Electrochemical Properties for Co-Doped Pyrite with High Conductivity
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Samples
2.2. Materials Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
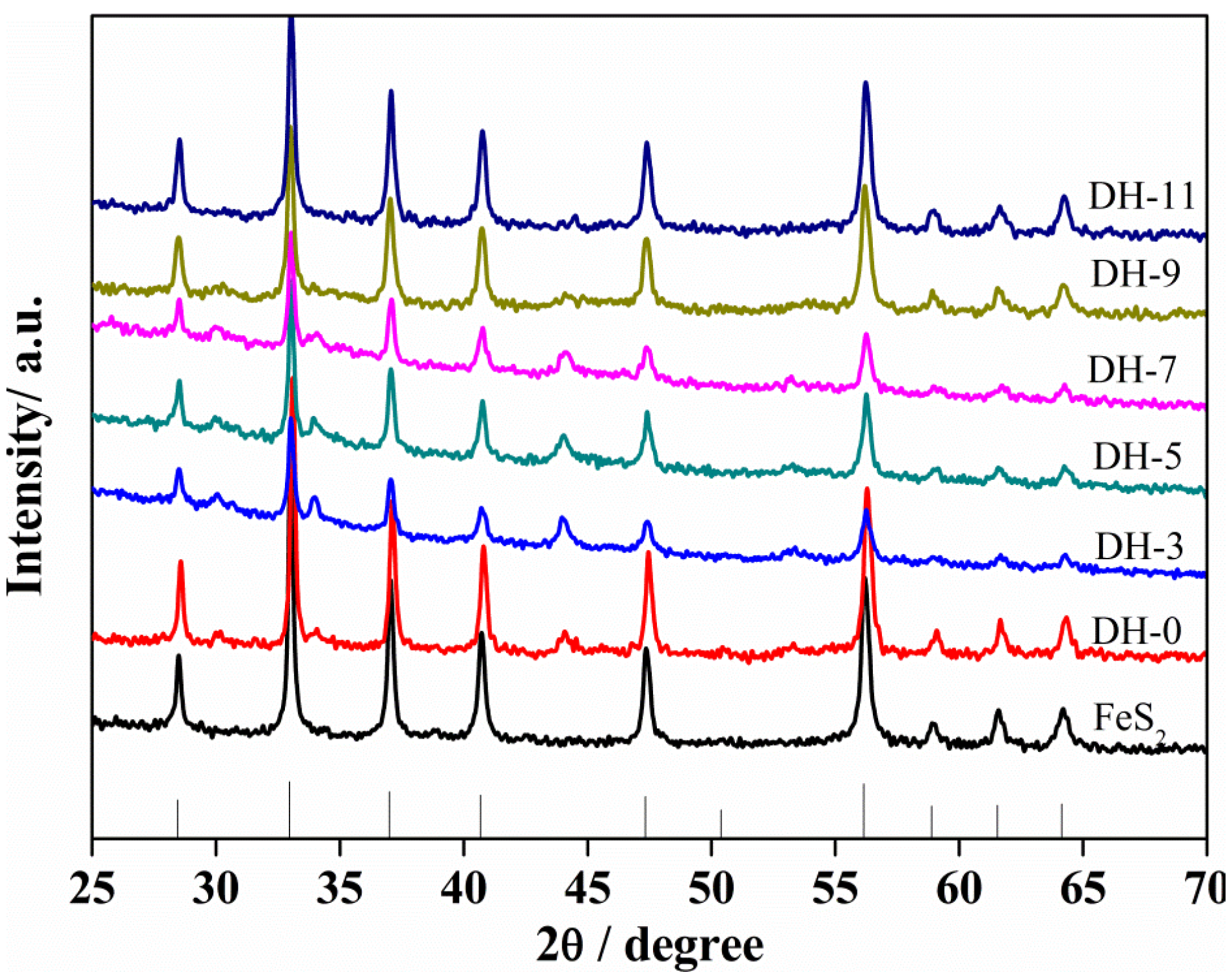


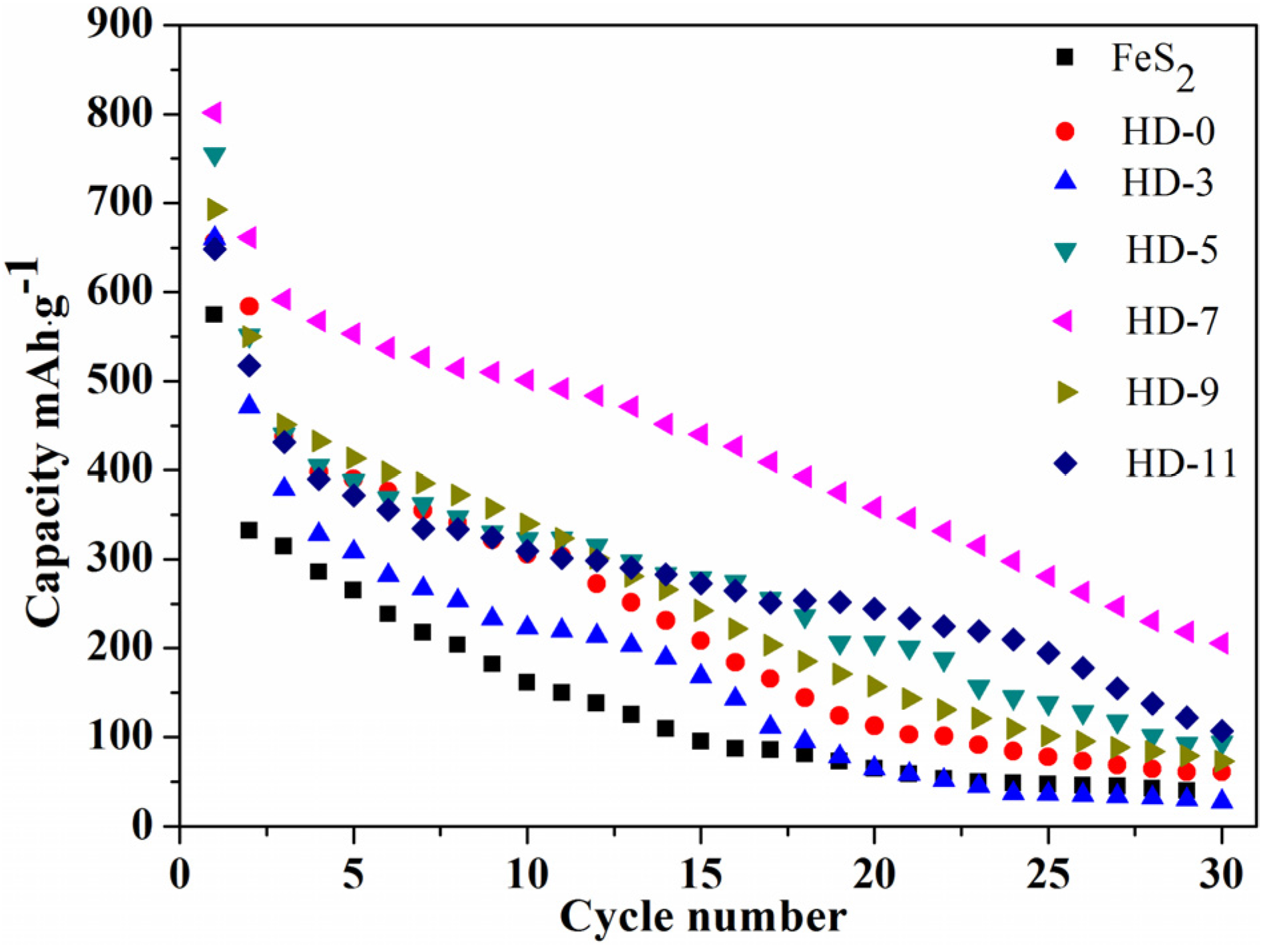
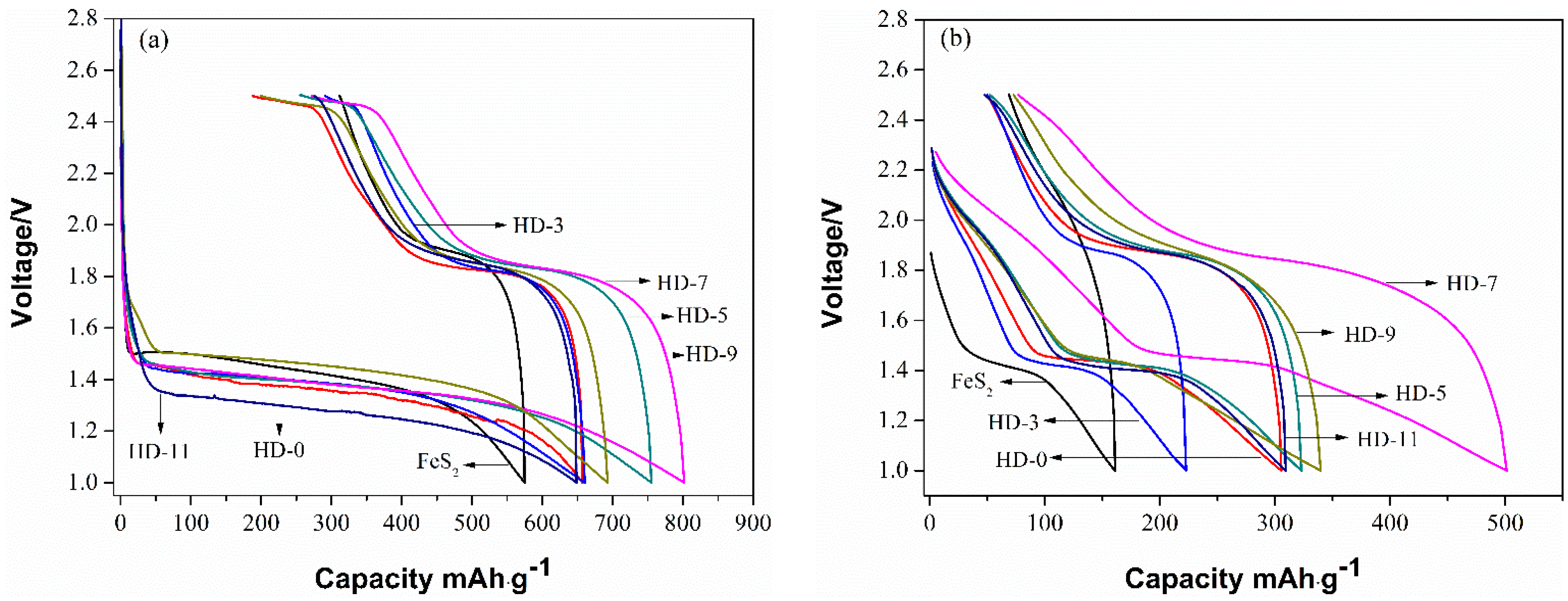
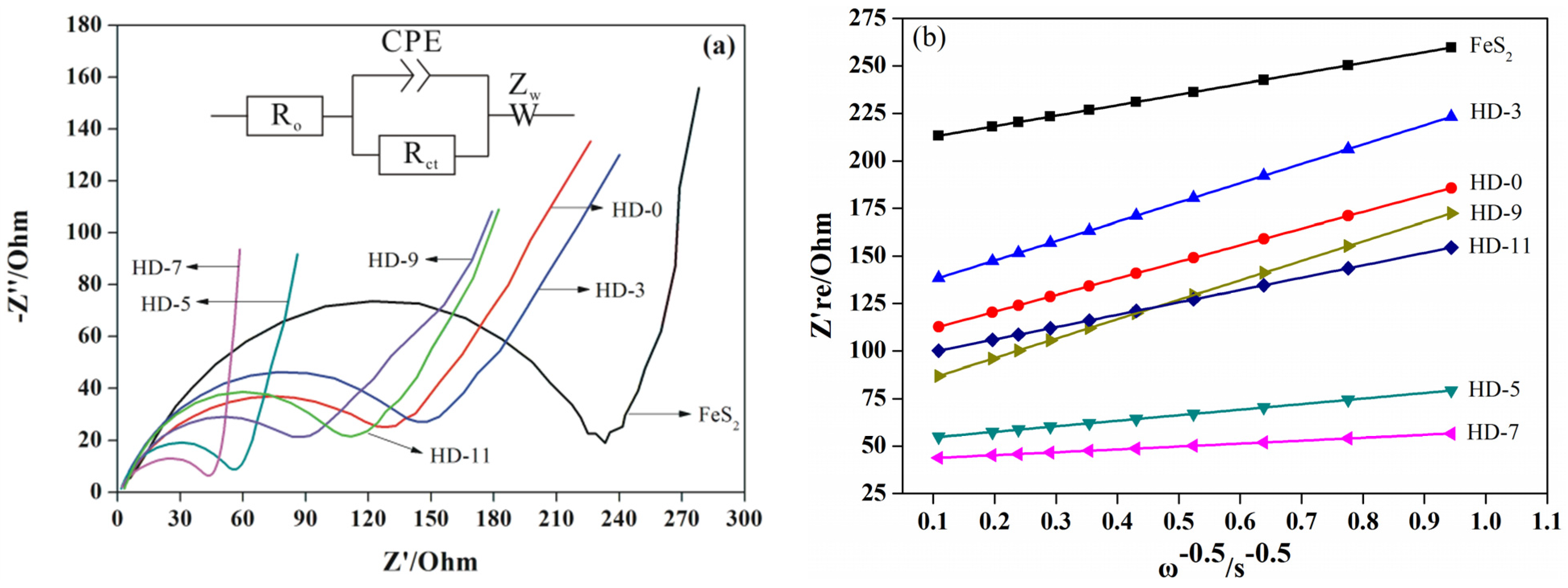
| Sample | Rct (Ω) | σw (Ω·cm2·s−0.5) | DLi (cm2·s−1) | σ (S·cm−1) |
|---|---|---|---|---|
| FeS2 | 234.0 | 55.6 | 3.7 × 10−7 | 9.7 × 10−6 |
| DH-0 | 118.8 | 176.5 | 3.6 × 10−8 | 1.9 × 10−5 |
| DH-3 | 137.5 | 101.6 | 1.1 × 10−7 | 1.6 × 10−5 |
| DH-5 | 52.3 | 29.3 | 1.3 × 10−7 | 3.3 × 10−5 |
| DH-7 | 43.9 | 15.2 | 4.9 × 10−6 | 5.1 × 10−5 |
| DH-9 | 87.9 | 102.4 | 1.1 × 10−7 | 2.6 × 10−5 |
| DH-11 | 105.1 | 65.1 | 2.7 × 10−7 | 2.2 × 10−5 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, S.S. The redox mechanism of FeS2 in non-aqueous electrolytes for lithium and sodium batteries. J. Mater. Chem. A 2015, 3, 7689–7694. [Google Scholar] [CrossRef]
- Kirkeminde, A.; Ruzicka, B.A.; Wang, R.; Puna, S.; Zhao, H.; Ren, S. Synthesis and optoelectronic properties of two-dimensional FeS2 nanoplates. ACS Appl. Mater. Interfaces 2012, 4, 1174–1177. [Google Scholar] [CrossRef] [PubMed]
