1. Introduction
While fuel flexibility and emissions performance create advantages for fluidized bed processes (combustion and gasification) for conversion of solid fuels, interactions of bed solids in the system can create operational constraints. Issues resulting from these interactions include bed agglomeration and ash deposition on heat transfer surfaces. A previous work [
1] has cited and summarized several published instances of agglomeration covering both combustion and gasification systems over several fuel types including coal and petroleum coke.
Unwanted bed solids interactions, notably agglomeration, can produce financial losses for commercial operations and can lead to operational issues and unavailability in commercial fluidized bed boilers. A means of evaluating the agglomeration potential of coal minerals, under reducing conditions, has been presented elsewhere [
2]. This technique breaks fuel into particle increments, according to particle density. The result is the isolation of particle groups with significant differences in ash melting behavior as compared with the overall (composite) fuel, and the potential for isolation and quantification of classes of particles that can form liquid phases at lower temperatures.
Application of this technique to reducing conditions can be useful in isolating low-melting particle classes for a fuel that might be used in a fluidized bed gasification system, where reducing conditions are expected. However, circulating fluidized bed (CFB) boiler technology is widely used for power generation, and both reducing and oxidizing conditions have been found in CFB boilers.
A CFB boiler typically splits the airflow into two zones. For purposes of the discussion here, the primary air zone is the region of the CFB boiler above the fluidization grid (where primary air enters), and the level at which the secondary air enters, is known as the secondary air zone. The air split, which is the relative fractions of primary and secondary air comprising the total airflow, may be in the range of 60% primary air (through the fluidization grid) and 40% secondary air. As such, the potential exists for the primary air zone to operate sub-stoichiometric. This and the nature of solids and gas flows in the primary air zone have led to the detection of both reducing and oxidizing conditions in the primary zones of CFB boilers. Oxygen-rich zones are expected in both the primary and secondary air zones. In the primary air zone, the region close to the distributor plate will be in an oxidizing environment. Also, in the secondary air zone, above the level at which secondary air is introduced, an oxidizing environment can be expected [
3].
Work reported by Leckner [
4] found both oxidizing and reducing conditions in the primary air zone of a CFB boiler. Where the air split was 100% primary air, conditions were largely oxidizing at 0.59 m above the bottom of the combustor. At 60% primary air, conditions alternated between oxidizing and reducing in this region, and at 40% primary air, reducing conditions predominated. Direct gas composition measurements in the primary air zone of a 235 MWe CFB boiler [
5] showed that the oxygen concentration varied with distance from the combustor wall, being highest (in the same range as the stack gas’s 3.9–4.7 vol.% O
2 composition) furthest from the wall. Closer to the wall, O
2 concentration decreased and CO concentration increased.
The work reported by Leckner [
4] indicated oxidizing conditions in the secondary air zone of the boiler examined in that work. Couturier
et al. [
6] found that oxygen concentrations in the secondary air zone of a CFB boiler were influenced by both axial and radial position in the system, with measured O
2 concentrations ranging as high as 15 vol.%.
At higher O
2 partial pressures, such as may be encountered near air inlets, fuel particle temperatures may be higher than average bed temperatures. This in turn can improve the prospects for liquid phase formation by the mineral constituents in the fuel particles. The presence of oxidizing gaseous environments in CFB boilers, as well as the potential for individual particle temperatures to exceed the measured bed temperature, suggested the utility of applying the technique previously published regarding reducing conditions [
2] to an oxidizing gaseous environment. Results as such are presented here.
2. Background
2.1. Role of Slag-Liquid Formation in Prediction of Fluid-Bed Agglomeration
Ash agglomeration occurs when particles that come into contact remain stuck after a collision. For particles to stick, the kinetic energy of the particles should get dissipated. The presence of a viscous slag-liquid layer causes the viscous dissipation of energy. If this viscous dissipation of energy due to this slag-liquid layer exceeds the kinetic energy of the particles, they remain in contact and form a larger particle [
7]. Therefore, the presence of a sticky slag-liquid layer (binder) between colliding particles is essential for the occurrence of agglomeration.
Suppression of this phenomenon can be assisted by an improved understanding of slag-liquid formation tendencies of fuel mineral particles having distinct chemical compositions. The tendency of a fuel to form slag-liquid, based on mineral matter transformations, has been extensively studied at a high temperature range of 1200 °C to 1600 °C. Van Dyk
et al. [
8] studied the mineral matter transformations of slag formation during high temperature combustion and gasification of coals using ash fusion tests (AFTs) and scanning electron microscopy (SEM). The effects of basic elements in coal on reactions that lead to slagging during combustion have been studied, but primarily in a high temperature regime [
9]. These studies of mineral matter transformations are relevant for slagging gasifiers such as entrained flow gasifiers, but similar studies at low fluidized bed operating temperatures are not as extensive. Studies on slag-liquid formation under fluidized bed conditions are more concentrated in the use of lignites or low rank coals [
10,
11] with a greater focus on gasification. More recently, studies investigating the mineral matter transformations that cause the formation of a sticky slag-liquid layer during fluidized bed combustion focus on the use of biomass fuels. These studies [
12,
13,
14,
15,
16] discuss the formation of slag-liquid due to the presence of K
2O, Al
2O
3 and SiO
2 containing minerals and in some cases CaO also. They propose gas phase reactions and condensation of minerals such as sodium and potassium sulfates onto particles causing the formation of the sticky slag layer. Different mechanisms of agglomeration of biomass fuels such as melt induced, coating induced and agglomeration by double layer formation have been discussed previously [
17,
18,
19]. This study focuses on the formation of the slag-liquid binding material during combustion of high rank coals such as bituminous coals in fluidized beds. These bituminous coals are being used in a number of fluidized bed combustion units in the US. The power plants using these coals experience agglomeration issues although these high rank coals do not contain high levels of sodium and potassium based minerals. These coals with high silica content and different mineral make-up are expected to have different slag formation tendencies and agglomeration mechanisms, that would be difficult to explain based on previous knowledge alone. Hence, this study is based on a detailed analysis on Pittsburgh No. 8 coal and it focuses on the formation of slag-liquid at the particle-level, in order to add a new perspective to the mechanism of agglomeration in fluidized bed combustors.
2.2. Heterogeneity in Ash Chemical Composition
An important consideration in the study of particle-particle sticking by an intermediate slag-liquid layer is the differences in chemical composition of different ash particles in the system. Mineral matter in fuel ash is distributive and the bulk chemical composition is not representative. Based on the mineral processing operations used, such as the grinding, the mineral content is distributed amongst particle classes and therefore each particle class differs in chemical composition. Thus, the bulk fuel contains classes of particles that may be rich in specific minerals such as a class that contains heavy, iron-based minerals or calcium-based minerals. The particles of such a class can be assumed to be reasonably homogeneous in composition, while each particle class would have a composition distinct from another. The term “particle-level” will thus be used to refer to the differences that exist at the level of these ash particle classes. The term “bulk” composition used in this paper refers to the overall composition of the whole coal that contains these specific mineral-rich particle classes. The studies of slag-liquid formation in fluidized bed combustors discussed above, have not elaborated on the compositional differences that exist at the particle-level and have used the bulk composition of the composite, whole coal.
