1. Introduction
Nuclear power has emerged as a reliable baseload source of electricity providing approximately 13% of the world’s electrical power [
1]. Nuclear energy is an important resource in managing atmospheric greenhouse gases and associated climate change with its overwhelmingly low carbon emission considering that energy generation accounts for 66% of worldwide greenhouse gas emissions [
1,
2,
3].
Zirconium (Zr) alloy (or Zircaloy2) has many useful properties for applications in nuclear facilities, such as low absorption cross-section of thermal electrons, high ductility, good fabricability, hardness, and corrosion resistance [
4,
5]. Therefore, these alloys are used widely as cladding and guide tubes in pressurized water-cooled reactors. For nuclear reactor applications, the existence of hafnium (Hf), which can be found in zirconium ore at 1–3 wt%, should be avoided because of its high thermal neutron cross section. Owing to their similar physical and chemical properties with almost identical ionic radii (0.074 mm for Zr
4+, 0.075 mm for Hf
4+), the separation of these elements is a challenge, leading to many intricate processing steps for producing Hf-free Zr in commercial nuclear reactor applications. In particular, a purification step is one of the core and harshest steps to determine the required Zr purity.
Because the tetrachlorides are the preferred compounds used in the reduction reactions for the production of Zr and Hf in a metallic form, extensive efforts have focused on the separation of HfCl
4 and ZrCl
4 [
6,
7,
8,
9]. Extraction is still the most popular and economic way for commercial Zr-Hf purification [
10,
11,
12,
13,
14,
15,
16]. On the other hand, regardless of its main virtue in mass production, the extraction-based Zr-Hf separation requires considerable amounts of relatively expensive, corrosive and environmentally harmful solvent chemicals. A large portion of the entire processing facility for the production of Zr and Hf metals is dedicated to handling a multiple-step solvent extraction process in the presence of a solvent. Furthermore, it also generates a huge volume of liquid waste, which is difficult to dispose of due to stringent environmental protection laws [
17,
18,
19,
20]. For example, isobutyl methylketone (MIBK), which is the most popular solvents used for Zr-Hf extraction, requires environmentally harmful cyanogen (CN) chemicals as an additive. These drawbacks of extraction-based separation have prompted research into the development of an environmentally benign technology to separate HfCl
4 and ZrCl
4.
Distillation has attracted considerable attention for Zr purification because of its potential for clean separation and many other advantages for a large scale production, such as fewer unit operations and chemical consumption, higher overall yield, and less effluent [
17,
18]. On the other hand, because distillation utilizes the volatility difference of the components associated with the vapor-liquid equilibrium (VLE), the close boiling points of ZrCl
4 and HfCl
4 with narrow and harsh conditions for the vapor-liquid phase existence limits the distillation applications: a large number of separation stages are required in the column to bring HfCl
4 to an acceptably low level; stringent temperature control and a confining pressure are needed to manage the VLE; and the column material must withstand high temperatures and pressures. Note that ZrCl
4 and HfCl
4 are solids at normal temperatures and sublime when heated under normal pressure. The physical properties of these components are described elsewhere [
21].
The extractive distillation technique can be an attractive alternative to overcome these limitations of conventional distillation, while still having advantages in distillation applications. A eutectic mixture of HfCl
4 and ZrCl
4 in certain fused salts is potentially useful for this purpose. Fused salts for extractive distillation should have some important properties, such as high solubility for ZrCl
4 and HfCl
4 at elevated temperatures, low vapor pressure, low viscosity, and high dissolution for many different materials [
22]. In addition, the lowest operating temperature of the column must be higher than the sublimation temperature of HfCl
4 at atmospheric pressure [
22]. Several candidate solutions have been described [
23,
24,
25,
26].
In this study, the feasibility of extractive distillation using molten salts was examined to separate ZrCl4 and HfCl4 for nuclear power reactor applications. The experimental data was chosen from the literature, and correlated with the Raoult’s law-type behavior model. The optimal design of extractive distillation column and its enhanced configuration was studied with its main design condition using a rigorous commercial process simulator, Aspen HYSYS, and their performance was compared with conventional distillation.
2. Vapor–Liquid Equilibrium Model for ZrCl4 and HfCl4 Mixture over Molten Salts
An alteration in the volatility of ZrCl
4 and HfCl
4 by adding a solvent is desired in view of their close vapor pressures. The essential parameters that should be considered for the design of extractive distillation are (i) the selection of a suitable solvent to dissolve the tetrachlorides; (ii) the operating temperature and pressure; and (iii) a reliable material of construction. Regarding these parameters, several molten salts have been recommended for extractive distillation [
27].
The volatility of HfCl
4 over a HfCl
4-KCl-NaCl solution is approximately 1.7 times larger than that of ZrCl
4 over the ZrCl
4-KCl-NaCl solution in the range, 63.0~67.5 mol% tetrachlorides (HfCl
4-ZrCl
4) [
22], which indicates the economic separation of HfCl
4 and ZrCl
4 by extractive distillation in a molten salt solution. In addition, the molten salt mixture of NaCl-KCl has preferential properties in that a homogeneous solution can be formulated at a relatively low temperature and the liquid phase exists over a reasonably wide range of compositions at atmospheric pressure [
22]. Note that a system with tetrachlorides only requires an exceedingly high pressure and higher temperature as well as a very narrow range of vapor-liquid phase.
This study targeted the molten salt mixture, 66.0 mol% tetrachlorides (ZrCl
4-HfCl
4) and 34.0 mol% salts (NaCl-KCl, 8:29 molar). The experiment results for this molten salt mixture showed that the molten salt system selected can be melted completely and consists of a liquid and vapor phase in the temperature range, 304 °C to 410 °C [
28].
