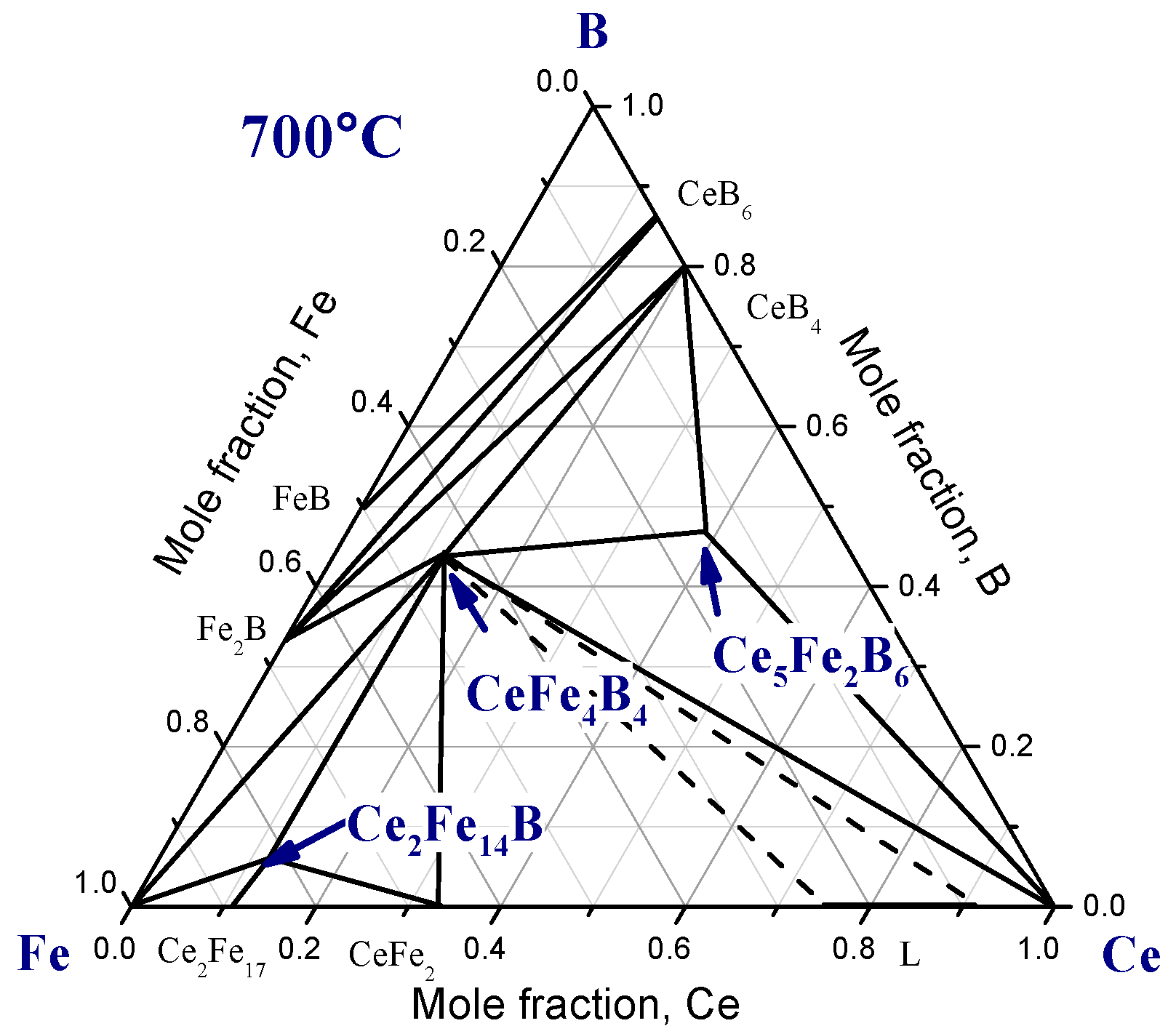


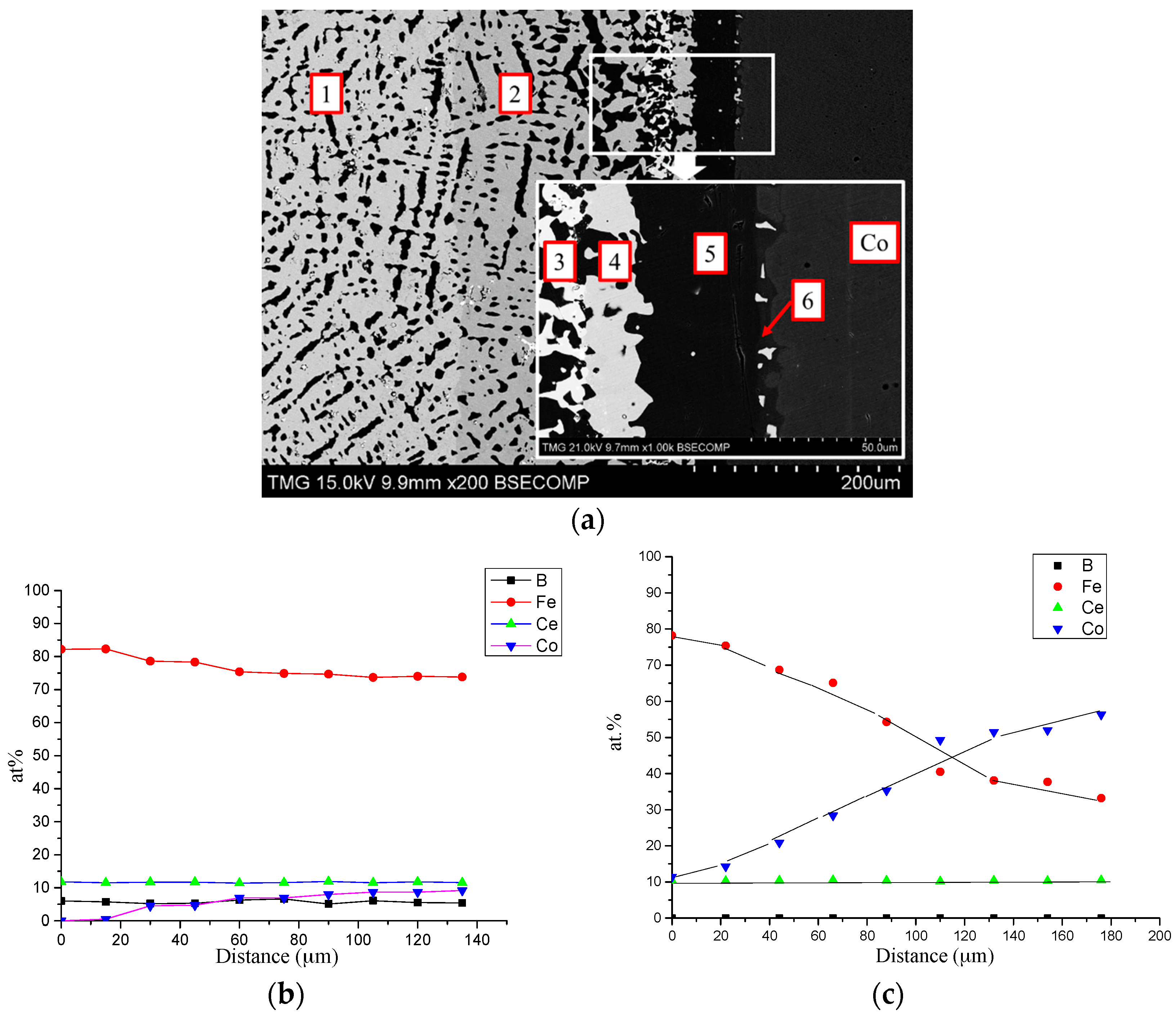
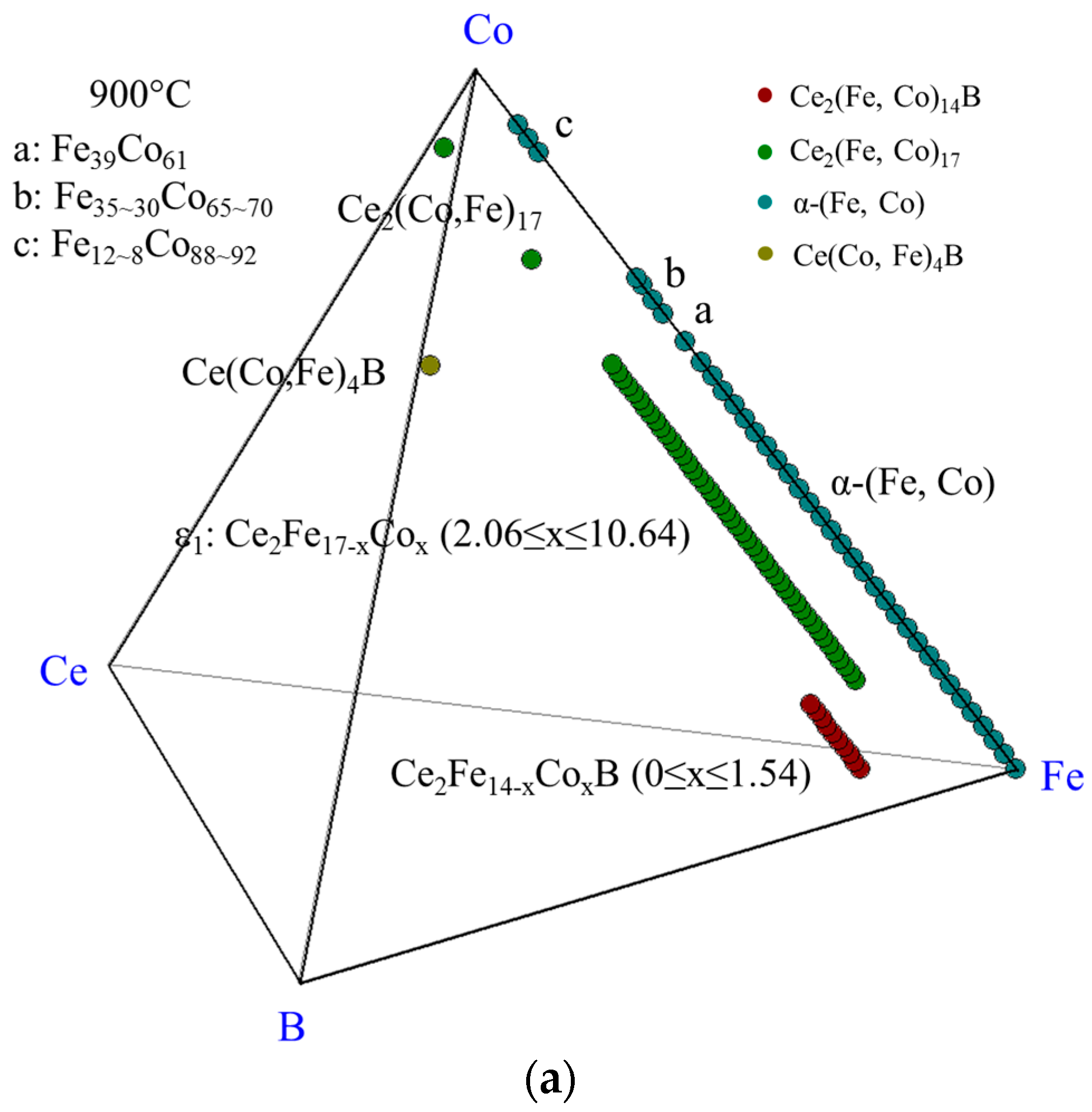
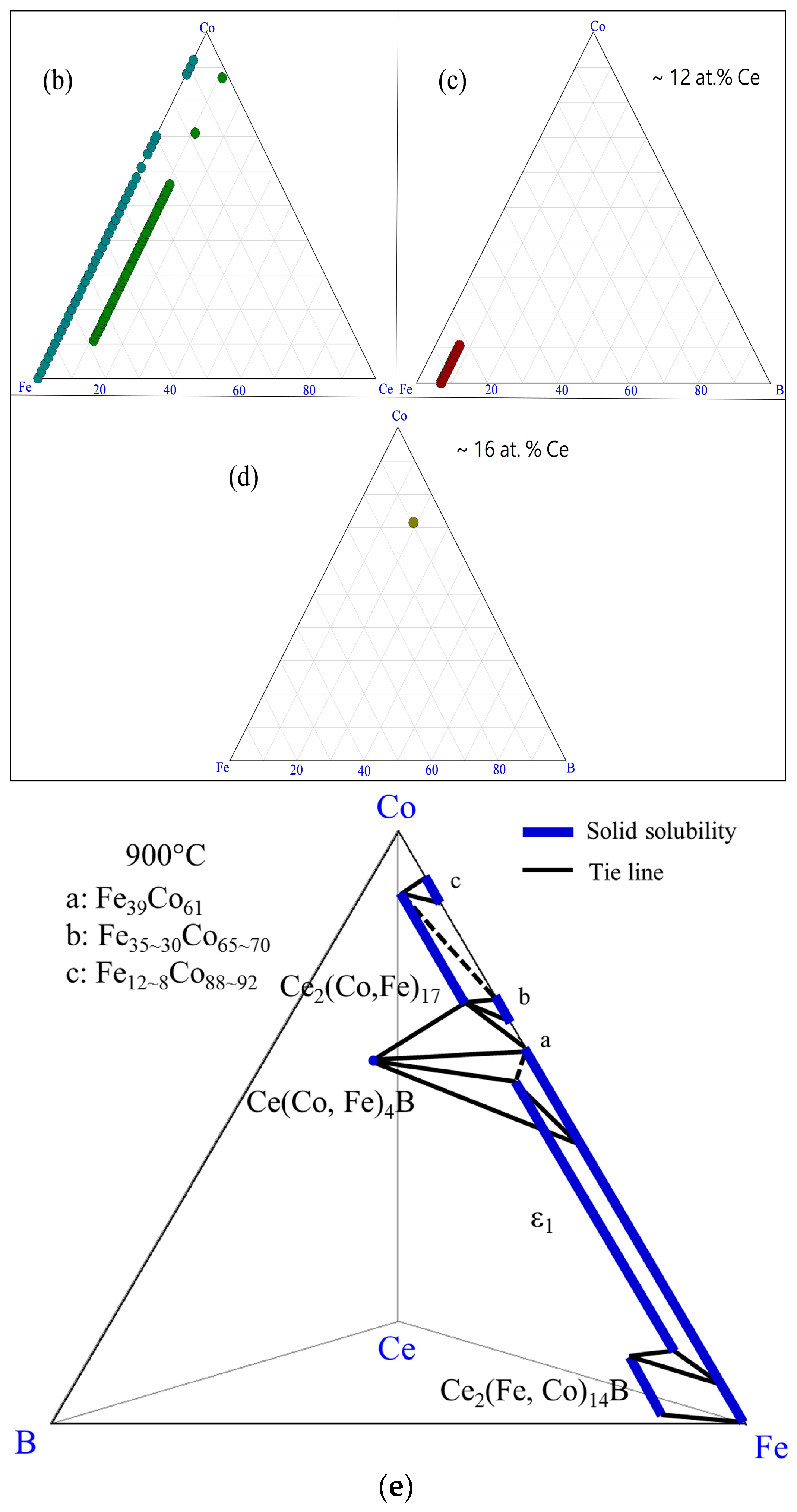
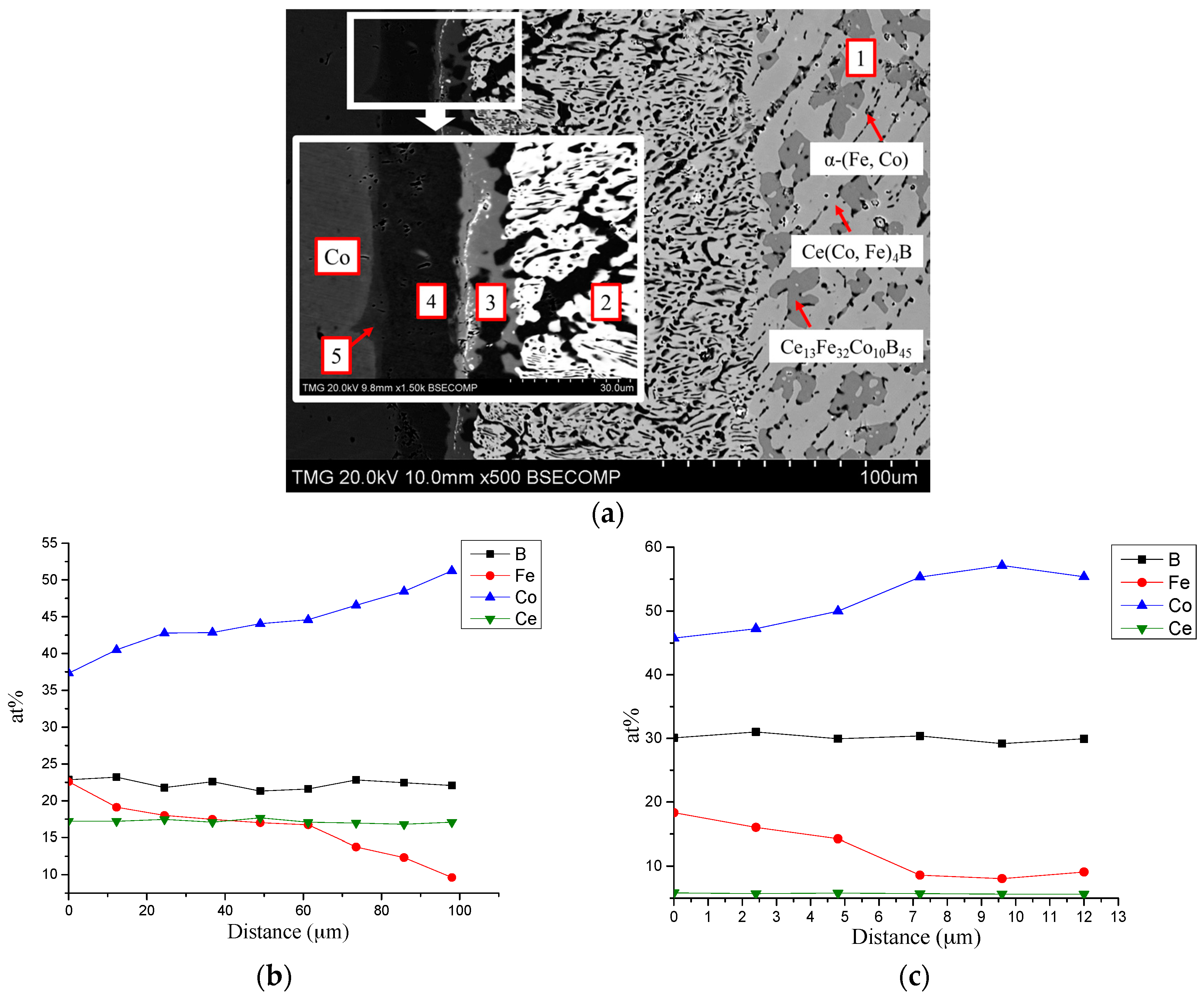

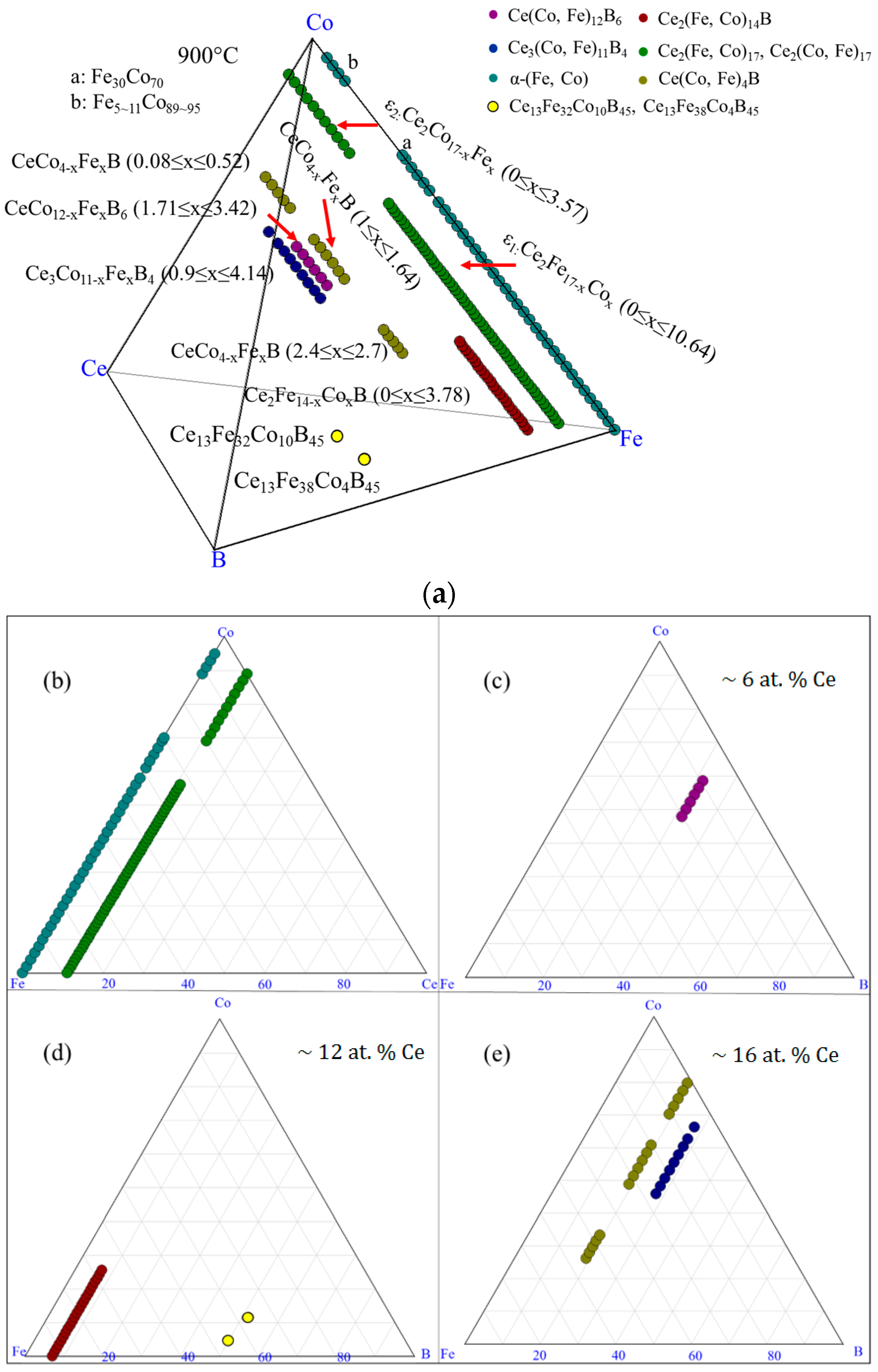
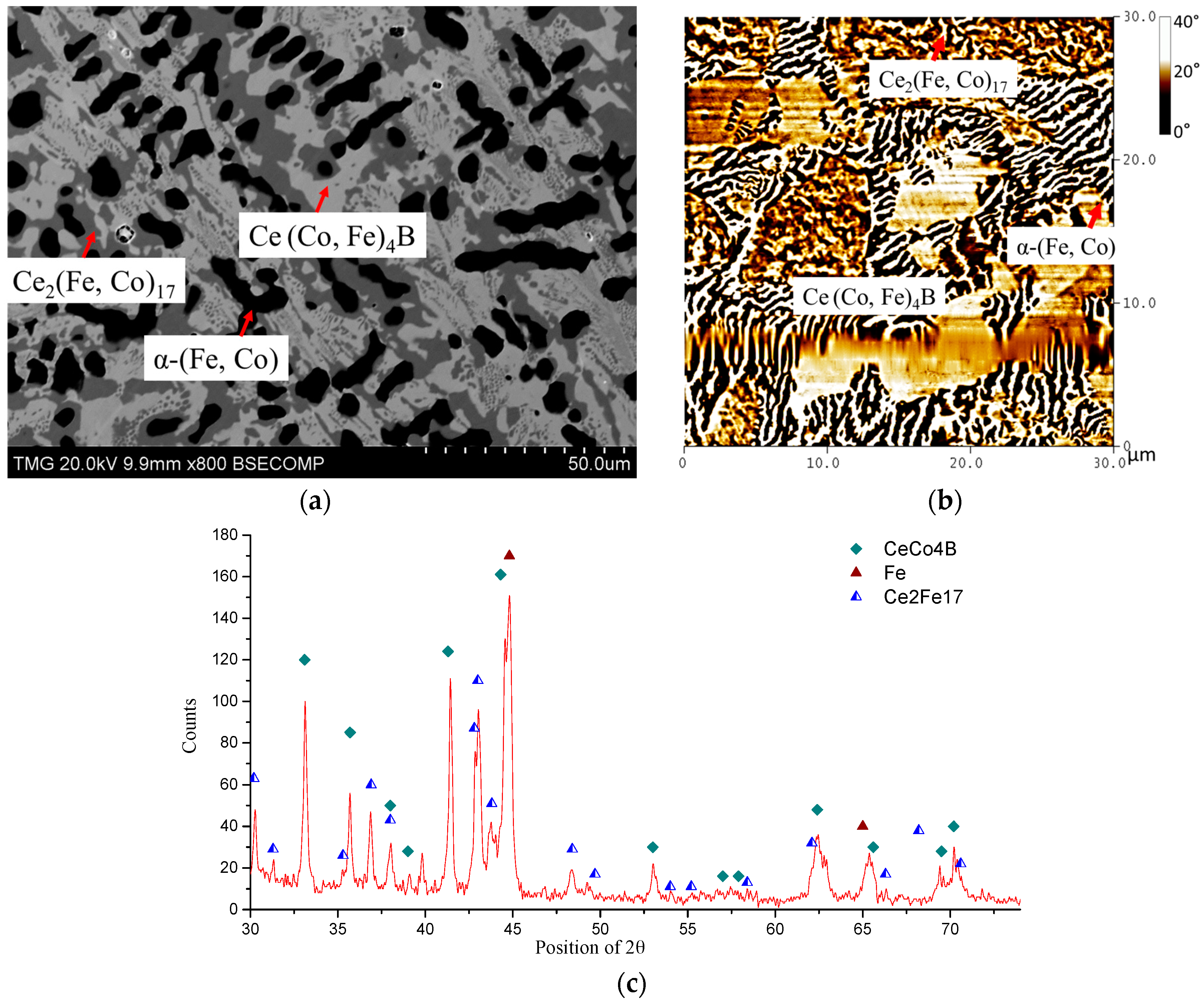
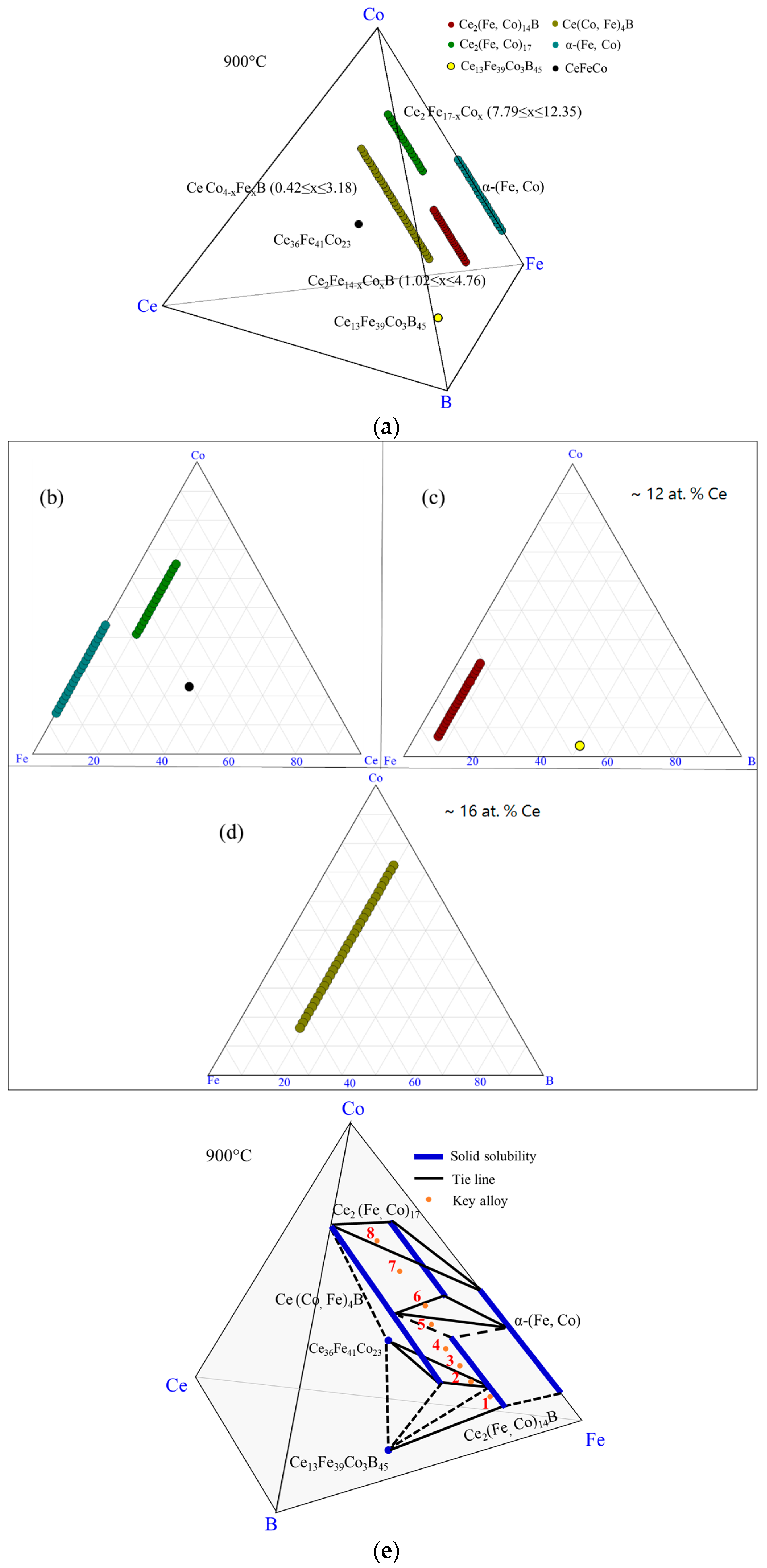
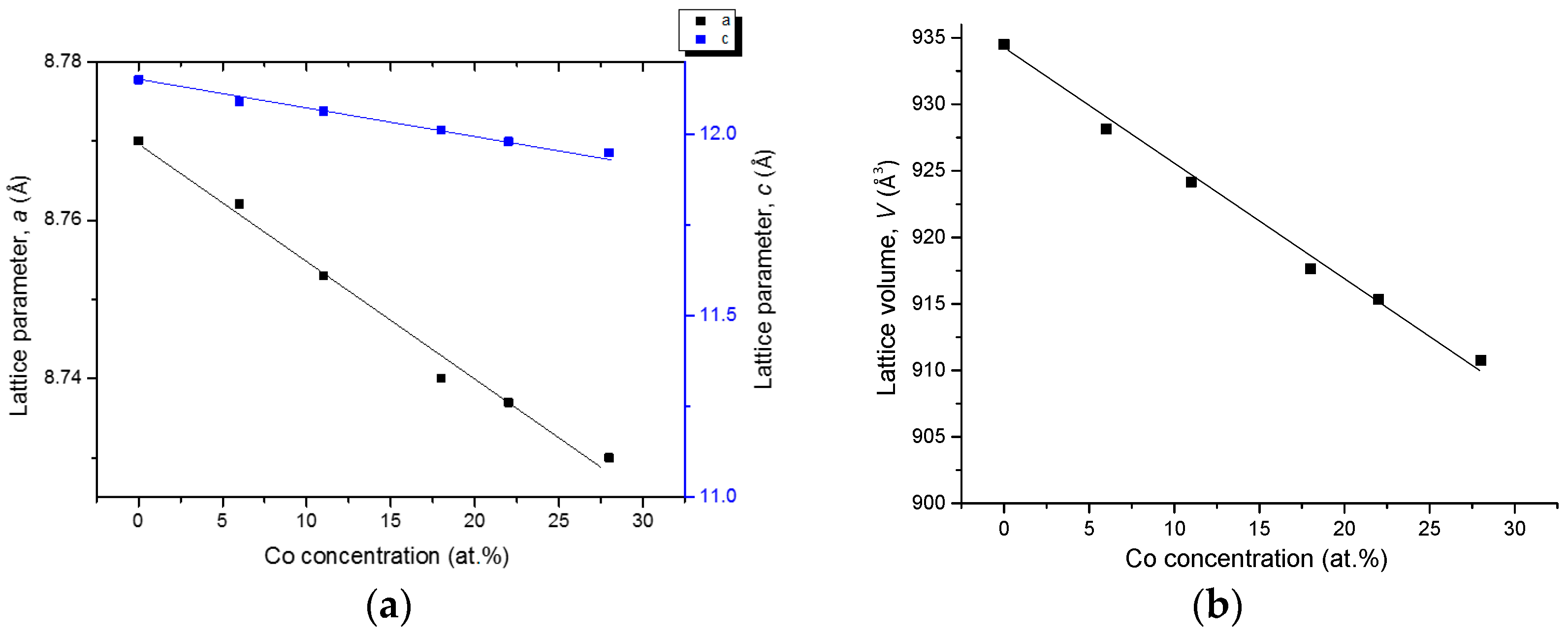
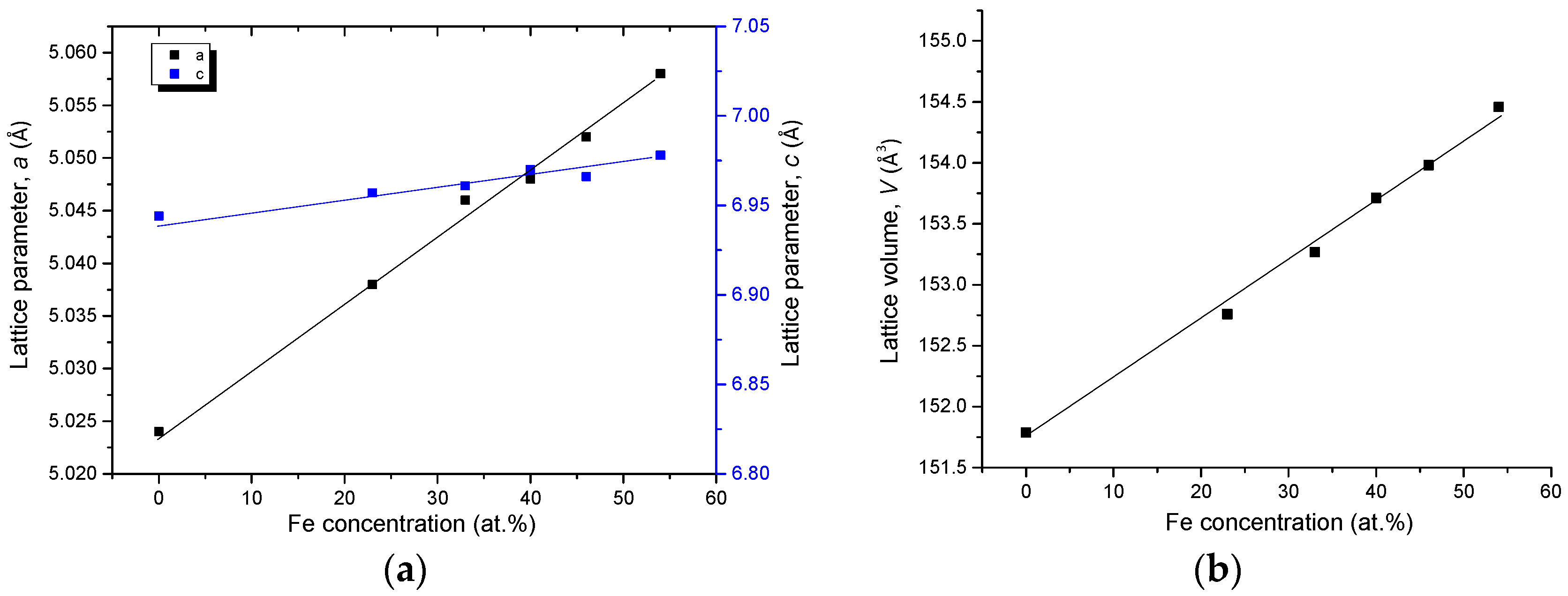
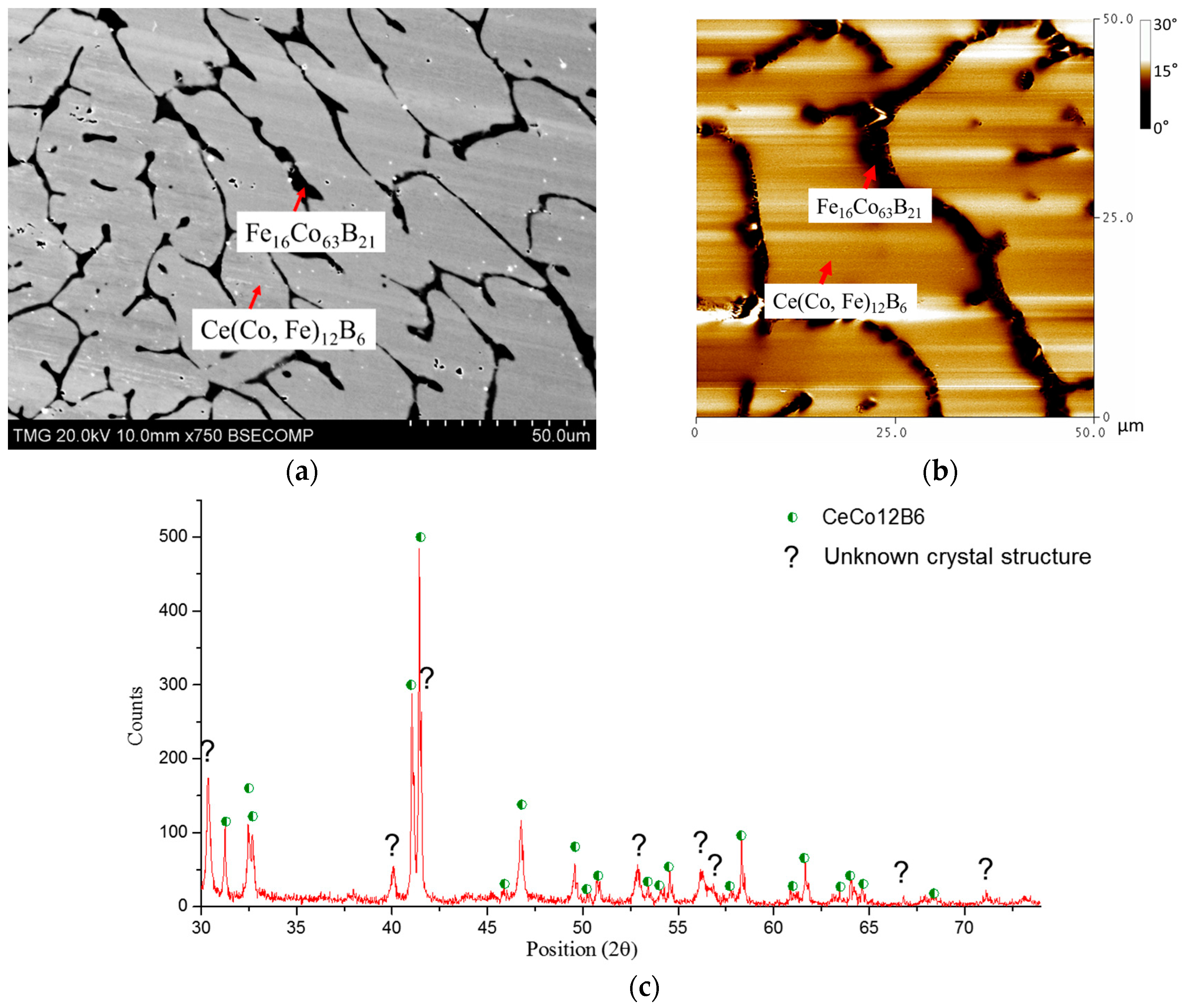
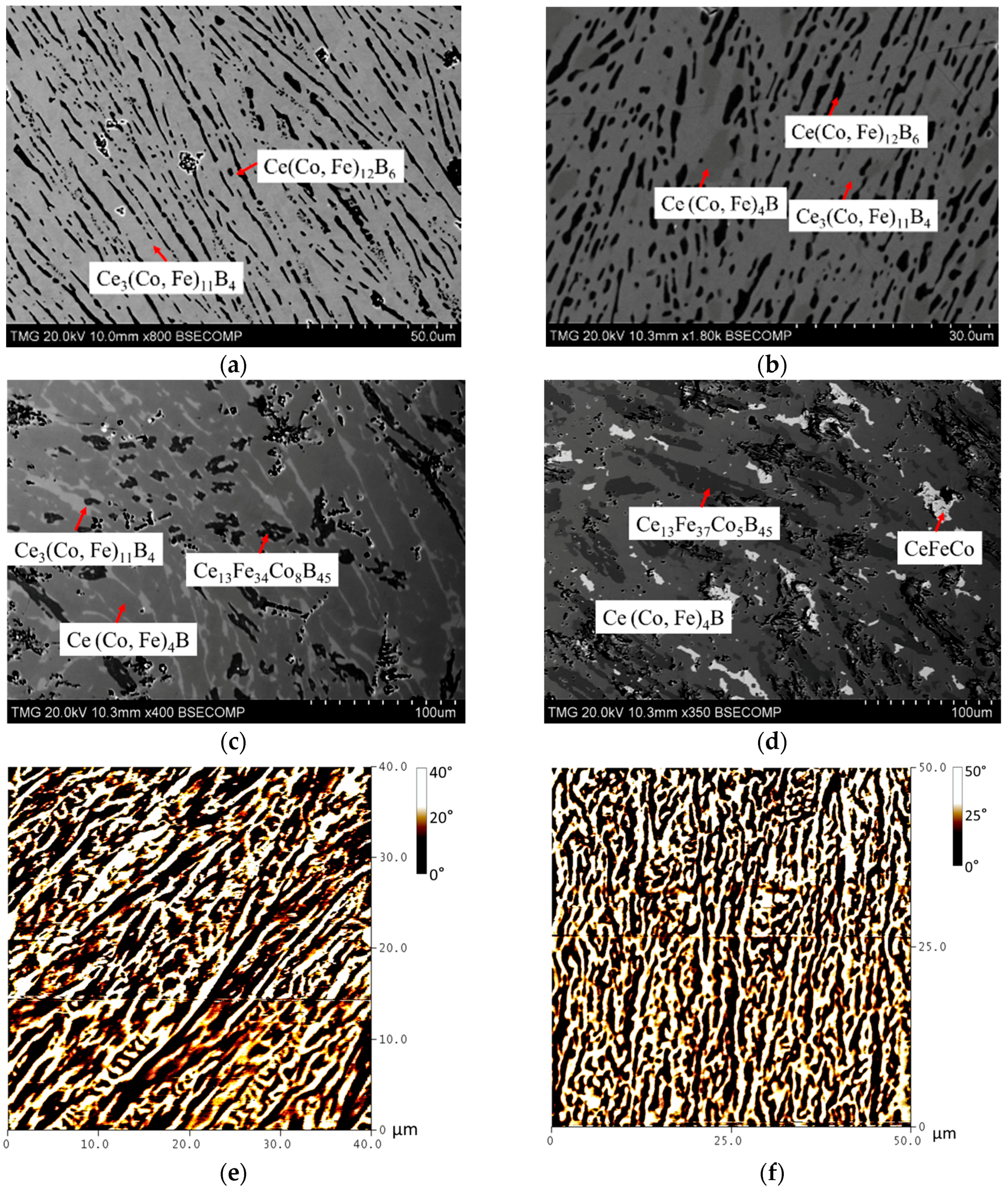
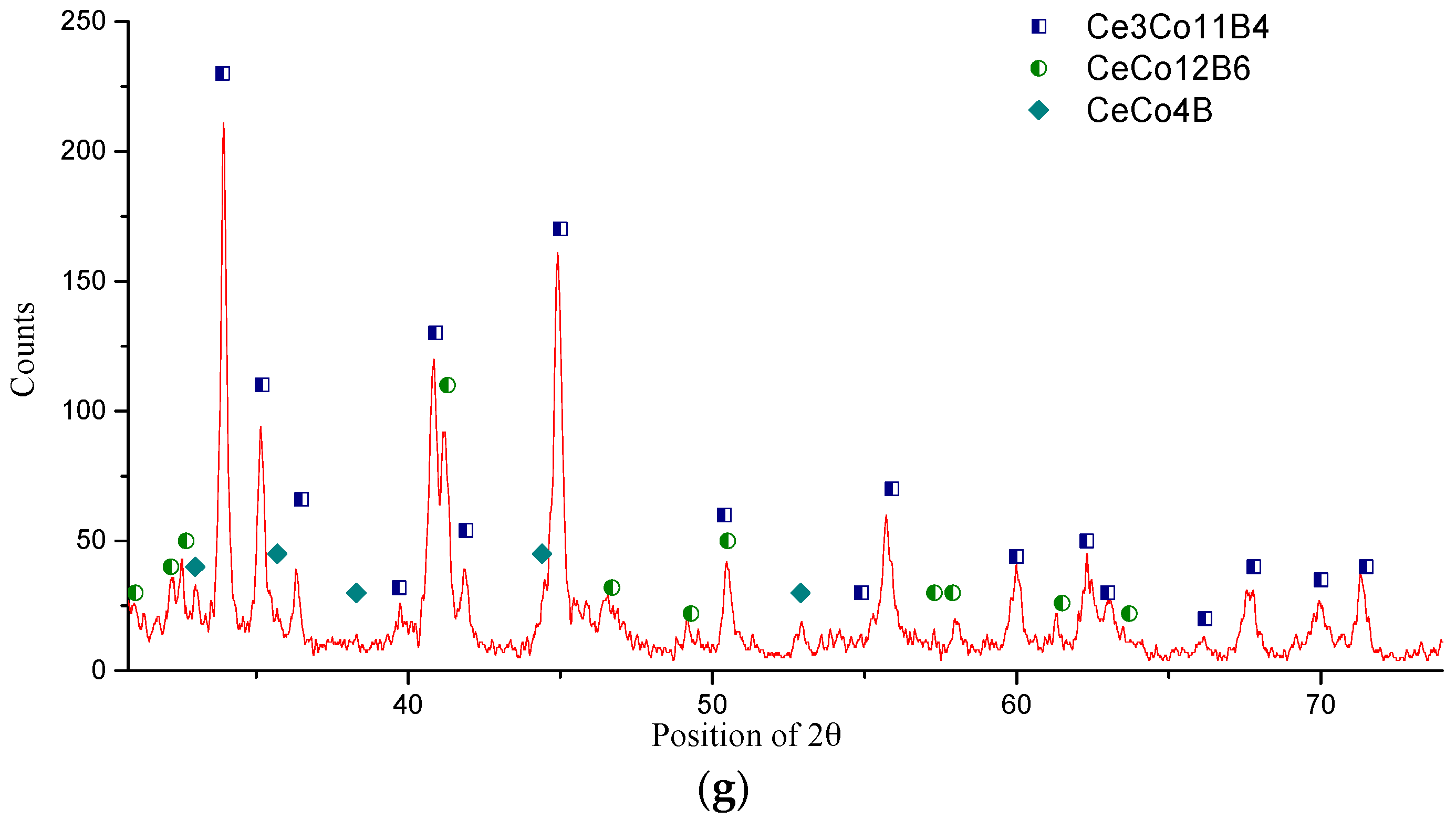
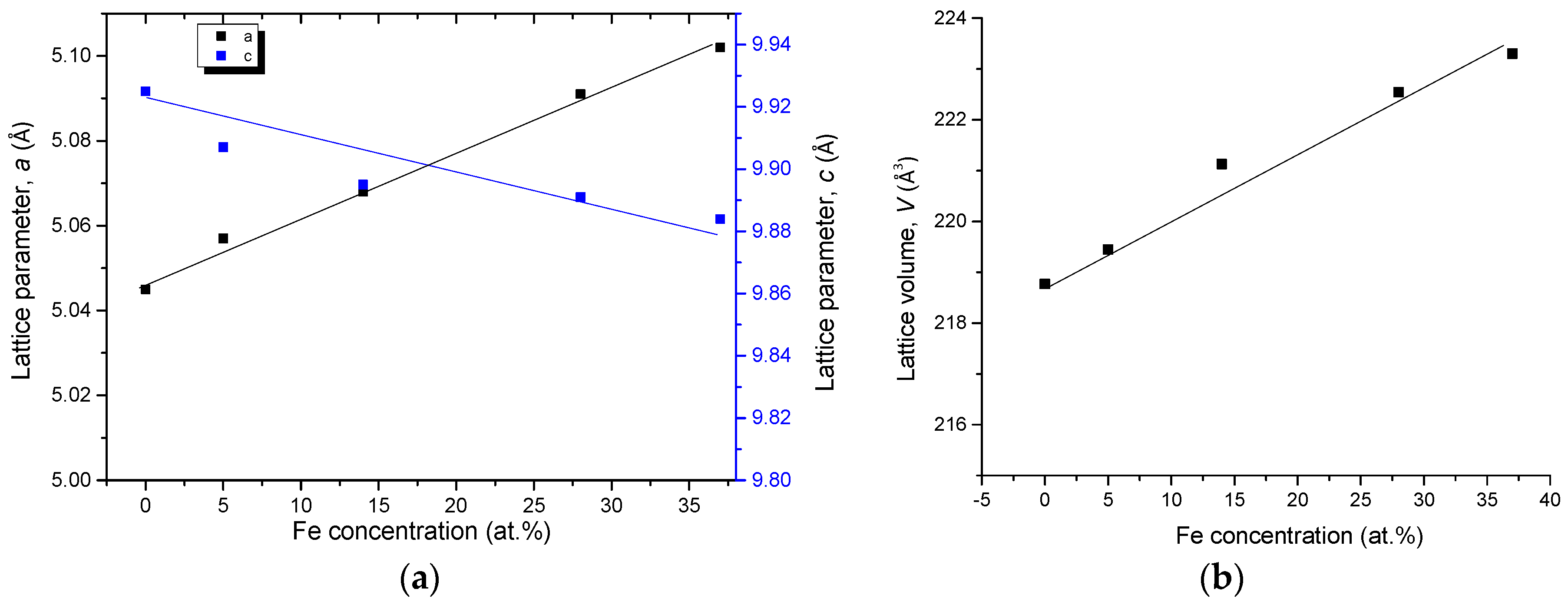
| Diffusion Couple | Layer | Composition (at. %) | Corresponding Phase | |||
|---|---|---|---|---|---|---|
| Ce | Fe | Co | B | |||
| DC1 (Ce2Fe14B/Co) | 1 | 12 | 82–73 | 0–9 | 6 | Ce2(Fe, Co)14B |
