Thermochemical conversion processes are a different approach to producing chemicals from various feedstock. Scrap tyres or any biomass can be converted to biofuel through thermochemical processes. These processes include incineration, hydrothermal liquefaction (HTL), gasification and pyrolysis that can also be completed in the presence or absence of catalysts.
4.1. Gasification
Gasification is a thermochemical process that converts organic material to a gas mixture (i.e., syngas) as well as minor products such as water, chars at elevated temperature (800–1000 °C) and partial pressure. Gasification at high temperature and a controlled environment results in converting a great proportion of the biomass into gas products. Gas products mainly include CH
4, C
2H
6, H
2, CO, CO
2, O
2 and char in addition to a small amount of tar. The syngas is predominantly used in gas turbines and engines to produce electricity [
60,
61]. Gasification reactors come in various styles such as fixed bed, fluidised bed, and entrained bed reactors. A few studies have been conducted on scrap tyre gasification [
62,
63,
64,
65,
66,
67,
68,
69].
In early work, the effects of temperature on gasification of tyre particles were investigated in a fluidised bed reactor by Raman et al. [
62]. They used shredded tyres with particles of 3 mm diameter and 5 mm length, at a temperature range of 900–1060 K with steam. Increasing the temperature led to the gas yield increasing from 0.21 to 0.76 N·m
3·kg
−1, but reducing the gas heating value from 39.6 to 22.2 MJ·m
3. The obtained gas was rich in olefins (13.5% to 34%), but depended on the operating temperature. Using steam in fluidised beds yielded higher gas product in comparison to other contactors. Gasification of tyre-char in the temperature range of 850–1000 °C and CO
2 pressure range of 0.3–1.0 atm showed that the gasification reaction is independent of the char particle size when it is less than 0.65 mm [
63].
Gasification of granulated tyre powder in a wider temperature range from 350 to 900 °C under air atmosphere was carried out by Leung and Wang [
64]. Equivalent ratio (ER), tyre feed rate, and particle size were the most effective parameters for increasing gas yield. At a feeding rate of 11 m
3·h
−1, gas with heating value of 6 MJ·m
−3 was obtained. Xiao et al. [
65] confirmed the results of previous research and stated that increasing both temperature and ER would increase the syngas production, but on the other side, the amount of carbon black decreased. It was reported that at 700 °C, increasing ER from 0.2 to 0.6 increased the syngas yield up to 5.5%, but the amount of carbon black decreased from 600 to 450 g·kg
−1.
Tyre gasification in a rotary kiln reactor yielded syngas with higher hydrogen content as well as methane and ethane [
66]. Increasing steam/tyre mass ratio in a rotary kiln reactor had a positive effect on enhancing CO and hydrogen content in gas products [
67]. Various agents such as steam, air/CO
2, and air/steam can be used in tyre gasification processing; steam resulted in a low heating value gas of 15.21 MJ·m
−3 [
68]. In regards to increasing temperature in tyre gasification, gas production is enhanced [
69].
Having reviewed the literature, we come to the conclusion that increasing temperature increases yield while other parameters influence the amount and quality of the syngas.
4.2. Hydrothermal Liquefaction
HTL is a thermochemical conversion method that enables a wide range of materials to be converted into liquid bio-oil (sometime called bio-crude). HTL operates at higher pressures than other thermochemical techniques with 200–300 bar not uncommon. The key advantage of HTL over pyrolysis is that the feedstock can be processed without any pre-drying [
70,
71,
72] but yields of liquid fuel are also quite high. However, the fuel properties of the bio-oil resulting from HTL are poor for transportation vehicles compared to that of diesel. To improve the fuel properties, some upgrading techniques such as separation, hydrogenation/hydrotreating, catalytic cracking, and hybrid processes should be applied to improve the fuel properties. Favourable feedstocks for this conversion method is wet or moist lignocellulosic materials such as wood, bagasse, and garbage, in addition to microalgae [
70,
73]. The use of different solvents like acetone, ethanol, water, dichloromethane, diethyl ether and black liquor produce yields in the range 20%–40% [
71]. In a recent study, Zhang et al. [
6] evaluated the potential of biofuel production of tyres by hydrothermal liquefaction. They carried out experiments in the temperature range of 200–430 °C in different atmospheres (air, hydrogen, nitrogen, carbon monoxide, and carbon dioxide) and examined the liquid, char, and gas products. They investigate the effects of variables such as temperature, reaction time, water (as solvent) to scrap tyre ratio, and gaseous environment among which temperature was found to be the most influential parameter on the products yield. The obtained 52.73 wt % oil yield with a heating value of approximately 45 MJ·kg
−1 at 400 °C. A key problem with HTL is that the very high pressures requires expensive equipment. To the knowledge of authors, there are only a limited number of research articles dedicated toward scrap tyre conversion into biofuel through gasification and liquefaction; however, great interests have been shown to scrap tyre pyrolysis as considerable oil is yielded.