For the design of an extractive distillation column, the vapor-liquid phase equilibrium behavior of the tetrachloride mixtures in the selected molten salt homogeneous solution needs to be known. To build up a proper vapor-liquid phase equilibrium model of the tetrachlorides in the selected molten salt mixture, the experimental data was taken from [
28], and correlated with Raoult’s law-type behavior with the Antoine vapor pressure model. For simplicity, the system was assumed to be a binary mixture composed of two hypothetical components: ZrCl
4 with the molten salts (
i.e., ZrCl
4-KCl-NaCl component) and HfCl
4 with the molten salts (
i.e., HfCl
4-KCl-NaCl component). Raoult’s law was chosen by considering the expected ideal solution behavior of the liquid phase due to the structural similarity and the ideal gas behavior under the low-pressure conditions. The extended Antoine equation [
29] was chosen for a rigorous prediction of the hypothetical component vapor pressure. The Antoine equation coefficients of each hypothetical component were then obtained by non-linear regression by minimizing the absolute average deviations between the predicted and experimental results for the total vapor pressure. The experimental data for the total vapor pressure was taken at the bubble point temperature, which consists only of the vapor or liquid phase. Note that in the experiment [
28], the amounts of ZrCl
4 and HfCl
4 varied for the different runs, whereas the total composition of ZrCl
4 and HfCl
4 was maintained at 66.0 mol% in the salt system.
The resulting Antoine equations for the hypothetical ZrCl
4 and HfCl
4 components are as follows:
where P and T denote the vapor pressure in kPa and temperature in K, respectively.
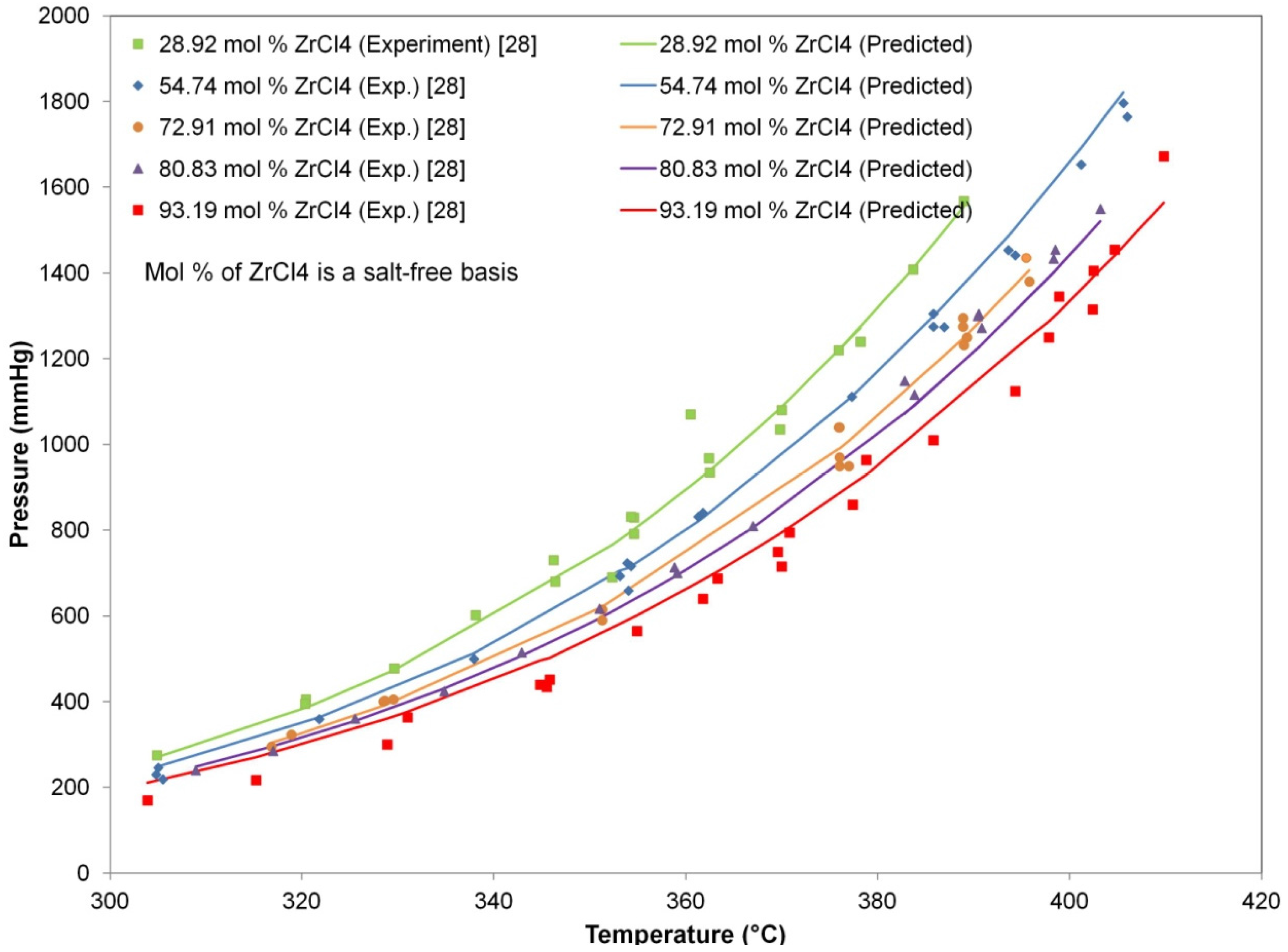
Figure 1 compares the predicted and experimental results of the total pressure for various compositions and temperatures. The total pressure predicted by Raoult’s law with the extended Antoine equation showed good agreement with the experimental values.
Figure 1.
Comparison of the predicted and experimental results of the total pressure for different ZrCl4 compositions in the molten salt system (34.0 mol% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
Figure 1.
Comparison of the predicted and experimental results of the total pressure for different ZrCl4 compositions in the molten salt system (34.0 mol% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
Table 1 lists the experimental data of the total pressure used for regression and the values predicted from the regression model. The resulting absolute average deviation (AAD) defined by Equation (3) was a small enough value of 4.15%, which indicates satisfactory prediction ability of the regression model for design purposes.
where N is the number of experimental data.
