Energetic and Exergetic Analysis of Combined Cycle Power Plant: Part-1 Operation and Performance
Abstract
:1. Introduction
2. Sabiya Power Plant Specification
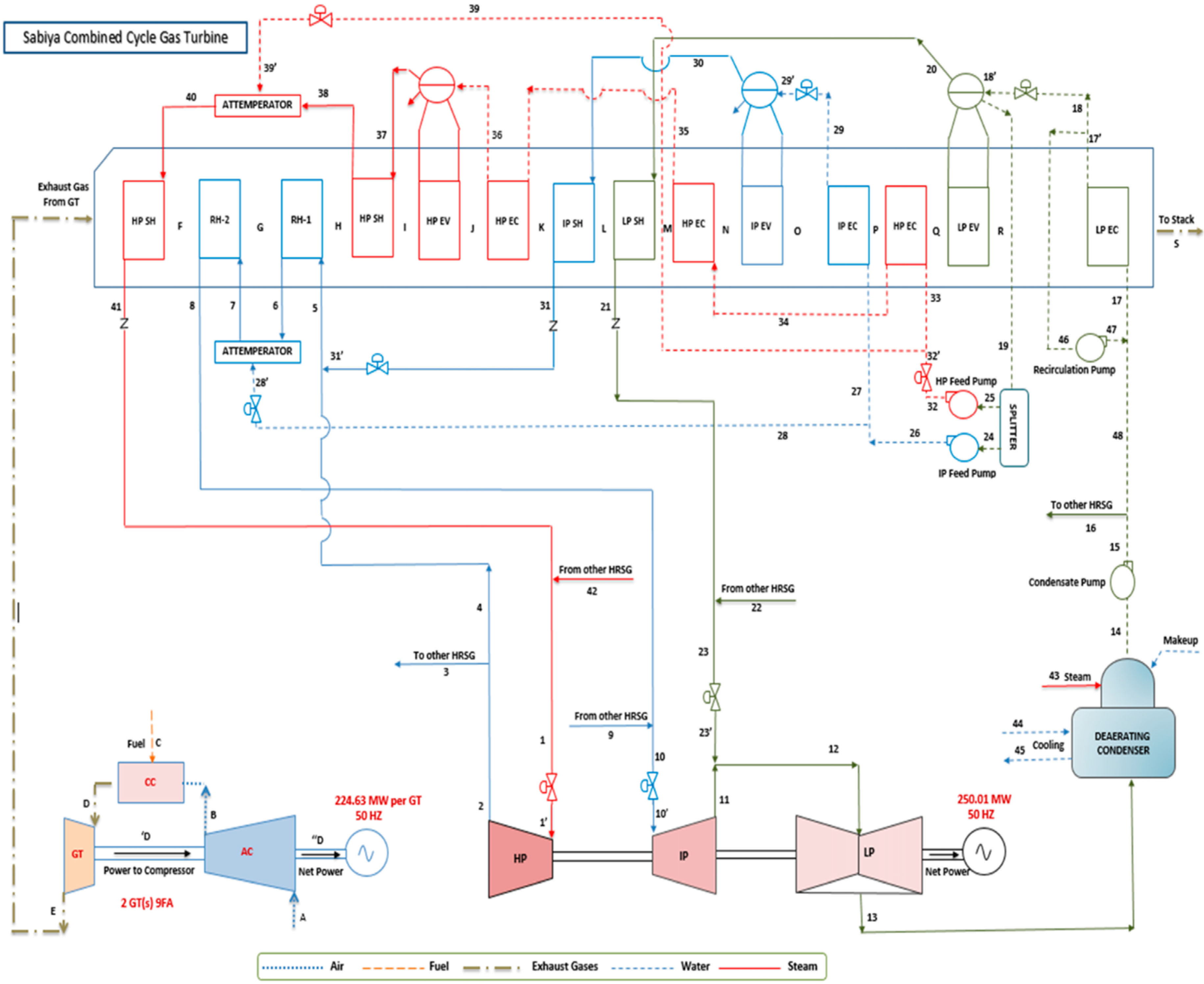
3. Performance Analysis
- The gas turbine model operated at a steady state.
- An ideal gas mixture concept is applied in both air and combustion products.
- Gas leakage is negligible.
- The kinetic and potential exergies of fluid streams are negligible.
- The combustion reaction is complete and N2 is inert.
- Heat transfer from the combustor is 2% of the natural gas lower heating value (LHV)
- Natural gas is supplied to the system as fuel (the composition is given in Table 1).
| Component | Molar Fraction (%) |
|---|---|
| Methane (CH4) | 93.34 |
| Ethane (C2H6) | 0.211 |
| Propane (C3H8) | 0.029 |
| Nitrogen (N2) | 6.42 |
| Component | Molar Fraction (%) |
|---|---|
| Nitrogen (N2) | 77.48 |
| Oxygen (O2) | 20.59 |
| Water Vapour (H2O) | 1.9 |
| Carbon dioxide (CO2) | 0.03 |
3.1. Energy Analysis
3.2. Exergy Analysis
| Component | Exergy Destruction | Exergetic Efficiency |
|---|---|---|
| Compressor & Pump | ||
| Gas & Steam Turbine | ||
| Combustion Chamber | ||
| Heat Exchanger | ||
| Cycle components |
3.3. Waste Exergy in Heat Recovery Steam Generator (HRSG)
- Exergy destroyed due to temperature difference between fluids.
- Exergy destroyed due to fluid friction as a result of movement along the pipes; and
- Energy release to the heat exchanger surroundings.