| 0 | 100–93 | 0–7 | 0 | α-(Fe, Co) | ||
| 2 | 11 | 79–34 | 10–55 | 0 | Ce2(Fe, Co)17 | |
| 0 | 93–57 | 7–43 | 0 | α-(Fe, Co) | ||
| 3 | 16 | 9 | 59 | 16 | Ce(Co, Fe)4B | |
| 0 | 57–39 | 43–61 | 0 | α-(Fe, Co) | ||
| 4 | 11 | 19 | 70 | 0 | Ce2(Co, Fe)17 | |
| 0 | 39 | 61 | 0 | α-(Fe, Co) | ||
| 5 | 0 | 35–30 | 65–70 | 0 | α-(Fe, Co) | |
| 6 | 0 | 12 | 88 | 0 | γ-(Fe, Co) | |
| 11 | 2 | 87 | 0 | Ce2(Co, Fe)17 | ||
| DC2 (Ce13Fe80B7/Co90Ce10) | 1 | 12 | 82–60 | 0–22 | 6 | Ce2(Fe, Co)14B |
| 0 | 100–82 | 0–18 | 0 | α-(Fe, Co) | ||
| 2 | 15 | 27–17 | 42–52 | 16 | Ce(Co, Fe)4B | |
| 0 | 82–37 | 18–63 | 0 | α-(Fe, Co) | ||
| 3 | 0 | 35–33 | 65–67 | 0 | α-(Fe, Co) | |
| 4 | 16 | 6–1 | 63–68 | 15 | Ce(Co, Fe)4B | |
| 11 | 14 | 75 | 0 | Ce2(Co, Fe)17 | ||