Table 1.
Experimental and predicted total pressure for the different ZrCl4 composition in the molten salt system (34.0 mol% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
Table 1.
Experimental and predicted total pressure for the different ZrCl4 composition in the molten salt system (34.0 mol% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
| Composition of ZrCl4, mol% (Salt-Free Basis) | Temperature (°C) | Pressure (mmHg) |
|---|
| Experiment [28] | Predicted |
|---|
| 28.92 | 383.7 | 1408 | 1410 |
| 389 | 1568 | 1555 |
| 375.9 | 1220 | 1218 |
| 346.2 | 730 | 675 |
| 354.6 | 830 | 802 |
| 362.5 | 935 | 939 |
| 360.5 | 1070 | 903 |
| 378.2 | 1240 | 1272 |
| 370 | 1080 | 1088 |
| 362.4 | 968 | 937 |
| 354.3 | 832 | 797 |
| 346.4 | 680 | 678 |
| 338.1 | 602 | 569 |
| 354.6 | 792 | 802 |
| 369.8 | 1035 | 1083 |
| 352.3 | 690 | 765 |
| 329.6 | 478 | 473 |
| 320.4 | 405 | 386 |
| 320.3 | 395 | 385 |
| 304.9 | 275 | 270 |
| 54.74 | 353.1 | 693 | 705 |
| 385.8 | 1305 | 1296 |
| 385.8 | 1275 | 1299 |
| 401.2 | 1653 | 1692 |
| 393.6 | 1454 | 1485 |
| 405.6 | 1797 | 1821 |
| 394.3 | 1442 | 1503 |
| 386.9 | 1274 | 1325 |
| 377.3 | 1112 | 1112 |
| 361.3 | 832 | 821 |
| 406 | 1764 | 1851 |
| 361.8 | 841 | 829 |
| 353.9 | 723 | 710 |
| 354 | 659 | 711 |
| 337.9 | 500 | 513 |
| 321.8 | 359 | 363 |
| 305 | 246 | 249 |
| 305.5 | 219 | 252 |
| 354.3 | 716 | 716 |
| 304.8 | 230 | 248 |
| 72.91 | 388.9 | 1295 | 1247 |
| 375.9 | 1040 | 989 |
| 388.9 | 1275 | 1247 |
| 395.5 | 1435 | 1399 |
| 376 | 1040 | 991 |
| 329.5 | 405 | 399 |
| 318.9 | 323 | 318 |
| 328.5 | 400 | 391 |
| 351.3 | 615 | 621 |
| 376 | 970 | 991 |
| 377 | 950 | 1009 |
| 389.3 | 1250 | 1256 |