- Cabán-Acevedo, M.; Faber, M.S.; Tan, Y.; Hamers, R.J.; Jin, S. Synthesis and properties of semiconducting iron pyrite (FeS2) nanowires. Nano Lett. 2012, 12, 1977–1982. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.; Ardel, G.; Livshits, V.; Burstein, L.; Golodnitsky, D.; Peled, E. Lithium polymer electrolyte pyrite rechargeable battery: Comparative characterization of natural pyrite from different sources as cathode material. J. Power Sources 2000, 88, 206–218. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Strauss, E.; Lang, J.; Lavi, Y. Li/CPE/FeS2 rechargeable battery. Electrochim. Acta 1998, 43, 1593–1599. [Google Scholar] [CrossRef]
- Huang, S.; Liu, X.; Li, Q.; Chen, J. Pyrite film synthesized for lithium-ion batteries. J. Alloy. Compd. 2009, 472, L9–L12. [Google Scholar] [CrossRef]
- Yersak, T.A.; Macpherson, H.A.; Kim, S.C.; Le, V.-D.; Kang, C.S.; Son, S.-B.; Kim, Y.-H.; Trevey, J.E.; Oh, K.H.; Stoldt, C.; et al. Solid state enabled reversible four electron storage. Adv. Energy Mater. 2013, 3, 120–127. [Google Scholar] [CrossRef]
- Li, L.; Caban-Acevedo, M.; Girard, S.N.; Jin, S. High-purity iron pyrite (FeS2) nanowires as high-capacity nanostructured cathodes for lithium-ion batteries. Nanoscale 2014, 6, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, X.; Mai, Y.; Xia, X.; Gu, C.; Tu, J. Enhanced electrochemical performance of FeS2 synthesized by hydrothermal method for lithium ion batteries. J. Appl. Electrochem. 2012, 42, 263–269. [Google Scholar] [CrossRef]
