Optimization of Electrochemically Deposited Highly Doped ZnO Bilayers on Ga-Rich Chalcopyrite Selenide for Cost-Effective Photovoltaic Device Technology
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
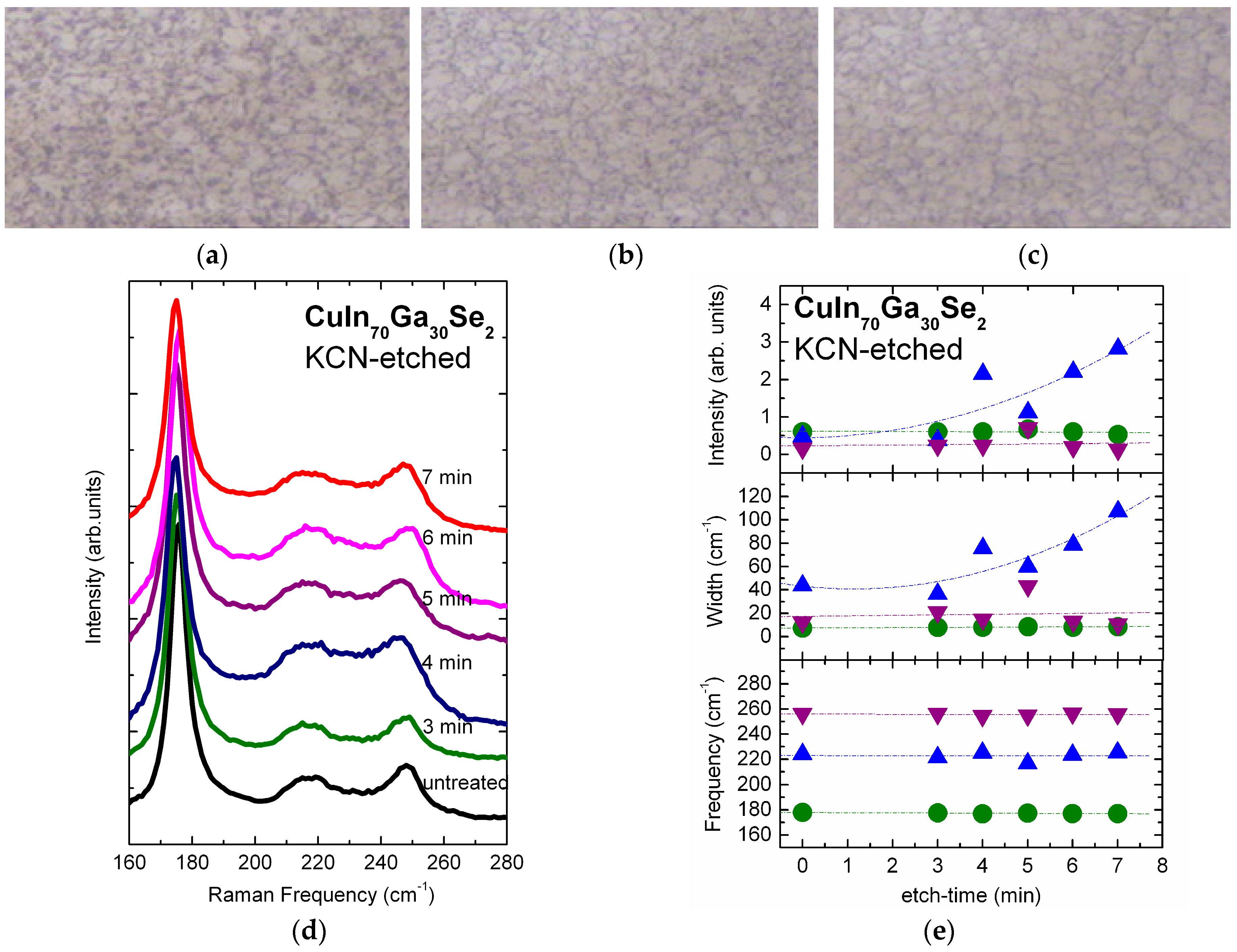
3.1. KCN-Etching of Cu(In,Ga)Se2 Surface
3.2. Structural Characterization of Al:ZnO on ZnSe/Cu(In,Ga)Se2/Mo/glass by XRD and SEM—Strain/Stress Analysis
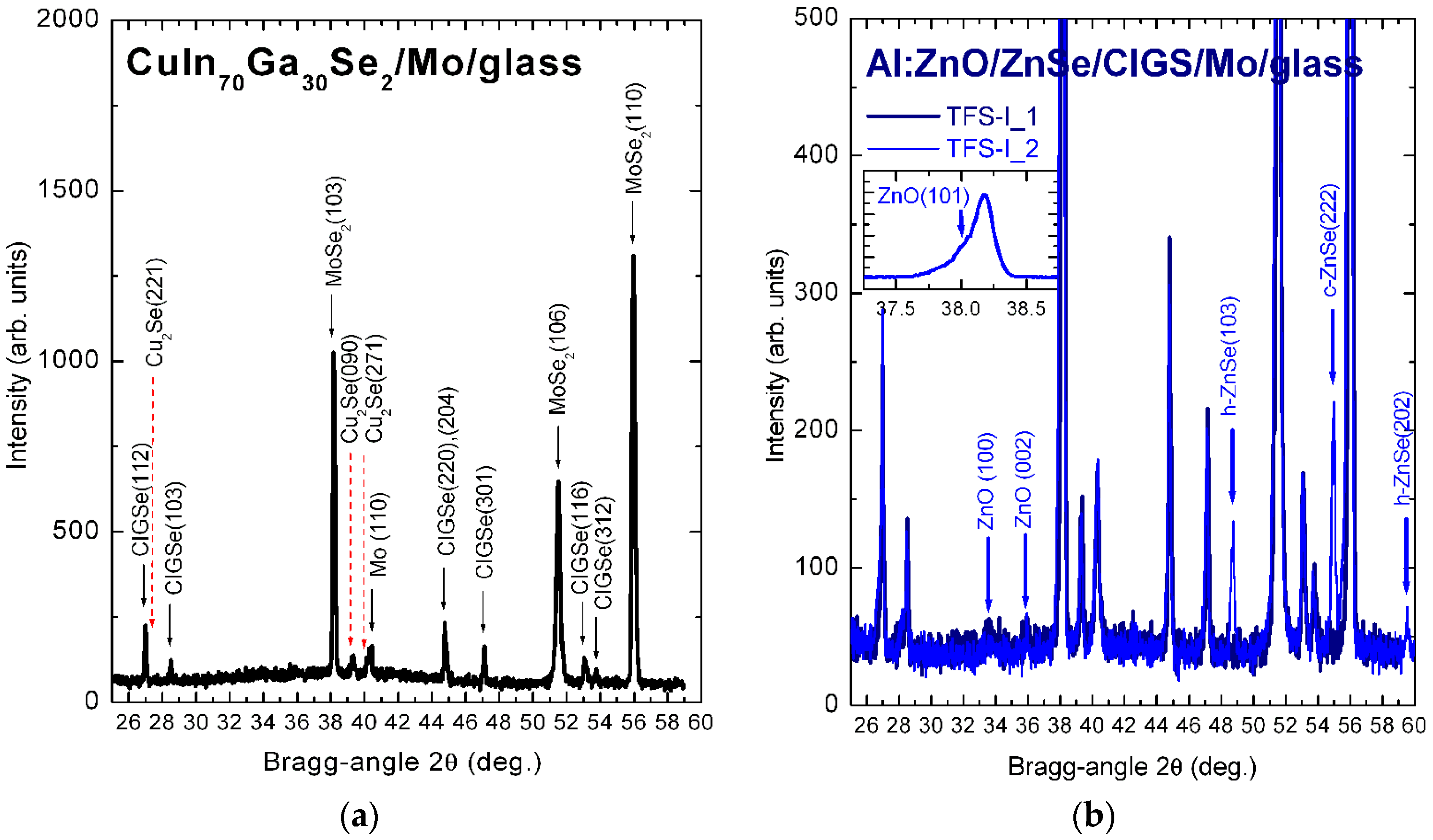
3.2.1. X-ray Diffractograms of CuIn70Ga30Se2
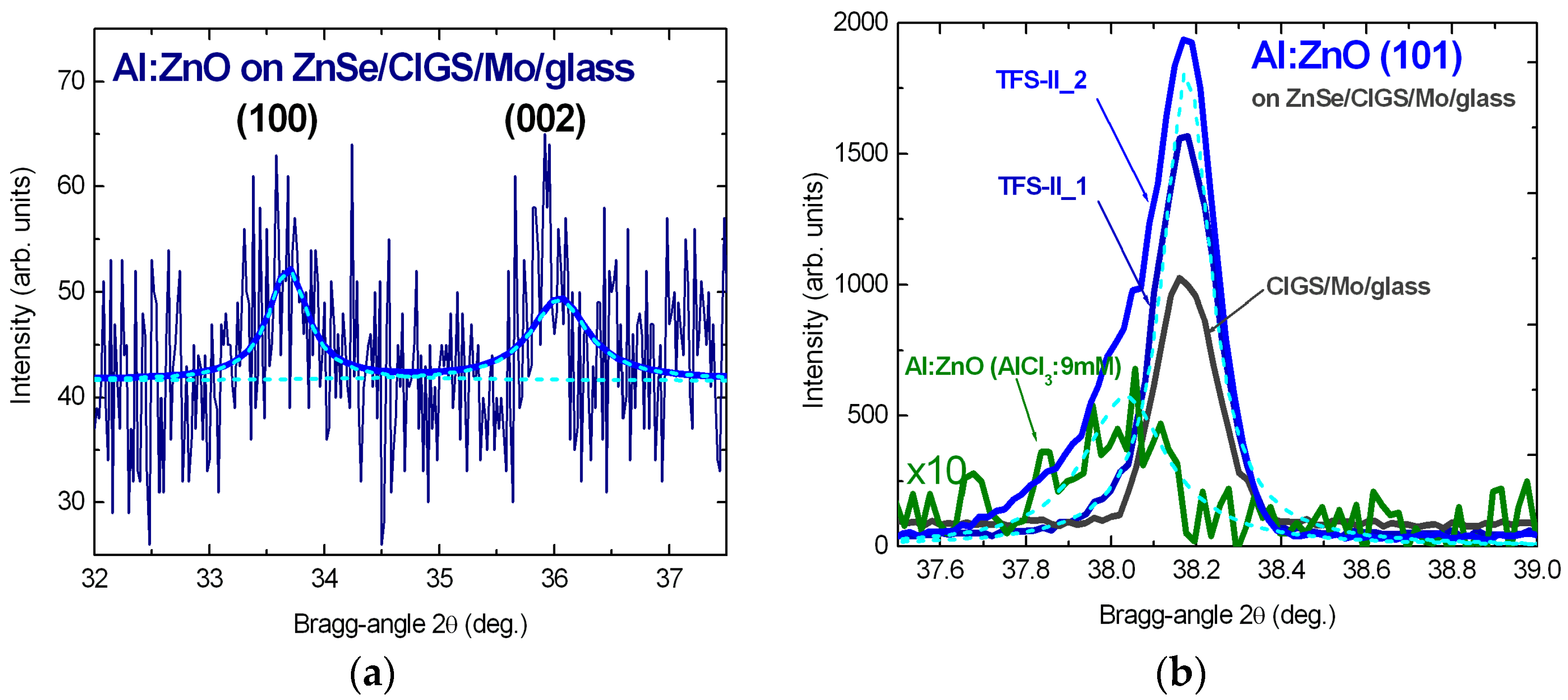
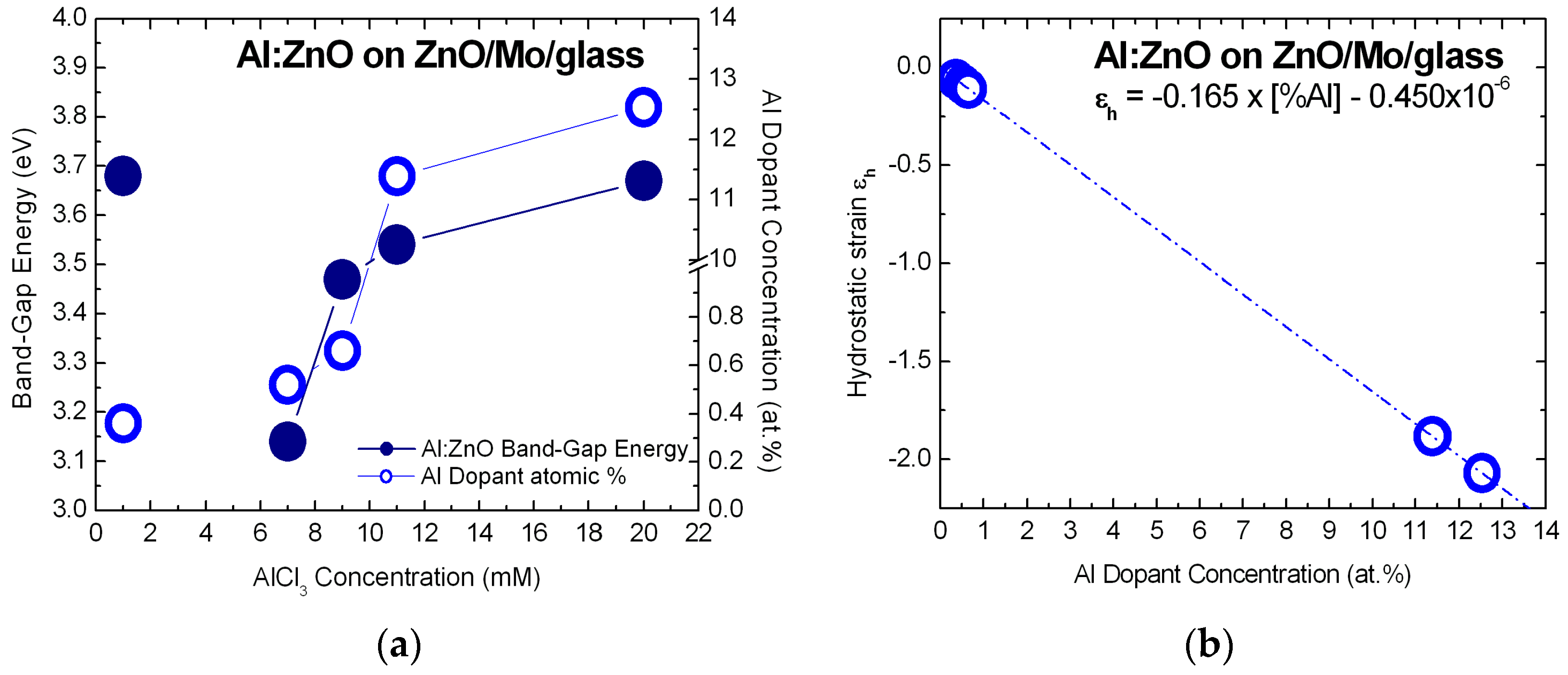
3.2.2. X-ray Diffractograms of ZnSe and Al:ZnO—Strain/Stress Analysis
- (a1)
- biaxial in-plane stress, in Equations (A12) and (A13), ,
- (a2)
- uniform pressure, in Equations (19) and (20), ,
- (b1)
- effective strain εa,eff, εc,eff, in Equations (13) and (14), ,
- (b2)
- effective hydrostatic strain εh(aepi,eff), εh(cepi,eff), in Equation (12), ,
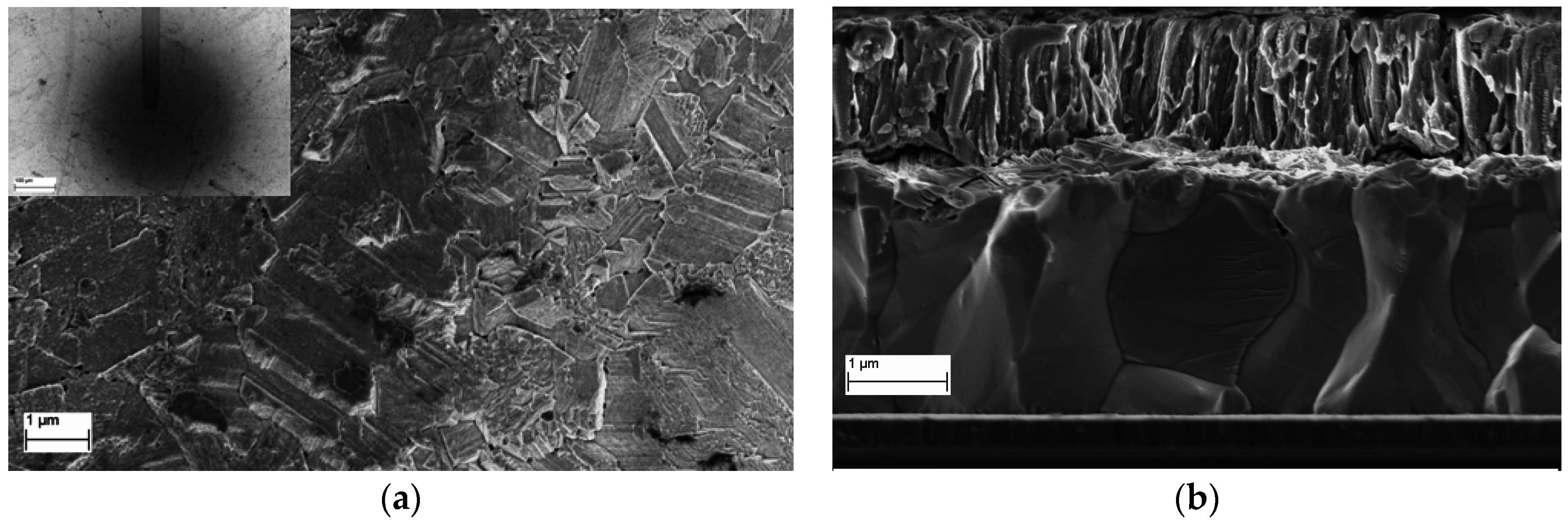
3.2.3. SEM Images of Al:ZnO
3.3. Optical Characterization of Al:ZnO on ZnSe/Cu(In,Ga)Se2/Mo/glass by NUV-VIS-NIR Spectrophotometry
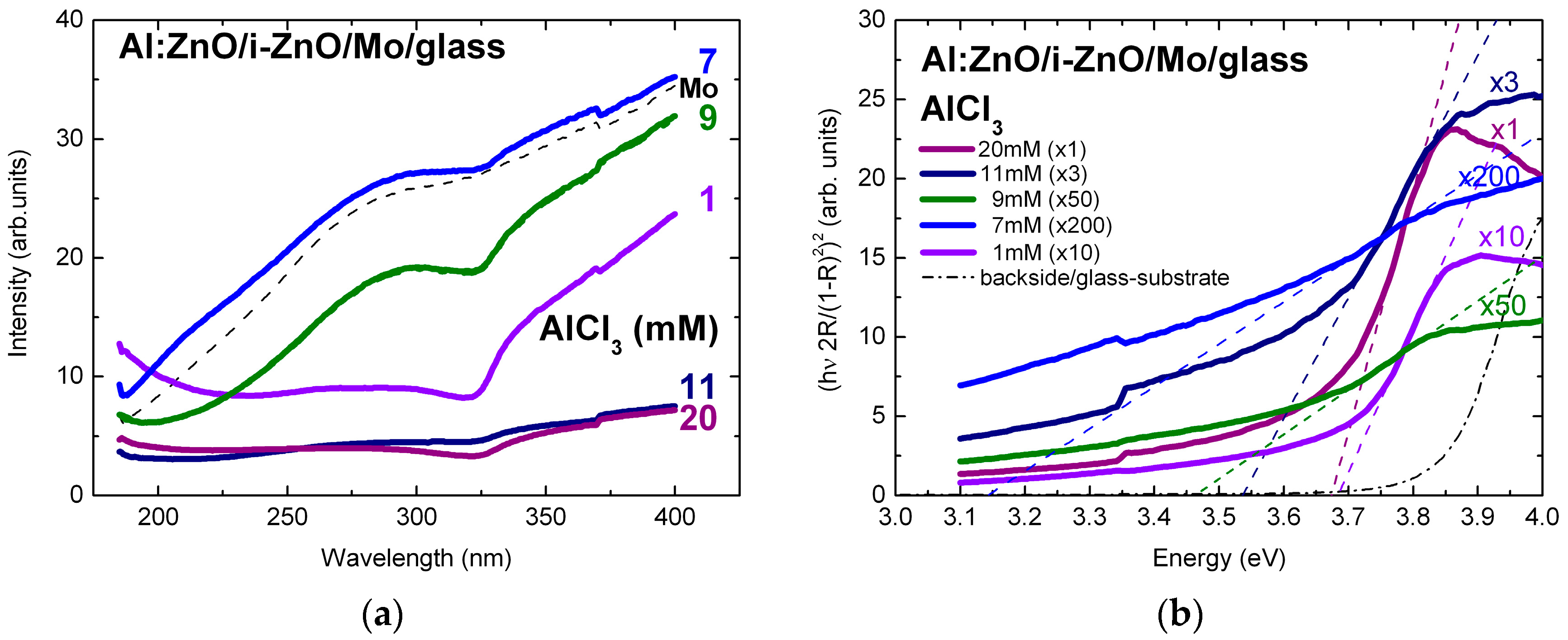
3.3.1. Optical Characterization of Al:ZnO/ZnO Reference Samples on Mo/glass
3.3.2. Optical Characterization of Al:ZnO on ZnSe/Cu(In,Ga)Se2/Mo/glass Solar Structures
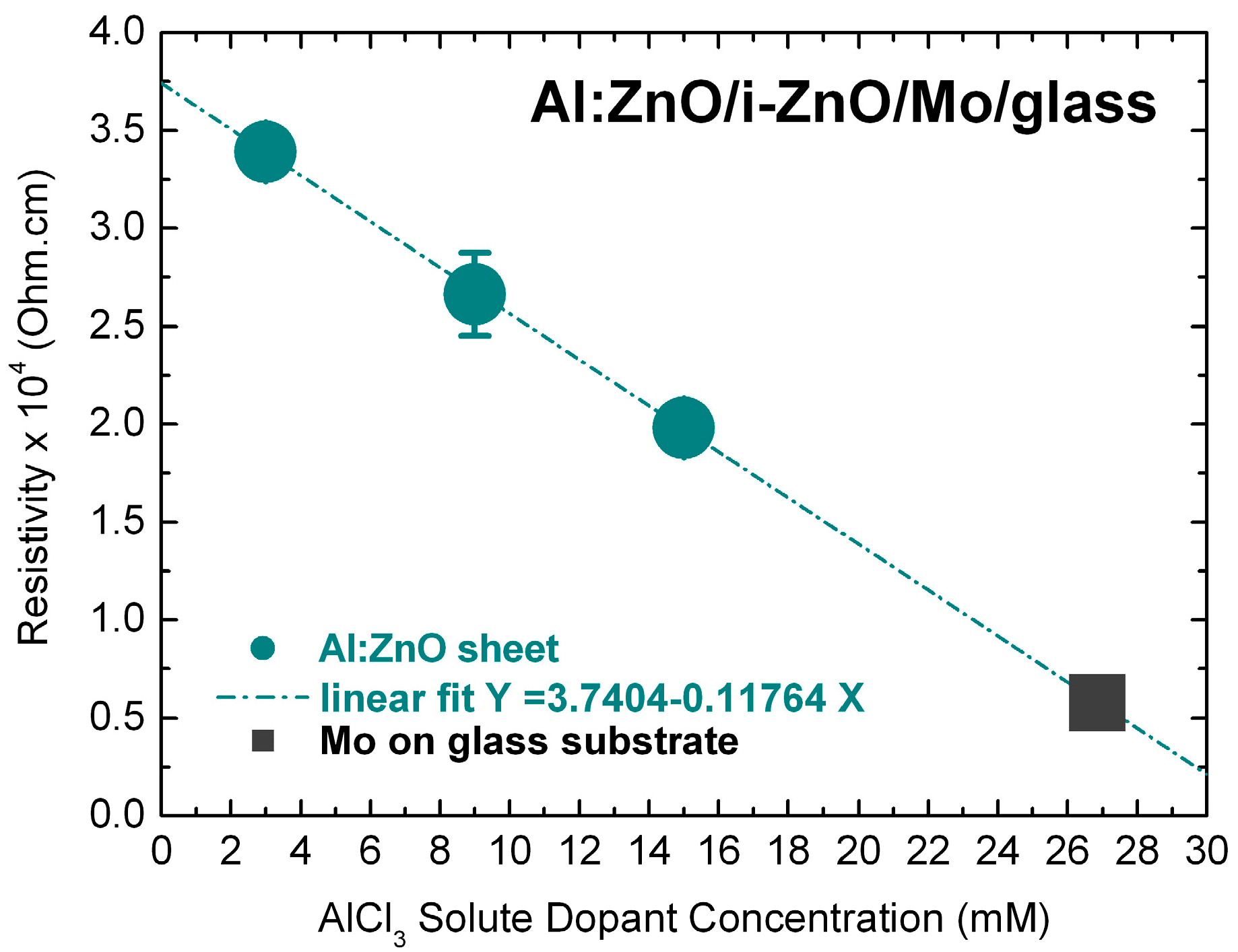
3.4. Electrical Characterization of Al:ZnO on ZnSe/Cu(In,Ga)Se2/Mo/glass by van-der-Pauw and I-V Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Strain/Stress Formalism
Appendix B
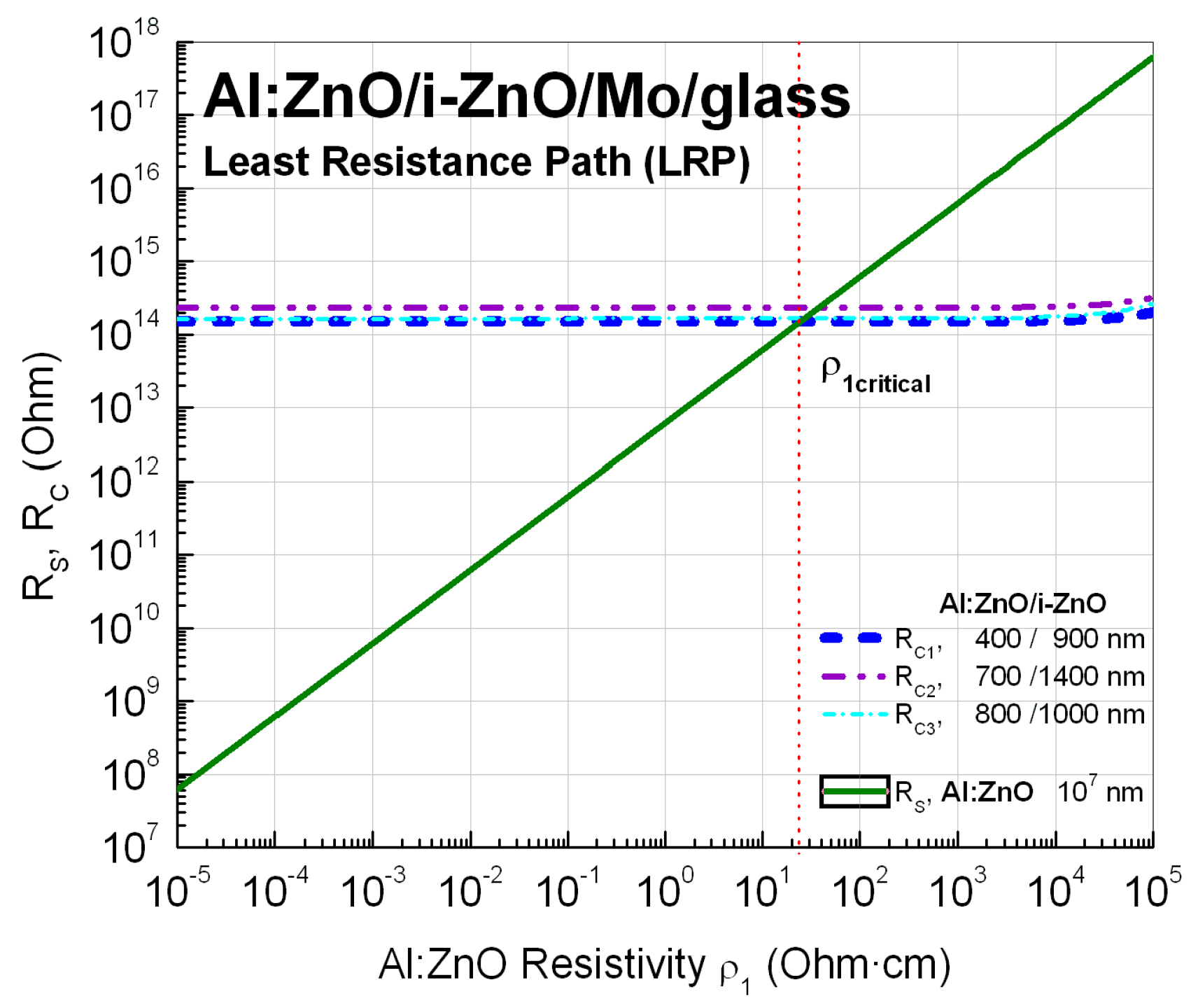
Appendix B.1. Sheet Resistance Measurements/Bulk Resistivity Calculations
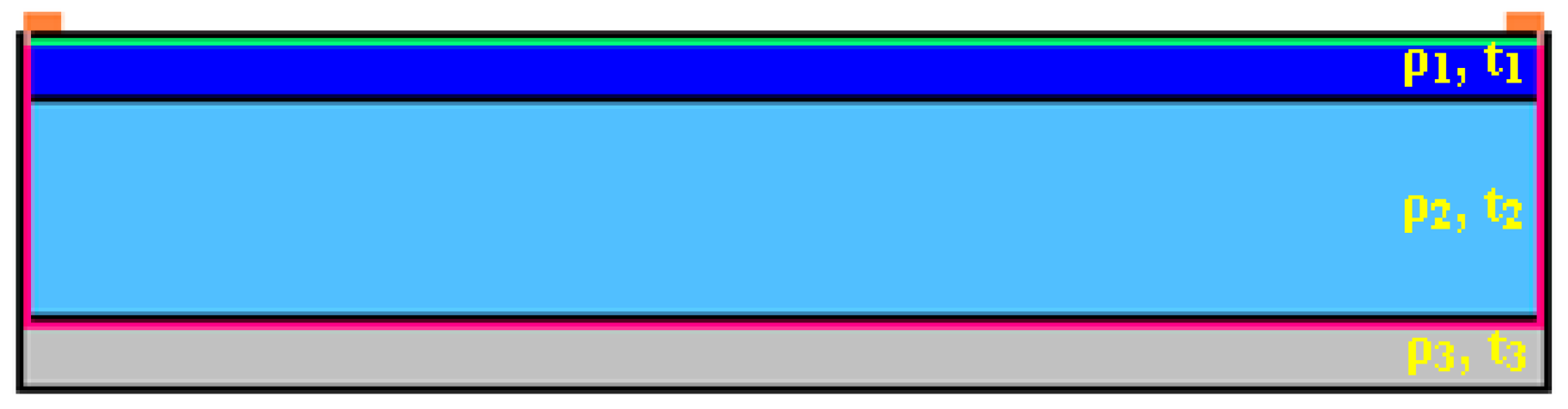
| Layer | C1 | C2 | C3 | Resistivity |
|---|---|---|---|---|
| Al:ZnO | t1 = 400 nm | 700 nm | 800 nm | ρ1: variable |
