Characteristics of Biochar Obtained by Hydrothermal Carbonization of Cellulose for Renewable Energy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrothermal Carbonization Process
2.3. Analytical Procedures
2.4. Thermogravimetric Analysis
| TGA Conditions | |
|---|---|
| Sample weight | 10 mg |
| Heating rate | 10 °C/min |
| Atmosphere | Air |
| Gas flow rate | 150 mL/min |
| Starting temperature | 45 °C |
| Final holding temperature | 900 °C |
3. Results and Discussion
3.1. Improvement of Cellulose Properties
| Hydrothermal Carbonization at (°C) | 150 | 180 | 200 | 220 | 250 | 280 |
|---|---|---|---|---|---|---|
| Proximate Analysis (wt. %, d.b. 1) | ||||||
| Volatile matter | 88.9 | 89.3 | 84.0 | 63.6 | 56.9 | 42.2 |
| Fixed carbon | 9.6 | 9.5 | 14.7 | 35.0 | 41.2 | 55.1 |
| Ash | 1.5 | 1.2 | 1.3 | 1.4 | 1.9 | 2.7 |
| Ultimate Analysis (wt. %, d.b. 1) | ||||||
| C | 43.9 | 45.6 | 51.2 | 63.5 | 69.4 | 76.5 |
| H | 6.6 | 6 | 5.7 | 4.7 | 4.6 | 4.5 |
| N | 0 | 0 | 0 | 0 | 0 | 0 |
| O | 48 | 47.2 | 41.8 | 30.4 | 24.1 | 16.3 |
| Calorific value (MJ/kg, d.b. 1) | 16.6 | 18.9 | 23 | 26.5 | 26.8 | 27.7 |
| Energy Recovery | ||||||
| Product yield (%) 2 | 92.5 | 80.5 | 62.4 | 58.4 | 50.4 | 46.2 |
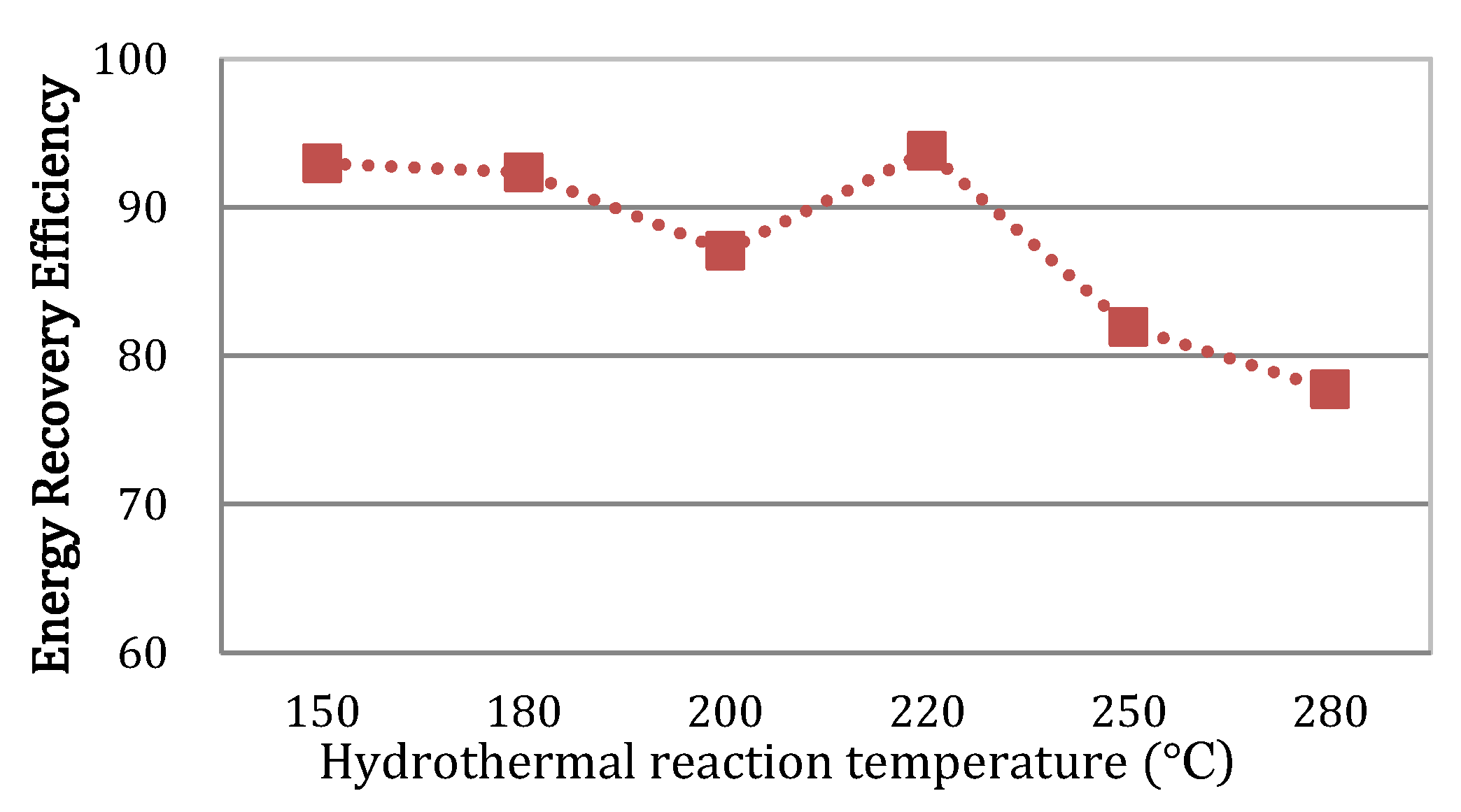