The gravity separation of coal into various density fractions done in previous studies [
20,
21] clearly showed these particle-level compositional differences along with variation in ash content of the separated fractions. Austin
et al. [
22] have also previously emphasized this, in their work on ash deposition in pulverized coal fired units. They mentioned the significance of particle-level heterogeneities in ash composition and used gravity separated Rosebud coal fractions in the study of deposition on heat transfer surfaces in pulverized coal fired boilers. This concept was further applied to the fluidized bed industry by Rozelle
et al. [
23]. in their models to predict the flow rates of fly ash and bottom ash from a CFB boiler. Moreover, in the study of ash particle sticking in entrained flow gasification, the effect of heterogeneity of fuel ash has been studied [
24].
These particle-level non-uniformities in chemical composition have not been considered in the determination of agglomeration tendencies of fuels. The techniques that have been used previously for estimation of agglomeration propensity, such as initial deformation temperature measurement (IDT) using AFT [
25] and base to acid ratio calculation [
26] have been applied using only the bulk coal composition. However, Van Dyk
et al. [
27] found on comparison to high temperature X-ray diffraction (HT-XRD) and FactSage
™ modeling results that a liquid phase was detected at temperatures below those predicted by AFT. Additionally, they stated that operational experiences have indicated that the slag formation tendencies as predicted by AFT were often not reliable. Stallmann
et al. [
28] also observed this, while Raask [
29] also identified limitations, and suggested that the margin of error may be significant, and the occurrence of errors in the determination of the point of onset of slag-liquid formation is possible. These may occur as particle to particle sintering by local liquid phase formation may not cause a visually detectable difference in the shape of the composite ash cone. Stallmann
et al. [
28] studied temperatures at which agglomerates begin to grow for six coals using sieve analyses and SEM, and found no correlation with the AFT measurements. Experimental estimation of sintering based on change in shrinkage or conductivity of ash pellets during heating has also been done previously to estimate the agglomeration potential [
30]. Gupta
et al. [
31] related the shrinkage to slag formation and particle stickiness. These techniques have also largely been applied to the bulk coal. The particle-level differences in size and composition have often been ignored in using only the composite fuel for analysis and prediction. A consideration of particle-level non-uniformities, in the prediction of ash behavior and agglomeration, is warranted.
This concept of heterogeneity becomes critical in the development of particle population models that quantify the response of different particle classes to stimulus, in order to obtain the resultant effect. Existing mathematical modeling techniques to predict agglomeration require further development as has been discussed elsewhere [
1]. Incorporation of the fuel ash compositional variations at the particle-level into these models for plant applications can lead to improved tools for eliminating agglomeration as an operating issue in CFB boilers.
This study focuses on understanding the slag-liquid formation tendencies of four density separated fractions of Pittsburgh No. 8 seam coal under an oxidizing gaseous environment using FactSage™ thermodynamic simulations. It studies the implications of particle-level differences to the onset of conditions favorable for ash agglomeration in fluidized bed combustors.
3. Research Objectives
The objectives of this research are to: (1) Obtain quantitative information on particle-level slag-liquid formation of Pittsburgh No. 8 seam coal, under an oxidizing gaseous environment; (2) understand the effect of heterogeneity in ash chemical composition on the slag-liquid formation tendencies; (3) contrast the particle-level slag-liquid formation tendencies obtained under these oxidizing conditions to those under reducing environment.
This information can be used to determine the extent of stickiness of ash particles in the development of models for prediction of agglomeration in fluidized bed combustors such as the Penn State Ash Agglomeration Model and the model by Li
et al. [
32]. It also helps in the determination of onset of conditions favorable to agglomerate growth at the particle-level.
4. Materials and Methods
Pittsburgh No. 8 seam coal was chosen for this study as a high rank coal with high iron content, and relatively simpler mineral assemblage, with low content of organically-bound alkali and alkaline earth metals. This coal was chosen due to the widespread use of bituminous coals in combustion units in the US. A better understanding of the cause of operational issues in these units would be helpful for the industry. The whole coal was separated into four density fractions using the float-sink gravity separation technique and then into seven particle size intervals. These gravity separated fractions are referred to as SG1, SG2, SG3 and SG4 in this paper, in increasing order of their specific gravity, while the whole coal is referred to as SG0. The fractions were characterized using proximate and ultimate analyses and the distinct ash composition of each of the fractions was determined using XRF previously [
20,
21]. The Inductively Coupled Plasma Atomic Electron Spectroscopy (ICP-AES) ash chemical composition in
Table 1 shows that each of the density fractions has a distinct composition. The same coal was also used in previous work [
2] under reducing gaseous environment, and it may be referred to for further details on the coal characterization and the methods used. In this study, each of the gravity fractions has been studied for two distinct size classes of 212 to 425 µm and also particles of 75 to 106 µm. The trends in the results for both the size fractions were very similar and so the results for only the smaller size fraction have been detailed here.
Table 1.
Inductively coupled plasma atomic electron spectroscopy (ICP-AES) ash analyses of particle classes (75 to 106 µm) of Pittsburgh seam coal [
20].
Table 1.
Inductively coupled plasma atomic electron spectroscopy (ICP-AES) ash analyses of particle classes (75 to 106 µm) of Pittsburgh seam coal [20].
| Species | SG0 | Wt. Percent in Fuel Ash (%) |
|---|
| SG1 (1.3 float) | SG2 (1.3 sink, 1.6 float) | SG3 (1.6 sink, 2.6 float) | SG4 (2.6 sink) |
|---|
| SiO2 | 46.4 | 49.9 | 55.3 | 51.8 | 12.5 |
| Al2O3 | 22.0 | 28.7 | 25.2 | 20.8 | 4.67 |
| Fe2O3 | 24.5 | 9.41 | 11.3 | 18.9 | 76.0 |
| CaO | 3.41 | 5.05 | 2.78 | 4.87 | 5.79 |
| TiO2 | 1.05 | 2.47 | 1.22 | 0.74 | 0.21 |
| K2O | 1.56 | 1.85 | 1.98 | 1.48 | 0.24 |
| MgO | 0.73 | 1.32 | 1.08 | 0.76 | 0.29 |
| Na2O | 0.42 | 0.76 | 0.91 | 0.52 | 0.13 |
| SrO | 0.11 | 0.34 | 0.10 | 0.04 | 0.29 |
| BaO | 0.08 | 0.17 | 0.07 | 0.03 | 0.03 |
| MnO | 0.03 | 0.02 | 0.03 | 0.02 | 0.07 |
In order to determine if the heterogeneity or particle-level behavior indicates onset of conditions favoring ash agglomeration at low operating temperatures, FactSage
™ was used as a tool to obtain the slag-liquid present in the bed at a given temperature at equilibrium. Each of the four gravity separated fractions were studied to incorporate the effect of chemical heterogeneity of bed ash in to the calculations, and to identify the bad actors through an understanding of particle-level mineral matter transformations. The ash generated from each of the four gravity separated fractions contained in 10 kg of the coal was equilibrated with an oxidizing gas composition. The effect of carbon content or additional bed sorbents on agglomeration is not considered in this study. The composition of the gaseous atmosphere used for this study was a typical flue gas composition containing 3% oxygen, 15% carbon dioxide, 10% water and 72% nitrogen. This gas composition was chosen based on the distribution of oxygen in the study by Hartge [
5] in a CFB combustor, which showed the oxygen content to be similar to the flue gas. The simulations were also repeated in air and the slag formation tendencies were not significantly different. The ratio of amount of coal to the gaseous atmosphere was determined based on the amount of carbon dioxide formed on complete combustion of whole coal. It was run at atmospheric pressure and the temperature was varied to depict fluidized bed operating conditions. The simulations were initially run at 800 °C. Then the temperature was raised by 10 °C until the presence of slag-liquid was detected. The simulations were then carried out at every 50 °C to obtain the amount of slag-liquid formed as a function of temperature.