| i-ZnO | t2 = 900 nm | 1400 nm | 1000 nm | ρ2 = 1.33 × 105 Ω·cm |
| Mo | t3 = 400 nm | 400 nm | 400 nm | ρ3 = 0.58 × 10−4 Ω·cm |
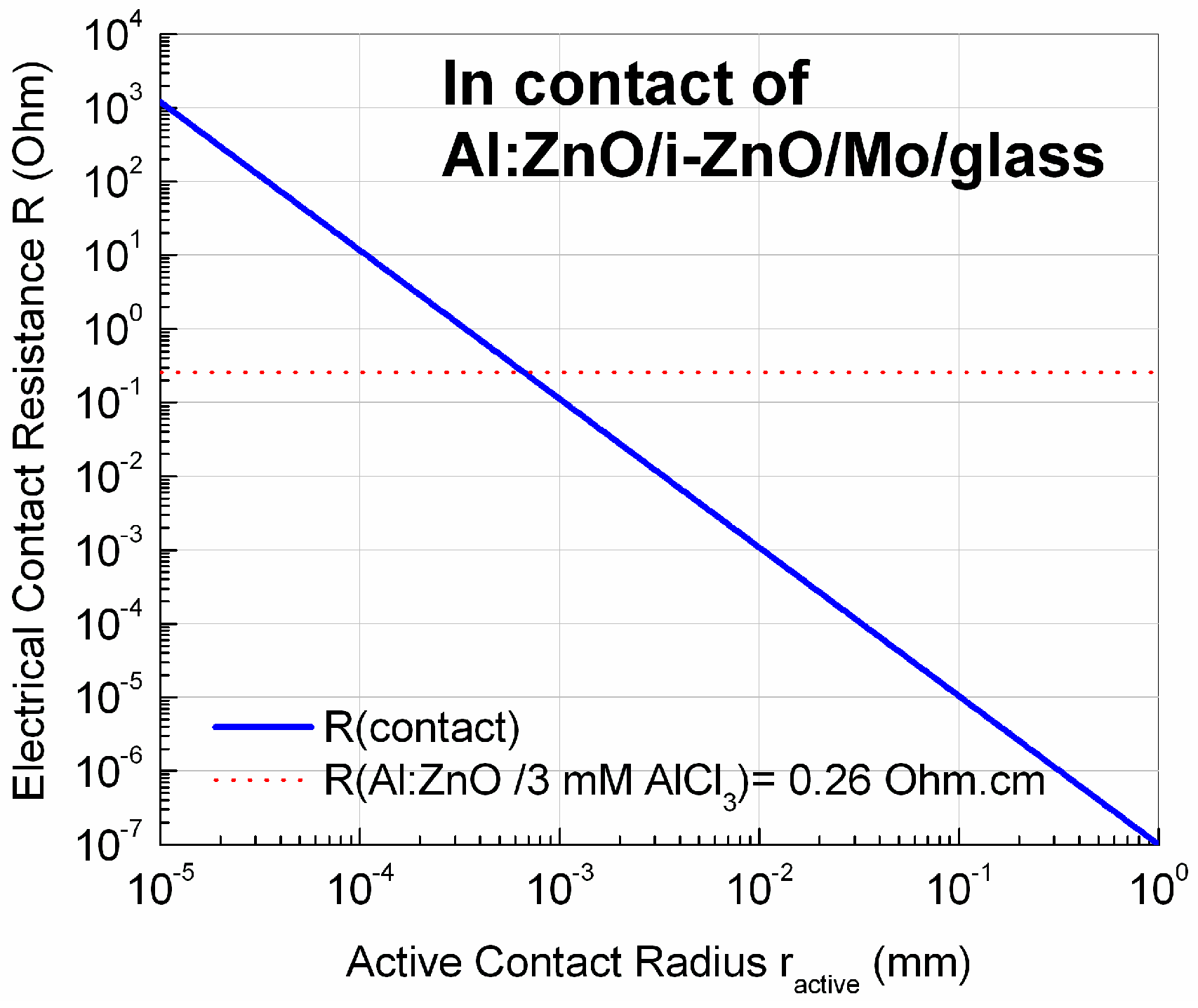
Appendix B.2. Electrical Contact Resistance (ECR)
References
- Rau, U.; Schock, H.W. Cu(In,Ga)Se2 solar cells. In Clean Energy from Photovoltaics; Archer, D., Hill, R., Eds.; Imperial College Press: London, UK, 2001; pp. 277–345. [Google Scholar]
- Ramanathan, K.; Contreras, M.A.; Perkins, C.L.; Asher, S.; Hasoon, F.S.; Keane, J.; Young, D.; Romero, M.; Metzg, W.; Noufi, R.; et al. Properties of 19.2% Efficiency ZnO/CdS/CuInGaSe2 Thin-film Solar Cells. Prog. Photovolt. Res. Appl. 2003, 11, 225–230. [Google Scholar] [CrossRef]
- Repins, I.; Contreras, M.A.; Egaas, B.; DeHart, C.; Scharf, J.; Perkins, C.L.; To, B.; Noufi, R. 19.9%-efficient ZnO/CdS/CuInGaSe2 Solar Cell with 81.2% Fill Factor. Prog. Photovolt. Res. Appl. 2008, 16, 235–239. [Google Scholar] [CrossRef]
- Jackson, P.; Hariskos, D.; Lotter, E.; Paetel, S.; Wuerz, R.; Menner, R.; Wischmann, W.; Powalla, M. New world record efficiency for Cu(In,Ga)Se2 thin-film solar cells beyond 20%. Prog. Photovolt. Res. Appl. 2011. [Google Scholar] [CrossRef]
- Savadogo, O. Chemically and electrochemically deposited thin films for solar energy materials. Sol. Energy Mater. Sol. Cells 1998, 52, 361–388. [Google Scholar] [CrossRef]
- Lincot, D. Electrodeposition of semiconductors. Thin Solid Films 2005, 487, 40–48. [Google Scholar] [CrossRef]
- Fauchais, P. Understanding plasma spraying. J. Phys. D Appl. Phys. 2004, 37, R86–R108. [Google Scholar] [CrossRef]
- Yoshida, T. Toward a new era of plasma spray processing. Pure Appl. Chem. 2006, 78, 1093–1107. [Google Scholar] [CrossRef]
