A Multi-Component Additive to Improve the Thermal Stability of Li(Ni1/3Co1/3Mn1/3)O2-Based Lithium Ion Batteries
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
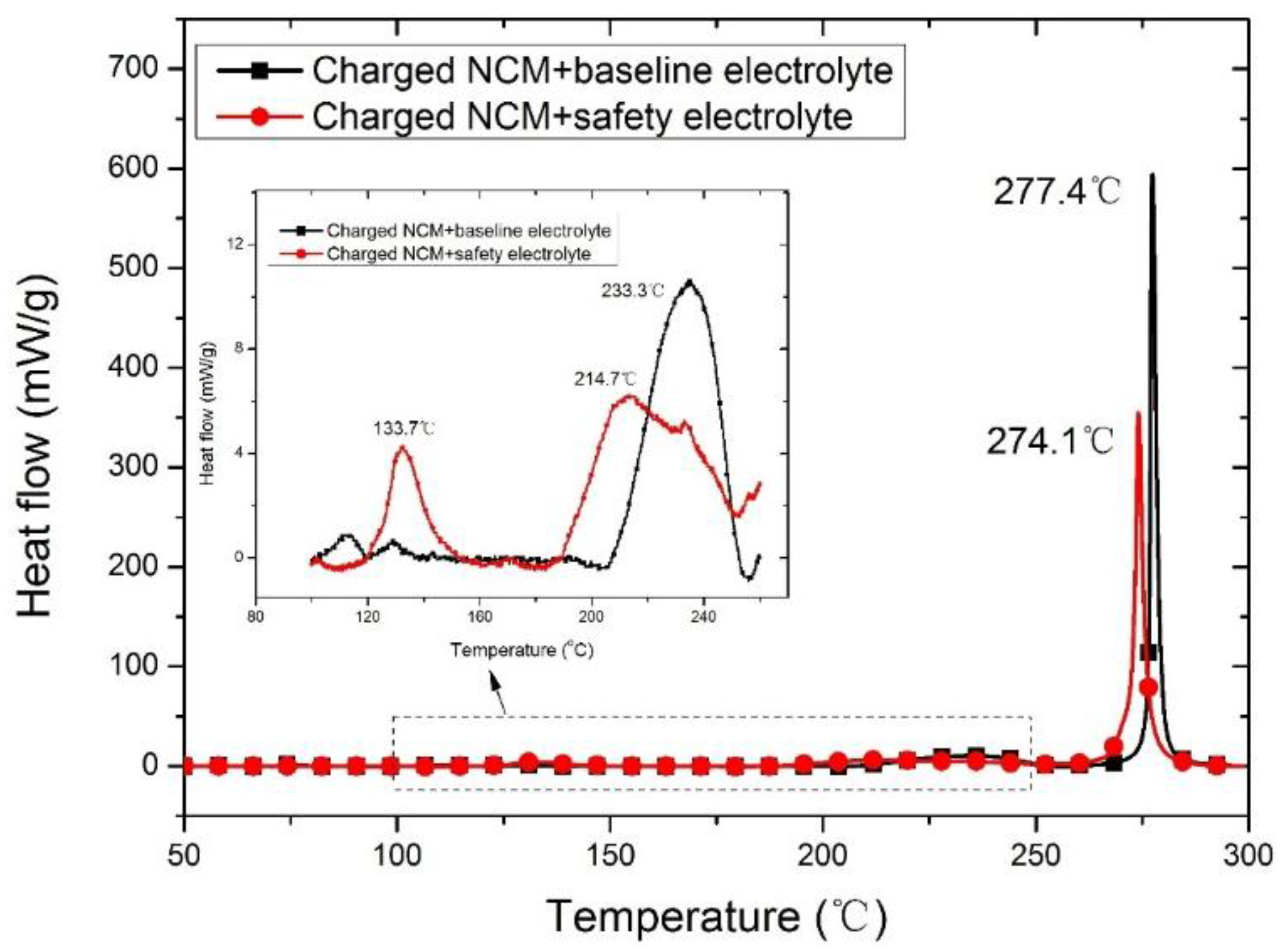
3.1. Thermal Stability
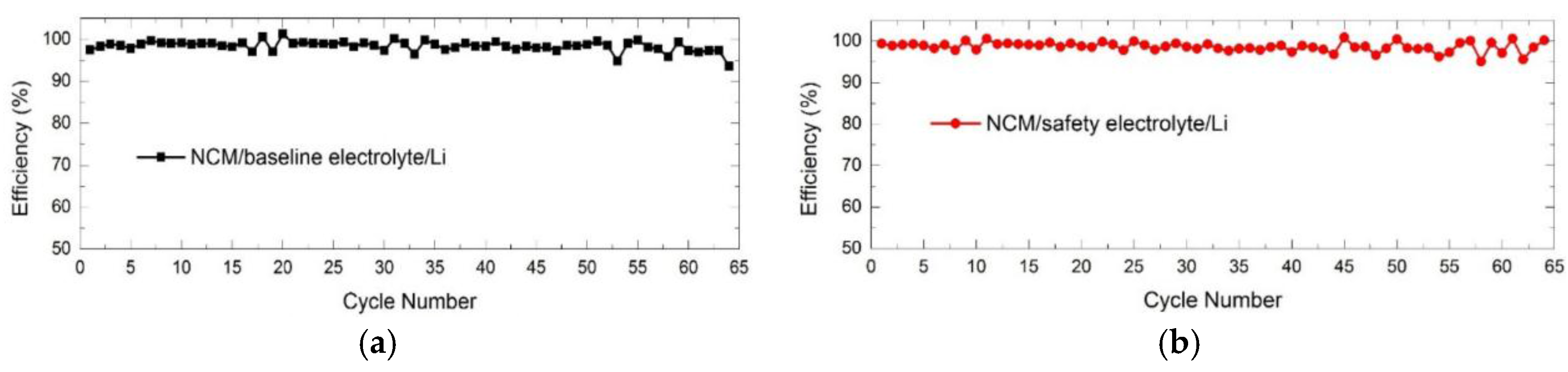
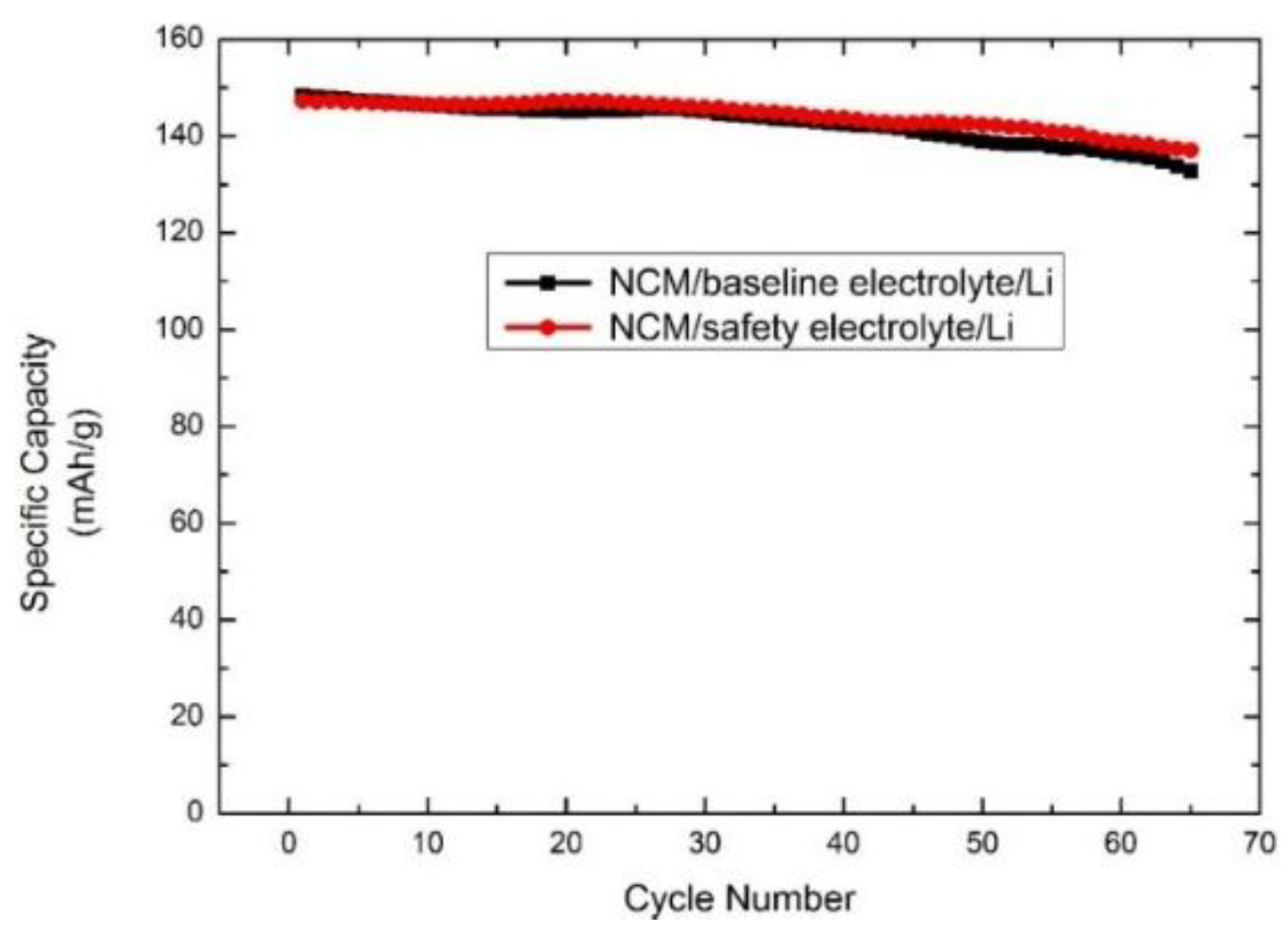
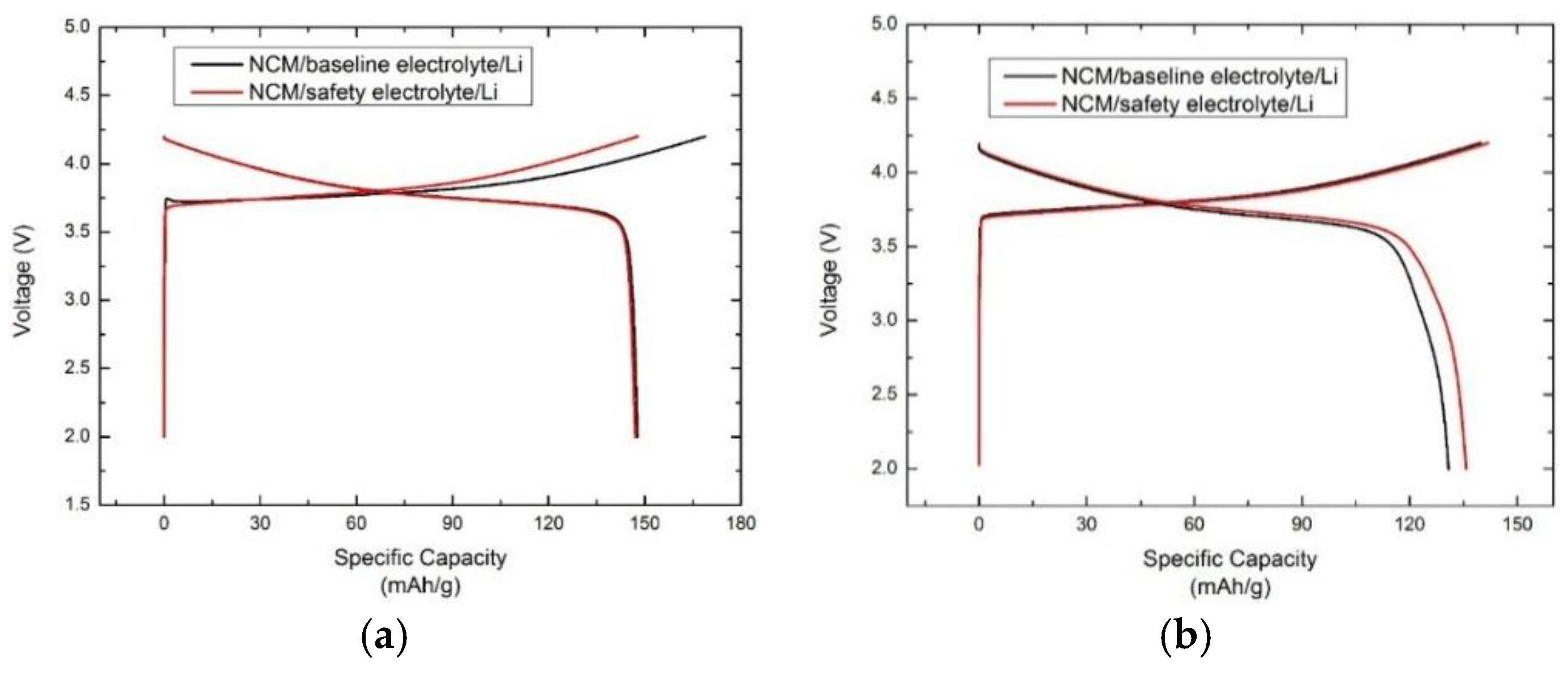
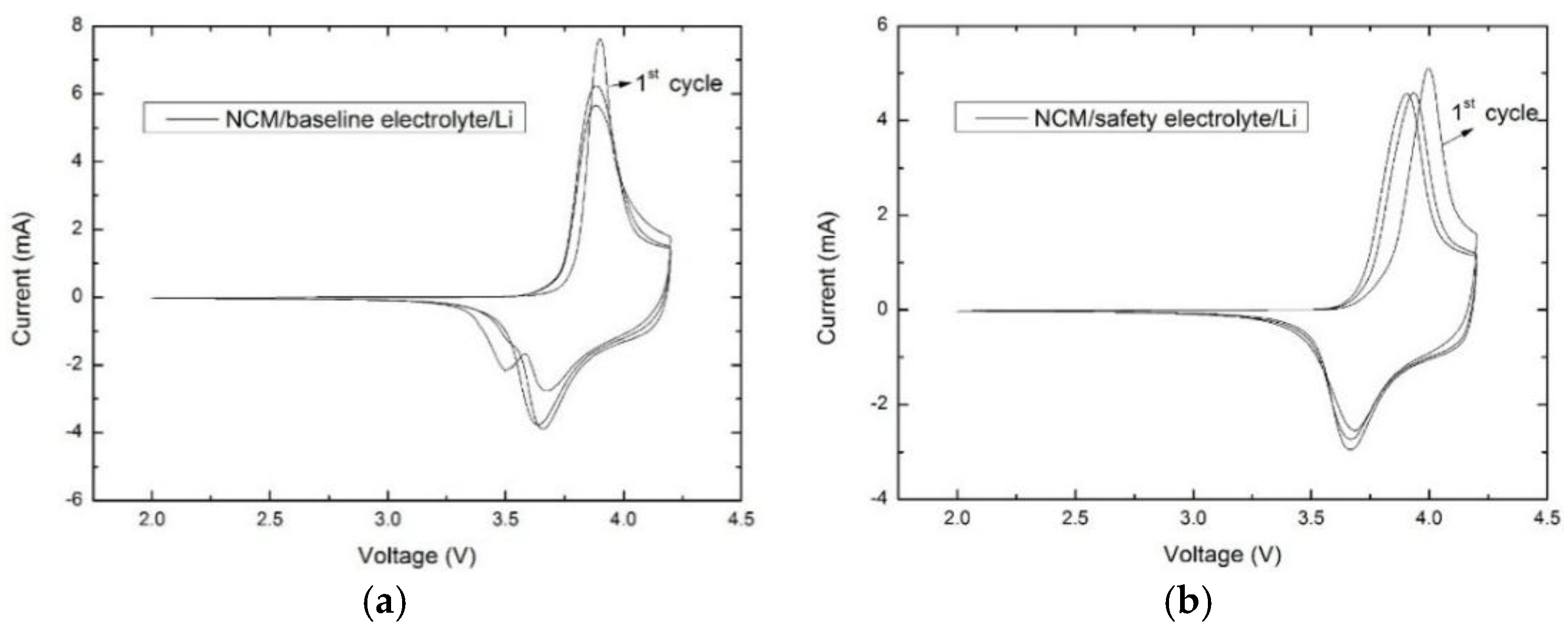
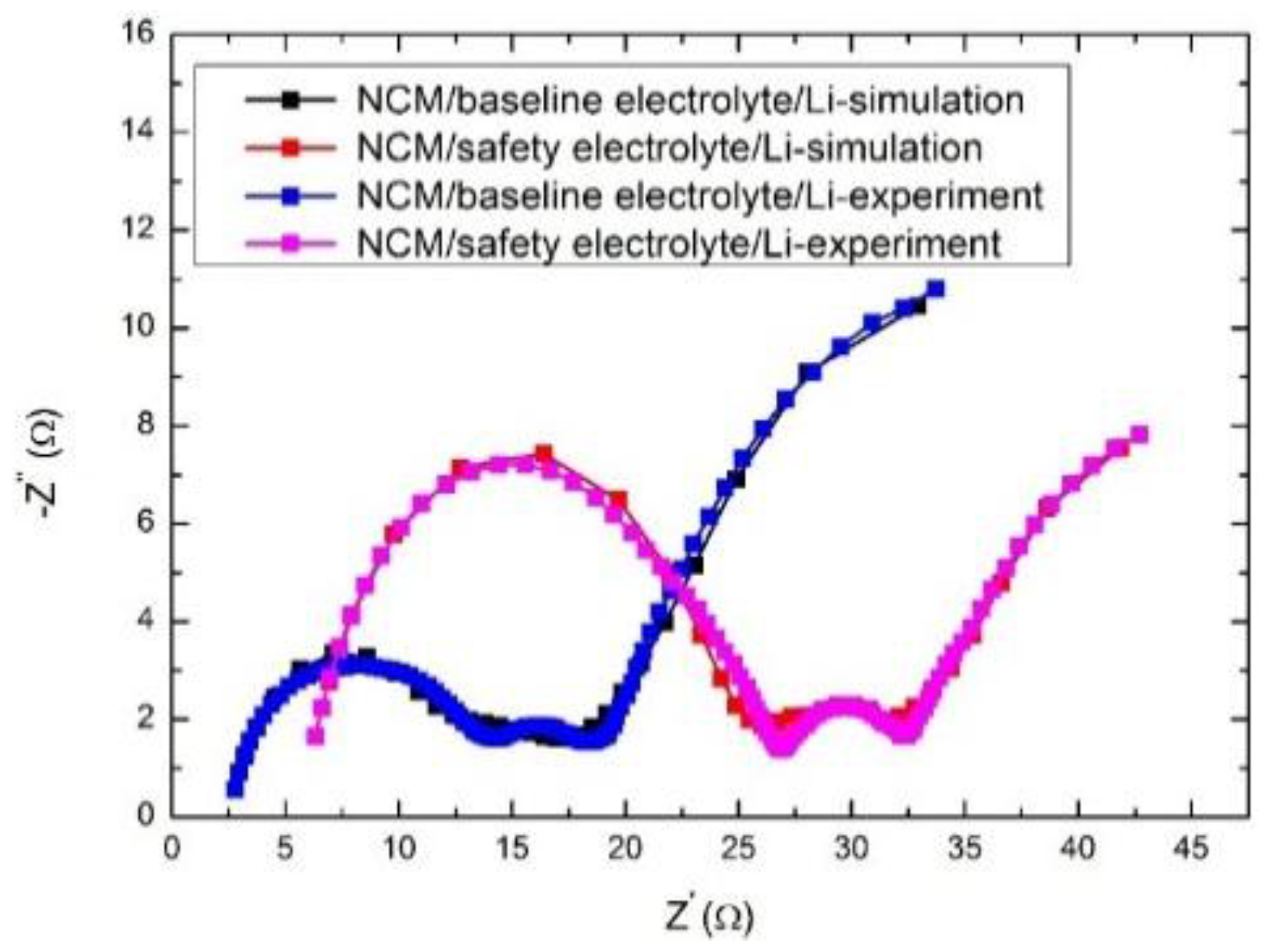
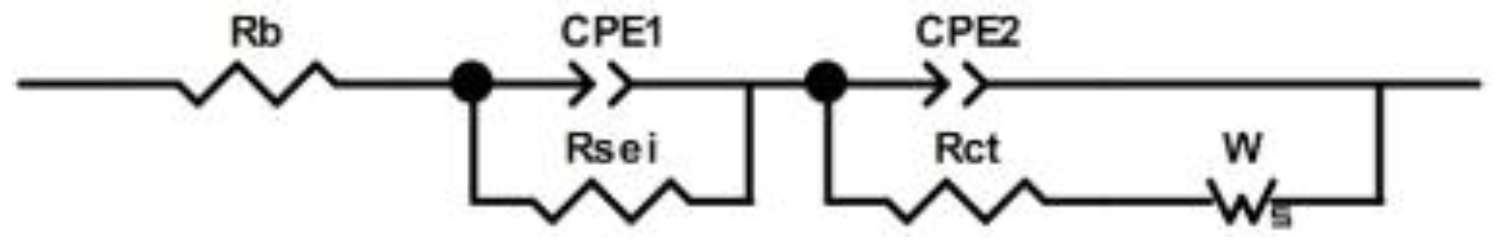
3.2. Electrochemical Performance
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hsieh, C.T.; Chen, Y.F.; Pai, C.T.; Mo, C.Y. Synthesis of lithium nickel cobalt manganese oxide cathode materials by infrared induction heating. J. Power Sources 2014, 269, 31–36. [Google Scholar] [CrossRef]
- Sa, Q.; Gratz, E.; He, M.; Lu, W.; Apelian, D.; Wang, Y. Synthesis of high performance LiNi1/3Mn1/3Co1/3O2 from lithium ion battery recovery stream. J. Power Sources 2015, 282, 140–145. [Google Scholar] [CrossRef]