The FactSage™ results were experimentally validated using ash generated at 650 °C in a TGA proximate analyzer (LECO Corporation, St. Joseph, MI, USA). Thermo-mechanical analysis (TMA) in a Shimadzu thermo-mechanical analyzer (Shimadzu Corporation, Kyoto, Japan), was used to experimentally study the onset temperature of slag-liquid formation of the density fractions. The minimum temperature at which the ash pellet begins to shrink is stated as the slag-liquid formation onset temperature. The onset temperature was recorded at an arbitrary value of 0.1% shrinkage for consistency between all the samples.
Further understanding of the mineral phases present and the transformations occurring with temperature rise was obtained using HT-XRD (Anton Paar GmbH, Ashland, VA, USA). XRD helped to gain qualitative understanding of the mineral phases present and was used in conjunction with the quantitative ICP-AES data and the FactSage™ simulation results to explain the differences in the slag-liquid formation tendencies of the four gravity separated fractions. Ash samples were heated from 700 °C to 1100 °C. The samples were previously ashed at 650 °C and hence XRD patterns were not recorded below this temperature. XRD patterns were obtained at every 100 °C temperature interval. A baseline pattern was also recorded before the ash was heated, to obtain the minerals present in the ash that had been generated at 650 °C. Air flow was maintained during the measurements to provide an oxidizing gaseous environment. All the experiments in this study were performed at atmospheric pressure conditions.
5. Uncertainty Analysis
In the method of gravity separation of the whole coal, errors can occur in the determination of the location of the layer that separates the float and sink fractions. A small sample loss during filtration can also introduce some error. The reproducibility of this separation technique was tested by conducting five repeated separations and calculating the yield of the 1.6 float and sink fractions. These repetitions resulted in an average yield of 4.5 wt.% of sinks with a standard deviation of 0.95.
The uncertainty in the measurement of the oxide content of the fractions using ICP-AES was determined through the analysis of a rock standard over time using the same technique and making observations across several previous measurements. The relative uncertainty depends on the oxide concentration. This uncertainty is shown in
Table 2. As an example, 2% relative uncertainty for a 20 wt.% oxide concentration means that the concentration would be 20% ± 0.4%.
Table 2.
Uncertainty in oxide content obtained from ICP-AES.
Table 2.
Uncertainty in oxide content obtained from ICP-AES.
| Concentration Range (wt.%) | Relative Uncertainty (wt.%) |
|---|
| >10 | ±2 to 3 |
| 1 to 10 | ±5 |
| 0.1 to 1 | ±10 |
| <0.1 | ±(>10) |
In order to determine the uncertainty of the results from the TMA, repeatability tests were performed on the SG4 fraction in an oxidizing atmosphere.
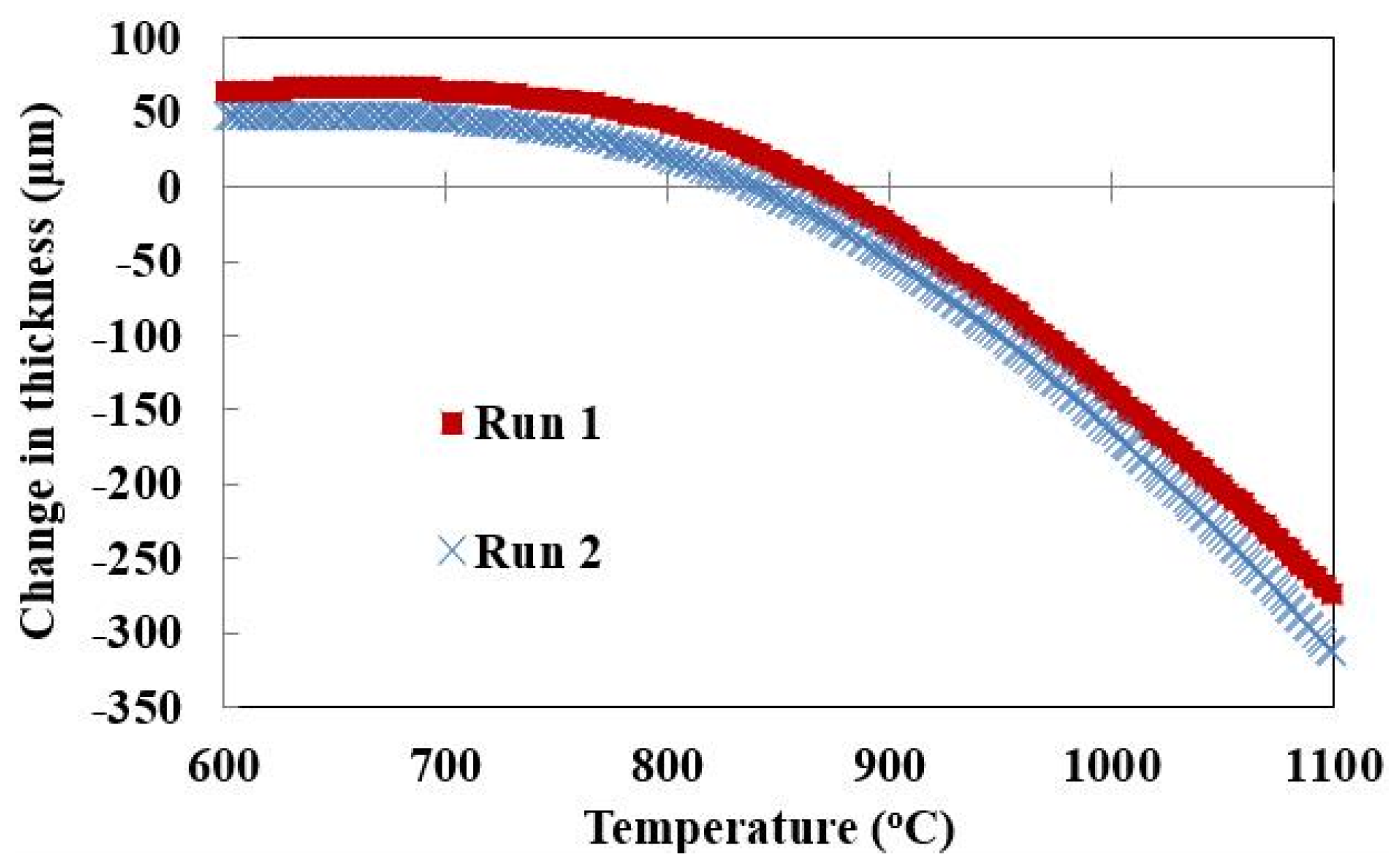
Figure 1 shows that the reproducibility in TMA data is reasonable for the requirements of this study. The pellet thickness changes as slag formation begins. The change in thickness from the original has been recorded in
Figure 1. At the initiation of slag formation, a change in the thickness is detected as seen by the drop from horizontal in the graph below. Near this point of initiation, the difference in the change in thickness between the two runs is an average of 17 µm. This translates to less than 10 °C difference in initiation temperature.
Figure 1.
Reproducibility analysis of thermo-mechanical analysis measurements.
Figure 1.