- Azimi, H.; Hou, Y.; Brabec, C.J. Towards low-cost, environmentally friendly printed chalcopyrite and kesterite solar cells. Energy Environ. Sci. 2014, 7, 1829–1849. [Google Scholar] [CrossRef]
- Xin, H.; Katahara, J.K.; Braly, I.L.; Hillhouse, H.W. 8% Efficient Cu2ZnSn(S,Se)4 Solar Cells from Redox Equilibrated Simple Precursors in DMSO. Adv. Energy Mater. 2014, 4, 1301823. [Google Scholar] [CrossRef]
- Werner, M.; Sutter-Fella, C.M.; Romanyuk, Y.E.; Tiwari, A.N. 8.3% efficient Cu2ZnSn(S,Se)4 solar cells processed from sodium-containing solution precursors in a closed reactor. Thin Solid Films 2015, 582, 308–312. [Google Scholar] [CrossRef]
- Lin, X.; Kavalakkatt, J.; Ennaoui, A.; Lux-Steiner, M.-C. Cu2ZnSn(S,Se)4 thin film absorbers based on ZnS, SnS and Cu3SnS4 nanoparticle inks: Enhanced solar cells performance by using a two-step annealing process. Sol. Energy Mater. Sol. Cells 2015, 132, 221–229. [Google Scholar] [CrossRef]
- Klenk, R. Characterisation and modelling of chalcopyrite solar cells. Thin Solid Films 2001, 387, 135–140. [Google Scholar] [CrossRef]
- Ellmer, K.; Klein, A. ZnO and Its Applications. In Transparent Conductive Zinc Oxide: Basics and Applications in Thin Film Solar Cells; Ellmer, K., Klein, A., Rech, B., Eds.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–33. [Google Scholar]
- Hariskos, D.; Spiering, S.; Powalla, M. Buffer layers in Cu(In,Ga)Se2 solar cells and modules. Thin Solid Films 2005, 480–481, 99–109. [Google Scholar] [CrossRef]
- Fischer, C.; Allsop, N.A.; Gledhill, S.E.; Köhler, T.; Krüger, M.; Sáez-Araoz, R.; Fu, Y.; Schwieger, R.; Richter, J.; Wohlfart, P.; et al. The spray-ILGARs (ion layer gas reaction) method for the deposition of thin semiconductor layers: Process and applications for thin film solar cells. Sol. Energy Mater. Sol. Cells 2011, 95, 1518–1526. [Google Scholar] [CrossRef]
