The Influence of Slight Protuberances in a Micro-Tube Reactor on Methane/Moist Air Catalytic Combustion
Abstract
:1. Introduction
2. Numerical Methods
2.1. Physical Model
2.2. Mathematical Model
- (1)
- Continuity equation:
- (2)
- Momentum equation:
- (3)
- Energy equation:
- (4)
- Composition equation:
- (5)
- The ideal gas state equation:
2.3. Grid Method and Boundary Conditions
3. Catalytic Combustion Mechanisms
4. Results and Discussion
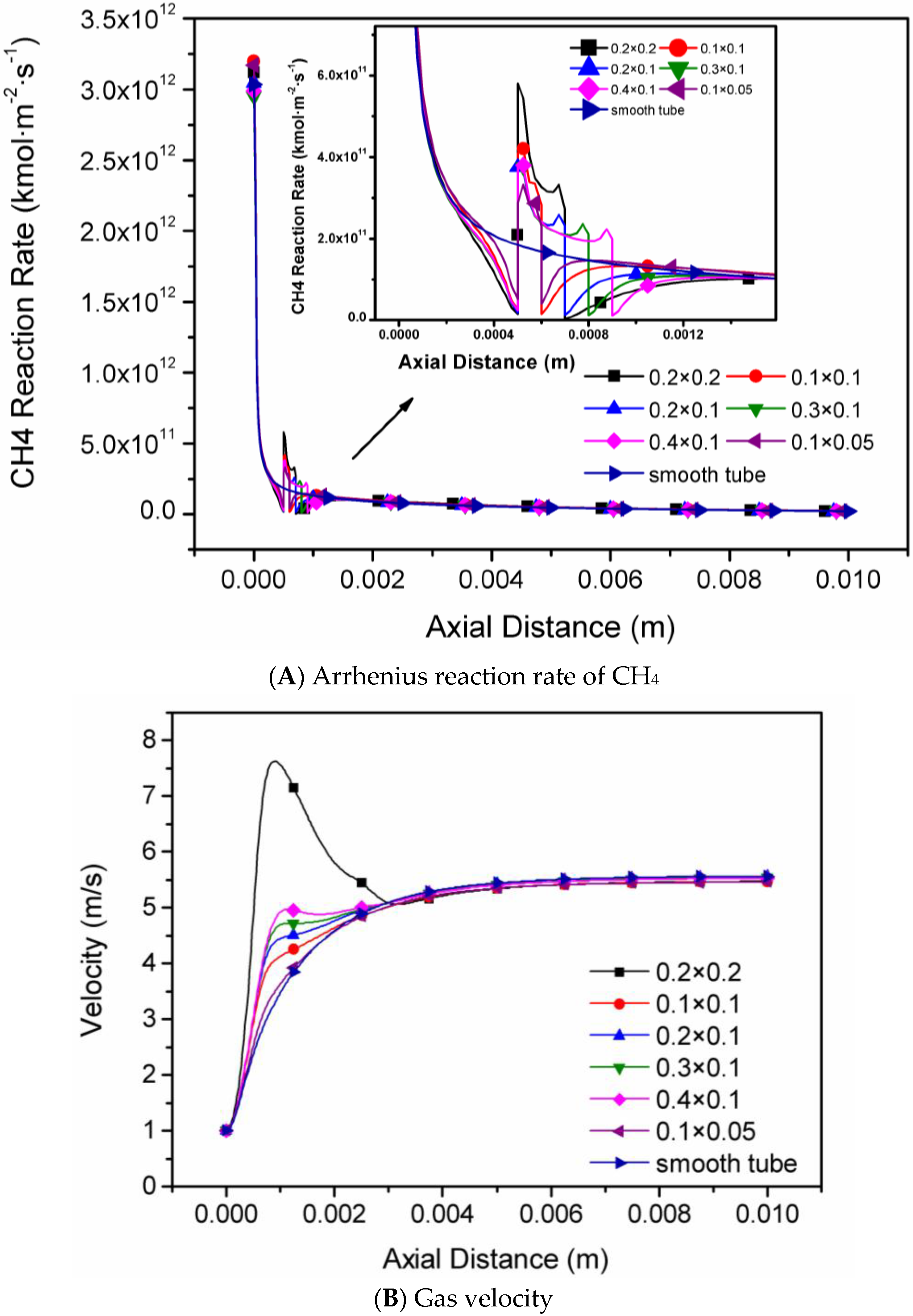
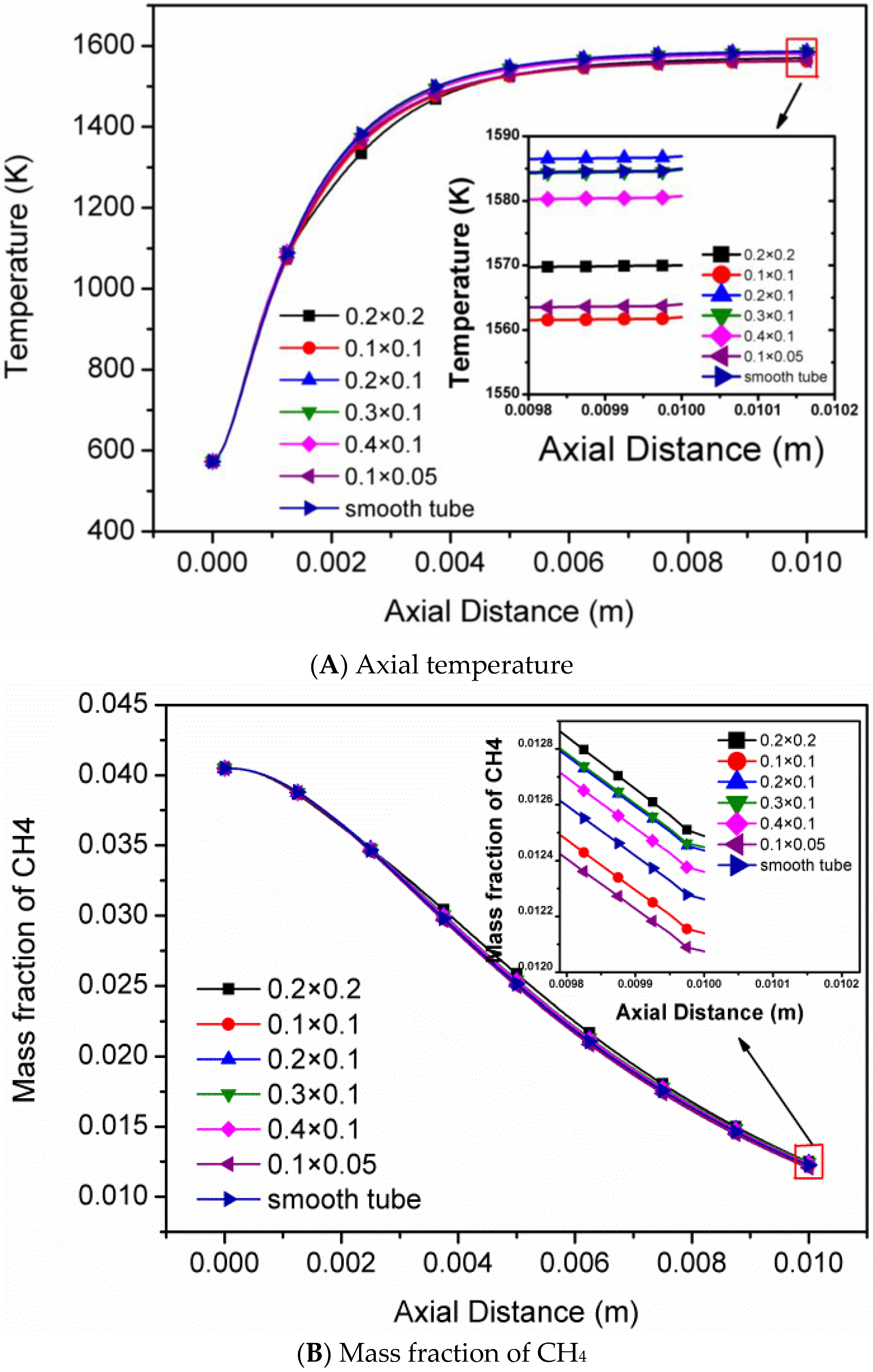
4.1. The Sizes of Single Rectangular Protuberance
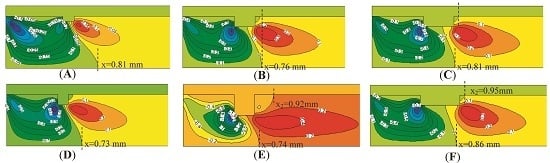
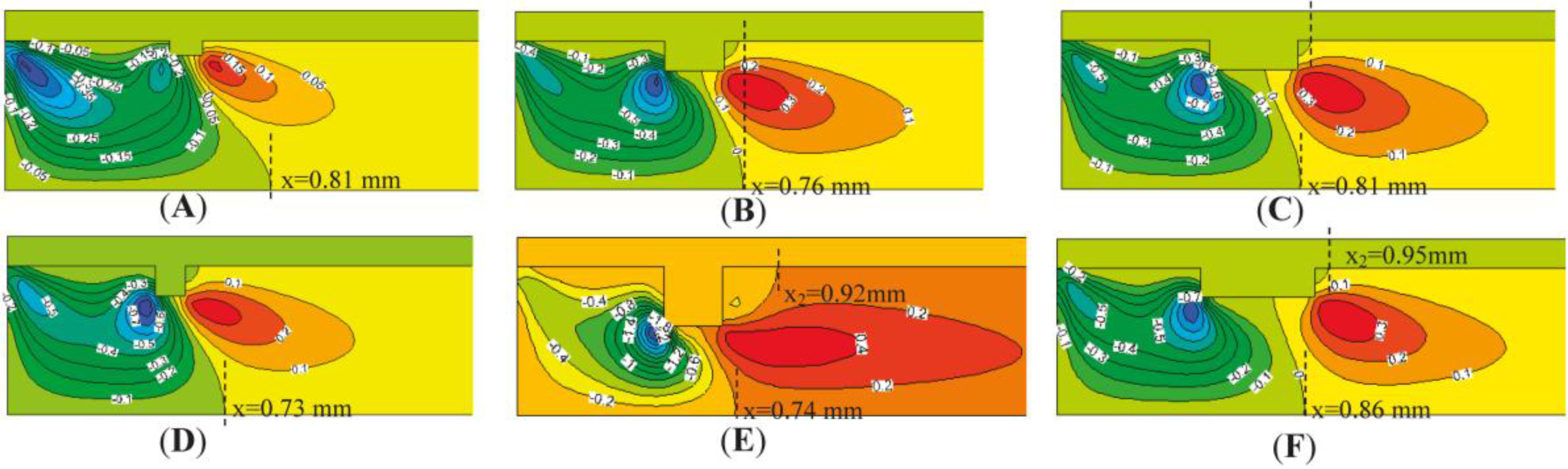
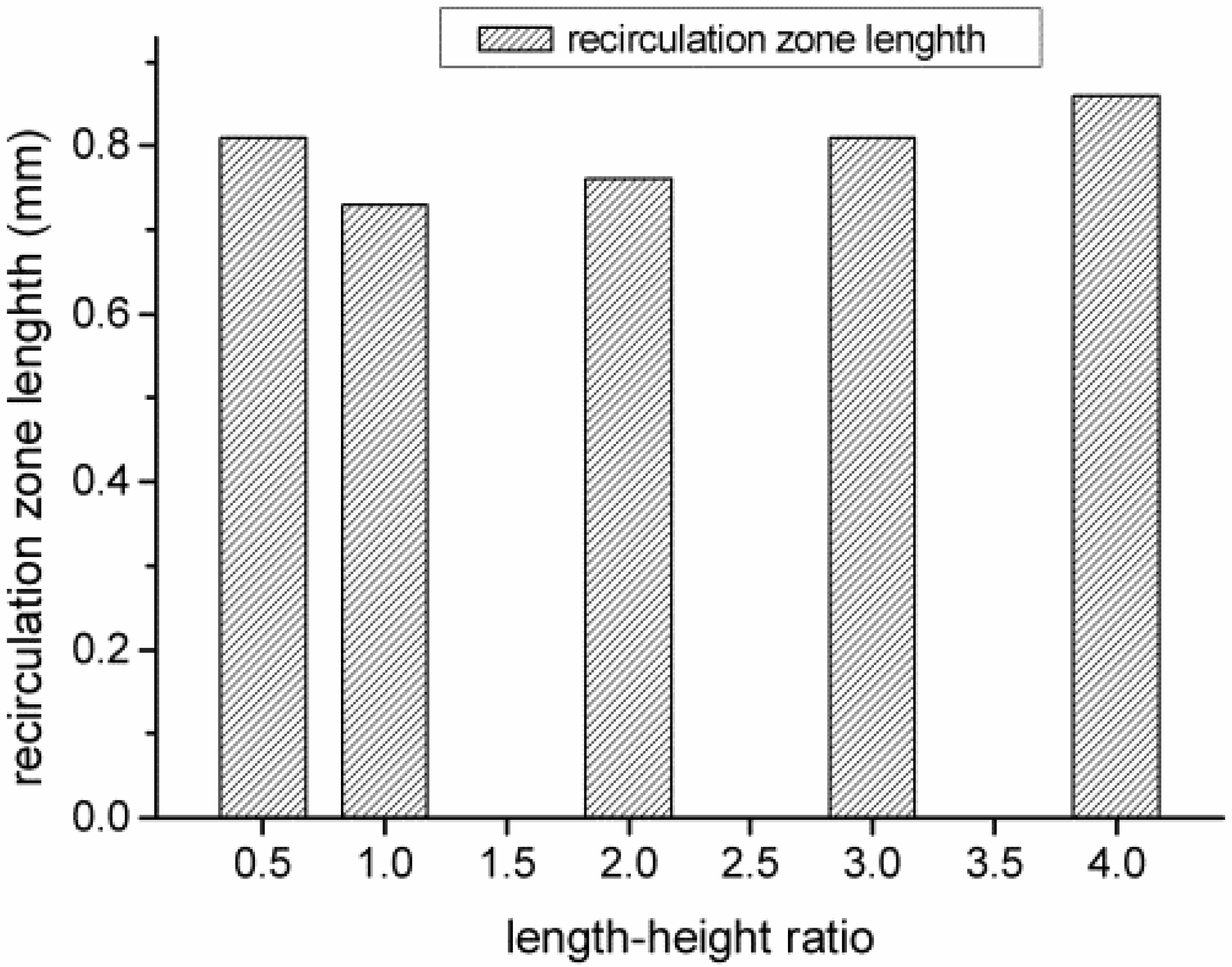
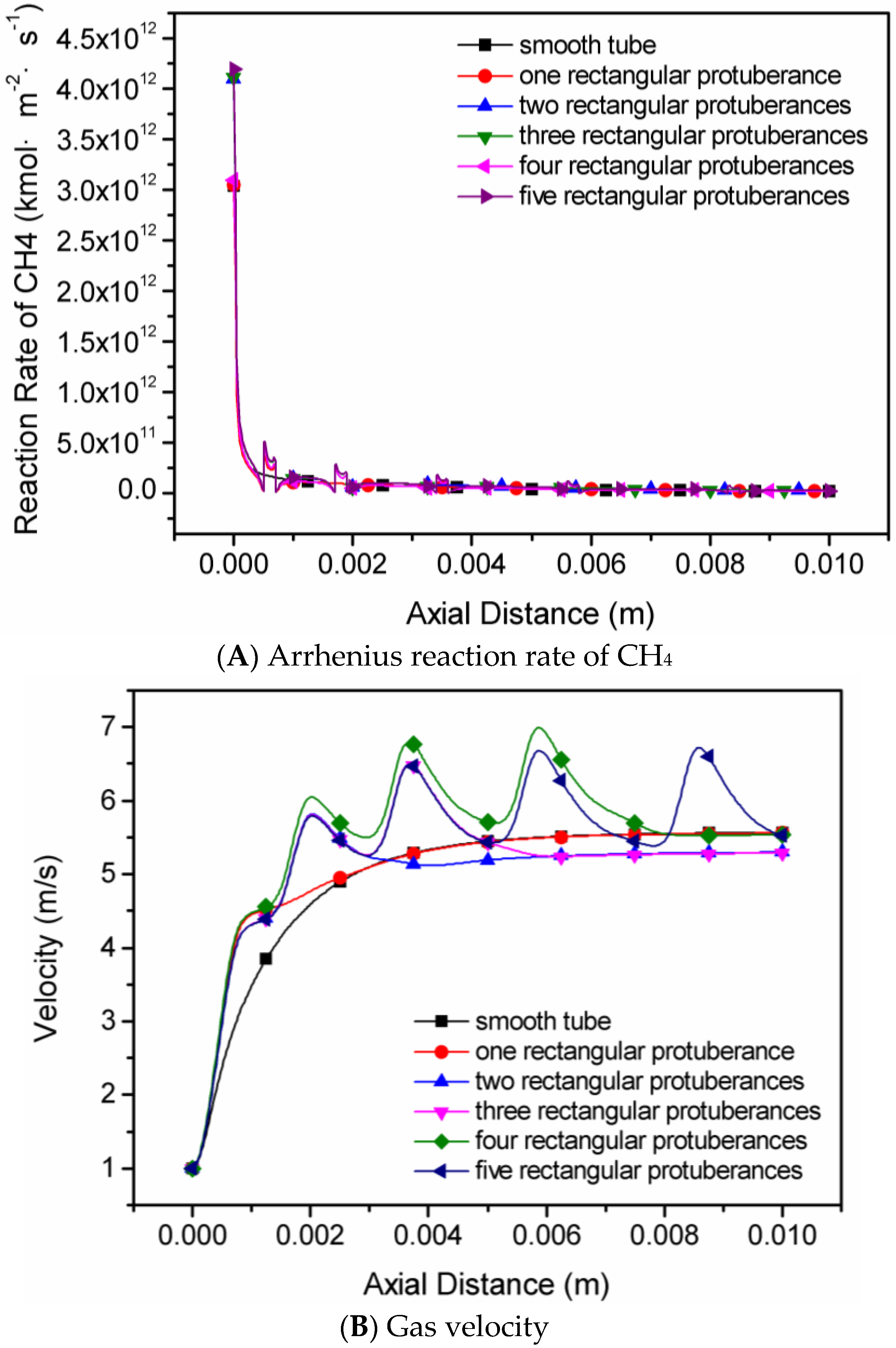
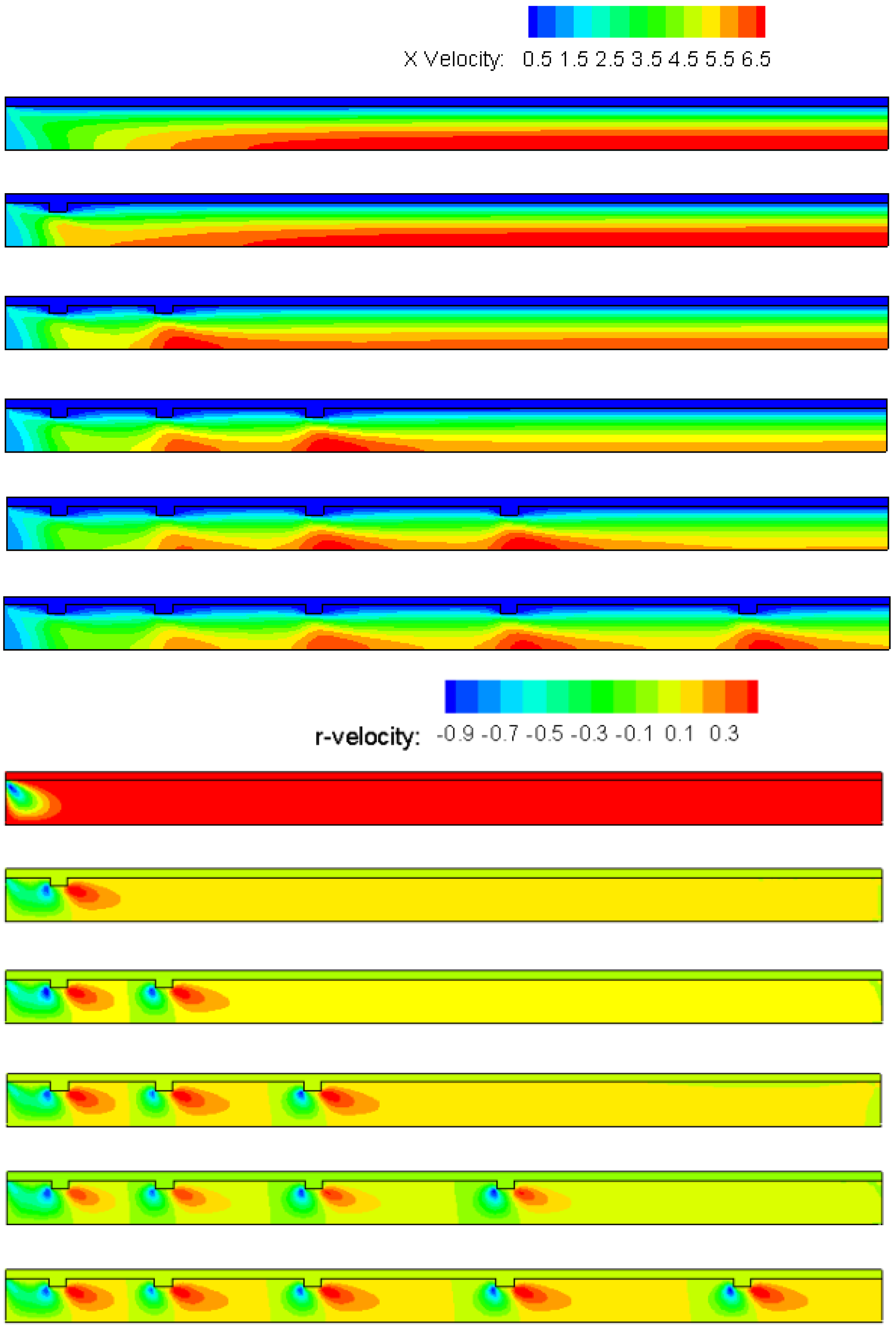