4. Simulation Tools
5. Results and Discussion
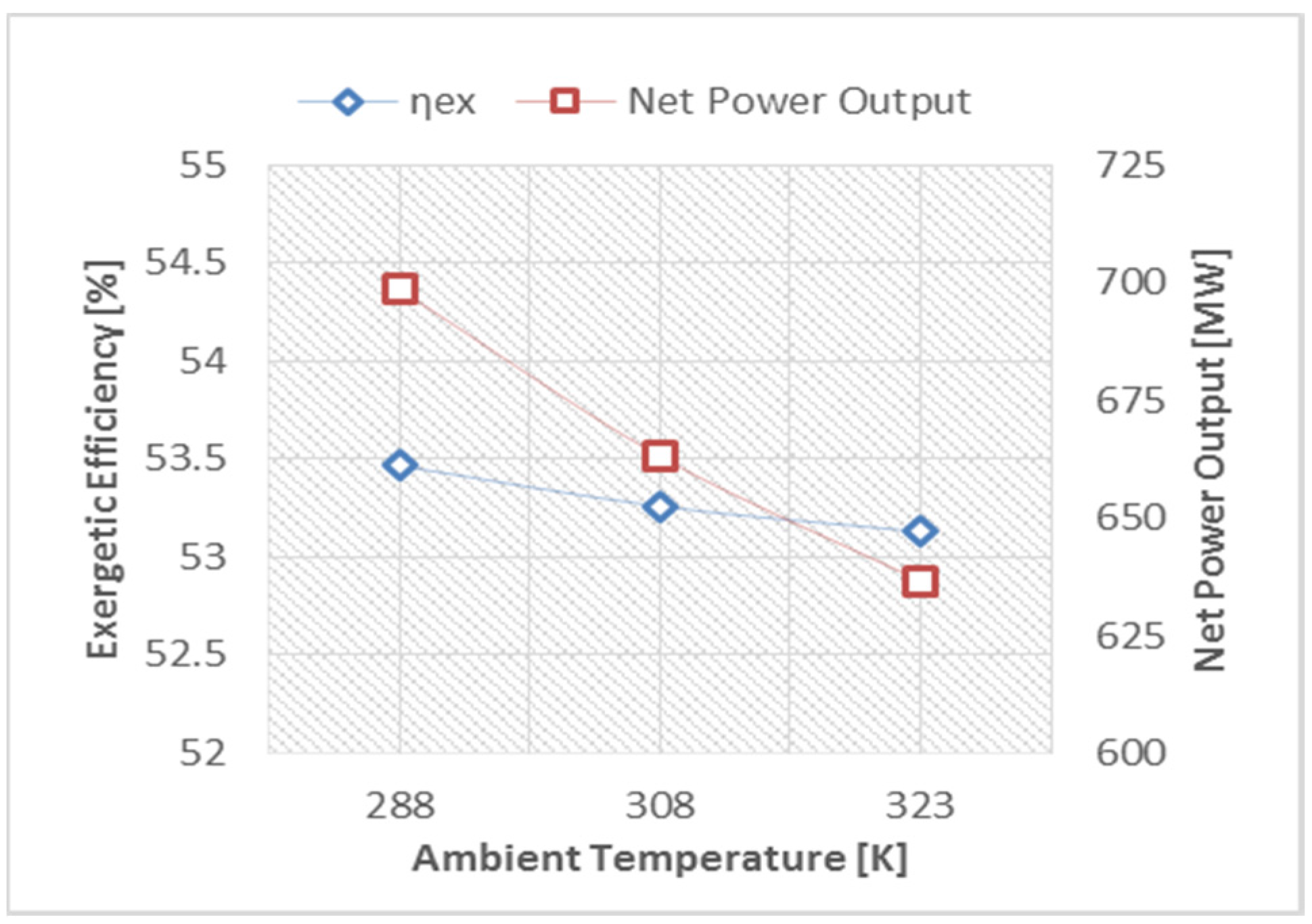
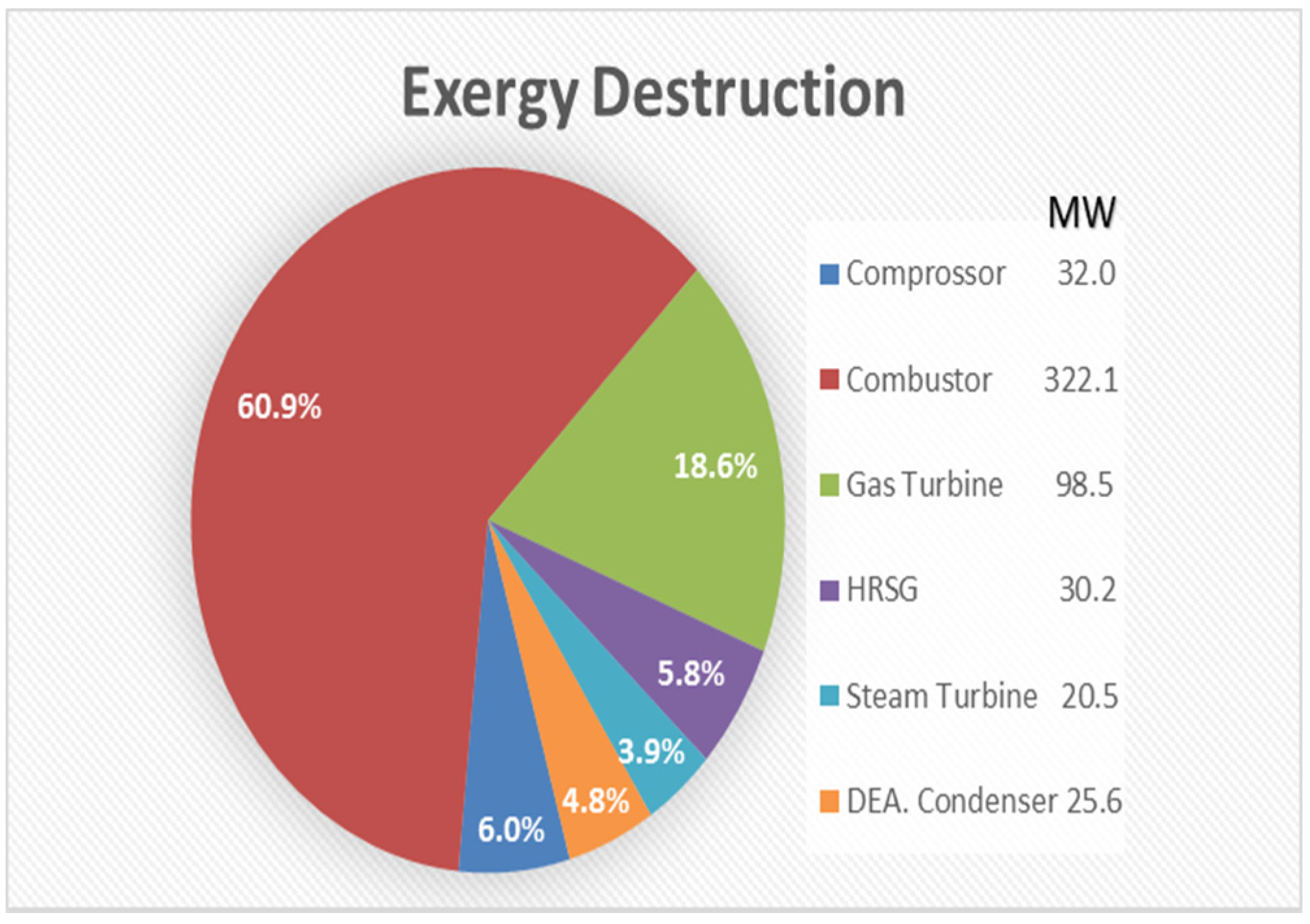
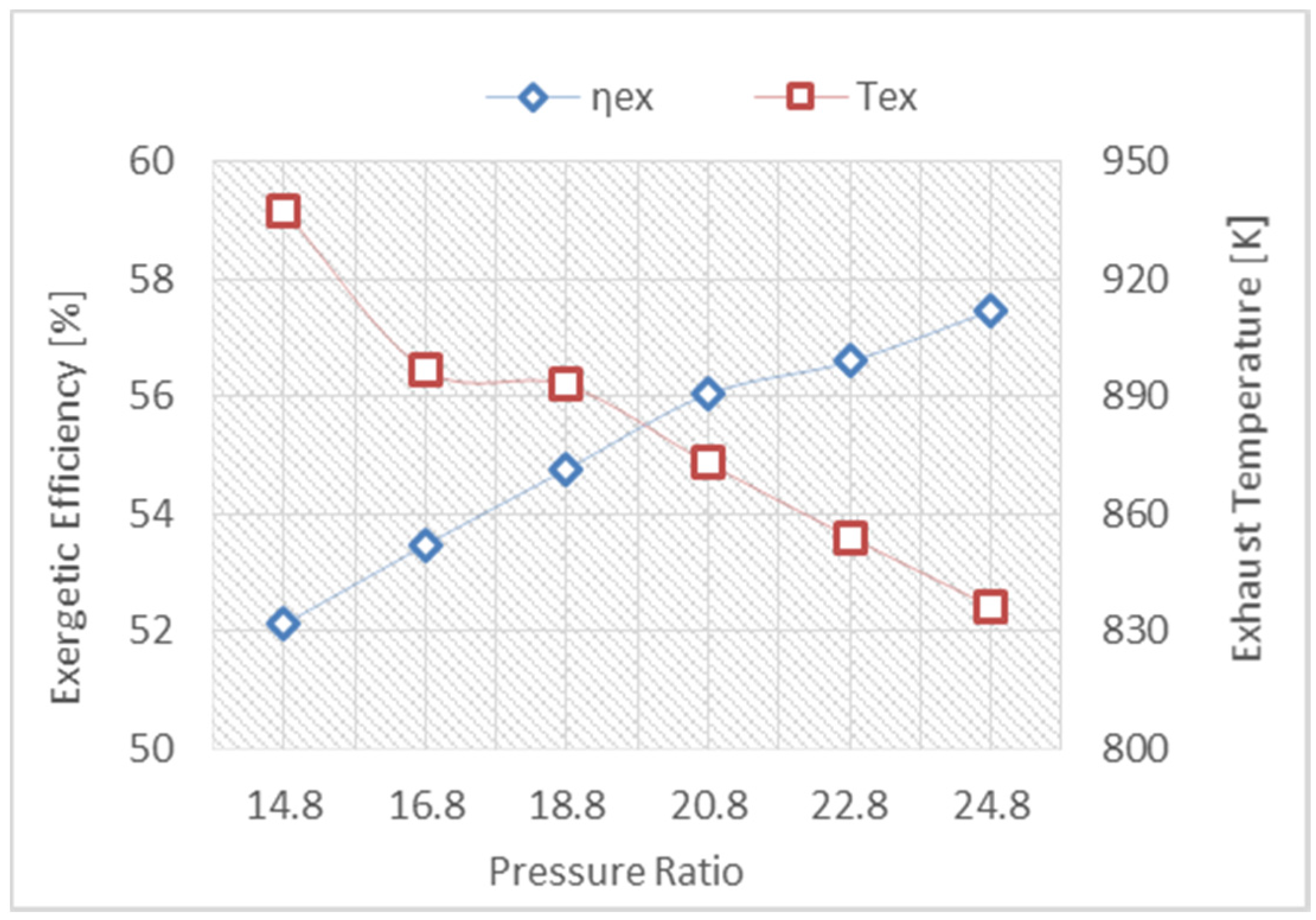

- Produce more power by using the organic Rankine cycle.
- Improve the primary mover performance by cooling the intake area using an absorption chiller.
- Alternatively, save amount of energy consumed to cool the plant building.
- Save a considerable amount of the fuel required by the industrial boiler to operate the desalination system.