- Sáez-Araoz, R.; Krammer, J.; Harndt, S.; Koehler, T.; Krueger, M.; Pistor, P.; Jasenek, A.; Hergert, F.; Lux-Steiner, M.-C.; Fischer, C.-H. ILGAR In2S3 buffer layers for Cd-free Cu(In,Ga)(S,Se)2 solar cells with certified efficiencies above 16%. Prog. Photovolt. Res. Appl. 2012, 20, 855–861. [Google Scholar] [CrossRef]
- Wang, L.; Lin, X.; Ennaoui, A.; Wolf, C.; Lux-Steiner, M.-C.; Klenk, R. Solution-processed In2S3 buffer layer for chalcopyrite thin film solar cells. EPJ Photovolt. 2016, 7, 70303. [Google Scholar] [CrossRef]
- Papadimitriou, D.; Roupakas, G.; Chatzitheodoridis, E.; Halampalakis, G.; Tselepis, S.; Sáez-Araoz, R.; Lux-Steiner, M.-C.; Nickel, N.H.; Alamé, S.; Vogt, P.; et al. Chemical and Electrochemical Processing of High Quality CIS/CIGS Absorber, Buffer, Window, and Anti-Reflective Coating for Low Cost Photovoltaic Technology. In Proceedings of the 29th European Photovoltaic Solar Energy Conference and Exhibition, PVSEC, Amsterdam, The Netherlands, 22–26 September 2014; pp. 1812–1815.
- Papadimitriou, D.; Roupakas, G.; Sáez-Araoz, R.; Lux-Steiner, M.-C.; Nickel, N.H.; Alamé, S.; Vogt, P.; Kneissl, M. Quality CuInSe2 and Cu(In,Ga)Se2 thin films processed by single step electrochemical deposition techniques. Mater. Res. Express 2015, 2, 1–15. [Google Scholar] [CrossRef]
- Papadimitriou, D.N. Structural, optical, electrical properties, and strain/stress of electrochemically deposited highly doped ZnO layers and nanostructured ZnO antireflective coatings for cost-effective photovoltaic device technology. Thin Solid Films 2016, 605, 215–231. [Google Scholar] [CrossRef]
