Effect of Sintering Temperature and Applied Load on Anode-Supported Electrodes for SOFC Application
Abstract
:1. Introduction
2. Material and Methods
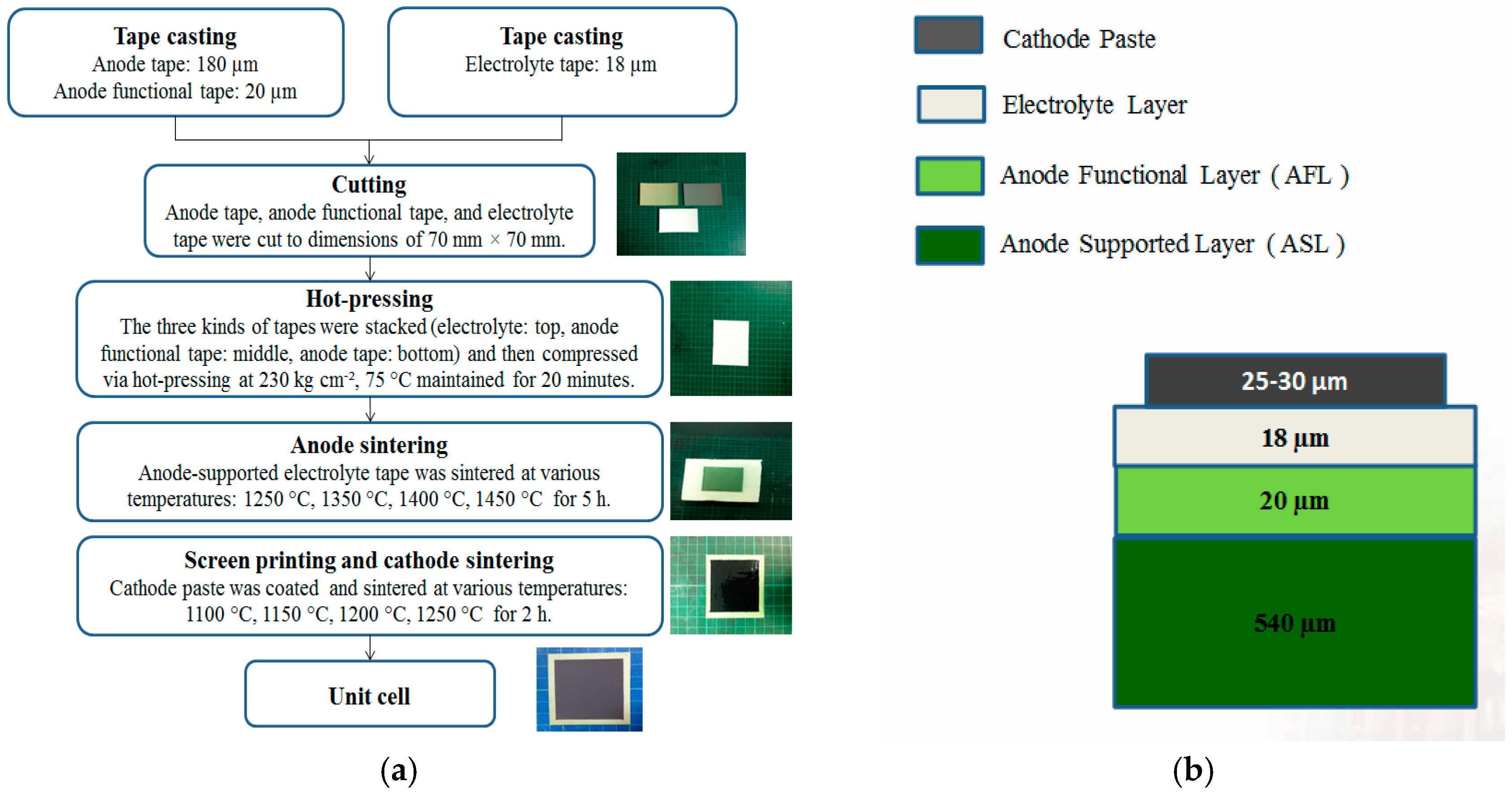
2.1. Cell Fabrication
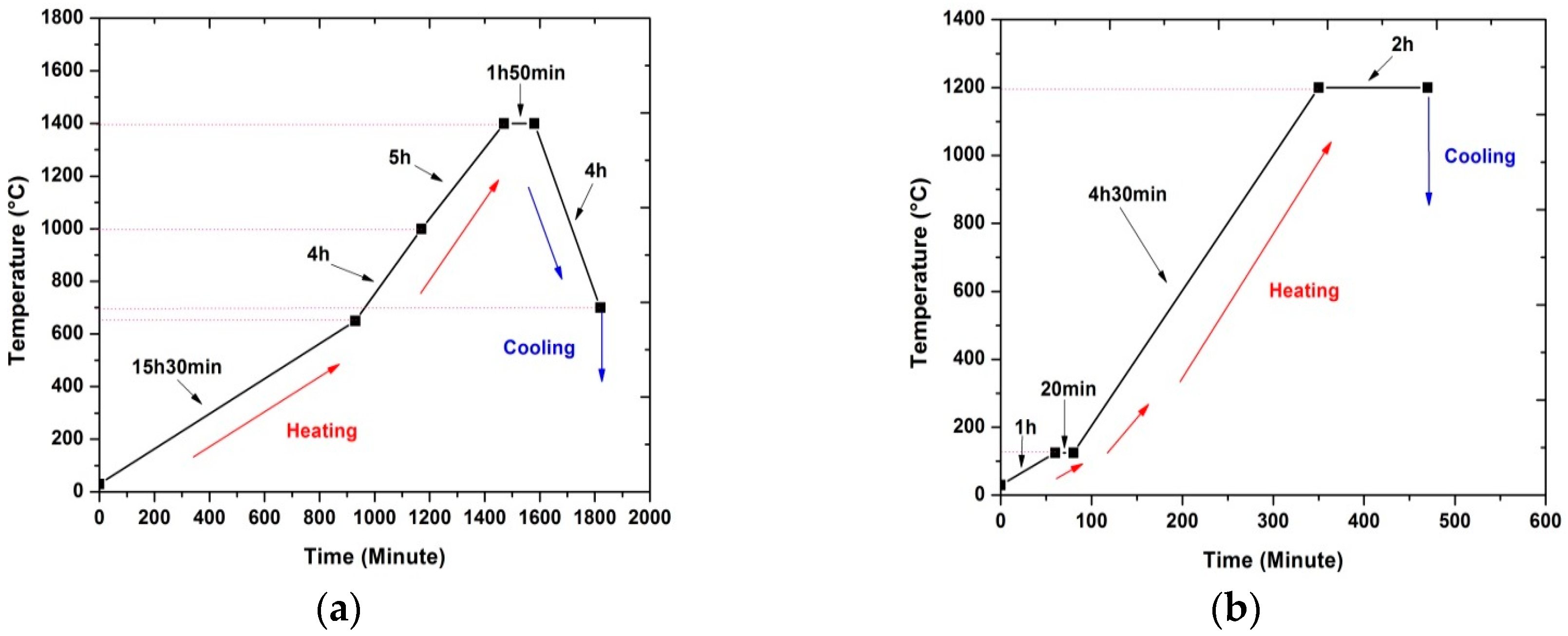
2.1.1. Heat-Treatment of the Anode-Supported Electrolyte
2.1.2. Heat-Treatment of the Cathode
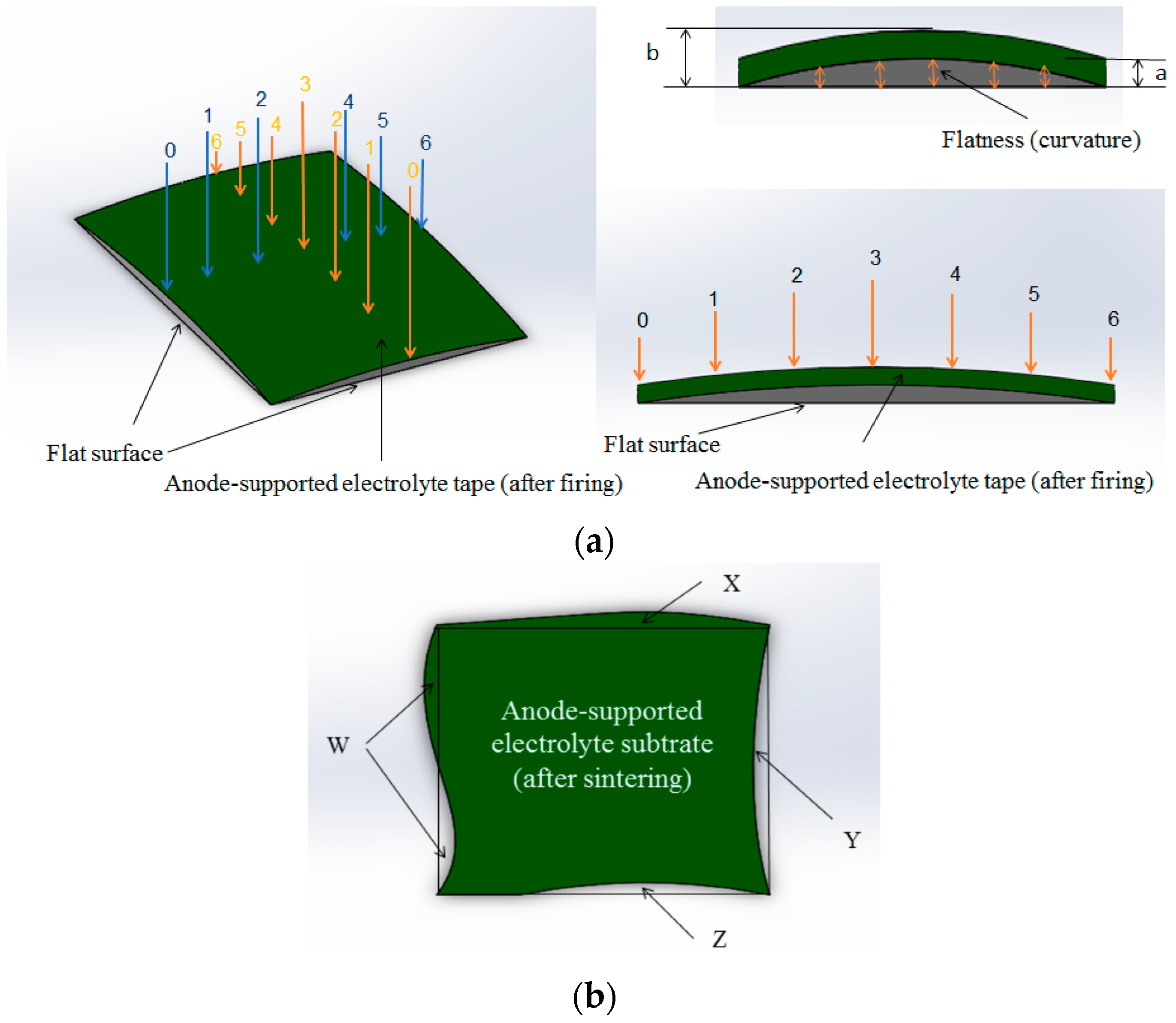
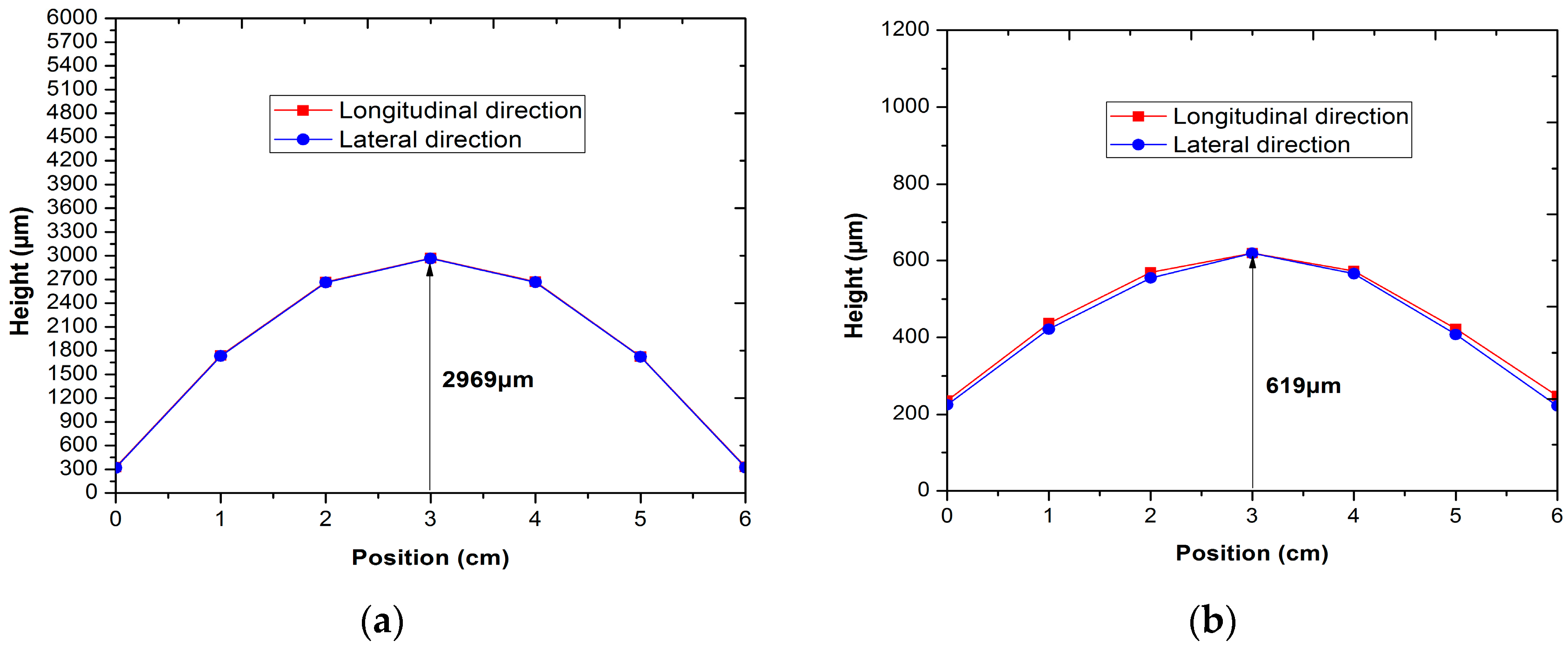
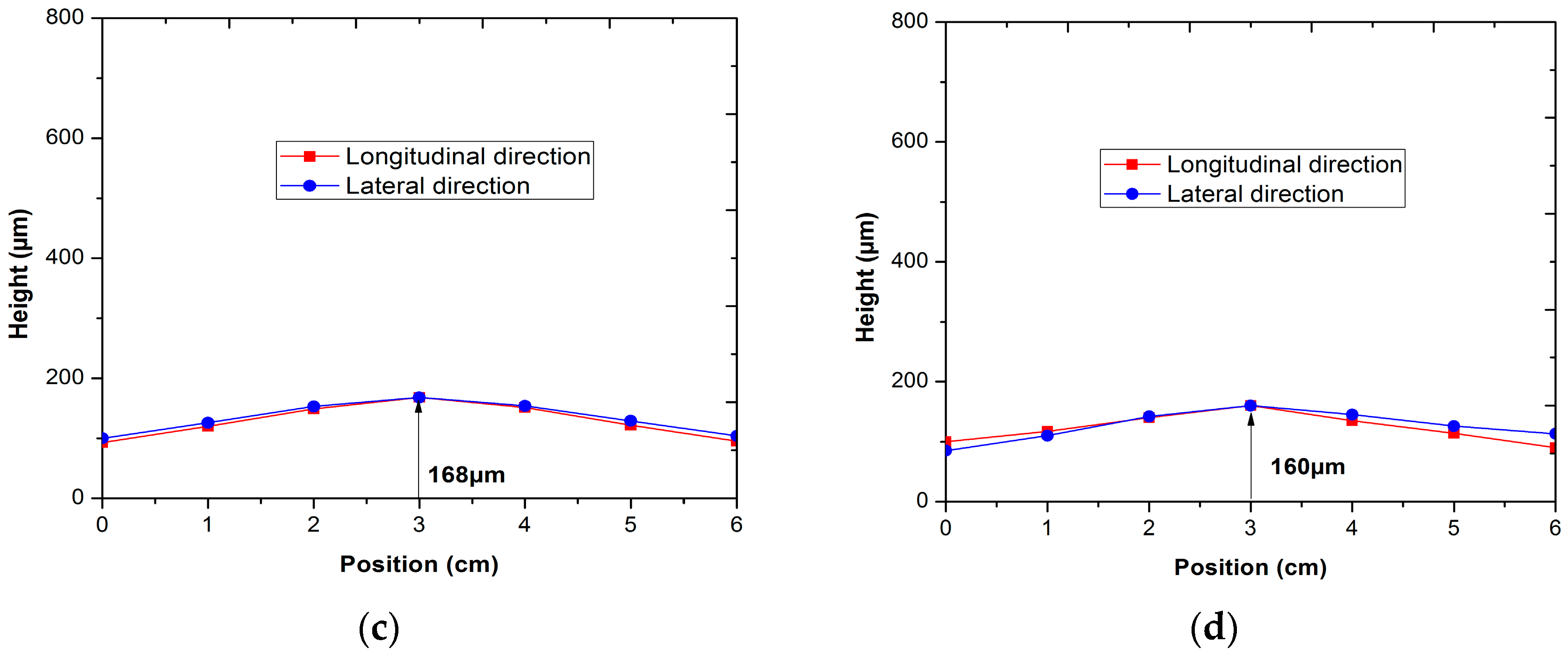
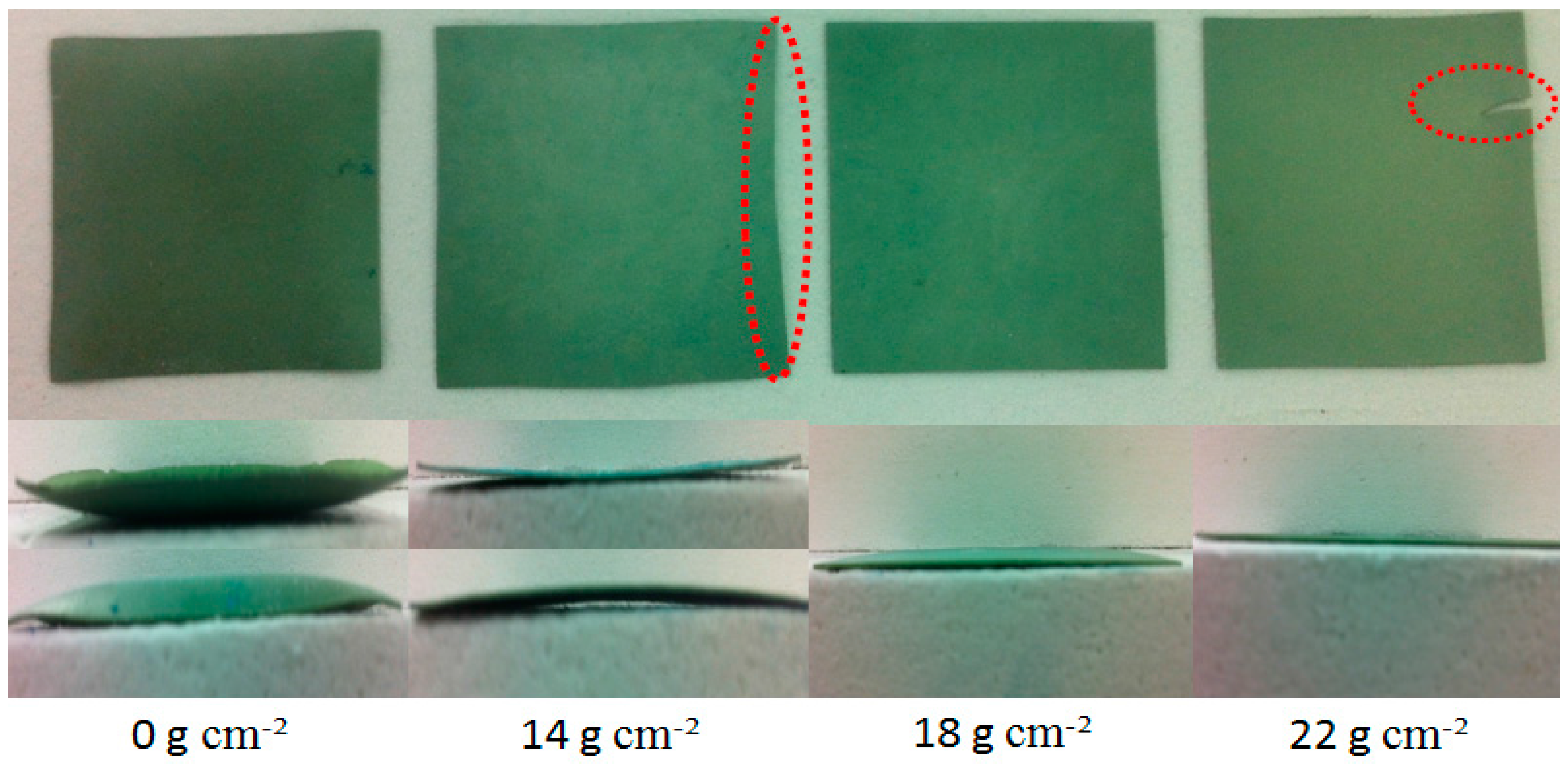
2.2. Evaluating the Effects of Flatness and Deformation on the Cell
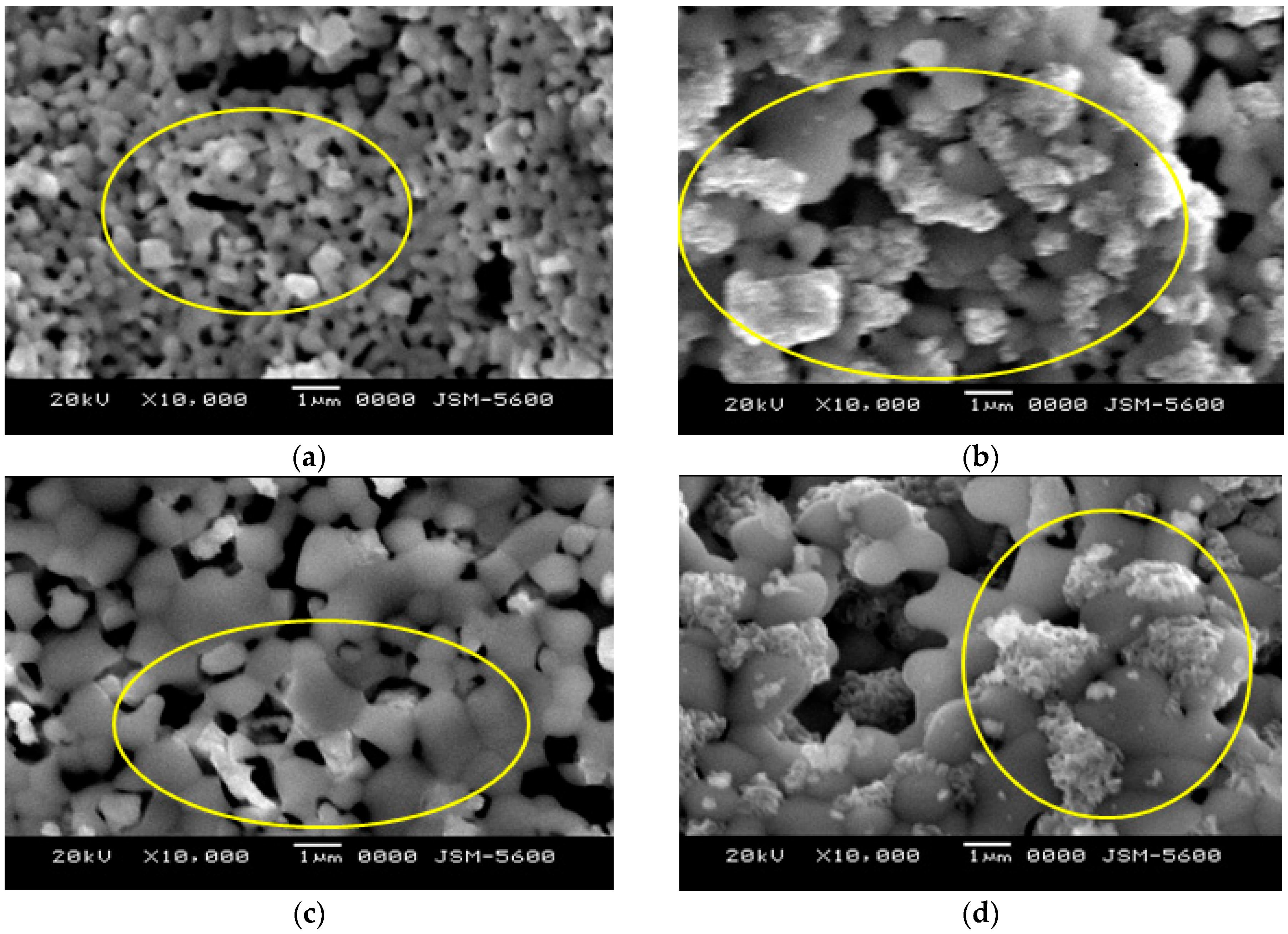
2.3. Surface Roughness and Microstructural Characterization
2.4. Cell Testing
3. Results and Discussion
3.1. Effect of Applied Loads on the Flatness and Deformation of the Anode-Supported Electrolyte
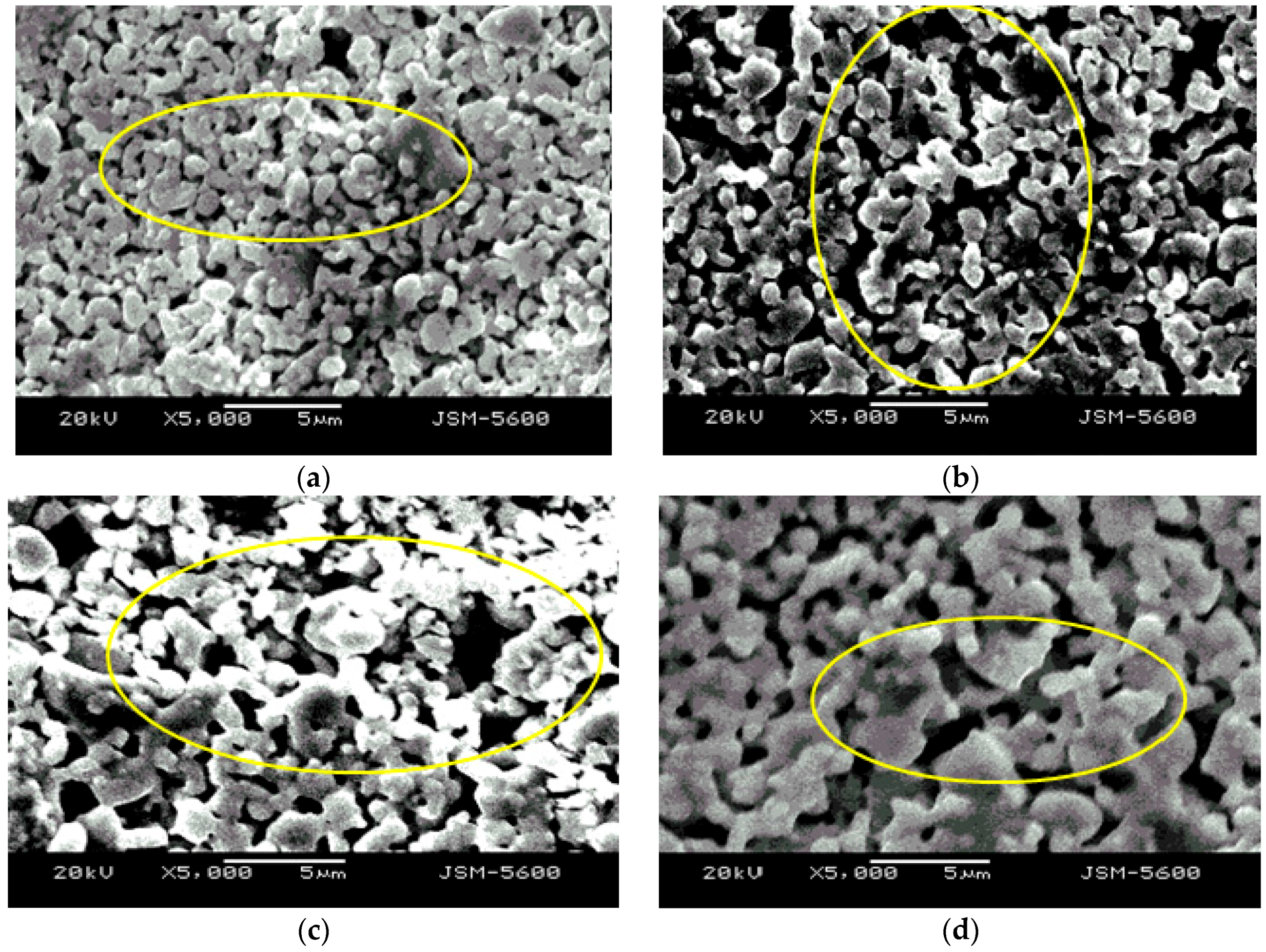
3.2. Effect of Co-Sintering Temperature on the Microstructural Properties
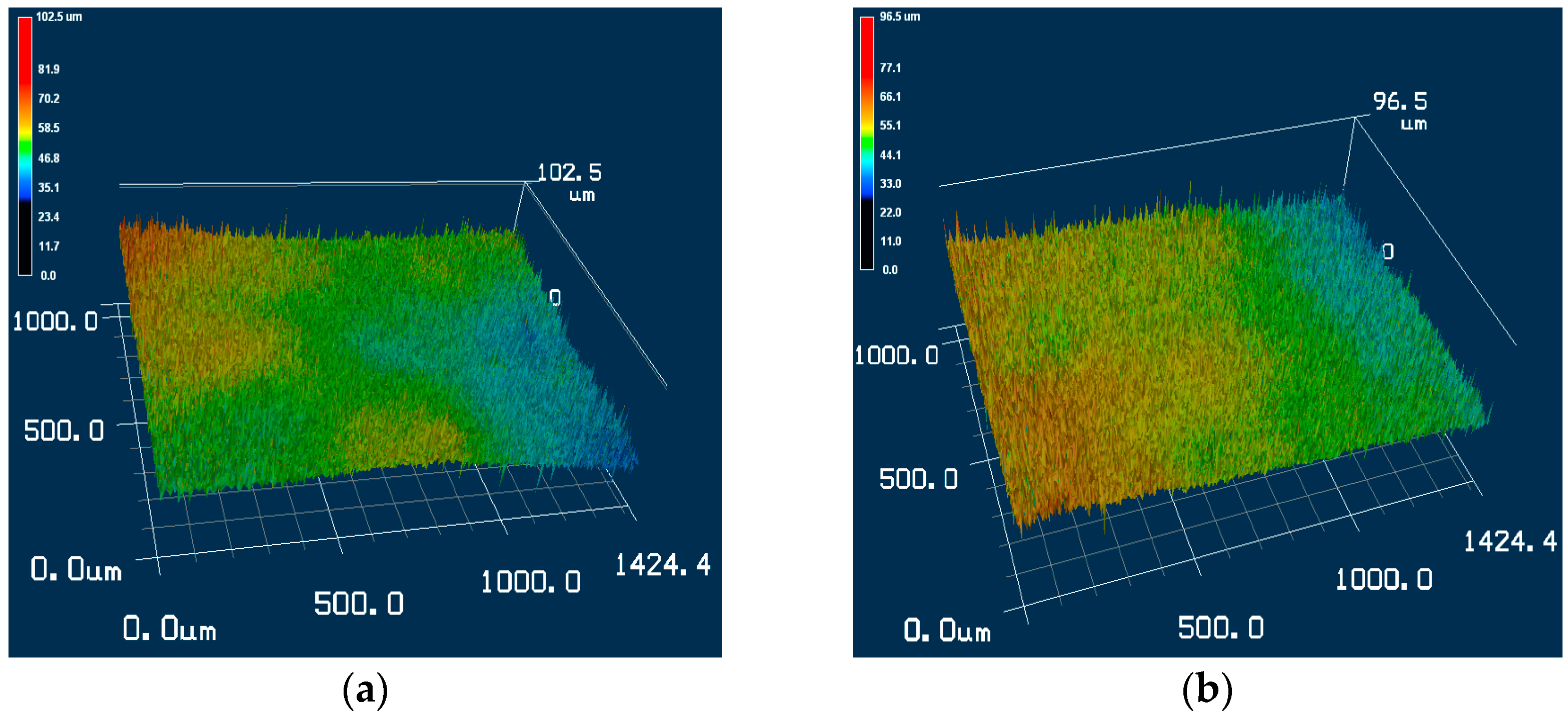
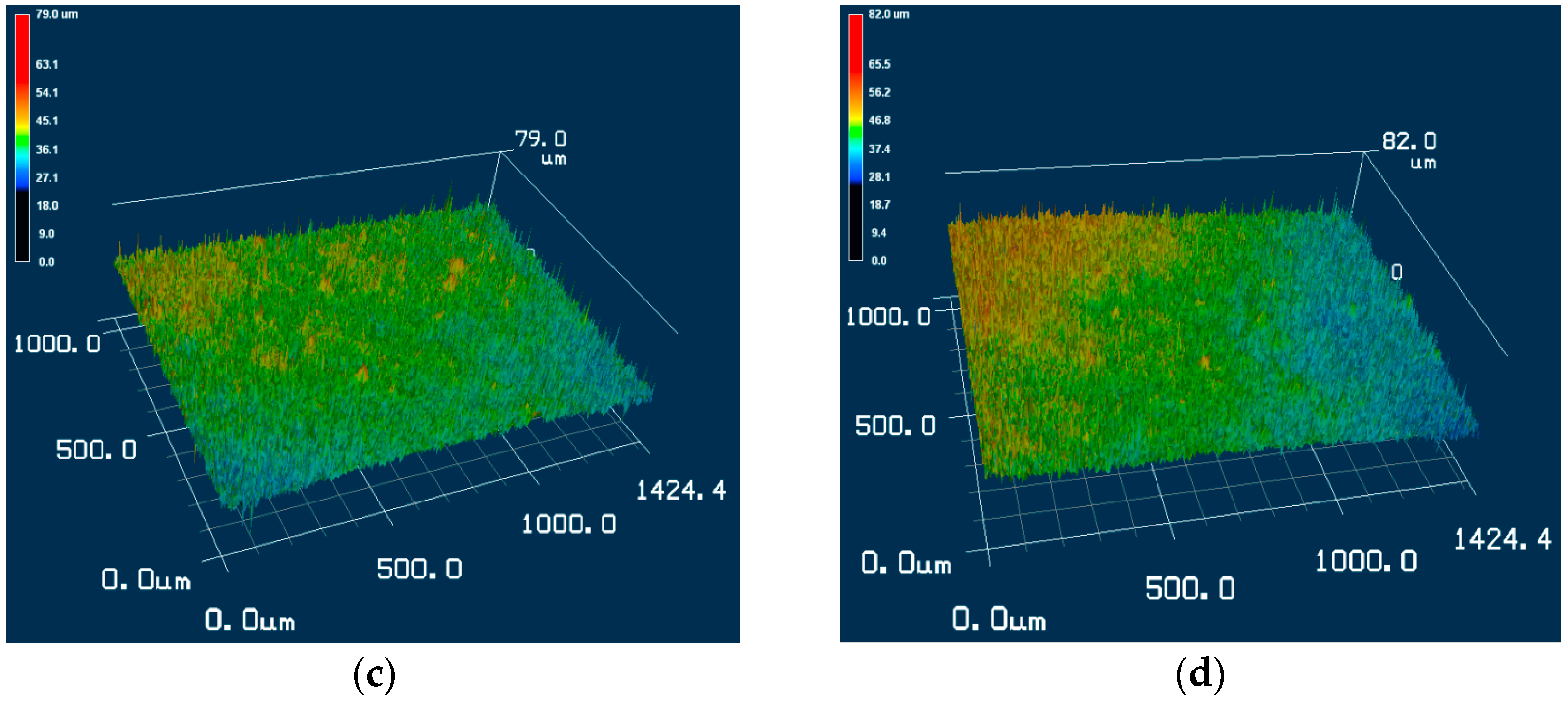
3.3. Surface Roughness