4.3. Pyrolysis
Pyrolysis involves thermal degradation of biomass which occurs at elevated temperature and low pressures. The process normally starts at around 400 °C and goes further up to even 1000 °C. Significantly, pyrolysis occurs in the absence of oxygen. This pre-condition requires that the feedstock is dried before entering the pyrolysis process. Pyrolysis is formed of two Greek words; “pyro” meaning heat and “lysis” meaning breakdown. Hence, pyrolysis causes larger molecules to breakdown into smaller molecules at elevated temperatures. The chemical reactions include thermal cracking, thermolysis, and depolymerisation. Pyrolysis was applied to produce charcoal more than 5000 years ago [
74]. The product yields and composition depend on material particle size, temperature, heating rate, and the condensation temperature of the volatile fraction. Researchers have attempted to advance this technology to make it technically and economically feasible and applied to produce biofuel from various feedstocks. Tyre pyrolysis has been the subject of many studies; a major reason is it is environmentally friendly and its lower emission except for a few equipment leakage and minor fugitive sources [
75,
76,
77,
78,
79,
80,
81]. Biomass pyrolysis is a very complex process and performed in two stages. Firstly, the volatile matter or vapour is produced and then secondary cracking starts at higher temperatures. The dominant parameters are temperature, reaction time, gaseous atmosphere type and pressure [
82].
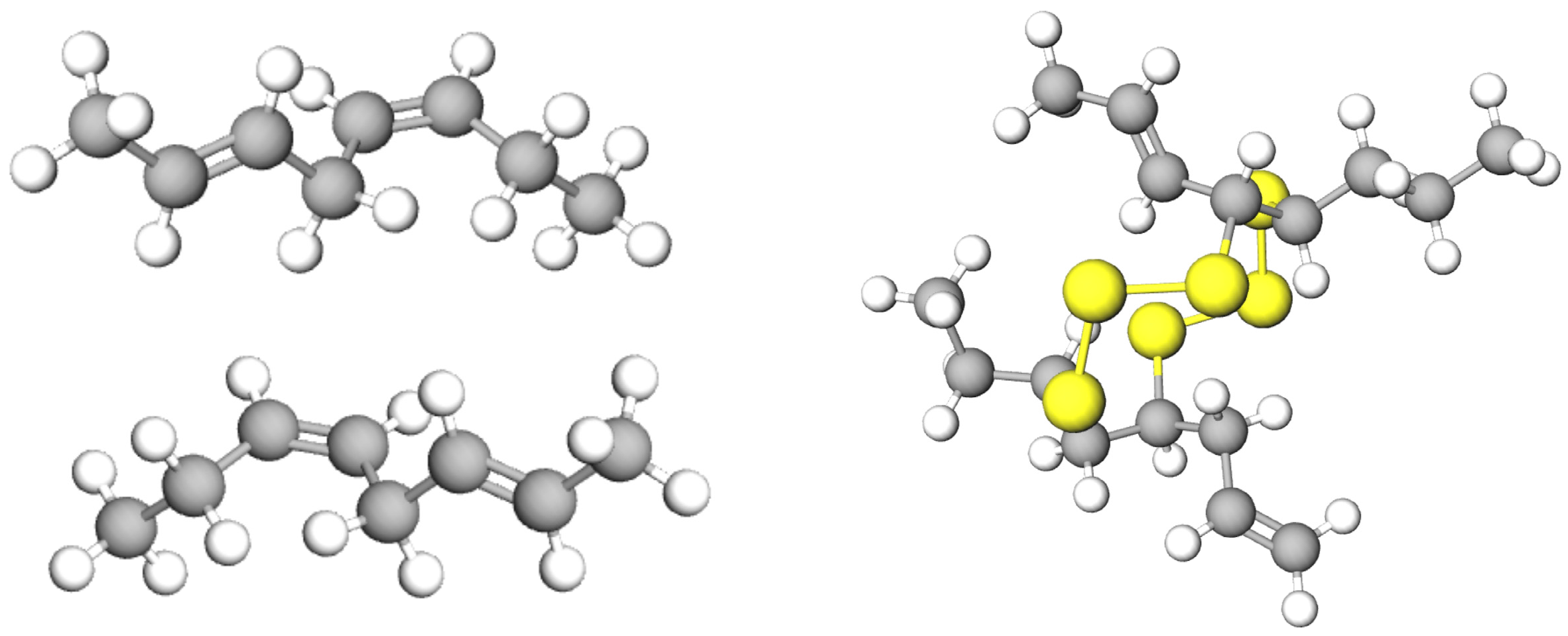
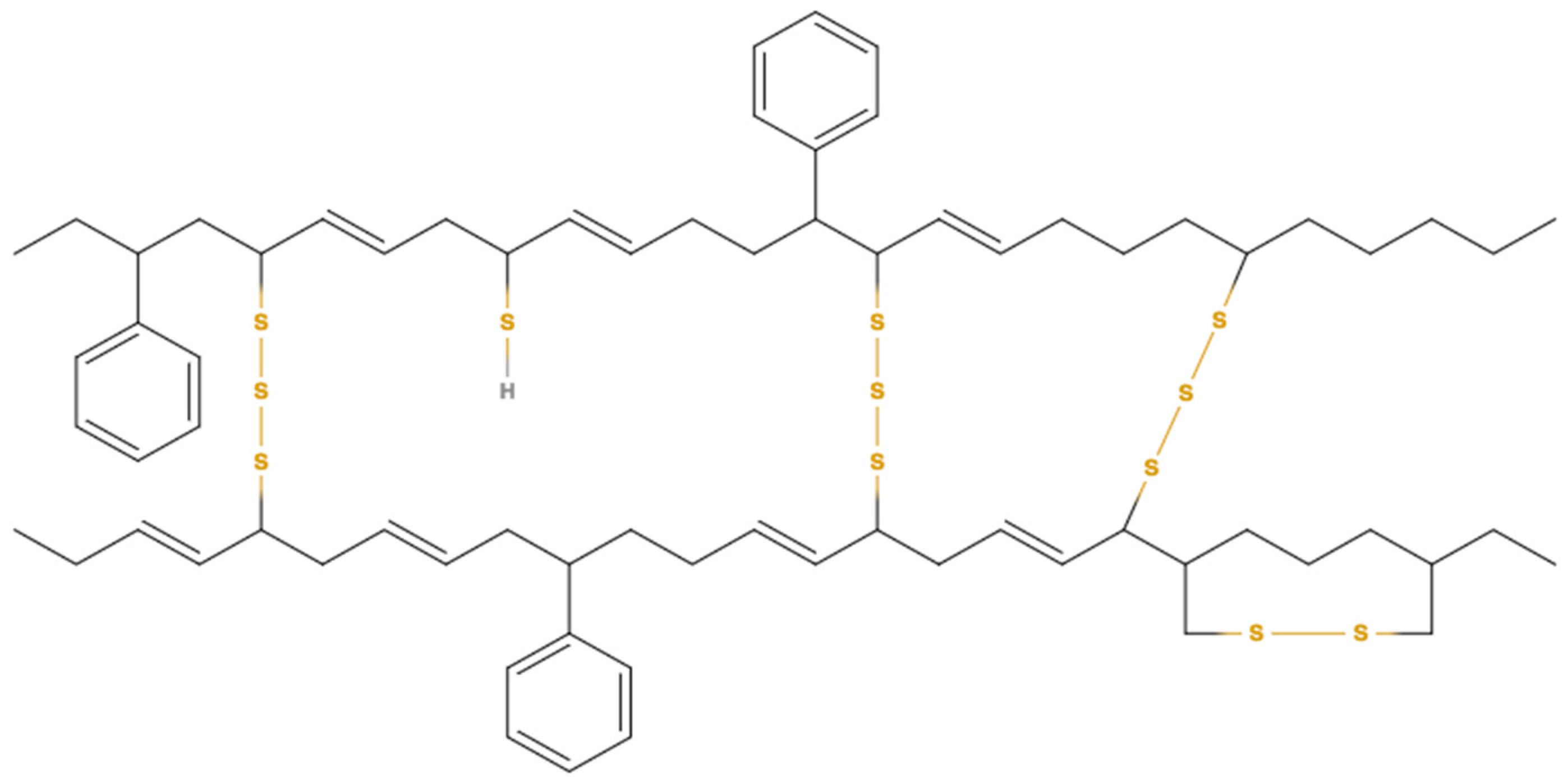
Pyrolysis of waste tyres is a favourable way of recycling that converts tyres into bio-oil. The tyre rubber is comprised of the highly complex polymeric structure. When the tyre is heated, the cracking process initially starts at S–S bonds, since S–S bonds need less dissociation energy compared to those of C–C and C–S bonds. The energy required for breaking down the S–S, C–C, and C–S bonds are 429, 607, and 699 kJ/mol, respectively [