- Huang, L.; Wang, F.; Luan, Z.; Meng, L. Pyrite (FeS2) thin films deposited by sol-gel method. Mater. Lett. 2010, 64, 2612–2615. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamamoto, A. Electrodeposition of pyrite (FeS2) thin films for photovoltaic cells. Sol. Energy Mater. Sol. Cells 2001, 65, 79–85. [Google Scholar] [CrossRef]
- Zhang, D.; Tu, J.P.; Mai, Y.J.; Zhang, J.; Qiao, Y.Q.; Wang, X.L. Preparation and characterization of FeS2/polyaniline composite electrode in lithium-ion battery. J. Aust. Ceram. Soc. 2012, 48, 189–193. [Google Scholar]
- Choi, Y.J.; Kang, W.G.; Ryu, H.S.; Nam, T.H.; Ahn, H.J.; Cho, K.K.; Kim, K.W.; Ryu, K.S. Effect of Fe addition on cycle performance of FeS2 cathode for Li/FeS2 battery. Mater. Technol. 2012, 27, 124–126. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Q.; Cheng, Y.; Li, K.; Yao, Y.; Zhang, Q.; Dong, C.; Wang, P.; Schwingenschlögl, U.; Yang, W.; et al. Doping monolayer graphene with single atom substitutions. Nano Lett. 2011, 12, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.-C.; Hsu, C.-M.; Chen, S.-Y.; Perng, Y.-H.; Chueh, Y.-L.; Chen, L.-J.; Chou, L.-H. Facile synthesis and characterization of high temperature phase FeS2 pyrite nanocrystals. Mater. Lett. 2012, 75, 152–154. [Google Scholar] [CrossRef]
- Lehner, S.W.; Savage, K.S.; Ayers, J.C. Vapor growth and characterization of pyrite (FeS2) doped with Co, Ni, and As: Variations in semiconducting properties. J. Cryst. Growth 2006, 286, 306–317. [Google Scholar] [CrossRef]
