Optimal Operation of Combined Photovoltaic Electrolyzer Systems
Abstract
:1. Introduction
2. Analysis and Modeling
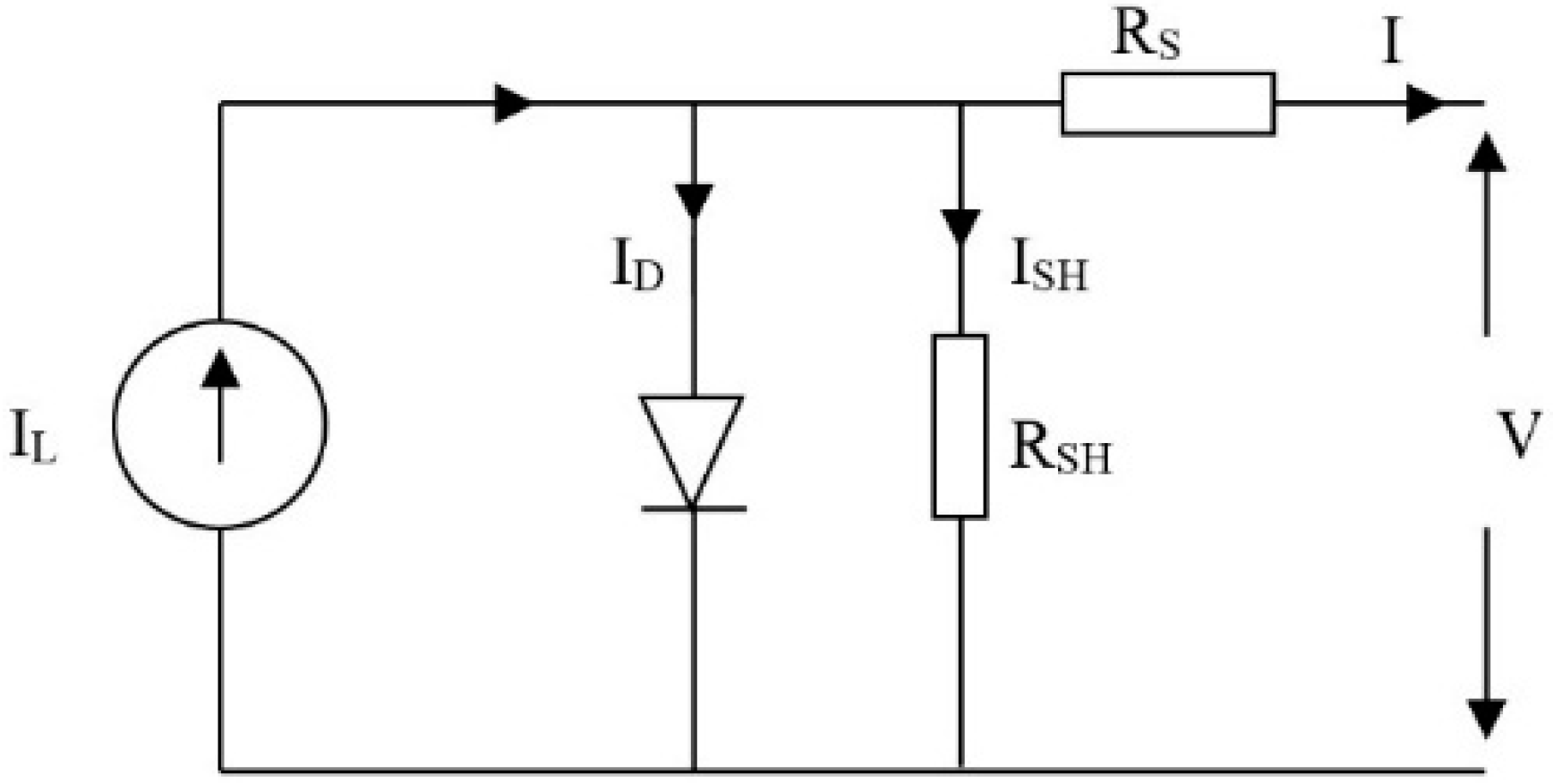
2.1. Photovoltaic System
2.2. Electrolyzer
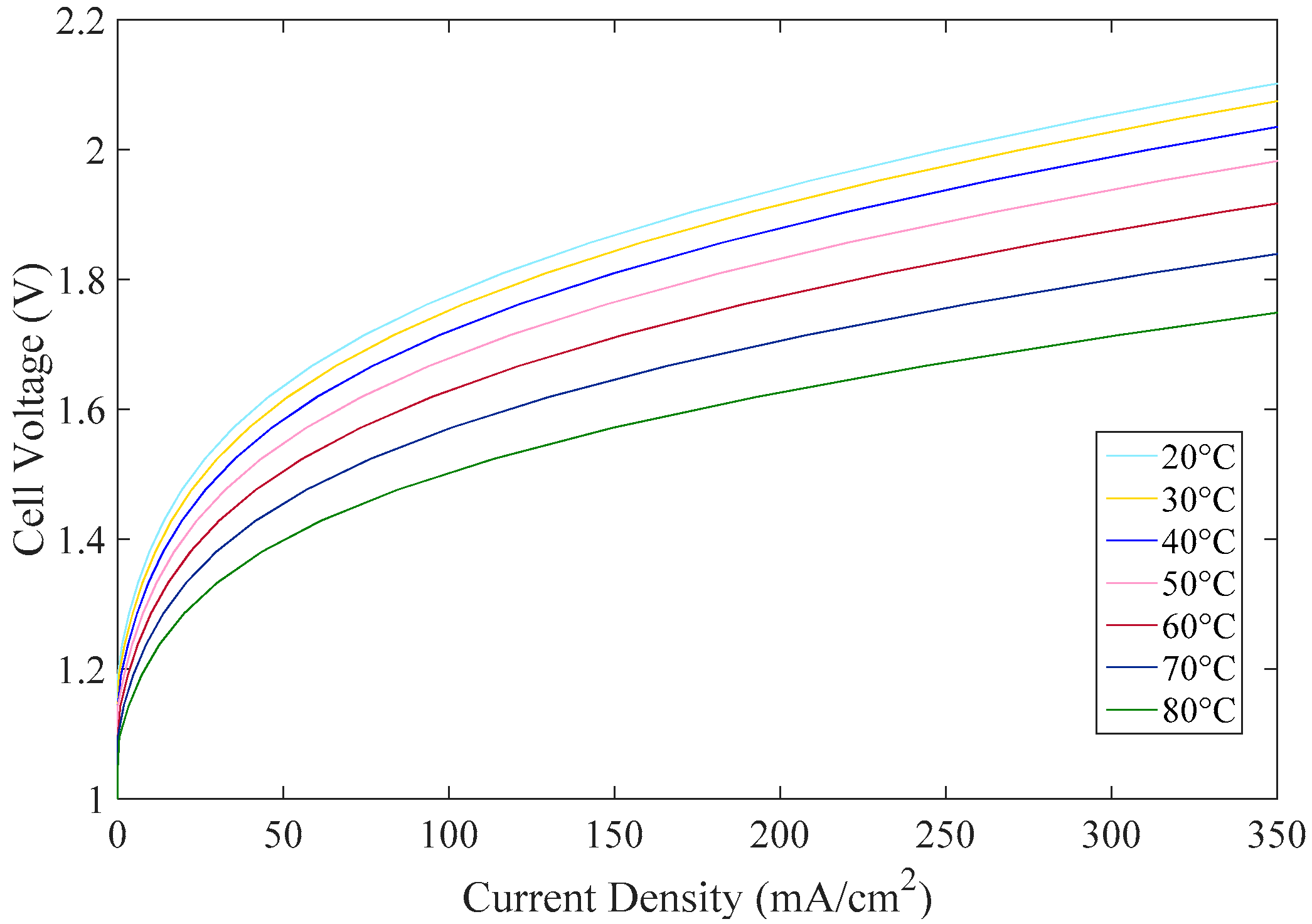
2.2.1. Electrochemical Model
2.2.2. Thermal Model
2.3. Storage
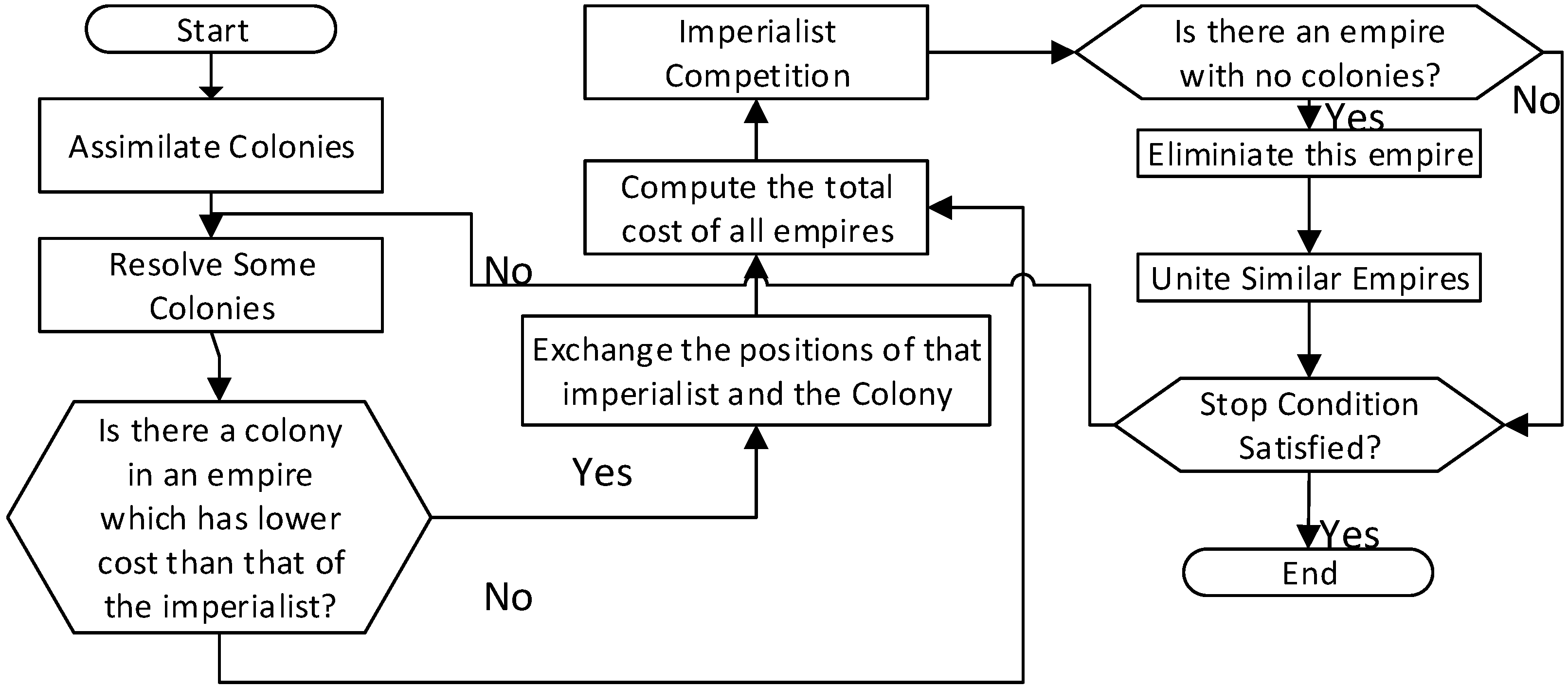
3. Imperialist Competitive Algorithm
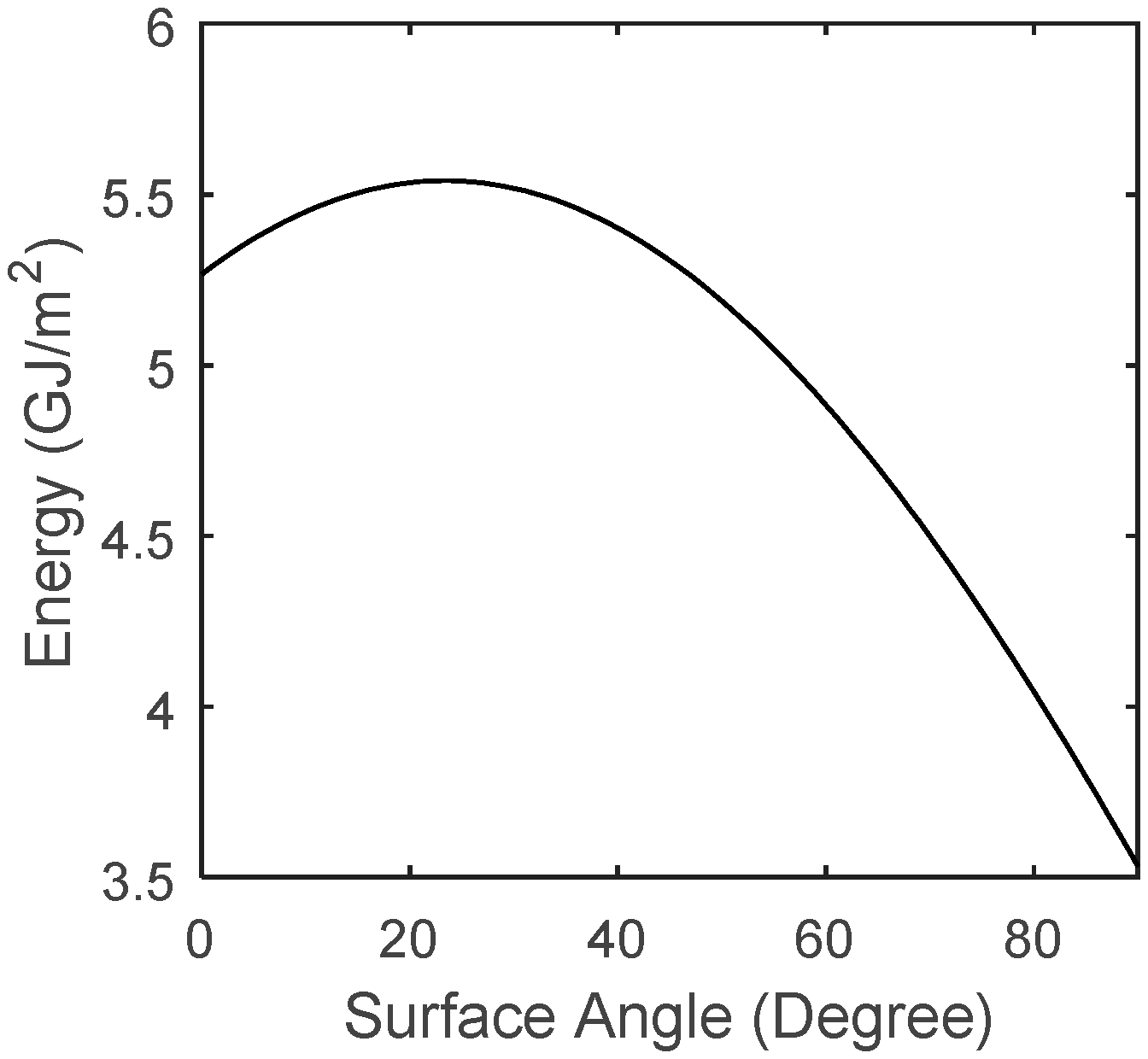
4. Solar Calculation
5. Hybrid System Simulation
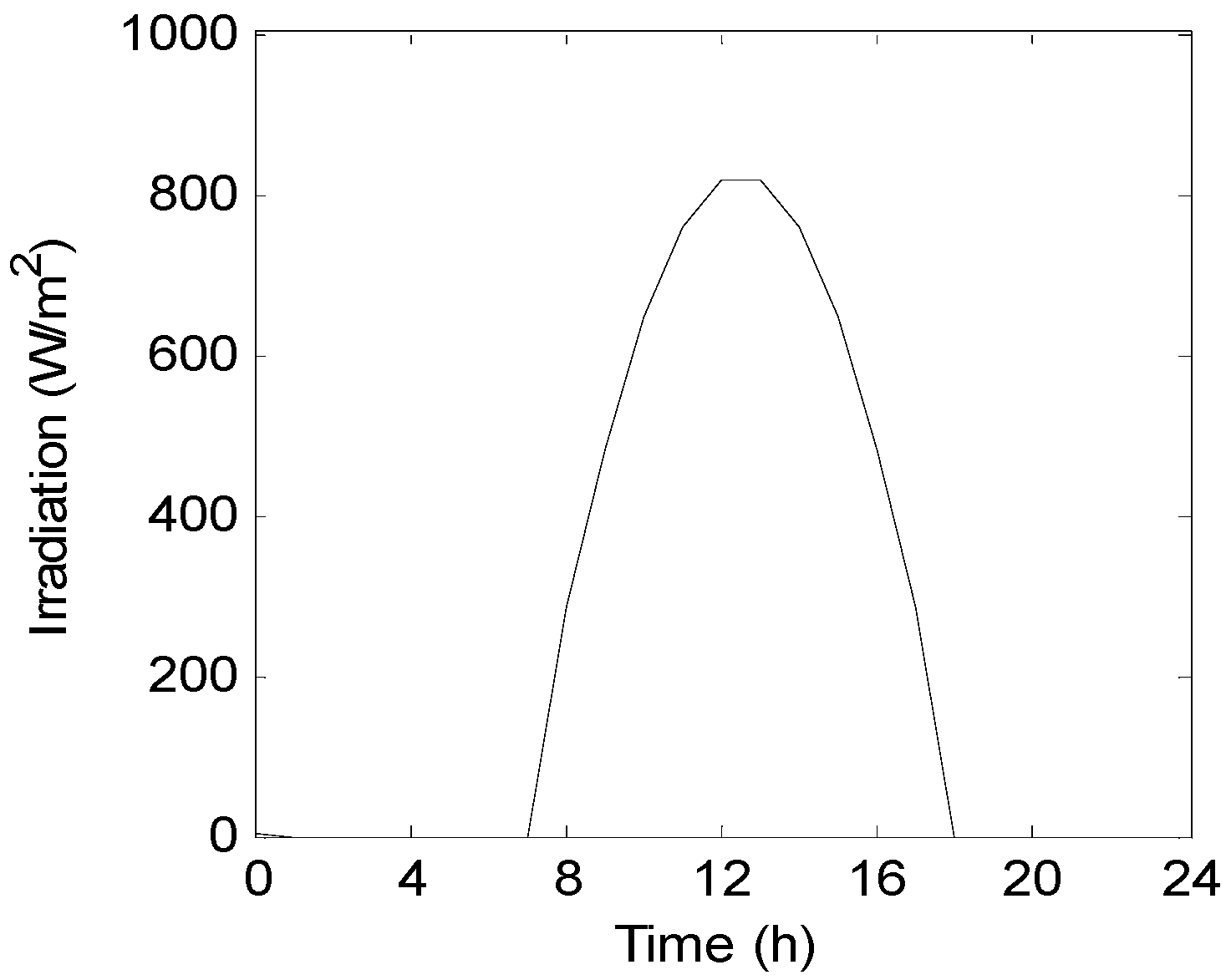
