Evaluation of Cell Disruption of Chlorella Vulgaris by Pressure-Assisted Ozonation and Ultrasonication
Abstract
:1. Introduction
2. Results
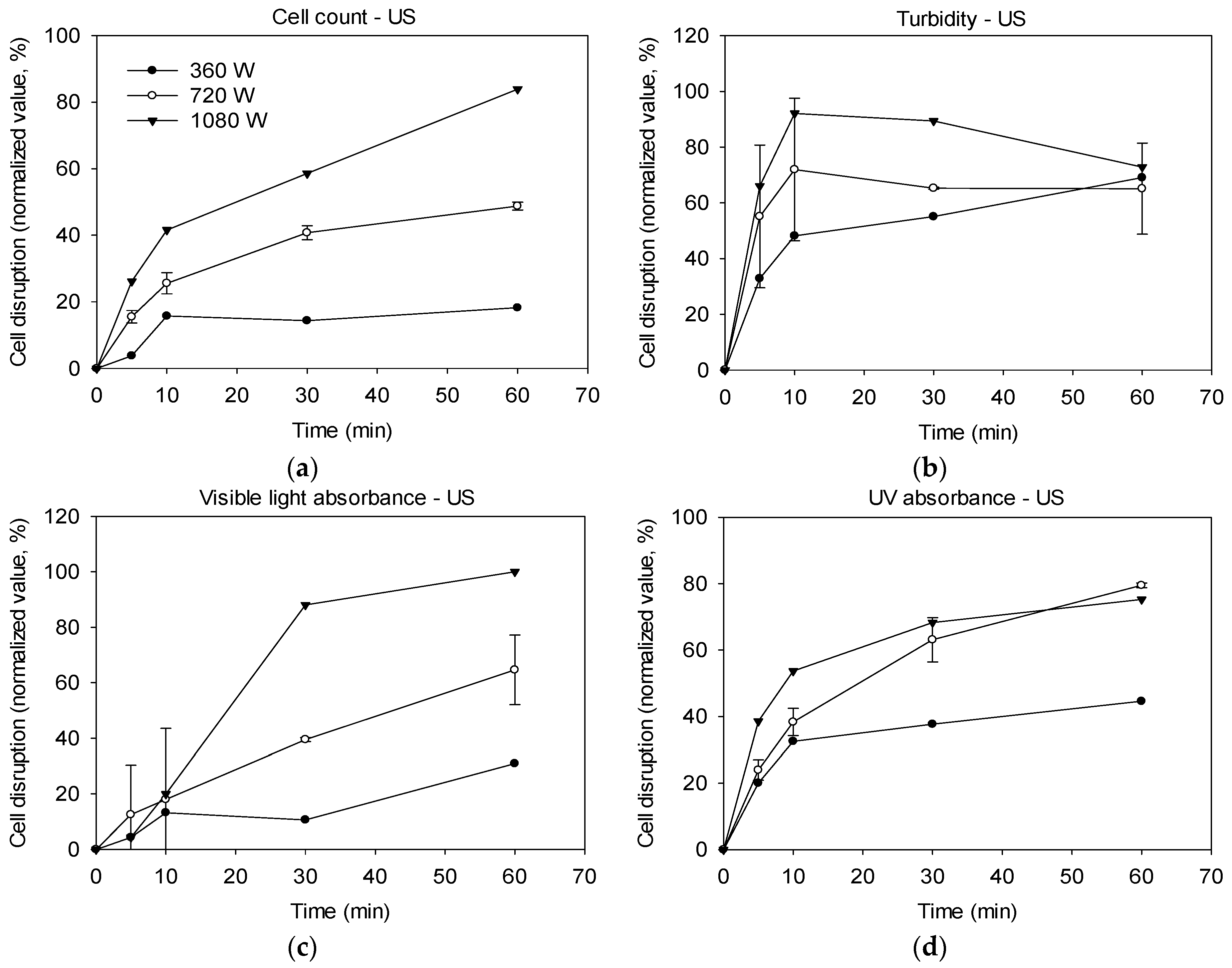
2.1. Cell Disruption by Ultrasonication
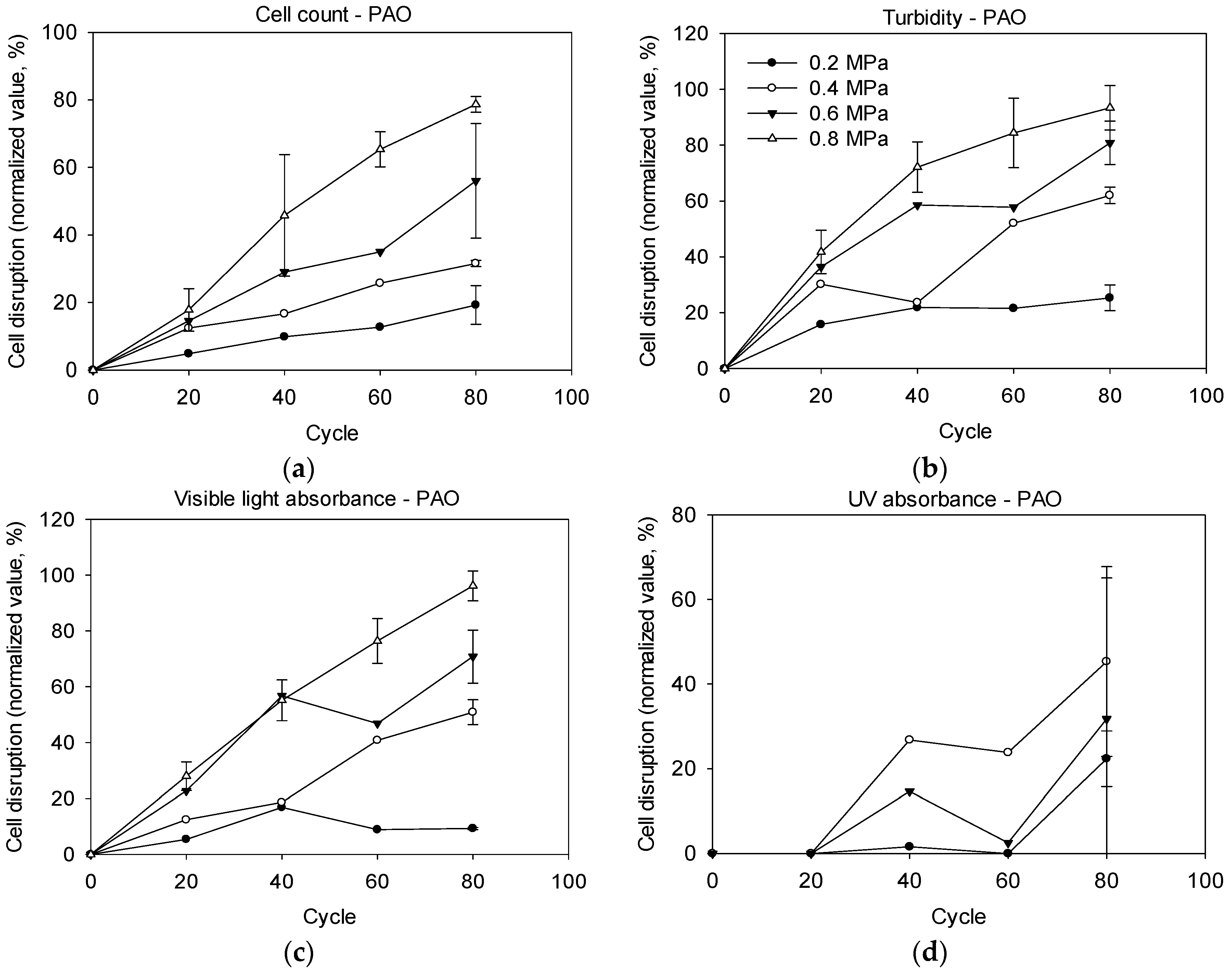
2.2. Cell Disruption by Pressure-Assisted Ozonation
2.3. Scanning Electron Microscope Observation
3. Discussion
3.1. Effectiveness of Ultrasound
3.2. Effectiveness of Pressure-Assisted Ozonation
3.3. Comparation of Different Works
4. Materials and Methods
4.1. Microalga Sample Preparation
4.2. Cell Disruption
4.3. Quantitative Evaluation of Cell Disruption
4.4. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| US | Ultrasonication |
| PAO | Pressure-assisted ozonation |
| UV | Ultra violet |
| SEM | Scanning electron microscope |
References
- Sun, J.; Sun, B.; Wang, S. The status and prospect of vehicle alternative fuel. Energy Res. Inf. 2014, 30, 125–128. [Google Scholar]
- Jiang, L.; Yu, H. Research status on gasification and pyrolysis characteristics of biomass components. Energy Res. Inf. 2015, 31, 9–13. [Google Scholar]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Rasoul-Amini, S.; Ghoshoon, M.B.; Ghasemi, Y. Chlorella vulgaris, a novel microalgal source for l-asparaginase production. Biocatal. Agric. Biotechnol. 2014, 3, 214–217. [Google Scholar] [CrossRef]
- Frumento, D.; Casazza, A.A.; Al Arni, S.; Converti, A. Cultivation of Chlorella vulgaris in tubular photobioreactors: A lipid source for biodiesel production. Biochem. Eng. J. 2013, 81, 120–125. [Google Scholar] [CrossRef]
- Kitada, K.; Machmudah, S.; Sasaki, M.; Goto, M.; Nakashima, Y.; Kumamoto, S.; Hasegawa, T. Supercritical CO2 extraction of pigment components with pharmaceutical importance from Chlorella vulgaris. J. Chem. Technol. Biotechnol. 2009, 84, 657–661. [Google Scholar] [CrossRef]
- Přibyl, P.; Cepák, V.; Zachleder, V. Production of lipids and formation and mobilization of lipid bodies in Chlorella vulgaris. J. Appl. Phycol. 2013, 25, 545–553. [Google Scholar] [CrossRef]
