Unconventional Gas: Experimental Study of the Influence of Subcritical Carbon Dioxide on the Mechanical Properties of Black Shale
Abstract
:1. Introduction
2. Experimental Methodology
2.1. Sample Preparation
2.2. Experimental Procedure
3. Results and Discussion
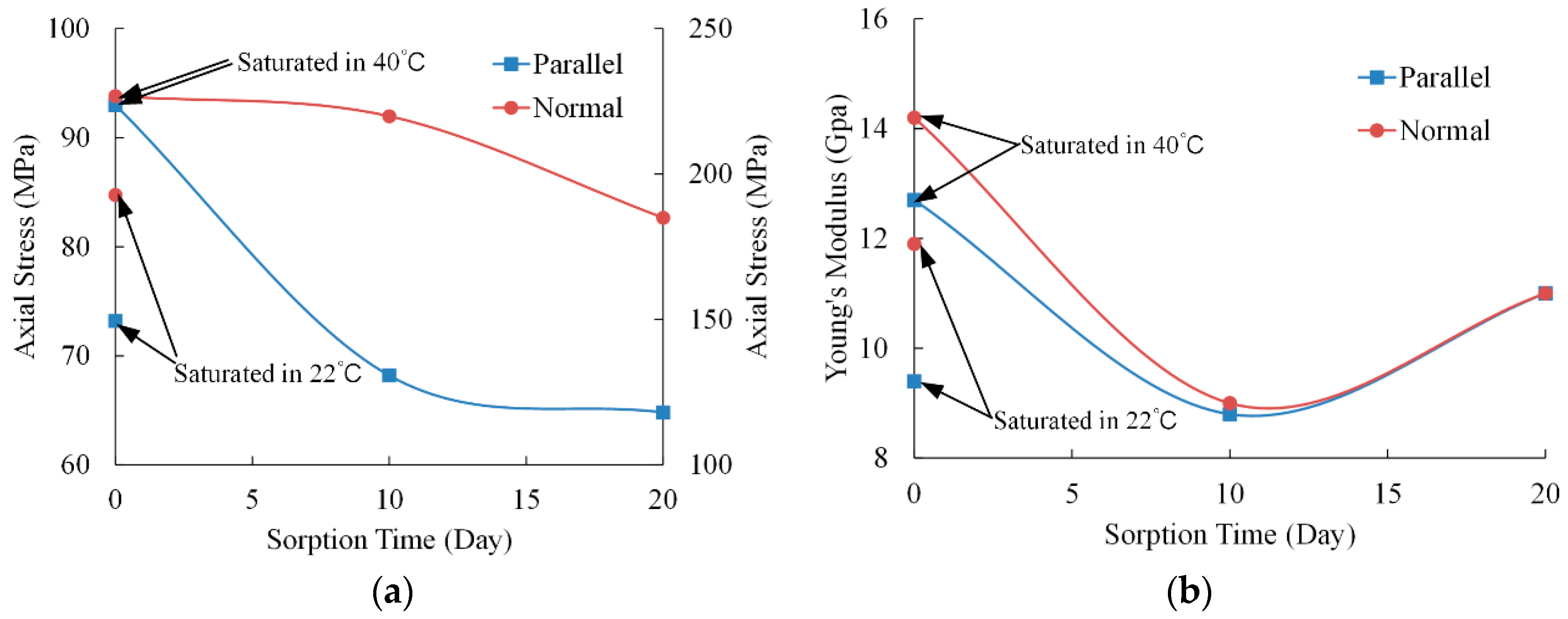
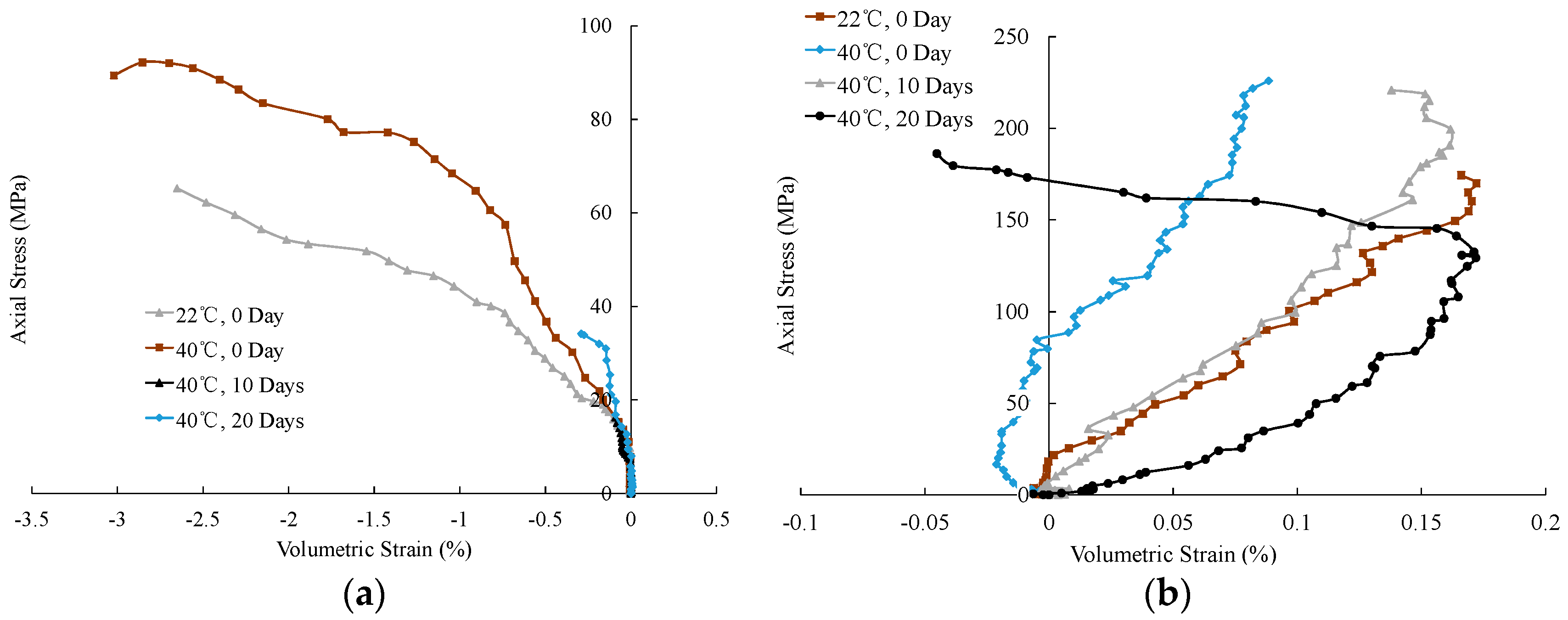
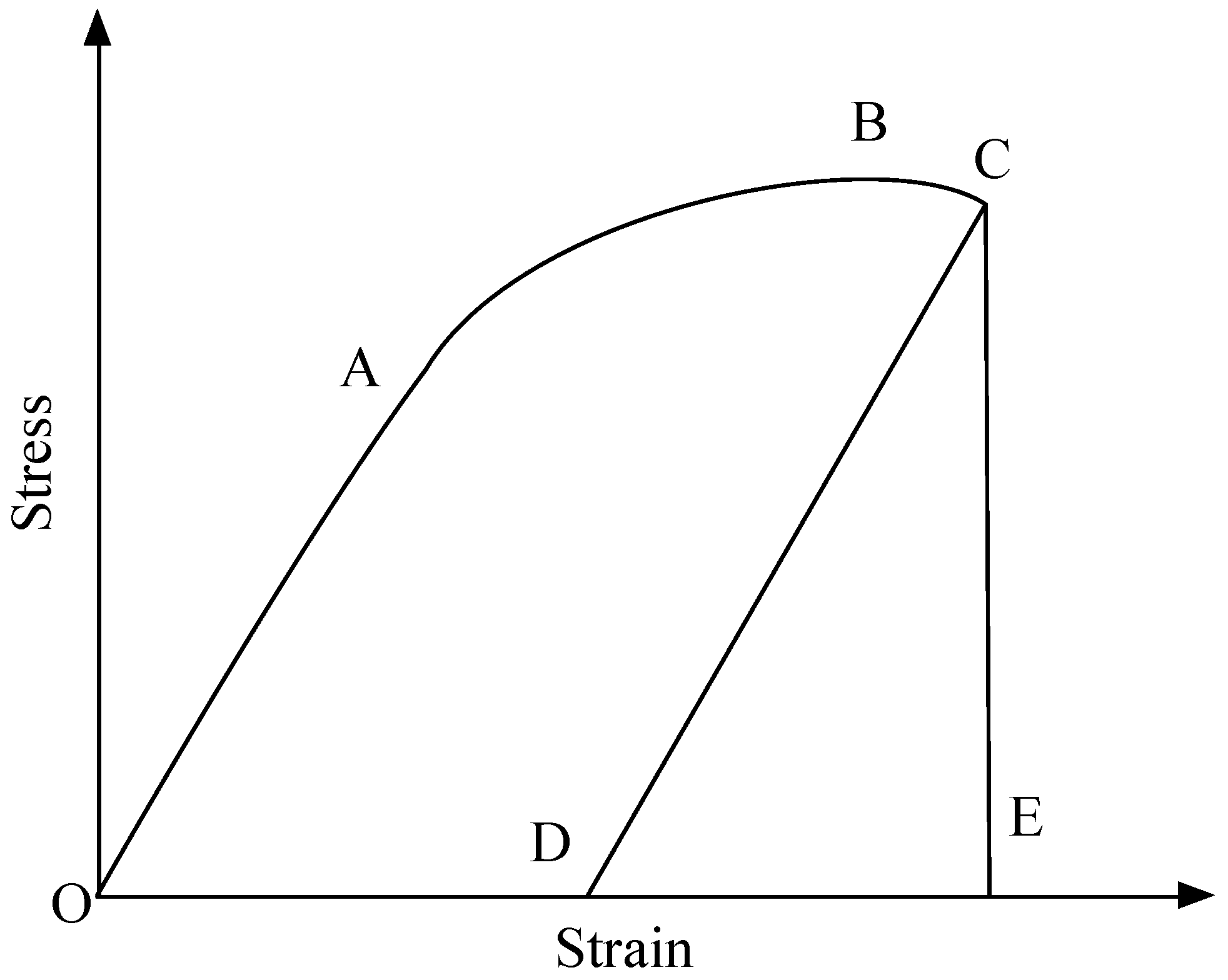
3.1. Mechanical Characteristics
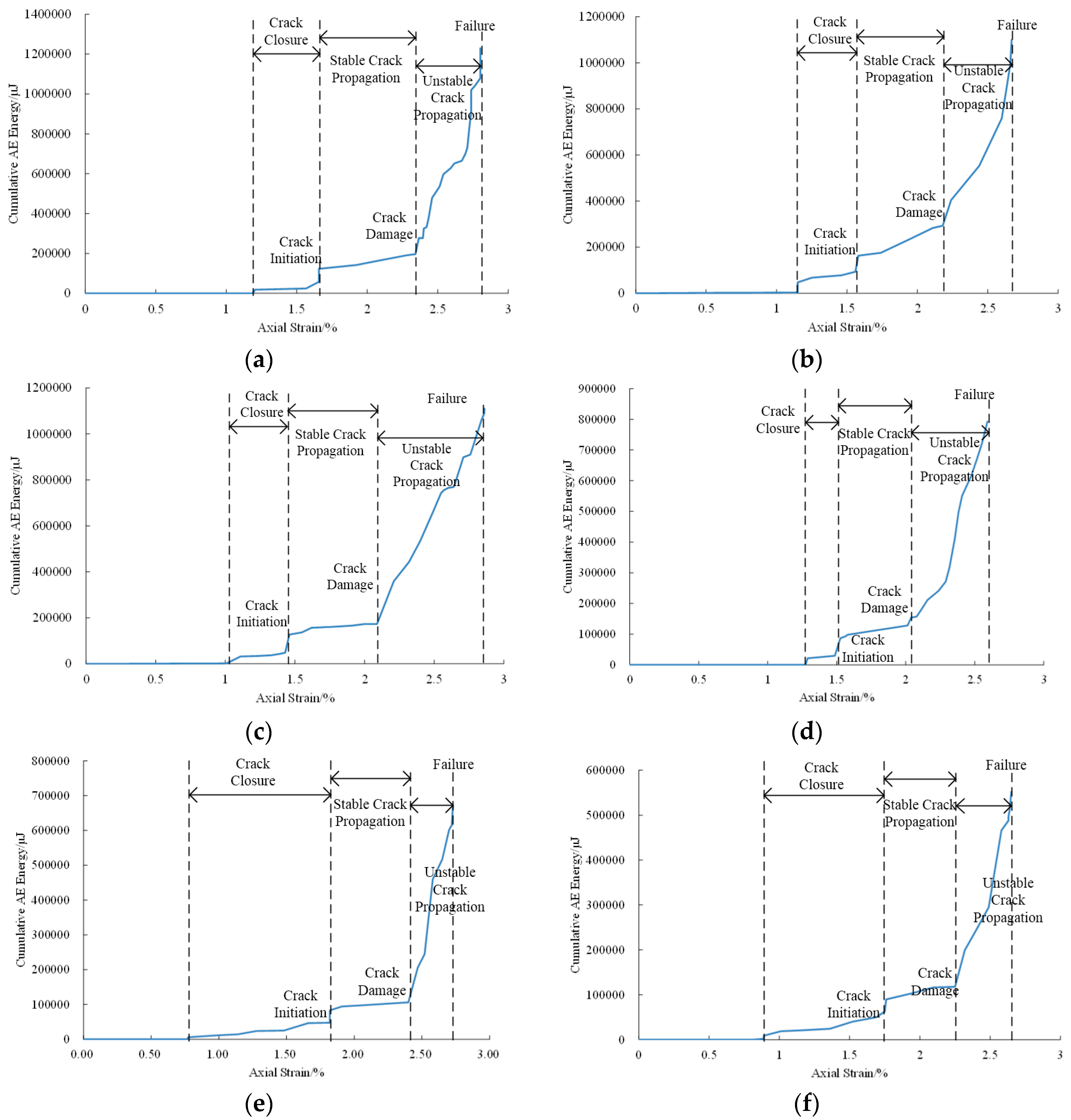
3.2. Acoustic Emission Response
3.3. Failure Pattern