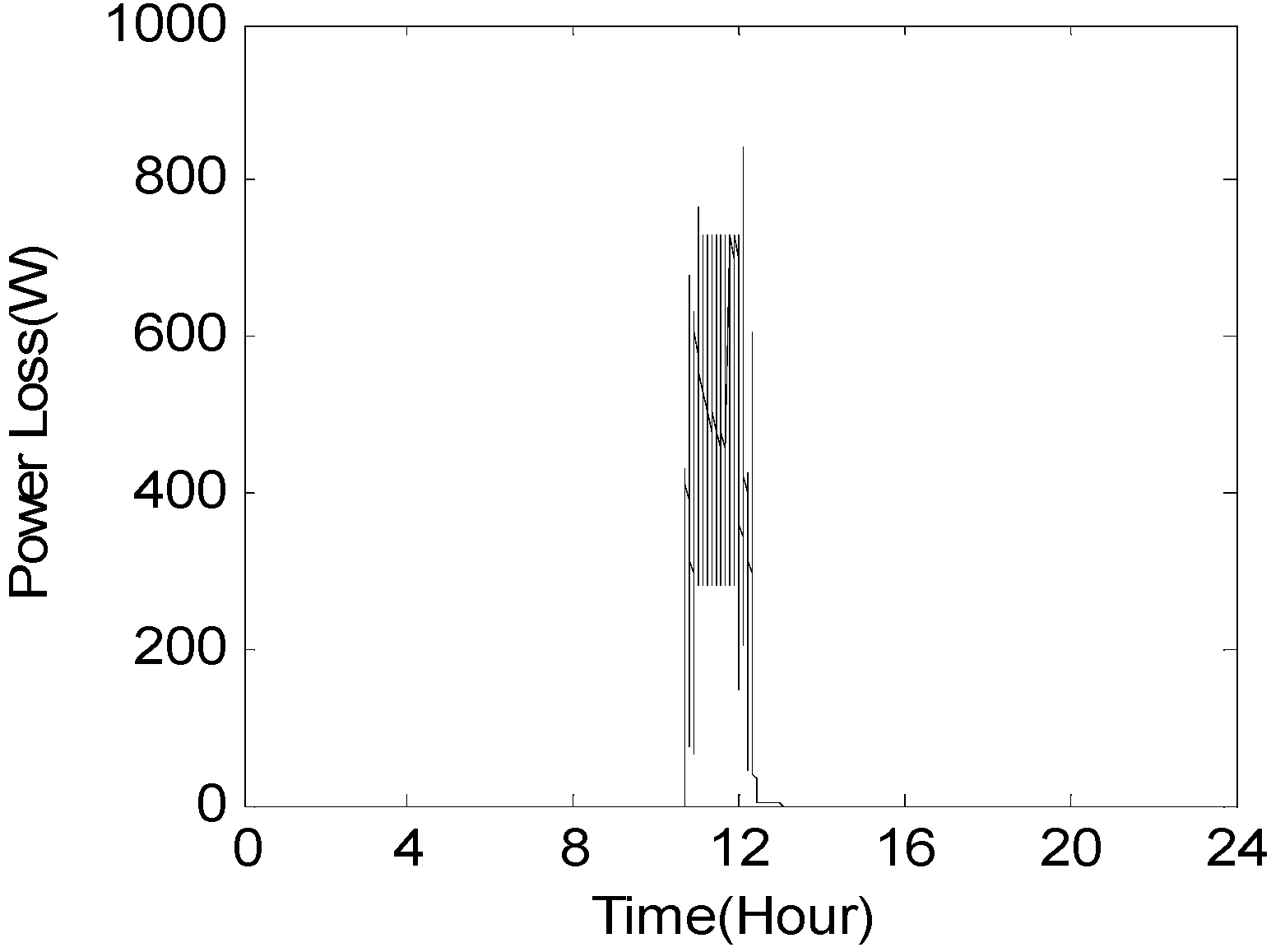
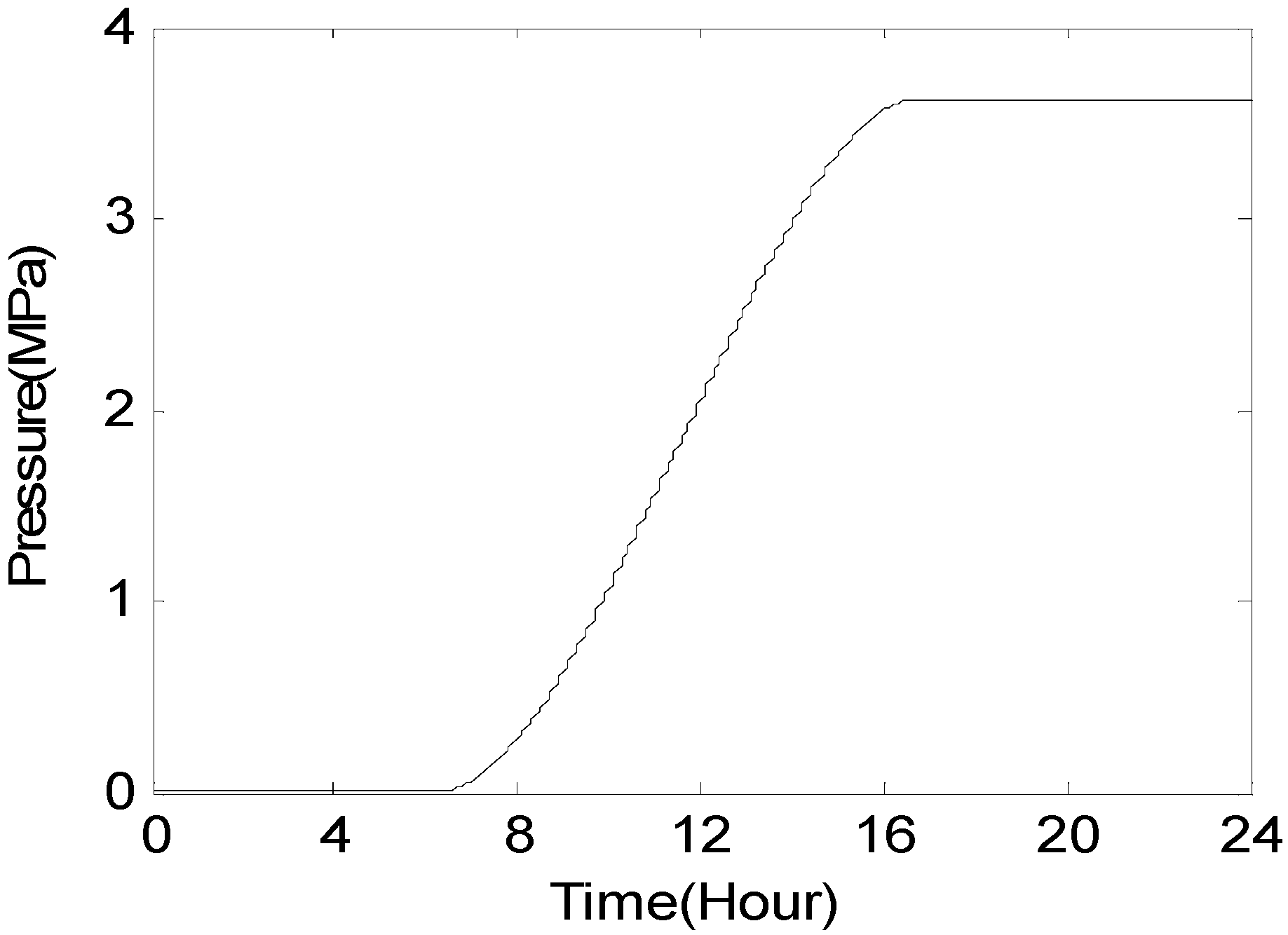
6. Results and Discussion
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Salmani, M.A.; Anzalchi, A.; Salmani, S. Virtual Power Plant: New Solution for Managing Distributed Generations in Decentralized Power Systems. In Proceedings of the 2010 International Conference on Management and Service Science (MASS), Wuhan, China, 24–26 August 2010; pp. 1–6.
- Anzalchi, A.; Sarwat, A.I. Analysis of Carbon Tax as an Incentive toward Building Sustainable Grid