On the Scale-up of Gas-Hydrate-Forming Reactors: The Case of Gas-Dispersion-Type Reactors
Abstract
:1. Introduction
2. General Consideration on Reactor Design
2.1. Modes of Reactor Operation
- (a)
- a batch operation during which neither the hydrate-forming gas nor water is discharged from, or supplied to, the reactor till the end of the operation, thereby causing a continuous decrease in pressure inside the reactor with the progress of hydrate formation;
- (b)
- a semi-batch operation during which only the hydrate-forming gas is continuously supplied to the reactor for compensating for the loss of the gas due to the hydrate formation, thereby maintaining an almost constant pressure inside the reactor; or
- (c)
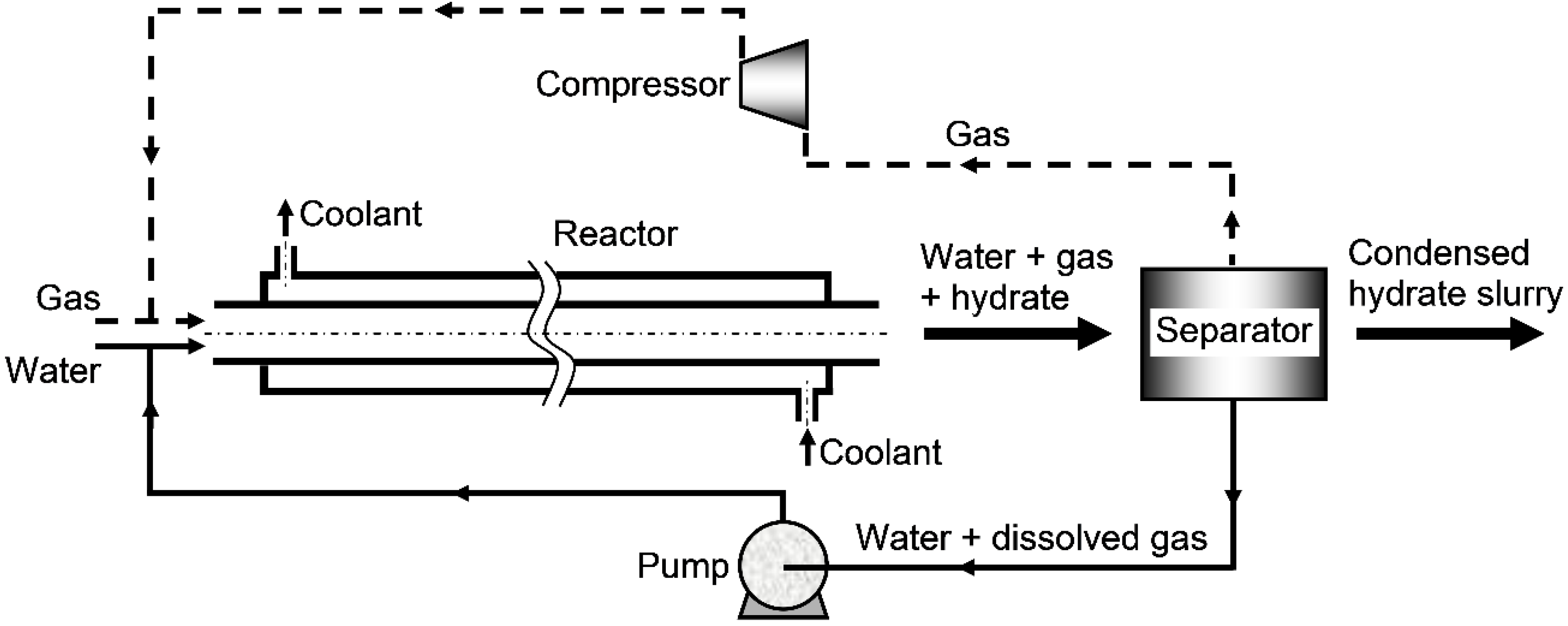
- a continuous operation during which both the hydrate-forming gas and water are continuously supplied to the reactor and a hydrate slurry (i.e., a mixture of the formed hydrate and the aqueous liquid) is continuously discharged from the reactor, thereby leaving the pressure and the gas–liquid–hydrate ratio inside the reactor practically invariant.
