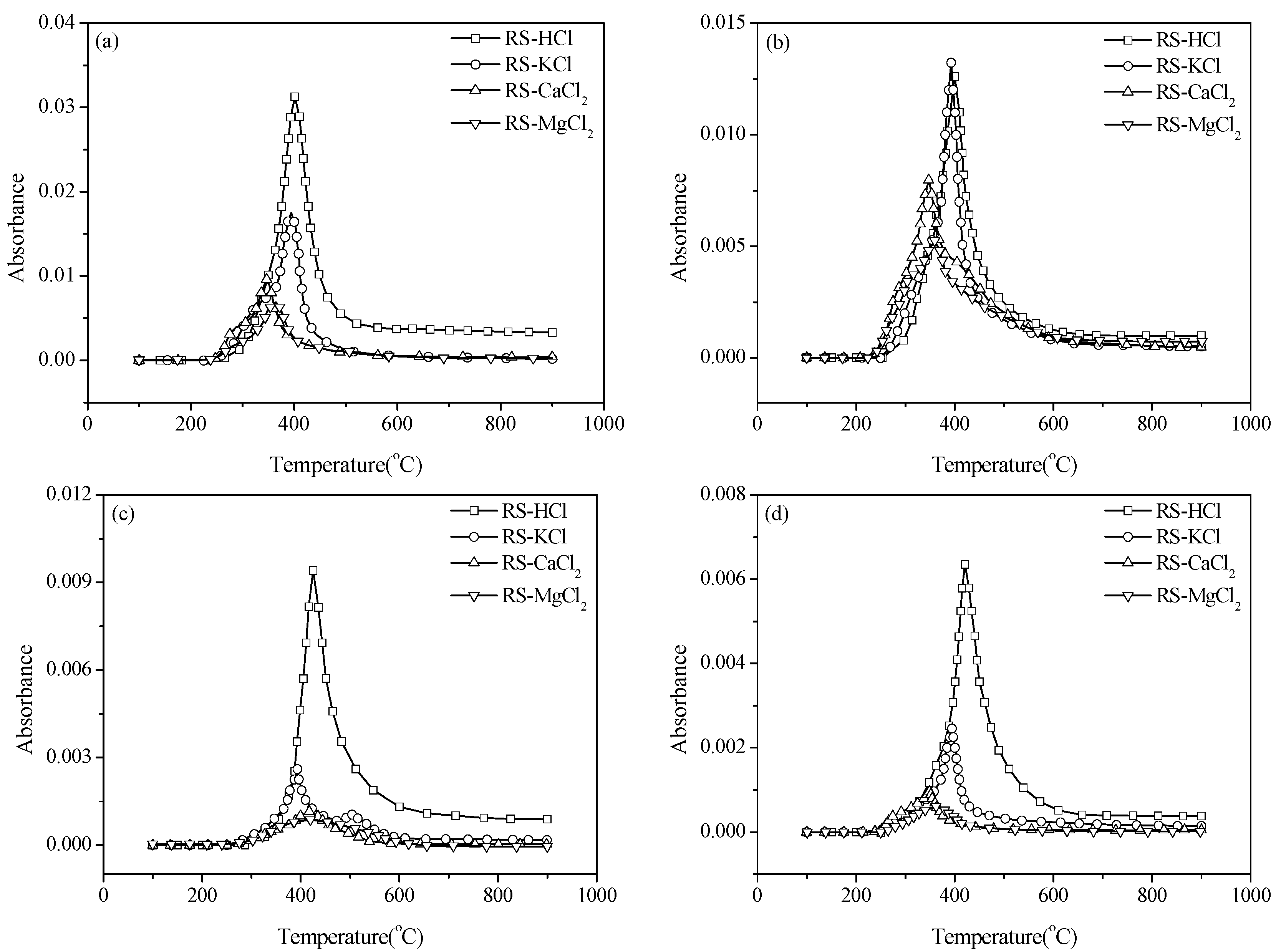
3.1. Thermal Gravity/Differential Thermal Gravity (TG/DTG) and Kinetic Analysis
Given that N-containing species are mainly accompanied by the release of volatile compounds, the pyrolysis characteristics of samples with different pretreatments should be studied. The experiments were carried out at a heating rate of 40 °C/min and the result is shown in
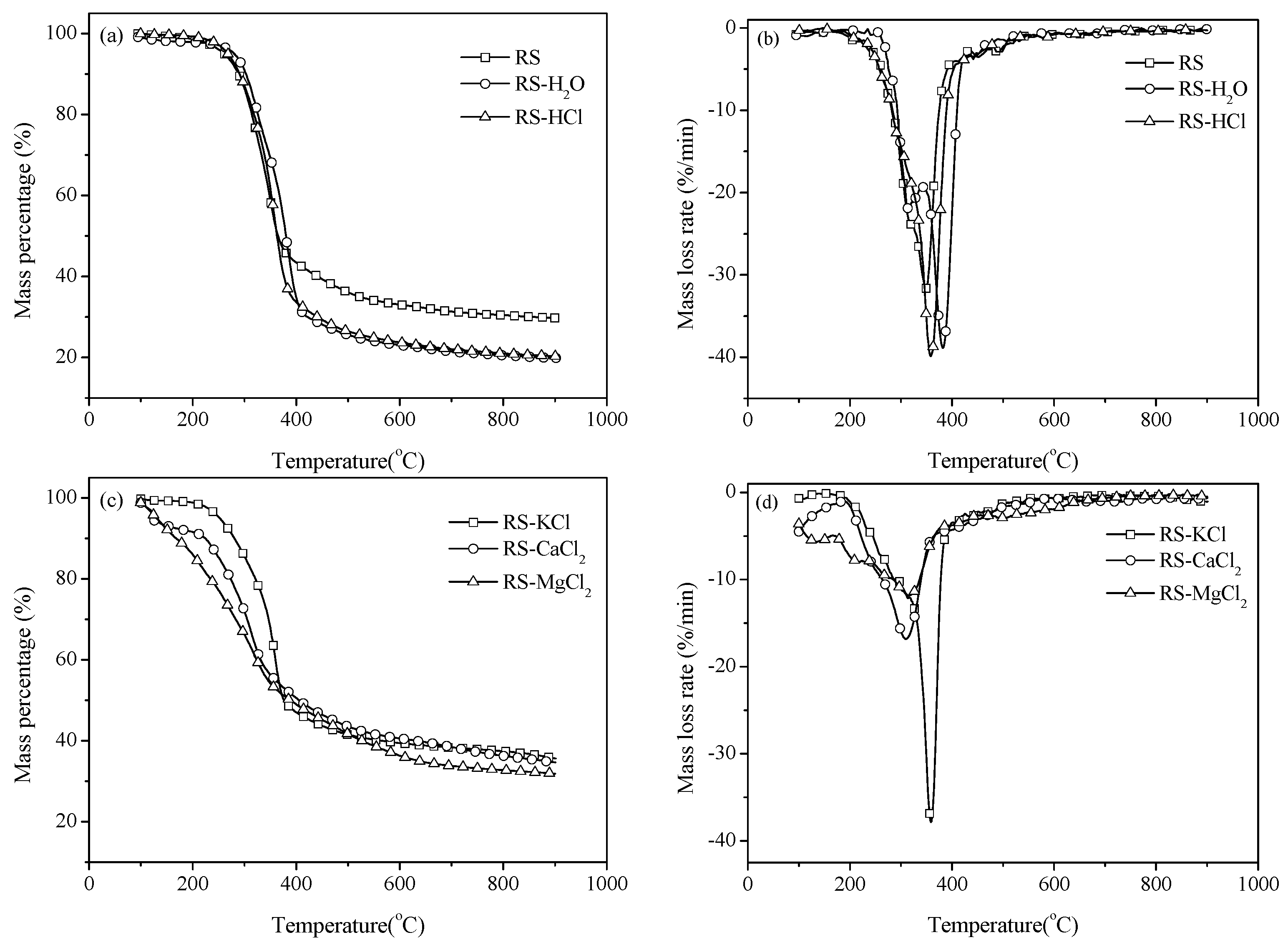
Figure 1. Weight loss mainly occurs at 250–400 °C, and the weight loss rate decreases when the temperature exceeds 400 °C (
Figure 1a). The residual mass percentages are 29.7%, 19.87%, and 20.28% for RS, RS-H
2O, and RS-HCl, respectively. This result illustrates that water washing and acid washing pretreatments can promote the generation of volatile compounds and decrease the solid product yield of pyrolysis. This result can be attributed to the change in physical structure because of the pretreatments. The average pore diameter of samples increases by water washing and acid washing, in accordance with the result of Long
et al. [
23]. This result can be attributed to the removal of minerals in the pore structure. The pore size also enlarges. These changes in pore structure are conducive to volatile release; thus, the residual solid mass percentage decreases. As shown in the DTG curves of
Figure 1b, the maximum weight loss rate increases with the removal of AAEMs and the corresponding peak temperature shifts to a high-temperature zone.