- Günerken, E.; D'Hondt, E.; Eppink, M.H.M.; Garcia-Gonzalez, L.; Elst, K.; Wijffels, R.H. Cell disruption for microalgae biorefineries. Biotechnol. Adv. 2015, 33, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Yoo, C.; Jun, S.Y.; Ahn, C.Y.; Oh, H.M. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Postma, P.R.; Miron, T.L.; Olivieri, G.; Barbosa, M.J.; Wijffels, R.H.; Eppink, M.H.M. Mild disintegration of the green microalgae Chlorella vulgaris using bead milling. Bioresour. Technol. 2015, 184, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, N.; Fernando, S.; Lacey, R.; Faulkner, W.B. Algal cell rupture using high pressure homogenization as a prelude to oil extraction. Renew. Energy 2012, 48, 300–308. [Google Scholar] [CrossRef]
- Yap, B.H.J.; Dumsday, G.J.; Scales, P.J.; Martin, G.J.O. Energy evaluation of algal cell disruption by high pressure homogenisation. Bioresour. Technol. 2015, 184, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Middelberg, A.P.J. Process-scale disruption of microorganisms. Biotechnol. Adv. 1995, 13, 491–551. [Google Scholar] [CrossRef]
- Spiden, E.M.; Scales, P.J.; Kentish, S.E.; Martin, G.J.O. Critical analysis of quantitative indicators of cell disruption applied to Saccharomyces cerevisiae processed with an industrial high pressure homogenizer. Biochem. Eng. J. 2013, 70, 120–126. [Google Scholar] [CrossRef]
- Hong, P.K.A.; Nakra, S.; Jimmy Kao, C.M.; Hayes, D.F. Pressure-assisted ozonation of PCB and PAH contaminated sediments. Chemosphere 2008, 72, 1757–1764. [Google Scholar] [CrossRef] [PubMed]
- Cha, Z.; Lin, C.F.; Cheng, C.J.; Hong, P.K.A. Removal of oil and oil sheen from produced water by pressure-assisted ozonation and sand filtration. Chemosphere 2010, 78, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Andy Hong, P.K.; Lin, C.F. Improved solubilization of activated sludge by ozonation in pressure cycles. Chemosphere 2012, 87, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.J.; Hong, P.K.A. Anaerobic digestion of activated sludge after pressure-assisted ozonation. Bioresour. Technol. 2013, 142, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hong, P.K.A.; Zhang, D.; Li, L. Comparison of cell rupturing by ozonation and ultrasonication for algal lipid extraction from Chlorella vulgaris. Environ. Technol. 2014, 35, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Bystryak, S.; Santockyte, R.; Peshkovsky, A.S. Cell disruption of S. cerevisiae by scalable high-intensity ultrasound. Biochem. Eng. J. 2015, 99, 99–106. [Google Scholar] [CrossRef]
