Distribution of Clay Minerals in Light Coal Fractions and the Thermal Reaction Products of These Clay Minerals during Combustion in a Drop Tube Furnace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Combustion Experiments
2.3. Sample Analysis
3. Results and Discussion
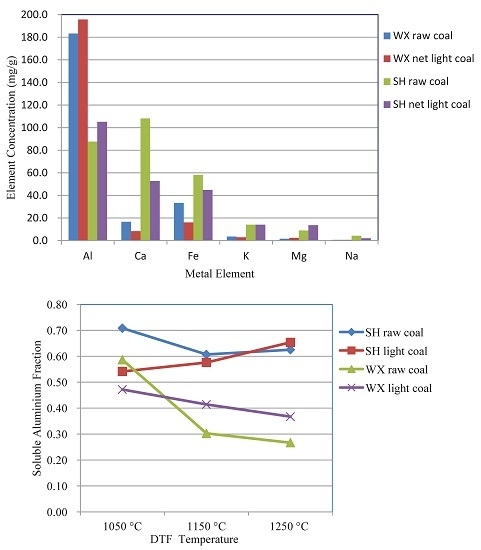
3.1. Mineral Matter Distribution in Light Coal
3.2. Phase Analyses of DTF Ashes and Their Boiling Acid Separation Residues
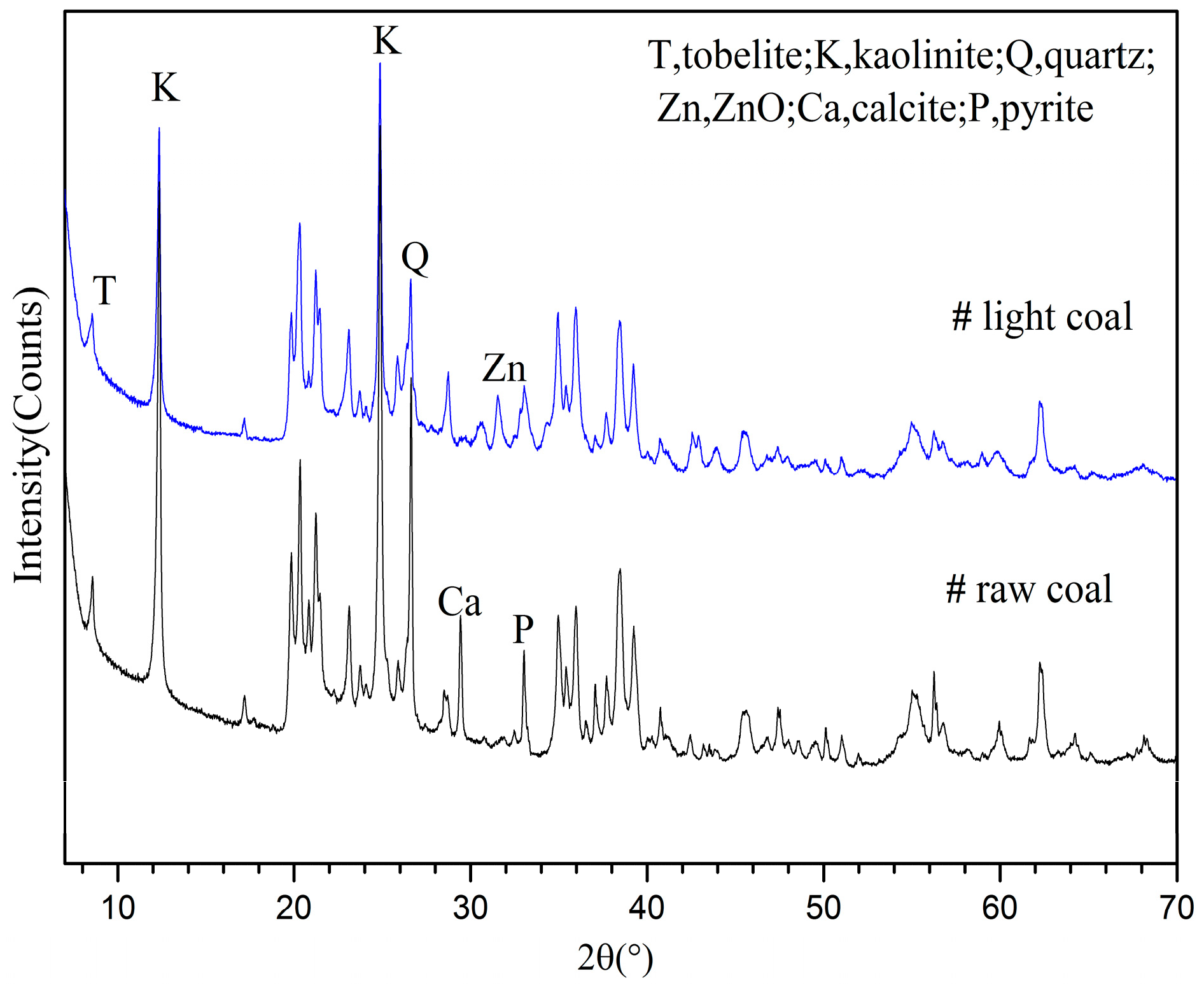
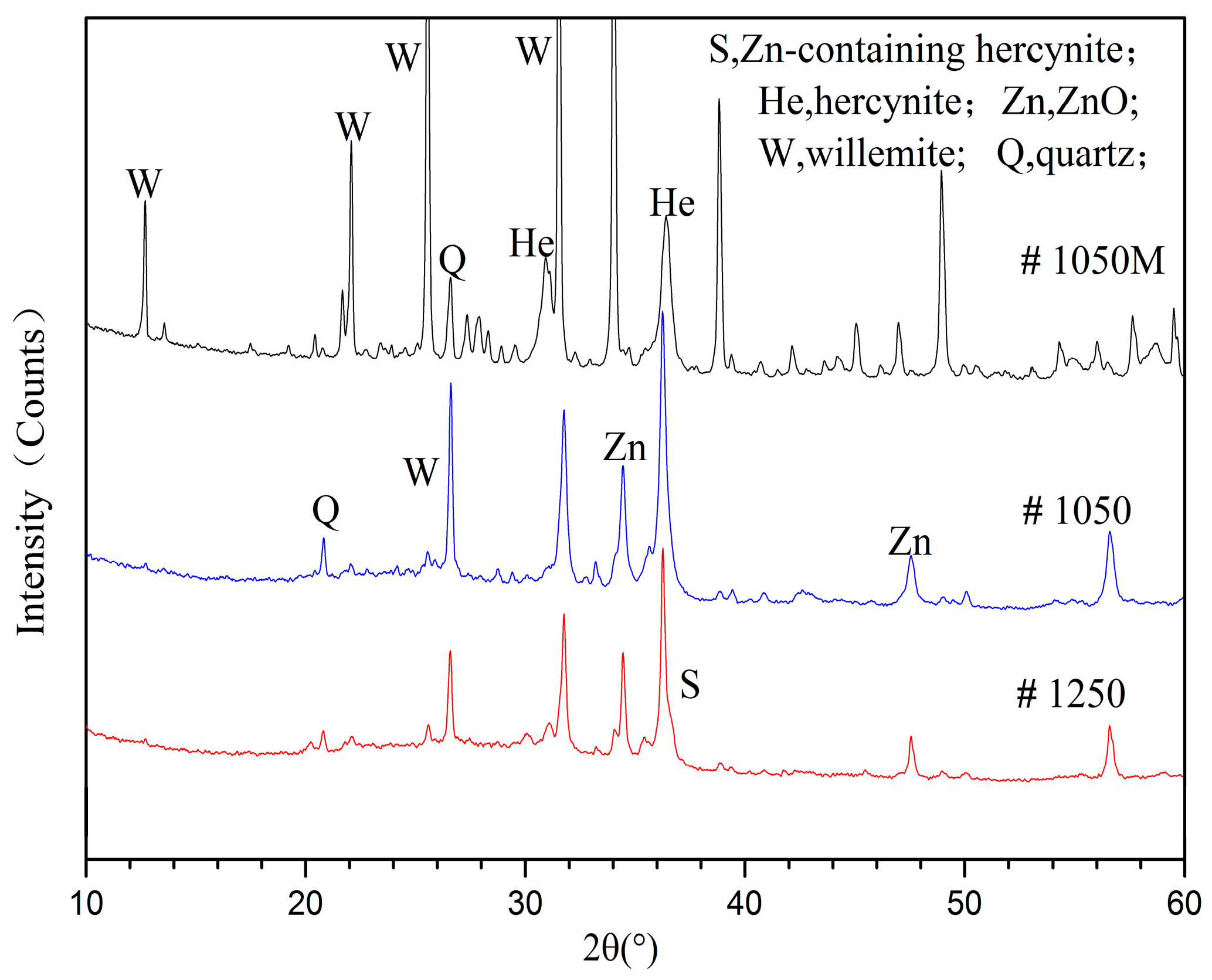
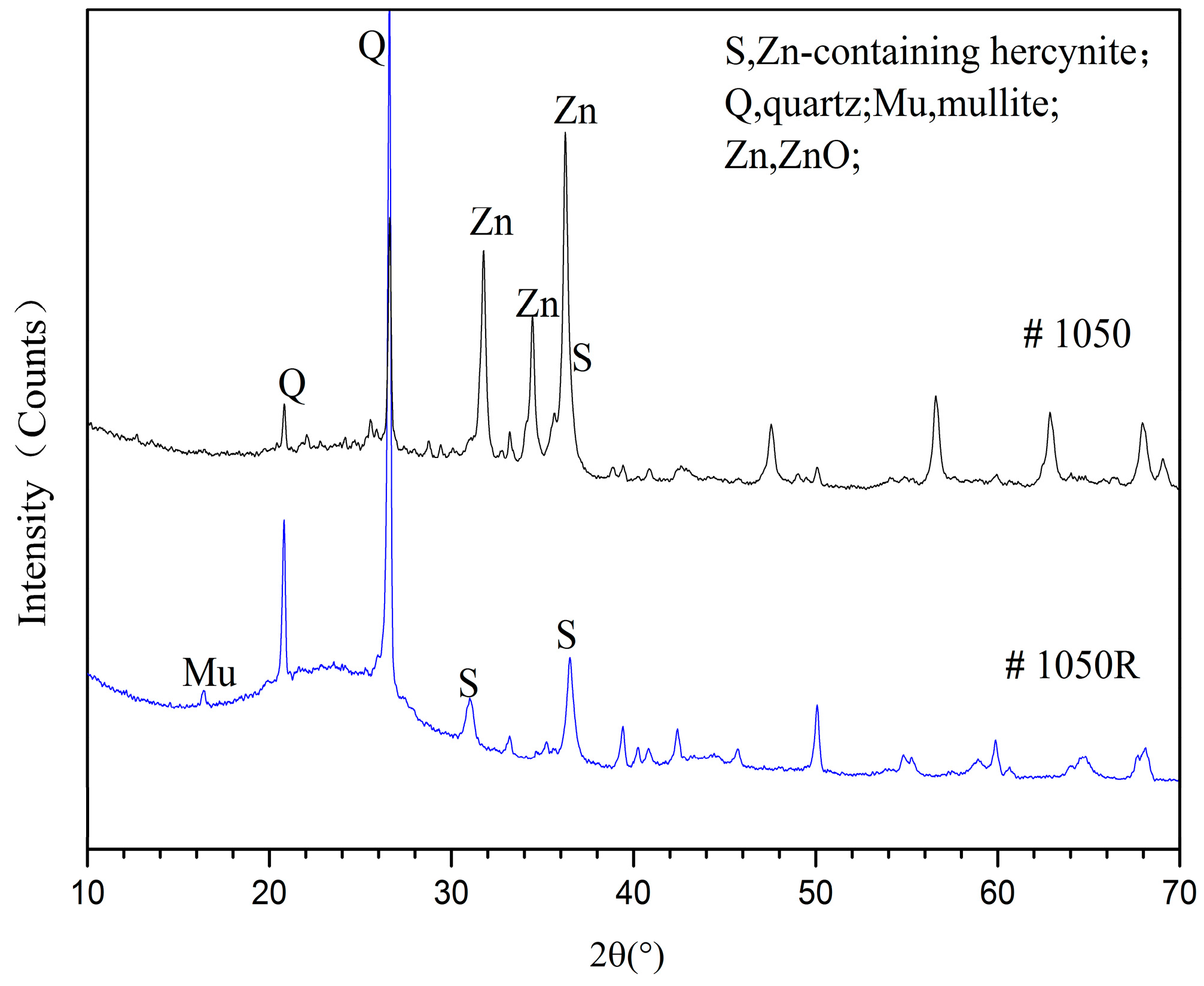
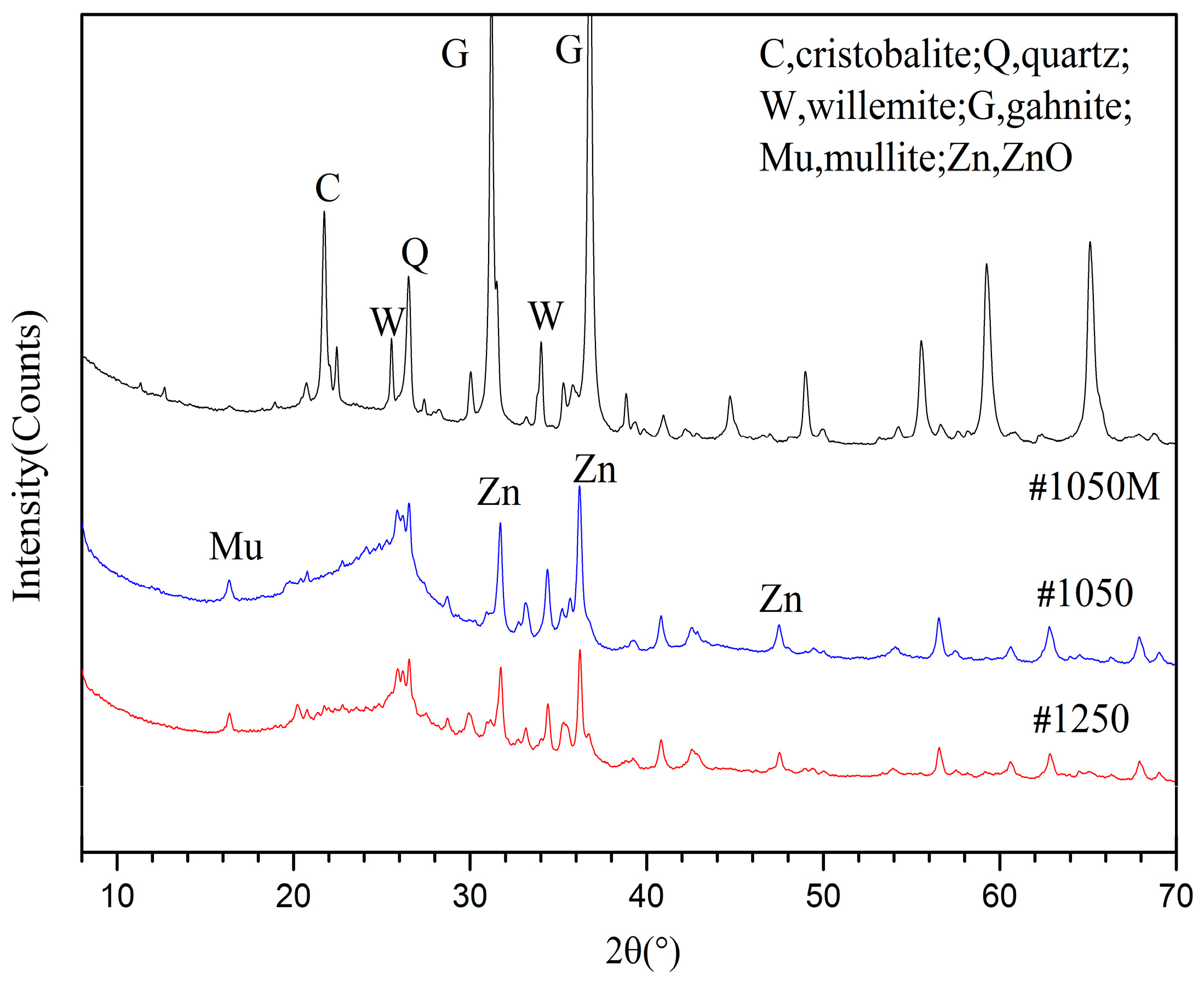
3.2.1. Phase Analyses of SH Light Coal DTF Ash
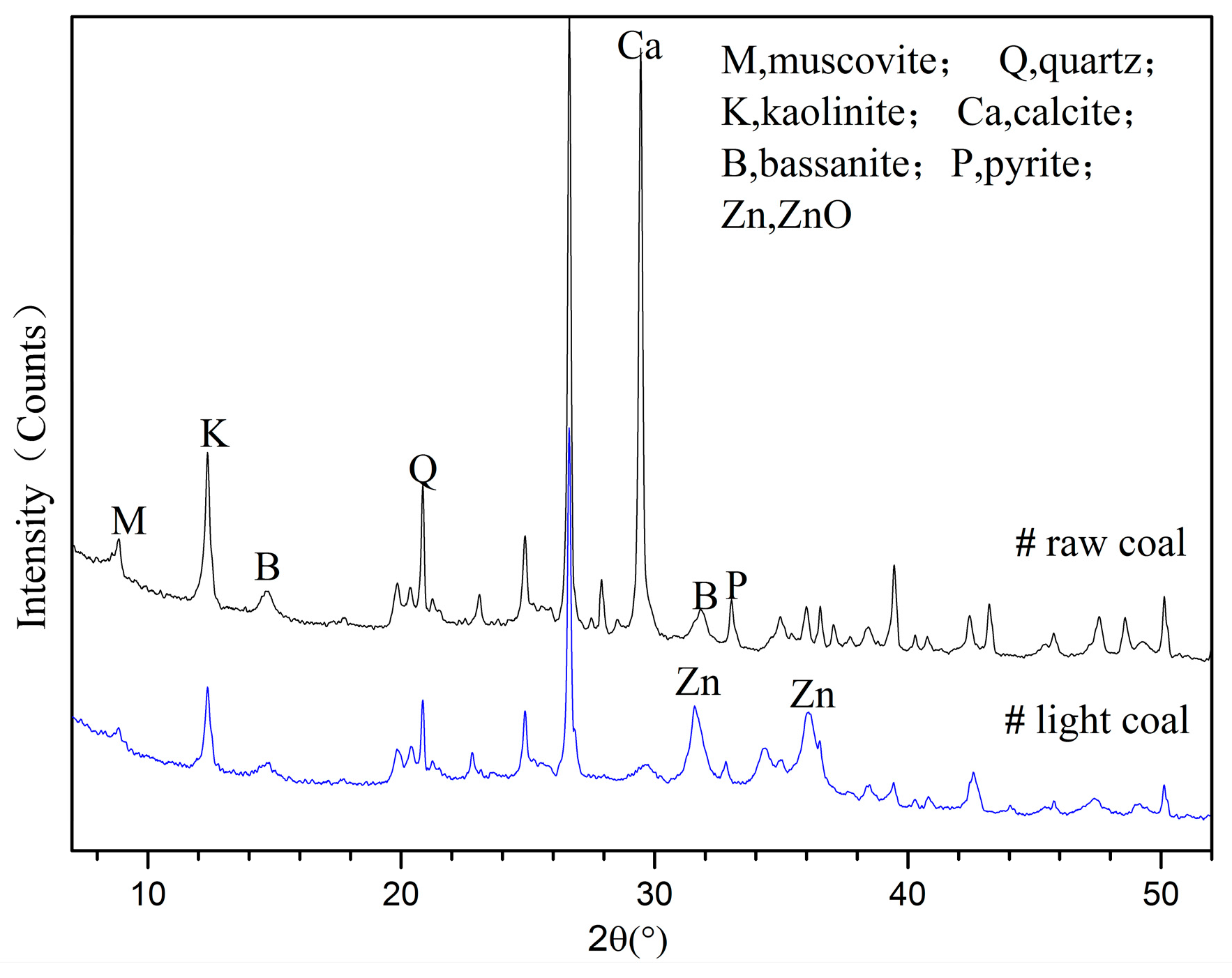
3.2.2. Phase Analyses of WX Light Coal DTF Ash
3.3. Clay Mineral Reaction Characteristics in Light Coal DTF Ash
3.3.1. Reactions of ZnO with Clay Minerals
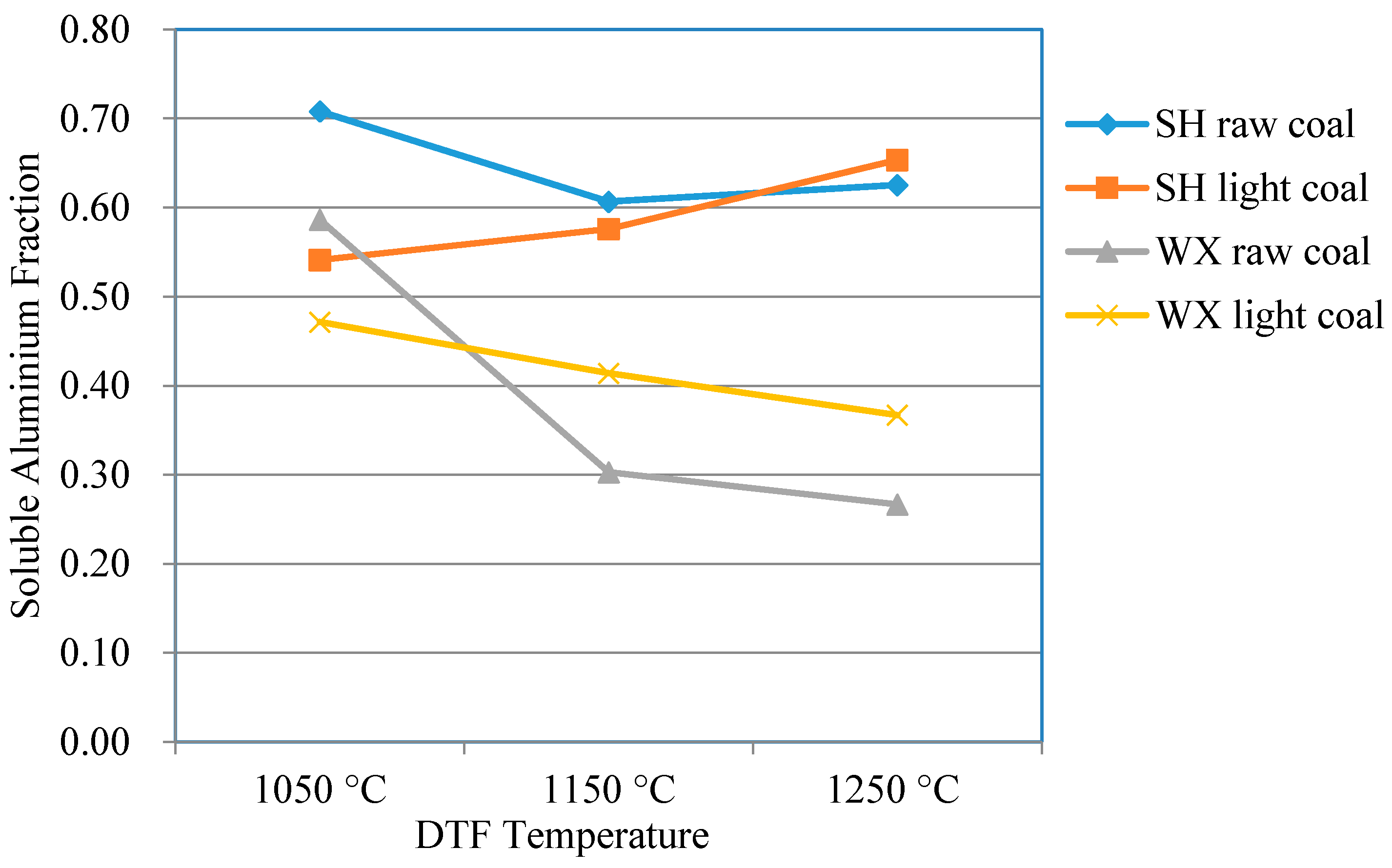
3.3.2. Comparison of Clay Mineral Reaction Products between Light Coals and Raw Coals
4. Conclusions
- (1)
- The content of clay minerals in the mineral matter of net light coals is higher than that in the relevant raw coals. Thermal reactions of clay minerals should be the focal point of investigations of production mechanisms for easily-slagging material in the ash of light coal fractions.
- (2)