2.2. Devices for Gas–Liquid Mixing and Heat Discharge
2.3. Structural Designs and Scale-up of Reactors
3. Specific Remarks on Reactor Scale-up
3.1. Stirred-Tank Reactors
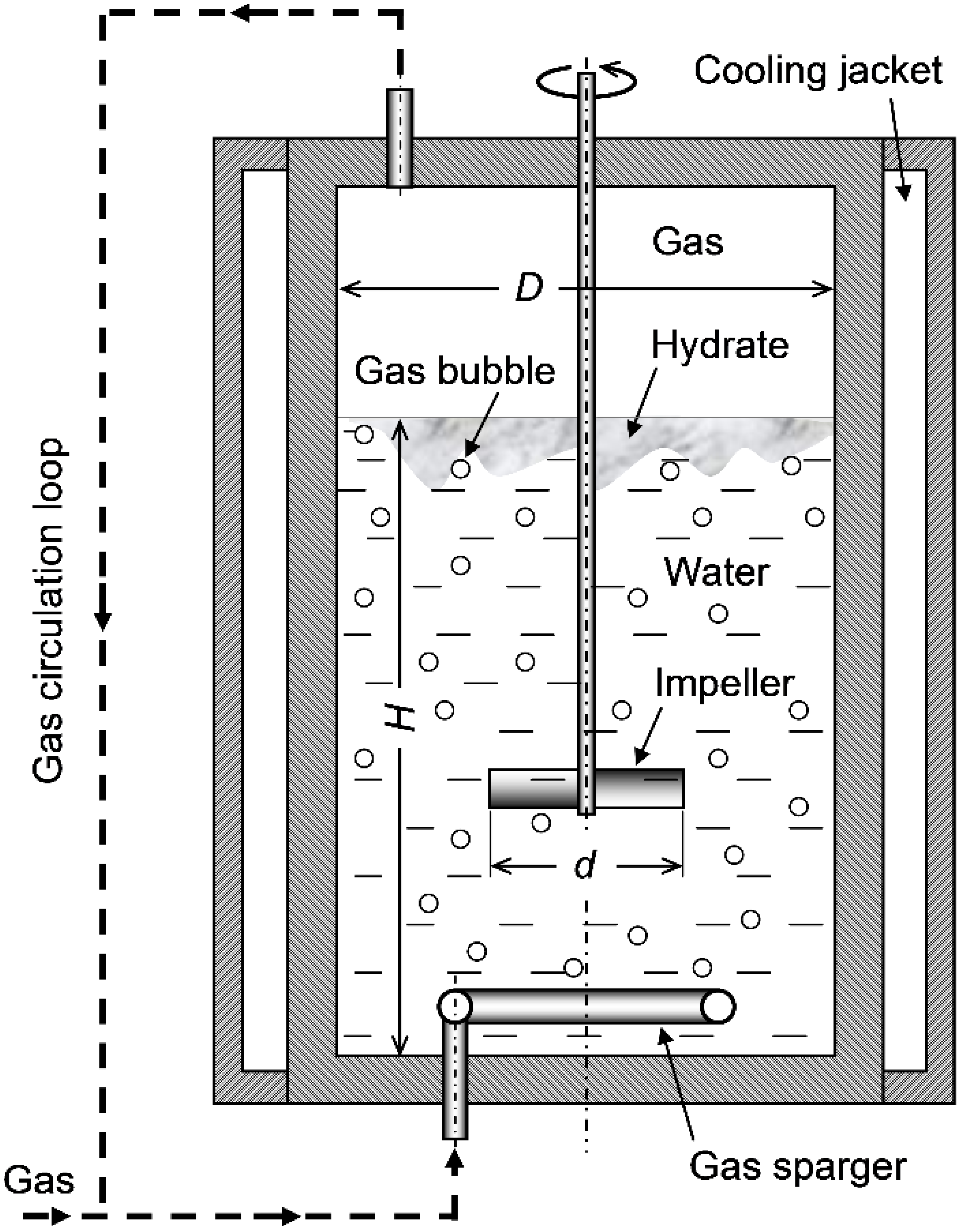
Case I: Size-Independent Liquid-to-Wall Thermal Conductance per Unit Volume
Case II: Size-Independent Gas–Liquid Flow Pattern Inside Tanks
3.2. Tubular Reactors
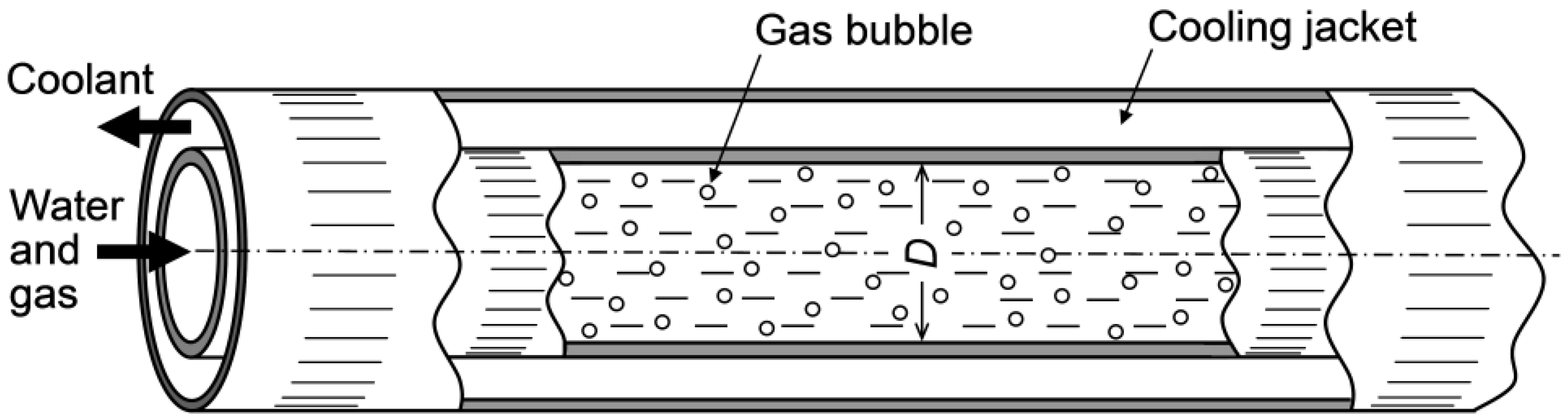
| J | case 1 | J 1/2 | 1 | 1 | J 3/5 | J | J |
| case 2 | 1 | J | 1 | J 1/5 | J 3 | J 3 | |
| case 3 | 1 | 1 | J | 1 | 1 | J | |
| 4 | case 1 | 2 | 1 | 1 | 2.30 | 4 | 4 |
| case 2 | 1 | 4 | 1 | 1.32 | 64 | 64 | |
| case 3 | 1 | 1 | 4 | 1 | 1 | 4 | |
| 16 | case 1 | 4 | 1 | 1 | 5.28 | 16 | 16 |
| case 2 | 1 | 16 | 1 | 1.74 | 4096 | 4096 | |
| case 3 | 1 | 1 | 16 | 1 | 1 | 16 |
4. Concluding Remarks
Acknowledgments
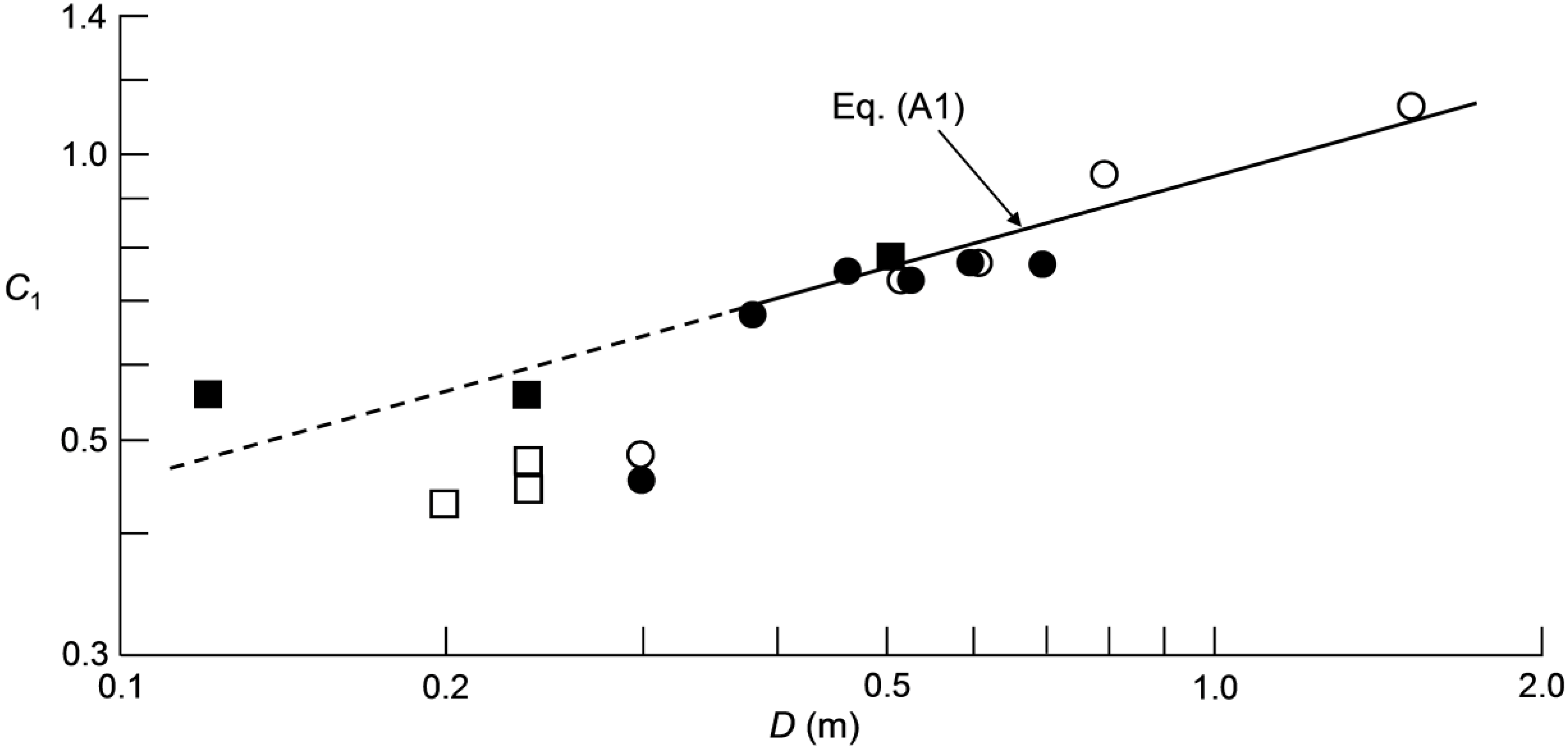
Appendix: Scale Effect on C1 and Its Consequence for Stirred-Tank Scale-up
Nomenclature
Latin letters
| A | inside surface area of a tube relevant to heat transfer, m2 |
| a | constant defined in Equation (A1), m−1/3 |
| C1 | empirical factor defined in Equation (1), dimensionless |
| C2 | constant given by Equation (4), J·K−1·m−7/3·s−1/3 |
| C3 | empirical factor defined in Equation (12), dimensionless |