| 5 | 0 | 11–0 | 89–100 | 0 | γ-(Fe, Co) | |
| 11 | 14–0 | 75–89 | 0 | Ce2(Co, Fe)17 | ||
| DC3 (Ce10Fe75B15/Co) | 1 | 12 | 82–65 | 0–17 | 6 | Ce2(Fe, Co)14B |
| 0 | 100–70 | 0–30 | 0 | α-(Fe, Co) | ||
| 13 | 38 | 4 | 45 | Ce13Fe38Co4B45 | ||
| 2 | 16 | 21–5 | 40–56 | 23 | Ce3(Co, Fe)11B4 | |
| 0 | 70–44 | 30–56 | 0 | α-(Fe, Co) | ||
| 3 | 6 | 11 | 53 | 30 | Ce(Co, Fe)12B5 | |
| 4 | 0 | 33–30 | 67–70 | 0 | α-(Fe, Co) | |
| DC4 (Ce15Fe43Co19B23/Co) | 1 | 16 | 45–40 | 23–28 | 16 | Ce(Co, Fe)4B |
| 0 | 17 | 83 | 0 | α-(Fe, Co) | ||
| 13 | 32 | 10 | 45 | Ce13Fe32Co10B45 | ||
| 2 | 17 | 23–9 | 37–51 | 23 | Ce3(Co, Fe)11B4 | |
| 0 | 65–53 | 35–47 | 0 | α-(Fe, Co) | ||
| 3 | 6 | 18–9 | 46–55 | 30 | Ce(Co, Fe)12B5 | |
| 0 | 53 | 47 | 0 | α-(Fe, Co) | ||