| Energy recovery efficiency (%) 3 | 93.0 | 92.4 | 87.1 | 93.8 | 81.9 | 77.7 |
| Energy densification 4 | 1.01 | 1.15 | 1.39 | 1.61 | 1.62 | 1.68 |
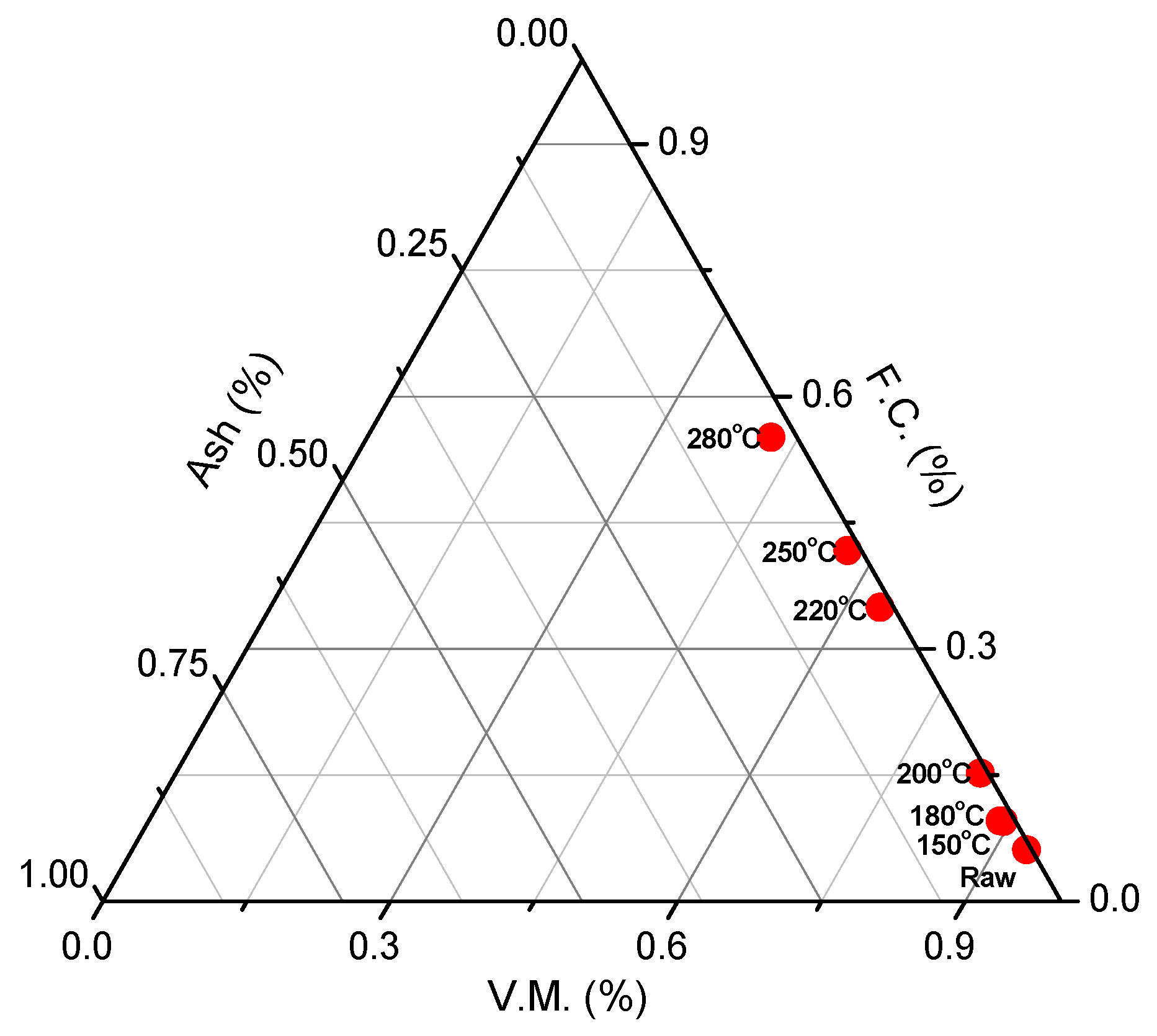
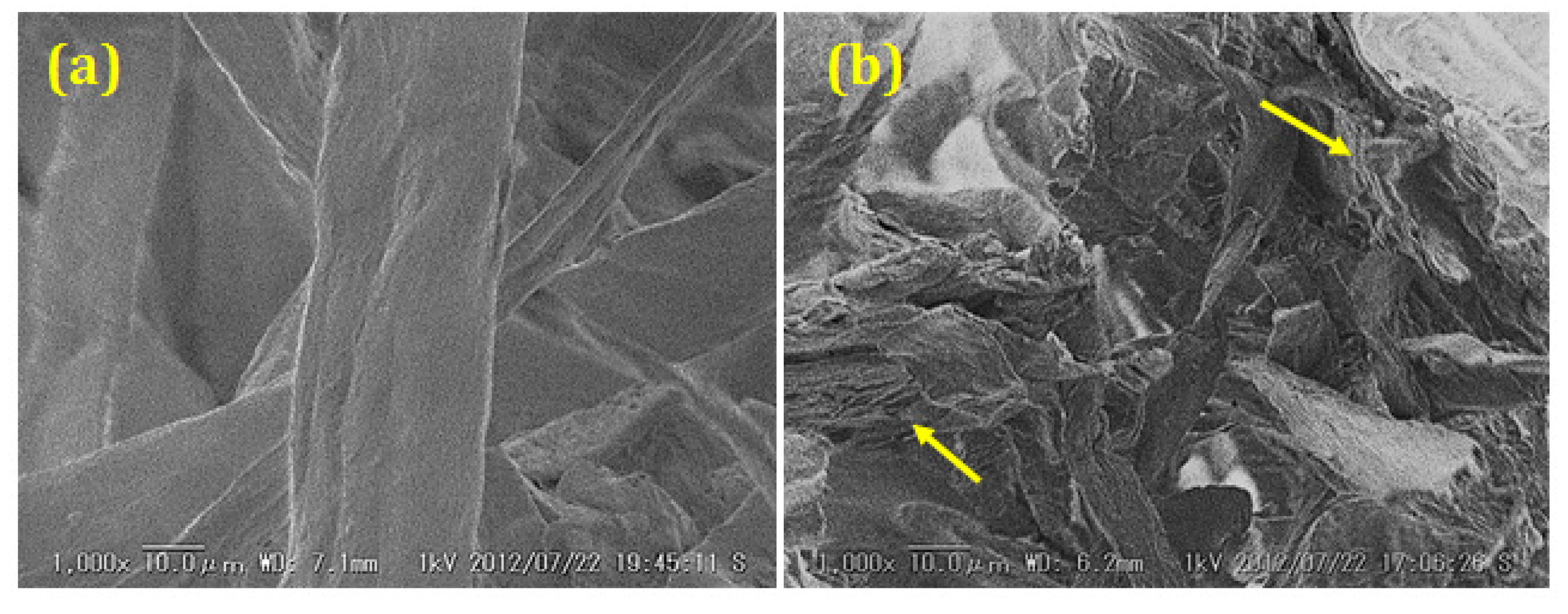
3.2. Coalification of Cellulose
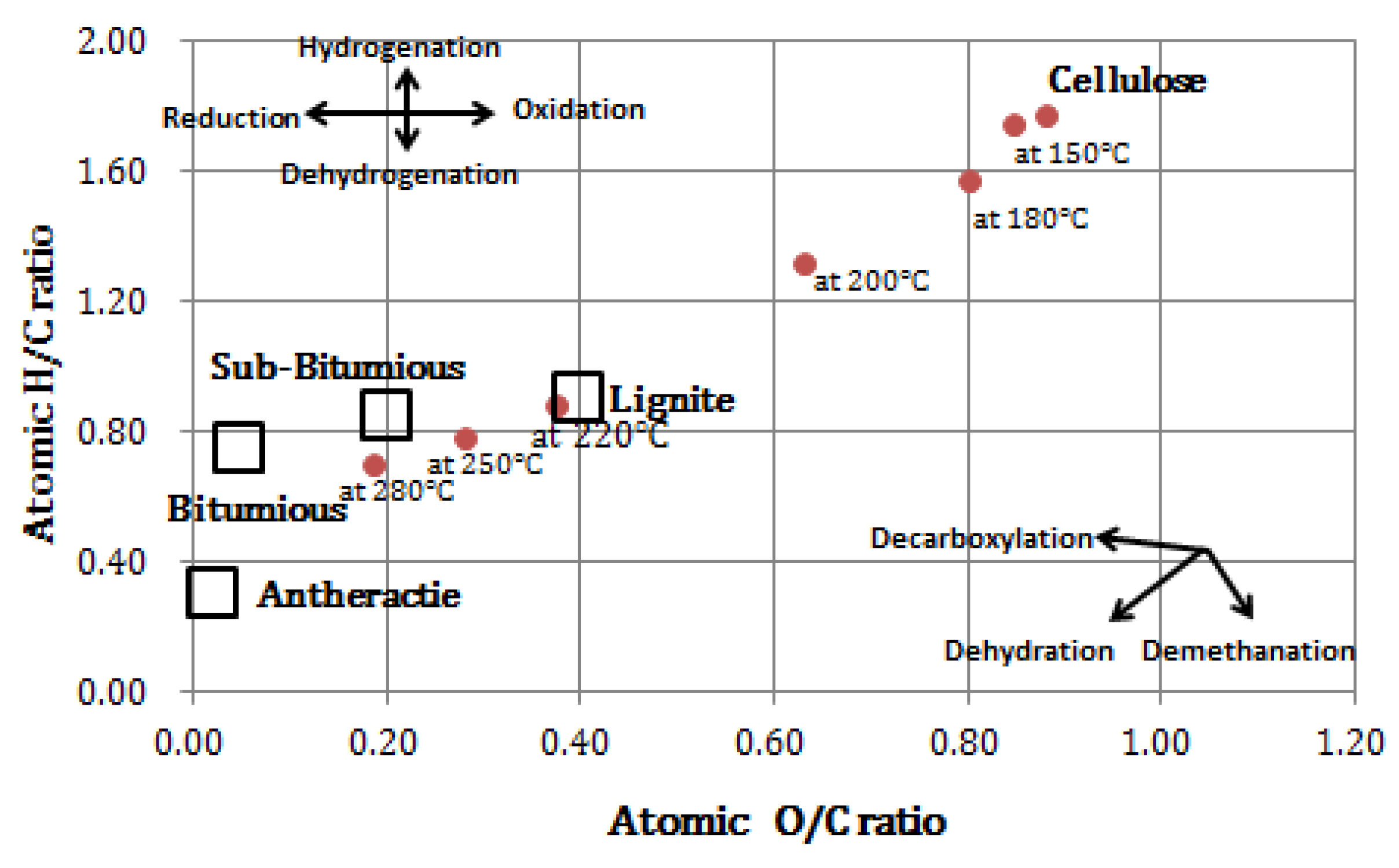
3.3. Changed Functional Groups of Cellulose and Related Compounds

3.4. Energy Recovery Efficiency
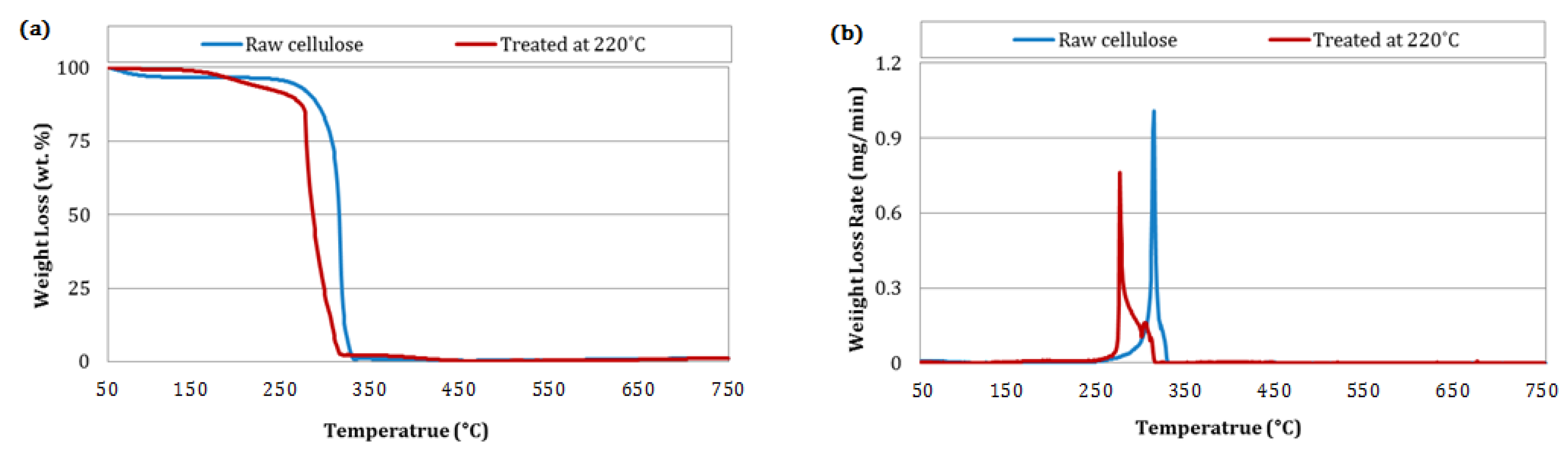
3.5. Combustion Behavior of Biochar
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal carbonization of biomass for energy and crop production. Appl. Bioenergy 2014, 1. [Google Scholar] [CrossRef]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef] [PubMed]
- Chandra, R.; Takeuchi, H.; Hasegawa, T. Hydrothermal pretreatment of rice straw biomass: A potential and promising method for enhanced methane production. Appl. Energy 2012, 94, 129–140. [Google Scholar] [CrossRef]
- Mosier, N.; Wyman, C.; Dale, B.; Elander, R.; Lee, Y.Y.; Holtzapple, M.; Ladisch, M. Features of promising technologies for pretreatment of lignocellulosic biomass. Bioresour. Technol. 2005, 96, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Barrena, R.; d’Imporzano, G.; Ponsá, S.; Gea, T.; Artola, A.; Vázquez, F.; Sánchez, A.; Adani, F. In search of a reliable technique for the determination of the biological stability of the organic matter in the mechanical-biological treated waste. J. Hazard Mater. 