- Ohm, W.; Riedel, W.; Askünger, Ü.; Heinemann, M.D.; Kaufmann, C.; Garzia, J.L.; Izqierdo, V.; Fontane, X.; Goislard, T.; Lux-Steiner, M.-C.; et al. An overview of technological aspects of Cu(In,Ga)Se2 solar cell architectures incorporating ZnO nanorod arrays. Phys. Status Solidi A 2015, 212, 76–87. [Google Scholar] [CrossRef]
- Wei, S.H.; Zhang, S.B.; Zunger, A. Effects of Ga addition to CuInSe2 on its electronic, structural, and defect properties. Appl. Phys. Lett. 1998, 72, 3199–3201. [Google Scholar] [CrossRef]
- Dharmadasa, I.M. Advances in Thin-Film Solar Cells; CRC Press: Boca Raton, FL, USA, 2012; pp. 95–122. [Google Scholar]
- Schulmeyer, T.; Kniese, R.; Hunger, R.; Jaegermann, W.; Powalla, M.; Klein, A. Influence of Cu(In,Ga)Se2 band gap on the valence band offset with CdS. Thin Solid Films 2004, 451–452, 420–423. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Papadimitriou, D.; Rega, N.; Siebentritt, S.; Lux-Steiner, M.-C. Raman and photoreflectance study of CuIn1-xGaxSe2 epitaxial layers. Thin Solid Films 2006, 511–512, 690–694. [Google Scholar] [CrossRef]
- Theodoropoulou, S.; Papadimitriou, D.; Anestou, K.; Cobet, C.; Esser, N. Optical properties of CuIn1−xGaxSe2 quaternary alloys for solar-energy conversion. Semicond. Sci. Technol. 2009, 24, 1–8. [Google Scholar] [CrossRef]
- Kaufmann, C.A.; Caballero, R.; Unold, T.; Hesse, R.; Klenk, R.; Schorr, S.; Nichterwitz, M.; Schock, H.-W. Depth profiling of Cu(In,Ga)Se2 thin films grown at low temperatures. Sol. Energy Mater. Sol. Cells 2009, 93, 859–863. [Google Scholar] [CrossRef]
- Caballero, R.; Kaufmann, C.A.; Efimova, V.; Rissom, T.; Hoffmann, V.; Schock, H.-W. Investigation of Cu(In,Ga)Se2 thin-film formation during the multi-stage co-evaporation process. Prog. Photovolt. Res. Appl. 2013, 21, 30–46. [Google Scholar] [CrossRef]
