Suppressing Heavy Metal Leaching through Ball Milling of Fly Ash
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Pretreatment
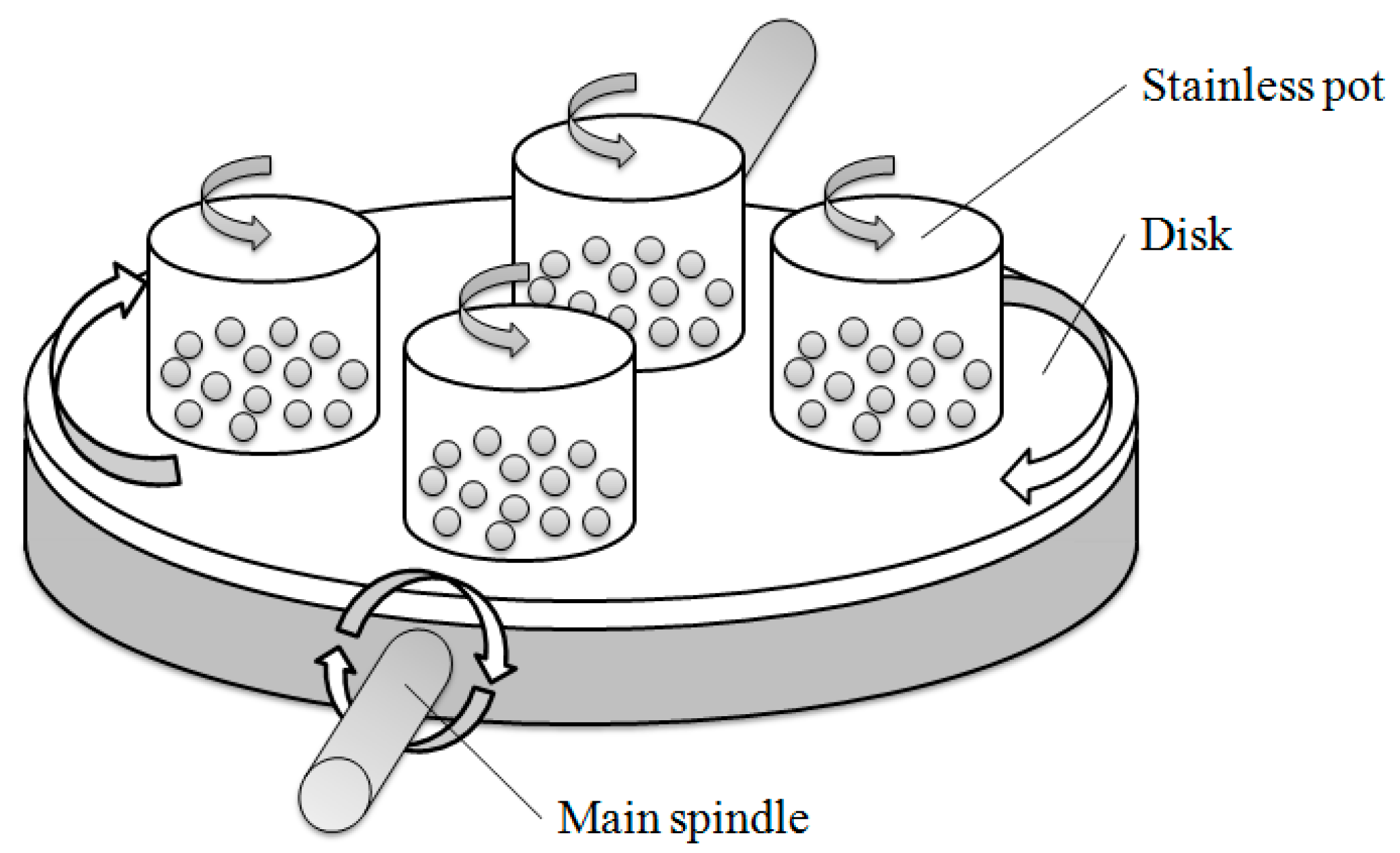
2.2. Ball Milling Experiments
2.3. Analytical Methods
3. Results and Discussion
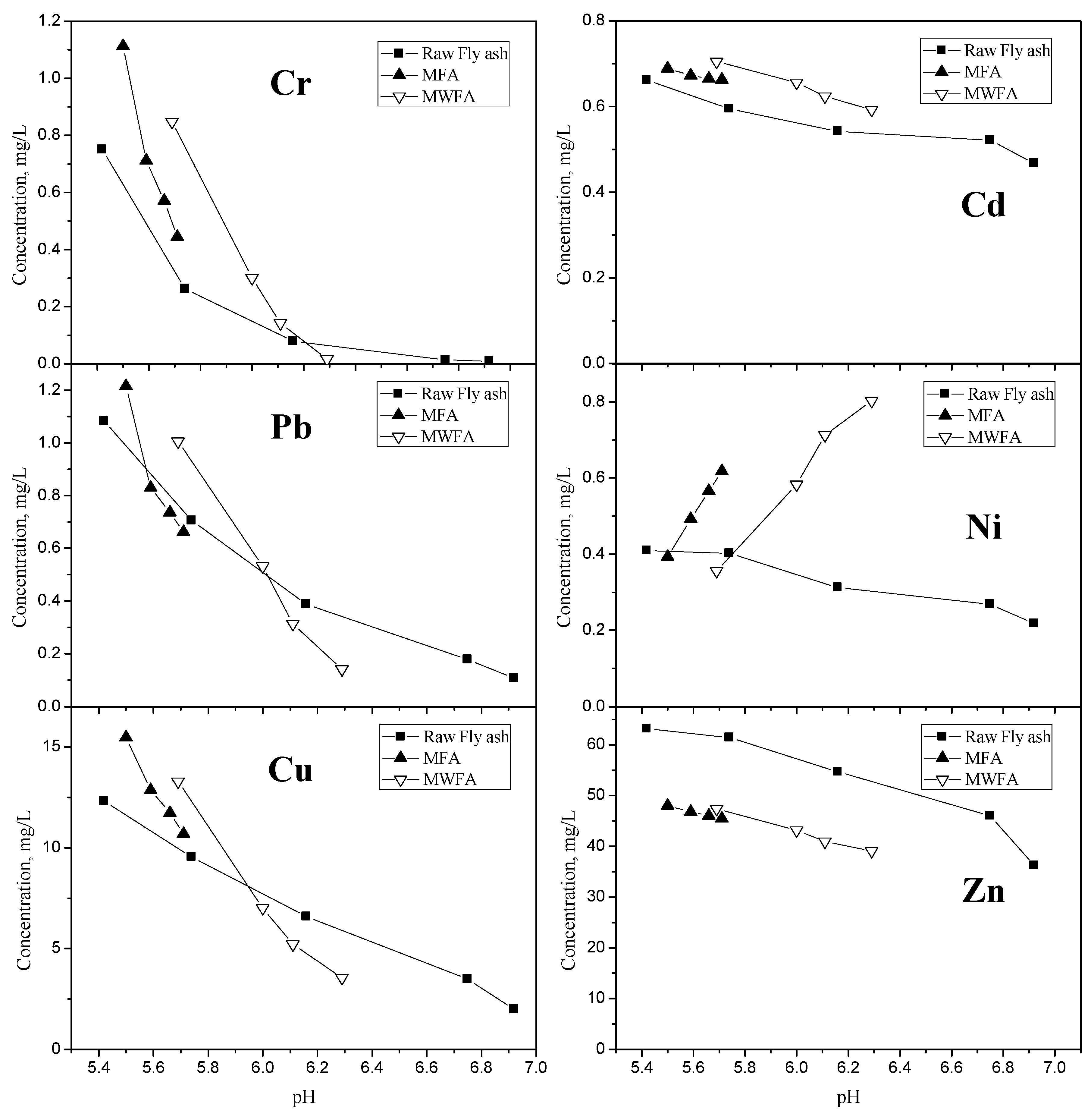
3.1. Characteristics of Milled and Washed fly Ash—Heavy Metals Leaching Tests
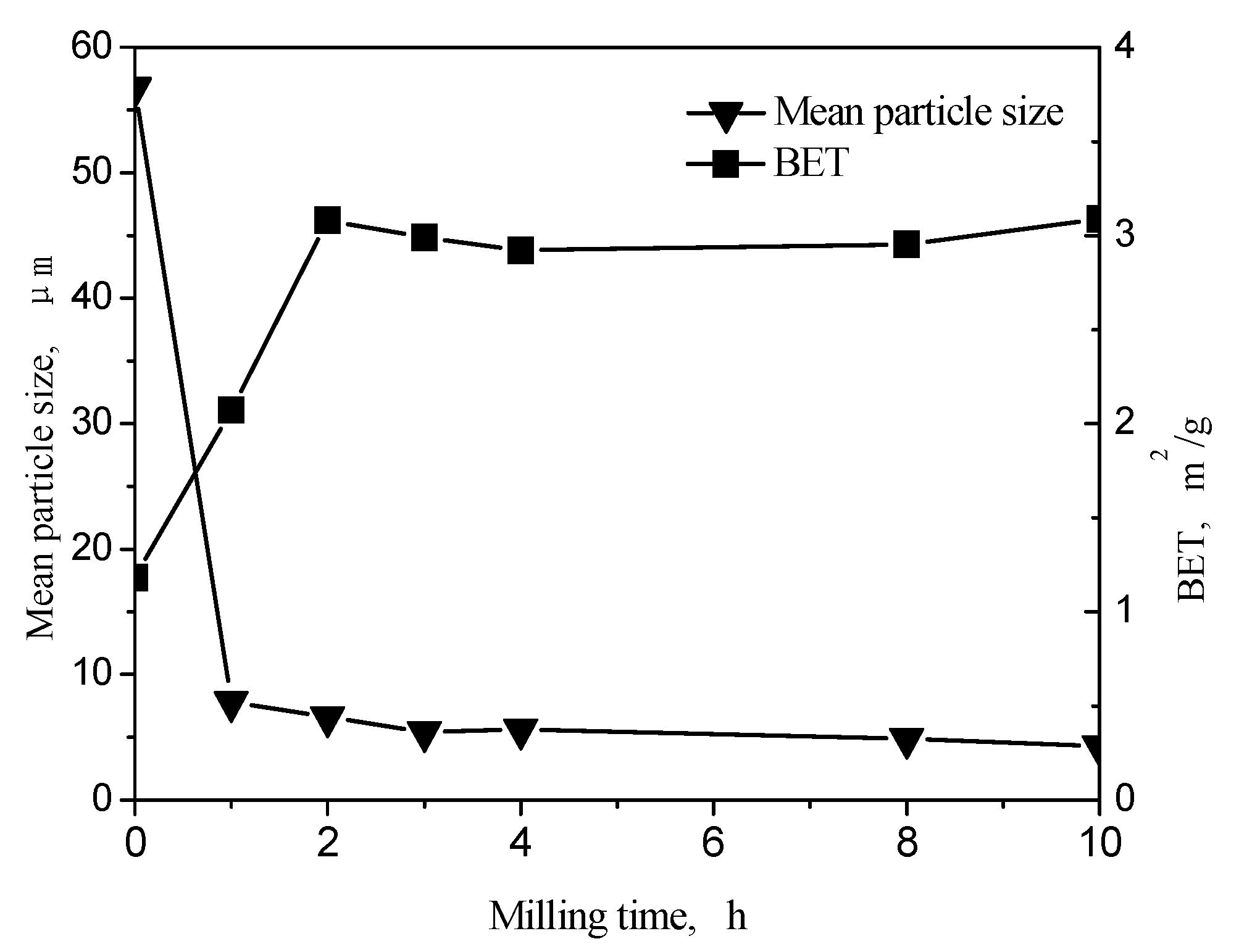
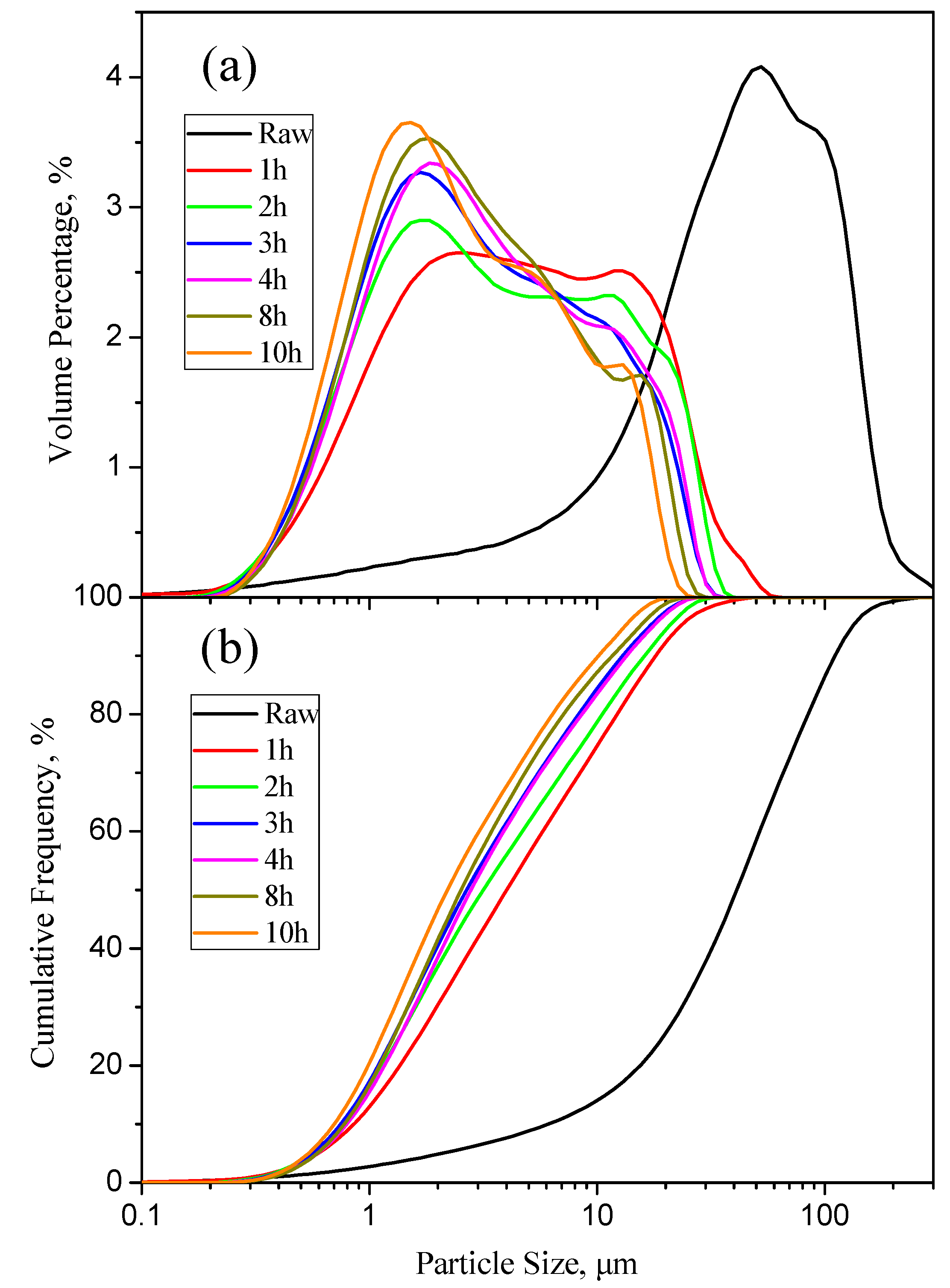
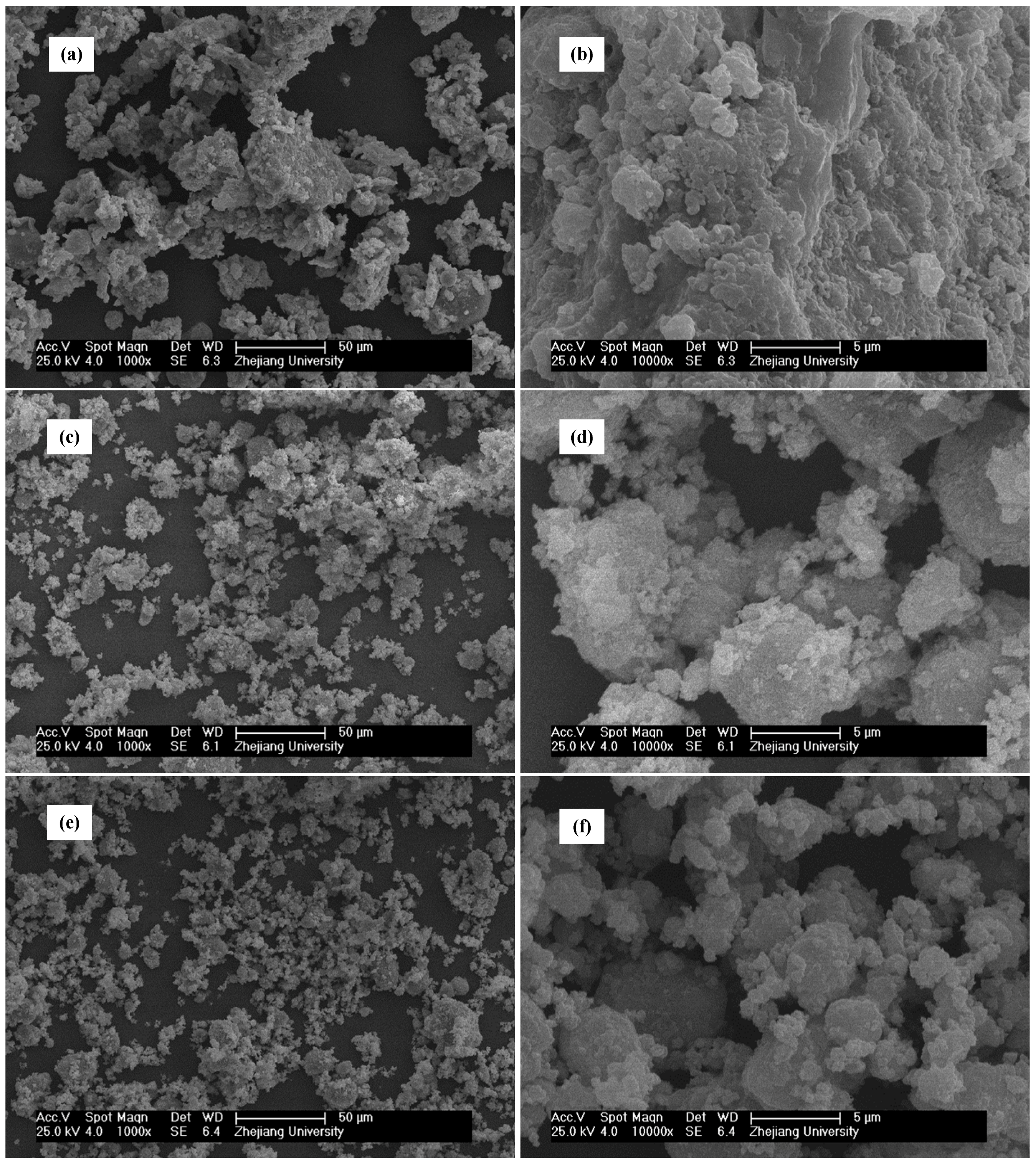
3.2. Variation in the Particle Size of Milled Fly Ash
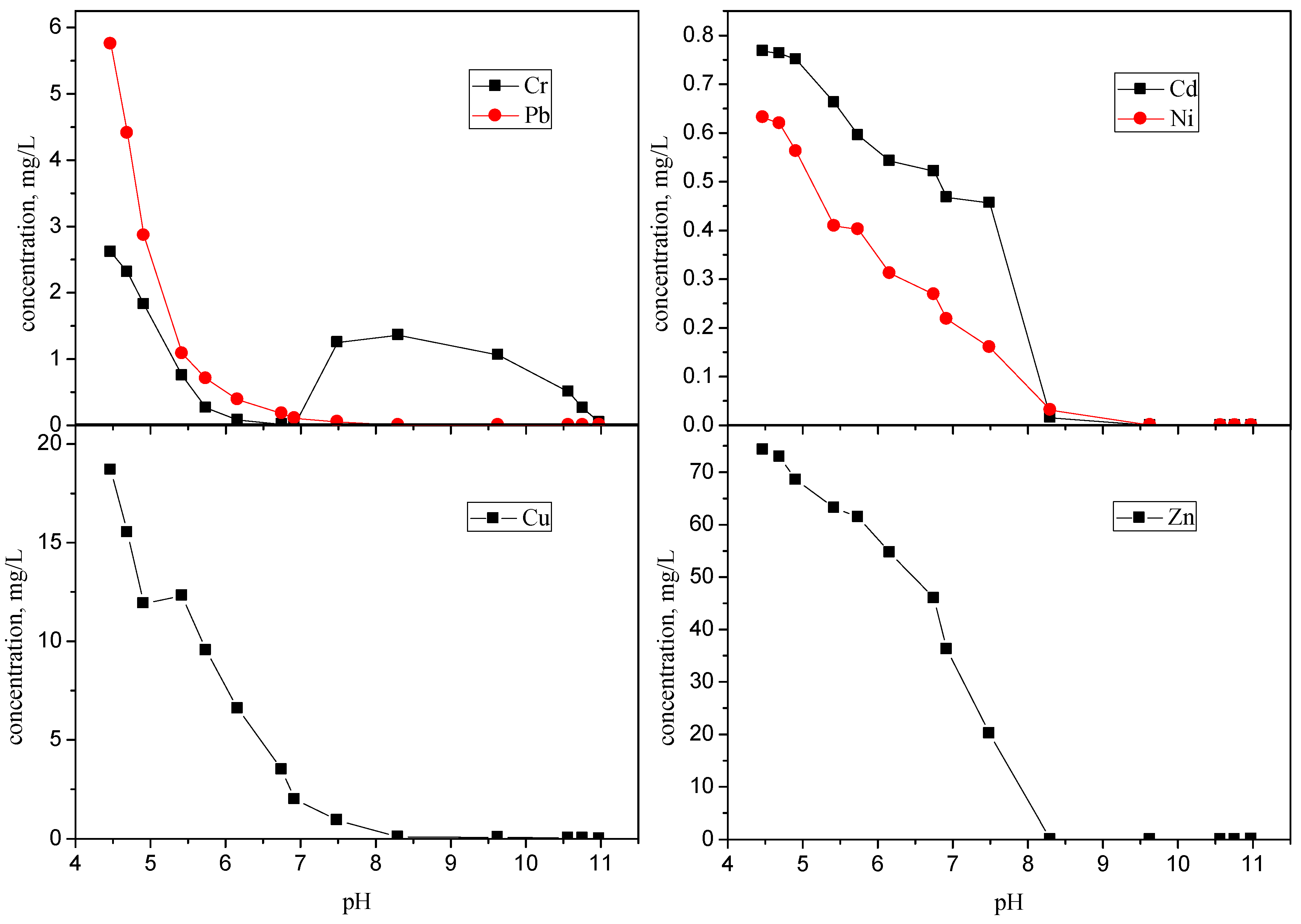
3.3. Leaching Test Results Related to pH and the Assessment of Stabilization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Q.W.; Matsumoto, H.; Saito, F.; Baron, M. Debromination of hexabromobenzene by its co-grinding with CaO. Chemosphere 2002, 48, 787–793. [Google Scholar] [CrossRef]
- Lu, S.; Huang, J.; Peng, Z.; Lia, X.; Yan, J. Ball milling 2,4,6-trichlorophenol with calcium oxide: Dechlorination experiment and mechanism considerations. Chem. Eng. J. 2012, 195, 62–68. [Google Scholar] [CrossRef]
- Wei, Y.; Yan, J.; Lu, S.; Li, X. Mechanochemical decomposition of pentachlorophenol by ball milling. J. Environ. Sci. 2009, 21, 1761–1768. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, J.; Yu, G.; Zhang, Q.; Deng, S.; Wang, B. Destruction of Perfluorooctane Sulfonate (PFOS) and Perfluorooctanoic Acid (PFOA) by Ball Milling. Environ. Sci. Technol. 2013, 47, 6471–6477. [Google Scholar] [CrossRef] [PubMed]