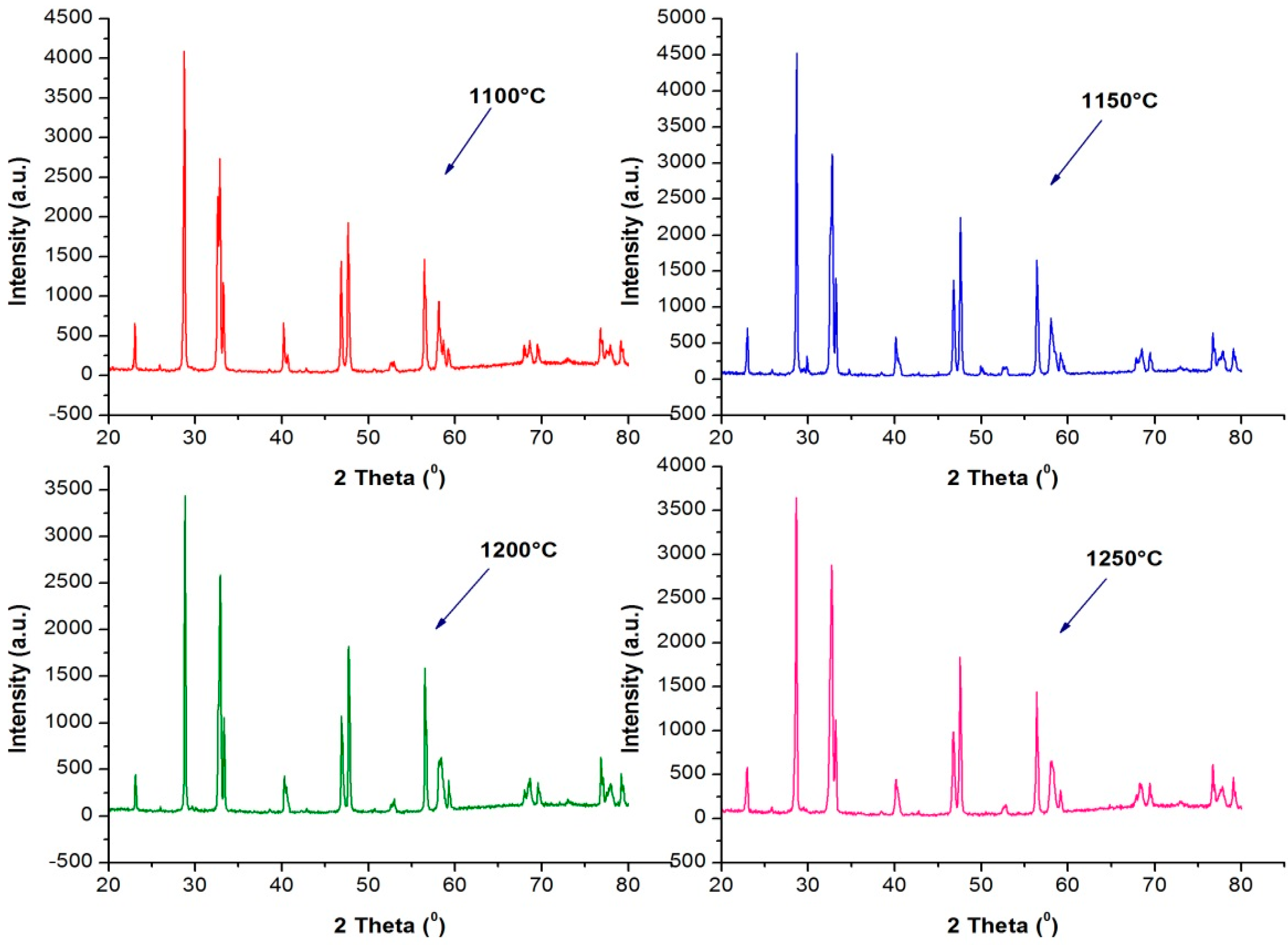
3.4. X-ray Diffraction Pattern
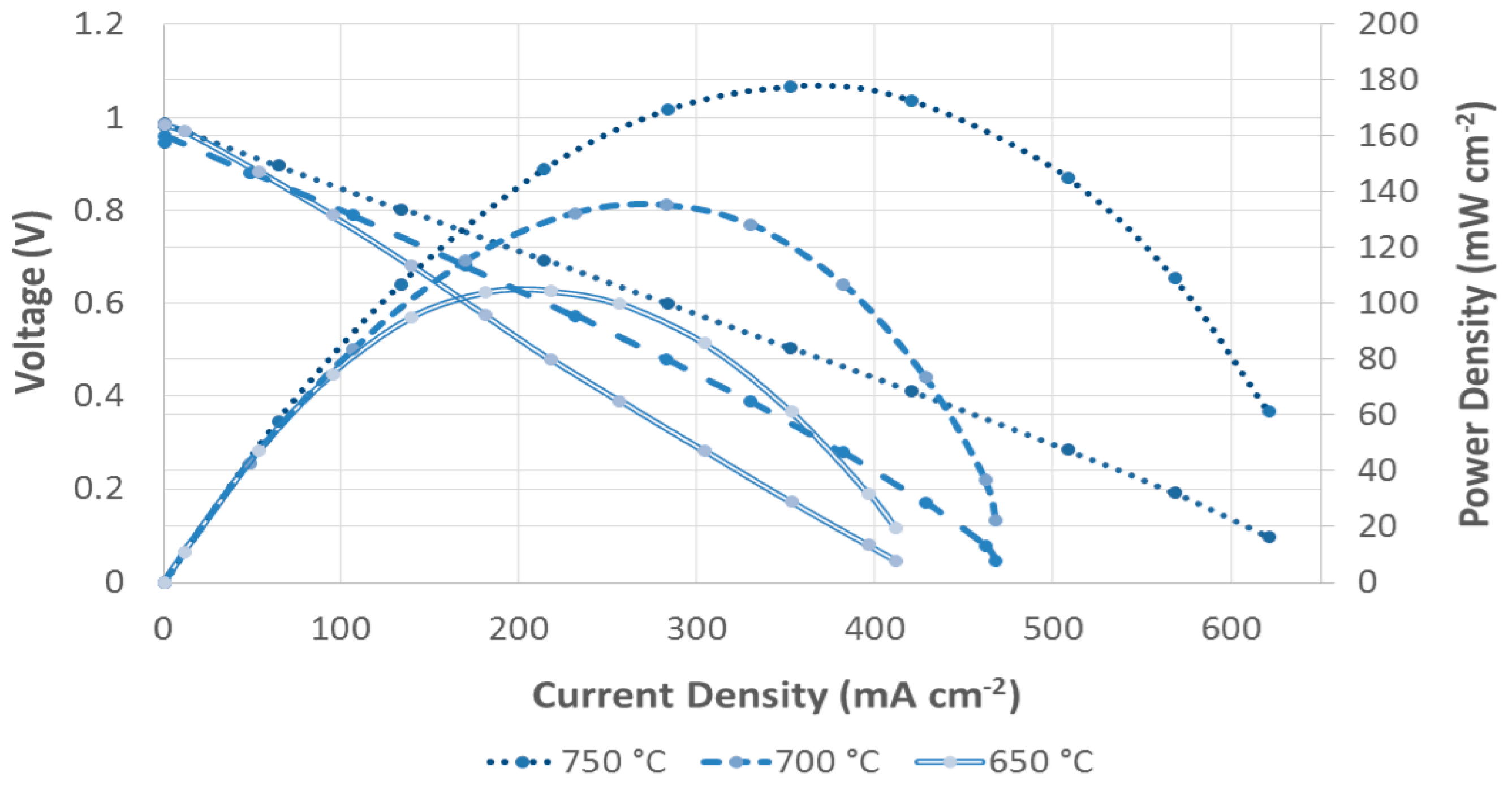
3.5. Cell Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SOFC | Solid oxide fuel cell |
| SEM | Scanning electron microscopy |
| CLSM | Confocal laser scanning microscopy |
| XRD | X-ray diffraction |
References
- Wang, Z.; Qian, J.; Cao, L.; Wang, S.; Wen, T. A study of multilayer tape casting method for anode-supported planar type solid oxide fuel cells (SOFCs). J. Alloys Compd. 2007, 437, 264–268. [Google Scholar] [CrossRef]
- Haile, S.M. Fuel cell materials and components. Acta Mater. 2003, 51, 5981–6000. [Google Scholar] [CrossRef]
- Jung, G.B.; Lo, K.F.; Chan, S.H. Effect of pretreatments on the anode structure of solid oxide fuel cells. J. Solid State Electrochem. 2007, 11, 1435–1440. [Google Scholar] [CrossRef]
- Minh, N.Q. Ceramic fuel cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Chen, C.C.; Nasrallah, M.M.; Anderson, H.U. Synthesis and characterization of YSZ thin film electrolytes. Solid State Ion. 1994, 70, 101–108. [Google Scholar] [CrossRef]