- For WX coal with a high ash melting point, increasing the DTF temperature resulted in more dehydroxylation products of the clay minerals in the ash of light coal compared to in the ash of raw coal. The dehydroxylation products will contribute to the solid-state reactions of ash particles.
- (3)
- For SH coal with a low ash melting point, increasing the DTF temperature had a more significantly positive impact on the production of high-NBO/T matter in the ash of light coal compared to in the ash of raw coal. In utility boiler furnaces, the clay minerals in light coal will play an important role in the occurrence of slagging.
- (4)
- Extraneous zinc oxide, formed by introduction from a zinc chloride solution, was found to react slightly with the clay minerals in the light coal fractions during DTF combustion. The organic components derived from highly volatile SH coal may contribute more to the interaction of zinc oxide with clay minerals than those derived from less-volatile WX coal contribute.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bryers, R.W. Fireside slagging, fouling, and high temperature corrosion of heat-transfer surface due to impurities in steam-raising fuels. Prog. Energy Combust. Sci. 1996, 22, 29–120. [Google Scholar] [CrossRef]
- Creelman, R.A.; Ward, C.R.; Schumacher, G.; Juniper, L. Relation between coal mineral matter and deposit mineralogy in pulverized fuel furnaces. Energy Fuels 2013, 27, 5714–5724. [Google Scholar] [CrossRef]
