Prediction of State-of-Health for Nickel-Metal Hydride Batteries by a Curve Model Based on Charge-Discharge Tests
Abstract
:1. Introduction
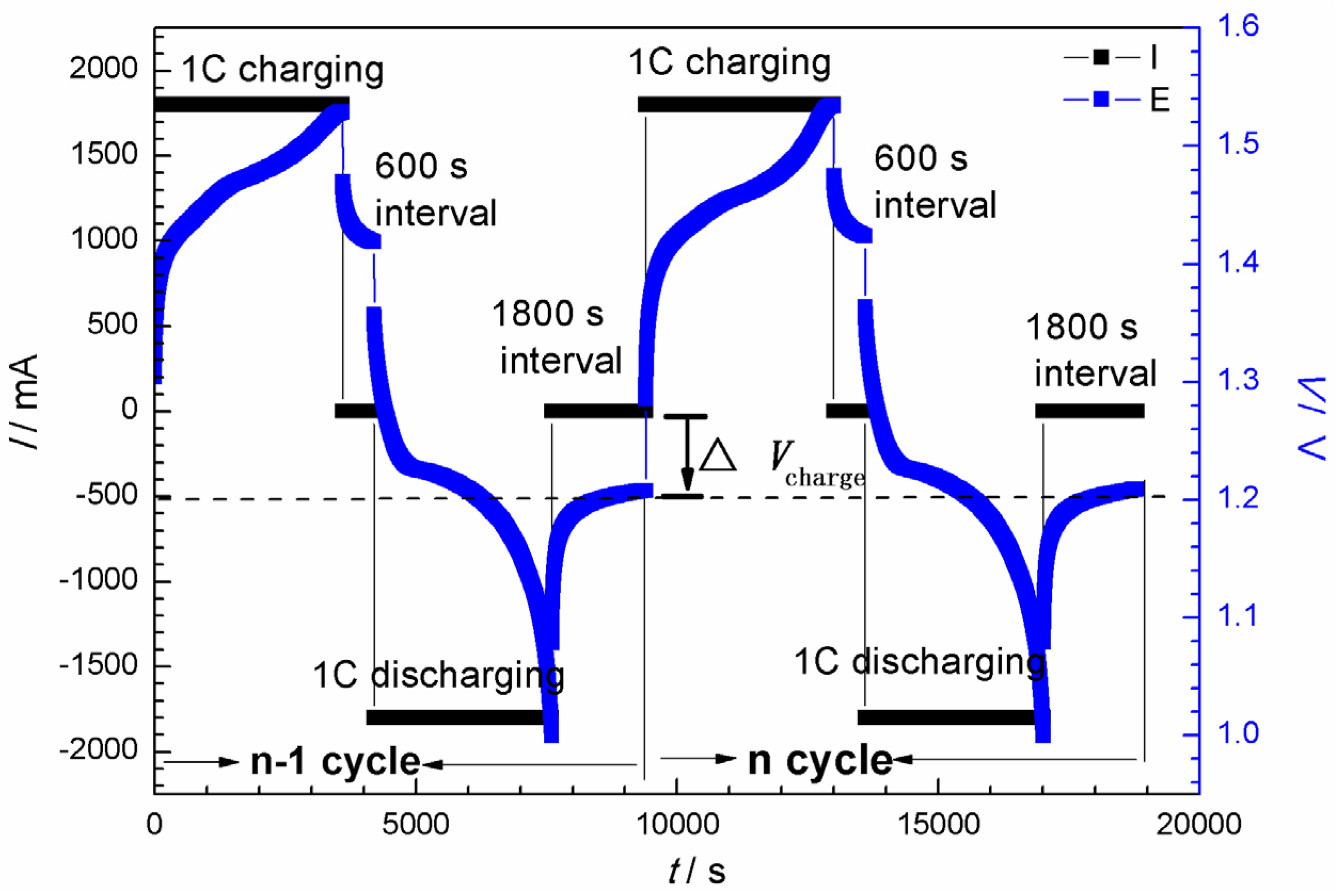
2. Experimental
3. Results and Discussion
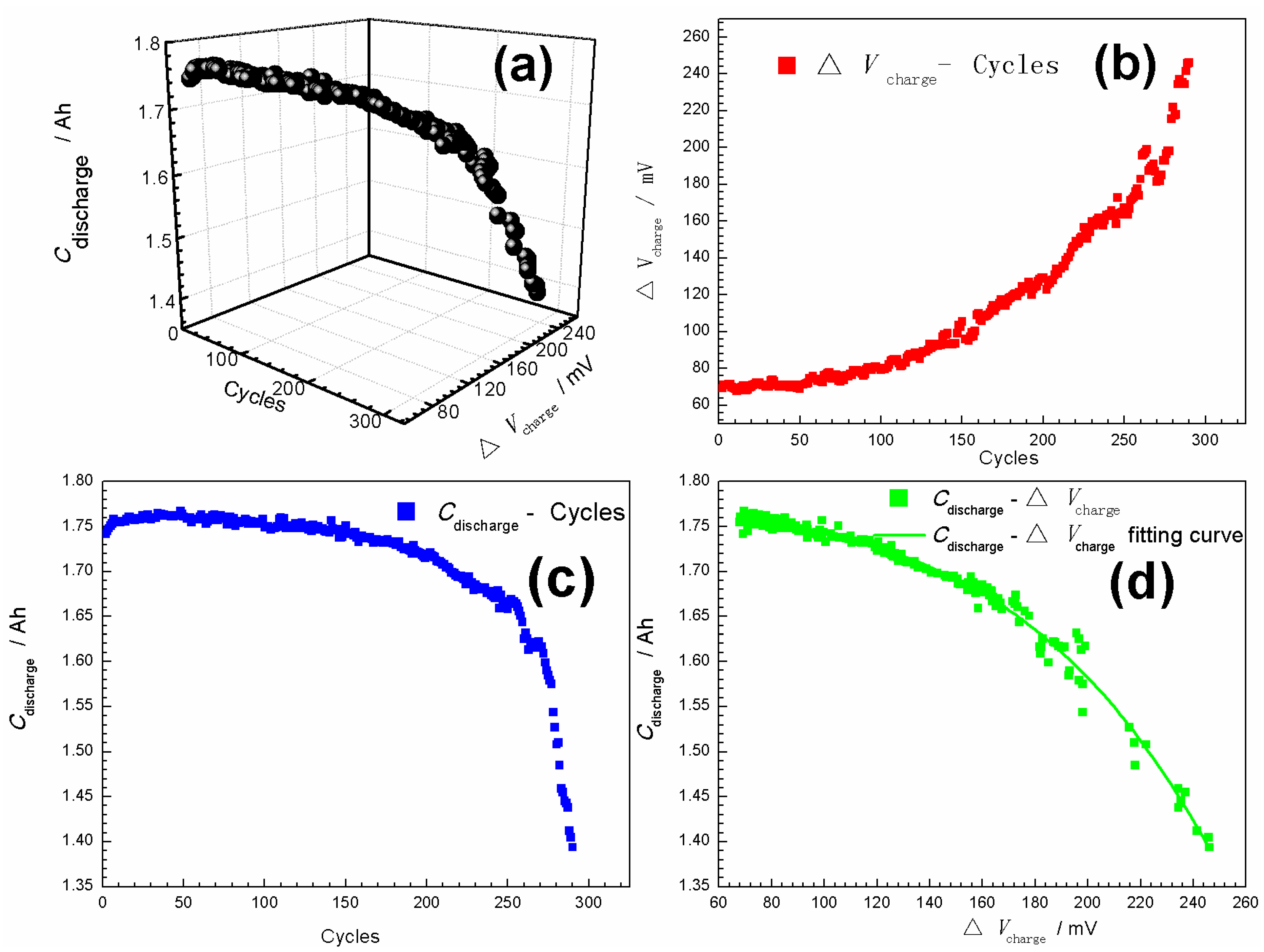
3.1. Curve Modeling for Cdischarge
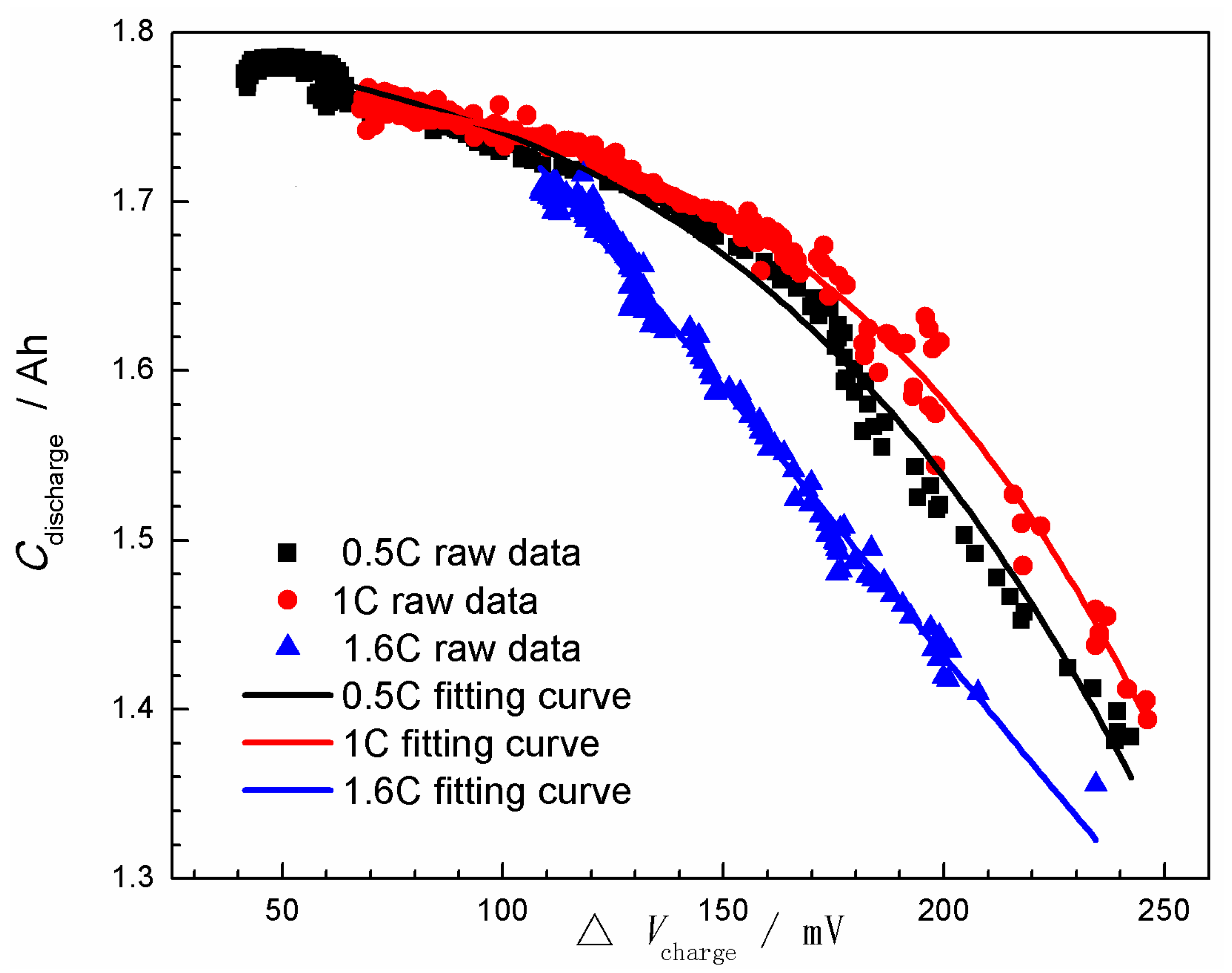