| 389 | 1232 | 1250 |
| 395.8 | 1380 | 1406 |
| 376 | 950 | 991 |
| 351.3 | 590 | 621 |
| 328.6 | 402 | 391 |
| 316.8 | 295 | 304 |
| 80.83 | 382.8 | 1149 | 1072 |
| 390.5 | 1305 | 1226 |
| 398.5 | 1455 | 1406 |
| 403.2 | 1550 | 1521 |
| 398.3 | 1433 | 1401 |
| 390.4 | 1300 | 1224 |
| 390.8 | 1272 | 1232 |
| 383.8 | 1117 | 1091 |
| 367 | 810 | 805 |
| 358.8 | 713 | 690 |
| 359.1 | 700 | 694 |
| 351 | 617 | 594 |
| 342.9 | 515 | 507 |
| 334.8 | 425 | 430 |
| 325.5 | 360 | 355 |
| 317 | 285 | 296 |
| 308.9 | 240 | 248 |
| 93.19 | 402.5 | 1405 | 1390 |
| 397.8 | 1250 | 1286 |
| 404.7 | 1455 | 1440 |
| 409.8 | 1672 | 1564 |
| 398.9 | 1345 | 1310 |
| 378.8 | 964 | 929 |
| 370.8 | 795 | 806 |
| 363.3 | 687 | 703 |
| 354.9 | 565 | 601 |
| 345.8 | 452 | 505 |
| 331 | 363 | 376 |
| 315.2 | 217 | 270 |
| 361.8 | 640 | 684 |
| 369.6 | 750 | 789 |
| 377.4 | 860 | 907 |
| 385.8 | 1010 | 1050 |
| 394.3 | 1125 | 1223 |
| 402.4 | 1315 | 1387 |
| 370 | 715 | 795 |
| 344.8 | 440 | 495 |
| 345.5 | 435 | 502 |
| 328.9 | 300 | 361 |
| 303.9 | 170 | 211 |
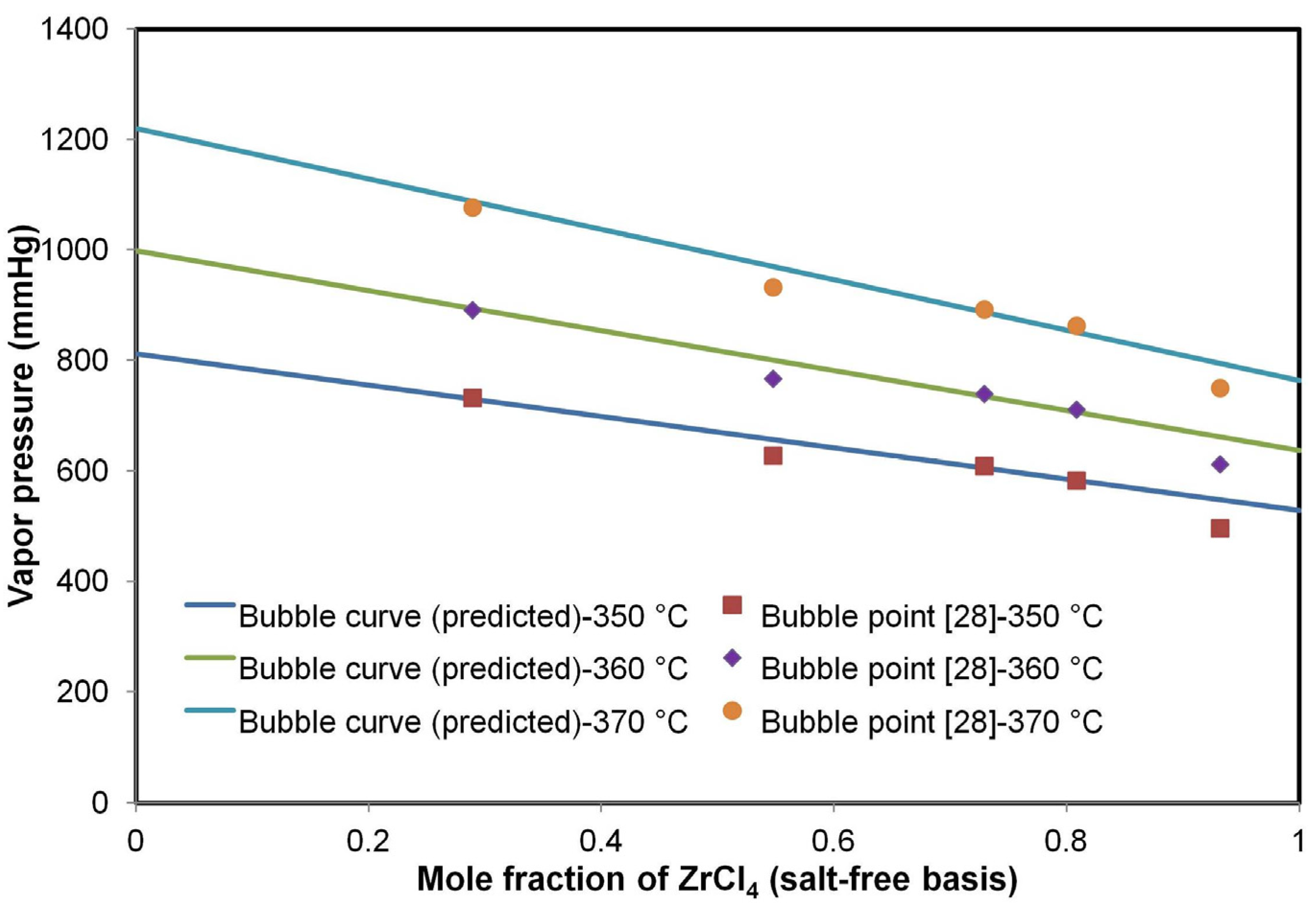
Figure 2 shows a P-x diagram of the molten salt system. The linear dependency of the total pressure to the liquid composition validates Raoult’s law for a molten salt system.
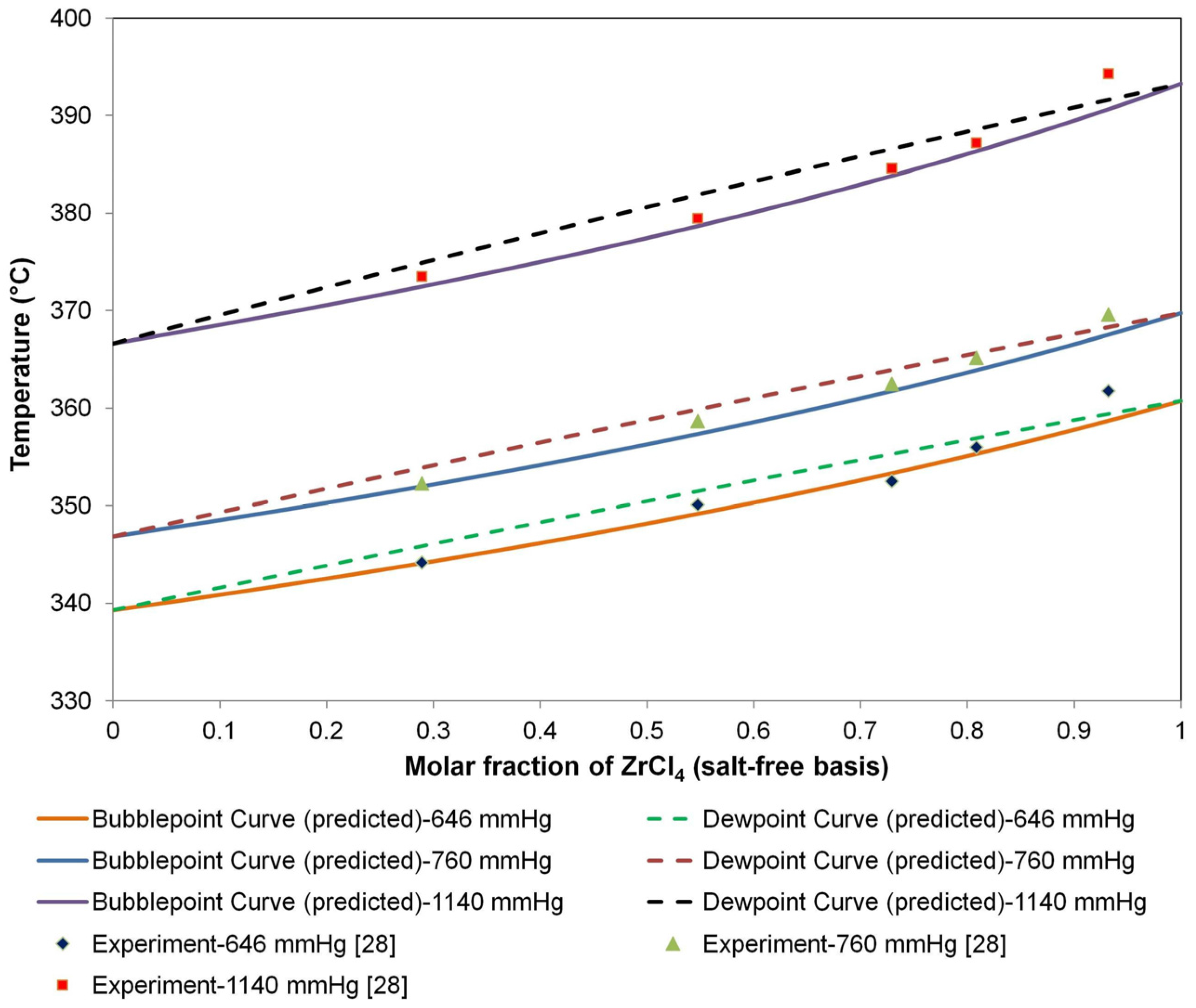
Figure 3 shows the T-x-y diagram of the molten salt system for different pressures. The predicted bubble point curves by Raoult’s law showed good agreement with the experimental data. The relatively wide region between the bubble and dew point curves also indicates the favorable properties of extractive distillation for the separation of HfCl
4 and ZrCl
4 in a molten salt solution.