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Energy Information Administration. Lower Electricity-Related CO2 Emissions Reflect Lower Carbon Intensity and Electricity Use, 2014. Available online: https://www.eia.gov/todayinenergy/detail.cfm?id=18511 (accessed on 23 October 2014).
- Lv, Q.; Long, X.; Kang, Y.; Xiao, L.; Wu, W. Numerical investigation on the expansion of supercritical carbon dioxide jet. In Proceedings of the 6th International Conference on Pumps and Fans with Compressors and Wind Turbines, Beijing, China, 19–22 September 2013.
- Du, Y.; Wang, R.; Ni, H.; Huang, Z.; Li, M. Dynamical analysis of high-pressure supercritical carbon dioxide jet in well drilling. J. Hydrodyn. Ser. B 2013, 25, 528–534. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Shen, Z.; Song, X.; Tang, G.; Hou, X. Experimental Study on the Efficiency of Cuttings Carrying with Supercritical CO2. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, Tianjin, China, 9–11 July 2012.
- Du, Y.K.; Wang, R.H.; Ni, H.J.; Li, M.K.; Song, W.Q.; Song, H.F. Determination of Rock-Breaking Performance of High-Pressure Supercritical Carbon Dioxide Jet. J. Hydrodyn. 2012, 24, 554–560. [Google Scholar] [CrossRef]
- Kolle, J.J. Coiled-Tubing Drilling with Supercritical Carbon Dioxide. In Proceedings of the CIM International Conference on Horizontal Well Technology, Calgary, AB, Canada; 2000. [Google Scholar]
- Gupta, A.P.; Gupta, A.; Langlinais, J. Feasibility of supercritical carbon dioxide as a drilling fluid for deep underbalanced drilling operation. In Proceedings of the SPE Annual Technical Conference and Exhibition, Society of Petroleum Engineers, Dallas, TX, USA, 9–12 October 2005.