- Kang, K.S.; Choi, S.; Song, J.; Woo, S.G.; Jo, Y.N.; Choi, J.; Yim, T.; Yu, J.S.; Kim, Y.J. Effect of additives on electrochemical performance of lithium nickel cobalt manganese oxide at high temperature. J. Power Sources 2014, 253, 48–54. [Google Scholar] [CrossRef]
- Wrodnigg, G.H.; Besenhard, J.O.; Winter, M. Cyclic and acyclic sulfites: new solvents and electrolyte additives for lithium ion batteries with graphitic anodes? J. Power Sources 2001, 97, 592–594. [Google Scholar] [CrossRef]
- Isken, P.; Dippel, C.; Schmitz, R.; Schmitz, R.; Kunze, M.; Passerini, S.; Winter, M.; Lex-Balducci, A. High flash point electrolyte for use in lithium-ion batteries. Electrochim. Acta 2011, 56, 7530–7535. [Google Scholar] [CrossRef]
- Wrodnigg, G.H.; Wrodnigg, T.M.; Besenhard, J.O.; Winter, M. Propylene sulfite as film-forming electrolyte additive in lithium ion batteries. Electrochem. Commun. 1999, 1, 148–150. [Google Scholar] [CrossRef]
- Schmitz, R.; Schmitz, R.; Müller, R.; Kazakova, O.; Kalinovich, N.; Röschenthaler, G.V.; Winter, M.; Passerini, S.; Lex-Balducci, A. Methyl tetrafluoro-2-(methoxy) propionate as co-solvent for propylene carbonate-based electrolytes for lithium-ion batteries. J. Power Sources 2012, 205, 408–413. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. C80 calorimeter studies of the thermal behavior of LiPF6 solutions. J. Solut. Chem. 2006, 35, 179–189. [Google Scholar] [CrossRef]
- Doughty, D.H.; Roth, E.P.; Crafts, C.C.; Nagasubramanian, G.; Henriksen, G.; Amine, K. Effects of additives on thermal stability of Li ion cells. J. Power Sources 2005, 146, 116–120. [Google Scholar] [CrossRef]
- Ritchie, A. Recent developments and likely advances in lithium rechargeable batteries. J. Power Sources 2004, 136, 285–289. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. Micro calorimeter study on the thermal stability of lithium-ion battery electrolytes. J. Loss Prev. Process Ind. 2006, 19, 561–569. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, S.; Allen, J.L.; Jow, T.R. Evaluation of fluorinated alkyl phosphates as flame retardants in electrolytes for Li-ion batteries: II. Performance in cell. J. Electrochem. Soc. 2003, 150, A170–A175. [Google Scholar] [CrossRef]
- Xiang, H.; Jin, Q.; Chen, C.; Ge, X.; Guo, S.; Sun, J. Dimethyl methylphosphonate-based nonflammable electrolyte and high safety lithium-ion batteries. J. Power Sources 2007, 174, 335–341. [Google Scholar] [CrossRef]
- Xiang, H.; Xu, H.; Wang, Z.; Chen, C. Dimethyl methylphosphonate (DMMP) as an efficient flame retardant additive for the lithium-ion battery electrolytes. J. Power Sources 2007, 173, 562–564. [Google Scholar] [CrossRef]
- Hyung, Y.E.; Vissers, D.R.; Amine, K. Flame-retardant additives for lithium-ion batteries. J. Power Sources 2003, 119, 383–387. [Google Scholar] [CrossRef]
- Yao, X.; Xie, S.; Chen, C.; Wang, Q.; Sun, J.; Li, Y.; Lu, S. Comparative study of trimethyl phosphite and trimethyl phosphate as electrolyte additives in lithium ion batteries. J. Power Sources 2005, 144, 170–175. [Google Scholar] [CrossRef]
- Mandal, B.K.; Padhi, A.K.; Shi, Z.; Chakraborty, S.; Filler, R. Thermal runaway inhibitors for lithium battery electrolytes. J. Power Sources 2006, 161, 1341–1345. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Yao, X.; Chen, C. 4-Isopropyl phenyl diphenyl phosphate as flame-retardant additive for lithium-ion battery electrolyte. Electrochem. Solid-State Lett. 2005, 8, A467–A470. [Google Scholar] [CrossRef]
- Choi, J.A.; Sun, Y.K.; Shim, E.G.; Scrosati, B.; Kim, D.W. Effect of 1-butyl-1-methylpyrrolidinium hexafluorophosphate as a flame-retarding additive on the cycling performance and thermal properties of lithium-ion batteries. Electrochim. Acta 2011, 56, 10179–10184. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Sun, J.; Chen, C. Cresyl diphenyl phosphate effect on the thermal stabilities and electrochemical performances of electrodes in lithium ion battery. J. Power Sources 2011, 196, 5960–5965. [Google Scholar] [CrossRef]
- Wang, Q.; Sun, J.; Chen, C. Enhancing the thermal stability of LiCoO2 electrode by 4-isopropyl phenyl diphenyl phosphate in lithium ion batteries. J. Power Sources 2006, 162, 1363–1366. [Google Scholar] [CrossRef]
- Liu, F.; Tian, W.; Yang, X.; Jia, G. Hydrogen-bonding and dielectric response of N,N-dimethylacetamide aqueous solutions under E/M fields using molecular dynamics. J. Mol. Liq. 2014, 197, 100–105. [Google Scholar] [CrossRef]
- Feng, L.H.; Wang, Q.S.; Ai, C.Y.; Sun, J.H. The Effect of Multi-component Electrolyte Additive on LiFePO4 Based Lithium Ion Batteries. In Proceedings of the 10th Asia-Oceania Symposium on Fire Science and Technology, Tsukuba, Japan, 5–7 October 2015.