Figure 2.
P-x diagram of the molten salt system (34.0% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
Figure 2.
P-x diagram of the molten salt system (34.0% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
Figure 3.
T-x-y diagram of the molten salt system (34.0% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
Figure 3.
T-x-y diagram of the molten salt system (34.0% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
3. Optimal Design of Extractive Distillation Column
To examine the feasible separation of ZrCl
4 and HfCl
4 for nuclear power reactor applications, a design study of the extractive distillation column was carried out for the molten salt system, 66.0 mol% tetrachlorides (ZrCl
4-HfCl
4) and 34.0 mol% salts (NaCl-KCl). A rigorous commercial simulator, ASPEN HYSYS 8.4, was used to simulate and design the extractive distillation column. Based on the vapor-liquid equilibrium behavior of the ZrCl
4 and HfCl
4 mixture over the molten salts, as discussed in the previous section, a modified ANTOINE fluid package model [
30] was selected from the Aspen HYSYS property library to simulate the vapor–liquid equilibrium and thermodynamic properties of the molten salt system. The modified ANTOINE fluid package model employs Raoult’s law for the vapor-liquid equilibrium behavior and Lee-Kesler model for the enthalpy calculation. The Antoine equation coefficients obtained from the regression were embedded into the modified ANTOINE fluid package model.
Table 2 lists the main properties of the two hypothetical components evaluated from the simulator.
Table 2.