- Kolle, J.J.; Marvin, M.H. Jet-Assisted Drilling with Supercritical Carbon Dioxide; Tempress Technologies Inc.: Houston, TX, USA, 2000. [Google Scholar]
- Middleton, R.S.; Carey, J.W.; Currier, R.P.; Hyman, J.D.; Kang, Q.; Karra, S.; Jiménez-Martínez, J.; Porter, M.L.; Viswanathan, H.S. Shale gas and non-aqueous fracturing fluids: Opportunities and challenges for supercritical CO2. Appl. Energy 2015, 147, 500–509. [Google Scholar] [CrossRef]
- Yang, Y.; Aplin, A.C. Permeability and petrophysical properties of 30 natural mudstones. J. Geophy. Res. Solid Earth 2007, 112. [Google Scholar] [CrossRef]
- Sarout, J.; Detournay, E. Chemoporoelastic analysis and experimental validation of the pore pressure transmission test for reactive shales. Int. J. Rock Mech. Min. Sci. 2011, 48, 759–772. [Google Scholar] [CrossRef]
- Josh, M.; Esteban, L.; Delle Piane, C.; Sarout, J.; Dewhurst, D.; Clennell, M. Laboratory characterisation of shale properties. J. Pet. Sci. Eng. 2012, 88, 107–124. [Google Scholar] [CrossRef]
- Britt, L.K.; Schoeffler, J. The geomechanics of a shale play: What makes a shale prospective. In Proceedings of the SPE Eastern Regional Meeting, Charleston, WV, USA, 23–25 September 2009.
- Heller, R.; Zoback, M. Adsorption of methane and carbon dioxide on gas shale and pure mineral samples. J. Unconv. Oil Gas Resour. 2014, 8, 14–24. [Google Scholar] [CrossRef]
- Chareonsuppanimit, P.; Mohammad, S.A.; Robinson, R.L., Jr.; Gasem, K.A. High-pressure adsorption of gases on shales: Measurements and modeling. Int. J. Coal Geol. 2012, 95, 34–46. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, R.M.; Chikatamarla, L. Adsorption-induced coal swelling and stress: Implications for methane production and acid gas sequestration into coal seams. J. Geophys. Res. Solid Earth 2007, 112. [Google Scholar] [CrossRef]
- Bustin, R.M.; Cui, X.; Chikatamarla, L. Impacts of volumetric strain on CO2 sequestration in coals and enhanced CH4 recovery. AAPG Bull. 2008, 92, 15–29. [Google Scholar] [CrossRef]
- Connell, L.; Detournay, C. Coupled flow and geomechanical processes during enhanced coal seam methane recovery through CO2 sequestration. Int. J. Coal Geol. 2009, 77, 222–233. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Viete, D.R. Effects of gaseous and super-critical carbon dioxide saturation on the mechanical properties of bituminous coal from the Southern Sydney Basin. Appl. Energy 2013, 110, 73–81. [Google Scholar] [CrossRef]
- Perera, M.S.A.; Ranjith, P.G.; Choi, S.K.; Airey, D. The effects of sub-critical and super-critical carbon dioxide adsorption-induced coal matrix swelling on the permeability of naturally fractured black coal. Energy 2011, 36, 6442–6450. [Google Scholar] [CrossRef]
- Verma, A.K.; Sirvaiya, A. Comparative analysis of intelligent models for prediction of Langmuir constants for CO2 adsorption of Gondwana coals in India. Geomech. Geophys. Geo-Energy Geo-Resour. 2016, 2, 97–109. [Google Scholar] [CrossRef]
- White, C.M.; Smith, D.H.; Jones, K.L.; Goodman, A.L.; Jikich, S.A.; LaCount, R.B.; DuBose, S.B.; Ozdemir, E.; Morsi, B.I.; Schroeder, K.T. Sequestration of carbon dioxide in coal with enhanced coalbed methane recovery a review. Energy Fuels 2005, 19, 659–724. [Google Scholar] [CrossRef]
- Liu, F.; Ellett, K.; Xiao, Y.; Rupp, J.A. Assessing the feasibility of CO2 storage in the New Albany Shale (Devonian–Mississippian) with potential enhanced gas recovery using reservoir simulation. Int. J. Greenhouse Gas Control 2013, 17, 111–126. [Google Scholar] [CrossRef]
- Kumar, A.; Noh, M.; Pope, G.; Sepehrnoori, K.; Bryant, S.; Lake, L. Reservoir simulation of CO2 storage in deep saline aquifers. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 17–21 April 2004.