- Monagheddu, M.; Mulas, G.; Doppiu, S.; Cocco, G.; Raccanelli, S. Reduction of polychlorinated dibenzodioxins and dibenzofurans in contaminated muds by mechanically induced combustion reactions. Environ. Sci. Technol. 1999, 33, 2485–2488. [Google Scholar] [CrossRef]
- Nah, I.W.; Hwang, K.; Shul, Y. Effect of metal and glycol on mechanochemical dechlorination of polychlorinated biphenyls (PCBs). Chemosphere 2008, 73, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Shu, S.; Junya, K.; Fumio, S.; Shimme, K.; Masuda, S.; Inoue, T. Effect of additives on dechlorination of PVC by mechanochemical treatment. J. Mater. Cycles Waste Manag. 2001, 1, 20–23. [Google Scholar]
- Mao, Q.; Peng, Z.; Lu, S.; Yan, J. Mechanochemical Degradation of OCDD/OCDF in Fly Ash from Medical Waste Incinerators. Acta Chim. Sin. 2012, 70, 659–666. [Google Scholar] [CrossRef]
- Peng, Z.; Ding, Q.; Sun, Y.; Jiang, C.; Gao, X.; Yan, J. Characterization of mechanochemical treated fly ash from a medical waste incinerator. J. Environ. Sci. 2010, 22, 1643–1648. [Google Scholar] [CrossRef]
- Mitoma, Y.; Miyata, H.; Egashira, N.; Simion, A.M.; Kakeda, M.; Simion, C. Mechanochemical degradation of chlorinated contaminants in fly ash with a calcium-based degradation reagent. Chemosphere 2011, 83, 1326–1330. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.H.; Peng, Z.; Lu, S.Y.; Li, X.D.; Ni, M.J.; Cen, K.F.; Dai, H.F. Degradation of PCDD/Fs by mechanochemical treatment of fly ash from medical waste incineration. J. Hazard. Mater. 2007, 147, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayana, C. Mechanical alloying and milling. Progress Mater. Sci. 2001, 46, 1–184. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; El-Bahnasawy, H.N.; Ahmed, H.A.; Eissa, N.A. Mechanical solid-state reduction of haematite with magnesium. J. Alloys Compd. 2001, 314, 286–295. [Google Scholar] [CrossRef]