- Cheung, Y.C.; Liu, X.X.; Wang, W.Q.; Wu, J.Y. Ultrasonic disruption of fungal mycelia for efficient recovery of polysaccharide-protein complexes from viscous fermentation broth of a medicinal fungus. Ultrason. Sonochemistry 2015, 22, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Monks, L.M.; Rigo, A.; Mazutti, M.A.; Vladimir Oliveira, J.; Valduga, E. Use of chemical, enzymatic and ultrasound-assisted methods for cell disruption to obtain carotenoids. Biocatal. Agric. Biotechnol. 2013, 2, 165–169. [Google Scholar] [CrossRef]
- Yusaf, T.; Al-Juboori, R.A. Alternative methods of microorganism disruption for agricultural applications. Appl. Energy 2014, 114, 909–923. [Google Scholar] [CrossRef]
- Wang, M.; Yuan, W.; Jiang, X.; Jing, Y.; Wang, Z. Disruption of microalgal cells using high-frequency focused ultrasound. Bioresour. Technol. 2014, 153, 315–321. [Google Scholar] [CrossRef] [PubMed]
- Show, K.Y.; Lee, D.J.; Tay, J.H.; Lee, T.M.; Chang, J.S. Microalgal drying and cell disruption—Recent advances. Bioresour. Technol. 2015, 184, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Pingret, D.; Fabiano-Tixier, A.-S.; Chemat, F. Degradation during application of ultrasound in food processing: A review. Food Control 2013, 31, 593–606. [Google Scholar] [CrossRef]
- Chisti, Y.; Moo-Young, M. Disruption of microbial cells for intracellular products. Enzym. Microbial. Technol. 1986, 8, 194–204. [Google Scholar] [CrossRef]
- Liu, D.; Zeng, X.-A.; Sun, D.-W.; Han, Z. Disruption and protein release by ultrasonication of yeast cells. Innov. Food Sci. Emerg. Technol. 2013, 18, 132–137. [Google Scholar] [CrossRef]
- Wileman, A.; Ozkan, A.; Berberoglu, H. Rheological properties of algae slurries for minimizing harvesting energy requirements in biofuel production. Bioresour. Technol. 2012, 104, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Gerde, J.A.; Montalbo-Lomboy, M.; Yao, L.; Grewell, D.; Wang, T. Evaluation of microalgae cell disruption by ultrasonic treatment. Bioresour. Technol. 2012, 125, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Matile, P.; Hortensteiner, S.; Thomas, H. Chlorophyll degradation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 67–95. [Google Scholar] [CrossRef] [PubMed]