| Rates | a/Ah | c/mV | k/mV−1 | R2 | |
|---|---|---|---|---|---|
| 0.5C | Prange | 1.5–2.0 | 200–400 | −0.02–0 | - |
| 1.8131 | 317.58 | −0.0146 | 0.9862 | ||
| 1.0C | Prange | 1.5–2.0 | 200–400 | −0.03–0 | - |
| 1.7828 | 320.26 | −0.0172 | 0.9882 | ||
| 1.7420 | 310.20 | −0.0164 | 0.9851 | ||
| 1.7680 | 223.30 | −0.0188 | 0.9796 | ||
| 1.7839 | 286.11 | −0.0166 | 0.9800 | ||
| 1.9433 | 313.77 | −0.0078 | 0.9865 | ||
| 1.7962 | 381.17 | −0.0078 | 0.9869 | ||
| 1.6C | Prange | 2.0–3.0 | 200–400 | −0.015–0 | - |
| 2.4479 | 258.21 | −0.0057 | 0.9898 | ||
| 2.6365 | 239.15 | −0.0048 | 0.9890 | ||
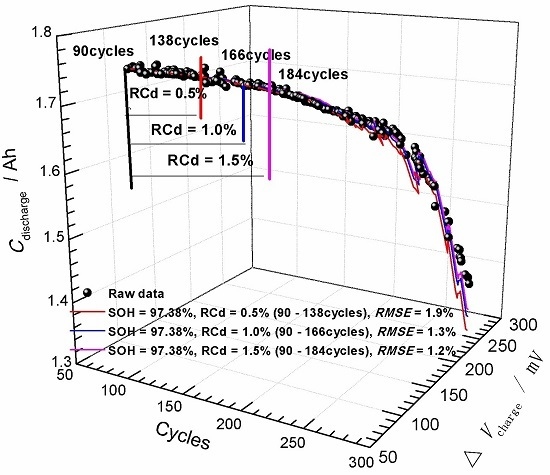