6. Conclusions
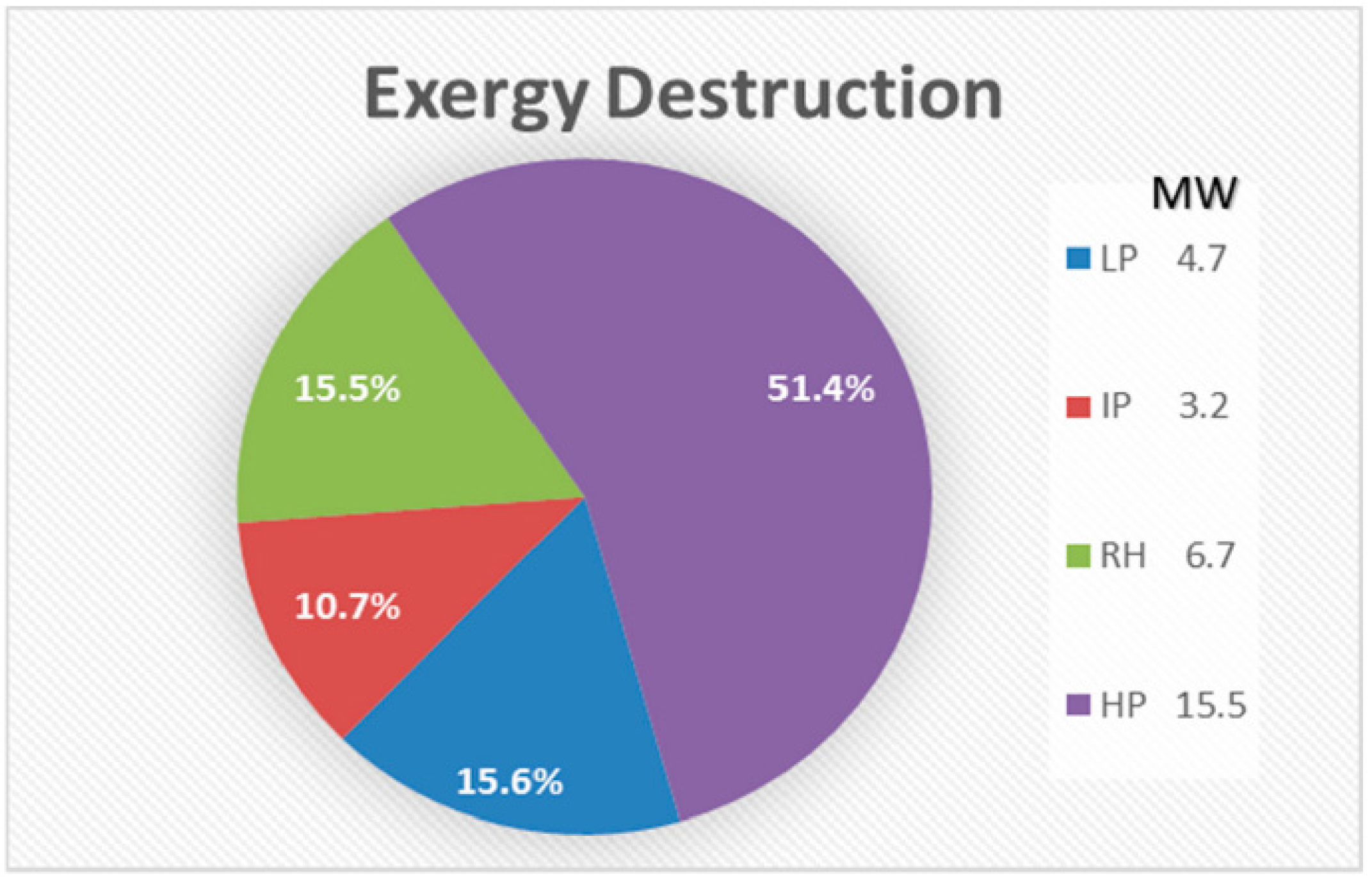
- The combustion chamber is the main source of irreversibility, with high exergy destruction attributable to three reasons: (i.) combined diffusion/fuel oxidation; (ii.) internal energy exchange–heat transfer; and (iii.) the mixing process.
- Inefficiency in the combustor can be reduced by improving the combustion process, adding an air preheater and reducing the air-to-fuel ratio.
- The steam turbine constitutes the lowest source of irreversibility as a result of the reheating system and the type of working fluid.
- The variation in ambient temperature has a significant effect on the CCPP’s exergetic efficiency and net power output, and it is recommended to use a cooling system at the point of intake of the gas turbine engine in order to achieve high power output and exergetic efficiency.
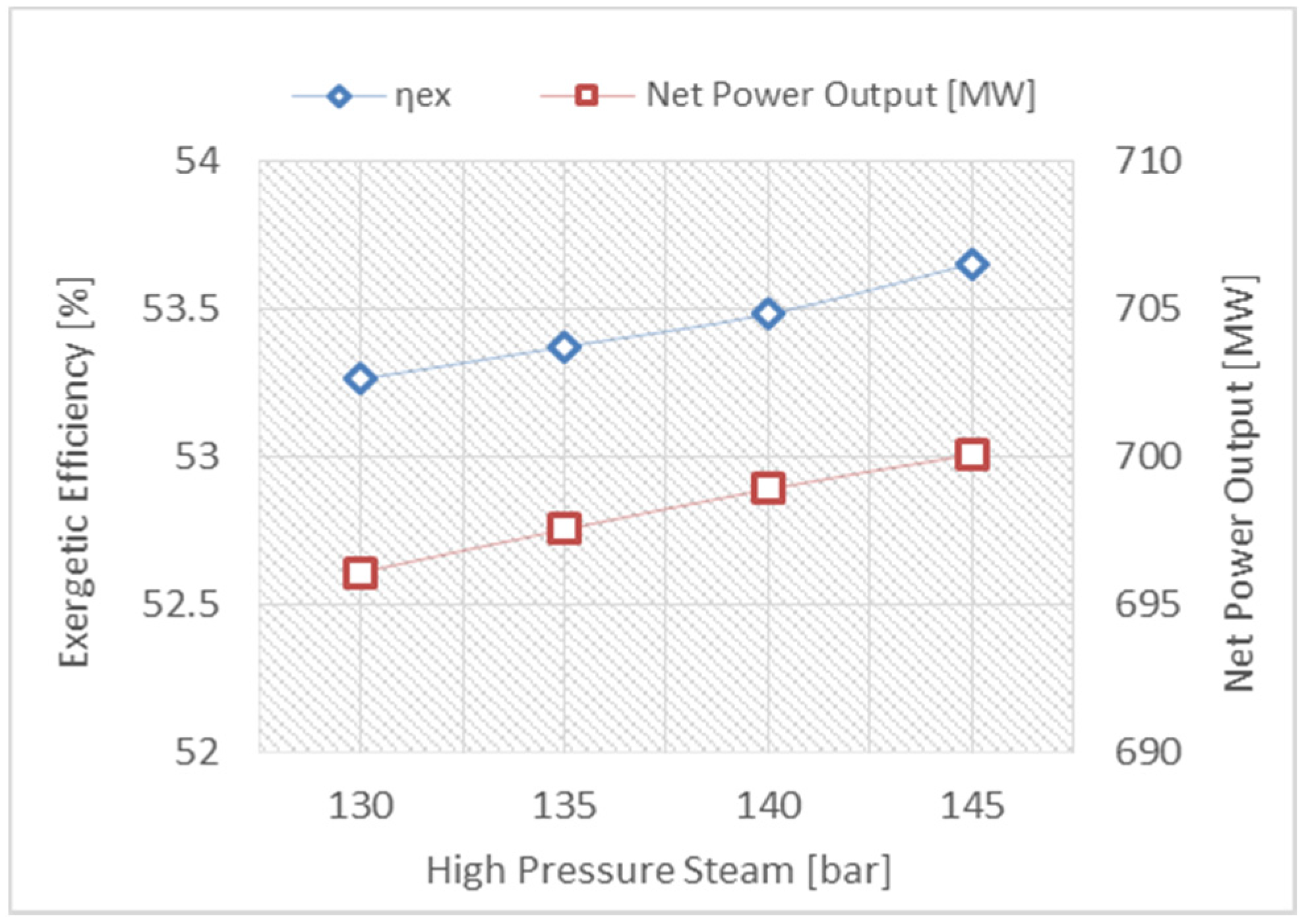
- The reheat gas turbine engine, with a high-pressure ratio, will achieve substantial improvement in the CCPP due to a reduction in the fuel consumption, producing more power and maintaining the exhaust temperature at a high level, which is compatible with the triple pressure reheat HRSG.