- Marjanovic, N.; Komljenovic, M.; Bascarevic, Z.; Nikolić, V. Improving reactivity of fly ash and properties of ensuing geopolymers through mechanical activation. Constr. Build. Mater. 2014, 57, 151–162. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, R. Mechanical activation of fly ash: Effect on reaction, structure and properties of resulting geopolymer. Ceramics Int. 2011, 37, 533–541. [Google Scholar] [CrossRef]
- Juhász, A.Z.; Oppczky, L. Mechanical Activation of Minerals by Grinding: Pulverising and Morphology of Particles; AkadémiaiKiadó: Budapest, Hungary, 1990. [Google Scholar]
- Hamzaoui, R.; Bouchenafa, O.; Guessasma, S.; Leklouc, N.; Bouaziz, A. The sequel of modified fly ashes using high energy ball milling on mechanical performance of substituted past cement. Mater. Des. 2016, 90, 29–37. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Matsushita, M.; Inoue, A. Phase transformations of ball-milled Nb50Zr10Al10Ni10Cu20 powders and the effect of annealing. J. Alloys Compd. 2001, 329, 239–252. [Google Scholar] [CrossRef]
- Hamzaoui, R.; Muslim, F.; Guessasma, S.; Bennabia, A.; Guillin, J. Structural and thermal behavior of proclay kaolinite using high energy ball milling process. Powder Technol. 2015, 271, 228–237. [Google Scholar] [CrossRef]
- Montinaro, S.; Concas, A.; Pisu, M.; Cao, G. Immobilization of heavy metals in contaminated soils through ball milling with and without additives. Chem. Eng. J. 2008, 142, 271–284. [Google Scholar] [CrossRef]