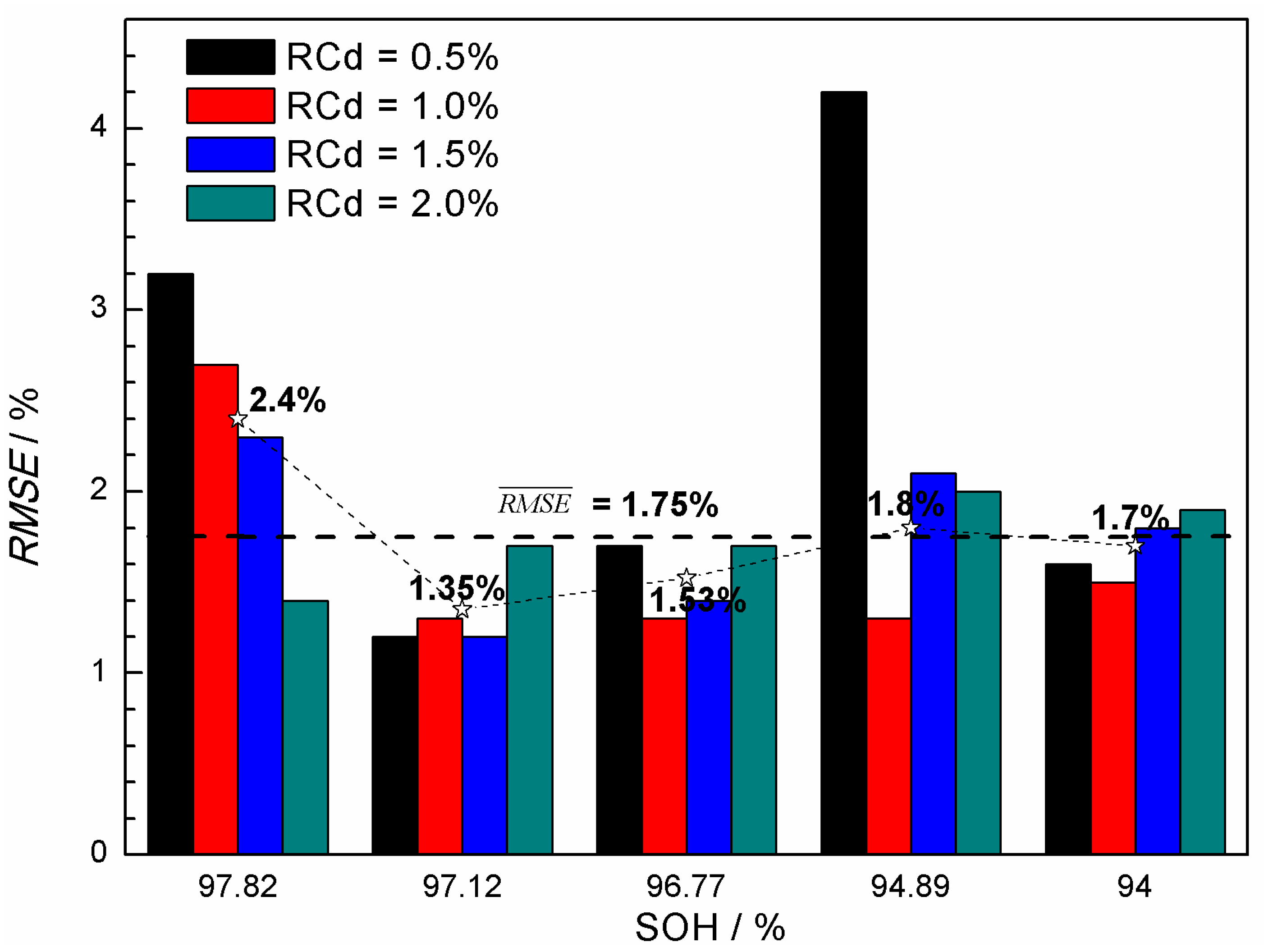
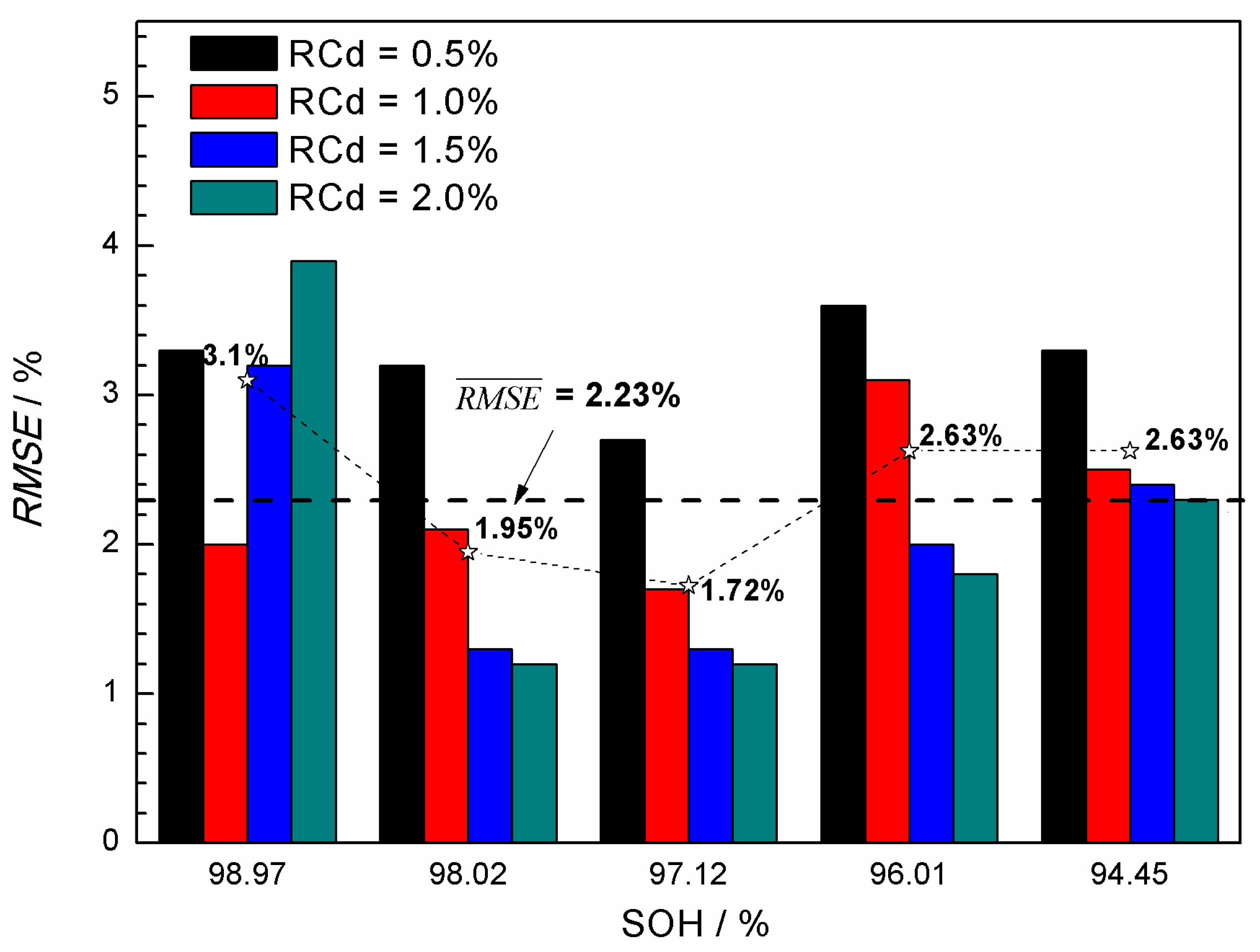
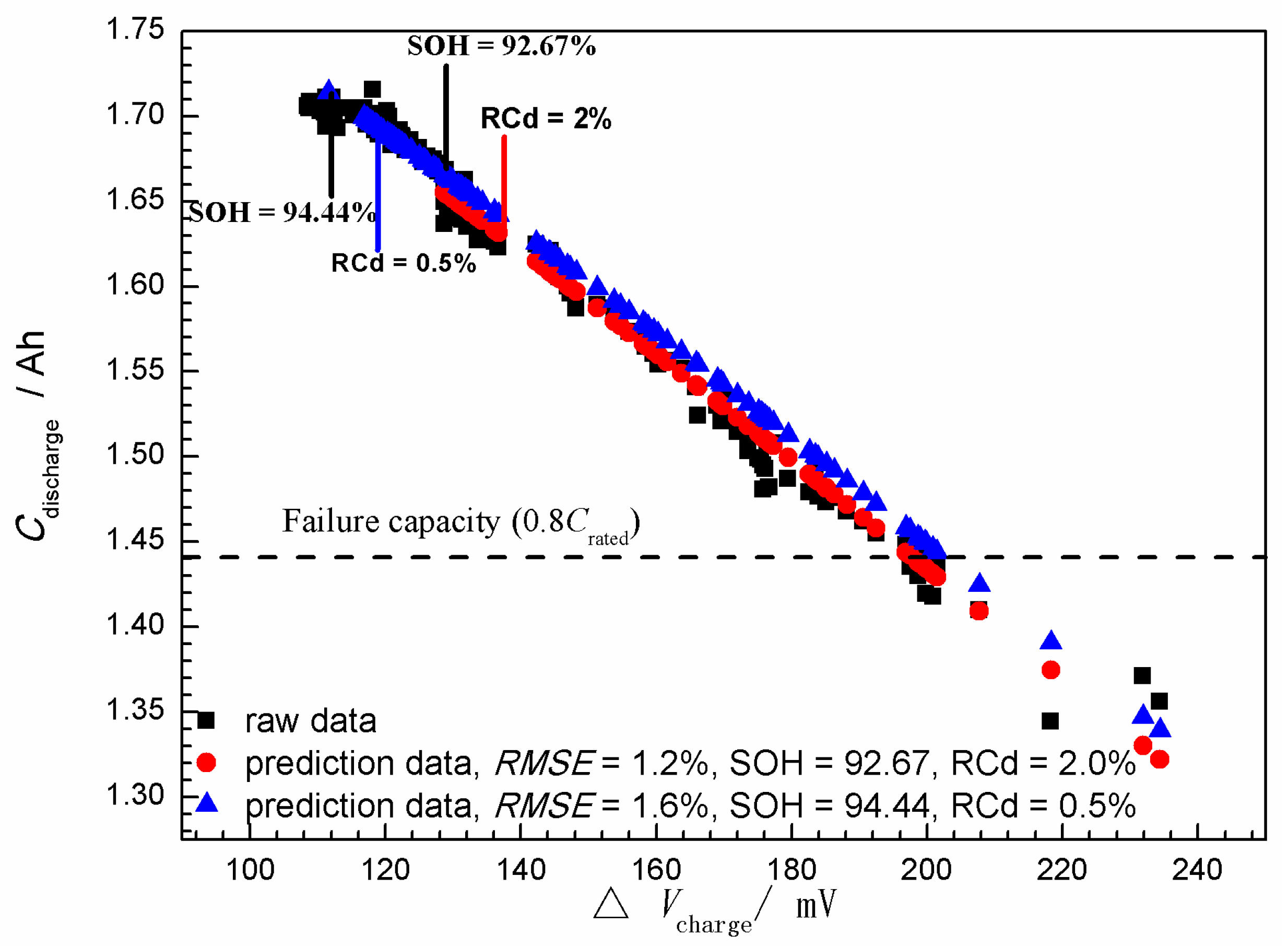
3.2. State-of-Health (SOH) Prediction of Ni-MH Batteries Based on the Curve Model for Cdischarge




4. Conclusions
- (1)
- Based on the analysis of charge-discharge data for the Ni-MH batteries, a nonlinear relationship between the Cdischarge (Ah) and ΔVcharge (mV) was found and described as Cdischarge = a/[1+ exp[−k × (ΔVcharge − c)]], where a (Ah), k (mV−1) and c (mV) are constants related to charge-discharge rates. Based on this equation, the curve model for the SOH prediction of Ni-MH batteries was constructed.