- Souza, S.D.; Visco, S.J.; Jonghe, L.C.D. Thin-film solid oxide fuel cell with high performance at low-temperature. Solid State Ion. 1997, 98, 57–61. [Google Scholar] [CrossRef]
- Kim, J.W.; Virkar, A.V.; Fung, K.Z.; Mehta, K.; Singhal, S.C. Polarization effects in intermediate temperature, anode-supported solid oxide fuel cells. J. Electrochem. Soc. 1999, 146, 69–78. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, S.M. Origin of cathodic degradation and new phase formation at the La0.9SrO0.1MnO3/YSZ interface. Solid State Ion. 1996, 90, 133–140. [Google Scholar] [CrossRef]
- Liu, Y.; Hashimoto, S.; Nishino, H.; Takei, K.; Mori, M. Fabrication and characterization of a co-fired La0.6Sr0.4Co0.2Fe0.8O3-δ cathode-supported Ce0.9Gd0.1O1.95 thin-film for IT-SOFCs. J. Power Sources 2007, 164, 56–64. [Google Scholar] [CrossRef]
- Bao, W.; Chang, Q.; Meng, G. Effect of NiO/YSZ compositions on the co-sintering process of anode-supported fuel cell. J. Membr. Sci. 2005, 259, 103–109. [Google Scholar] [CrossRef]