The TG and DTG curves of samples with external AAEMs are shown in
Figure 1c,d. The weight loss of RS-KCl is similar to that of RS. However, the weight loss curves of RS-CaCl
2 and RS-MgCl
2 obviously shift to a low-temperature zone. This result indicates that the volatile can be released at low temperatures in the presence of CaCl
2 and MgCl
2. As shown in
Figure 1d, the maximum weight loss rate of RS-KCl shows an insignificant difference with that of RS. However, the values of RS-CaCl
2 and RS-MgCl
2 are significantly smaller than that of RS. Thus, alkaline earth metals can suppress the volatile release rate.
Figure 1.
Thermal gravity (TG) and differential thermal gravity (DTG) curves of samples pyrolysis with a heating rate of 40 °C/min. (a) TG curves; (b) DTG curves; (c) TG curves with additives; (d) DTG curves with additives.
Figure 1.
Thermal gravity (TG) and differential thermal gravity (DTG) curves of samples pyrolysis with a heating rate of 40 °C/min. (a) TG curves; (b) DTG curves; (c) TG curves with additives; (d) DTG curves with additives.
As mentioned above, the pyrolysis characteristics for the six samples under different pretreatment conditions significantly differ to each other. Therefore, to obtain a further understanding of the effect of AAEMs on RS pyrolysis, experiments with different heating rates (30 °C/min, 40 °C/min and 50 °C/min) were conducted and the kinetic parameters were estimated according to the DAEM method.
