Low Temperature Performance of Selective Catalytic Reduction of NO with NH3 under a Concentrated CO2 Atmosphere
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
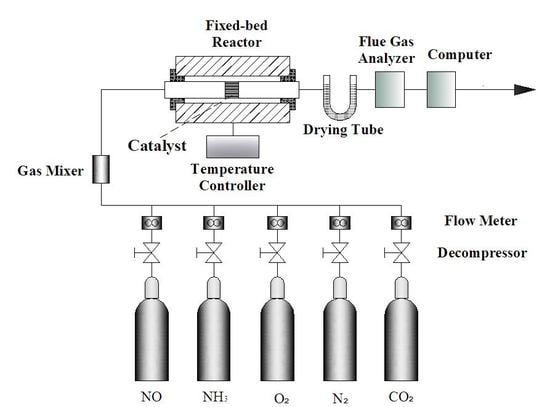
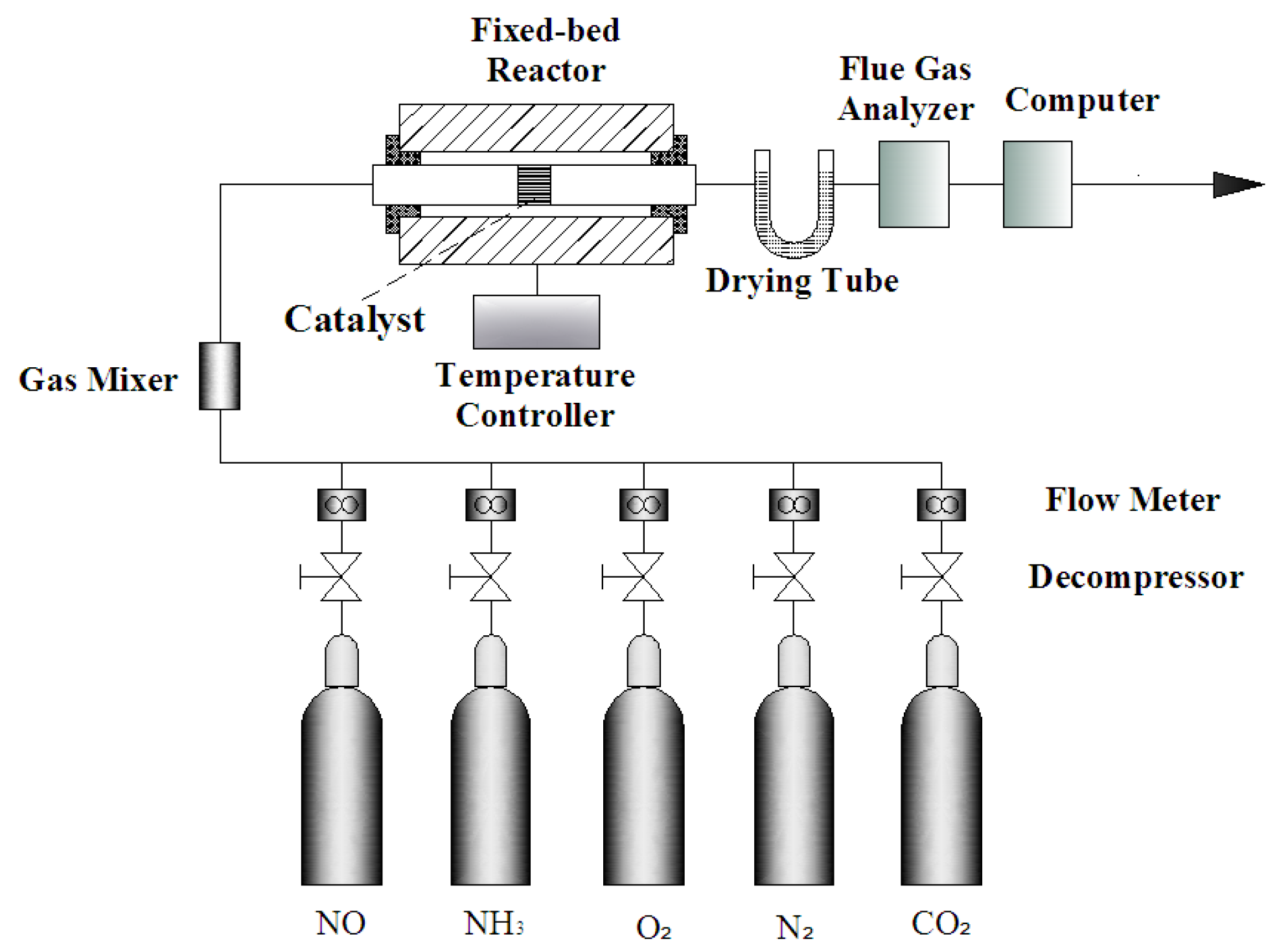
2.2. Experimental System
2.3. Catalyst Characterization
3. Results and Discussion
3.1. Comparison of CO2 and N2 Atmosphere with Different Catalysts





3.2. Selective Catalytic Reduction of NOx with NH3 (NH3-SCR) Test under CO2 and N2 Atmosphere with Different Process Conditions



4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, Z.; Woo, S. Recent Advances in Catalytic DeNOx Science and Technology. Catal. Rev. 2006, 48, 43–89. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Zhang, L.; Cao, Y.; Zou, W.; Li, L.; Ma, K.; Tang, C.; Gao, F.; Dong, L. Improved low temperature NH3-SCR performance of FeMnTiOx mixed oxide with CTAB-assisted synthesis. Chem. Commun. 2015, 51, 3470–3473. [Google Scholar] [CrossRef] [PubMed]
- Busca, G.; Lietti, L.; Ramis, G.; Berti, F. Chemical and mechanistic aspects of the selective catalytic reduction of NOx by ammonia over oxide catalysts: A review. Appl. Catal. B Environ. 1998, 18, 1–36. [Google Scholar] [CrossRef]
- Amiridis, M.D.; Duevel, R.V.; Wachs, I.E. The effect of metal oxide additives on the activity of V2O5/TiO2 catalysts for the selective catalytic reduction of nitric oxide by ammonia. Appl. Catal. B Environ. 1999, 20, 111–122. [Google Scholar] [CrossRef]
- Liu, F.; Asakura, K.; He, H.; Shan, W.; Shi, X.; Zhang, C. Influence of sulfation on iron titanate catalyst for the selective catalytic reduction of NOx with NH3. Appl. Catal. B Environ. 2011, 103, 369–377. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Deng, B.Y.; Zhang, Z.Q.; Wu, Z.L.; Tang, X.J.; Yao, S.L.; Lu, H. Effect of Zr Addition on the Low-Temperature SCR Activity and SO2 Tolerance of Fe-Mn/Ti Catalysts. J. Phys. Chem. C 2014, 118, 14866–14875. [Google Scholar] [CrossRef]