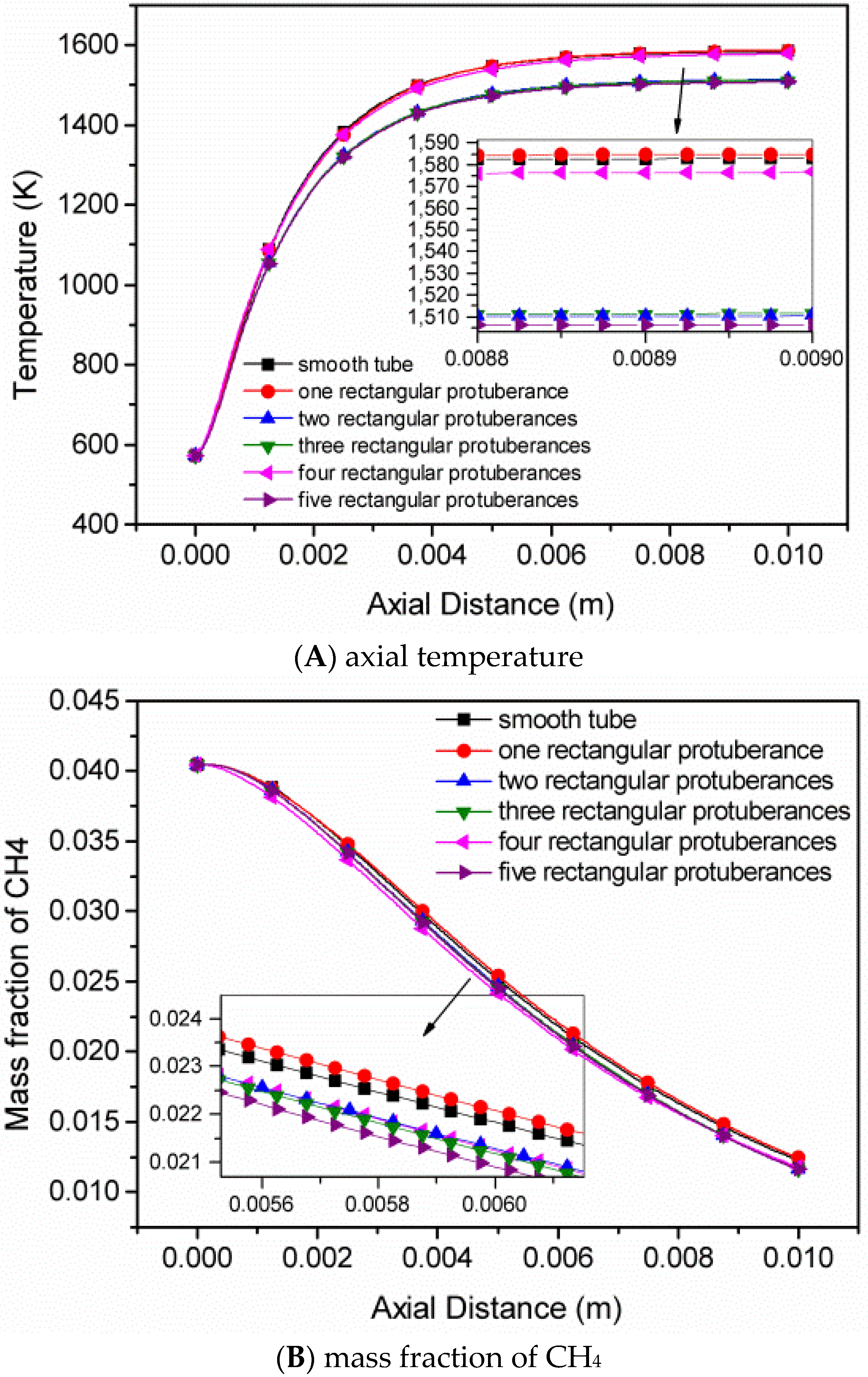
4.2. The Number of Rectangular Protuberances
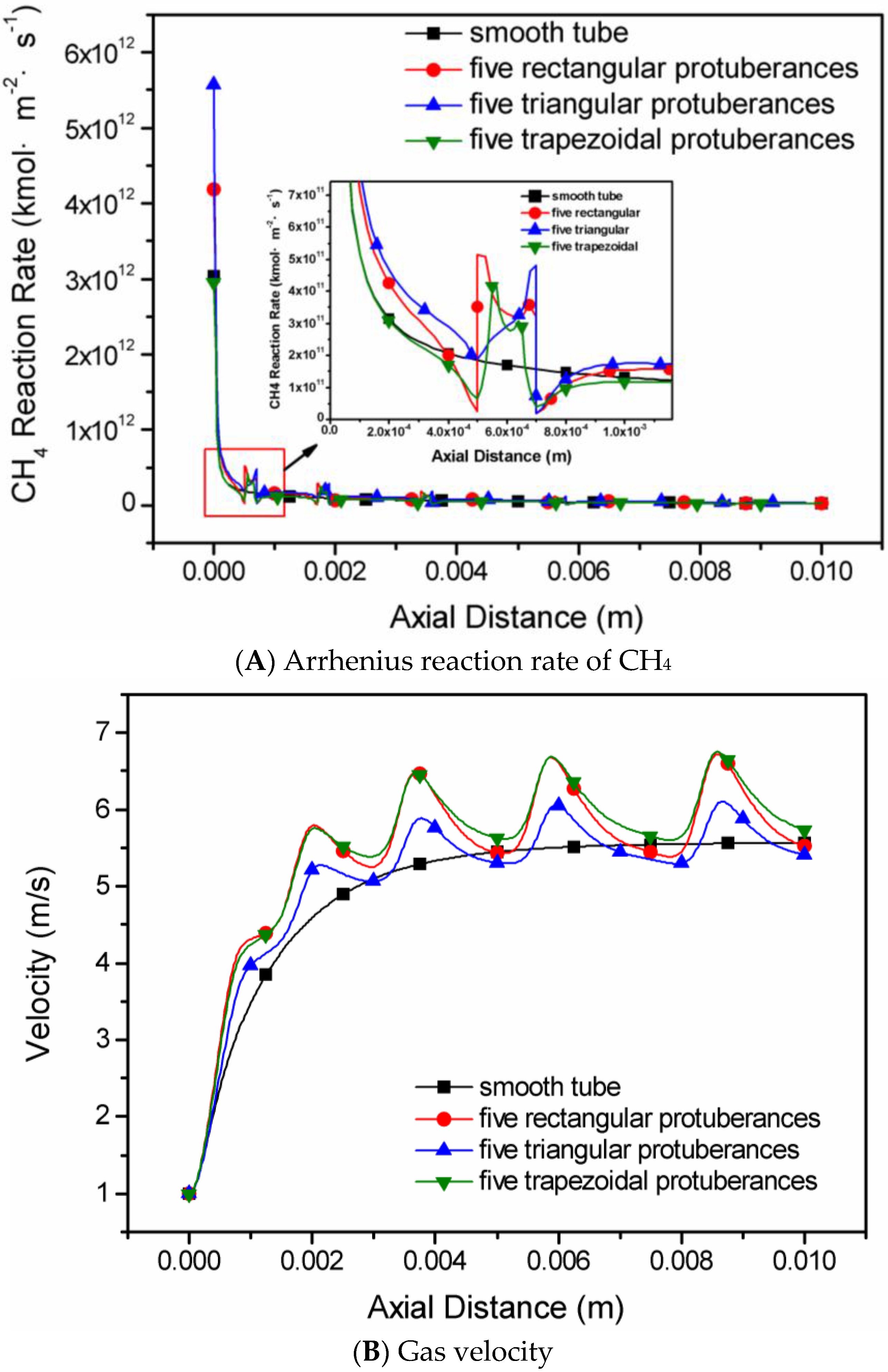
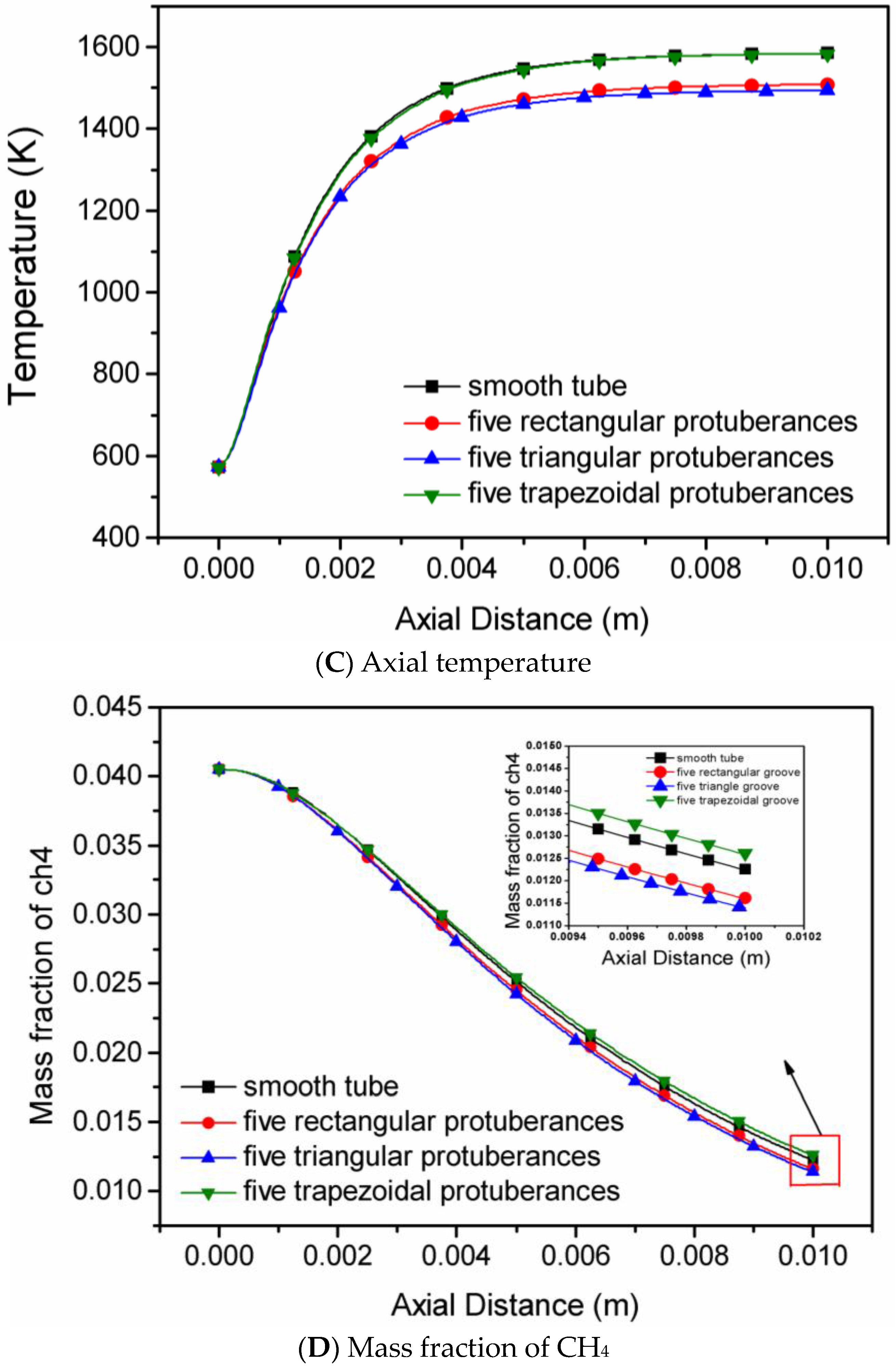
4.3. The Shape of Protuberances
5. Conclusions
- (1)
- The Arrhenius reaction rate of CH4 and flow velocity increase with the increase of protuberance height and area, while the axial temperature and the methane conversion rate are affected little in each micro-tube with one single rectangular protuberance.
- (2)
- Cavities and recirculation zones are formed due to the addition of slight protuberances on the inner wall. For a number of rectangular protuberances from 0–5, the influence of protuberance number on the reaction rate decreases as the mixture gas flows towards the exit. The conversion rate of methane is the highest in the micro-tube with five rectangular protuberances.
- (3)
- The shape of slight protuberances has a greater influence than protuberance number. The methane conversion rate in the micro-tubes decreases progressively in the order five triangular slight protuberances > five rectangular protuberances > five trapezoidal protuberances > smooth tube.