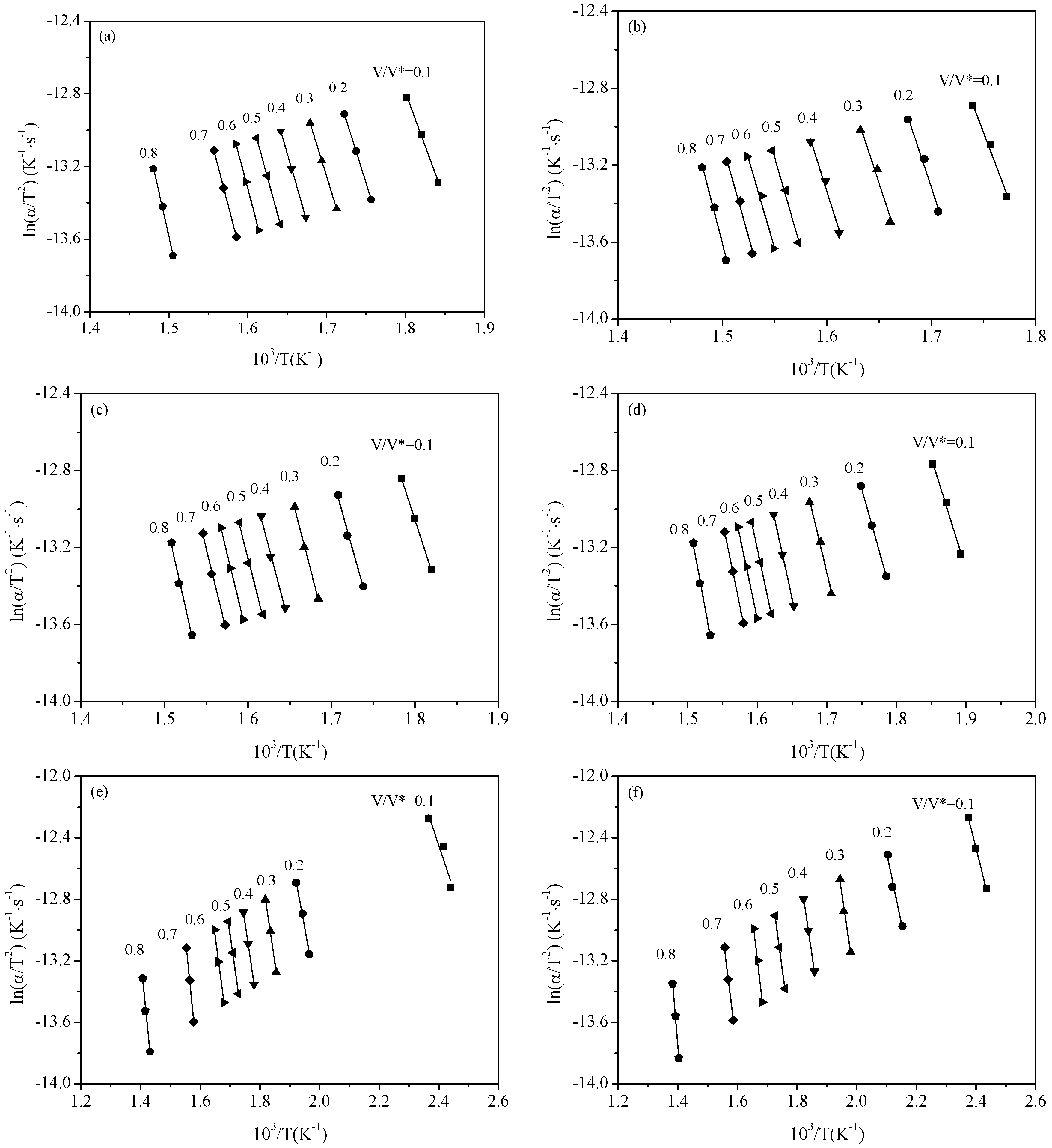
Figure 2 shows the Arrhenius plots of ln(α
/T2)
vs. 1/
T for the six samples. From
Figure 2, it can be observed that ln(α
/T2) is in a linear relationship with 1/
T for each sample under different reacted fraction
V/V*.
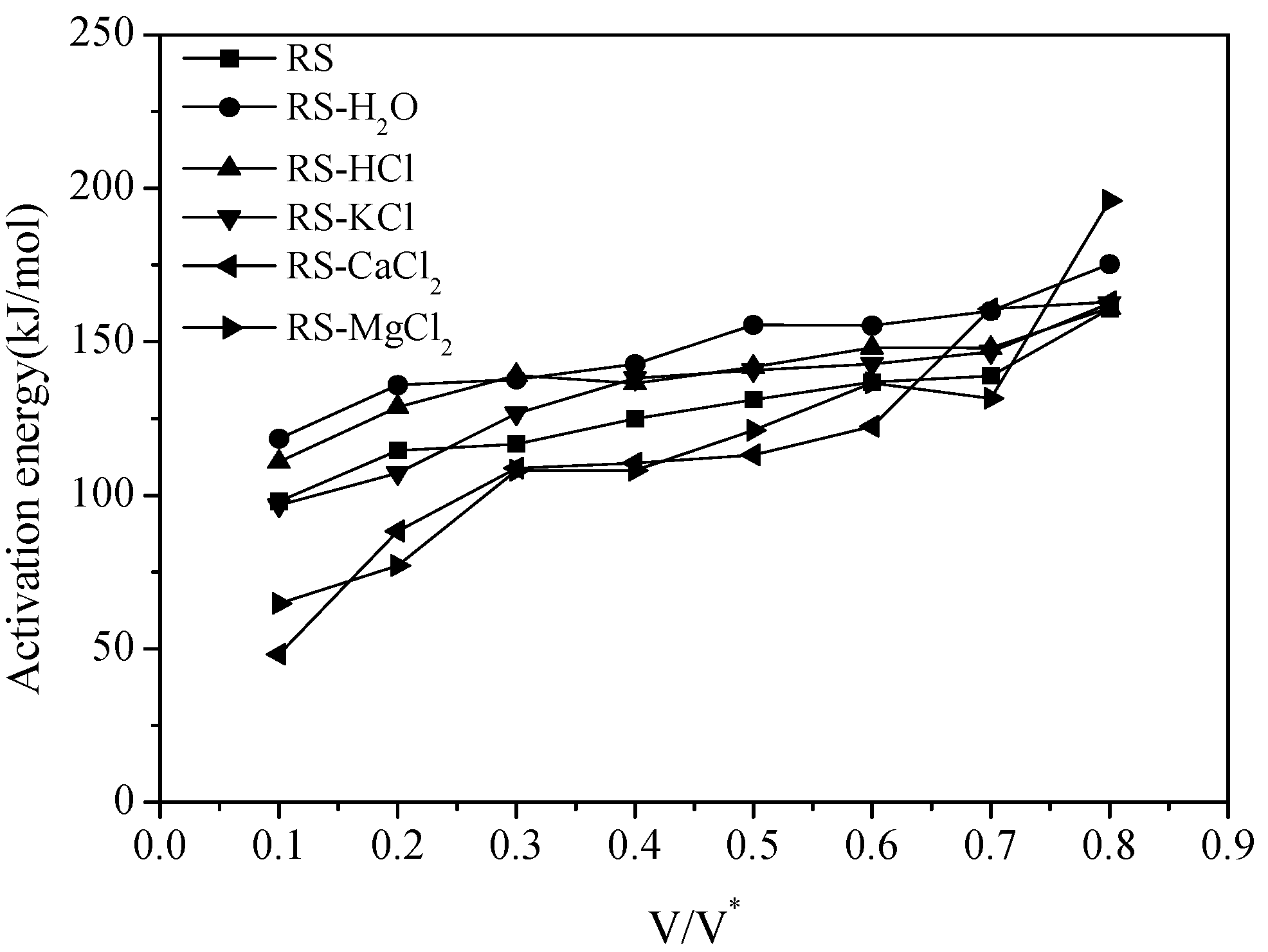
The activation energy values can be calculated from the slope in each Arrhenius plot and the result is shown in
Figure 3. The activation energy values of all samples increase with the increase of the reacted fraction
V/V*. As can be seen from
Figure 3, the activation energy values of RS-H
2O and RS-HCl are both higher than that of RS. The increase of activation energy requires high reaction temperatures, which explains the slight shift of the volatile release curve of RS-H
2O and RS-HCl toward the high-temperature zone. The activation energy values of RS-CaCl
2 and RS-MgCl
2 are lower that of RS when the reacted fraction
V/V* below 0.6. Thus, the pyrolysis reaction of samples with the addition of CaCl
2 and MgCl
2 can be obtained at low temperatures (
Figure 1c).
Figure 2.
Arrhenius plots of α/T2 vs. 1/T at selected V/V* values. (a) Rice straw (RS); (b) RS-H2O; (c) RS-HCl; (d) RS-KCl; (e) RS-CaCl2; (f) RS-MgCl2.
Figure 2.
Arrhenius plots of α/T2 vs. 1/T at selected V/V* values. (a) Rice straw (RS); (b) RS-H2O; (c) RS-HCl; (d) RS-KCl; (e) RS-CaCl2; (f) RS-MgCl2.
Figure 3.
Activation energy curves with different V/V*.
Figure 3.
Activation energy curves with different V/V*.
3.2. Effect of Inherent Alkaline Earth Metallic Species (AAEMs) on N-Containing Species Release