| 4 | 0 | 31 | 69 | 0 | α-(Fe, Co) | |
| 5 | 0 | 14–11 | 86–89 | 0 | γ-(Fe, Co) | |
| Key Alloys Number | Actual Global Composition (at. %) | WDS Composition (at. %) | Corresponding Phases | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ce | Fe | Co | B | Ce | Fe | Co | B | By WDS | By XRD | |
| KA 1 | 14 | 73 | 7 | 6 | 12 | 76 | 6 | 6 | Ce2(Fe, Co)14B | Ce2(Fe, Co)14B |
| 13 | 39 | 3 | 45 | Ce13Fe39Co3B45 | N/A * | |||||
| KA 2 | 15 | 66 | 12 | 7 | 12 | 71 | 11 | 6 | Ce2(Fe, Co)14B | Ce2(Fe, Co)14B |
| 16 | 54 | 15 | 15 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 36 | 41 | 23 | 0 | CeFeCo | CeFeCo | |||||
| KA 3 | 14 | 58 | 20 | 8 | 12 | 64 | 18 | 6 | Ce2(Fe, Co)14B | Ce2(Fe, Co)14B |
| 16 | 46 | 23 | 15 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 0 | 86 | 14 | 0 | α-(Fe, Co) | α-(Fe, Co) | |||||