2009, 162, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.S.; Park, J.I.; Park, J.M. High-rate slurry-phase decomposition of food wastes: Indirect performance estimation from dissolved oxygen. Process Biochem. 2005, 40, 1301–1306. [Google Scholar] [CrossRef]
- Liu, Z.; Balasubramanian, R. Upgrading of waste biomass by hydrothermal carbonization (HTC) and low temperature pyrolysis (LTP): A comparative evaluation. Appl. Energy 2014, 114, 857–864. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Byrappa, K.; Adschiri, T. Hydrothermal technology for nanotechnology. Prog. Cryst. Growth Charact. Mater. 2007, 53, 117–166. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal carbonization of anaerobically digested sludge for solid fuel production and energy recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y. Hydrothermal liquefaction of microalgae in an ethanol-water co-solvent to produce biocrude oil. Energy Fuels 2014, 28, 5178–5183. [Google Scholar] [CrossRef]
- Manara, P.; Zabaniotou, A. Towards sewage sludge based biofuels via thermochemical conversion—A review. Renew. Sustain. Energy Rev. 2012, 16, 2566–2582. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Kim, D.; Prawisudha, P.; Yoshikawa, K. Hydrothermal upgrading of Korean MSW for solid fuel production: Effect of MSW composition. J. Combust. 2012, 2012. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R., Jr.; Mines, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Garrote, G.; Dominguez, H.; Parajo, J.C. Hydrothermal processing of lignocellulosic materials. J. Wood Wood Prod. 1999, 57, 191–202. [Google Scholar] [CrossRef]
- Japanese Industrial Standard (JIS M 8814). Coal and Coke Determination of Gross Calorific Value by the Bomb Calorimetric Method, and Calculation of Met Calorific Value; Japanese Industrial Standards Committee: Tokyo, Japan, 2003. [Google Scholar]
- Sevilla, M.; Fuertes, A.B. The production of carbon materials by hydrothermal carbonization of cellulose. Carbon 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- Quitain, A.T.; Faisal, M.; Kang, K.; Daimon, H.; Fujie, K. Low-molecular-weight carboxylic acids produced from hydrothermal treatment of organic wastes. J. Hazard. Mater. 2002, 93, 209–220. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal carbonization (HTC) of lignocellulosic biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal carbonization of municipal waste streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef] [PubMed]