- Fons, P.; Niki, S.; Uchino, M.; Yamaa, A.; Ouangi, H. A High Resolution X-ray Diffraction and TEM Study of the CuxSe Surface Phase of Cu-rich CuInSe2. Jpn. J. Appl. Phys. 2000, 39, 189–191. [Google Scholar] [CrossRef]
- Papadimitriou, D. Application of optical spectroscopic techniques in the characterization of elastic strain effects in semiconductor heterostructures and nanostructures and in semiconductor-based thin-film solar cells. Phys. Status Solidi B 2015, 252, 30–55. [Google Scholar] [CrossRef]
- Xue, C.; Papadimitriou, D.; Esser, N. Optical characterization of epitaxial CuxGaySe2-layers by photoreflectance spectroscopy. Thin Solid Films 2004, 451–452, 189–192. [Google Scholar] [CrossRef]
- Papadimitriou, D.; Esser, N.; Xue, C. Structural properties of chalcopyrite thin films studied by Raman spectroscopy. Phys. Status Sol. 2005, 242, 2633–2643. [Google Scholar] [CrossRef]
- Tanino, H.; Maeda, T.; Fujikake, H.; Nakanishi, H.; Endo, S.; Irie, T. Raman spectra of CuInSe2. Phys. Rev. B 1992, 45, 13323–13330. [Google Scholar] [CrossRef]
- Ohrendorf, F.W.; Haeuseler, H. Lattice Dynamics of Chalcopyrite Type Compounds. Part I. Vibrational Frequencies. Cryst. Res. Technol. 1999, 34, 339–349. [Google Scholar] [CrossRef]
- Xue, C.; Papadimitriou, D.; Raptis, Y.S.; Richter, W.; Esser, N.; Siebentritt, S.; Lux-Steiner, M.Ch. Micro-Raman Study of Orientation Effects of CuxSe-Crystallites on Cu-rich CuGaSe2 Thin Films. J. Appl. Phys. 2004, 96, 1963–1966. [Google Scholar] [CrossRef]
- Joint Committee on Powder Diffraction Standards—International Centre for Diffraction Data; JCPDS-ICDD: Newtown Square, PA, USA, 1950–2000; Card number: CuInSe2 (40–1487), CuIn70Ga30Se2 (35-1102), Cu2Se (27–1131, 37–1187), Cu2-xSe (71–0044), ZnO (36–1451), ZnSe hexagonal (15-0105), ZnSe cubic (37–1463), Mo (42–1120), MoSe2 (29-0914, 01–077–1715).