| cp, g | specific heat at constant pressure of hydrate-forming gas (evaluated at bulk temperature), J kg−1 K −1 |
| cp, l | specific heat at constant pressure of liquid (evaluated at bulk temperature), J·kg−1·K−1 |
| D | inside diameter of stirred-tank or tubular reactor, m |
| d | impeller diameter, m |
, impeller-to-tank diameter ratio, dimensionless | |
| f | friction factor for water flow through a tube, dimensionless |
| Fl | , Flow number for buoyant gas flow versus stirrer-driven flow, dimensionless |
| Fr | , Froude number for stirrer-driven flow, dimensionless |
| g | acceleration due to gravity, m2·s −1 |
| H | depth of liquid pool in a stirred-tank reactor, m |
, height-to-diameter ratio of liquid pool in a stirred-tank reactor, dimensionless | |
| h | convection coefficient for heat transfer from aqueous phase to reactor wall, W·m−2·K−1 |
| J | factor of magnification in water mass flow rate, , accompanying reactor scale-up, dimensionless |
| kl | thermal conductivity of liquid (evaluated at bulk temperature), W·m−1·K−1 |
| L | axial tube length relevant to heat transfer, m |
mass flow rate of hydrate-forming gas through a tube, kg·s−1 | |
mass flow rate of liquid through a tube, kg·s−1 | |
| N | rotational speed (revolutions per second) of stirrer shaft, s−1 |