- (2)
- The (Cdischarge, ΔVcharge) data groups obtained from the charge-discharge cycle test at different rates (0.5C, 1.0C, and 1.6C) were employed to verify the validity of the curve model for the SOH prediction. It was found that the curve model based on the nonlinear relationship between the Cdischarge and ΔVcharge can be effectively applied to predict the SOH of the Ni-MH batteries. The data groups used for the SOH prediction have significantly influence on the prediction accuracy, and the best prediction RMSE can reach 1.2%.
- (3)
- Generally, using (Cdischarge, ΔVcharge) data groups with the SOH in an intermediate range and with the RCd value in the range of 1.5%–2.0% may result in lower prediction RMSE.
Acknowledgments
Conflicts of Interest
References
- Leblanc, P.; Blanchard, P.; Senyarich, S. Self-Discharge of Sealed Nickel-Metal Hydride Batteries Mechanisms and Improvements. J. Electrochem. Soc. 1998, 145, 844–847. [Google Scholar] [CrossRef]
- Yao, M.; Okuno, K.; Iwaki, T.; Tanase, S.; Harada, K.; Kato, M.; Emura, K.; Sakai, T. High-power nickel/metal-hydride battery using new micronetwork substrate: Discharge rate capability and cycle-life performance. J. Power Sources 2007, 171, 1033–1039. [Google Scholar] [CrossRef]
- Fetcenko, M.; Ovshinsky, S.; Reichman, B.; Young, K.; Fierro, C.; Koch, J.; Zallen, A.; Mays, W.; Ouchi, T. Recent advances in Ni-MH battery technology. J. Power Sources 2007, 165, 544–551. [Google Scholar] [CrossRef]
- Castro, E.; Cuscueta, D.; Milocco, R.; Ghilarducci, A.; Salva, H. An EIS based study of a Ni–MH battery prototype. Modeling and identification analysis. Int. J. Hydrog. Energy 2010, 35, 5991–5998. [Google Scholar] [CrossRef]
- Serrao, L.; Chehab, Z.; Guezennee, Y.; Rizzoni, G. An aging model of Ni-MH batteries for hybrid electric vehicles. In Proceedings of the 2005 IEEE Conference Vehicle Power and Propulsion, Chicago, IL, USA, 7–9 September 2005; pp. 78–85.
- Chehab, Z.; Serrao, L.; Guezennec, Y.G.; Rizzoni, G. Aging Characterization of Nickel: Metal Hydride Batteries Using Electrochemical Impedance Spectroscopy. In Proceedings of the International Mechanical Engineering Congress and Exposition, Chicago, IL, USA, 5–10 November 2006; pp. 343–349.