- Shen, Y.S.; Su, Y.; Ma, Y.F. Transition metal ions regulate the catalytic performance of Ti0.8M0.2Ce0.2O2+x for the NH3-SCR of NO: The acidic mechanism. RSC Adv. 2015, 5, 7597–7603. [Google Scholar] [CrossRef]
- Wan, Y.P.; Zhao, W.R.; Tang, Y.; Li, L.; Wang, H.J.; Cui, Y.L.; Gu, J.L.; Li, Y.S.; Shi, J.L. Ni-Mn bi-metal oxide catalysts for the low temperature SCR removal of NO with NH3. Appl. Catal. B Environ. 2014, 118–149, 114–122. [Google Scholar] [CrossRef]
- Zeng, Z.; Lu, P.; Li, C.T.; Zeng, G.M.; Jiang, X.; Zhai, Y.B.; Fan, X.P. Selective catalytic reduction (SCR) of NO by urea loaded on activated carbon fibre (ACF) and CeO2/ACF at 30 °C: The SCR mechanism. Environ. Technol. 2012, 33, 1331–1337. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.H.; Qiu, M.Y.; Yang, S.S.; Zhu, D.D.; Yu, H.B.; Li, Y. Facile preparation of MnO2 doped Fe2O3 hollow nanofibers for low temperature SCR of NO with NH3. J. Mater. Chem. A 2014, 2, 20486–20493. [Google Scholar] [CrossRef]
- Chang, H.; Li, J.; Yuan, J.; Chen, L.; Dai, Y.; Arandiyan, H.; Xu, J.; Hao, J. Ge, Mn-doped CeO2-WO3 catalysts for NH3-SCR of NOx: Effects of SO2 and H2 regeneration. Catal. Today 2013, 201, 139–144. [Google Scholar] [CrossRef]
- Grzybek, T.; Klinik, J.; Motak, M.; Papp, H. Nitrogen-promoted active carbons as catalytic supports: 2. The influence of Mn promotion on the structure and catalytic properties in SCR. Catal. Today 2008, 137, 235–241. [Google Scholar] [CrossRef]
- Tian, X.; Xiao, Y.; Zhou, P.; Zhang, W.; Luo, X. Investigation on performance of V2O5-WO3-TiO2-cordierite catalyst modified with Cu, Mn and Ce for urea-SCR of NO. Mater. Res. Innov. 2014, 18, 202–206. [Google Scholar] [CrossRef]
- Peng, Y.; Li, J.; Si, W.; Li, X.; Shi, W.; Luo, J.; Fu, J.; Crittenden, J.; Hao, J. Ceria promotion on the potassium resistance of MnOx/TiO2 SCR catalysts: An experimental and DFT study. Chem. Eng. J. 2015, 269, 44–50. [Google Scholar] [CrossRef]