- Azencott, H.R.; Peter, G.F.; Prausnitz, M.R. Influence of the Cell Wall on Intracellular Delivery to Algal Cells by Electroporation and Sonication. Ultrasound Med. Biol. 2007, 33, 1805–1817. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Tao, W. The mechanisms of ozonation on cyanobacteria and its toxins removal. Sep. Purif. Technol. 2009, 66, 187–193. [Google Scholar] [CrossRef]
- Cheng, Y.-L.; Juang, Y.-C.; Liao, G.-Y.; Ho, S.-H.; Yeh, K.-L.; Chen, C.-Y.; Chang, J.-S.; Liu, J.-C.; Lee, D.-J. Dispersed ozone flotation of Chlorella vulgaris. Bioresour. Technol. 2010, 101, 9092–9096. [Google Scholar] [CrossRef] [PubMed]
- Blanca Roncero, M.; Colom, J.F.; Vidal, T. Why oxalic acid protects cellulose during ozone treatments? Carbohydr. Polym. 2003, 52, 411–422. [Google Scholar] [CrossRef]
- Doucha, J.; Lívanský, K. Influence of processing parameters on disintegration of Chlorella cells in various types of homogenizers. Appl. Microbiol. Biotechnol. 2008, 81, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Yin, J.; Gao, Z.; Huang, H.; Ji, X.; Dou, C. Disruption of Chlorella vulgaris Cells for the Release of Biodiesel-Producing Lipids: A Comparison of Grinding, Ultrasonication, Bead Milling, Enzymatic Lysis, and Microwaves. Appl. Biochem. Biotechnol. 2011, 164, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.C.; Hung, T.C.; Chen, J.Y.; Su, C.H.; Wu, W.T. Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresour. Technol. 2010, 101, 8750–8754. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, L.; Song, C. Effect of nitrogen and phosphorus levels on the lipid production of Chlorella vulgaris and evaluation of ultrasound-assisted lipid extraction. Fresenius Environ. Bull. 2013, 22, 2848–2854. [Google Scholar]
- Indigo Colorimetric Method, Standard Methods, 21st ed.; 4500-O3 B; American Public Health Association: Washington, DC, USA, 2005.