| KA 4 | 15 | 54 | 24 | 7 | 12 | 60 | 22 | 6 | Ce2(Fe, Co)14B | Ce2(Fe, Co)14B |
| 16 | 40 | 28 | 16 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 0 | 80 | 20 | 0 | α-(Fe, Co) | α-(Fe, Co) | |||||
| KA 5 | 14 | 46 | 32 | 8 | 12 | 54 | 28 | 6 | Ce2(Fe, Co)14B | Ce2(Fe, Co)14B |
| 16 | 33 | 35 | 16 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 0 | 76 | 24 | 0 | α-(Fe, Co) | α-(Fe, Co) | |||||
| KA 6 | 12 | 42 | 40 | 6 | 11 | 48 | 41 | 0 | Ce2(Fe, Co)17 | Ce2(Fe, Co)17 |
| 16 | 23 | 45 | 16 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 0 | 66 | 34 | 0 | α-(Fe, Co) | α-(Fe, Co) | |||||
| KA 7 | 12 | 32 | 50 | 6 | 11 | 37 | 52 | 0 | Ce2(Fe, Co)17 | Ce2(Fe, Co)17 |
| 17 | 13 | 54 | 16 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 0 | 57 | 43 | 0 | α-(Fe, Co) | α-(Fe, Co) | |||||
| KA 8 | 12 | 22 | 60 | 6 | 11 | 24 | 65 | 0 | Ce2(Fe, Co)17 | Ce2(Fe, Co)17 |
| 17 | 7 | 60 | 16 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 0 | 56 | 44 | 0 | α-(Fe, Co) | α-(Fe, Co) | |||||
| Key Alloys Number | Actual Global Composition (at. %) | WDS Composition (at. %) | Corresponding Phases | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ce | Fe | Co | B | Ce | Fe | Co | B | By WDS | By XRD | |
| KA 9 | 9 | 7 | 56 | 28 | 6 | 4 | 60 | 30 | Ce(Co, Fe)12B6 | Ce(Co, Fe)12B6 |
| 0 | 16 | 63 | 21 | Fe16Co63B21 | N/A * | |||||
| KA 10 | 16 | 8 | 51 | 25 | 17 | 5 | 55 | 23 | Ce3(Co, Fe)11B4 | Ce3(Co, Fe)11B4 |