- Nomura, Y.; Fujiwara, K.; Terada, A.; Nakai, S.; Hosomi, M. Prevention of lead leaching from fly ashes by mechanochemical treatment. Waste Manag. 2010, 30, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.; Okada, T.; Nakai, S.; Hosomi, M. Inhibition of heavy metal elution from fly ashes by mechanochemical treatment and cementation. Kagaku Kogaku Ronbunshu 2006, 32, 196–199. [Google Scholar] [CrossRef]
- Li, M.; Sun, C.; Gau, S.; Chuang, C.J. Effects of wet ball milling on lead stabilization and particle size variation in municipal solid waste incinerator fly ash. J. Hazard. Mater. 2010, 174, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Li, M.; Gau, S.; Chuang, C.J. Effect of the milling solution on lead stabilization in municipal solid waste incinerator fly ash during the milling processes. Waste Manag. 2011, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Nomura, Y.F.K.T. Detoxification of fly ash by mechanochemical treatment with blast furnace slag and the usability of the residues as cement materials. J. Jpn. Soc. Waste Manag. Experts 2006, 5, 355–360. [Google Scholar] [CrossRef]
- Suryanarayana, C. Bibliography on Mechanical Alloying and Milling; Cambridge International Science Publishing: Cambridge, UK, 1995. [Google Scholar]
- Orumwense, O.A.; Forssberg, E. Surface and structural-changes in wet ground minerals. Powder Technol. 1991, 68, 23–29. [Google Scholar] [CrossRef]
- Lu, L.; Lai, M.O.; Zhang, S. Diffusion in mechanical alloying. J. Mater. Proc. Technol. 1997, 67, 100–104. [Google Scholar] [CrossRef]