- Besra, L.; Zha, S.; Liu, M. Preparation of NiO-YSZ/YSZ bi-layers for solid oxide fuel cells by electrophoretic deposition. J. Power Sources 2006, 160, 207–214. [Google Scholar] [CrossRef]
- Leng, Y.J.; Chan, S.H.; Khor, K.A.; Jiang, S.P. Performance evaluation of anode-supported solid oxide fuel cells with thin film YSZ electrolyte. Int. J. Hydrog. Energy 2004, 29, 1025–1033. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Chroneos, A.; Yildiz, B.; Tarancon, A.; Parfitt, D.; Kilner, J.A. Oxygen diffusion in solid oxide fuel cell cathode and electrolyte materials: Mechanistic insights from atomistic simulations. Energy Environ. Sci. 2011, 4, 2774–2789. [Google Scholar] [CrossRef]
- De Jonghe, L.C.; Jacobson, C.P.; Visco, S.J. Supported electrolyte thin film synthesis of solid oxide fuel cells. Annu. Rev. Mater. Res. 2003, 33, 169–182. [Google Scholar] [CrossRef]
- Rupasov, D.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W.; Istomin, S.Y.; Antipov, E.V. Oxygen diffusion in Sr0.75Y0.25CoO2.625: A molecular dynamics study. Phys. Rev. B 2009, 79, 1–4. [Google Scholar] [CrossRef]
