Second Generation Ethanol Production from Brewers’ Spent Grain
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening of Ethanologenic Microorganisms for Ethanol Production in a Synthetic Medium
| Strains | Glucose (g/L) | Ethanol (g/L) |
|---|---|---|
| 40.0 | 0.0 | |
| Saccharomyces cerevisiae NRRL Y 12,908 | 0.99 | 10.52 ± 0.02 |
| Saccharomyces cerevisiae NRRL YB 2293 | 0.0 | 10.82 ± 0.03 |
| Saccharomyces cerevisiae NRRL Y 11,878 | 0.0 | 9.25 ± 0.05 |
| Saccharomyces cerevisiae NRRL Y 2034 | 0.0 | 9.56 ± 0.03 |
| Zigosaccharomyces rouxi NRRL Y 2547 | 0.0 | 9.01 ± 0.05 |
2.2. Chemical Pretreatment of BSG and Saccharification of the Pretreated BSG by a Cocktail of Commercial Enzymes
| Component | Untreated BSG | Pretreated BSG |
|---|---|---|
| Cellulose | 14.42 | 86.49 |
| Hemicellulose | 34.21 | 3.87 |
| Lignin | 3.93 | 2.31 |
| Others (ash, protein and extractives) | 47.43 | 7.33 |
| Waste Type | Waste Pretreatment | Enzymatic Hydrolysis | Yield of Glucose after Enzymatic Hydrolysis | Microorganism Used in Fermentation Step | Ethanol Production | References |
|---|---|---|---|---|---|---|
| BSG | 1.25% (v/v) H2SO4 in a ratio of 1:8 (w/w) at 120 °C for 17 min following by treatement with 2% (v/v) NaOH in a 1:20 (w/w) ratio at 120 °C for 90 min | 2.24% (v/v) cellulase (Novozymes) and 1% (v/v) β-glucosidase (Novozymes), using 8% (w/v) * substrate at 45 °C and 120 rpm for 72 h | 75 g/L glucose corresponding to 97% efficiency of cellulose conversion into glucose | Saccharomyces cerevisiae NRRL YB 2293 | 12.0 g/L ethanol, corresponding to 0.26 g ethanol/g substrate in BSG hydrolysate without adding any nutrients supplementation and 12.79 g/L ethanol, corresponding to 0.28 g ethanol/g in the yeast extract-supplemented BSG | This study |
| BSG | 20% (w/v) BSG, pretreated with 0.16 N HNO3 at 121 °C for 15 min, partially neutralized to pH 5–6 with NaOH | Novozymes Biomass sample kit (cocktail of 42.0 (U/g) ** cellulase, 1.5 (U/g) β-glucosidase, 3.0 (U/g) hemicellulase and 2.5 (U/g) xylanase) for 18 h at 50 °C, 130 rpm | 27 g/L glucose, 16.7 g/L xylose and 11.9 g/L arabinose | Pichia stipitis NCYC 1540 and | 8.3 g/L corresponding to ethanol conversion yields of 0.32 g ethanol/g substrate | [18] |
| BSG | 12.5% (w/v) BSG, pretreated with 2.5 M NaOH at 121 °C for 30 min, neutralized to pH 5–6 with H2SO4 | 17.0 (U/g) of xylanase and 3.21 (U/g) of endoglucanase from Fusarium oxysporum for 24 h at 30 °C, 1400 rpm | 52 g/L glucose | Fusarium oxysporum F3 | Ethanol conversion yields of 0.065 g ethanol/g substrate | [19] |