83].
Figure 3 shows a tyre molecule with probable positions of thermal crackings that may occur at positions 1–4.
The reactor is an important part of the pyrolysis process. A wide range of reactors including fixed-bed (batch), fluidized-bed, rotary kiln, screw kiln, and vacuum have been used by researchers for scrap tyre pyrolysis among which fixed-bed (batch) reactors is extensively applied in waste tyre pyrolysis. Hita et al. [
84] conducted a critical review of tyre pyrolysis based on the reactor types. The heating system is typically an external electric furnace and nitrogen, or another inert gas, is used as a carrier gas. Using a thermogravimetric analysis (TG), it is observed that the tyre thermal degradation temperature commences at around 350 °C. Thus, the pyrolysis temperature range should consider the degradation temperature, and it is normally defined in the range of 450–700 °C. The investigation of rotary kilns fluidised bed and moving bed reactors are more favourable because they are continuous processes even though there are some commercial batch reactors for tyre pyrolysis.
4.3.1. Fixed-Bed Reactor Pyrolysis
Research into waste tyre pyrolysis in a fixed-bed reactor commenced in 1983, and only the effect of temperature on product yield was initially investigated [
85]. It was observed that increasing the temperature in a larger fixed-bed reactor up to 475 °C only slightly increased the oil and gas yields. In subsequent research, the temperature increased up to 720 °C by passing the vapour through a secondary reactor and other effective parameters such as residence time were also examined [
86]. Increasing the temperature and residence time resulted in an increase in gas yield from 10 to 20 wt %, but the oil yield decreased from 51.5 to 41.5 wt %.