| 6 | 14 | 51 | 29 | Ce(Co, Fe)12B6 | Ce(Co, Fe)12B6 | |||||
| KA 11 | 17 | 18 | 43 | 22 | 17 | 14 | 46 | 23 | Ce3(Co, Fe)11B4 | Ce3(Co, Fe)11B4 |
| 6 | 32 | 32 | 30 | Ce(Co, Fe)12B6 | Ce(Co, Fe)12B6 | |||||
| KA 12 | 17 | 33 | 27 | 23 | 17 | 28 | 32 | 23 | Ce3(Co, Fe)11B4 | Ce3(Co, Fe)11B4 |
| 6 | 46 | 18 | 30 | Ce(Co, Fe)12B6 | Ce(Co, Fe)12B6 | |||||
| 17 | 39 | 27 | 17 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| KA 13 | 17 | 42 | 18 | 23 | 17 | 37 | 23 | 23 | Ce3(Co, Fe)11B4 | Ce3(Co, Fe)11B4 |
| 16 | 47 | 20 | 17 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 13 | 34 | 8 | 45 | Ce13Fe34Co8B45 | N/A * | |||||
| KA 14 | 17 | 50 | 10 | 23 | 16 | 55 | 12 | 17 | Ce(Co, Fe)4B | Ce(Co, Fe)4B |
| 13 | 37 | 5 | 45 | Ce13Fe37Co5B45 | N/A * | |||||
| 35 | 45 | 20 | 0 | CeFeCo | CeFeCo | |||||
| KA 15 | 15 | 20 | 49 | 16 | 17 | 16 | 44 | 23 | Ce3(Co, Fe)11B4 | Ce3(Co, Fe)11B4 |
| 16 | 26 | 42 | 16 | Ce(Co, Fe)4B | Ce(Co, Fe)4B | |||||
| 0 | 68 | 32 | 0 | α-(Fe, Co) | α-(Fe, Co) | |||||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Kevorkov, D.; Medraj, M. Phase Equilibria and Magnetic Phases in the Ce-Fe-Co-B System. Materials 2017, 10, 16. https://doi.org/10.3390/ma10010016
Wang T, Kevorkov D, Medraj M. Phase Equilibria and Magnetic Phases in the Ce-Fe-Co-B System. Materials. 2017; 10(1):16. https://doi.org/10.3390/ma10010016
Chicago/Turabian StyleWang, Tian, Dmytro Kevorkov, and Mamoun Medraj. 2017. "Phase Equilibria and Magnetic Phases in the Ce-Fe-Co-B System" Materials 10, no. 1: 16. https://doi.org/10.3390/ma10010016