- Li, X.; Feng, Z.; Han, G.; Elsworth, D.; Marone, C.; Saffer, D.; Cheon, D.-S. Breakdown pressure and fracture surface morphology of hydraulic fracturing in shale with H2O, CO2 and N2. Geomech. Geophys. Geo-Energy Geo-Resour. 2016, 2, 63–76. [Google Scholar] [CrossRef]
- Busch, A.; Bertier, P.; Gensterblum, Y.; Rother, G.; Spiers, C.J.; Zhang, M.; Wentinck, H.M. On sorption and swelling of CO2 in clays. Geomech. Geophys. Geo-Energy Geo-Resour. 2016, 2, 111–130. [Google Scholar] [CrossRef]
- Choi, C.S.; Song, J.J. Swelling and Mechanical Property Change of Shale and Sandstone in Supercritical CO2. In Proceedings of the 7th Asian Rock Mechanics Symposium, Seoul, Korea, 15–19 October 2012; International Society for Rock Mechanics: Seoul, Korea, 2012. [Google Scholar]
- Gibbs, J.W. On the equilibrium of heterogeneous substances. Am. J. Sci. 1878, III, 441–458. [Google Scholar] [CrossRef]
- Griffith, A.A. The phenomena of rupture and flow in solids. Philos. Trans. R Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1921, 221, 163–198. [Google Scholar] [CrossRef]
- Rebinder, P. Influence of changes in the surface energy on cleavage, hardness, and other properties of crystals. In Proceedings of the Sixth Conference of Russian Physicists, Moscow, Russia; 1928. [Google Scholar]
- Likhtman, V.I.; Shchukin, E.D.; Rebinder, P.A. Physicochemical Mechanics of Metals; Israel Program for Scientific Translations: Jerusalem, Israel, 1964; p. 244. [Google Scholar]
- Aziz, N.I.; Ming-Li, W. The effect of sorbed gas on the strength of coal-an experimental study. Geotech. Geol. Eng. 1999, 17, 387–402. [Google Scholar] [CrossRef]
- Brochard, L.; Vandamme, M.; Pellenq, R.-M. Poromechanics of microporous media. J. Mech. Phys. Solids 2012, 60, 606–622. [Google Scholar] [CrossRef]
- Vandamme, M.; Brochard, L.; Lecampion, B.; Coussy, O. Adsorption and strain: the CO2-induced swelling of coal. J. Mech. Phys. Solids 2010, 58, 1489–1505. [Google Scholar] [CrossRef] [Green Version]
- Viete, D.; Ranjith, P. The effect of CO2 on the geomechanical and permeability behaviour of brown coal: Implications for coal seam CO2 sequestration. Int. J. Coal Geol. 2006, 66, 204–216. [Google Scholar] [CrossRef]
- Chenevert, M.E. Shale alteration by water adsorption. J. Pet. Technol. 1970, 22, 1141–1148. [Google Scholar] [CrossRef]
- Hale, A.H.; Mody, F.K.; Salisbury, D.P. Experimental investigation of the influence of chemical potential on wellbore stability. In Proceedings of the IADC/SPE Drilling, New Orleans, LA, USA, 18–21 February 1992.