The effect of heating rate on the tyre pyrolysis products was evaluated by Williams et al. [
87]. 50 g of tyre was used to examine the effect of heating rate (5–80 °C·min
−1) and temperature (300 °C to 720 °C) in a 200 cm
3 fixed-bed batch reactor on product yield. At 300 °C, tyre resisted degrading. They observed that by increasing the temperature up to 720 °C, both oil and gas yield increased up to 58.5 wt % and 14.8 wt %, respectively (at a heating rate of 80 °C·min
−1). Only a small change was reported in the products when the heating rate increased from 5 to 80 °C·min
−1. The gas products mostly contained H
2, CO, CO
2, C
4H
6, CH
4, and C
2H
6. Fast pyrolysis of the tyre (heating rate of 1200 °C·min
−1) showed a direct dependency between pyrolysis temperature and product yields [
88]. Increasing the temperature from 500 to 900 °C can enhance the gas yield from 5.0 to 23 wt % under fast pyrolysis conditions.
Some studies demonstrated that increasing the temperature resulted in higher oil yield [
84,
87,
89,
90]. Mastral et al. [
89] investigated the effect of various parameters including temperature, heating rate, reaction time, gas velocity, and hydrogen pressure on tyre pyrolysis product yields in a fixed-bed reactor. Increasing the temperature led to higher yields of bio-oil; 45 wt % oil at 600 °C while the oil yield was 36 wt % at 400 °C. Effect of temperature on car tyre pyrolysis in a fixed bed reactor at the temperature range of 300–700 °C showed both liquid and gas product yields increased [
90]. The maximum oil of 38.5 wt % was yielded at 700 °C which was only 0.5 wt % higher than the oil obtained at 500 °C.
Another group of researchers reported that the oil yield decreased continuously when the temperature goes up [
12,
91]. Mastral et al. [
91] investigated tyre pyrolysis in a swept fixed-bed reactor and observed a continuous slight decrease in the oil yield from 60.5 wt % at 450 °C to 57.5 wt % at 900 °C. The effect of thermal cracking on conversion products is stronger at higher temperatures and this effect led to lower oil yields. During pyrolysis of small pieces of car tyres (2–3 cm) in the temperature range 300–700 °C, no changes were observed in oil product above 500 °C [
92]. Removing the fabric and steel of the tyres and performing pyrolysis tests in a fixed bed reactor, Aydın and IIkilic [
81] found that the maximum oil yield obtained 40.26 wt % at 500 °C. At higher temperatures, they observed only a small fall in the oil yield (39.74 wt % at 700 °C). On the other hand, the maximum gas yield of 18.68 wt % obtained by temperature rising at 700 °C. They also examined the effect of nitrogen flow rate and did not observe considerable changes in the product yield. In the temperature range of 375–500 °C and a heating rate of 10 °C·min
−1, an optimum temperature of 425 °C to obtain maximum oil yield was reported by Kar [
93]. The maximum oil yield of 60.02 wt % obtained at 425 °C and when temperature increased further up to 500 °C, the oil yield declined to 54.12 wt %. By increasing the temperature during the experiments, the gas product yield continuously increased from 2.99 wt % at 375 °C to 20.22 wt % at 500 °C.
Passenger car and truck tyre pyrolysis was carried out in a fixed bed reactor to investigate the effect of different compositions on product yield [
13]. At temperatures of 550, 650 and 850 °C under the N
2 atmosphere, similar gas composition from both car and truck tyres were obtained; C
1–C
4 hydrocarbons were observed in both. The physical properties of pyrolytic oils were similar, but the aromatic and sulfur content from car tyres was higher than that of obtained from a truck tyre. The pyrolysis of bicycle, motorcycle, and truck tyres at 375 °C to 575 °C in a fixed-bed reactor yielded 45 wt % to 56.5 wt % oil products [
15]. At higher temperatures of up to 575 °C, the oil yield decreased up to 8 wt % and the maximum gas product of 20 wt % yielded.
4.3.2. Fluidised-Bed Reactor Pyrolysis
Tyre pyrolysis in fluidised bed reactors has been extensively studied by researchers, and the influence of different parameters have been evaluated [
94,
95,
96,
97,
98]. In very early research, waste plastic was pyrolysed and it was observed that fluidising sand bed is insensitive to the size of inlet material. Then the design was adapted to test the pyrolysis of whole tyres. Each piece of scrap tyre weighed at least 2.7 kg [
94]. Pyrolysis products comprised of 20–40 wt % oil and 10–30 wt % gas. In a pilot plant at 700 °C, the tyre pieces tested each weighed 6–20 kg. It was successful in obtaining 19.6 wt % oil in addition to 16 wt % gas products. A feasibility study of fluidized-bed pyrolysis of scrap tyres at lower temperatures was performed by Kaminsky and Mennerrich [
96]. They carried out experimental tests in a laboratory-scale (1 kg·h
−1) fluidized-bed reactor which was indirectly heated to between 500 and 600 °C. They investigated the influence of steam as the fluidising gas. Increasing the temperature resulted in increasing the gas, oil and char yields. No significant finding was reported when steam was used as a fluidizing gas at 500 °C. By pyrolysing the truck tyre, the effect of temperature in a fluidized bed reactor was also examined [
98]. It was reported that increasing the temperature from 600 to 700 °C resulted in more oil (30 wt %) gas (20 wt %) and carbon black (20 wt %) yields. Changing the tyre feed rate in fluidized bed reactor demonstrated no change in the products when the feeding rate increased from 0.21 to 0.52 kg·h
−1. However, increasing the temperature from 700 to 880 °C, resulted in an increase in gas yield; however the oil decreased, and the char remained constant [
99].