with Renewable Energy Utilization. In Proceedings of the 2015 Seventh Annual IEEE on Green Technologies Conference, New Orleans, LA, USA, 15–17 April 2015; pp. 103–109.
- Moghadasi, A.H.; Heydari, H.; Farhadi, M. Pareto optimality for the design of SMES solenoid coils verified by magnetic field analysis. IEEE Trans. Appl. Supercond. 2011, 21, 13–20. [Google Scholar] [CrossRef]
- Moghadasi, A.; Torabi, S.M.; Salehifar, M. Combined Operation of the Unified Power Quality Conditioner with SFCL and SMES. In Proceedings of the 2010 First Power Quality Conference, Tehran, Iran, 14–15 September 2010; pp. 1–7.
- Moghadasi, A.H.; Sundararajan, A.; Sarwat, A.I. Power Management and Control Strategy in Standalone DC Microgrid along with SMES Solenoid Coil. Int. J. Enhanc. Res. Sci. Technol. Eng. 2014, 3, 102–112. [Google Scholar]
- Wei, L.; Moghadasi, A.H.; Sundararajan, A.; Sarwat, A.I. Defending Mechanisms for Protecting Power Systems against Intelligent Attacks. In Proceedings of the 2015 10th IEEE Conference on System of Systems Engineering (SoSE), San Antonio, TX, USA, 17–20 May 2015.
- Gholami, H.; Sarwat, A.I.; Hosseinian, H.; Khalilnejad, A. Evaluation of optimal dual axis concentrated photovoltaic thermal system with active ventilation using Frog Leap algorithm. Energy Convers. Manag. 2015, 105, 782–790. [Google Scholar] [CrossRef]
- Gholami, H.; Khalilnejad, A.; Gharehpetian, G.B. Electrothermal performance and environmental effects of optimal photovoltaic–thermal system. Energy Convers. Manag. 2015, 95, 326–333. [Google Scholar] [CrossRef]
- Calderon, M.; Calderon, A.J.; Ramiro, A.; Gonzalez, J.F.; Gonzalez, I. Evaluation of a hybrid photovoltaic-wind system with hydrogen storage performance using exergy analysis. Int. J. Hydrog. Energy 2011, 36, 5751–5762. [Google Scholar] [CrossRef]
- Castañeda, M.; Fernández, L.M.; Sánchez, H.; Cano, A.; Jurado, F. Sizing methods for stand-alone hybrid systems based on renewable energies and hydrogen. In Proceedings of the 2012 16th IEEE Mediterranean Electrotechnical Conference, Yasmine Hammamet, Tunisia, 25–28 March 2012; pp. 832–835.