- Moosavi, E.S.; Seyed, A.; Dastgheib, S.A.; Karimzadeh, R. Adsorption of Thiophenic Compounds from Model Diesel Fuel Using Copper and Nickel Impregnated Activated Carbons. Energies 2012, 5, 4233–4250. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, P.; Li, C.; Zeng, Z.; Zeng, G.; Hu, L.; Mai, L.; Li, Z. Experimental study on a room temperature urea-SCR of NO over activated carbon fibre-supported CeO2-CuO. Environ. Technol. 2013, 34, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Guo, Q.; Hou, Y.; Ma, G.; Han, X.; Huang, Z. Catalytic role of vanadium(V) sulfate on activated carbon for SO2 oxidation and NH3-SCR of NO at low temperatures. Catal. Commun. 2014, 56, 23–26. [Google Scholar] [CrossRef]
- Guo, Q.; Jing, W.; Hou, Y.; Huang, Z.; Ma, G.; Han, X.; Sun, D. On the nature of oxygen groups for NH3-SCR of NO over carbon at low temperatures. Chem. Eng. J. 2015, 270, 41–49. [Google Scholar] [CrossRef]
- Garcia-Cuello, V.S.; Giraldo, L.; Moreno-Pirajan, J.C. Textural Characterization and Energetics of Porous Solids by Adsorption Calorimetry Textural Characterization and Energetics of Porous Solids by Adsorption Calorimetry. Energies 2011, 4, 928–947. [Google Scholar] [CrossRef]
- Habib, M.A.; Badr, H.M.; Ahmed, S.F.; Ben-Mansour, R.; Mezghani, K.; Imashuku, S.; la O’, G.J.; Shao-Horn, Y.; Mancini, N.D.; Mitsos, A.; et al. A review of recent developments in carbon capture utilizing oxy-fuel combustion in conventional and ion transport membrane systems. Int. J. Energy Res. 2011, 35, 741–764. [Google Scholar] [CrossRef]
- Scheffknecht, G.; Al-Makhadmeh, L.; Schnell, U.; Maier, J. Oxy-fuel coal combustion—A review of the current state-of-the-art. Int. J. Greenh. Gas Control 2011, 5, S16–S35. [Google Scholar] [CrossRef]
- Zheng, B.B.; Xu, J.P. Carbon Capture and Storage Development Trends from a Techno-Paradigm Perspective. Energies 2014, 7, 5221–5250. [Google Scholar] [CrossRef]