- Shen, M.; Qiu, K.; Zhang, L.; Huang, Z.; Wang, Z.; Liu, J. Influence of coal blending on ash fusibility in reducing atmosphere. Energies 2015, 8, 4735–4754. [Google Scholar] [CrossRef]
- Benson, S.A.; Jones, M.L.; Harb, J.N. Ash Formation and Deposition. In Fundamentals of Coal Combustion for Clean and Efficient Use; Smoot, L.D., Ed.; Elsevier: New York, NY, USA, 1993; pp. 299–373. [Google Scholar]
- Zhang, Z.; Wu, X.; Zhou, T.; Chen, Y.; Hou, N.; Piao, G.; Kobayashid, N.; Itayad, Y.; Morid, S. The effect of iron-bearing mineral melting behaviour on ash deposition during coal combustion. Proc. Combust. Inst. 2011, 33, 2853–2861. [Google Scholar] [CrossRef]
- Reinmöller, M.; Klinger, M.; Schreiner, M.; Gutte, H. Relationship between ash fusion temperatures of ashes from hard coal, brown coal, and biomass and mineral phases under different atmospheres: A combined FactSageTM computational and network theoretical approach. Fuel 2015, 151, 118–123. [Google Scholar] [CrossRef]
- Gupta, R.P.; Wall, T.F.; Kajigaya, I.; Miyamae, S.; Tsumita, Y. Computer-controlled scanning electron microscopy of minerals in coal—Implications for ash deposition. Prog. Energy Combust. Sci. 1998, 24, 523–543. [Google Scholar] [CrossRef]