Alkali metals and alkaline metals can be respectively removed by water washing and acid washing (
Table 1). To study the effect of inherent AAEMs on the release of N-containing species, pyrolysis experiments were conducted by using RS. RS-H
2O and RS-HCl as samples have a fixed heating rate of 40 °C/min. The release curves of NH
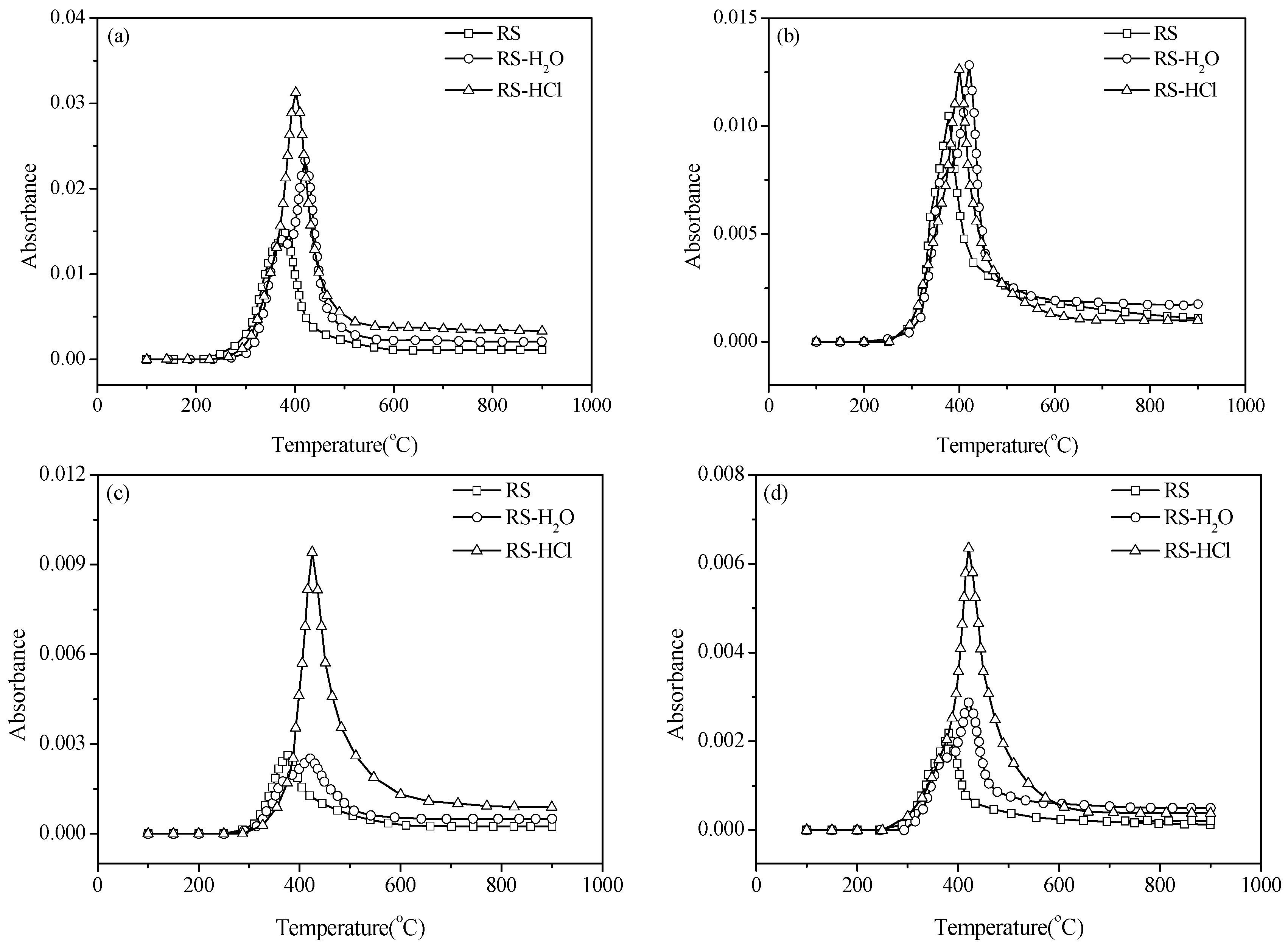
3, HCN, nitric oxide (NO), and HNCO are illustrated in
Figure 4. The formation of N-containing species is significantly affected by the demineralization pretreatments. The NH
3 release curves of RS-H
2O and RS-HCl shift to a high-temperature zone compared with the RS sample. This result is mainly caused by the volatile release behavior without inherent AAEMs.
Figure 4.
Release of N-compounds for RS, RS-H2O, and RS-HCl. (a) ammonia (NH3); (b) hydrogen cyanide (HCN); (c) isocyanic acid (HNCO); (d) nitric oxide (NO).
Figure 4.
Release of N-compounds for RS, RS-H2O, and RS-HCl. (a) ammonia (NH3); (b) hydrogen cyanide (HCN); (c) isocyanic acid (HNCO); (d) nitric oxide (NO).
The NH
3 yield of RS-H
2O is slightly lower than that of RS at <375 °C, whereas the yield of RS-HCl is almost the same as that of RS. The NH
3 yields of RS-DW and RS-HCl both increase obviously in a high-temperature zone (>375 °C), and the NH
3 release curve of RS-HCl has the highest peak value. Corresponding peak temperatures of RS, RS-H
2O, and RS-HCl are 398, 420, and 401 °C, respectively. HCN, HNCO, and NO release curves shift to a high-temperature zone for RS-DW and RS-HCl compared with RS, which have similar trends with NH
3. The peak value of the HCN release curve is also increased by pretreatment, but the increase is smaller than that of NH