- (4)
- In all simulated methane/moist air catalytic combustion conditions, the micro-tube with five triangular protuberances has the peak efficiency and is recommended for high efficiency reactors.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chia, L.C.; Feng, B. The development of a micropower (micro-thermophotovoltaic) device. J. Power Sources 2007, 165, 455–480. [Google Scholar] [CrossRef]
- Yang, W.M.; Chua, K.J.; Pan, J.F.; Jiang, D.Y.; An, H. Development of micro-thermophotovoltaic power generator with heat recuperation. Energy Convers. Manag. 2014, 78, 81–87. [Google Scholar] [CrossRef]
- Beckers, J.; Gaudillère, C.; Farrusseng, D.; Rothenberg, G. Marrying gas power and hydrogen energy: A catalytic system for combining methane conversion and hydrogen generation. Green Chem. 2009, 11, 921–925. [Google Scholar] [CrossRef]
- Svetovoy, V.; Postnikov, A.; Uvarov, I.; Sanders, R.; Krijnen, G. Overcoming the fundamental limit: Combustion of a hydrogen-oxygen mixture in micro- and nano-bubbles. Energies 2016, 9, 94. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, Z.; Liu, J. Numerical analysis on combustion characteristic of leaf spring rotary engine. Energies 2015, 8, 8086–8109. [Google Scholar] [CrossRef]
- Minotti, A.; Teofilatto, P. Swirling combustor energy converter: H2/Air simulations of separated chambers. Energies 2015, 8, 9930–9945. [Google Scholar] [CrossRef]
- Baigmohammadi, M.; Tabejamaat, S.; Zarvandi, J. Numerical study of the behavior of methane-hydrogen/air pre-mixed flame in a micro reactor equipped with catalytic segmented bluff body. Energy 2015, 85, 117–144. [Google Scholar] [CrossRef]
- Wiswall, J.T.; Li, J.; Wooldridge, M.S.; Im, H.G. Effects of platinum stagnation surface on the lean extinction limits of premixed methane/air flames at moderate surface temperatures. Combust. Flame 2011, 158, 139–145. [Google Scholar] [CrossRef]
- Li, Y.-H.; Hsu, H.-W.; Lien, Y.-S.; Chao, Y.-C. Design of a novel hydrogen-syngas catalytic mesh combustor. Int. J. Hydrogen Energy 2009, 34, 8322–8328. [Google Scholar] [CrossRef]
- Kaisare, N.S.; Deshmukh, S.R.; Vlachos, D.G. Stability and performance of catalytic microreactors: Simulations of propane catalytic combustion on Pt. Chem. Eng. Sci. 2008, 63, 1098–1116. [Google Scholar] [CrossRef]
- Persson, K.; Pfefferle, L.D.; Schwartz, W.; Ersson, A.; Järås, S.G. Stability of palladium-based catalysts during catalytic combustion of methane: The influence of water. Appl. Catal. B Environ. 2007, 74, 242–250. [Google Scholar] [CrossRef]
- Mantzaras, J. Understanding and modeling of thermofluidic processes in catalytic combustion. Catal. Today 2006, 117, 394–406. [Google Scholar] [CrossRef]
- Salciccioli, M.; Stamatakis, M.; Caratzoulas, S.; Vlachos, D.G. A review of multiscale modeling of metal-catalyzed reactions: Mechanism development for complexity and emergent behavior. Chem. Eng. Sci. 2011, 66, 4319–4355. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhang, Y. Effect of CH4-air ratios on gas explosion flame microstructure and propagation behaviors. Energies 2012, 5, 4132–4146. [Google Scholar] [CrossRef]
- Lang, J.; Cheng, S.; Zhou, Y.; Zhao, B.; Wang, H.; Zhang, S. Energy and environmental implications of hybrid and electric vehicles in China. Energies 2013, 6, 2663–2685. [Google Scholar] [CrossRef]
- Korup, O.; Goldsmith, C.F.; Weinberg, G.; Geske, M.; Kandemir, T.; Schlögl, R.; Horn, R. Catalytic partial oxidation of methane on platinum investigated by spatial reactor profiles, spatially resolved spectroscopy, and microkinetic modeling. J. Catal. 2013, 297, 1–16. [Google Scholar] [CrossRef]
- Chin, Y.-H.; Buda, C.; Neurock, M.; Iglesia, E. Selectivity of chemisorbed oxygen in C–H bond activation and CO oxidation and kinetic consequences for CH4–O2 catalysis on Pt and Rh clusters. J. Catal. 2011, 283, 10–24. [Google Scholar] [CrossRef]
- Li, J.; Zhong, B. Experimental investigation on heat loss and combustion in methane/oxygen micro-tube combustor. Appl. Therm. Eng. 2008, 28, 707–716. [Google Scholar] [CrossRef]
- Burch, R.; Urbano, F.J. Investigation of the active state of supported palladium catalysts in the combustion of methane. Appl. Catal. A Gen. 1995, 124, 121–138. [Google Scholar] [CrossRef]
- Schicks, J.M.; Spangenberg, E.; Giese, R.; Steinhauer, B.; Klump, J.; Luzi, M. New approaches for the production of hydrocarbons from hydrate bearing sediments. Energies 2011, 4, 151–172. [Google Scholar] [CrossRef]
- Moccia, V.; D’Alessio, J. Burning behaviour of high-pressure CH4-H2-air mixtures. Energies 2013, 6, 97–116. [Google Scholar] [CrossRef]
- Boushaki, T.; Dhué, Y.; Selle, L.; Ferret, B.; Poinsot, T. Effects of hydrogen and steam addition on laminar burning velocity of methane-air premixed flame: Experimental and numerical analysis. Int. J. Hydrogen Energy 2012, 37, 9412–9422. [Google Scholar] [CrossRef] [Green Version]