Reproducibility analysis of thermo-mechanical analysis measurements.
6. Results
The FactSage™ results helped to predict and quantify slag-liquid formation tendencies with temperature under oxidizing environments as distinct from reducing gaseous atmospheres. The effect of heterogeneity in ash chemical composition on these tendencies became evident through the study of each of the individual density fractions as well as the bulk coal ash.
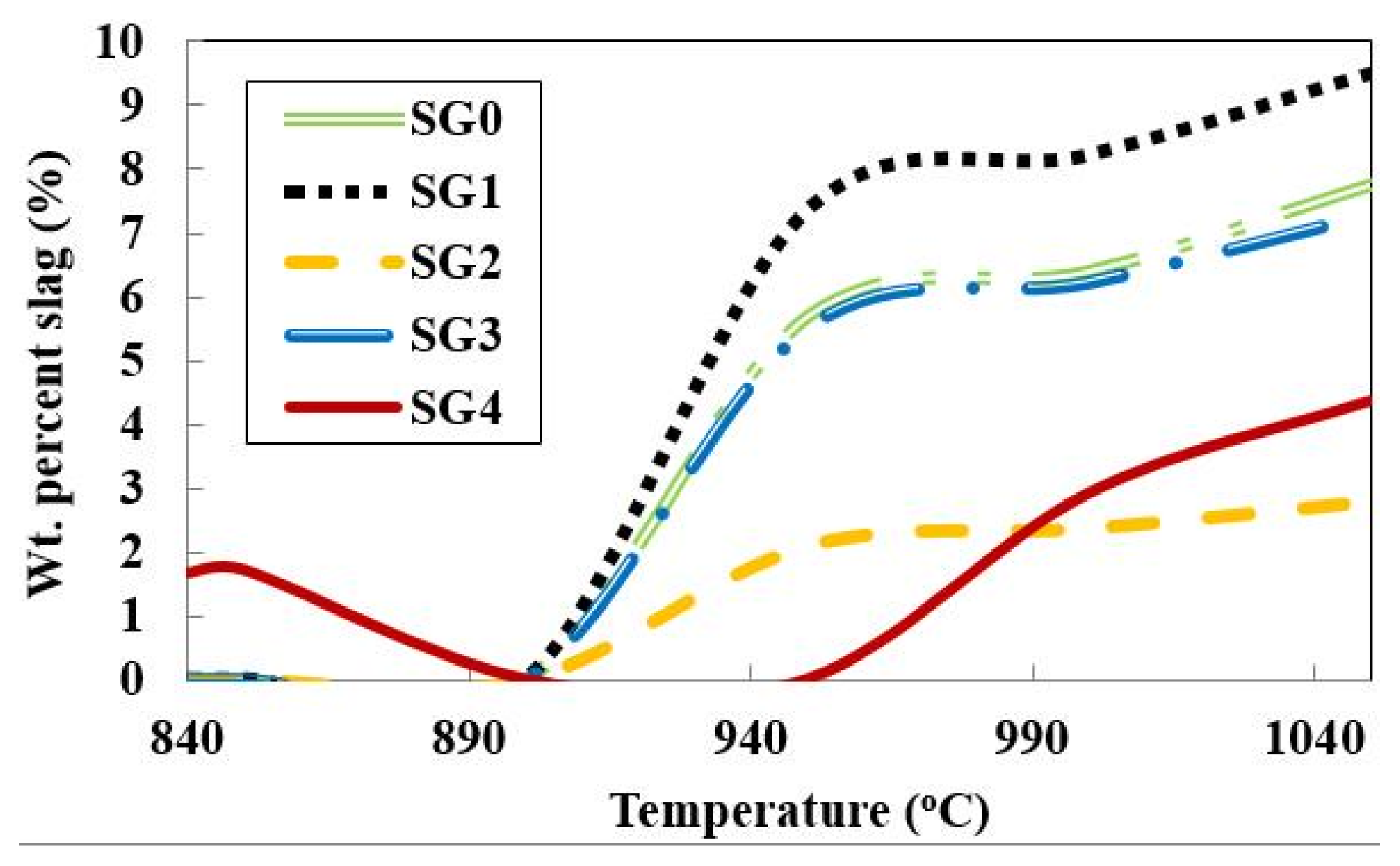
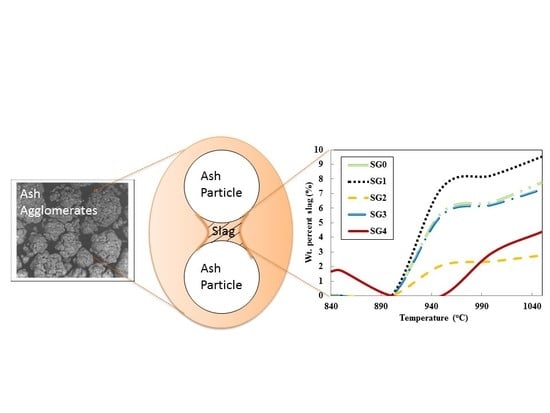
Figure 2 shows the changes in slag-liquid amount with temperature. Each of the four density fractions forms distinct slag-liquid amounts and it is also distinct from that of the bulk (SG0). This shows that heterogeneity in ash chemical composition is of significance in the slag-liquid formation tendencies and subsequent particle agglomeration. To further illustrate the importance of differences in the behavior of ash particles, the temperature of onset of slag-liquid formation was also predicted. A lower slag-liquid onset temperature is seen for the SG4 fraction in
Table 3, as compared to the other fractions and whole coal. This emphasizes the importance of heterogeneity in ash chemical composition.
Figure 2.
Slag-liquid formation tendencies of whole coal and density fractions of Pittsburgh No. 8 seam coal.
Figure 2.
Slag-liquid formation tendencies of whole coal and density fractions of Pittsburgh No. 8 seam coal.
Table 3.
Temperature of slag-liquid formation onset for each gravity fraction and whole coal (FactSage™ predictions).
Table 3.
Temperature of slag-liquid formation onset for each gravity fraction and whole coal (FactSage™ predictions).
| Sample | Density of Fraction (g/cc) | Slag-Liquid Formation Onset Temperature (°C) |
|---|
| SG0 | Whole | 920 |
| SG1 | <1.3 | 940 |
| SG2 | 1.3–1.6 | 920 |
| SG3 | 1.6–2.6 | 920 |
| SG4 | >2.6 | 850 |
Thermo-mechanical analysis helped to validate the trends in the FactSage
™ results and supports the significance of particle-level heterogeneities in chemical composition. As seen in
Figure 3 and
Table 4 the slag formation for the SG4 fraction is initiated at a lower temperature than the other density fractions and the bulk coal.
Figure 3.
Thermo-mechanical analysis (TMA) results for whole coal and density fractions of Pittsburgh No. 8 seam coal.
Figure 3.
Thermo-mechanical analysis (TMA) results for whole coal and density fractions of Pittsburgh No. 8 seam coal.
The composition of the slag-liquid phase obtained at initiation using FactSage
™ for each of the density fractions is shown in
Table 5. A higher CaO content along with lower alumina content is seen in the SG4 fraction. The slag of the remaining fractions contains mainly sodium aluminosilicates at initiation.
Table 4.
Temperature of slag-liquid formation onset for each gravity fraction and whole coal (Thermo-mechanical analysis (TMA) results).
Table 4.