3. The peak value of the HNCO release curve for RS-H
2O is almost identical with that of RS. However, the peak value for RS-HCl is obviously higher than those of RS and RS-H
2O. This result indicates that the alkaline earth metals of RS have a greater impact on the formation of HNCO than alkali metals. The release characteristic of NO is similar to HNCO except that the peak value of the NO release curve for RS-H
2O is higher than that of RS.
Figure 4 shows that AAEMs can inhibit the conversion of fuel N to gas N-compounds but the inhibition of minerals is weakened by the demineralization pretreatments.
Given that the nitrogen in biomass is mainly protein N, water washing and acid washing pretreatments can change the contents of N in the samples. The N contents of samples were determined accurately by elemental analysis, and the values are 1.03%, 0.86%, and 0.62% for RS, RS-H
2O, and RS-HCl, respectively (
Table 1). The N content of RS-H
2O and RS-HCl accounts for 83.5% and 60.2% of that of RS. The change in N content in the sample is significant; thus, the change in the study of N-species formation yield and selectivity should be considered. However, no relevant research has been conducted for this topic. Therefore, the effect of inherent AAEMs on the release of N-containing species is not comprehensive without considering the change of N content and composition. The calculation of the relative yield of nitrogen should be conducted at the same total-N content. In other words, the relative yield of N-species of RS-H
2O and RS-HCl should be multiplied by 1.20 and 1.66, respectively.