- Hudson, M.R.; Queen, W.L.; Mason, J.A.; Fickel, D.W.; Lobo, R.F.; Brown, C.M. Unconventional, Highly Selective CO2 Adsorption in Zeolite SSZ-13. J. Am. Chem. Soc. 2012, 134, 1970–1973. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Min, K.M.; Lee, J.K.; Hong, S.B.; Cho, B.K.; Nam, I.-S. Effect of CO2 on the DeNOx Activity of a Small Pore Zeolite Copper Catalyst for NH3/SCR. ChemCatchem 2014, 6, 1186–1189. [Google Scholar]
- Magnusson, M.; Fridell, E.; Ingelsten, H.H. The influence of sulfur dioxide and water on the performance of a marine SCR catalyst. Appl. Catal. B Environ. 2012, 111, 20–26. [Google Scholar] [CrossRef]
- Forzatti, P.; Nova, I.; Tronconi, E.; Kustov, A.; Thogersen, J.R. Effect of operating variables on the enhanced SCR reaction over a commercial V2O5-WO3/TiO2 catalyst for stationary applications. Catal. Today 2012, 184, 153–159. [Google Scholar] [CrossRef]
- Huang, H.L.; Shan, W.P.; Yang, S.J.; Zhang, J.H. Novel approach for a cerium-based highly-efficient catalyst with excellent NH3-SCR performance. Catal. Sci. Technol. 2014, 4, 3611–3614. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, P.S.; Cho, B.K.; Nam, I.S.; Oh, S.H. Enhanced NOx reduction and byproduct removal by (HC plus OHC)/SCR over multifunctional dual-bed monolith catalyst. Catal. Today 2012, 184, 95–106. [Google Scholar] [CrossRef]
- Lei, Z.; Han, B.; Yang, K.; Chen, B. Influence of H2O on the low-temperature NH3-SCR of NO over V2O5/AC catalyst: An experimental and modeling study. Chem. Eng. J. 2013, 215, 651–657. [Google Scholar] [CrossRef]
- Pan, S.; Luo, H.; Li, L.; Wei, Z.; Huang, B. H2O and SO2 deactivation mechanism of MnOx/MWCNTs for low-temperature SCR of NOx with NH3. J. Mol. Catal. A Chem. 2013, 377, 154–161. [Google Scholar] [CrossRef]
- Smith, M.A.; Depcik, C.D.; Hoard, J.W.; Bohac, S.V.; Assanis, D.N. Modeling of SCR NH3 Storage in the Presence of H2O. In Proceedings of the ASME 2011 Internal Combustion Engine Division Fall Technical Conference, Morgantown, WV, USA, 2–5 October 2011.