Temperature of slag-liquid formation onset for each gravity fraction and whole coal (Thermo-mechanical analysis (TMA) results).
| Sample | Density of Fraction (g/cc) | Slag-Liquid Formation Onset Temperature (°C) |
|---|
| SG0 | Whole | 870 |
| SG1 | <1.3 | 870 |
| SG2 | 1.3–1.6 | 880 |
| SG3 | 1.6–2.6 | 920 |
| SG4 | >2.6 | 850 |
Table 5.
Slag phase composition of the whole coal and various density fractions at slag-liquid formation onset temperature.
Table 5.
Slag phase composition of the whole coal and various density fractions at slag-liquid formation onset temperature.
| Phase in Slag | Wt.% |
|---|
| SG0 | SG1 | SG2 | SG3 | SG4 |
|---|
| SiO2 | 62.48 | 67.28 | 62.50 | 62.48 | 63.22 |
| CaO | 2.64 | 2.73 | 2.66 | 2.26 | 13.81 |
| Al2O3 | 20.08 | 18.56 | 20.03 | 20.07 | 8.24 |
| TiO2 | 2.26 | 1.98 | 2.64 | 7.43 | 8.34 |
| K2O | 0.12 | 0.39 | 0.13 | 0.12 | 2.96 |
| Na2O | 7.43 | 7.56 | 7.47 | 3.38 | 0.99 |
| MnO | 3.38 | 0.00 | 2.97 | 2.64 | 1.46 |
| FeO | 0.03 | 0.03 | 0.03 | 1.57 | 0.98 |
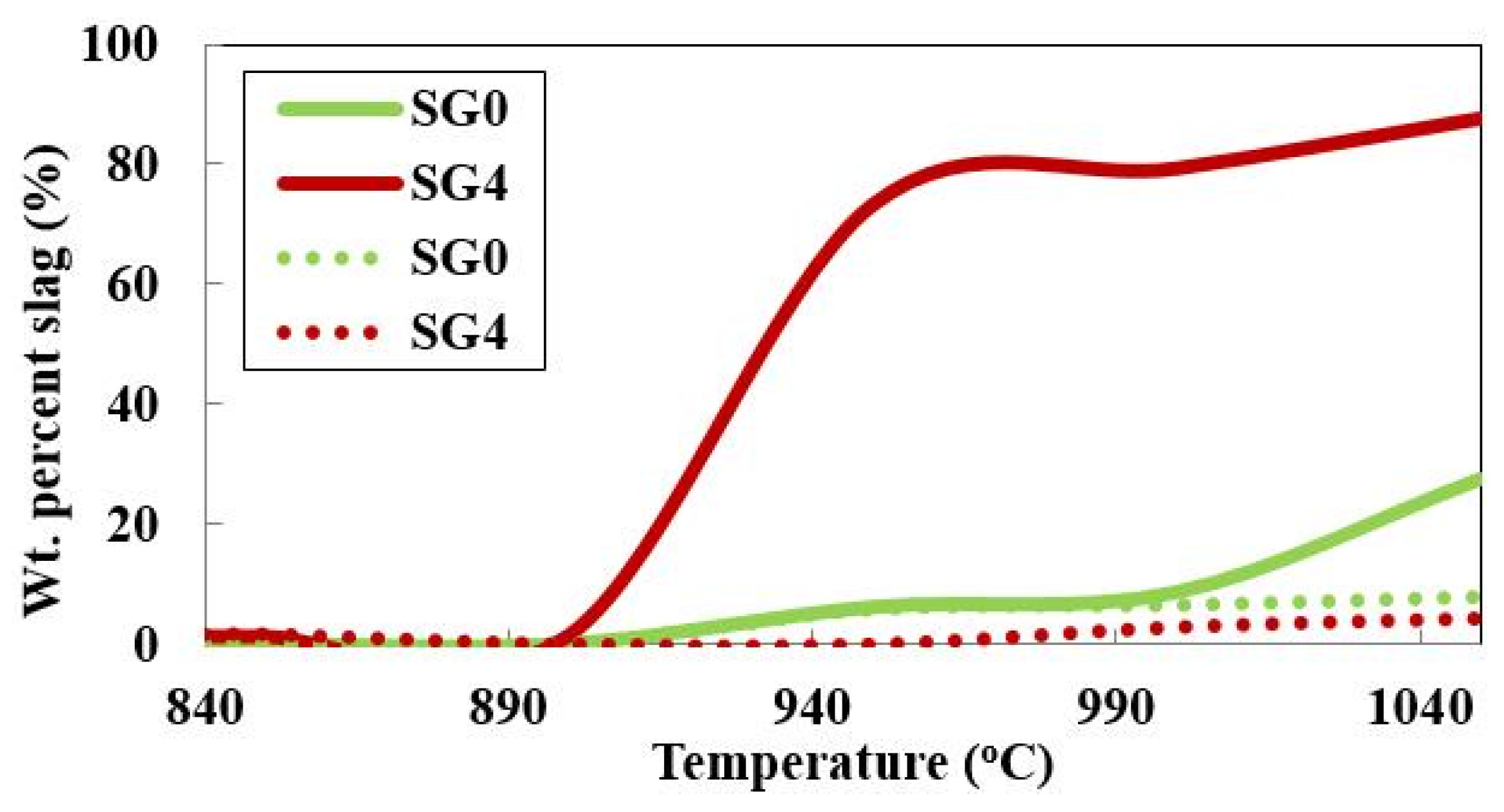
The amount of slag-liquid formed is lower under oxidizing gaseous atmospheres than under reducing conditions. There is a major difference in the slag-liquid amount for the SG4 fraction under these two gaseous atmospheres as seen in
Figure 4. These differences are not detectable through the analysis of bulk coal alone and differences in slag-liquid tendencies in the SG0 (bulk coal) fraction are seen only above around 1000 °C.
Figure 4.
SG4 fraction showing significant difference in slag-liquid amounts in reducing (solid) than oxidizing (dotted) atmospheres unlike whole coal.
Figure 4.
SG4 fraction showing significant difference in slag-liquid amounts in reducing (solid) than oxidizing (dotted) atmospheres unlike whole coal.