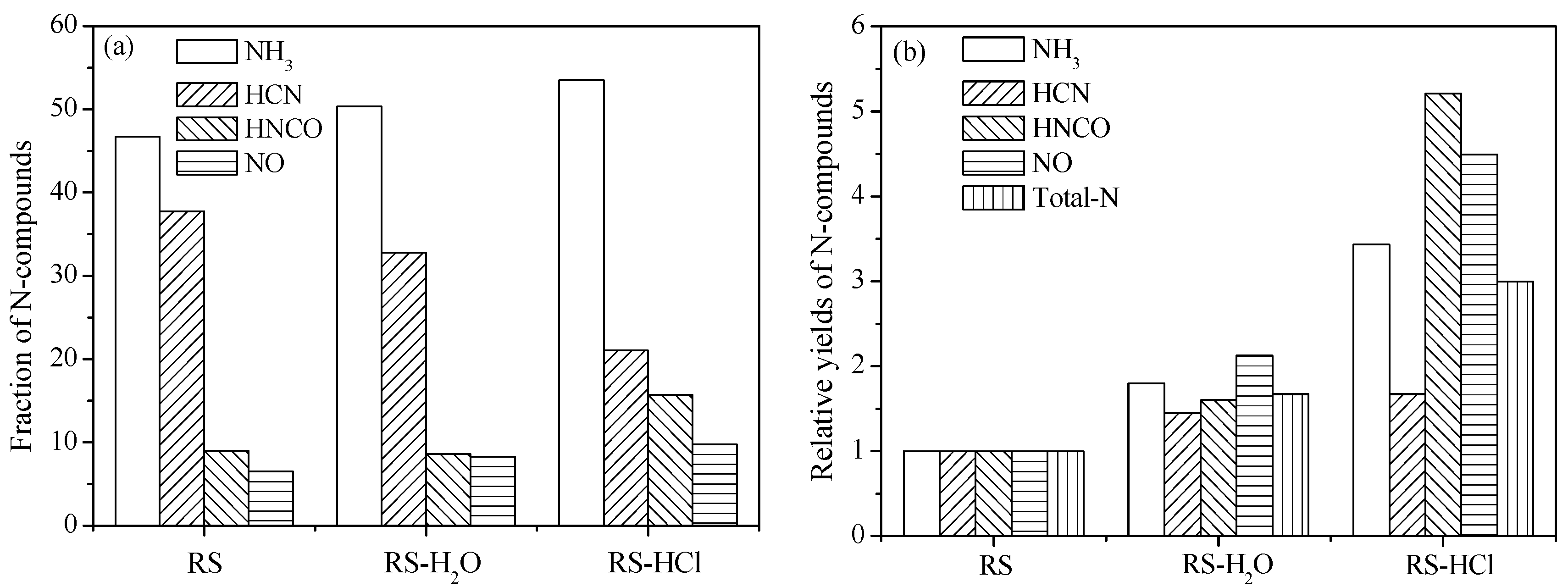
Figure 5a shows the N-containing species formation selectively of RS, RS-H
2O, and RS-HCl. The fraction of NH
3 is enhanced by demineralization pretreatment, whereas the fraction of HCN is decreased. Thus, AAEMs removal can improve NH
3 formation selectivity and inhibit HCN selectivity. The HNCO fraction of RS-H
2O is slightly lower than that of RS, thus indicating that alkali metals (K, Na) have little effect on HNCO formation. The HNCO fraction of RS-HCl is obviously higher than those of RS and RS-H
2O. The results show that the formation of HNCO is mainly affected by the influence of alkaline earth metals (Ca, Mg). Furthermore, NO formation selectivity is improved by water washing and acid washing, similar to NH
3.
Figure 5.
Selectivity and relative yields of N-compounds. (a) Selectivity; (b) yield.
Figure 5.
Selectivity and relative yields of N-compounds. (a) Selectivity; (b) yield.
N-compound yields during RS pyrolysis were set to units, and the relative yields of RS-H
2O and RS-HCl were calculated (
Figure 5b). The four types of N-containing species yields were enhanced by demineralization pretreatment. The total-N relative yields of RS-H
2O and RS-HCl are 1.67 and 3.0 times higher than that of RS. The relative yields of NH
3, HNCO, and NO for RS-HCl increases obviously compared with that of RS-H
2O, whereas HCN relative yield is slightly enhanced.
3.3. Effect of External Alkaline Earth Metallic Species on N-Containing Species Release
Although the effect of AAEMs on N-containing species transformation can be drawn qualitatively, the inhibition ability cannot be compared with each other for different mass percent of AAEMs in RS. Therefore, the same mass percent (10 wt %) of KCl, CaCl
2, and MgCl
2 were added into the RS-HCl sample by solution impregnation. The pyrolysis of RS-KCl, RS-CaCl
2, and RS-MgCl
2 were conducted by using the same condition as that of RS-HCl (
Figure 6). In the presence of AAEM, the NH
3 release curve remains a single peak structure, the curve becomes narrow, and the peak value decreases significantly compared with the release curve of RS-HCl. This result indicates that NH
3 release was suppressed by the addition of AAEM. The peak values of RS-CaCl
2 and RS-MgCl
2 are obviously lower than that of RS-KCl.
The HCN release curve of RS-KCl is slightly narrow, and the peak value is almost at the same level. Therefore, the effect of KCl on HCN release behavior is insignificant. The release peak temperature of RS-CaCl2 and RS-MgCl2 shifts to a low-temperature zone for 52.6 and 40.7 °C, respectively, and the release peak value of RS-MgCl2 is lower than that of RS-CaCl2. Compared with RS-HCl, the HNCO release of RS-KCl is significantly inhibited and the release curve is changed from a single peak to a double peak structure. The peak temperature is 393.2 and 491.7 °C for RS-HCl and RS-KCl, respectively, but the second release peak value is relatively small. The release curves of RS-CaCl2 and RS-MgCl2 are still a single peak structure and are lower than that of RS-KCl.