- Cho, J.; Jung, H.; Park, Y.; Kim, G.; Lim, H.S. Electrochemical properties and thermal stability of LiaNi1−x COxO2 cathode materials. J. Electrochem. Soc. 2000, 147, 15–20. [Google Scholar] [CrossRef]
- Hsieh, C.T.; Mo, C.Y.; Chen, Y.F.; Chung, Y.J. Chemical-wet synthesis and electrochemistry of LiNi1/3Co1/3Mn1/3O2 cathode materials for Li-ion batteries. Electrochim. Acta 2013, 106, 525–533. [Google Scholar] [CrossRef]
- Wu, F.; Wang, M.; Su, Y.; Chen, S.; Xu, B. Effect of TiO2-coating on the electrochemical performances of LiCo1/3Ni1/3Mn1/3O2. J. Power Sources 2009, 191, 628–632. [Google Scholar] [CrossRef]
- Liu, S.; Xiong, L.; He, C. Long cycle life lithium ion battery with lithium nickel cobalt manganese oxide (NCM) cathode. J. Power Sources 2014, 261, 285–291. [Google Scholar] [CrossRef]
- Wang, Q.; Ping, P.; Sun, J.; Chen, C. Improved thermal stability of lithium ion battery by using cresyl diphenyl phosphate as an electrolyte additive. J. Power Sources 2010, 195, 7457–7461. [Google Scholar] [CrossRef]
- Zeng, Z.; Jiang, X.; Wu, B.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. Bis (2,2,2-trifluoroethyl) methylphosphonate: an novel flame-retardant additive for safe lithium-ion battery. Electrochim. Acta 2014, 129, 300–304. [Google Scholar] [CrossRef]
- Xiang, H.F.; Jin, Q.Y.; Wang, R.; Chen, C.H.; Ge, X.W. Nonflammable electrolyte for 3-V lithium-ion battery with spinel materials LiNi0.5Mn1.5O4 and Li4Ti5O12. J. Power Sources 2008, 179, 351–356. [Google Scholar] [CrossRef]
- Xu, K.; Zhang, S.; Allen, J.L.; Jow, T.R. Nonflammable electrolytes for Li-ion batteries based on a fluorinated phosphate. J. Electrochem. Soc. 2002, 149, A1079–A1082. [Google Scholar] [CrossRef]
- Shaju, K.; Rao, G.S.; Chowdari, B. Performance of layered Li(Ni1/3Co1/3Mn1/3)O2 as cathode for Li-ion batteries. Electrochim. Acta 2002, 48, 145–151. [Google Scholar] [CrossRef]
- Yabuuchi, N.; Makimura, Y.; Ohzuku, T. Solid-state chemistry and electrochemistry of LiCo1/3Ni1/3Mn1/3O2 for advanced lithium-ion batteries II. Preparation and characterization. J. Electrochem. Soc. 2007, 154, A1434–A1440. [Google Scholar] [CrossRef]