7. Discussion
7.1. High Temperature X-Ray Diffraction (HT-XRD) Based Mineral Matter Transformations of Pittsburgh No. 8 Coal under Fluidized Bed Conditions that Support FactSage™ and Thermo-Mechanical Analysis (TMA) Results
FactSage
™ and TMA results (
Figure 2 and
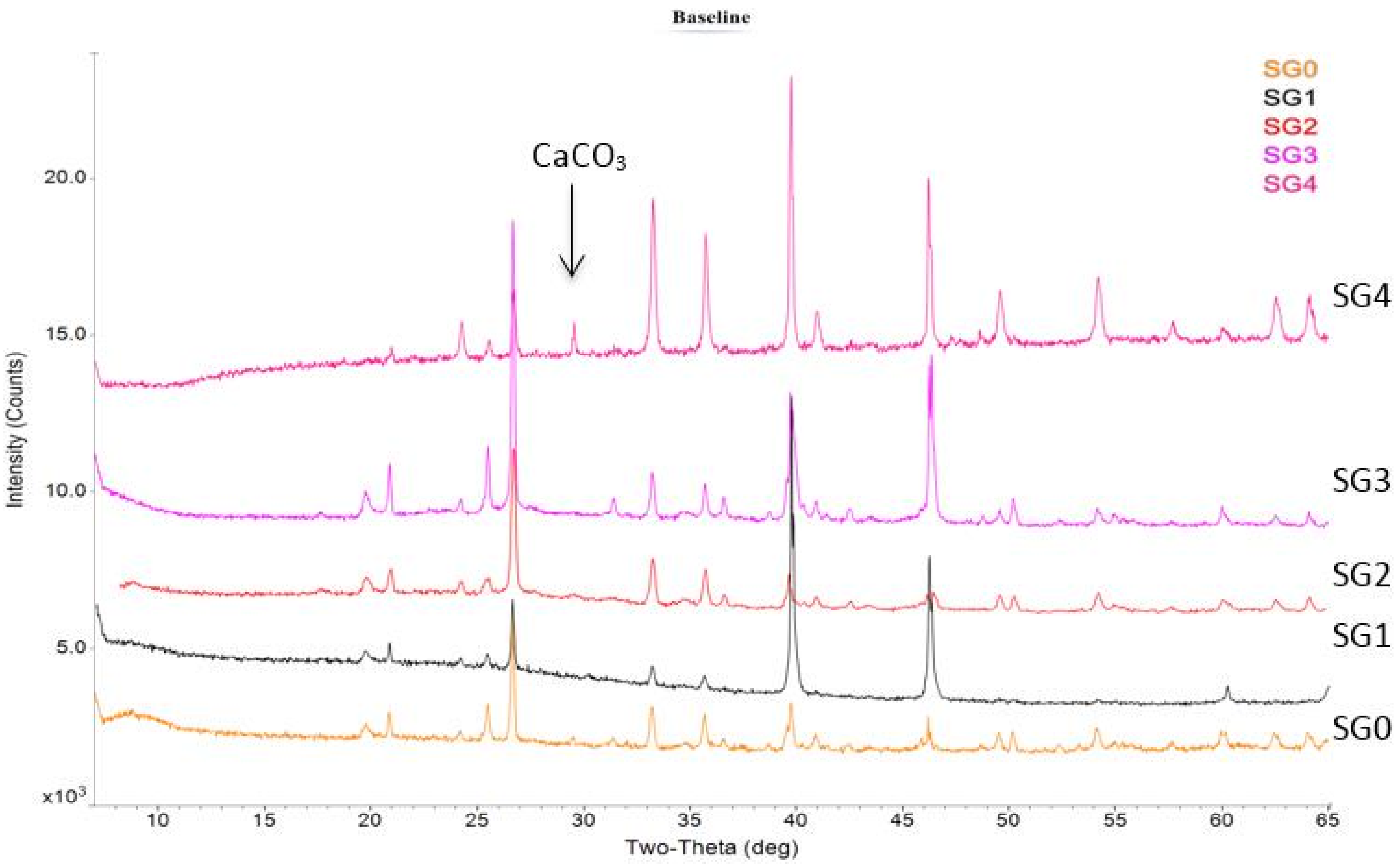
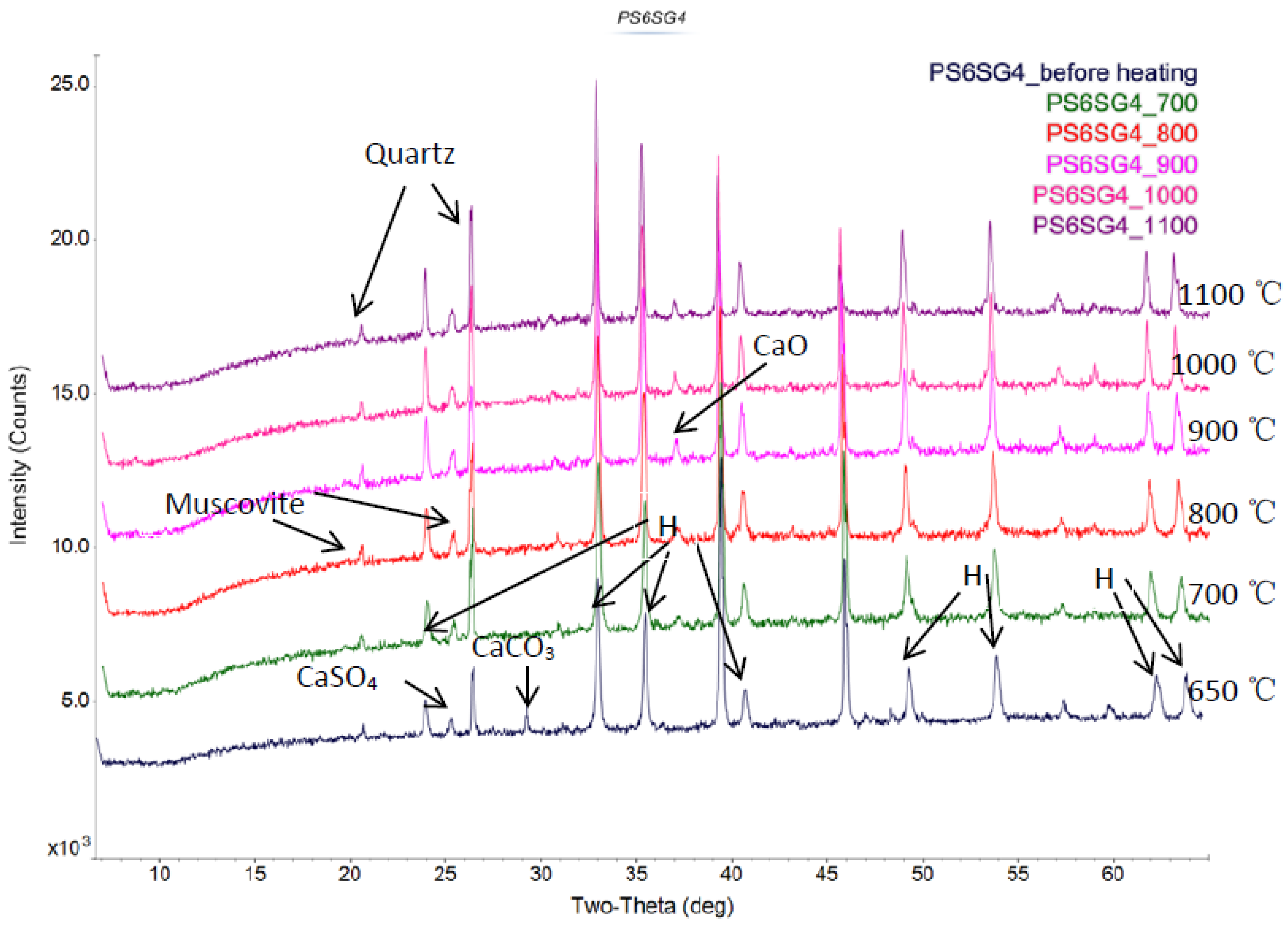
Figure 3) show that slag-liquid formation in SG4 fraction is initiated at a lower temperature (850 °C) than the other fractions and the bulk coal. In order to understand the mineral matter transformations associated with this low temperature slag-liquid formation, HT-XRD was used. Using HT-XRD, the significant presence of crystalline CaCO
3 is seen only in the SG4 fraction at 650 °C (
Figure 5). This CaCO
3 decomposes to form CaO as shown in
Figure 6 at higher temperatures and is fully converted above 900 °C. The presence of calcium oxide from calcium carbonate decomposition is seen in the SG4 fraction and it is expected to play a role in the slag-liquid formation. The unique behavior of the SG4 fraction in an oxidizing environment was likely to be related to this mineral transformation. Hence, the literature was surveyed to identify the possibility of low temperature liquid phase formation in the presence of CaO, which is significantly present only in the SG4 fraction as shown in
Figure 7. Since the formation of eutectics between two or more phases leads to lowering of melting point below each of the individual phases, the possibility of formation of eutectics involving CaO was investigated. A eutectic of 23.25% CaO, 14.75% alumina and 62% silica that leads to liquid phase formation at 1170 °C, was reported in the literature [
33]. In the presence of additional components, such as FeO, Na
2O and K
2O, this eutectic temperature is expected to be even lower. This is likely to be the cause of lower slag formation temperatures of particle classes such as SG4, as their chemical composition favors this eutectic formation. The analysis of the ash composition obtained from ICP-AES given in
Table 1 revealed that the proportion of these three oxides in SG4 fraction was distinct from the other fractions. It was indeed close to that of the reported eutectic composition, as illustrated in the ternary composition diagram (
Figure 8).