| Nt | number of tubes arranged in parallel in a tubular reactor, dimensionless |
| NuD | , Nusselt number for heat transfer from aqueous phase to reactor wall, dimensionless |
| Po | , Power number for stirring liquid by an impeller or impellers, dimensionless |
mean Po value over an estimated Red range relevant to the operation of scaled-up reactor | |
| Prl | , Prandtl number of liquid (evaluated at bulk temperature), dimensionless |
| ReD | , Reynolds number for liquid flow through a tube, dimensionless |
| Red | , Reynolds number for rotational liquid flow induced by a stirrer, dimensionless |
| U | overall coefficient of heat transfer across tube wall (based on inside tube-surface area), W·m−2·K−1 |
| uav | average axial velocity of liquid flowing through a tube, m·s−1 |
| ug | , superficial gas velocity in a stirred-tank reactor, m·s−1 |
volumetric flow rate of hydrate-forming gas, m3·s−1 | |
, volumetric gas flow rate per unit liquid volume in a stirred-tank reactor, s−1 | |
| Vil | , bulk-to-wall viscosity ratio of liquid, dimensionless |
power required for driving a stirrer or for pumping liquid through a tube against the friction working on the tube wall over axial length L, W | |
| Δp | pressure drop along the liquid flow through a tube over its axial length L, Pa |