| Treatment | Time (min) | Energy Dissipated (kJ·mL−1) | Disruption Rate (%) | Treatment Condition |
|---|---|---|---|---|
| PAO | 40 | 8.16 | 80.3 | 0.8 MPa, 80 cycle, power of 1020 W |
| US | 60 | 19.44 | 83.8 | Power of 1080 W |
| Treatment * | Disruption rate (%) ** | Operation Condition | |||
|---|---|---|---|---|---|
| Cell Count | Turbidity | Visible Light Absorbance | UV Absorbance | ||
| CBO | 34.6 | 25.6 | 60.6 | 0.0 | Bubbling ozonation for 40 min under ambient pressure |
| PAO | 80.3 | 87.7 | 92.4 | 7.6 | 0.8 MPa, 80 cycle |
| Method | Conditions | Outcome | References |
|---|---|---|---|
| Bead milling | Glass beads 0.25–0.5 mm | 85% cell disintegration | [36] |
| Algae dry weight 158 g·L−1 | |||
| Beads filling 70% | |||
| Feed rate 62 kg·h−1 | |||
| Time 60 min | |||
| Bead milling | ZrO2 beads 1 mm | 97% cell disintegration | [10] |
| Beads filling 65% | |||
| Biomass concentration 87.5 g·kg−1 | |||
| Agitator speed 9 m·s−1 | |||
| Time 10 min | |||
| Microwave treatment | 2450 MHz, | 17% (w/w) lipid yield | [37] |
| 100 °C | |||
| Biomass concentration 5 g·L−1 | |||
| Time 25 min | |||
| Autoclaving | 125 °C | 10% (w/w) lipid yield | [9] |
| 1.5 MPa | |||
| Biomass concentration 5 g·L−1 | |||
| Time 5 min | |||
| Enzymatic lysis | Cellulase 5 mg·L−1 | 24% (w/w) lipid yield | [37] |
| 55 °C | |||
| pH 4.8 | |||
| Biomass concentration 5 g·L−1 | |||
| Time 600 min | |||
| Enzymatic lysis | Immobilized cellulose 140 mg/m2 | 60% cell wall hydrolysis | [38] |
| 50 °C | |||
| pH 4.6 | |||
| Biomass concentration 20 g·L−1 | |||
| Time 50 h | |||
| PAO | Aqueous ozone concentrations 2.4 mg·L−1 | 80.3% cell disruption; 24% (w/w) lipid yield | This work and [19] |
| 0.8 MPa | |||
| 80 cycles | |||
| Time 40 min |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Qin, S.; Zhang, D.; Li, L.; Mu, Y. Evaluation of Cell Disruption of Chlorella Vulgaris by Pressure-Assisted Ozonation and Ultrasonication. Energies 2016, 9, 173. https://doi.org/10.3390/en9030173
Huang Y, Qin S, Zhang D, Li L, Mu Y. Evaluation of Cell Disruption of Chlorella Vulgaris by Pressure-Assisted Ozonation and Ultrasonication. Energies. 2016; 9(3):173. https://doi.org/10.3390/en9030173
Chicago/Turabian StyleHuang, Yuanxing, Shengnan Qin, Daofang Zhang, Liang Li, and Yan Mu. 2016. "Evaluation of Cell Disruption of Chlorella Vulgaris by Pressure-Assisted Ozonation and Ultrasonication" Energies 9, no. 3: 173. https://doi.org/10.3390/en9030173