- Siebentritt, S. Wide gap chalcopyrites: Material properties and solar cells. Thin Solid Films 2002, 403–404, 1–8. [Google Scholar] [CrossRef]
- Wei, S.-H.; Zunger, A. Band offsets at the CdS/CuInSe2 heterojunction. Appl. Phys. Lett. 1993, 63, 2549–2551. [Google Scholar] [CrossRef]
- Schmid, D.; Ruckh, M.; Schock, H.W. Photoemission studies on Cu(In,Ga)Se2 thin films and related binary selenides. Appl. Surf. Sci. 1996, 103, 409–429. [Google Scholar] [CrossRef]
- Rau, U.; Schmidt, M. Electronic properties of ZnO/CdS/Cu(In,Ga)Se2 solar cells-aspects of heterojunction formation. Thin Solid Films 2001, 387, 141–146. [Google Scholar] [CrossRef]
- Hofmann, A.; Pettenkofer, C. Surface orientation dependent band alignment for CuInSe2–ZnSe–ZnO. Appl. Phys. Lett. 2011, 98, 113503. [Google Scholar] [CrossRef]
- Fearheiley, M.L.; Bachmann, K.J.; Shing, Y.-H.; Vasquez, S.A.; Herrington, C.R. The Lattice Constants of CulnSe2. J. Electron. Mater. 1985, 14, 677–683. [Google Scholar] [CrossRef]
- Fiedeler, U. Rekombination und Diffusion in CuGaSe2—Solarzellen: Photolumineszenz und Quanteneffizienzuntersuchungen an MOCVD Gewachsenen Absorbern. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2002. [Google Scholar]
- Fuertes-Marrón, D. Structural and Electronic Characterisation of Thin-Film Solar Cells Based on CVD-Grown CuGaSe2. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2003. [Google Scholar]
- Rega, N. Photolumineszenz von Epitaktischen Cu(In,Ga)Se2-Schichten. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2004. [Google Scholar]
- Riha, S.C.; Johnson, D.C.; Prieto, A.L. Cu2Se Nanoparticles with Tunable Electronic Properties Due to a Controlled Solid-State Phase Transition Driven by Copper Oxidation and Cationic Conduction. J. Am. Chem. Soc. 2011, 133, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, K.; Gahtori, B.; Bathula, S.; Jayasimhadri, M.; Singh, N.-K.; Sharma, S.; Haranath, D.; Srivastava, A.K.; Dhar, A. Enhanced thermoelectric performance of spark plasma sintered copper-deficient nanostructured copper selenide. J. Phys. Chem. Solids 2015, 81, 100–105. [Google Scholar] [CrossRef]
- Gabor, A.M.; Tuttle, J.R.; Albin, D.S.; Contreras, M.A.; Noufi, R.; Hermann, A.M. High-efficiency CuIn1-xGaxSe2 solar cells made from (Inx,Ga1-x)2Se3 precursor films. Appl. Phys. Lett. 1994, 65, 198–200. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-Ray Diffraction; Addison-Wesley: Reading, MA, USA, 1978. [Google Scholar]
- Kolb, E.D.; Laudise, R.A. Hydrothermal Crystallization of Zinc Selenide. J. Cryst. Growth 1970, 7, 199–202. [Google Scholar] [CrossRef]
- Kolb, E.D.; Coriell, A.S.; Laudise, R.A.; Hutson, A.R. The Hydrothermal Growth of Low Carrier Concentration ZnO at High Water and Hydrogen Pressures. Mater. Res. Bull. 1967, 2, 1099–1106. [Google Scholar] [CrossRef]
- Laudise, R.A.; Kolb, E.D.; Caporaso, A.J. Hydrothermal Growth of Large Sound Crystals of Zinc Oxide. J. Am. Ceram. Soc. 1964, 47, 9–12. [Google Scholar] [CrossRef]
- Hanada, T. Basic Properties of ZnO, GaN, and Related Materials. In Oxide and Nitride Semiconductors: Processing, Properties, and Applications; Yao, T., Hong, S.-K., Eds.; Springer Series; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–20. [Google Scholar]
- Kisielowski, C.; Krüger, J.; Ruvimov, S.; Suski, T.; Ager, J.W., III; Jones, E.; Liliental-Weber, Z.; Rubin, M.; Weber, E.R.; Bremser, M.D.; et al. Strain-related phenomena in GaN thin films. Phys. Rev. B 1996, 54, 17745–17753. [Google Scholar] [CrossRef]
- Azuhata, T.; Takesada, M.; Yagi, T.; Shikanai, A.; Chichibu, S.F.; Torii, K.; Nakamura, A.; Sota, T.; Cantwell, G.; Eason, D.B.; et al. Brillouin scattering study of ZnO. J. Appl. Phys. 2003, 94, 968–972. [Google Scholar] [CrossRef]
- Rössler, U. ZnO: Bulk Modulus, Compressibility in New Data and Updates for Several Semiconductors with Chalcopyrite Structure, for Several II-VI Compounds, and Diluted Magnetic IV-VI Compounds, Landolt-Börnstein—Group III Condensed Matter; Springer: Berlin/Heidelberg, Germany, 2013; pp. 159–169. [Google Scholar]
- Van de Walle, C.G.; Martin, R.M. Theoretical Study of Band Offsets at Semiconductor Interfaces. In Electronic Structure of Semiconductor Heterojunctions; Margaritondo, G., Ed.; Perspectives in Condensed Matter Physics 1; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1988; pp. 268–279. [Google Scholar]
- Schlachter, S.I.; Fietz, W.H.; Grube, K.; Goldacker, W. High Pressure Studies of Tc and Lattice Parameters of MgB2, Advances in Cryogenic Engineering. In Proceedings of the International Cryogenic Materials Conference—ICMC, Xi’an, China, 16–20 June 2002; Volume 48, pp. 809–816.
- Adachi, S. Properties of Group-IV, III-V and II-VI Semiconductors. John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 45. [Google Scholar]
- Berlincourt, D.; Jaffe, H.; Shiozawa, L.R. Electroelastic Properties of the Sulfides, Selenides, and Tellurides of Zinc and Cadmium. Phys. Rev. 1963, 129, 1009. [Google Scholar] [CrossRef]
- Klingshirn, C.; Fallert, J.; Zhou, H.; Sartor, J.; Thiele, C.; Maier-Flaig, F.; Schneider, D.; Kalt, H. 65 years of ZnO research—Old and very recent results. Phys. Status Solidi B 2010, 247, 1424–1447. [Google Scholar] [CrossRef]
- Özgür, Ü.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Doğan, S.; Avrutin, V.; Cho, S.-J.; Morkoç, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 041301. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Synthesis, Characterization, and Applications of ZnO Nanowires. J. Nanomater. 2012. [Google Scholar] [CrossRef]
- Park, S.-H.; Seo, S.-Y.; Kim, S.-H.; Han, S.-W. Surface roughness and strain effects on ZnO nanorod growth. Appl. Phys. Lett. 2006, 88, 251903. [Google Scholar] [CrossRef]
- Gu, F.; Gai, L.; Shao, W.; Li, C.; Schmidt-Mende, L. Heteroepitaxial growth of ZnO branches selectively on Ti02 nanorod tips with improved light harvesting performance. Chem. Commun. 2011, 47, 8400–8402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, M.A.; Jones, K.M.; Gedvilas, L.; Matson, R. Preferred Orientation in Polycrystalline Cu(In,Ga)Se2 and Its Effect on Absorber Thin-Films and Devices. In Proceedings of the 16th European Photovoltaic Solar Energy Conference and Exhibition, Glasgow, UK, 1–5 May 2000.