- Tri Mursito, A.; Hirajima, T.; Sasaki, K. Upgraging and dewatering of raw tropical peat by hydrothermal treatment. Fuel 2010, 89, 635–641. [Google Scholar] [CrossRef]
- Tri Mursito, A.; Hirajima, T.; Sasaki, K.; Kumagai, S. The effect of hydrothermal dewatering of Pontianak tropical peat on organics in wastewater and gaseous products. Fuel 2010, 89, 3394–3924. [Google Scholar] [CrossRef]
- Xiao, L.P.; Shi, Z.J.; Xu, F.; Sun, R.C. Hydrothermal carbonization of lignocellulose biomass. Bioresour. Technol. 2012, 118, 619–623. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Li, G.; Kong, L.; Wang, H.; Huang, J.; Xu, J. Application of hydrothermal reaction in resource recovery of organic wastes. Resour. Conserv. Recycl. 2008, 52, 691–699. [Google Scholar] [CrossRef]
- Freitas, J.C.; Bonagamba, T.J.; Emmerich, F.G. 13C high-resolution solid-state NMR study of peat carbonization. Energy Fuels 1999, 13, 53–59. [Google Scholar] [CrossRef]
- Falco, C.; Perez Caballero, F.; Babonneau, F.; Gervais, C.; Laurent, G.; Titirici, M.M.; Baccile, N. Hydrothermal carbon from biomass: Structural differences between hydrothermal and pyrolyzed carbons via 13C solid state NMR. Langmuir 2011, 27, 14460–14471. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ro, K.S.; Chappell, M.; Li, Y.; Mao, J. Chemical structures of swine-manure chars produced under different carbonization conditions investigated by Advanced Solid-State 13C Nuclear Magnetic Resonance (NMR) Spectroscopy. Energy Fuels 2010, 25, 388–397. [Google Scholar] [CrossRef]
- Lu, X.; Pellechia, P.J.; Flora, J.R.; Berge, N.D. Influence of reaction time and temperature on product formation and characteristics associated with the hydrothermal carbonization of cellulose. Bioresour. Technol. 2013, 138, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, D-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Yoshikawa, K.; Park, K.Y. Characteristics of Biochar Obtained by Hydrothermal Carbonization of Cellulose for Renewable Energy. Energies 2015, 8, 14040-14048. https://doi.org/10.3390/en81212412
Kim D, Yoshikawa K, Park KY. Characteristics of Biochar Obtained by Hydrothermal Carbonization of Cellulose for Renewable Energy. Energies. 2015; 8(12):14040-14048. https://doi.org/10.3390/en81212412
Chicago/Turabian StyleKim, Daegi, Kunio Yoshikawa, and Ki Young Park. 2015. "Characteristics of Biochar Obtained by Hydrothermal Carbonization of Cellulose for Renewable Energy" Energies 8, no. 12: 14040-14048. https://doi.org/10.3390/en81212412