- Alleso, M.; Chieng, N.; Rehder, S.; Rantanen, J.; Rades, T.; Aaltonen, J. Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: Amorphous naproxen-cimetidine mixtures prepared by mechanical activation. J. Control. Release 2009, 136, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Grassi, G.; Grassi, M. Drug Mechanochemical Activation. J. Pharm. Sci. 2009, 98, 3961–3986. [Google Scholar] [CrossRef] [PubMed]
- Elamin, A.A.; Ahlneck, C.; Alderborn, G.; Nyström, C. Increased metastable solubility of milled griseofulvin, depending on the formation of a disordered surface-structure. Int. J. Pharm. 1994, 111, 159–170. [Google Scholar] [CrossRef]
- Gbureck, U.; Grolms, O.; Barralet, J.E.; Grover, L.M.; Thull, R. Mechanical activation and cement formation of beta-tricalcium phosphate. Biomaterials 2003, 24, 4123–4131. [Google Scholar] [CrossRef]
- Huang, Z.; Lu, J.; Li, X.; Tong, Z. Effect of mechanical activation on physico-chemical properties and structure of cassava starch. Carbohydr. Polym. 2007, 68, 128–135. [Google Scholar] [CrossRef]
- Huang, Z.; Xie, X.; Chen, Y.; Lu, J.; Tong, Z. Ball-milling treatment effect on physicochemical properties and features for cassava and maize starches. Comptes Rendus Chim. 2008, 11, 73–79. [Google Scholar] [CrossRef]
- Murty, B.S.; Ranganathan, S. Novel materials synthesis by mechanical alloying/milling. Int. Mater. Rev. 1998, 43, 101–141. [Google Scholar] [CrossRef]
- Sakai, S.; Yamada, Y.; Zenke, T.; Kawakamia, K. Novel chitosan derivative soluble at neutral pH and in-situ gellable via peroxidase-catalyzed enzymatic reaction. J. Mater. Chem. 2009, 19, 230–235. [Google Scholar] [CrossRef]

| Methods | Preparation of Extracting Reagent | pH (Extracting Reagent) | L/S | Shaking Conditions |