- Zheng, J.M.; Wu, X.B.; Yang, Y. A comparison of preparation method on the electrochemical performance of cathode material Li[Li0.2Mn0.54Ni0.13Co0.13]O2 for lithium ion battery. Electrochim. Acta 2011, 56, 3071–3078. [Google Scholar] [CrossRef]
- Oljaca, M.; Blizanac, B.; Du Pasquier, A.; Sun, Y.; Bontchev, R.; Suszko, A.; Wall, R.; Koehlert, K. Novel Li(Ni1/3Co1/3Mn1/3)O2 cathode morphologies for high power Li-ion batteries. J. Power Sources 2014, 248, 729–738. [Google Scholar] [CrossRef]
- Tan, L.; Liu, H. High rate charge–discharge properties of LiNi1/3Co1/3Mn1/3O2 synthesized via a low temperature solid-state method. Solid State Ion. 2010, 181, 1530–1533. [Google Scholar] [CrossRef]
- He, Y.B.; Liu, M.; Huang, Z.D.; Zhang, B.; Yu, Y.; Li, B.; Kang, F.; Kim, J.K. Effect of solid electrolyte interface (SEI) film on cyclic performance of Li4Ti5O12 anodes for Li ion batteries. J. Power Sources 2013, 239, 269–276. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Rong, H.; Wang, Y.; Liu, J.; Xing, L.; Xu, M.; Li, W. A novel electrolyte with the ability to form a solid electrolyte interface on the anode and cathode of a LiMn2O4/graphite battery. J. Mater. Chem. A 2013, 1, 12954–12961. [Google Scholar] [CrossRef]
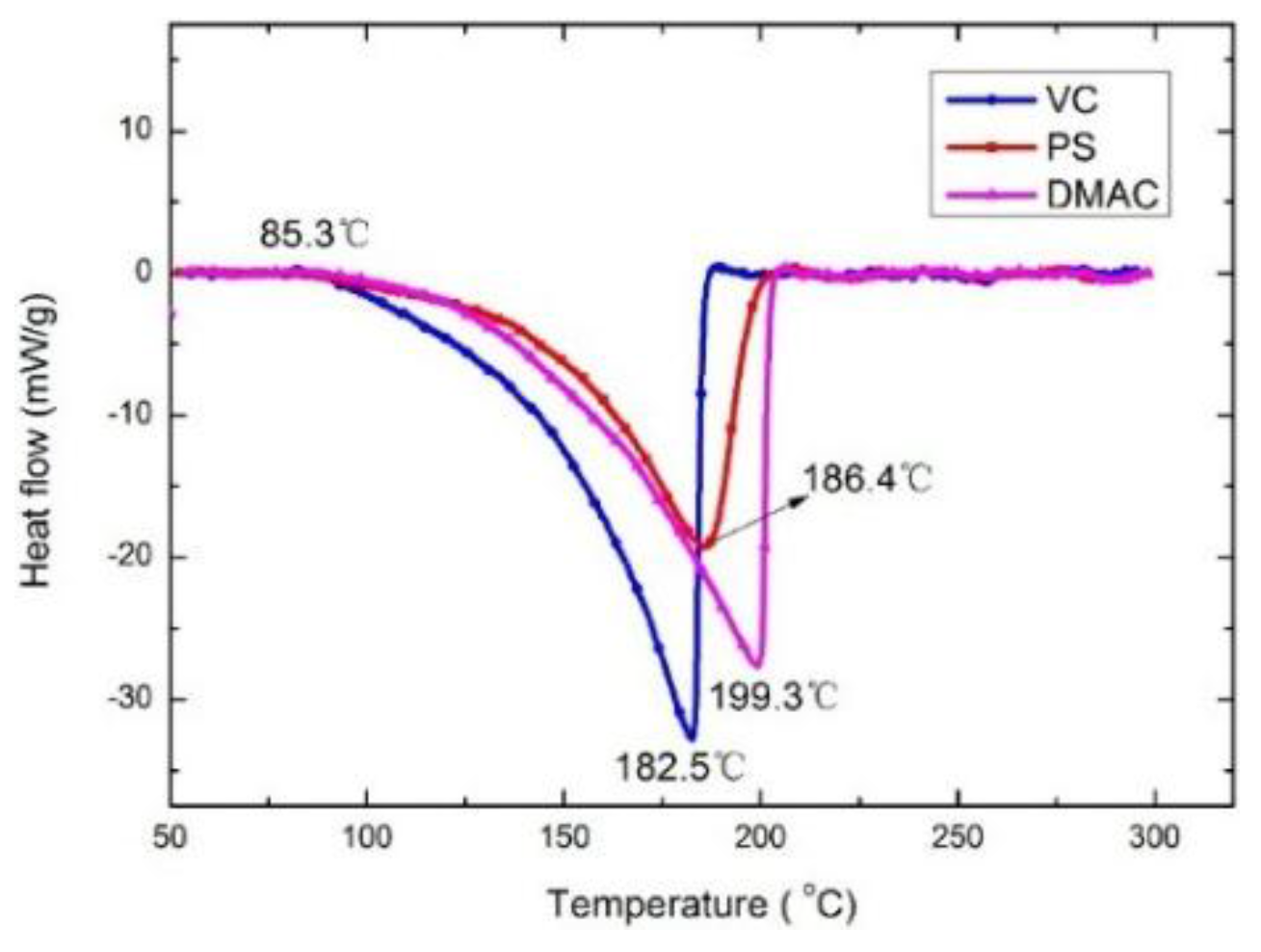
| Electrolyte | Exothermic onset Temperature (°C) | Exothermic Peak (°C) | Heat Generation (J/g) |
|---|---|---|---|
| Baseline Electrolyte | 181.8 | 201.4 | −298.2 |
| Safety Electrolyte | 217.2 | 244.2 | −162.1 |
| Half Cell | Rb (Ω) | Rsei (Ω) | Rct (Ω) |
|---|---|---|---|
| NCM/baseline electrolyte/Li | 2.487 | 8.172 | 7.608 |
| NCM/safety electrolyte/Li | 5.651 | 17.56 | 10.380 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Feng, L.; Sun, J. A Multi-Component Additive to Improve the Thermal Stability of Li(Ni1/3Co1/3Mn1/3)O2-Based Lithium Ion Batteries. Energies 2016, 9, 424. https://doi.org/10.3390/en9060424
Wang Q, Feng L, Sun J. A Multi-Component Additive to Improve the Thermal Stability of Li(Ni1/3Co1/3Mn1/3)O2-Based Lithium Ion Batteries. Energies. 2016; 9(6):424. https://doi.org/10.3390/en9060424
Chicago/Turabian StyleWang, Qingsong, Lihua Feng, and Jinhua Sun. 2016. "A Multi-Component Additive to Improve the Thermal Stability of Li(Ni1/3Co1/3Mn1/3)O2-Based Lithium Ion Batteries" Energies 9, no. 6: 424. https://doi.org/10.3390/en9060424