| BSG | 20% (w/v) BSG, pretreated with 0.16 N HNO3 at 121 °C for 15 min, partially neutralized to pH 5–6 with NaOH | Novozymes Biomass sample kit (cocktail of 42.0 (U/g) cellulase, 1.5 (U/g) β-glucosidase, 3.0 (U/g) hemicellulase and 2.5 (U/g) xylanase) for 18 h at 50 °C, 130 rpm | 27 g/L glucose, 16.7 g/L xylose and 11.9 g/L arabinose | Pichia stipitis NCYC 1540 | 14.8 g/L ethanol | [20] |
| BSG | 25% (w/v) BSG, pretreated with 5% (w/v) NaOH at 50 °C for 12 h | 51 Filter Paper Uunit (FPU)/g Cellic®CTec2 (Novozymes) for 24 h | 41.7 g/L glucose and 14.6 xylose | Saccharomyces cerevisiae strain NCYC479 | 17.3 g/L ethanol, corresponding to ca. 81% of theoretical ethanol yield | [21] |
| BSG | BSG treated with 7% (w/v) H2SO4 at 96 °C for 3 h | Enzymatic cocktail produced by Fusarium oxysporum F3 under submerged conditions | - | Fusarium oxysporum F3 | 109 g/Kg of substrate, corresponding to 60% of theoretical ethanol yield | [22] |
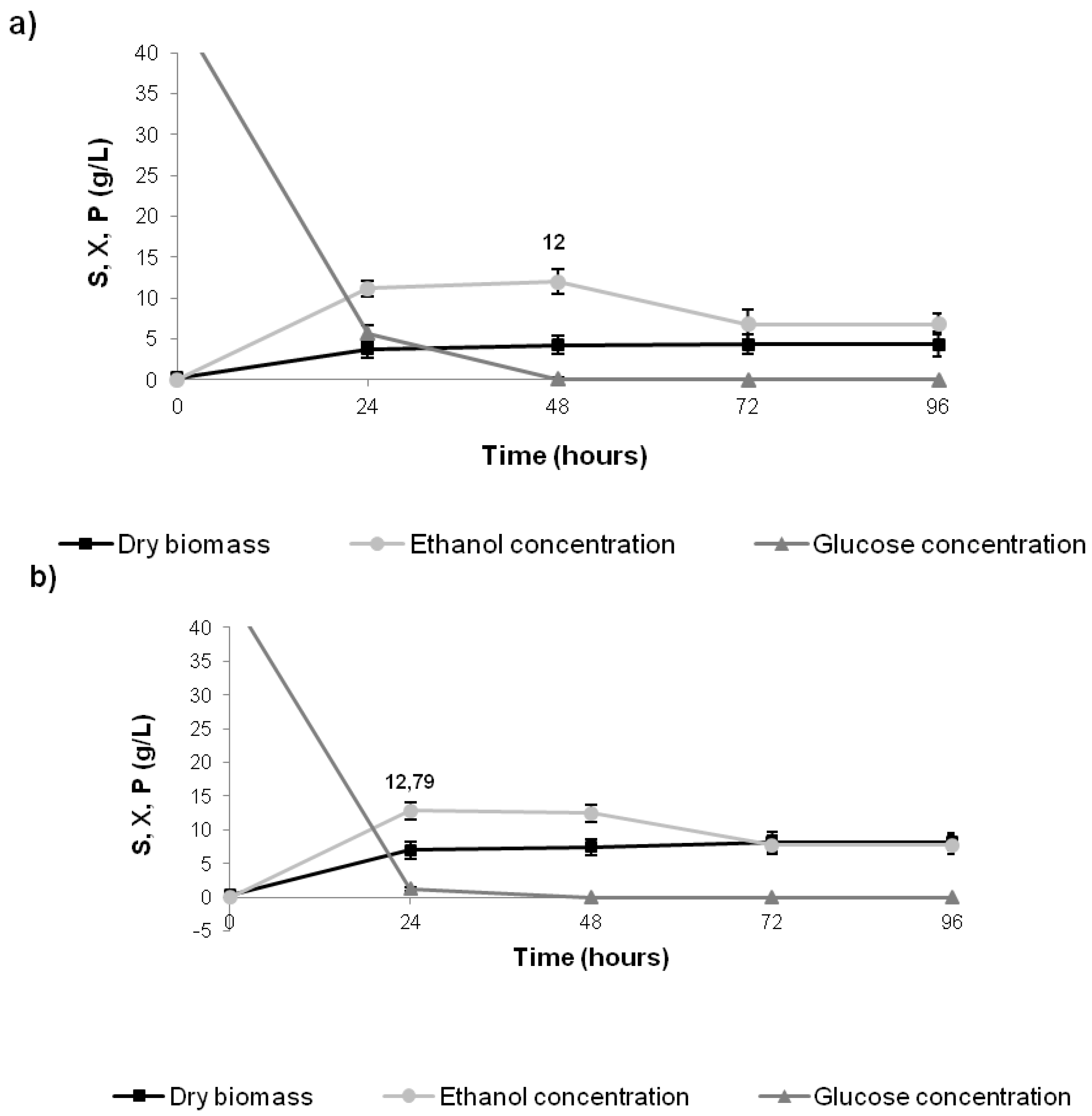
2.3. Ethanol Production Using BSG Hydrolysate as Fermentation Medium
| Medium composition | Glucose Consumption (g/L) | Ethanol (g/L) | YP/S (g/g) a | YP/X (g/g) b | QP (g/L h) c | η (%) d |
|---|---|---|---|---|---|---|
| BSG hydrolysate | 45.0 | 12.0 | 0.26 | 2.8 | 0.25 | 51 |
| BSG hydrolysate + yeast extract | 45.0 | 12.79 | 0.28 | 1.7 | 0.53 | 55 |
3. Experimental Section
3.1. Microorganisms and Cultivation Conditions for Screening in Synthetic Medium
3.2. Chemical Pretreatment of BSG and Enzymatic Hydrolysis of Pretreated Material
3.3. Inoculum and Fermentation Conditions for Cultivation in BSG Hydrolysate
3.4. Analytical Methods
3.5. Analysis of Fermentative Parameters
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amore, A.; Giacobbe, S.; Liguori, R.; Faraco, V. The second generation ethanol production. Rend. Accad. Naz. Sci. XL Mem. Sci. Fis. Naur. 2014, XXXVII, 113–136. [Google Scholar]
- Prasad, S.; Singh, A.; Joshi, H.C. Ethanol as an alternative fuel from agricultural, industrial and urban residues. Conserv. Recycl. 2007, 50, 1–39. [Google Scholar] [CrossRef]
- Liguori, R.; Amore, A.; Faraco, V. Waste valorization by biotechnological conversion into added value products. Appl. Microbiol. Biotechnol. 2013, 97, 6129–6147. [Google Scholar] [CrossRef] [PubMed]
- Dusselier, M.; Mascal, M.; Sels, B.F. Top chemical opportunities from carbohydrate biomass: A chemist’s view of the biorefinery. Top. Curr. Chem. 2014, 353, 1–40. [Google Scholar] [PubMed]