- Russell, N.V.; Mendez, L.B.; Wigley, F.; Williamson, J. Ash deposition of a Spanish anthracite: Effect of included and excluded mineral matter. Fuel 2002, 81, 657–663. [Google Scholar] [CrossRef]
- Hurley, J.P.; Schobert, H.H. Ash formation during pulverized subbituminous coal combustion. 2. Inorganic transformations during middle and late stages of burnout. Energy Fuels 1993, 7, 542–553. [Google Scholar] [CrossRef]
- Wu, H.; Wall, T.; Liu, G.; Bryant, G. Ash liberation from included minerals during combustion of pulverized coal: The relationship with char structure and burnout. Energy Fuels 1999, 13, 1197–1202. [Google Scholar] [CrossRef]
- Spears, D.A.; Martinez-Tarazon, M.R. Geochemical and mineralogical characteristics of a power station feed-coal, Eggborough, England. Int. J. Coal Geol. 1993, 22, 1–20. [Google Scholar] [CrossRef]
- Shimogori, M.; Mine, T.; Ohyatsu, N.; Takarayama, N.; Matsumura, Y. Effects of fine ash particles and alkali metals on ash deposition characteristics at the initial stage of ash deposition determined in 1.5 MW pilot plant tests. Fuel 2012, 97, 233–240. [Google Scholar] [CrossRef]
- Russell, N.V.; Wigley, F.; Williamson, J. The roles of lime and iron oxide on the formation of ash and deposits in PF combustion. Fuel 2002, 81, 673–681. [Google Scholar] [CrossRef]