- Wong, R. Swelling and softening behaviour of La Biche shale. Can. Geotech. J. 1998, 35, 206–221. [Google Scholar] [CrossRef]
- Ghorbani, A.; Zamora, M.; Cosenza, P. Effects of desiccation on the elastic wave velocities of clay-rocks. Int. J. Rock Mech. Min. Sci. 2009, 46, 1267–1272. [Google Scholar] [CrossRef]
- Lyu, Q.; Ranjith, P.; Long, X.; Kang, Y.; Huang, M. Effects of coring directions on the mechanical properties of Chinese shale. Arabian J. Geosci. 2015, 8, 10289–10299. [Google Scholar] [CrossRef]
- Meier, T.; Rybacki, E.; Backers, T.; Dresen, G. Influence of bedding angle on borehole stability: A laboratory investigation of transverse isotropic oil shale. Rock Mech. Rock Eng. 2015, 48, 1535–1546. [Google Scholar] [CrossRef]
- Li, Y.; Fu, Y.; Tang, G.; She, C.; Guo, J.; Zhang, J. Effect of weak bedding planes on wellbore stability for shale gas wells. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, Tianjin, China, 9–11 July 2012.
- Ranjith, P.G.; Perera, M.S.A. A new triaxial apparatus to study the mechanical and fluid flow aspects of carbon dioxide sequestration in geological formations. Fuel 2011, 90, 2751–2759. [Google Scholar] [CrossRef]
- Emadi, H.; Soliman, M.; Samuel, R.; Ziaja, M.; Moghaddam, R.; Hutchison, S. Experimental study of the swelling properties of unconventional shale oil and the effects of invasion on compressive strength. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 30 September–2 October 2013.
- Lyu, Q.; Ranjith, P.; Long, X.; Kang, Y.; Huang, M. A review of shale swelling by water adsorption. J. Nat. Gas Sci. Eng. 2015, 27, 1421–1431. [Google Scholar] [CrossRef]
- de Jong, S.M.; Spiers, C.J.; Busch, A. Development of swelling strain in smectite clays through exposure to carbon dioxide. Int. J. Greenhouse Gas Control 2014, 24, 149–161. [Google Scholar] [CrossRef]
- Lajtai, E. Microscopic fracture processes in a granite. Rock Mech. Rock Eng. 1998, 31, 237–250. [Google Scholar] [CrossRef]
- Rickman, R.; Mullen, M.J.; Petre, J.E.; Grieser, W.V.; Kundert, D. A practical use of shale petrophysics for stimulation design optimization: All shale plays are not clones of the Barnett Shale. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 24–24 September 2008.
- Gong, Q.; Zhao, J. Influence of rock brittleness on TBM penetration rate in Singapore granite. Tunn. Undergr. Space Technol. 2007, 22, 317–324. [Google Scholar] [CrossRef]
- Hucka, V.; Das, B. Brittleness determination of rocks by different methods. Int. J. Rock Mech. Min. Sci. Geomech. Abstr. 1974, 11, 389–392. [Google Scholar] [CrossRef]
- Rathnaweera, T.; Ranjith, P.; Perera, M.; Haque, A.; Lashin, A.; Al Arifi, N.; Chandrasekharam, D.; Yang, S.; Xu, T.; Wang, S. CO2-induced mechanical behaviour of Hawkesbury sandstone in the Gosford basin: An experimental study. Mater. Sci. Eng. A 2015, 641, 123–137. [Google Scholar] [CrossRef]


| Direction | Temperature | Sorption Time | UCS | Average UCS | ∆UCS | E | Average E | ∆E |
|---|---|---|---|---|---|---|---|---|