Scrap tyre pyrolysis in a semi-continuous fluidised-bed at a temperature of 450–600 °C was carried out by Williams and Brindle [
100]. The maximum oil yield was 55 wt % at 450 °C and increasing the temperature to 600 °C reduced the oil yield to 43.5 wt %. An increase in gas yield was observed from 2.5 to 14.0 wt %.
Investigating the effects of temperature (360–810 °C), residence time (1–5 s), and heating rate in a circulating fluidized-bed reactor on scrap tyre pyrolysis detected that higher temperature and longer residence time would effect on product gas; more methane, ethane, hydrogen, and carbon monoxide and less heavy hydrocarbon gases (C
xH
y) were obtained. No significant changes were reported on the effect of particle size on the gas composition [
101].
4.3.3. Moving-Bed Reactor Pyrolysis
Tyre pyrolysis in a rotary kiln reactor has been compared with other types of rectors and the influence of parameters such as temperature, residence time and inert gas flow have been studied. Scrap tyre analysis at a temperature range of 550–680 °C yielded 38.12 wt % of oil product at 550 °C [
102]. While the temperature increased, the amount of char produced during the pyrolysis remained almost unchanged. Li et al. [
103] studied tyre pyrolysis in a continuous rotary kiln reactor at a temperature range of 450–650 °C. Increasing the temperature, they observed no change in the char fraction. The maximum oil yield of 45.1 wt % was obtained at 500 °C with high calorific value (40–42 MJ·kg
−1) that enables it to be a used as liquid fuel.
A comparison of tyre pyrolysis in fixed-bed and moving-bed reactors and evaluating the influence of reactor type on yields and product characteristics was conducted by Aylón et al. [
104]. Both reactors worked at 600 °C and used an inert atmosphere. The continuous moving-bed reactor with 15 kg·h
−1 waste tyre throughput demonstrated that all tyre conversion was achieved as well as the fixed-bed reactor in spite of its longer residence time. Due to the longer residence time and faster heating rate in the moving-bed reactor, more primary pyrolysis products and more cracking was observed. Similar char was obtained for both fixed-bed and moving-bed reactors 38 wt % char yield. On the other hand, moving-bed reactor reduced the oil yield from 54.6 to 43.2 wt %, and increased the product gas yield from 7.5 to 17.1 wt %.
In a further stage, the effects of various parameters were examined for their effect on product yield including temperature, solid residence time and inner gas flow rate in a screw kiln reactor [
105]. They observed maximum oil yield of 48.4 wt % at 600 °C with a throughput of 8 kg·h
−1. On the other side, they compared their result with a small scaled fixed-bed reactor and observed 56.4 wt % oil yield that could be caused by the differences in heating rate and gas residence time between the two reactors.
4.3.4. Vacuum Reactor Pyrolysis
Another technique which is applicable in tyre pyrolysis is vacuum reactors. Vacuum reactor pyrolysis is based on fluidised bed technology that can pyrolyze larger particles of tyres (up to 50 mm) at low pressures. However, due to the operation in a vacuum condition and complicated mechanical structure of the reactor, the maintenance is costly. This reactor works at a lower temperature in comparison to fluidised-bed reactors but yields less oil [
106]. Roy and colleagues conducted several vacuum tyre pyrolysis tests to investigate the products yields and quality [
107,
108,
109,
110,
111]. They pyrolysed recycled scrap tyres at 415 °C and used a pressure of less than 2 kPa (absolute pressure) and developed a vacuum pyrolysis pilot plant with two different particle sizes [
107]. The maximum oil yield was 58.4 wt %, in addition to 6% of the gas product when tyre particles were 150 mm to 400 mm. They tested a 200 kg·h
−1 vacuum pyrolysis pilot plant and concluded that it was feasible for a large amount of scrap tyre to be processed.
Pyrolysis of car and truck tyres in a vacuum reactor at 480–520 °C concluded in obtaining 33% to 39% carbon black that can be used as a replacement to commercial carbon blacks for pavement fillers. It was also reported that 43%–56% of the light oil fraction was rich in dl-limonene which is a positive additive to gasoline [
108]. Pyrolysis of the crushed tyre in a vacuum reactor showed that when the temperature increased from 450 to 600 °C, the maximum oil yield of 48.8 wt % and gas yield of 16.3 wt % was achieved [
14]. Increasing the pressure from 25 to 50 kPa as well as the temperature 425–500 °C resulted in an increase in liquid products and improvement in residual carbon black surface area [
112].
The quality of light naphtha distilled from pyrolytic oil that was obtained in vacuum tyre pyrolysis showed that 50 wt % oil yield contained 20 wt % light naphtha. This light naphtha was found to be rich in aromatic and olifinic compounds with higher octane number and capable of adding to hydrofiner feedstock without influencing the process requirement [
109]. Car tyre pyrolysis in a vacuum reactor at 500 °C obtained 61 wt % oil that of which contained 18 wt % naphtha [
110].
The effect of pressure on vacuum pyrolysis of scrap tyre was also investigated [
113]. It was observed that changing the pressure did not significantly influence the oil, gas, and carbon black yield. By increasing the pressure from 0.8 to 28 kPa, the oil yield decreased from 62.2 to 61.7 wt %, and the gas yield increased from 1.0 to 3.2 wt %. However, the oil composition was found to be significantly affected by the pressure. Pakdel et al. [
111] pyrolyzed waste tyres of cars and trucks in a continues feed reactor under vacuum condition at a temperature range of 440–570 °C to produce dl-limonene, a major product which is formed during rubber decomposition. They found that the dl-limonene yield decreased with increasing both temperature and pressure.