Acronyms
| NTU | number of transfer units defined by Equation (20), dimensionless |
| STC | specific thermal conductance defined by Equation (7), W·m−3·K−1 |
Greek letters
| μl | dynamic viscosity of liquid (evaluated at bulk temperature), Pa·s |
| μls | dynamic viscosity of liquid (evaluated at reactor-wall temperature), Pa·s |
| νl | kinematic viscosity of liquid (evaluated at bulk temperature), m2·s−1 |
| ρl | mass density of liquid (evaluated at bulk temperature), kg·m−3 |
| τg | , superficial gas residence time in the liquid phase inside a stirred-tank reactor, s |
Conflicts of Interest
References
- Sloan, E.D., Jr.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008; Section 1.2.7. [Google Scholar]
- Barduhn, A.J. Desalination by crystallization processes. Chem. Eng. Prog. 1967, 63, 98–103. [Google Scholar]
- Ida, H.; Kohda, K. Highly efficient natural gas hydrate production technology. JFE Giho 2004, 6, 76–80. (In Japanese) [Google Scholar]
- Iwasaki, T.; Katoh, Y.; Nagamori, S.; Takahashi, S.; Oya, N. Continuous natural gas hydrate pellet production (NGHP) by process development unit (PDU). In Proceedings of the 5th International Conference on Gas Hydrates, Trondheim, Norway, 12–16 June 2005. Paper No. 4003.