Physical properties of the hypothetical ZrCl4 and HfCl4 components over the molten salt system (34.0 mol% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4) estimated from Aspen HYSYS.
Table 2.
Physical properties of the hypothetical ZrCl4 and HfCl4 components over the molten salt system (34.0 mol% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4) estimated from Aspen HYSYS.
| Properties | ZrCl4 | HfCl4 |
|---|
| Eutectic point (°C) | 218 | 234 |
| Acentricity factor | 1.126 | 1.383 |
| Critical Point | | |
| Temperature (°C) | 406.4 | 421.9 |
| Pressure (bar) | 21.15 | 20.15 |
| Volume (mL/mol) | 658.1 | 699.8 |
Based on the Hf impurity in the natural state, a crude zirconium feed mixture of 23401.5 kg/h consisting of 98.4 wt% ZrCl
4 and 1.6 wt% HfCl
4 (salt-free basis), was assumed for the column design [
9,
31]. The column was designed to obtain an ultra-purified ZrCl
4 with less than 40 ppm HfCl
4 impurity and more than 85% ZrCl
4 recovery (salt-free basis). The column was also assumed to be operated at atmospheric pressure. In the extractive distillation column, a ZrCl
4 rich molten salt product was obtained from the bottom and the HfCl
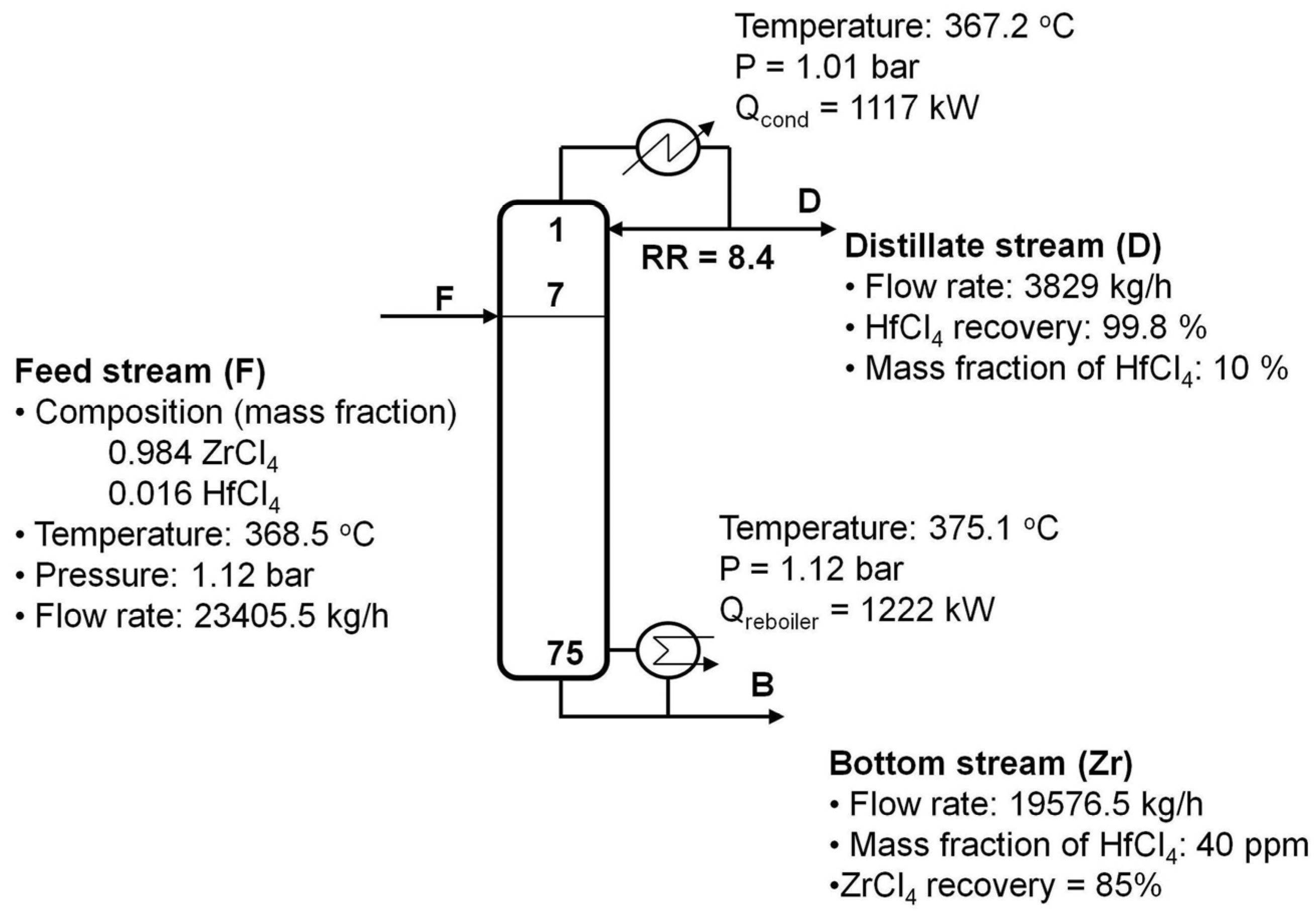
4 impurities were removed from the top of the column. For optimal design of the column, the column was initially set up using a short-cut column design facility to obtain an initial estimate for the required number of trays and the reflux ratio. The column was then simulated rigorously to determine the optimal design conditions.
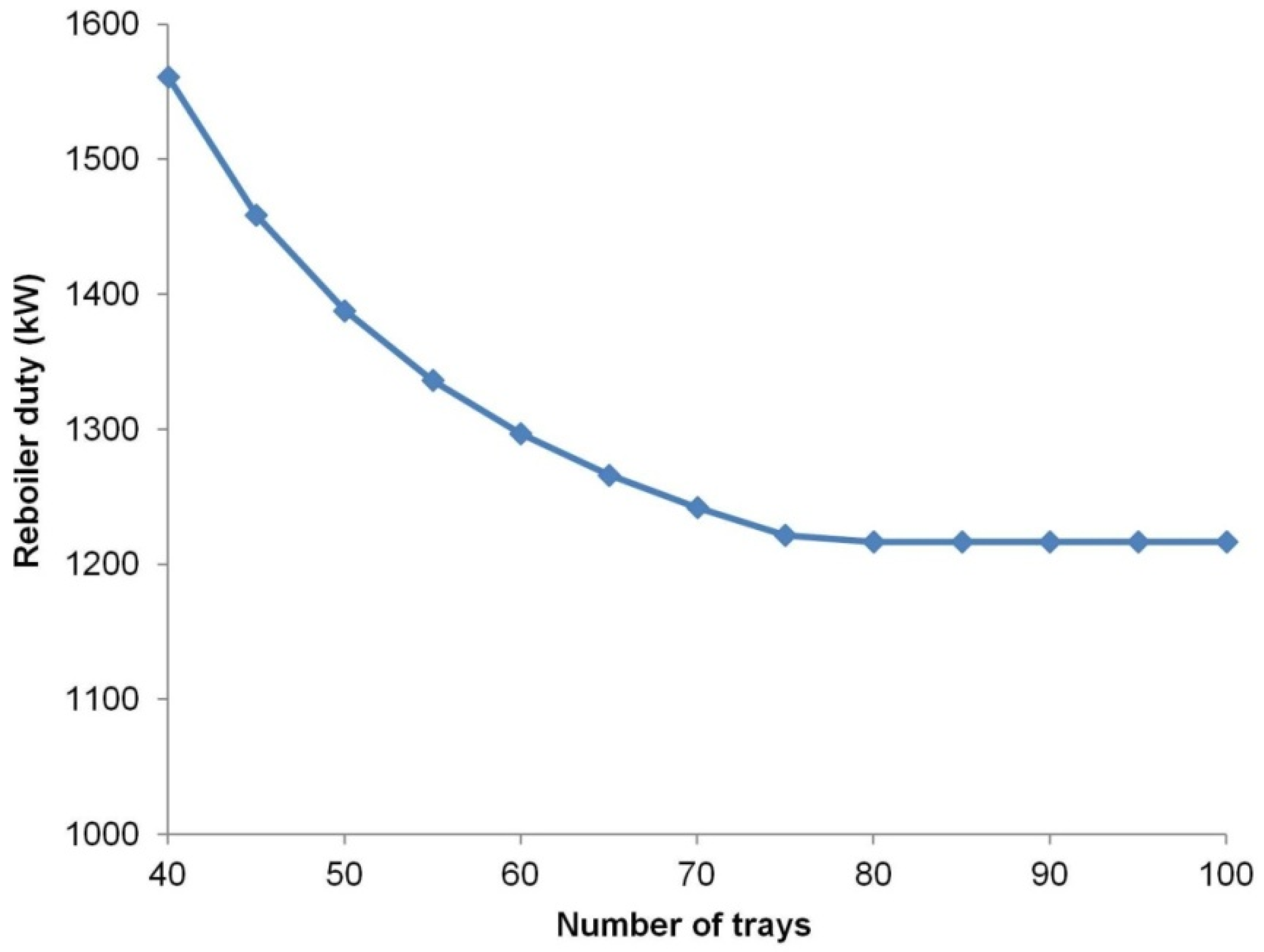
For optimization of the column structure, the number of stages was varied while keeping the product specifications.