Figure 6.
Release of N-compounds with alkaline earth metallic specie (AAEM) additives (a) NH3; (b) HCN; (c) HNCO; (d) NO.
Figure 6.
Release of N-compounds with alkaline earth metallic specie (AAEM) additives (a) NH3; (b) HCN; (c) HNCO; (d) NO.
The NO release change of RS-KCl, RS-CaCl2, and RS-MgCl2 is comparable to that of NH3, which remains a single peak structure. However, the curve is narrow and the peak value is low. The temperatures corresponding to the release curve peak shift to a low-temperature zone for 26.8, 71.9, and 58.8 °C compared with that of RS-HCl. The NO release curves of RS-CaCl2 and RS-MgCl2 are similar to each other, and both curves are lower than that of RS-KCl. The N-containing species release is suppressed for all AAEM additives when the pyrolysis temperature above 600 °C.
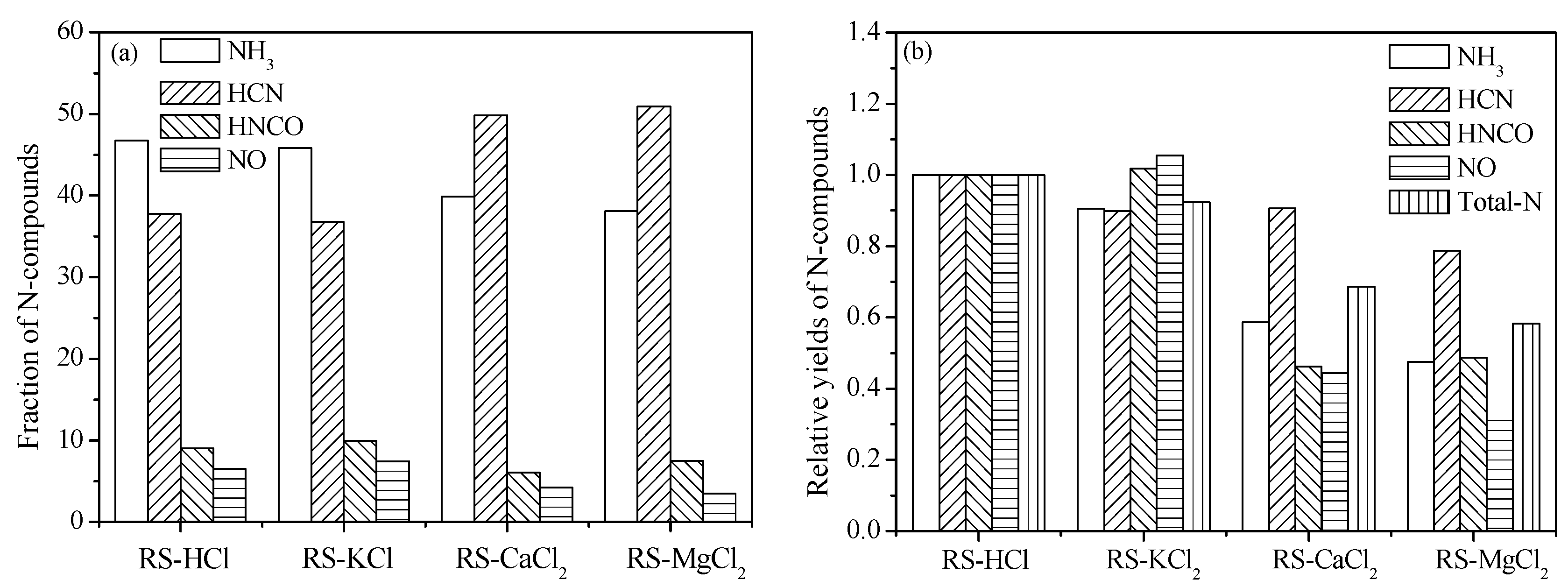
Figure 7a shows the fraction of N-containing compounds in the presence of AAEMs additives. Fractions of NH
3 and HCN are slightly decreased, and the values of HNCO and NO increased mildly. Therefore, the effect of KCl on N-containing species formation selectivity is relatively little. The fractions of NH
3, HNCO, and NO decrease and the value of HCN increases obviously in the presence of CaCl
2 and MgCl
2. The formation selectivity of HCN is promoted by adding alkaline earth metals.
To obtain the inhibition ability quantitatively on the N-containing species of three types of AAEM additives, the relative yields of N-containing species should be calculated at the same nitrogen content. Thus, yields of RS-KCl, RS-CaCl
2, and RS-MgCl
2 were multiplied by 1.111 because the mass percent of AAEM is 10% in samples. The N-containing species yields of samples with additives were calculated and displayed in
Figure 7b. For RS-KCl, the relative yields of NH
3 and HCN are decreased and the values of HNCO and NO increase compared with that of RS-HCl. This result is seemly contradictory with the result of inherent K metals possibly because different K forms with K metals contain inorganic salts and organic potassium [
24], whereas KCl is only considered an additive. The relative yields of all four types of N-containing species decreases in the presence of CaCl
2 and MgCl
2. The total-N relative yield of RS-KCl, RS-CaCl
2, and RS-MgCl
2 is 92.3%, 68.7%, and 58.3% of RS-HCl, respectively. Therefore, MgCl
2 shows the strongest inhibition ability on N-containing species formation followed by CaCl
2 and KCl.