- Persson, K.; Ersson, A.; Jansson, K.; Fierro, J.L.G.; Järås, S.G. Influence of molar ratio on Pd-Pt catalysts for methane combustion. J. Catal. 2006, 243, 14–24. [Google Scholar] [CrossRef]
- ViÇes, F.; Lykhach, Y.; Staudt, T.; Lorenz, M.P.A.; Papp, C.; Steinrueck, H.-P.; Libuda, J.; Neyman, K.M.; Gçrling, A. Methane activation by platinum: Critical role of edge and corner sites of metal nanoparticles. Chem. A Eur. J. 2010, 16, 6530–6539. [Google Scholar]
- Trevo, D.J.; Cox, D.M.; Kaldor, A. Methane activation on unsupported platinum clusters. J. Am. Chem. Soc. 1990, 112, 3742–3749. [Google Scholar] [CrossRef]
- Luntz, A.C.; Bethune, D.S. Activation of methane dissociation on a Pt(111) surface. J. Chem. Phys. 1989, 90, 1274–1280. [Google Scholar] [CrossRef]
- Gremminger, A.T.; Pereira de Carvalho, H.W.; Popescu, R.; Grunwaldt, J.-D.; Deutschmann, O. Influence of gas composition on activity and durability of bimetallic Pd-Pt/Al2O3 catalysts for total oxidation of methane. Catal. Today 2015, 258, 470–480. [Google Scholar] [CrossRef]
- Richecoeur, F.; Kyritsis, D.C. Experimental study of flame stabilization in low Reynolds and Dean number flows in curved mesoscale ducts. Proc. Combust. Inst. 2005, 30, 2419–2427. [Google Scholar] [CrossRef]
- Pizza, G.; Mantzaras, J.; Frouzakis, C.E.; Tomboulides, A.G.; Boulouchos, K. Suppression of combustion instabilities of premixed hydrogen/air flames in microchannels using heterogeneous reactions. Proc. Combust. Inst. 2009, 32, 3051–3058. [Google Scholar] [CrossRef]
- Brambilla, A.; Schultze, M.; Frouzakis, C.E.; Mantzaras, J.; Bombach, R.; Boulouchos, K. An experimental and numerical investigation of premixed syngas combustion dynamics in mesoscale channels with controlled wall temperature profiles. Proc. Combust. Inst. 2015, 35, 3429–3437. [Google Scholar] [CrossRef]
- Avcı, A.K.; Trimm, D.L.; Karakaya, M. Microreactor catalytic combustion for chemicals processing. Catal. Today 2010, 155, 66–74. [Google Scholar] [CrossRef]
- Tonkovich, A.Y.; Perry, S.; Wang, Y.; Qiu, D.; LaPlante, T.; Rogers, W.A. Microchannel process technology for compact methane steam reforming. Chem. Eng. Sci. 2004, 59, 4819–4824. [Google Scholar] [CrossRef]
- Yang, W.M.; Chou, S.K.; Shu, C.; Li, Z.W.; Xue, H. Experimental study of micro-thermophotovoltaic systems with different combustor configurations. Energy Convers. Manag. 2007, 48, 1238–1244. [Google Scholar] [CrossRef]
- Baigmohammadi, M.; Tabejamaat, S.; Farsiani, Y. An experimental study of methane-oxygen-carbon dioxide premixed flame dynamics in non-adiabatic cylinderical meso-scale reactors with the backward facing step. Chem. Eng. Proc. Process Intensif. 2015, 95, 105–123. [Google Scholar] [CrossRef]
- Li, J.; Chou, S.K.; Yang, W.M.; Li, Z.W. A numerical study on premixed micro-combustion of CH4-air mixture: Effects of combustor size, geometry and boundary conditions on flame temperature. Chem. Eng. J. 2009, 150, 213–222. [Google Scholar] [CrossRef]
- Yang, W.; Deng, C.; Zhou, J.; Liu, J.; Wang, Y.; Cen, K. Experimental and numerical investigations of hydrogen-air premixed combustion in a converging-diverging micro tube. Int. J. Hydrogen Energy 2014, 39, 3469–3476. [Google Scholar] [CrossRef]
- Ran, J.; Li, L.; Du, X.; Wang, R.; Pan, W.; Tang, W. Numerical investigations on characteristics of methane catalytic combustion in micro-channels with a concave or convex wall cavity. Energy Convers. Manag. 2015, 97, 188–195. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, B.; Gu, F. Effects of changes in pipe cross-section on the explosion-proof distance and the propagation characteristics of gas explosions. J. Nat. Gas Sci. Eng. 2015, 25, 236–241. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, G.-B.; Wu, F.-H.; Cheng, T.-S.; Chao, Y.-C. Effects of catalyst segmentation with cavities on combustion enhancement of blended fuels in a micro channel. Combust. Flame 2012, 159, 1644–1651. [Google Scholar] [CrossRef]
- Wan, J.-L.; Fan, A.-W.; Yao, H.; Liu, W.; Gou, X.-L.; Zhao, D.-Q. The impact of channel gap distance on flame splitting limit of H2/air mixture in microchannels with wall cavities. Int. J. Hydrogen Energy 2014, 39, 11308–11315. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, J.; Yan, Y.; Pan, W.; Yang, Z.; Chen, Y.; Ji, X. Numerical investigation on the transient characteristics of hydrogen production from catalytic autothermal reforming of methane in a micro combustor with multiple cylinders. J. Nat. Gas Sci. Eng. 2014, 19, 251–257. [Google Scholar] [CrossRef]