- Clarke, R.E.; Giddey, S.; Ciacchi, F.T.; Badwal, S.P.S.; Paul, B.; Andrews, J. Direct coupling of an electrolyzer to a solar PV system for generating hydrogen. Int. J. Hydrog. Energy 2009, 34, 2531–2542. [Google Scholar] [CrossRef]
- Pelacchi, P.; Poli, D. The influence of wind generation on power system reliability and the possible use of hydrogen storages. Electr. Power Syst. Res. 2010, 80, 249–255. [Google Scholar] [CrossRef]
- Abaspour, A.; Parsa, N.T.; Sadeghi, M. A new feedback Linearization-NSGA-II based control design for PEM fuel cell. Int. J. Comput. Appl. 2014, 97, 25–32. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Venkateswaran, P.S.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Ganeshan, I.S.; Manikandan, V.V.S.; Sundhar, V.R.; Sajiv, R.; Shanthi, C.; Kottayil, S.K.; Ramachandran, T. Regulated hydrogen production using solar powered electrolyzer. Int. J. Hydrog. Energy 2015. [Google Scholar] [CrossRef]
- Anzalchi, A.; Sarwat, A. Artificial Neural Network Based Duty Cycle Estimation for Maximum Power Point Tracking in Photovoltaic Systems. In Proceedings of the IEEE on SoutheastCon 2015, Fort Lauderdale, FL, USA, 9–12 April 2015.
- Martinez-Frias, J.; Pham, A.Q.; Aceves, S.M. A natural gas-assisted steam electrolyzer for high-efficiency production of hydrogen. Int. J. Hydrog. Energy 2003, 28, 483–490. [Google Scholar] [CrossRef]
- Rajesh, R.; Mabel, M.C. A comprehensive review of photovoltaic systems. Renew. Sustain. Energy Rev. 2015, 51, 231–248. [Google Scholar] [CrossRef]
- Zahedi, A. Maximizing solar PV energy penetration using energy storage technology. Renew. Sustain. Energy Rev. 2011, 15, 866–870. [Google Scholar] [CrossRef]
- Ulleberg, Ø. Modeling of advanced alkaline electrolyzers: A system simulation approach. Int. J. Hydrog. Energy 2003, 28, 21–33. [Google Scholar] [CrossRef]
- Kiaee, M.; Cruden, A.; Chladek, P.; Infield, D. Demonstration of the operation and performance of a pressurized alkaline electrolyzer operating in the hydrogen fueling station in Porsgrunn, Norway. Energy Convers. Manag. 2015, 94, 40–50. [Google Scholar] [CrossRef]
- Zhou, T.; Francois, B. Modeling and control design of hydrogen production process or an active hydrogen/wind hybrid power system. Int. J. Hydrog. Energy 2009, 34, 21–30. [Google Scholar] [CrossRef]
- Khalilnejad, A.; Riahy, G.H. A hybrid wind-PV system performance investigation for the purpose of maximum hydrogen production and storage using advanced alkaline electrolyzer. Energy Convers. Manag. 2014, 80, 398–406. [Google Scholar] [CrossRef]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- Torreglosa, J.P.; Garcia, P.; Fernández, L.M.; Jurado, F. Energy dispatching based on predictive controller of an off-grid wind turbine/photovoltaic/hydrogen/battery hybrid system. Renew. Energy 2015, 74, 326–336. [Google Scholar] [CrossRef]
- Brka, A.; Al-Abedli, Y.M.; Kothapalli, G. The interplay between renewables penetration, costing and emissions in the sizing of stand-alone hydrogen systems. Int. J. Hydrog. Energy 2015, 40, 125–135. [Google Scholar] [CrossRef]
- Zhang, H.C.; Su, S.; Lin, G.; Chen, J. Efficiency calculation and configuration design of a PEM electrolyzer system for hydrogen production. Int. J. Electrochem. Sci. 2012, 7, 4143–4157. [Google Scholar]
- Opu, M.S. Effect of operating parameters on performance of alkaline water electrolysis. Int. J. Therm. Environ. Eng. 2015, 9, 53–60. [Google Scholar]
- Stetson, N.T.; McWhorter, S.; Ahn, C.C. Introduction to hydrogen storage. Compend. Hydrog. Energy 2016, 3–25. [Google Scholar]
- Zhevago, N.K. Other methods for the physical storage of hydrogen. Compend. Hydrog. Energy 2016, 189–218. [Google Scholar]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Khalghani, M.R.; Khooban, M.H. A novel self-tuning control method based on regulated bi-objective emotional learning controller’s structure with TLBO algorithm to control DVR compensator. Appl. Soft Comput. 2014, 24, 912–922. [Google Scholar] [CrossRef]
- Khalghani, M.R.; Khooban, M.H.; Mahboubi-Moghaddam, E.; Vafamand, N.; Goodarzi, M. A self-tuning load frequency control strategy for microgrids: Human brain emotional learning. Int. J. Electr. Power Energy Syst. 2016, 75, 311–319. [Google Scholar] [CrossRef]
- Khalghani, M.R.; Shamsi-Nejad, M.A.; Farshad, M.; Khooban, M.H. Modifying power quality’s indices of load by presenting an adaptive method based on Hebb learning algorithm for controlling DVR. Automatika J. Control Meas. Electron. Comput. Commun. 2014, 55, 153–161. [Google Scholar] [CrossRef]
- Nazari-Shirkouhi, S.; Eivazy, H.; Ghodsi, R.; Rezaie, K.; Atashpaz-Gargari, E. Solving the integrated product mix-outsourcing problem by a novel meta-heuristic algorithm: Imperialist competitive algorithm. Expert Syst. Appl. 2010, 37, 7615–7626. [Google Scholar] [CrossRef]
- Wikimedia Commons. Available online: https://commons.wikimedia.org/wiki/File:Imperialist-competitive-algorithm-flowchart.jpg (accessed on 10 May 2009).