4.3.5. Other Reactors
Apart from the frequently used pyrolysis reactor designs (i.e., fixed bed, moving bed, and fluidised bed reactors), other innovative designs have been attempted for pyrolysing scrap tyres. One of these designs is the spouted-bed reactor [
112,
114,
115]. Olazar et al. [
114,
115] developed a fluidised-bed reactor which operates with an isothermal process to generate a higher heat transfer rate. A conical spouted bed reduces the residence time of the gas. This reactor had a high production capacity. They obtained a maximum oil yield at 425 °C. Tyre pyrolysis in a spouted bed at a higher temperature resulted in more oil yields. At a temperature range of 425–600 °C, the oil fraction was 55 wt % at 600 °C while at 425 °C only 44 wt % of oil was obtained [
116].
Conesa et al. [
117] studied tyre pyrolysis in a drop tube reactor at a pilot plant scale with a system for condensation of semivolatile matter at temperatures of 450, 750, and 1000 °C. In this reactor, after reaching the desirable temperatture, 5 g tyre was fed into the reactor. Gas was condensed after one hour with dry ice (solid carbon monoxide) placed in the condesation jacket. The char was collected after cooling down the reactor. In this research, it was observed that the amount of solid product increased at higher temperatures. At 1000 °C, the gas products mostly contained methane and benzene. The liquid phase which mainly included styrene, limonene and isoprene decreased from 37.8 wt % at 450 °C to 10.9 wt % at 750 °C.
Pyrolysis of waste car tyres was performed in a captive sample reactor known as a wire mesh microreactor [
118]. The influence of the temperature between 390 and 980 °C was investigated, at a heating rate of 70–90 K·s
−1 under helium atmosphere. The wire mesh microreactor has several advantages; temperature, heating rate and residence time can be independently controlled. Also, the residence time can be very low. The gaseous product is quickly cooled, and the heat transfer is very effective. In these experiments, 200 mg of scrap tyre was cut into small pieces of less than 500 mm and so the temperature could be rapidly increased to the intended temperature (890 °C). A remarkable increase in gas yield from 22 wt % at 450 °C to 73 wt % at 860 °C was reported.
Pyrolysis of powdered tyre (200–600 μm) in a plasma reactor was performed by Huang and Tang [
119]. Using a plasma reactor, they found that using radio frequency (RF) input power of between 1600 and 2000 W and reactor absolute pressure of between 3000 and 8000 Pa might lead to a temperature of 900–1500 °C. The tyre particles passed through the plasma reactor in nitrogen gas flow. The gas products comprised of hydrogen, methane, carbon monoxide, and carbon dioxide. At 1500 °C, the highest gas amount yielded about 78 wt %.
A two-stage pyrolysis reactor was applied to examine the sulfur content of the pyrolytic oil [
120]. A fluidized-bed reactor and an auger reactor were in series. It was observed that less sulfur was formed at 500 °C compared to 600 °C. In the two-stage reactor, 5.0 wt % oil with 0.55 wt % sulfur in auger reactor at 330 °C, and 48 wt % oil in a fluidized-bed reactor was obtained at 510 °C. A summary of tyre pyrolysis conditions and products is presented in
Table 5.
4.4. Catalytic Pyrolysis
Catalysts accelerate the rate of chemical reactions and remains unconsumed at the end of the process [
124]. Pyrolysis reactions can be enhanced in the presence of some catalysts. Pyrolytic oil has some disadvantages including high instability, repolymerisation, ageing and high corrosive property of bio-oil. Using catalysts, on the other hand, changes the product compositions. Catalytic pyrolysis can be carried out in fixed-bed or fluidised bed reactors. In the case of tyre pyrolysis, the catalyst is mixed with scrap tyre pieces in the solid phase. In the next phase of pyrolysis, the vapour contains both catalyst and tyre. Therefore, when the mixed vapour is condensed at the end of the process, the compositions of the products are different. Zeolite is a microporous solid catalyst which is extensively used in catalytic pyrolysis of tyre rubber. Methylsulfonylmethane (MSM), perlites, and zeolites and their family including ZSM-5 and ultrastable Y-type zeolite (USY) are other common catalysts used in tyre pyrolysis [
125,
126]. Several types of research have been conducted to investigate the catalytic pyrolysis of scrap tyres.
The effect of temperature (500–700 °C) and Pt-supported catalyst on polar-aromatic content in waste tyre pyrolysis was investigated by Dũng et al. [
127]. They reported that increased pyrolysis temperature led to increasing the content of polar aromatics and the oil yield. Also, they used two zeolite-based catalyst-HBETA and HMOR and observed that the polar-aromatic decreased 30% and 50% by using HMOR and HBETA, respectively. More reduction in polar-aromatic content is due to the stronger site density, smaller crystallites and the 3D structure of the HBETA. The role of Pt was to convert polar-aromatics to saturated hydrocarbons, and as a result reducing the amount of polar-aromatics. The effect of adding ITQ-21 and ITQ-24 catalysts as additives into HMOR was investigated in the same apparatus [
128]. The results showed that increasing the catalyst-to-tyre ratio, increased the gasoline and kerosene fraction. On the other hand, adding ITQ zeolites, reduced the gasoline production. Both ITQ-21 and ITQ-24 additives increased the oil yield from 40.3 to 41.0 wt % and 42.4 wt %, respectively. Furthermore, adding ITQ-21 increased the kerosene production whereas the addition of ITQ-24 decreased the kerosene yield. The distinctive influence of the two ITQ zeolites is due to the difference in their acid properties and topology.