- Salkind, A.J.; Fennie, C.; Singh, P.; Atwater, T.; Reisner, D.E. Determination of state-of-charge and state-of-health of batteries by fuzzy logic methodology. J. Power Sources 1999, 80, 293–300. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Sean, W.Y.; Ke, J.C. Online estimation of internal resistance and open-circuit voltage of lithium-ion batteries in electric vehicles. J. Power Sources 2011, 196, 3921–3932. [Google Scholar] [CrossRef]
- Ng, K.S.; Moo, C.S.; Chen, Y.P.; Hsieh, Y.C. Enhanced coulomb counting method for estimating state-of-charge and state-of-health of lithium-ion batteries. Appl. Energy 2009, 86, 1506–1511. [Google Scholar] [CrossRef]
- Waag, W.; Fleischer, C.; Sauer, D.U. Critical review of the methods for monitoring of lithium-ion batteries in electric and hybrid vehicles. J. Power Sources 2014, 258, 321–339. [Google Scholar] [CrossRef]
- Huet, F. A review of impedance measurements for determination of the state-of-charge or state-of-health of secondary batteries. J. Power Sources 1998, 70, 59–69. [Google Scholar] [CrossRef]
- Blanke, H.; Bohlen, O.; Buller, S.; de Doncker, R.W.; Fricke, B.; Hammouche, A.; Linzen, D.; Thele, M.; Sauer, D.U. Impedance measurements on lead-acid batteries for state-of-charge, state-of-health and cranking capability prognosis in electric and hybrid electric vehicles. J. Power Sources 2005, 144, 418–425. [Google Scholar] [CrossRef]
- Liu, G.; Ouyang, M.; Lu, L.; Li, J.; Han, X. Online estimation of lithium-ion battery remaining discharge capacity through differential voltage analysis. J. Power Sources 2015, 274, 971–989. [Google Scholar] [CrossRef]
- Sauer, D.U.; Wenzl, H. Comparison of different approaches for lifetime prediction of electrochemical systems—Using lead-acid batteries as example. J. Power Sources 2008, 176, 534–546. [Google Scholar] [CrossRef]
- Zhu, J.; Sun, Z.; Wei, X.; Dai, H. A new lithium-ion battery internal temperature on-line estimate method based on electrochemical impedance spectroscopy measurement. J. Power Sources 2015, 274, 990–1004. [Google Scholar] [CrossRef]
- Wenzl, H.; Baring-Gould, I.; Kaiser, R.; Liaw, B.Y.; Lundsager, P.; Manwell, J.; Ruddell, A.; Svoboda, V. Life prediction of batteries for selecting the technically most suitable and cost effective battery. J. Power Sources 2005, 144, 373–384. [Google Scholar] [CrossRef]
- Ramadass, P.; Haran, B.; White, R.; Popov, B.N. Capacity fade of Sony 18650 cells cycled at elevated temperatures: Part II. Capacity fade analysis. J. Power Sources 2002, 112, 614–620. [Google Scholar] [CrossRef]
- Bundy, K.; Karlsson, M.; Lindbergh, G.; Lundqvist, A. An electrochemical impedance spectroscopy method for prediction of the state of charge of a nickel-metal hydride battery at open circuit and during discharge. J. Power Sources 1998, 72, 118–125. [Google Scholar] [CrossRef]
- Meissner, E.; Richter, G. Battery monitoring and electrical energy management: Precondition for future vehicle electric power systems. J. Power Sources 2003, 116, 79–98. [Google Scholar] [CrossRef]
- Santhanagopalan, S.; Guo, Q.; Ramadass, P.; White, R.E. Review of models for predicting the cycling performance of lithium ion batteries. J. Power Sources 2006, 156, 620–628. [Google Scholar] [CrossRef]
- Gao, L.; Liu, S.; Dougal, R.A. Dynamic lithium-ion battery model for system simulation. IEEE Trans. Compon. Packag. Technol. 2002, 25, 495–505. [Google Scholar] [CrossRef]
- Rao, R.; Vrudhula, S.; Rakhmatov, D.N. Battery modeling for energy aware system design. Computer 2003, 36, 77–87. [Google Scholar] [CrossRef]
- González-Longatt, F.M. Circuit based battery models: A review. In Proceedings of the 2nd Congreso IberoAmericano De Estudiantes deIngeniería Eléctrica, Puerto la Cruz, Venezuela, 3–7 April 2006; pp. 1–5.
- Chen, M.; Rincon-Mora, G.A. Accurate electrical battery model capable of predicting runtime and IV performance. IEEE Trans. Energy Convers. 2006, 21, 504–511. [Google Scholar] [CrossRef]
- Zhu, W.H.; Zhu, Y.; Tatarchuk, B.J. A simplified equivalent circuit model for simulation of Pb-acid batteries at load for energy storage application. Energy Convers. Manag. 2011, 52, 2794–2799. [Google Scholar] [CrossRef]
- Pascoe, P.E.; Anbuky, A.H. A unified discharge voltage characteristic for VRLA battery capacity and reserve time estimation. Energy Convers. Manag. 2004, 45, 277–302. [Google Scholar] [CrossRef]
- Pascoe, P.E.; Anbuky, A.H. A VRLA battery simulation model. Energy Convers. Manag. 2004, 45, 1015–1041. [Google Scholar] [CrossRef]
- Chiang, Y.H.; Sean, W.Y. Dynamical estimation of state-of-health of batteries by using adaptive observer. In Proceedings of the 2nd International Conference on Power Electronics and Intelligent Transportation System, 19–20 December 2009; pp. 110–115.