- Yousefi, M.; Darus, A.N.; Mohammadi, H. Second law based optimization of a plate fin heat exchanger using imperialist competitive algorithm. Int. J. Phys. Sci. 2011, 6, 4749–4759. [Google Scholar]
- Hadidi, A.; Hadidi, M.; Nazari, A. A new design approach for shell-and-tube heat exchangers using imperialist competitive algorithm (ICA) from economic point of view. Energy Convers. Manag. 2013, 67, 66–74. [Google Scholar] [CrossRef]
- Kaveh, A.; Talatahari, S. Imperialist competitive algorithm for engineering design problems. Asian J. Civil Eng. Build. Hous. 2010, 11, 675–697. [Google Scholar]


| Parameter | PV Area | Voc | Isc | Vmpp | Impp | Rsh | Rs |
|---|---|---|---|---|---|---|---|
| Vlaue | 0.85 | 22.2 | 5.45 | 17.2 | 4.95 | 813 | 1.2 |
| Unit | m2 | V | A | V | A | Ω | Ω |
| Parameter | Urev | A | s | t1 | t2 | t3 | r1 | r2 |
|---|---|---|---|---|---|---|---|---|
| Value | 1.229 | 0.25 | 0.185 | 1.002 | 8.424 | 247.3 | 8.05 × 10−5 | −2.5 × 10−5 |
| Unit | V | m2 | V | A−1m2 | A−1m2°C | A−1m2°C2 | Ωm2 | Ωm2°C−1 |
| Parameter | f1 | f2 |
|---|---|---|
| Value | 200 | 0.985 |
| Unit | mA2·cm−4 | mA2 cm−4 |
| Step | ICA Generic Pseudocode | Description |
|---|---|---|
| 1 | Formulate the problem | by defining the objective function: f(x), where x = (x1, x2, …., xd) Produce a random solution in the search space and create an initial set of Empires |
| 2 | Assimilation is performed | wherein the colonies move towards the imperialist states in different in directions |
| 3 | Revolution follows where random changes are triggered | altering certain characteristics of some countries in the space |
| 4 | A colony gaining a position better than that of the imperialist has chance to control that Empire | by replacing the existing imperialist |
| 5 | All imperialist countries compete against each other | to possess one another‘s colonies |
| 6 | Empires deemed weak in these imperialist competitions lose their power gradually | finally it will be eliminated |
| 7 | If the exit condition is satisfied go to step 8, else go to step 3 | - |
| 8 | End | - |
| Step | Name | Description |
|---|---|---|
| 1 | Intialization | Select random points on the function and initialize them |
| 2 | Assimilation | Perform the assimilation process |
| 3 | Exchange | If there is a colony with lower cost than that of the imperialist, exchange positions |
| 4 | Annexation | Annex the weakest colony of the weakest empire to the empire that has the most likelihood to possess it |
| 5 | Elimination | Eliminate the powerless empires |
| 6 | Repeation | Repeat steps (a) through (e) until there is just one empire left |
| Parameter | PV Nominal Power | Area of PV Array | Hydrogen Production Rate | Average Faraday Efficiency |
|---|---|---|---|---|
| Value | 12.3 | 226 | 0.081 | 0.39 |
| Unit | kW | m2 | mol/s | - |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalilnejad, A.; Sundararajan, A.; Abbaspour, A.; Sarwat, A. Optimal Operation of Combined Photovoltaic Electrolyzer Systems. Energies 2016, 9, 332. https://doi.org/10.3390/en9050332
Khalilnejad A, Sundararajan A, Abbaspour A, Sarwat A. Optimal Operation of Combined Photovoltaic Electrolyzer Systems. Energies. 2016; 9(5):332. https://doi.org/10.3390/en9050332
Chicago/Turabian StyleKhalilnejad, Arash, Aditya Sundararajan, Alireza Abbaspour, and Arif Sarwat. 2016. "Optimal Operation of Combined Photovoltaic Electrolyzer Systems" Energies 9, no. 5: 332. https://doi.org/10.3390/en9050332