- Wee, H.L.; Wu, H.; Zhang, D. Heterogeneity of ash deposits formed in a utility boiler during PF combustion. Energy Fuels 2007, 21, 441–450. [Google Scholar] [CrossRef]
- Barroso, J.; Ballester, J.; Pina, A. Study of coal ash deposition in an entrained flow reactor: Assessment of traditional and alternative slagging indices. Fuel Process. Technol. 2007, 88, 865–876. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Y.; Wei, C.; Yao, B.; Zheng, C. Mineralogy and microstructure of ash deposits from the Zhuzhou coal-fired power plant in China. Int. J. Coal Geol. 2010, 81, 309–319. [Google Scholar] [CrossRef]
- Lee, B.; Kim, S.I.; Kim, S.M.; Oh, D.H.; Gupta, S.; Jeon, C. Ash deposition characteristics of Moolarben coal and its blends during coal combustion. Korean J. Chem. Eng. 2016, 33, 147–153. [Google Scholar] [CrossRef]
- Tian, S.; Zhuo, Y.; Chen, C. Characterization of the products of the clay mineral thermal reactions during pulverization coal combustion in order to study the coal slagging propensity. Energy Fuels 2011, 25, 4896–4905. [Google Scholar] [CrossRef]
- Ward, C.R.; French, D. Determination of glass content and estimation of glass composition in fly ash using quantitative X-ray diffractometry. Fuel 2006, 85, 2268–2277. [Google Scholar] [CrossRef]