|---|---|---|---|---|
| HJ/T300-2007 | Dilute 17. 25 mL glacial acetic acid with deionised water to 1 L | 2. 64 ± 0. 05 | 20:1 | Rotary shaking at 30 ± 2 r/min for 18 ± 2 h, at room temperature |
| TCLP | Dilute 5. 7 mL glacial acetic acid with deionised water to 1 L | 2. 88 ± 0. 05 | 20:1 | Rotary shaking at 30 ± 2 r/min for 18 ± 2 h, at room temperature |
| Cations, Anions, and Heavy Metals | Raw Fly Ash | Milled Fly Ash (10 h of Milling) | Washed Fly Ash |
|---|---|---|---|
| O a | 34.71 ± 0.3 | 35.69 ± 0.3 | 37.97 ± 0.3 |
| Ca a | 16.98 ± 0.15 | 15.95 ± 0.15 | 17.88 ± 0.15 |
| Si a | 11.18 ± 0.10 | 13.26 ± 0.11 | 13.18 ± 0.11 |
| Al a | 5.80 ± 0.08 | 5.84 ± 0.08 | 6.39 ± 0.09 |
| Cl a | 7.43 ± 0.13 | 6.12 ± 0.12 | 1.37 ± 0.06 |
| C a | 7.50 ± 0.13 | 7.61 ± 0.13 | 7.96 ±0.13 |
| Mg a | 3.41 ± 0.07 | 3.02 ± 0.07 | 3.92 ± 0.07 |
| Fe a | 2.86 ± 0.07 | 2.98 ± 0.07 | 3.48 ± 0.08 |
| P a | 1.46 ± 0.04 | 1.24 ± 0.04 | 1.87 ± 0.04 |
| S a | 1.28 ± 0.04 | 1.06 ± 0.04 | 1.61 ± 0.04 |
| Na a | 4.44 ± 0.09 | 4.25 ± 0.09 | 1.91 ± 0.06 |
| K a | 1.68 ± 0.06 | 1.67 ± 0.06 | 0.897 ± 0.043 |
| Ti a | 0.554 ± 0.03 | 0.55 ± 0.03 | 0.634 ± 0.031 |
| Zn b | 4160 ± 210 | 4110 ± 210 | 5060 ± 250 |
| Cu b | 996 ± 50 | 980 ± 50 | 1170 ± 60 |
| Mn b | 770 ± 39 | 780 ± 39 | 931 ± 47 |
| Pb b | 677 ± 34 | 685 ± 34 | 815 ± 41 |
| Cr b | 408 ± 20 | 453 ± 24 | 484 ± 24 |
| Ni b | 77 ± 4 | 83 ± 4 | 94 ± 5 |
| Cd b | 26 ± 6 | 32 ±6 | 20 ± 9 |
| Heavy Metals | Milling Time | Cu | Pb | Cr | Ni | Cd | Zn | pH |
|---|---|---|---|---|---|---|---|---|
| FA | 0 h a | 15.48 | 1.21 | 1.11 | 0.39 | 0.69 | 48.00 | 5.50 |