- Horiguchi, K.; Watanabe, S.; Moriya, H.; Nakai, S.; Yoshimitsu, A.; Taoda, A. Completion of natural gas hydrate (NGH) overland transportation demonstration project. In Proceedings of the 7th International Conference on Gas Hydrates, Edinburgh, Scotland, UK, 17–21 July 2011. Paper No. P5.053.
- Nauman, E.B. Chemical Reactor Design, Optimization, and Scaleup, 2nd ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Mori, Y.H. Recent advances in hydrate-based technologies for natural gas storage—A review. J. Chem. Ind. Eng. 2003, 54, 1–17. [Google Scholar]
- Gudmundsson, J.S.; Parlaktuna, M.; Levik, O.I.; Andersson, V. Laboratory for continuous production of natural gas hydrates. Ann. N.Y. Acad. Sci. 2000, 912, 851–858. [Google Scholar] [CrossRef]
- Hutchinson, A.J.L. System for Forming and Storing Hydrocarbon Hydrates. U.S. Patent US2356407, 22 August 1944. [Google Scholar]
- Rogers, R.E.; Yevi, C.Y.; Swalm, M. Hydrates for storage of natural gas. In Proceedings of the 2nd International Conference on Natural Gas Hydrates, Toulouse, France, 2–6 June 1996; pp. 423–429.
- Ohmura, R.; Kashiwazaki, S.; Shiota, S.; Mori, Y.H. Structure-I and structure-H hydrate formation using water spraying. Energy Fuels 2002, 16, 1141–1147. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kondo, Y.; Kimura, T.; Fujimoto, T. Production Method for Hydrate and Device for Processing the Same. U.S. Patent US6653516 B1, 25 November 2003. [Google Scholar]
- Brinchi, L.; Castellani, B.; Rossi, F.; Cotana, F.; Morini, E.; Nicolini, A.; Filipponi, M. Experimental investigations on scaled-up methane hydrate production with surfactant promotion: Energy considerations. J. Pet. Sci. Technol. 2014, 120, 187–193. [Google Scholar]
- Kutergin, O.B.; Mel’nikov, V.P.; Nesterov, A.N. Surfactant effect on the mechanism and kinetics of gas hydrate formation. Dokl. Akad. Nauk 1992, 323, 549–553. (In Russian) [Google Scholar]
- Okutani, K.; Kuwabara, Y.; Mori, Y.H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: An experimental study using methane and sodium alkyl sulfates. Chem. Eng. Sci. 2008, 63, 183–194. [Google Scholar] [CrossRef]
- Ando, N.; Kodama, T.; Kondo, W.; Mori, Y.H. Clathrate hydrate formation from a methane + ethane + propane mixture in an unstirred surfactant-containing system. Energy Fuels 2012, 26, 1798–1804. [Google Scholar] [CrossRef]
- Mel’nikov, V.P.; Nesterov, A.N.; Feklistov, V.V. Device for gas hydrate formation. Russian Patent RU2166348 C1, 05 October 2001. [Google Scholar]
- Watanabe, K.; Imai, S.; Mori, Y.H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: An experimental study using HFC-32 and sodium dodecyl sulfate. Chem. Eng. Sci. 2005, 60, 4846–4857. [Google Scholar] [CrossRef]
- Seo, Y.-T.; Moudrakovski, I.L.; Ripmeester, J.A.; Lee, J.-W.; Lee, H. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels. Environ. Sci. Technol. 2005, 39, 2315–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linga, P.; Daraboina, N.; Ripmeester, J.A.; Englezos, P. Enhanced rate of gas hydrate formation in a fixed bed column filled with sand compared to a stirred vessel. Chem. Eng. Sci. 2012, 68, 617–623. [Google Scholar] [CrossRef]