Figure 7.
Selectivity and relative yields of N-compounds with AAEM additives. (a) Selectivity; (b) yield.
Figure 7.
Selectivity and relative yields of N-compounds with AAEM additives. (a) Selectivity; (b) yield.
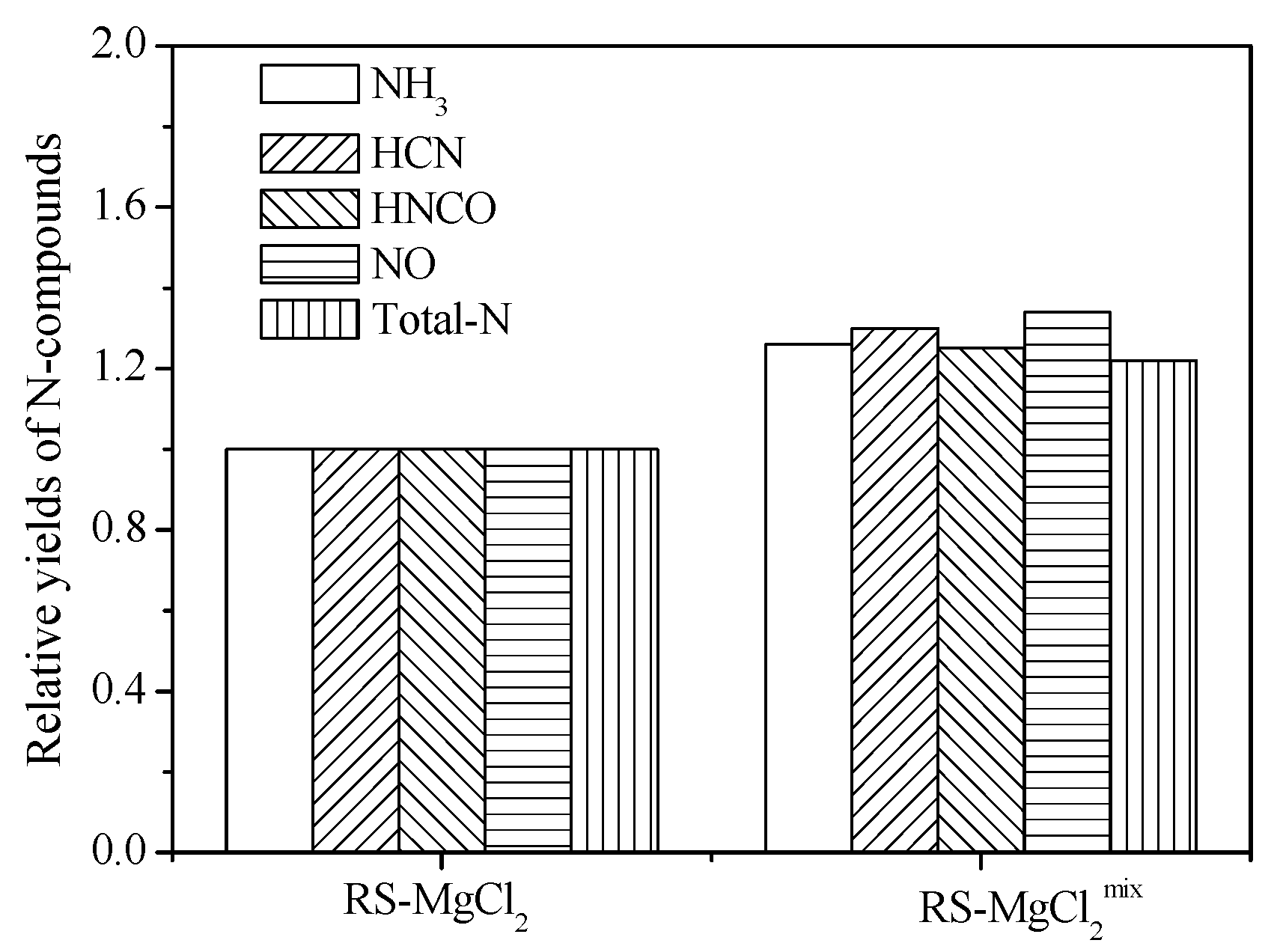
For further information on the AAEM inhibition of N-species release during RS pyrolysis, two adding methods (solution impregnation and solid phase physical mixing) were studied. Solid powders of MgCl
2 were added into RS-HCl samples with a mass content of 10%, and the sample was signed as RS-MgCl
2mix. The N-species relative yields of RS-MgCl
2mix were compared with that of RS-MgCl
2, which was set as units.
Figure 8 shows that the relative yields of RS-MgCl
2mix are obviously higher than that of RS-MgCl
2. Thus, the inhibition ability of RS-MgCl
2mix is lower than that of RS-MgCl
2 because of two reasons. First, MgCl
2 can enter the interior pores of the sample by diffusion via solution impregnation. The uniform dispersion in the interior and surface of the sample particle can be expected. Material mixing uniformity by solution impregnation is better than that of dry powder mixing. Second, the inhibition mechanism may be different that the reaction of N-species on the surface of MgCl
2 during RS-MgCl
2mix pyrolysis. Mg
2+ can be directly combined with chemical bonds of N in RS by solution impregnation. The specific inhibition mechanism of AAEMs needs to be further studied.
Figure 8.
Relative yields of N-compounds for different adding methods.
Figure 8.
Relative yields of N-compounds for different adding methods.