- Diaz-Chao, P.; Ferrer, I.J.; Sanchez, C. Co distribution through n-type pyrite thin films. Thin Solid Films 2008, 516, 7116–7119. [Google Scholar] [CrossRef]
- Jiao, J.Q.; Chen, L.P.; Kuang, D.B.; Gao, W.; Feng, H.J.; Xia, J. Synthesis of FeS2 and Co-doped FeS2 films with the aid of supercritical carbon dioxide and their photoelectrochemical properties. RSC Adv. 2011, 1, 255–261. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Han, Y.; Du, H.; Peng, W.; Huan, Q.; Song, D.; Si, Y.; Wang, Y.; Yuan, H. CoS2 hollow spheres: Fabrication and their application in lithium-ion batteries. J Phys. Chem. C 2011, 115, 8300–8304. [Google Scholar] [CrossRef]
- Faber, M.S.; Park, K.; Cabán-Acevedo, M.; Santra, P.K.; Jin, S. Earth-abundant cobalt pyrite (CoS2) thin film on glass as a robust, high-performance counter electrode for quantum dot-sensitized solar cells. J. Phys. Chem. Lett. 2013, 4, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Faber, M.S.; Dziedzic, R.; Lukowski, M.A.; Kaiser, N.S.; Ding, Q.; Jin, S. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures. J. Am. Chem. Soc. 2014, 136, 10053–10061. [Google Scholar] [CrossRef] [PubMed]