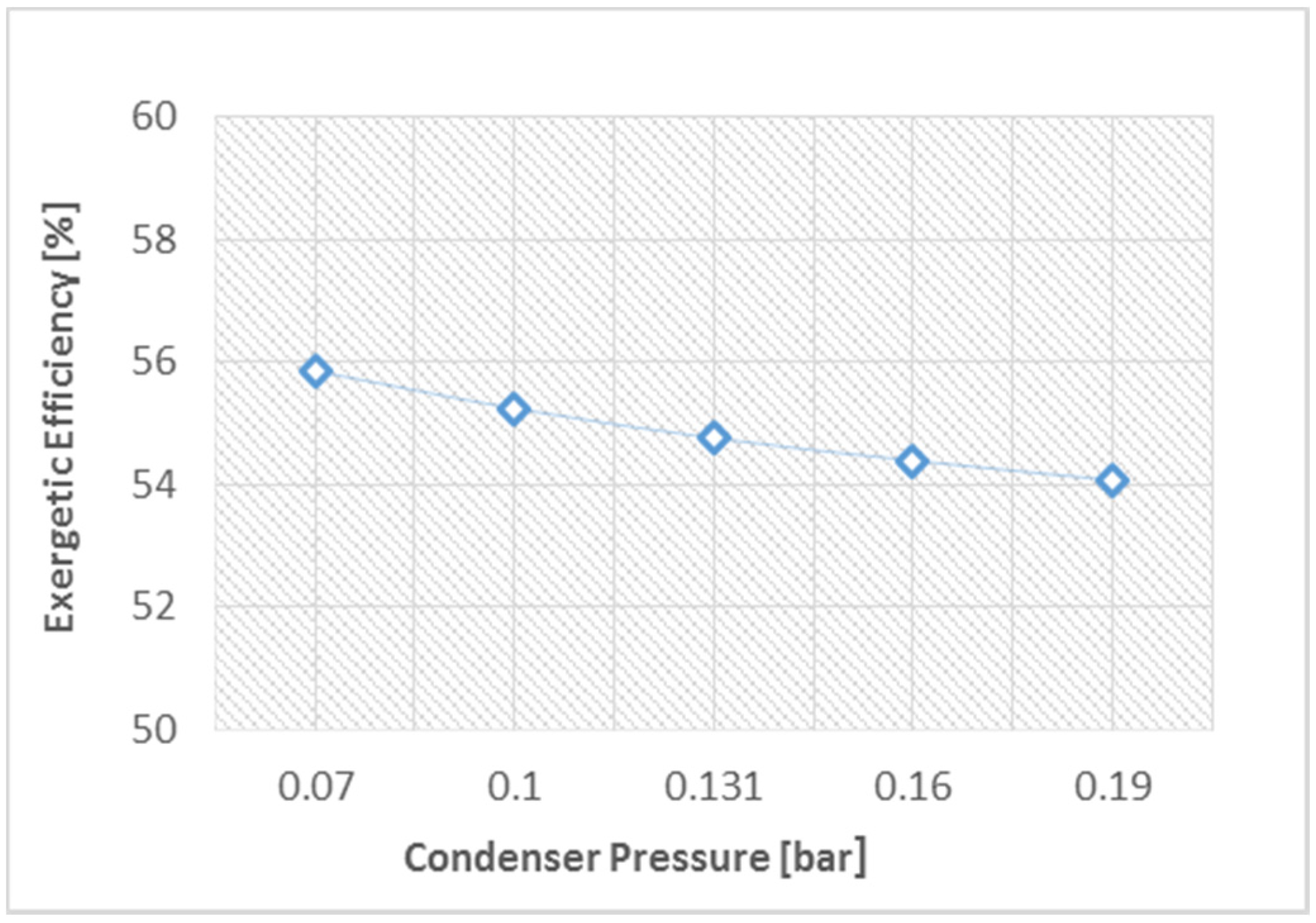
- Adjusting the steam pressure at a high value and the condenser pressure at a low value can be proposed in order to improve the CCPP’s efficiency.