Figure 4 presents the reboiler duty as the number of stages in the column.
Based on the sensitivity analysis, the optimal number of trays in the column was selected to be 75. In addition, the column was designed with a maximum flooding of 80% to prevent flooding in the column. To determine the maximum flooding of a particular column, the rating mode was simulated using the column internal specifications. A high reflux ratio was required to achieve the design specifications, which resulted in a somewhat high reboiler duty as 62.4 W per 1 kg/h Zr production.
Figure 5 presents a flow sheet of the resulting extractive distillation column along with its main design condition. All the composition and flow rates shown in
Figure 5 were based on a salt free basis. Note that HfCl
4 and ZrCl
4 dissolve in the molten salt as being the overhead and bottom products, respectively.
Figure 4.
Sensitivity analysis between the number of stages and the reboiler duty for the molten salt extractive distillation column.
Figure 4.
Sensitivity analysis between the number of stages and the reboiler duty for the molten salt extractive distillation column.
Figure 5.
Optimal design of extractive distillation column the molten salt system (34.0% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
Figure 5.
Optimal design of extractive distillation column the molten salt system (34.0% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4).
The bottom stream with high purity ZrCl
4 molten salt was then run through a stripper column to collect ZrCl
4 as a solid product and the solvent salt was returned to the feed storage [
32,
33,
34]. The HfCl
4-rich liquid comes off the overhead stream and is then collected in a HfCl
4 stripper unit to obtain the salt-free HfCl
4. The main drawback of the extractive distillation approach is that it requires these post treatment units for the removal of the solvent salt from the tetrachlorides, which might give a rise to significant cost. Several approaches for the removal of the solvent salt from the tetrachlorides are described elsewhere [
24].
The highly corrosive solvent nature might also have an impact on the selection of the material. Therefore, the corrosion effect is another important consideration in all such molten salt systems; thus, reducing the operation temperature and avoiding the use of excess molten salt is important. This also provides lower operating costs and allows the use of less expensive construction materials. Generally, the construction material should be chosen considering the high temperature and corrosion phenomenon. In the present study, Zr cladding was recommended for enduring the high temperature and corrosion condition in an economic manner [
35,
36]. Furthermore, a fuel furnace should be used as the high temperature generation source to boil up the bottom section of the column. Simultaneously, in view of economic advantage, the latent heat from the top vapor condensation can be utilized to generate high-pressure steam.
4. Enhanced Configuration by Heat-Pump Assisted Self-Heat Integration Technique
Heat pump technology, which allows use of the heat of condensation released at the condenser for evaporation in the reboiler, is an economic way to conserve energy when the temperature difference between the overhead and bottom of the column is small enough and the heat load is high [
35]. A heat pump on the top of the column does not change the vapor and liquid traffic inside the column. The methods used widely are the top vapor recompression, closed cycle heat pump and bottom flashing heat pump. Many studies have been developed to improve the heat pump technology for different applications [
37,
38,
39]. The heat pump can be used both in grassroots or retrofitting designs because they are easy to introduce and plant operation is normally simpler than other heat integration cases [
40]. In this study, the focus was mainly on the feasibility of the enhanced heat pump-assisted configuration by partial bottom flashing [
9,
41,
42].
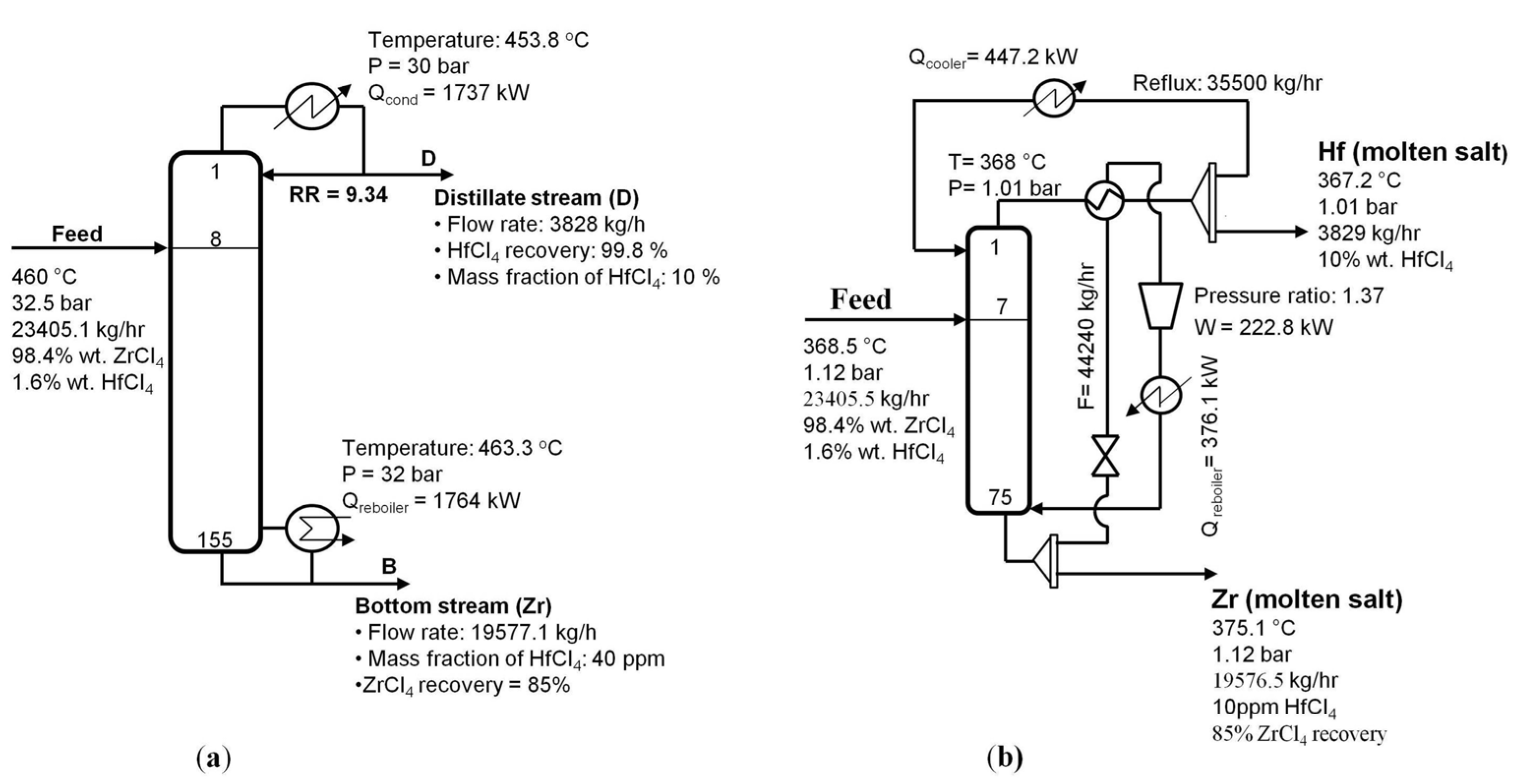
Figure 6b presents the resulting flow sheet of the extractive distillation applying the heat pump technique, comparing with the conventional distillation [
9] (
Figure 6a).