- Vargas, S.; Frandsem, F.J.; Dam-Johansen, K. Rheological properties of high-temperature melts of coal ashes and other silicates. Prog. Energy Combust. Sci. 2001, 27, 237–429. [Google Scholar] [CrossRef]
- Lu, P. Fundermentals of Inorganic Materials Science (Chemistry and Physics of Silicates); Wuhan University of Technology Press: Wuhan, China, 2006; pp. 77–106. (In Chinese) [Google Scholar]
- Mysen, B.O. Element partitioning between mineral and melt, melt composition, and melt structure. Chem. Geol. 2004, 213, 1–16. [Google Scholar] [CrossRef]
- Berry, E.E.; Hemmings, R.T.; Cornelus, B.J. Speciation in size and density fractionated fly ash III the influence of HCl leaching on the glassy constituents of a high-Ca fly ash. Mater. Res. Soc. Symp. Proc. 1988, 113, 55–63. [Google Scholar] [CrossRef]
- Toplis, M.J.; Dingwell, D.B. Shear viscosities of CaO-Al2O3-SiO2 liquids: Implications for the structural role of aluminium and the degree of polymerization of synthetic and natural aluminosilicate melts. Geochim. Cosmochim. Acta 2004, 68, 5169–5188. [Google Scholar] [CrossRef]
- Kuo, Y.; Huang, K.; Wang, C.; Wang, J. Effect of Al2O3 mole fraction and cooling method on vitrification of an artificial hazardous material. Part 1: Variation of crystalline phases and slag structures. J. Hazard. Mater. 2009, 169, 626–634. [Google Scholar] [CrossRef] [PubMed]