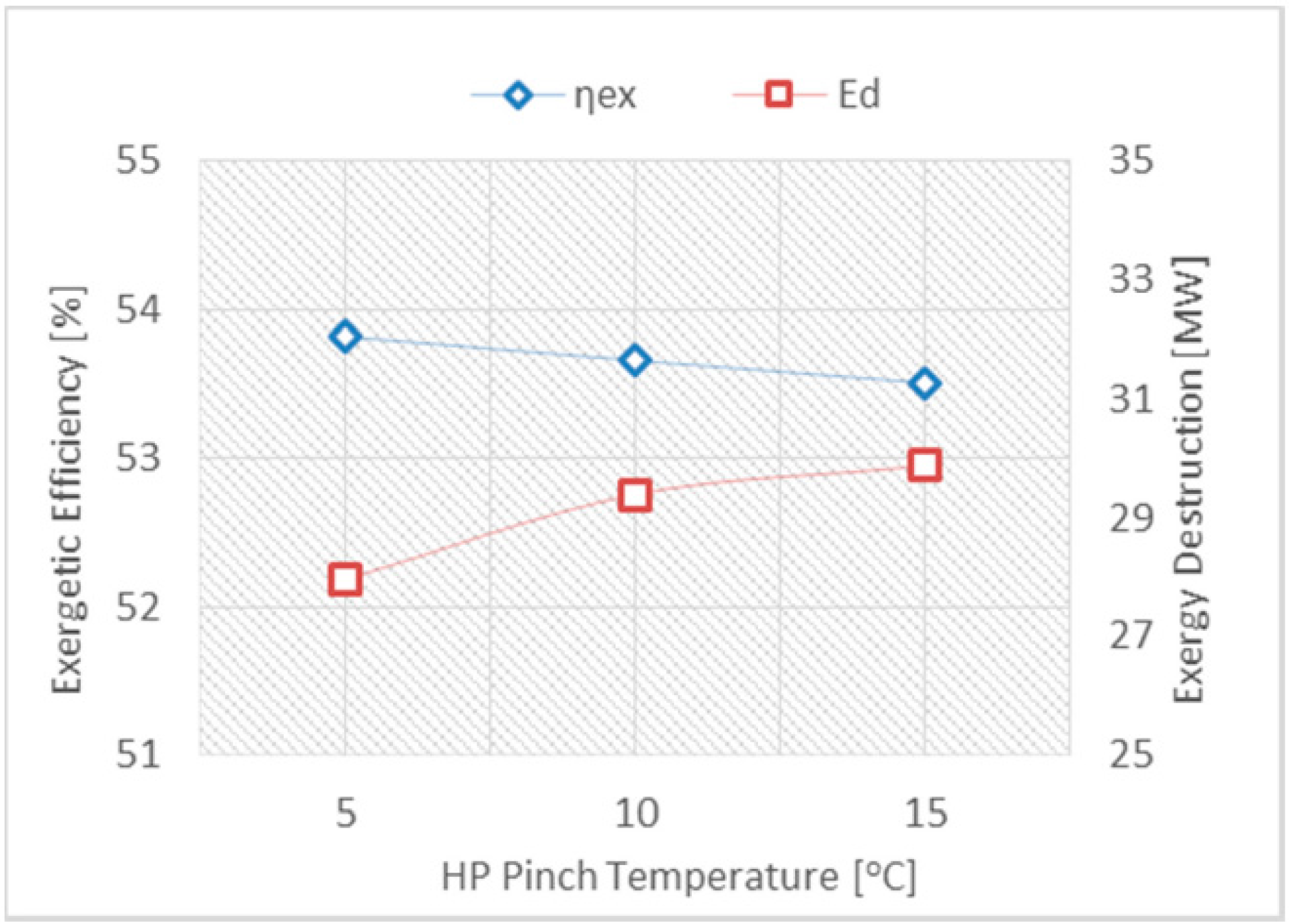
- Regulating the HP pinch temperature at a low level will augment the CCPP’s efficiency, while a high approach temperature is always preferable in order to recover more energy from the exhaust gases from the gas turbine.
- The temperature difference between the streams represents the major source of irreversibilities in the HRSG, and can be reduced either by preheating or adjusting the stream pressure.
- A large number of components in high pressure stream is not desirable, and can create an adverse effect on the performance, based on the optimum design criteria of the thermal system.
Acknowledgments
Author Contributions
Conflicts of Interest
Nomenclature
Rate of exergy flow in stream | |
Specific molar chemical exergy | |
| g | Acceleration of gravity |
| h | Specific enthalpy |
Latent heat of vaporisation | |
Low heating value in molar basis | |
Mass flow rate | |
Number of mole | |
Pressure of the stream | |
Heat transfer rate | |
Gas constant | |
Universal gas constant | |
| s | Specific entropy |
Entropy generation | |
| T | Temperature of the stream |
| y | Mole fraction |
| v | Velocity |
| V | Specific volume |
| Z | Altitude |
Power |
Greek letters
Exergetic Efficiency | |
Difference | |
Fuel-to-air ratio |
Superscripts
| ac | Actual |
| c | Cold Stream |
| ch | Chemical |
| cv | Control volume |
| d | Destruction |
| e | Outlet |
| f | Fuel |
| h | Hot Stream |
| i | Inlet |
| k | Component |
| ke | Kinetic energy |
| L | Loss |
| o | Reference state |
| p | Product |
| ph | Physical |
| pe | Potentials |
| S | Steam |
| st | Stoichiometric |
| sur | Surrounding |
Abbreviations
| AFR | Air to fuel ratio |
| AC | Air compressor |
| ATT | Attemperator |
| CC | Combustion chamber |
| CCPP | Combined Cycles Power Plant |
| EC | Economizer |
| EV | Evaporator |
| GE | General Electric |
| GT | Gas Turbine |
| HP | High-Pressure |
| IP | Intermediate-Pressure |
| ISO | International Standards Organization |
| LP | Low-Pressure |
| MEW | Ministry of Electricity and Water |
| RH | Reheat |
| SH | Super-Heater |
| ST | Steam Turbine |
Appendix A: Molar and Energy Analysis
Appendix B: Properties and Exergy Flow Rates
| Point | Location inlet outlet | Fluid | Mass flow rate (kg/s) | Temperature (K) | Pressure (bar) | Enthalpy (kJ/kg) | Entropy (kJ/kg. K) | Exergy rate (MW) | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | HP-Valve | HP SH-2 | Steam | 149.65 | 838.44 | 119.90 | 3521.05 | 6.70 | 238.28 |
| 1′ | HP ST | HP-Valve | Steam | 149.65 | 838.06 | 118.90 | 3521.05 | 6.71 | 238.12 |
| 2 | Splitter | HP ST | Steam | 149.65 | 625.82 | 27.89 | 3126.91 | 6.79 | 175.37 |
| 3 | To other HRSG | Splitter | Steam | 72.00 | 625.82 | 27.89 | 3126.91 | 6.79 | 84.37 |
| 4 | Mixer | Splitter | Steam | 77.65 | 625.82 | 27.89 | 3126.91 | 6.79 | 91.00 |
| 5 | RH-1 | Mixer | Steam | 88.36 | 620.05 | 26.71 | 3116.11 | 6.80 | 102.55 |
| 6 | IP ATT | RH-1 | Steam | 88.36 | 785.00 | 25.21 | 3488.76 | 7.35 | 121.25 |
| 7 | RH-2 | IP ATT | Steam | 88.36 | 765.00 | 25.21 | 3444.20 | 7.30 | 118.77 |
| 8 | Mixer | RH-2 | Steam | 88.36 | 838.00 | 24.01 | 3608.43 | 7.52 | 127.50 |
| 9 | Mixer | From other HRSG | Steam | 78.00 | 838.00 | 25.15 | 3607.43 | 7.50 | 112.98 |
| 10 | IP-Valve | Mixer | Steam | 166.36 | 837.75 | 23.91 | 3607.96 | 7.53 | 239.92 |
| 10′ | IP ST | IP-Valve | Steam | 166.36 | 837.74 | 23.89 | 3607.96 | 7.53 | 239.89 |
| 11 | Mixer | IP ST | Steam | 166.36 | 605.76 | 4.60 | 3132.86 | 7.62 | 156.59 |
| 12 | LP ST | Mixer | Steam | 184.31 | 603.55 | 4.60 | 3128.31 | 7.61 | 173.05 |
| 13 | DEA-Condenser | LP ST | Steam | 184.31 | 324.34 | 0.13 | 2516.56 | 7.82 | 49.17 |
| 14 | Condensate Pump | DEA-Condenser | Water | 192.99 | 322.19 | 0.13 | 205.30 | 0.69 | 1.51 |
| 15 | Splitter | Condensate Pump | Water | 192.99 | 322.33 | 22.31 | 207.81 | 0.69 | 1.95 |
| 16 | To other HRSG | Splitter | Water | 96.49 | 322.33 | 22.31 | 207.81 | 0.69 | 0.98 |