| (°C) | (Day) | (MPa) | (MPa) | % | (GPa) | (GPa) | % | |
| parallel | 40 | 0 | 92.2 | 93.0 | - | 12.8 | 12.7 | - |
| 93.7 | (0.75) | 12.6 | (0.10) | |||||
| 22 | 0 | 71.7 | 73.2 | 21.3% | 9.0 | 9.4 | 26.0% | |
| 74.6 | (1.45) | 9.8 | (0.40) | |||||
| 40 | 10 | 67.5 | 68.2 | 26.7% | 9.0 | 8.8 | 30.7% | |
| 68.8 | (0.65) | 8.6 | (0.20) | |||||
| 40 | 20 | 65.3 | 64.8 | 30.3% | 10.7 | 11.0 | 13.8% | |
| 64.2 | (0.50) | 11.2 | (0.26) | |||||
| normal | 40 | 0 | 225.9 | 226.8 | - | 13.9 | 14.2 | - |
| 227.6 | (0.85) | 14.4 | (0.26) | |||||
| 22 | 0 | 191.3 | 192.8 | 15.0% | 12.2 | 11.9 | 15.9% | |
| 194.2 | (1.46) | 11.6 | (0.30) | |||||
| 40 | 10 | 220.8 | 219.9 | 3.0% | 8.6 | 9.0 | 36.7% | |
| 218.9 | (0.95) | 9.3 | (0.40) | |||||
| 40 | 20 | 186.3 | 185.0 | 18.4% | 11.2 | 11.0 | 22.6% | |
| 183.7 | (1.30) | 10.7 | (0.26) |
| Direction | Temperature | Sorption Time | Strain (DE) | Strain (OE) | Brittleness Index | Average Brittleness Index |
|---|---|---|---|---|---|---|
| (°C) | (Day) | (%) | (%) | (%) | (%) | |
| parallel | 40 | 0 | 1.28 | 2.21 | 57.92 | 63.87 |
| 22 | 0 | 1.82 | 2.83 | 64.31 | ||
| 40 | 10 | 1.82 | 2.89 | 62.98 | ||
| 40 | 20 | 1.89 | 2.69 | 70.26 | ||
| normal | 40 | 0 | 1.92 | 2.58 | 74.42 | 74.74 |
| 22 | 0 | 2.11 | 2.78 | 75.90 | ||
| 40 | 10 | 2.06 | 2.81 | 73.31 | ||
| 40 | 20 | 2.23 | 2.96 | 75.34 |
| Direction | Temperature (°C) | Sorption Time (Day) | Strain Variations in Crack Closure (%) | Strain Variations in Unstable Crack Propagation (%) | Peak Cumulative AE Energy (μJ) |
|---|---|---|---|---|---|
| parallel | 40 | 0 | 1.27–1.52 | 2.01–2.59 | 793168 |
| 22 | 0 | 1.01–1.46 | 2.09–2.86 | 1104306 | |
| 40 | 10 | 1.15–1.58 | 2.18–2.67 | 1110382 | |
| 40 | 20 | 1.20–1.66 | 2.34–2.80 | 1230716 | |
| normal | 40 | 0 | 0.77–1.57 | 2.14–2.49 | 436088 |
| 22 | 0 | 0.92–1.75 | 2.33–2.64 | 511007 | |
| 40 | 10 | 0.89–1.75 | 2.25–2.65 | 552154 | |
| 40 | 20 | 0.78–1.82 | 2.40–2.73 | 672011 |
| Parallel (40 °C, 0 day) | Parallel (20 °C, 0 day) | ||
 |  |  |  |
| Parallel (40 °C, 10 days) | Parallel (40 °C, 20 days) | ||
 |  |  |  |
| Normal (40 °C, 0 day) | Normal (20 °C, 0 day) | ||
 |  |  |  |
| Normal (40 °C, 10 days) | Normal (40 °C, 20 days) | ||
 |  |  |  |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, Q.; Long, X.; Ranjith, P.G.; Kang, Y. Unconventional Gas: Experimental Study of the Influence of Subcritical Carbon Dioxide on the Mechanical Properties of Black Shale. Energies 2016, 9, 516. https://doi.org/10.3390/en9070516
Lyu Q, Long X, Ranjith PG, Kang Y. Unconventional Gas: Experimental Study of the Influence of Subcritical Carbon Dioxide on the Mechanical Properties of Black Shale. Energies. 2016; 9(7):516. https://doi.org/10.3390/en9070516
Chicago/Turabian StyleLyu, Qiao, Xinping Long, Pathegama Gamage Ranjith, and Yong Kang. 2016. "Unconventional Gas: Experimental Study of the Influence of Subcritical Carbon Dioxide on the Mechanical Properties of Black Shale" Energies 9, no. 7: 516. https://doi.org/10.3390/en9070516