- Belver, C.; Munoz, M.A.B.; Vicente, M.A. Chemical activation of a Kaolinite under acid and alkaline conditions. Chem. Mater. 2002, 14, 2033–2043. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Sun, J.; Bai, X.; Zheng, C. Mineralogy, chemical composition, and microstructure of ferrospheres in fly ashes from coal combustion. Energy Fuels 2006, 20, 1490–1497. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Zyryanov, V.; Zhang, J.; Zheng, C. Physical-chemical characteristics and elements enrichment of magnetospheres from coal fly ashes. Fuel 2014, 135, 15–26. [Google Scholar] [CrossRef]
- Terry, B. Acid decomposition of silicate minerals. Part I: Reactivities and modes of dissolution of silicates. Hydrometallurgy 1983, 10, 135–150. [Google Scholar] [CrossRef]
- Behrens, H.; Haack, M. Cation diffusion in soda-lime-silicate glass melts. J. Non-Cryst. Solids 2007, 353, 4743–4752. [Google Scholar] [CrossRef]
- Jones, F.; Bankiewicz, D.; Hupa, M. Ocurrence and sources of zinc in fuels. Fuel 2014, 117, 763–775. [Google Scholar] [CrossRef]
- Tian, S.; Zhuo, Y.; Shu, X.; Chen, C.; Li, D. Distribution of mineral matter in Shenhua coal by combined ZnCl2 and HCl fractionation. J. Tsinghua Univ. Sci. Technol. 2008, 48, 240–243. (In Chinese) [Google Scholar]
- Determination of Fusibility of Coal Ash; GB/T 219-2008; China National Standard: Beijing, China, 2008.