- Oertel, J.; Ellmer, K.; Bohne, W.; Röhrich, J.; Tributsch, H. Growth of n-type polycrystalline pyrite (FeS2) films by metalorganic chemical vapour deposition and their electrical characterization. J. Cryst. Growth 1999, 198–199, 1205–1210. [Google Scholar] [CrossRef]
- Wang, F.; Huang, L.; Luan, Z.; Huang, J.; Meng, L. Effect of sulfurization temperature on the surface roughening, electrical and optical properties of FeS2 films deposited by sol-gel method. Mater. Chem. Phys. 2012, 132, 505–508. [Google Scholar] [CrossRef]
- Gong, M.G.; Kirkeminde, A.; Ren, S.Q. Symmetry-defying iron pyrite (FeS2) nanocrystals through oriented attachment. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Fong, R.; Dahn, J.R.; Jones, C.H.W. Electrochemistry of pyrite-based cathodes for ambient-temperature lithium batteries. J. Electrochem. Soc. 1989, 136, 3206–3210. [Google Scholar] [CrossRef]
- Shao-Horn, Y.; Horn, Q.C. Chemical, structural and electrochemical comparison of natural and synthetic FeS2 pyrite in lithium cells. Electrochim. Acta 2001, 46, 2613–2621. [Google Scholar] [CrossRef]
- Bao, W.; Zhuang, Q.; Xu, S.; Cui, Y.; Shi, Y.; Qiang, Y. Investigation of electronic and ionic transport properties in α-MoO3 cathode material by electrochemical impedance spectroscopy. Ionics 2013, 19, 1005–1013. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, X.; Guo, R. Improved electrochemical performance of La0.7Sr0.3MnO3 and carbon co-coated LiFePO4 synthesized by freeze-drying process. Electrochim. Acta 2010, 55, 922–926. [Google Scholar] [CrossRef]