Figure 6.
Schematic diagram of (
a) conventional distillation [
9] and (
b) enhanced extractive distillation configuration using a partial bottom flashing heat pump.
Figure 6.
Schematic diagram of (
a) conventional distillation [
9] and (
b) enhanced extractive distillation configuration using a partial bottom flashing heat pump.
The bottom column outlet stream can be divided into two streams, where one stream is the bottom product of high purity ZrCl
4 and another stream is expanded in a valve to decrease its temperature, which allows heat exchange with the top stream in a heat exchanger. This heat exchanger enables boiling the bottom column outlet stream and condensing the top column outlet stream. After the heat exchanger, the bottom stream must be recompressed to the column pressure using a compressor. This stream is finally recycled to the bottom section of the column. In the present study, the pressure ratio of the compressor was selected at 1.37 to obtain a minimum approach in the heat exchanger of 10 °C. Simultaneously, a reboiler needs to produce the remaining boil-up. As a result, the use of a partial bottom flashing heat pump reduced the energy requirement in the condenser and reboiler duty significantly by 60.0% and 69.2%, respectively, compared to the case without a heat pump. The required energy consumption was reduced to 30.6 W per 1 kg/h Zr production, which is equivalent to a 51.0% reduction.
Table 3 provides a comparative summary of the key results.
Table 3.
Comparison of different distillation approaches for ultra-purification of Zr-Hf tetrachlorides.
Table 3.
Comparison of different distillation approaches for ultra-purification of Zr-Hf tetrachlorides.
| Column specification and performance | Conventional distillation [9] | Extractive distillation | Heat pump assisted extractive distillation |
|---|
| Number of stages | 155 | 75 | 75 |
| Column diameter (m) | 0.85 | 2.0 | 2.0 |
| Column temperature * (°C) | 463.3 | 375.1 | 375.1 |
| Column pressure * (bar) | 32.0 | 1.12 | 1.12 |
| Compressor duty (kW) | - | 0.0 | 222.8 |
| Condenser duty (kW) | 1737 | 1117 | 447.2 |
| Reboiler duty (kW) | 1764 | 1222 | 376.1 |
| Condenser duty saving (%) | - | 35.7 | 74.2 (60.0) |
| Reboiler duty saving (%) | - | 30.7 | 78.7 (69.2) |
| Total energy saving (%) | - | 33.2 | 70.1 (55.3) |
The performance of the proposed extractive distillation columns was compared with the conventional distillation column [
9], as shown in
Table 3. The proposed extractive distillation columns with and without a heat pump reduced the energy requirements by 70.1% and 33.2%, respectively, compared to the conventional distillation column approach. This result also showed that the proposed extractive distillation approach has many advantages over the conventional distillation for ultra-high purity Zr-Hf separation: milder design and operating conditions, such as a smaller number of theoretical stages, lower operating temperature and atmospheric operating pressure.
5. Conclusions
The feasibility of extractive distillation using molten salt was examined for the environmentally benign separation of ZrCl4 and HfCl4 for nuclear power reactor applications. Raoult’s law with the extended ANTOINE vapor pressure model predicted the total vapor pressure of ZrCl4 and HfCl4 over the molten mixture of NaCl-KCl. Adding a proper molten salt mixture leads to the increase in the relative volatility of ZrCl4 and HfCl4. This also allowed milder pressure and temperature conditions as well as a wider range for vapor–liquid phase existence for distillation applications.
The optimal design of the extractive distillation column was carried out on the molten salt system (34.0% NaCl and KCl (8:29 M); 66.0 mol% ZrCl4 and HfCl4) through a rigorous simulation to obtain an ultra-purified ZrCl4 of less than 40 ppm HfCl4 and more than 85% ZrCl4 recovery. The resulting extractive distillation column showed many preferential properties for commercial separation, such as relatively mild operating temperatures, atmospheric operation pressures and smaller number of required stages. This also required lower energy requirement for separation: 33.2% energy saving was achieved compared to the conventional distillation case. An enhanced configuration by a heat-pump-assisted self-heat integration was also proposed to enhance the energy efficiency. The proposed heat-pump-assisted configuration achieved a significant decrease in the net energy requirement: a 70.1% energy saving was achieved compared to the conventional distillation case.
Extractive distillation using a proper molten salt system can be a promising option as an environmentally benign and economically effective way of obtaining ultra-purified Zr separation for nuclear power reactor applications, with a high potential of competing with an existing commercial separation process using extraction technology.