- Proximate Analysis of Coal; GB/T 212-1991; China National Standard: Beijing, China, 1991.
- Tian, S.; Zhuo, Y.; Shu, X.; Chen, C. The chemical transformation of calcium in Shenhua coal during combustion in a muffle furnace. In Proceedings of the 7th International Symposium on Coal Combustion, Harbin, China, 17–19 July 2011.
- Waerenborgh, J.C.; Figueiredo, M.O.; Cabral, J.M.; Pereira, L. Powder XRD structure refinements and 57Fe Mossbauer effects study of synthetic Zn1−xFexAl2O4 (0 < x ≤ 1) spinels annealed at different temperatures. Phys. Chem. Miner. 1994, 21, 460–468. [Google Scholar]
- Fukushima, J.; Hayashi, Y.; Takizawa, H. Structure and magnetic properties of FeAl2O4 synthesized by microwave magnetic field irradiation. J. Asian Ceram. Soc. 2013, 1, 41–45. [Google Scholar] [CrossRef]
- Mollah, M.; Promreuk, S.; Schennach, R.; Cocke, D.; Guler, R. Cristobalite formation from thermal treatment of Texas lighite fly ash. Fuel 1999, 78, 1277–1282. [Google Scholar] [CrossRef]



| Sample | Vad | Aad | Cad | Mad | St,ad | Qgr,v (J/g) | DT | ST | HT | FT |
|---|---|---|---|---|---|---|---|---|---|---|
| WX coal | 13.9 | 27.9 | 57 | 1.2 | 1.55 | 25,092 | >1450 | - | - | - |
| SH coal | 40.4 | 9.7 | 39.7 | 10.2 | 0.43 | 24,302 | 1130 | 1150 | 1170 | 1180 |
| Sample | SiO2 | Al2O3 | CaO | Fe2O3 | K2O | MgO | Na2O | SO3 | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| WX coal | 51.32 | 36.78 | 2.48 | 4.2 | 0.5 | 0.39 | 0.14 | 1.94 | 1.32 | 0.53 |
| SH coal | 48.17 | 18.04 | 14.37 | 6.99 | 1.95 | 2.4 | 0.7 | 5.85 | 0.22 | 0.84 |
| Sample | LOI | Sample | LOI |
|---|---|---|---|
| SHL 1050 | 0.15 | WXL 1050 | 0.57 |
| SHL 1150 | 0.09 | WXL 1150 | 0.41 |
| SHL 1250 | 0.06 | WXL 1250 | 0.28 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, S.; Zhuo, Y.; Zhan, Z.; Shu, X.; Kang, Z. Distribution of Clay Minerals in Light Coal Fractions and the Thermal Reaction Products of These Clay Minerals during Combustion in a Drop Tube Furnace. Energies 2016, 9, 428. https://doi.org/10.3390/en9060428
Tian S, Zhuo Y, Zhan Z, Shu X, Kang Z. Distribution of Clay Minerals in Light Coal Fractions and the Thermal Reaction Products of These Clay Minerals during Combustion in a Drop Tube Furnace. Energies. 2016; 9(6):428. https://doi.org/10.3390/en9060428
Chicago/Turabian StyleTian, Sida, Yuqun Zhuo, Zhonghua Zhan, Xinqian Shu, and Zhizhong Kang. 2016. "Distribution of Clay Minerals in Light Coal Fractions and the Thermal Reaction Products of These Clay Minerals during Combustion in a Drop Tube Furnace" Energies 9, no. 6: 428. https://doi.org/10.3390/en9060428