- Subba Reddy, C.V.; Chen, M.; Jin, W.; Zhu, Q.Y.; Chen, W.; Mho, S.-I. Characterization of (PVDF + LiFePO4) solid polymer electrolyte. J. Appl. Electrochem. 2007, 37, 637–642. [Google Scholar] [CrossRef]
- Moya, A.A. Electrochemical impedance of ion-exchange systems with weakly charged membranes. Ionics 2013, 19, 1271–1283. [Google Scholar] [CrossRef]
- Schmidt, J.P.; Chrobak, T.; Ender, M.; Illig, J.; Klotz, D.; Ivers-Tiffée, E. Studies on LiFePO4 as cathode material using impedance spectroscopy. J. Power Sources 2011, 196, 5342–5348. [Google Scholar] [CrossRef]
- Andre, D.; Meiler, M.; Steiner, K.; Walz, H.; Soczka-Guth, T.; Sauer, D.U. Characterization of high-power lithium-ion batteries by electrochemical impedance spectroscopy. II: Modelling. J. Power Sources 2011, 196, 5349–5356. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wang, S. Electrochemical Properties for Co-Doped Pyrite with High Conductivity. Energies 2015, 8, 9584-9593. https://doi.org/10.3390/en8099584
Liu Y, Wang S. Electrochemical Properties for Co-Doped Pyrite with High Conductivity. Energies. 2015; 8(9):9584-9593. https://doi.org/10.3390/en8099584
Chicago/Turabian StyleLiu, Yongchao, and Shengping Wang. 2015. "Electrochemical Properties for Co-Doped Pyrite with High Conductivity" Energies 8, no. 9: 9584-9593. https://doi.org/10.3390/en8099584