| 2 h | 12.86 | 0.83 | 0.71 | 0.49 | 0.67 | 46.79 | 5.59 | |
| 6 h | 11.73 | 0.74 | 0.57 | 0.56 | 0.66 | 46.05 | 5.66 | |
| 10 h | 10.69 | 0.66 | 0.44 | 0.618 | 0.663 | 45.45 | 5.71 | |
| WFA | 0 h a | 13.29 | 1.006 | 0.85 | 0.356 | 0.705 | 47.40 | 5.69 |
| 2 h | 7.01 | 0.53 | 0.30 | 0.58 | 0.65 | 43.15 | 6.00 | |
| 6 h | 5.21 | 0.31 | 0.14 | 0.71 | 0.62 | 40.96 | 6.11 | |
| 10 h | 3.55 | 0.14 | 0.016 | 0.80 | 0.59 | 39.11 | 6.29 |
| pH | Cr | Pb | Cd | Ni | Cu | Zn |
|---|---|---|---|---|---|---|
| 10.98 | 0.044 | 0.0069 | ND | ND | 0.016 | 0.075 |
| 10.76 | 0.26 | 0.0052 | ND | ND | 0.037 | 0.010 |
| 10.57 | 0.50 | 0.0031 | ND | ND | 0.050 | 0.0045 |
| 9.63 | 1.06 | 0.0020 | ND | 0.0003 | 0.077 | 0.0009 |
| 8.30 | 1.36 | 0.0019 | 0.015 | 0.031 | 0.10 | 0.0087 |
| 7.49 | 1.25 | 0.05 | 0.45 | 0.16 | 0.93 | 20.16 |
| 6.92 | 0.009 | 0.11 | 0.46 | 0.22 | 1.98 | 36.20 |
| 6.75 | 0.013 | 0.18 | 0.52 | 0.27 | 3.49 | 45.98 |
| 6.16 | 0.079 | 0.39 | 0.54 | 0.31 | 6.59 | 54.67 |
| 5.74 | 0.26 | 0.70 | 0.60 | 0.40 | 9.54 | 61.44 |
| 5.42 | 0.75 | 1.08 | 0.66 | 0.41 | 12.31 | 63.18 |
| 4.91 | 1.82 | 2.86 | 0.75 | 0.56 | 11.92 | 68.56 |
| 4.69 | 2.31 | 4.40 | 0.76 | 0.62 | 15.52 | 72.96 |
| 4.47 | 2.61 | 5.75 | 0.77 | 0.63 | 18.69 | 74.26 |
| Heavy Metals | Steps | Cu | Cr | Pb | Cd | Ni | Zn | Al | Fe | Mn |
|---|---|---|---|---|---|---|---|---|---|---|
| FA | 1st | 15.48 | 1.11 | 1.21 | 0.69 | 0.39 | 48.00 | 17.61 | 5.34 | 5.21 |
| 2nd | 5.48 | 1.17 | 1.61 | 0.096 | 0.28 | 22.92 | 292.90 | 57.19 | 3.55 | |
| 3rd | 1.82 | 0.34 | 0.52 | 0.019 | 0.096 | 4.03 | 127.30 | 10.63 | 1.18 | |
| Sum | 22.79 | 2.62 | 3.34 | 0.80 | 0.77 | 74.95 | 437.81 | 73.17 | 9.95 | |