Ruthenium (Ru) is a popular catalyst due to its various functionality, high stability in air, and abundance of solvents. Using Ru as a catalyst in tyre pyrolysis has been considered in some research. Using Ru/SBA-1 in tyre pyrolysis resulted in higher gaseous products yields of up to around two times greater than that of non-catalytic pyrolysis [
129]. The highest activity of this catalyst is attributed to the small ruthenium particle size and high sulfur content. In experiments, the content of light fraction increased from 50 to 70 wt %, while the content of poly- and polar aromatic hydrocarbon (PPAH) were reduced. The presence of ruthenium reduced the PPAHs and led to good light oil production. Using MCM-41 and Ru/MCM-41 catalysts on tyre pyrolysis yielded less oil, but more gas products comparing to Ru/SBA-1 catalyst [
130]. The gas yield increased from 11 (non-catalytic) to 15 wt % and 30 wt % when using MCM-41 and Ru/MCM-41, respectively. On the other hand, the oil yield was the maximum around 42 wt % when no catalyst was used. MCM-41 decreased the oil yield to 38 wt % and Ru/MCM-41 also decreased to 25 wt %. Using Ru/MCM-41 resulted in light olefins yields of up to four times greater than for non-catalytic pyrolysis. Pyrolysis of 10 g of tyre at 500 °C in the presence of MCM-48 and Ru/MCM-48 catalysts revealed more reduction in oil products [
131]. Ru/MCM-48 increased the gas yield from 14 wt % (non-catalytic pyrolysis) to 23 wt % and 25 wt % for MCM-48 and Ru/MCM-48, respectively. Also, the amount of light olefin produced was twice greater than for non-catalytic pyrolysis.
Several researchers have investigated the effect of zeolite and its family on tyre pyrolysis. Shen et al. [
132] studied the two-stage pyrolysis of the waste tyre by the use of USY catalyst. They used shredded car tyre (8.0–10 mm) at 500 °C in a fixed bed reactor. USY catalyst increased the product gas yield, whereas the oil yield decreased. Also, the oil yield decreased to a large degree when catalyst-tyre ratio increased from 0.25 to 1.0. It was also shown that the high catalyst-tyre ratio increased the light oil fraction (boiling point < 220 °C). By increasing the catalyst-tyre ratio to 0.5, a remarkable change in benzene and toluene concentration was reported. In a similar experiment, they carried out tyre pyrolysis using USY and ZSM-5 catalysts to investigate the effect of catalyst temperature on products yield [
133]. USY and ZSM-5 catalysts in tyre pyrolysis reduced the oil yield but increased the yield of gas products. However, the oil yield with the ZSM-5 catalyst is greater than that of USY catalyst. They reported 54.1 wt % oil yield for non-catalytic pyrolysis, 40.4 wt % for ZSM-5 and 32.6 wt % for USY catalysts. The reason provided for the lower oil yield was the cracking of large molecules to smaller ones.
Three types of different zeolite catalysts including ZSM-5, Y-Zeolite (CBV-400), and Y-Zeolite (CBV-800) in tyre pyrolysis yielded more oil products compared to USY [
134]. Using this catalyst increased the yield of gas products by up to 20 wt %. When no catalyst was used, the maximum oil yield was 55.8 wt % and the minimum gas of 6.1 wt % was obtained. The use of three catalysts led to an oil reduction among which the ZSM-5 yielded the maximum oil yield of 35.8 wt % in comparison to Y-zeolite (CBV-400) and Y-zeolite (CBV-780). The different oil yields is due to the difference in catalysts pore size and the ratio of silica/alumina which influenced the number of catalytically active sites on the catalyst surface.
Investigating the effect of catalyst temperature on oil products shows that by increasing the catalyst temperature from 430 to 600 °C, two different behaviours were observed while two catalysts were used. Using ZSM-5 resulted in an increase in benzene, toluene and xylene. For the Y-zeolite, benzene and toluene increased but caused a reduction in the amount of xylene [
135,
136].
Investigation of scrap tyre pyrolysis with the presence of Fe-supported zeolites catalysts concluded that introducing 5% Fe on zeolite catalyst only slightly changed the oil and gas yield. Using 5%, Fe on HZSM-5 increased the gas products by three wt % with the expense of 5 wt % of oil yield [
137]. The effect of different catalysts including ZSM-5, USY, Beta, SAPO-11, and ZSM-22 in a batch reactor at 500 °C was studied by Li et al. [
138]. The oil remained almost unchanged (in some cases only a little lower), and reported the maximum oil yield of 55.65 wt % using ZSM-5. SAPO-11 increased the gas yield up to 10.45 wt % whereas in the absence of catalyst only 4.5 wt % gas obtained.