- Hu, C.; Youn, B.D.; Chung, J. A multiscale framework with extended Kalman filter for lithium-ion battery SOC and capacity estimation. Appl. Energy 2012, 92, 694–704. [Google Scholar] [CrossRef]
- Sepasi, S.; Ghorbani, R.; Liaw, B.Y. A novel on-board state-of-charge estimation method for aged Li-ion batteries based on model adaptive extended Kalman filter. J. Power Sources 2014, 245, 337–344. [Google Scholar] [CrossRef]
- Saha, B.; Goebel, K.; Poll, S.; Christophersen, J. Prognostics methods for battery health monitoring using a Bayesian framework. IEEE Trans. Instrum. Meas. 2009, 58, 291–296. [Google Scholar] [CrossRef]
- Chang, W.Y. Estimation of the state of charge for a LFP battery using a hybrid method that combines a RBF neural network, an OLS algorithm and AGA. Int. J. Electr. Power Energy Syst. 2013, 53, 603–611. [Google Scholar] [CrossRef]
- Gorman, C.; Ingersoll, D.; Jungst, R.; Paez, T.L. Artificial neural network simulation of battery performance. In Proceedings of the Thirty-First Hawaii International Conference, Kohala Coast, HI, USA, 6–9 January 1998; pp. 115–121.
- Andre, D.; Nuhic, A.; Soczka-Guth, T.; Sauer, D. Comparative study of a structured neural network and an extended Kalman filter for state of health determination of lithium-ion batteries in hybrid electricvehicles. Eng. Appl. Artif. Intell. 2013, 26, 951–961. [Google Scholar] [CrossRef]
- Rufus, F.; Lee, S.; Thakker, A. Health monitoring algorithms for space application batteries. In Proceedings of the Prognostics and Health Management, Denver, CO, USA, 6–9 October 2008; pp. 1–8.
- Widodo, A.; Shim, M.C.; Caesarendra, W.; Yang, B.S. Intelligent prognostics for battery health monitoring based on sample entropy. Expert Syst. Appl. 2011, 38, 11763–11769. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, E.W.; Tsui, K.L.; Pecht, M. Battery management systems in electric and hybrid vehicles. Energies 2011, 4, 1840–1857. [Google Scholar] [CrossRef]
- Singh, P.; Fennie, C.; Reisner, D. Fuzzy logic modelling of state-of-charge and available capacity of nickel/metal hydride batteries. J. Power Sources 2004, 136, 322–333. [Google Scholar] [CrossRef]
- Singh, P.; Kaneria, S.; Broadhead, J.; Wang, X.; Burdick, J. Fuzzy logic estimation of SOH of 125Ah VRLA batteries. In Proceedings of the International Telecommunications Energy Conference, Chicago, IL, USA, 19–23 September 2004; pp. 524–531.
- Micea, M.V.; Ungurean, L.; Carstoiu, G.N.; Groza, V. Online state-of-health assessment for battery management systems. IEEE Instrum. Meas. 2011, 60, 1997–2006. [Google Scholar] [CrossRef]
- Wang, F.; Fan, B.; Liu, S.; Qian, G.; Huang, X.; Han, S. Attenuation test and duplication of power battery’s cycle life. J. Automot. Saf. Energy 2012, 3, 71–76. [Google Scholar]
- Kang, L.; Zhao, X.; Ma, J. A new neural network model for the state-of-charge estimation in the battery degradation process. Appl. Energy 2014, 121, 20–27. [Google Scholar] [CrossRef]
- Xing, Y.; Ma, E.W.; Tsui, K.-L.; Pecht, M. An ensemble model for predicting the remaining useful performance of lithium-ion batteries. Microelectron. Reliab. 2013, 53, 811–820. [Google Scholar] [CrossRef]
- He, W.; Williard, N.; Osterman, M.; Pecht, M. Prognostics of lithium-ion batteries based on Dempster-Shafer theory and the Bayesian Monte Carlo method. J. Power Sources 2011, 196, 10314–10321. [Google Scholar] [CrossRef]
- Eom, S.W.; Kim, M.K.; Kim, I.J.; Moon, S.I.; Sun, Y.K.; Kim, H.S. Life prediction and reliability assessment of lithium secondary batteries. J. Power Sources 2007, 174, 954–958. [Google Scholar] [CrossRef]
- Remmlinger, J.; Buchholz, M.; Meiler, M.; Bernreuter, P.; Dietmayer, K. State-of-health monitoring of lithium-ion batteries in electric vehicles by on-board internal resistance estimation. J. Power Sources 2011, 196, 5357–5363. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Qiu, Y.; Guo, X. Prediction of State-of-Health for Nickel-Metal Hydride Batteries by a Curve Model Based on Charge-Discharge Tests. Energies 2015, 8, 12474-12487. https://doi.org/10.3390/en81112322
Yang H, Qiu Y, Guo X. Prediction of State-of-Health for Nickel-Metal Hydride Batteries by a Curve Model Based on Charge-Discharge Tests. Energies. 2015; 8(11):12474-12487. https://doi.org/10.3390/en81112322
Chicago/Turabian StyleYang, Huan, Yubing Qiu, and Xingpeng Guo. 2015. "Prediction of State-of-Health for Nickel-Metal Hydride Batteries by a Curve Model Based on Charge-Discharge Tests" Energies 8, no. 11: 12474-12487. https://doi.org/10.3390/en81112322