| MFA a | 1st | 10.69 | 0.44 | 0.66 | 0.66 | 0.62 | 45.45 | 23.27 | 6.35 | 7.38 |
| 2nd | 8.14 | 1.81 | 1.32 | 0.11 | 0.23 | 28.62 | 343.90 | 71.59 | 3.53 | |
| 3rd | 2.83 | 0.58 | 0.41 | 0.023 | 0.047 | 4.91 | 159.80 | 33.23 | 1.18 | |
| Sum | 21.67 | 2.83 | 2.40 | 0.80 | 0.90 | 74.07 | 526.97 | 111.17 | 12.09 | |
| WFA | 1st | 13.29 | 0.85 | 1.006 | 0.70 | 0.35 | 47.40 | 35.06 | 4.15 | 4.76 |
| 2nd | 7.78 | 1.35 | 2.49 | 0.15 | 0.30 | 29.87 | 318.90 | 57.55 | 4.57 | |
| 3rd | 2.25 | 0.39 | 0.80 | 0.030 | 0.11 | 7.48 | 150.10 | 14.33 | 1.36 | |
| Sum | 23.32 | 2.60 | 4.30 | 0.89 | 0.77 | 84.75 | 504.06 | 76.03 | 10.70 | |
| WMFA a | 1st | 3.55 | 0.016 | 0.14 | 0.59 | 0.80 | 39.11 | 2.19 | 0.12 | 9.15 |
| 2nd | 17.58 | 2.59 | 1.71 | 0.39 | 0.33 | 48.27 | 387.40 | 57.47 | 5.65 | |
| 3rd | 4.95 | 0.97 | 0.48 | 0.056 | 0.077 | 9.26 | 204.60 | 25.68 | 1.90 | |
| Sum | 26.09 | 3.58 | 2.33 | 1.03 | 1.21 | 96.64 | 594.18 | 83.27 | 16.71 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Lu, S.; Mao, Q.; Buekens, A.; Chang, W.; Wang, X.; Yan, J. Suppressing Heavy Metal Leaching through Ball Milling of Fly Ash. Energies 2016, 9, 524. https://doi.org/10.3390/en9070524
Chen Z, Lu S, Mao Q, Buekens A, Chang W, Wang X, Yan J. Suppressing Heavy Metal Leaching through Ball Milling of Fly Ash. Energies. 2016; 9(7):524. https://doi.org/10.3390/en9070524
Chicago/Turabian StyleChen, Zhiliang, Shengyong Lu, Qiongjing Mao, Alfons Buekens, Wei Chang, Xu Wang, and Jianhua Yan. 2016. "Suppressing Heavy Metal Leaching through Ball Milling of Fly Ash" Energies 9, no. 7: 524. https://doi.org/10.3390/en9070524