- Santos, M.; Jiménez, J.J.; Bartolome, B.; Gómez-Cordovés, C.; del Nozal, M.J. Variability of brewers’ spent grain within a brewery. Food Chem. 2003, 80, 17–21. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Balat, M. Global trends on the processing bio-fuel. Energy Explor. Exploit. 2007, 25, 195–218. [Google Scholar] [CrossRef]
- Pimentel, D.; Marklein, A.; Toth, M.A.; Karpoff, M.; Paul, G.S.; McCormack, R.; Kyriazis, J.; Krueger, T. Biofuel impacts on world food supply: Use of fossil fuel, land and water resources. Energies 2008, 1, 41–78. [Google Scholar] [CrossRef]
- Chundawat, S.; Beckham, G.; Himmel, M.; Dale, B. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu. Rev. Chem. Biomol. Eng. 2011, 2, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Cheng, J. Hydrolysis of lignocellulosic materials for ethanol production: A review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Fernandes, M.; Dragone, G.; Mancilha, I.M.; Roberto, I.C. Brewers’ spent grain as raw material for lactic acid production by Lactobacillus delbrueckii. Biotechnol. Lett. 2007, 29, 1973–1976. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, N. Chemical composition of brewers spent grain—A review. Int. J. Sci. 2014, 3, 2109–2112. [Google Scholar]
- Murdock, F.R.; Hodgson, A.S.; Riley, R.E., Jr. Nutritive value of brewers grains for lactating dairy cows. J. Dairy Sci. 1981, 64, 1826–1832. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Acid hydrolysis and fermentation of brewers’ spent grain to produce xylitol. J. Sci. Food Agric. 2005, 85, 2453–2460. [Google Scholar] [CrossRef]
- Clayton, D.; Easty, D.; Einspahr, D.; Lonsky, W.; Malcolm, E.; McDonough, T.; Schroeder, L.; Thompson, N. Pulp and Paper Manufacture, 3rd ed.; Grace, T.M., Malcolm, E.W., Eds.; The Joint Textbook Committee of the Paper Industry: Montreal, QC, Canada, 1989; Volume 5, pp. 1–128. [Google Scholar]
- Knill, C.J.; Kennedy, J.F. Degradation of cellulose under alkaline conditions. Carbohydr. Polym. 2003, 51, 281–300. [Google Scholar] [CrossRef]
- White, J.S.; Yohannan, B.K.; Walker, G.M. Bioconversion of brewer’s spent grains to bioethanol. FEMS Yeast Res. 2008, 8, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Evaluation of Fusarium oxysporum as an enzyme factory for the hydrolysis of brewer’s spent grain with improbe biodegradability for ethanol production. Ind. Crop. Prod. 2008, 28, 213–224. [Google Scholar] [CrossRef]
- Yohannan, B.K.; White, J.S.; Bennett, J.; Walker, G.M. Distiller’s spent grains: A substrate for bioethanol? In Distilled Spirits: New Horizons: Energy, Environment and Enlightenment, Proceedings of the 3rd Worldwide Conference on Distilled Spirits, Edinburgh, Scotland, UK, 7–10 September 2008.