Figure 8 illustrates that this eutectic would not be possible in the remaining particle classes due to the lower CaO weight percent relative to SiO
2 and Al
2O
3. The compositions of both the particle size classes studied have been presented in this figure for contrast. The formation of this eutectic has been identified as an important mineral matter transformation that occurs at the particle-level, contributing to formation of sticky SG4 particles even under the low temperatures occurring in fluidized bed combustors. This is further supported by the composition of the slag phase at the onset temperature, shown in
Table 4. The SG4 slag is rich in CaO, unlike the remaining fractions. All these observations substantiate and explain the FactSage
™ and TMA results. Besides this, the lower Al
2O
3 content of the SG4 fraction compared to the other fractions also supports the predicted low initiation temperature, since increasing Al
2O
3 content has been found to increase slag-liquid formation temperature [
34].
Figure 5.
Presence of significant amount of CaCO3 seen only in SG4 fraction using X-ray diffraction (XRD) on ash samples prepared at 650 °C.
Figure 5.
Presence of significant amount of CaCO3 seen only in SG4 fraction using X-ray diffraction (XRD) on ash samples prepared at 650 °C.
Figure 6.
High temperature X-ray diffraction (HT-XRD) of SG4 fraction showing decomposition of CaCO3 to CaO.
Figure 6.
High temperature X-ray diffraction (HT-XRD) of SG4 fraction showing decomposition of CaCO3 to CaO.
Figure 7.
Presence of CaO only in SG4 density fraction at 1100 °C.
Figure 7.
Presence of CaO only in SG4 density fraction at 1100 °C.
Figure 8.
Ternary composition diagram of CaO-SiO2-Al2O3 showing SG4 fractions (crosses) with compositions closer to the eutectic (circled square) (remaining fractions: squares).
Figure 8.
Ternary composition diagram of CaO-SiO2-Al2O3 showing SG4 fractions (crosses) with compositions closer to the eutectic (circled square) (remaining fractions: squares).
Besides the onset temperature of slag formation, some differences are also observed in the amount of slag-liquid formed from the different fractions. The slag formed in SG1, SG2 fractions consists mainly of aluminosilicates with some alkali-alkaline earth metal contents, while that from the SG4 fraction is mainly composed of calcium oxides with aluminosilicates. The reduced amounts of slag seen in SG4 fraction as compared to the SG1 and SG3 fractions (FactSage
™ results,
Figure 2) are believed to be due to lower contents of calcium phases and aluminosilicates (
Table 1). The decreased slag-liquid amount in the 900 °C to 950 °C range for the SG4 fraction is believed to be due to changes in the CaO-SiO
2-Al
2O
3 ratio after the liquid phase formation occurs near the eutectic composition, along with crystallization of anorthite and andradite phases.
7.2. Importance of Heterogeneity in Ash Composition at Particle-Level on Mineral Matter Transformations
As discussed earlier in the paper, conventional techniques for the prediction of agglomeration such as AFT can be inadequate and these predictions have been based only on bulk coal composition.
The distinct slag-liquid formation tendencies of the four density fractions shown in
Figure 2 demonstrate the importance of particle-level heterogeneity in chemical composition. As has been discussed in detail, the FactSage
™ results in
Table 2 as well as the TMA results in
Table 3 showed that the initial sintering temperature of the SG4 particles is significantly lower, at 850 °C, than that of the bulk coal. The AFT of bulk Pittsburgh seam coal shows an initial deformation temperature of 1430 °C under oxidizing conditions [
22]. This would not indicate agglomeration risk at fluidized bed operating temperatures. However, the study of particle density fractions reveals the possibility of onset of slag formation at fluidized bed temperatures, at the particle-level.
Furthermore, if only the bulk coal properties are analyzed, the difference in slag-liquid formation tendencies under reducing and oxidizing gaseous atmospheres are not easily detected. As seen in
Figure 4 negligible difference is seen in the slag-liquid formation tendencies of whole coal under the two different gaseous atmospheres. However, an appreciable difference is found in the slag-liquid amount for the particles of the SG4 density fraction.
The concept of heterogeneity in ash chemical composition has been previously used to study deposition in pulverized coal fired units [
22]. However, literature extending this to agglomeration in fluidized beds is not extensive and the effects of this heterogeneity under an oxidizing environment have not been quantified previously. The quantification provided in this study will aid the development of particle population models to study agglomeration and mitigate it. It helps to better understand the reason for operational issues due to fluidized bed agglomeration at temperatures much lower than predicted by bulk analysis alone.
7.3. Comparative Analysis of Oxidizing and Reducing Gaseous Environments
Comparison of mineral matter transformations and slag-liquid formation tendencies under oxidizing and reducing environment is important, since both these conditions exist around ash particles in fluidized bed combustors, as discussed. A few studies that compare the effect of gaseous atmosphere on mineral matter transformations are found in the literature. One of the early studies on this topic was made by Huffman
et al. [
9] which was targeted more towards application in pulverized coal fired units and focused on higher temperature conditions (focus above 1000 °C). Their study showed that partial ash melting is accelerated in reducing environments. The study presented in this paper is also in line with their observation and shows that the amount of slag-liquid is lower in an oxidizing environment. The work of Huffman, however, used conventional, qualitative techniques such as AFT to determine the slag formation behavior. They acknowledge the need for more quantitative estimates of slag-liquid amounts. The present study has provided this quantitative information through thermodynamic simulations under fluidized bed operating conditions, along with experimental validation. Similar work using thermodynamic simulations to compare slag-liquid formation tendencies has been done by Jones
et al. [
35] for a waste fired fluidized bed boiler. However, the chemical composition and slag-liquid formation tendencies for biomass based fuel are different and involve gas phase reactions and condensation on to particles. Although more liquid phase formation tendencies were witnessed under reducing atmospheres once again, it is difficult to directly extrapolate mineral matter transformation results for biomass based fuels to high rank coals. Another study identified that liquid phase formation and vaporization of volatile metals along with characteristics of char produced vary with bulk gas composition under an oxy-combustion environment [
36]. The present study focuses on the effect of gaseous environment on slag-liquid formation of specific particle classes. The SG4 fraction showed distinct behavior under the two varying gaseous atmospheres. This density fraction is rich in iron and calcium oxides, as seen from the ICP-AES analysis. Conversion between the multiple oxidation states of the iron phases, explain the differences in oxidizing and reducing environments. In a reducing environment, more slag was continually formed and the amount of slag was very high especially at higher temperatures. This was due to the formation of eutectics involving reduced forms of iron oxide such as FeO [
2]. Under oxidizing environment, the dominant iron phase is Fe
2O
3. In this case, the presence of CaO dominates the slag-liquid formation in the SG4 fraction. Although the dominant cause of slag formation is different in the two gaseous environments, the onset of slag-liquid formation is the lowest, at 850 °C, for the highest density, mineral-rich SG4 fraction, in both the gaseous environments. The onset temperature is lower than that predicted by bulk coal analysis (oxidizing: 920 °C; reducing: 910 °C) and is not significantly different in the two gaseous environments.