- Seymour, I.D.; Tarancón, A.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in PrBaCo2O5.5 double perovskites. Solid State Ion. 2012, 216, 41–43. [Google Scholar] [CrossRef]
- Timakul, P.; Jinawath, S.; Aungkavattana, P. Fabrication of electrolyte materials for solid oxide fuel cells by tape-casting. Ceram. Int. 2008, 34, 867–871. [Google Scholar] [CrossRef]
- Mücke, R.; Menzler, N.H.; Buchkremer, H.P.; Stover, D. Co-firing of thin zirconia films during SOFC manufacturing. J. Am. Ceram. Soc. 2009, 92, S95–S102. [Google Scholar] [CrossRef]
- Moon, H.; Kim, S.D.; Park, E.W.; Hyun, S.H.; Kim, H.S. Characteristics of SOFC single cells with anode active layer via tape casting and co-firing. Int. J. Hydrog. Energy 2008, 33, 2826–2833. [Google Scholar] [CrossRef]
- Park, H.G.; Moon, H.; Park, S.C.; Lee, J.J.; Yoon, D.; Hyun, S.H.; Kim, D.H. Performance improvement of anode-supported electrolytes for planar solid oxide fuel cells via a tape-casting/lamination/co-firing technique. J. Power Sources 2010, 195, 2463–2469. [Google Scholar] [CrossRef]