- Niu, J.; Ran, J.; Li, L.; Du, X.; Wang, R.; Ran, M. Effects of trapezoidal bluff bodies on blow out limit of methane/air combustion in a micro-channel. Appl. Therm. Eng. 2016, 95, 454–461. [Google Scholar] [CrossRef]
- Veeraragavan, A.; Cadou, C.P. Flame speed predictions in planar micro/mesoscale combustors with conjugate heat transfer. Combust. Flame 2011, 158, 2178–2187. [Google Scholar] [CrossRef]
- Veeraragavan, A. On flame propagation in narrow channels with enhanced wall thermal conduction. Energy 2015, 93, 631–640. [Google Scholar] [CrossRef]
- Kang, X.; Veeraragavan, A. Experimental investigation of flame stability limits of a mesoscale combustor with thermally orthotropic walls. Appl. Therm. Eng. 2015, 85, 234–242. [Google Scholar] [CrossRef]
- Veeraragavan, A.; Beri, J.; Gollan, R.J. Use of the method of manufactured solutions for the verification of conjugate heat transfer solvers. J. Comput. Phys 2016, 307, 308–320. [Google Scholar] [CrossRef]
- Saiki, Y.; Suzuki, Y. Effect of wall surface reaction on a methane-air premixed flame in narrow channels with different wall materials. Proc. Combust. Inst. 2013, 34, 3395–3402. [Google Scholar] [CrossRef]
- Ran, J.-Y.; Wu, S.; Yang, L.; Zhang, L. The wall heat transfer phenomenon of premixed CH4/air catalytic combustion in a Pt coated microtube. J. Heat Transfer 2014, 136, 021201. [Google Scholar] [CrossRef]
- Gosiewski, K.; Pawlaczyk, A.; Warmuzinski, K.; Jaschik, M. A study on thermal combustion of lean methane-air mixtures: Simplified reaction mechanism and kinetic equations. Chem. Eng. J. 2009, 154, 9–16. [Google Scholar] [CrossRef]
- Federic, J.A.; Wetzel, E.D.; Geil, B.R.; Vlachos, D.G. Single channel and heat recirculation catalytic microburners: An experimental and computational fluid dynamics study. Proc. Combust. Inst. 2009, 32, 3011–3018. [Google Scholar] [CrossRef]
- Li, Y.-H.; Chen, G.-B.; Wu, F.-H.; Cheng, T.-S.; Chao, Y.-C. Combustion characteristics in a small-scale reactor with catalyst segmentation and cavities. Proc. Combust. Inst. 2013, 34, 2253–2259. [Google Scholar] [CrossRef]
- Deutschmann, O.; Maier, L.I.; Riedel, U.; Stroemman, A.H.; Dibble, R.W. Hydrogen assisted catalytic combustion of methane on platinum. Catal. Today 2000, 59, 141–150. [Google Scholar] [CrossRef]
- Schwiedernoch, R.; Tischer, S.; Deutschmann, O.; Warnatz, J. Experimental and numerical investigation of the ignition of methane combustion in a platinum-coated honeycomb monolith. Proc. Combust. Inst. 2002, 29, 1005–1011. [Google Scholar] [CrossRef]
- Zhai, X.; Ding, S.; Cheng, Y.; Jin, Y.; Cheng, Y. CFD simulation with detailed chemistry of steam reforming of methane for hydrogen production in an integrated micro-reactor. Int. J. Hydrogen Energy 2010, 35, 5383–5392. [Google Scholar] [CrossRef]
- Ran, J.; Qi, W.; Wang, R. Numerical study of the micro-channel rough surface on methane catalytic combustion. J. Eng. Thermophys. 2014, 35, 1244–1247. [Google Scholar]
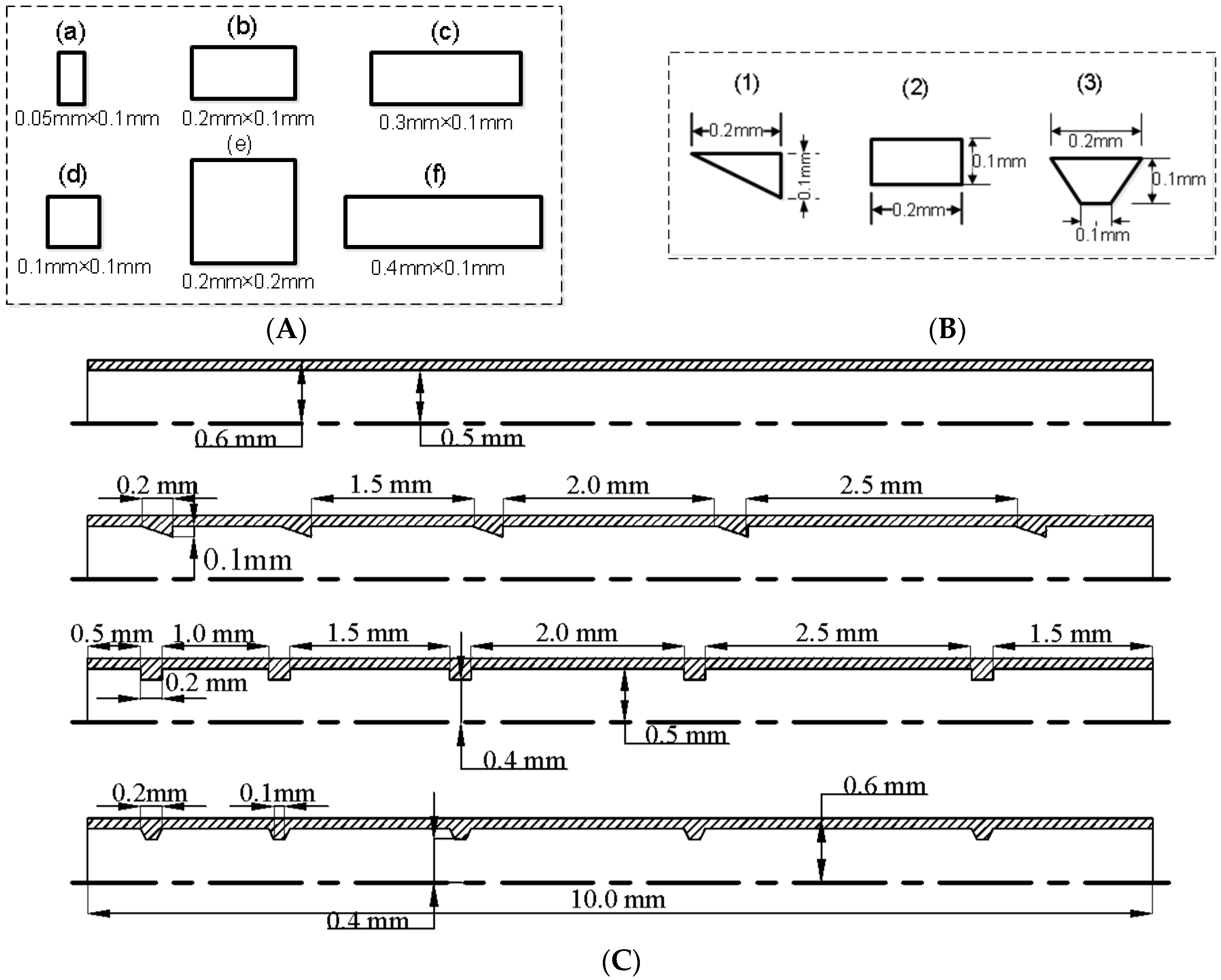
| Working Conditions | Protuberance Number | Protuberance Shape | Protuberance Size | Distance from Entrance (mm) |
|---|---|---|---|---|
| Case 1 | 1 | Rectangular | Six different sizes see Figure 1A | 0.5 |
| Case 2 | 1, 2, 3, 4, 5 | Rectangular | One fixed size: 0.2 mm × 0.1 mm | 0.5, 1.7, 3.4, 5.6 and 8.3 for the rectangular protuberances numbered 1, 2, 3, 4 and 5, respectively |
| Case 3 | 5 | Five rectangular protuberances; | The dimensions of each protuberance shape are fixed. see Figure 1B | See Figure 1C: 0.5, 1.7, 3.4, 5.6 and 8.3 for the protuberances numberd 1, 2, 3, 4 and 5, respectively |
| Five triangular protuberances; | ||||
| Five trapezoidal protuberances |
| Protuberance Type | Protuberance Sizes (mm × mm) | Outlet Temperature (K) | Outlet Velocity (m/s) | Methane Conversion Rate (%) |
|---|---|---|---|---|
| (a) | 0.1 × 0.05 | 1565 | 5.46 | 87.88 |
| (b) | 0.1 × 0.1 | 1563 | 5.47 | 87.82 |
| (c) | 0.2 × 0.2 | 1571 | 5.48 | 87.48 |
| (d) | 0.2 × 0.1 | 1588 | 5.56 | 87.57 |
| (e) | 0.3 × 0.1 | 1586 | 5.54 | 87.50 |
| (f) | 0.4 × 0.1 | 1582 | 5.53 | 87.60 |
| (g) | Smooth tube | 1585 | 5.57 | 87.56 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, R.; Ran, J.; Du, X.; Niu, J.; Qi, W. The Influence of Slight Protuberances in a Micro-Tube Reactor on Methane/Moist Air Catalytic Combustion. Energies 2016, 9, 421. https://doi.org/10.3390/en9060421
Wang R, Ran J, Du X, Niu J, Qi W. The Influence of Slight Protuberances in a Micro-Tube Reactor on Methane/Moist Air Catalytic Combustion. Energies. 2016; 9(6):421. https://doi.org/10.3390/en9060421
Chicago/Turabian StyleWang, Ruirui, Jingyu Ran, Xuesen Du, Juntian Niu, and Wenjie Qi. 2016. "The Influence of Slight Protuberances in a Micro-Tube Reactor on Methane/Moist Air Catalytic Combustion" Energies 9, no. 6: 421. https://doi.org/10.3390/en9060421