- Babu, P.; Kumar, R.; Linga, P. A new porous material to enhance the kinetics of clathrate process: Application to precombustion carbon dioxide capture. Environ. Sci. Technol. 2013, 47, 13191–13198. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, S.; Tsuda, H.; Mori, Y.H. Hydrate formation using water spraying onto a cooled solid surface in a guest gas. AIChE J. 2006, 52, 2978–2987. [Google Scholar] [CrossRef]
- Murakami, T.; Kuritsuka, H.; Fujii, H.; Mori, Y.H. Forming a structure-H hydrate using water and methylcyclohexane jets impinging on each other in a methane atmosphere. Energy Fuels 2009, 23, 1619–1625. [Google Scholar] [CrossRef]
- Fujita, S.; Watanabe, K.; Mori, Y.H. Clathrate-hydrate formation by water spraying onto a porous metal plate exuding a hydrophobic liquid coolant. AIChE J. 2009, 55, 1056–1064, Errata: AIChE J. 2009, 55, 1916. [Google Scholar]
- Yamamura, K.; Fukuzaki, J.; Mori, Y.H. Clathrate hydrate formation using liquid jets impinging on each other: An observational study using paired water jets or water and methylcyclohexane jets. Chem. Eng. Sci. 2011, 66, 1844–1858. [Google Scholar] [CrossRef]
- Linga, P.; Kumar, R.; Lee, J.D.; Ripmeester, J.; Englezos, P. A new apparatus to enhance the rate of gas hydrate formation: Application to capture of carbon dioxide. Int. J. Greenh. Gas Control 2010, 4, 630–637. [Google Scholar] [CrossRef]
- Wichterle, K. Heat transfer in agitated vessels. Chem. Eng. Sci. 1994, 9, 1480–1483. [Google Scholar] [CrossRef]
- Rushton, J.H.; Costich, E.W.; Everett, H.J. Power characteristics of mixing impellers. 1. Chem. Eng. Progr. 1950, 46, 395–404. [Google Scholar]
- Rushton, J.H.; Costich, E.W.; Everett, H.J. Power characteristics of mixing impellers. 2. Chem. Eng. Progr. 1950, 46, 467–476. [Google Scholar]
- Mueller, S.G.; Dudukovic, M.P. Gas holdup in gas–liquid stirred tanks. Ind. Eng. Chem. Res. 2010, 49, 10744–10750. [Google Scholar] [CrossRef]
- Lee, B.W.; Dudukovic, M.P. Determination of flow regime and gas holdup in gas–liquid stirred tanks. Chem. Eng. Sci. 2014, 109, 264–275. [Google Scholar] [CrossRef]
- Dorstewitz, F.; Mews, D. The influence of heat transfer on the formation of hydrate layers in pipes. Int. J. Heat Mass Transf. 1994, 37, 2131–2137. [Google Scholar] [CrossRef]
- Yang, D.; Le, L.A.; Martinez, R.J.; Currier, R.P.; Spencer, D.F.; Depper, G. Heat transfer during CO2 hydrate formation in a continuous flow reactor. Energy Fuels 2008, 22, 2649–2659. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 6th ed.; Wiley: Hoboken, NJ, USA, 2011; Section 8.1.4. [Google Scholar]
- Taitel, Y.; Dukler, A.E. A model for predicting flow regime transitions in horizontal and near horizontal gas–liquid flow. AIChE J. 1976, 22, 47–55. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori, Y.H. On the Scale-up of Gas-Hydrate-Forming Reactors: The Case of Gas-Dispersion-Type Reactors. Energies 2015, 8, 1317-1335. https://doi.org/10.3390/en8021317
Mori YH. On the Scale-up of Gas-Hydrate-Forming Reactors: The Case of Gas-Dispersion-Type Reactors. Energies. 2015; 8(2):1317-1335. https://doi.org/10.3390/en8021317
Chicago/Turabian StyleMori, Yasuhiko H. 2015. "On the Scale-up of Gas-Hydrate-Forming Reactors: The Case of Gas-Dispersion-Type Reactors" Energies 8, no. 2: 1317-1335. https://doi.org/10.3390/en8021317