7.4. Implications to Bed Ash Agglomeration
The study of four individual particle classes explained differences in the chemistry and slag-liquid formation tendencies of heterogeneous bed ash. The authors believe that this heterogeneous behavior of bed ash helps to explain the occurrence of agglomeration at unexpectedly low temperatures in fluidized bed combustors.
Studies such as the one by Atakül
et al. [
37] showed that agglomeration occurs 125–200 °C lower than characteristic ash fusion temperatures in a fluidized bed combustor. They identified the temperature at which agglomeration was initiated based on the detection of bed temperature fluctuations and pressure drop measurements, as well as pictures of the active bed surface.
This study of particle-level slag-liquid formation done using density fractions of Pittsburgh No. 8 coal showed that the highest density fraction, SG4, has a slag onset temperature of 850 °C, in an oxidizing environment. The contribution of this mineral rich SG4 fraction to the bed ash is high although its amount in the feed is low [
21]. The SG4 density class is heavier and would be more difficult to fluidize, for a given size class, than all the other density fractions. Therefore, it is likely that these sticky and heavy SG4 particles initiate agglomeration at low fluidized bed operating temperatures.
Once initiated, as particle size increases, the ratio of the particle velocity to the minimum fluidization velocity of the larger particles can decrease. This will increase the possibility of particle segregation. The study on particle segregation conducted by the Westinghouse Electric Corporation in fluidized beds containing dolomite and char particles showed the occurrence of segregation in most situations, when the gas velocity was lowered [
38]. Hence it is possible that once agglomeration is initiated by low melting particles, such as those of the SG4 density class, the change in bed hydrodynamics would lead to further agglomeration due to decreasing particle velocities and decreasing bed voidage. At subsequent higher temperatures caused by temperature imbalances, the other particle classes such as SG1 would become adequately sticky for the propagation of agglomeration in the bed. At least about 3% of the ash in the SG2 fraction and 5%–6% of ash in SG3 also forms slag-liquid, even under oxidizing fluidized bed conditions. The SG1 and SG2 fractions also form a large amount of the coal feed while the SG3 fraction contains high ash amounts. Thus, low slag-liquid amounts could lead to adequate stickiness of particles and create conditions favorable for agglomeration. A report [
17] studied agglomeration of biomass in a laboratory scale and plant scale reactor in which they analyzed the wall thickness of agglomerates produced, using SEM. They found that initiation of agglomeration by the melt-induced mechanism can occur before the formation of a complete coating around particles. Thus, before a complete coating can be formed around bed particles due to sintering effects of the bed siliceous materials, considerable agglomeration can occur due to the small slag-liquid amounts formed by partial melting of the biomass ash. The Penn State study of ash agglomeration through an in-depth analysis of slag-liquid formation behavior at the particle level helps to better understand this critical phenomenon of particle-level onset of agglomeration by partial melting.
Besides segregation, another mechanism of propagation of agglomerate growth in a bed can be due to temperature surges that can result in a continuous system around burning char particles in oxygen rich zones. A third proposition on the propagation of agglomeration in the system is that, once few particles of larger sizes form, due to decrease in their velocity, they can deposit on to heat transfer surfaces and gradually decrease efficiency of heat transfer. The subsequent temperature rise would once again lead to propagation of agglomeration and deposition in the fluidized bed. In this study, we have identified onset conditions for particle-particle sticking and proposed situations that may lead to propagation of agglomeration in the bed.
The suggestion that agglomeration is initiated around a few particles and then propagates throughout the fluidized bed combustor is supported by previous literature that studied agglomerates formed in fluidized bed combustion units. A Department of Energy report [
39] that surveyed thirteen fluidized bed combustion units using SEM to observe the agglomerates that were formed, indicated the presence of dark cores with rims. The agglomerates were also found to have a high calcium sulfate and iron oxide content. These observations further support the onset of conditions favorable for agglomeration by sticking of particles with composition similar to the SG4 fraction, which could have a dark iron core at locations of initiation of agglomerate formation with rims of aluminosilicate-rich slag.
The quantitative information on slag-liquid formation presented here through an in-depth analysis at the particle-level can help to develop agglomeration models based on particle-particle collisions. The heterogeneities in ash chemical composition can then be accounted for in these agglomeration models and can help to improve the prediction capabilities.
8. Conclusions
This study of slag-liquid formation tendencies of Pittsburgh No. 8 seam coal under oxidizing conditions shows that onset of conditions that favor fluid bed agglomeration occur at temperatures as low as 850 °C. It also provides quantitative information of particle-level slag-liquid amounts, for the development of particle population models for predicting ash agglomeration in FBCs.
FactSage™ thermodynamic simulations in conjunction with TMA and HT-XRD experiments of four gravity separated fractions helped to quantify the slag-liquid amounts as a function of temperature. The results showed that a maximum of 10% slag-liquid was formed from the four density fractions studied, in the operating range of 850 to 1050 °C, in an oxidizing environment. The slag-liquid amount generated was lower than that formed under reducing conditions.
Each of the four density fractions studied showed distinct slag-liquid formation tendencies. This emphasized the role of heterogeneity in ash chemical composition in the study of ash agglomeration. The slag-liquid formation for the whole coal, SG1, SG2 and SG3 fraction occurred at relatively higher temperatures closer to 900 °C. The SG4 fraction formed low temperature eutectics based on CaO, Al2O3 and SiO2. This SG4 particle class showed the onset of slag formation at 850 °C, which was distinctly lower than the remaining fractions. Presence of slag-liquid would not be detected at 850 °C using bulk analysis alone, thereby highlighting the role of heterogeneity in ash chemical composition in the onset of agglomeration.
The SG4 fraction also showed appreciable differences in behavior under oxidizing conditions compared to reducing environments. Low temperature slag-liquid formation was due to eutectics involving FeO in reducing gaseous conditions that led to high amounts of slag-liquid. On the other hand, the amount of slag-liquid formed in oxidizing conditions was lower and was primarily due to CaO based eutectics. The higher CaO content of about 6% in combination with a very low Al2O3 and SiO2 content of about 5% and 13% respectively, is believed to be a bad actor under oxidizing conditions. Although the chemistry dominating the slag-liquid formation was distinct, the onset temperature was low under both the gaseous environments. The distinct mineral matter transformations under the varying atmospheric conditions, resulting slag-liquid amounts and subsequent agglomeration was not measurable using bulk analysis alone.
Based on this study, it is believed that particle-level initiation of ash agglomeration begins around particles of specific chemical composition such as the SG4 particles and then propagates throughout the bed, as a result of changes in particle temperatures and hydrodynamics.
This study provides the basis and also quantitative information on slag-liquid amounts that is required for the development of particle collision based models to predict ash agglomeration in fluidized bed combustors.