- Moon, H.; Kang, D.W.; Park, H.G.; Hyun, S.H. Stress and camber analysis of anode-supported electrolytes by tape-casting and co-firing techniques. Int. J. Hydrog. Energy 2011, 36, 10991–10997. [Google Scholar] [CrossRef]
- Malzbender, J.; Steinbrech, R.W.; Singheiser, L. A review of advanced techniques for characterizing SOFC behavior. Fuel Cells 2009, 9, 785–793. [Google Scholar] [CrossRef]
- Shen, Z.; Zhu, X.; Le, S.; Sun, W.; Sun, K. Co-sintering anode and Y2O3 stabilized ZrO2 thin electrolyte film for solid oxide fuel cell fabricated by co-tape casting. Int. J. Hydrog. Energy 2012, 37, 10337–10345. [Google Scholar] [CrossRef]
- Menzler, N.H.; Malzbender, J.; Schoderbock, P.; Kauert, R.; Buchkremer, H.P. Sequential tape casting of anode-supported solid oxide fuel cells. Fuel Cells 2014, 14, 96–106. [Google Scholar] [CrossRef]
- Cologna, M.; Contino, A.R.; Montinaro, D.; Sglavo, V.M. Effect of Al and Ce doping on the deformation upon sintering in sequential tape cast layers for solid oxide fuel cells. J. Power Sources 2009, 193, 80–85. [Google Scholar] [CrossRef]