| 17 | LP EC | Mixer | Water | 193.05 | 367.77 | 18.26 | 397.73 | 1.24 | 7.91 |
| 17′ | Splitter | LP EC | Water | 193.05 | 411.17 | 12.26 | 581.20 | 1.72 | 17.01 |
| 18 | LP EV -Value | LP EC | Water | 96.55 | 411.15 | 12.26 | 581.20 | 1.72 | 8.51 |
| 18′ | LP EV | LP EV -Value | Water | 96.55 | 411.27 | 5.31 | 581.20 | 1.72 | 8.46 |
| 19 | Splitter | LP EV | Water | 87.58 | 426.56 | 5.71 | 646.91 | 1.88 | 9.47 |
| 20 | LP SH | LP EV | Steam | 8.97 | 426.52 | 5.21 | 2749.92 | 6.81 | 7.10 |
| 21 | Mixer | LP SH | Steam | 8.97 | 584.85 | 4.91 | 3088.99 | 7.51 | 8.32 |
| 22 | Mixer | From other HRSG | Steam | 8.97 | 582.00 | 4.85 | 3083.24 | 7.51 | 8.28 |
| 23 | LP-Valve | Mixer | Steam | 17.95 | 583.39 | 4.85 | 3086.11 | 7.51 | 16.59 |
| 23′ | Mixer | LP-Valve | Steam | 17.95 | 583.28 | 4.75 | 3086.11 | 7.52 | 16.54 |
| 24 | IP Feed pump | Splitter | Water | 11.43 | 426.56 | 5.71 | 646.91 | 1.88 | 1.24 |
| 25 | HP Feed pump | Splitter | Water | 76.15 | 426.56 | 5.71 | 646.91 | 1.88 | 8.23 |
| 26 | Splitter | IP Feed pump | Water | 11.43 | 426.89 | 29.38 | 649.79 | 1.88 | 1.27 |
| 27 | IP EC | Splitter | Water | 11.11 | 426.89 | 29.38 | 649.79 | 1.88 | 1.23 |
| 28 | IP ATT -Value | Splitter | Water | 0.32 | 426.89 | 29.38 | 649.79 | 1.88 | 0.04 |
| 29 | IP EV -Value | IP EC | Water | 11.11 | 499.14 | 28.98 | 971.52 | 2.57 | 2.58 |
| 29′ | IP EV | IP EV -Value | Water | 11.11 | 499.13 | 28.88 | 971.52 | 2.57 | 2.58 |
| 30 | IP SH | IP EV | Steam | 11.11 | 504.71 | 28.78 | 2803.15 | 6.20 | 10.91 |
| 31 | RH -Value | IP SH | Steam | 10.71 | 588.15 | 27.88 | 3037.76 | 6.65 | 12.05 |
| 31′ | Mixer | RH -Value | Steam | 10.71 | 588.13 | 27.87 | 3037.76 | 6.65 | 12.05 |
| 32 | HP EC -Value | HP Feed pump | Water | 76.15 | 428.68 | 158.30 | 665.44 | 1.88 | 9.55 |
| 32′ | Splitter | HP EC -Value | Water | 76.15 | 429.06 | 132.30 | 665.44 | 1.89 | 9.41 |
| 33 | HP EC-1 | Splitter | Water | 76.15 | 429.06 | 132.30 | 665.44 | 1.89 | 9.31 |
| 34 | HP EC-2 | HP EC-1 | Water | 75.36 | 506.45 | 132.25 | 1007.74 | 2.62 | 19.21 |
| 35 | HP EC-3 | HP EC-2 | Water | 75.36 | 583.98 | 131.85 | 1401.50 | 3.34 | 33.21 |
| 36 | HP EV | HP EC-3 | Steam | 75.36 | 604.01 | 131.80 | 1530.68 | 3.56 | 38.23 |
| 37 | HP SP-1 | HP EV | Steam | 74.65 | 604.79 | 131.30 | 2659.79 | 5.43 | 82.02 |
| 38 | HP ATT | HP SP-1 | Steam | 74.65 | 693.29 | 128.30 | 3101.54 | 6.12 | 100.00 |
| 39 | HP ATT -Value | Splitter | Water | 0.78 | 429.06 | 132.30 | 665.44 | 1.89 | 0.10 |
| 40 | HP SP-2 | IP ATT | Steam | 74.65 | 672.29 | 128.20 | 3028.95 | 6.02 | 96.87 |
| 41 | Mixer | HP SP-2 | Steam | 74.65 | 841.65 | 125.20 | 3524.21 | 6.69 | 119.42 |
| 42 | Mixer | From other HRSG | Steam | 75.00 | 838.00 | 122.00 | 3517.91 | 6.69 | 119.42 |
| Point | Location inlet outlet | Fluid | Mass flow rate (kg/s) | Temperature (K) | Pressure (bar) | Enthalpy (kJ/kg) | Entropy (kJ/kg. K) | Exergy rate (MW) |
|---|---|---|---|---|---|---|---|---|
| A | AC Air | Air | 577.22 | 288.00 | 1.01 | 15.01 | 6.85 | 0.00 |
| B | CC AC | Air | 577.22 | 684.64 | 17.02 | 426.47 | 6.93 | 223.91 |
| C | CC Fuel | Fuel | 14.05 | 368.00 | 18.91 | 142.27 | ---- | 663.47 |
| D | GT CC | Exhaust Gases | 591.27 | 1550.00 | 16.52 | 1532.68 | 8.22 | 717.15 |
| E | HP SH-3 GT | Exhaust Gases | 591.27 | 897.81 | 1.05 | 702.14 | 8.34 | 205.62 |
| F | RH-2 HP SH-3 | Exhaust Gases | 591.27 | 845.64 | 1.05 | 639.61 | 8.27 | 180.77 |
| G | RH-1 RH-2 | Exhaust Gases | 591.27 | 825.00 | 1.05 | 615.07 | 8.24 | 171.17 |
| H | HP SH-2 RH-1 | Exhaust Gases | 591.27 | 777.80 | 1.05 | 559.38 | 8.17 | 149.98 |
| I | HP EV HP SH-2 | Exhaust Gases | 591.27 | 730.00 | 1.05 | 503.61 | 8.10 | 129.51 |
| J | HP EC-3 HP EV | Exhaust Gases | 591.27 | 613.00 | 1.04 | 369.78 | 7.90 | 84.29 |
| K | IP SH HP EC-3 | Exhaust Gases | 591.27 | 598.37 | 1.04 | 353.32 | 7.87 | 79.09 |
| L | LP SH IP SH | Exhaust Gases | 591.27 | 594.59 | 1.04 | 349.07 | 7.86 | 77.69 |