- Wilkinson, S.; Smart, K.A.; Cook, D.J. Optimisation of alkaline reagent based chemical pre-treatment of Brewers spent grains for bioethanol production. Ind. Crop. Prod. 2014, 62, 219–227. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Enhanced ethanol production from brewer’s spent grain by a Fusarium oxysporum consolidated system. Biotechnol. Biofuels 2009, 2. [Google Scholar] [CrossRef]
- Niemi, P.; Faulds, C.B.; Sibakov, J.; Holopainen, U.; Poutanen, K.; Buchert, J. Effect of a milling pre-treatment on the enzymatic hydrolysis of carbohydrates in brewer’s spent grain. Bioresour. Technol. 2012, 116, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Xiros, C.; Topakas, E.; Katapodis, P.; Christakopoulos, P. Hydrolysis and fermentation of brewer’s spent grain by Neurospora crassa. Bioresour. Technol. 2008, 99, 5427–5435. [Google Scholar] [CrossRef] [PubMed]
- Kolothumannil, C.T.; Ingledew, W.M. Fuel alcohol production: Effects of free amino nitrogen on fermentation of very-high-gravity wheat mashes. Appl. Environ. Microbiol. 1990, 56, 2046–2050. [Google Scholar] [PubMed]
- Mussatto, S.I.; Dragone, G.; Rocha, G.J.M.; Roberto, I.C. Optimum operating conditions for brewers’ spent grain soda pulping. Carbohydr. Polym. 2006, 64, 22–28. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Wine, R.H. Use of detergents in the analysis of fibrous feeds. IV Determination of plant cell-wall constituents. Assoc. Off. Agric. Chem. J. 1967, 50, 50–55. [Google Scholar]
- Van Soest, P.J. Development of a comprehensive system of feed analysis and its application to forage. J. Anim. Sci. 1967, 26, 119–120. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liguori, R.; Soccol, C.R.; Porto de Souza Vandenberghe, L.; Woiciechowski, A.L.; Faraco, V. Second Generation Ethanol Production from Brewers’ Spent Grain. Energies 2015, 8, 2575-2586. https://doi.org/10.3390/en8042575
Liguori R, Soccol CR, Porto de Souza Vandenberghe L, Woiciechowski AL, Faraco V. Second Generation Ethanol Production from Brewers’ Spent Grain. Energies. 2015; 8(4):2575-2586. https://doi.org/10.3390/en8042575
Chicago/Turabian StyleLiguori, Rossana, Carlos Ricardo Soccol, Luciana Porto de Souza Vandenberghe, Adenise Lorenci Woiciechowski, and Vincenza Faraco. 2015. "Second Generation Ethanol Production from Brewers’ Spent Grain" Energies 8, no. 4: 2575-2586. https://doi.org/10.3390/en8042575