- He, C.R.; Wang, W.G.; Wang, J.; Xue, Y. Effect of alumina on the curvature, Young’s modulus, thermal expansion coefficient and residual stress of planar solid oxide fuel cells. J. Power Sources 2011, 196, 7639–7644. [Google Scholar] [CrossRef]
- Sun, B.; Rudkin, R.A.; Atkinson, A. Effect of thermal cycling on residual stress and curvature of anode-supported SOFCs. Fuel Cells 2009, 9, 805–813. [Google Scholar] [CrossRef]
- Lia, W.; Hasinska, K.; Seabaugh, M.; Swartz, S.; Lannutti, J. Curvature in solid oxide fuel cells. J. Power Sources 2004, 138, 145–155. [Google Scholar] [CrossRef]
- Jung, G.B.; Fang, L.H.; Chiou, M.J.; Nguyen, X.-V.; Su, A.; Lee, W.-T.; Chang, S.W.; Kao, I.C.; Yu, J.W. Effects of pretreatment methods on electrodes and SOFC performance. Energies 2014, 7, 3922–3933. [Google Scholar] [CrossRef]
- Jung, G.B.; Fang, L.H.; Lin, C.Y.; Nguyen, X.-V.; Yeh, C.C.; Lee, C.Y.; Yu, J.W.; Chan, S.H.; Lee, W.-T.; Chang, S.W.; et al. Electrochemical performance and long-term durability of a reversible solid oxide fuel cell. Int. J. Electrochem. Sci. 2015, 10, 9089–9104. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, X.-V.; Chang, C.-T.; Jung, G.-B.; Chan, S.-H.; Huang, W.C.-W.; Hsiao, K.-J.; Lee, W.-T.; Chang, S.-W.; Kao, I.-C. Effect of Sintering Temperature and Applied Load on Anode-Supported Electrodes for SOFC Application. Energies 2016, 9, 701. https://doi.org/10.3390/en9090701
Nguyen X-V, Chang C-T, Jung G-B, Chan S-H, Huang WC-W, Hsiao K-J, Lee W-T, Chang S-W, Kao I-C. Effect of Sintering Temperature and Applied Load on Anode-Supported Electrodes for SOFC Application. Energies. 2016; 9(9):701. https://doi.org/10.3390/en9090701
Chicago/Turabian StyleNguyen, Xuan-Vien, Chang-Tsair Chang, Guo-Bin Jung, Shih-Hung Chan, Wilson Chao-Wei Huang, Kai-Jung Hsiao, Win-Tai Lee, Shu-Wei Chang, and I-Cheng Kao. 2016. "Effect of Sintering Temperature and Applied Load on Anode-Supported Electrodes for SOFC Application" Energies 9, no. 9: 701. https://doi.org/10.3390/en9090701