| M | HP EC-2 LP SH | Exhaust Gases | 591.27 | 590.00 | 1.04 | 343.92 | 7.86 | 76.03 |
| N | IP EV HP EC-2 | Exhaust Gases | 591.27 | 545.00 | 1.04 | 293.73 | 7.77 | 61.33 |
| O | IP EC IP EV | Exhaust Gases | 591.27 | 513.87 | 1.03 | 259.33 | 7.70 | 51.96 |
| P | HP EC-1 IP EC | Exhaust Gases | 591.27 | 508.38 | 1.03 | 253.28 | 7.69 | 50.30 |
| Q | LP EV HP EC-1 | Exhaust Gases | 591.27 | 468.55 | 1.03 | 209.65 | 7.60 | 39.62 |
| R | LP EC LP EV | Exhaust Gases | 591.27 | 429.50 | 1.03 | 167.23 | 7.51 | 30.54 |
| S | ------- LP EC | Exhaust Gases | 591.27 | 373.88 | 1.03 | 107.33 | 7.36 | 20.46 |
References
- Bejan, A.; Moran, M.J. Thermal Design and Optimization; John Wiley & Sons: Hoboken, NJ, USA, 1996. [Google Scholar]
- Dincer, I.; Rosen, M.A. Exergy: Energy, Environment and Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Aydin, H. Exergetic sustainability analysis of LM6000 gas turbine power plant with steam cycle. Energy 2013, 57, 766–774. [Google Scholar] [CrossRef]
- Tawney, R.K.; Ugolini, D.J.; Wengert, T.J.; Narula, R.G. Steam cycle selection considerations for combined cycleplant. In Proceedings of the Joint Power Generation Conference, Boston, MA, USA, 21–25 October 1990.
- Kotas, T.J. The Exergy Method of Thermal Plant Analysis; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Facchini, B.; Fiaschi, D.; Manfrida, G. Exergy analysis of combined cycles using latest generation gas turbines. J. Eng. Gas Turbines Power 2000, 122, 233–238. [Google Scholar] [CrossRef]
- Moran, M.J.; Shapiro, H.N.; Boettner, D.D.; Bailey, M.B. Fundamentals of Engineering Thermodynamics; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Rahim, M.A. Combined cycle power plant performance analyses based on the single-pressure and multipressure heat recovery steam generator. J. Energy Eng. 2011, 138, 136–145. [Google Scholar] [CrossRef]
- Rahim, M.A. Thermodynamic evaluation and parametric study of a combined cycle power plant: Application for Ankara City. J. Energy Eng. 2013, 140, 1–10. [Google Scholar] [CrossRef]
- Ersayin, E.; Ozgener, L. Performance analysis of combined cycle power plants: A case study. Renew. Sustain. Energy Rev. 2015, 43, 832–842. [Google Scholar] [CrossRef]
- Ameri, M.; Ahmadi, P.; Khanmohammadi, S.H. Exergy analysis of a 420 MW combined cycle power plant. Int. J. Energy Res. 2008, 32, 175–183. [Google Scholar] [CrossRef]
- Sue, D.-C.; Chuang, C.-C. Engineering design and exergy analyses for combustion gas turbine based power generation system. Energy 2004, 29, 1183–1205. [Google Scholar] [CrossRef]
- Petrakopoulou, F.; Tsatsaronis, G.; Morosuk, T.; Carassai, A. Conventional and advanced exergetic analyses applied to a combined cycle power plant. Energy 2012, 41, 146–152. [Google Scholar] [CrossRef]
- Ministry of Electricity and Water (MEW), State of Kuwait. Private Communication.
- López Paniagua, I.; Rodríguez Martín, J.; González Fernandez, C.; Jiménez Alvaro, Á.; Nieto Carlier, R. A new simple method for estimating exergy destruction in heat exchangers. Entropy 2013, 15, 474–489. [Google Scholar] [CrossRef]
- Jin, Z.; Dong, Q.; Liu, M. Exergy study of fouling factors in heat exchanger networks. J. Heat Transf. 2010, 132, 011802. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almutairi, A.; Pilidis, P.; Al-Mutawa, N. Energetic and Exergetic Analysis of Combined Cycle Power Plant: Part-1 Operation and Performance. Energies 2015, 8, 14118-14135. https://doi.org/10.3390/en81212418
Almutairi A, Pilidis P, Al-Mutawa N. Energetic and Exergetic Analysis of Combined Cycle Power Plant: Part-1 Operation and Performance. Energies. 2015; 8(12):14118-14135. https://doi.org/10.3390/en81212418
Chicago/Turabian StyleAlmutairi, Abdulrahman, Pericles Pilidis, and Nawaf Al-Mutawa. 2015. "Energetic and Exergetic Analysis of Combined Cycle Power Plant: Part-1 Operation and Performance" Energies 8, no. 12: 14118-14135. https://doi.org/10.3390/en81212418