Catalytic pyrolysis of tyre rubber has been known to reduce oil yield, but recent studies showed that some catalysts could increase a number of oil products. Perlite is one of those catalysts that increases the oil yield [
93]. Perlite is a volcanic rack capable of expanding up to 35 times bigger than its initial size at a temperature range of 850–1100 °C [
139]. Car tyre pyrolysis with the presence of expanded perlite showed an increase in oil yield up to 65.11 wt % while pyrolysis without catalyst yielded around 60 wt % of oil product. There were no significant changes in gas production when using perlite in these experiments [
93].
Tyre pyrolysis over calcium carbide (CaC
2) catalyst showed around 60% increase in the oil yield comparing to non-catalytic pyrolysis [
140]. CaC
2 contains pi (π) electrons, and vibration of these electrons enhances the vibrational energy of tyres up to resonance and forms polymer radicals. Hence, they are expected to assist greater oil yields. The result showed that in the presence of a catalyst, the oil increases from 22.8% (non-catalytic) to 38.4%, and the gas product decreased from 37% (non-catalytic) to 29.6%. Demirbas et al. [
141] in a recent study, investigated the tyre pyrolysis with the presence of sodium carbonate (Na
2CO
3). They used a stainless-steel vertical reactor and carried out their experiments in temperatures range of 400–600 °C. Up to 85% of the produced oil could be used as fuel in combustion engines. Also, they obtained 49.2 wt % oil using 10% catalyst at 485 °C, whereas the oil yield was 39.6 wt % in the same condition without using a catalyst. Tyre pyrolysis at 430 °C with the catalyst mixture of ZSM-5 and lubricant base oil (LBO) was carried out by Qu et al. [
142]. The mixture of two catalysts significantly increased the degradation rate. It was reported that the oil yield when using ZSM-5 and ZSM-5/LBO was 33.6 wt % and 48 wt %, respectively. Co-pyrolysis with LBO increased the interaction between tyre and catalysts and consequently enhanced the oil yield and quality.
Using 1.0 g MgCl
2 as a catalyst in tyre pyrolysis process at 387.5 °C could increase the oil yield up to 45 wt % while at the same condition and higher temperature (475 °C) without catalyst the yield was only 19.8 wt % [
143].
Studying the effect of effect of zeolite and calcium carbide (CaC
2) catalysts as well as the pyrolysis temperature on the product yields of tyre pyrolysis, it was stated that zeolite mostly affected the gasoline production and increased it, while the calcium carbide enhanced the diesel production. However, adding either of the catalysts reduced the amount of fuel oil and heavy oil. They also examined the effect of temperature and reported that by increasing the temperature up to 570 °C, both gasoline and diesel yields increased [
144].
Whole tyre pyrolysis was carried out with the presence of a commercial catalyst (CoMo/Al
2O
3 SiO
2) and (NiMo–Al
2O
3) at 360 °C and 5 MPa of hydrogen pressure and an improvement in fuel characteristic was observed [
145]. However, the pyrolytic oil could be used as a component of fuel oils due to the high amount of aromatic hydrocarbons.
Table 6 summarizes the tyre pyrolysis with/without presence of catalysts.
4.5. Pyrolytic Oil Properties
The fuel properties of petroleum-derived fuels including diesel and gasoline are compared to tyre pyrolysis oils. Fuels, like any other material, are specified with some particular properties. These properties can be attributed to fuels from different facets. Physical and chemical properties, primarily, specify the density, thermal capacity, and chemical compositions. Many other properties are attributed to combustion or economics of the fuels. Flash point, for instance, is a term that defines the lowest temperature at which fuel can be ignited in the proximity of air. Other parameters like fuel viscosity, carbon residue, and sulfur content are other important properties that closely related to combustion of the fuels in engines and burners. The higher the fuel density, the more fuel per unit volume can be injected into the engine, and consequently the engine performance and gas emission (carbon monoxide and carbon dioxide) is affected. Viscosity is a restrictive factor in fuel injection and created ignition delay. Low viscosity is desirable for fuel handling and transportation, and leads to greater pump and engine performance. The boiling point range is defined because petroleum products do not have a single boiling point. Initial boiling point and final boiling points also describe the first drop of distillation, and the when the maximum temperature observed.
Table 7 summarises the fuel properties of a selected number of pyrolytic oils from the research in this study and compare with those of gasoline and diesel.
Looking at the properties of pyrolytic oil, one comes to the understanding that with upgrading, the bio-oil properties can be quite similar to diesel and it can be used as an alternative fuel at either full load or blended with diesel. However, there are some problems with these fuels that may affect combustion, engine performance, and emissions. Of eminent concern is the viscosity which is two to three times greater than for diesel. The carbon content is in the same range of diesel fuel, but the nitrogen content is comparatively greater than diesel fuel which leads to worse NO
x emissions. The impurities in the pyrolytic oil can be filtered with filtration methods with 99% efficiency [
147]. Desulfurization and hydrotreating are techniques used by Ramirez et al. [
71] to upgrade pyrolytic oil due to the higher amount sulfur and water content. A stability study of tyre pyrolysis fuel over a 12-month period illustrated no changes in viscosity that at room temperature in the absence of light and air. Increasing the temperature showed only a slight reduction in viscosity and so the pyrolytic oil would not be problematic for atomization in diesel engines [
16].