- Chang, H.; Chen, X.; Li, J.; Ma, L.; Wang, C.; Liu, C.; Schwank, J.W.; Hao, J. Improvement of Activity and SO2 Tolerance of Sn-Modified MnOx-CeO2 Catalysts for NH3-SCR at Low Temperatures. Environ. Sci. Technol. 2013, 47, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, L.; Wang, J.; Wang, R. Ag/bauxite catalysts: Improved low-temperature activity and SO2 tolerance for H2-promoted NH3-SCR of NOx. Appl. Catal. B Environ. 2015, 165, 700–705. [Google Scholar] [CrossRef]
- Cha, J.S.; Choi, J.C.; Ko, J.H.; Park, Y.K.; Park, S.H.; Jeong, K.E.; Kim, S.S.; Jeon, J.K. The low-temperature SCR of NO over rice straw and sewage sludge derived char. Chem. Eng. J. 2010, 156, 321–327. [Google Scholar] [CrossRef]
- Singh, S.; Nahil, M.A.; Sun, X.; Wu, C.; Chen, J.; Shen, B.; Williams, P.T. Novel application of cotton stalk as a waste derived catalyst in the low temperature SCR-deNOx process. Fuel 2013, 105, 585–594. [Google Scholar] [CrossRef]
- Smirniotis, P.G.; Sreekanth, P.M.; Peña, D.A.; Jenkins, R.G. Manganese Oxide Catalysts Supported on TiO2, Al2O3, and SiO2: A Comparison for Low-Temperature SCR of NO with NH3. Ind. Eng. Chem. Res. 2006, 45, 6436–6443. [Google Scholar] [CrossRef]
- Shen, B.X.; Ma, H.Q.; He, C.; Zhang, X.P. Low temperature NH3–SCR over Zr and Ce pillared clay based catalysts. Fuel Process. Technol. 2014, 119, 121–129. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Wu, Z. Low-temperature selective catalytic reduction of NO on MnOx/TiO2 prepared by different methods. J. Hazard. Mater. 2009, 162, 1249–1254. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Fang, D.; He, F.; Chen, J.; Fu, Z.; Chen, X. Performance and mechanism about MnOx species included in MnOx/TiO2 catalysts for SCR at low temperature. Catal. Commun. 2012, 28, 77–81. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Performance and kinetics study for low-temperature SCR of NO with NH3 over MnOx-CeO2 catalyst. J. Catal. 2003, 217, 434–441. [Google Scholar] [CrossRef]
- Kijlstra, W.S.; Brands, D.S.; Poels, E.K.; Bliek, A. Mechanism of the selective catalytic reduction of NO by NH3 over MnOx/Al2O3: I. Adsorption and desorption of the single reaction components. J. Catal. 1997, 171, 208–218. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, R.T. Removal of NO by reversible adsorption on Fe-Mn based transition metal oxides. Langmuir 2001, 17, 4997–5003. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, B.; Zhuo, Y.; Chen, C.; Xu, X. Effects of water vapor, CO2 and SO2 on the NO reduction by NH3 over sulfated CaO. Korean J. Chem. Eng. 2011, 28, 1785–1790. [Google Scholar] [CrossRef]
- Garcia-Bordeje, E.; Pinilla, J.L.; Lazaro, M.J.; Moliner, R. NH3-SCR of NO at low temperatures over sulphated vanadia on carbon-coated monoliths: Effect of H2O and SO2 traces in the gas feed. Appl. Catal. B Environ. 2006, 66, 281–287. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gou, X.; Wu, C.; Zhang, K.; Xu, G.; Si, M.; Wang, Y.; Wang, E.; Liu, L.; Wu, J. Low Temperature Performance of Selective Catalytic Reduction of NO with NH3 under a Concentrated CO2 Atmosphere. Energies 2015, 8, 12331-12341. https://doi.org/10.3390/en81112319
Gou X, Wu C, Zhang K, Xu G, Si M, Wang Y, Wang E, Liu L, Wu J. Low Temperature Performance of Selective Catalytic Reduction of NO with NH3 under a Concentrated CO2 Atmosphere. Energies. 2015; 8(11):12331-12341. https://doi.org/10.3390/en81112319
Chicago/Turabian StyleGou, Xiang, Chunfei Wu, Kai Zhang, Guoyou Xu, Meng Si, Yating Wang, Enyu Wang, Liansheng Liu, and Jinxiang Wu. 2015. "Low Temperature Performance of Selective Catalytic Reduction of NO with NH3 under a Concentrated CO2 Atmosphere" Energies 8, no. 11: 12331-12341. https://doi.org/10.3390/en81112319