- Kubelka, P.; Munk, F. Ein Beitrag zur Optik der Farbanstriche. Z. Tech. Phys. 1931, 12, 593–601. [Google Scholar]
- Sandoval, C.; Kim, A.D. Deriving Kubelka-Munk theory from radiative transport. J. Opt. Soc. Am. A 2014, 31, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Sol. B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Kröger, F.A. The Chemistry of Imperfect Crystals; North-Holland Pub. Co.: Amsterdam, The Netherlands, 1964. [Google Scholar]
- Avadhut, Y.S.; Weber, J.; Hammarberg, E.; Feldmann, C.; der Günne, J.S. Structural investigation of aluminium doped ZnO nanoparticles by solid-state NMR spectroscopy. Phys. Chem. Chem. Phys. 2012, 14, 11610–11625. [Google Scholar] [CrossRef] [PubMed]
- Serier, H.; Gaudon, M.; Ménétrier, M. Al-doped ZnO powdered materials: Al solubility limit and IR absorption properties. Solid State Sci. 2009, 11, 1192–1197. [Google Scholar] [CrossRef]
- Sanzaro, S.; la Magna, A.; Smecca, E.; Mannino, G.; Pellegrino, G.; Fazio, E.; Neri, F.; Alberti, A. Controlled Al3+ Incorporation in the ZnO Lattice at 188 °C by Soft Reactive Co-Sputtering for Transparent Conductive Oxides. Energies 2016, 9, 433. [Google Scholar] [CrossRef]
- Ennaoui, A. High Efficiency CIGSS Thin Film based Solar Cells and Mini-modules. Moroc. J. Condens. Matter 2000, 3, 8–15. [Google Scholar]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Acres, R.G.; Harmer, S.L.; Beattie, D.A. Synchrotron XPS studies of solution exposed chalcopyrite, bornite, and heterogeneous chalcopyrite with bornite. Int. J. Miner. Process. 2010, 94, 43–51. [Google Scholar] [CrossRef]
- Ahmed, E. Growth and Characterization of Cu(In,Ga)Se2 Thin Films for Solar Cell Applications. Ph.D. Thesis, University of Salford, Salford, UK, 1995. [Google Scholar]
- Ramanathan, K.; Keane, J.; Noufi, R. Properties of High-Efficiency CIGS Thin-Film Solar Cells. In Proceedings of the 31st IEEE Photovoltaics Specialists Conference and Exhibition, Lake Buena Vista, FL, USA, 3–7 January 2005.
- Messmann-Vera, L.-A. Einfluss der Undotierten ZnO-Schicht auf die elektrischen Eigenschaften von ZnO/Cu(In,Ga)Se2-Solarzellen, Master’s Thesis, Technische Universität Berlin, Berlin, Germany, 2010. [Google Scholar]
- Desal, P.D.; Chu, T.K.; James, H.M.; Ho, C.Y. Electrical Resistivity of Selected Elements. J. Phys. Chem. Ref. Data 1984, 13, 1069–1096. [Google Scholar]
- Scofield, J.H.; Duda, A.; Albin, D.; Ballard, B.L.; Predecki, P.K. Sputtered Molybdenum Bilayer Back Contact for Copper Indium Diselenide-Based Polycrystalline Thin-Film Solar Cells. Thin Solid Films 1995, 260, 26–31. [Google Scholar] [CrossRef]
- Chen, S.-F.; Wang, S.-J.; Lee, W.-D.; Chen, M.-H.; Wei, C.-N.; Bor, H.-Y.Y. Preparation and Characterization of Molybdenum Thin Films by Direct-Current Magnetron Sputtering. Atlas J. Mater. Sci. 2015, 2, 54–59. [Google Scholar] [CrossRef]
- Ellmer, K.; Bikowski, A. Intrinsic and extrinsic doping of ZnO and ZnO alloys. J. Phys. D Appl. Phys. 2016, 49, 413002. [Google Scholar] [CrossRef]
- Ellmer, K. Resistivity of polycrystalline zinc oxide films: Current status and physical limit. J. Phys. D Appl. Phys. 2001, 34, 3097–3108. [Google Scholar] [CrossRef]
- Ellmer, K. Electrical Properties. In Transparent Conductive Zinc Oxide Basics and Applications in Thin Film Solar Cells; Ellmer, K., Klein, A., Rech, B., Eds.; Springer Series in Materials Science; Springer: Berlin/Heidelberg, Germany, 2007; Volume 104, pp. 35–78. [Google Scholar]
- Denhoff, M.W. An accurate calculation of spreading resistance. J. Phys. D Appl. Phys. 2006, 39, 1761–1765. [Google Scholar] [CrossRef]
- Berger, H.H. Contact Resistance and Contact Resistivity. J. Electrochem. Soc. 1972, 119, 507–514. [Google Scholar] [CrossRef]
- Marlow, G.S.; Das, M.B. The Effects of Contact Size and non-zero Metal Resistance on the Determination of Specific Contact Resistance. Solid State Electron. 1982, 25, 91–94. [Google Scholar] [CrossRef]
- Gall, D. Electron mean free path in elemental metals. J. Appl. Phys. 2016, 119, 085101. [Google Scholar] [CrossRef]
- Kogut, L.; Komvopoulos, K. Electrical contact resistance theory for conductive rough surfaces. J. Appl. Phys. 2003, 94, 3153–3162. [Google Scholar] [CrossRef]
- Ellmer, K.; Diesner, K.; Wendt, R.; Fiechter, S. Relations between Texture and Electrical Parameters of Thin Polycrystalline Zinc Oxide Films. Solid State Phenom. 1996, 51–52, 541–546. [Google Scholar] [CrossRef]
- Cebulla, R.; Wendt, R.; Ellmer, K. Al-Doped Zinc Oxide Films Deposited by Simultaneous RF and DC Excitation of a Magnetron Plasma: Relationships between Plasma Parameters and Structural and Electrical Film Properties. J. Appl. Phys. 1998, 83, 1087–1095. [Google Scholar] [CrossRef]
- Lee, C.; Lim, K.; Song, J. Highly textured ZnO thin films doped with indium prepared by the pyrosol method. Sol. Energy Mater. Sol. Cells 1996, 43, 37–45. [Google Scholar] [CrossRef]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimitriou, D.N.; Roupakas, G.; Roumeliotis, G.G.; Vogt, P.; Köhler, T. Optimization of Electrochemically Deposited Highly Doped ZnO Bilayers on Ga-Rich Chalcopyrite Selenide for Cost-Effective Photovoltaic Device Technology. Energies 2016, 9, 951. https://doi.org/10.3390/en9110951
Papadimitriou DN, Roupakas G, Roumeliotis GG, Vogt P, Köhler T. Optimization of Electrochemically Deposited Highly Doped ZnO Bilayers on Ga-Rich Chalcopyrite Selenide for Cost-Effective Photovoltaic Device Technology. Energies. 2016; 9(11):951. https://doi.org/10.3390/en9110951
Chicago/Turabian StylePapadimitriou, Dimitra N., Georgios Roupakas, Georgios G. Roumeliotis, Patrick Vogt, and Tristan Köhler. 2016. "Optimization of Electrochemically Deposited Highly Doped ZnO Bilayers on Ga-Rich Chalcopyrite Selenide for Cost-Effective Photovoltaic Device Technology" Energies 9, no. 11: 951. https://doi.org/10.3390/en9110951





