1. Introduction
Thermoelectric materials are of technological interest owing to their ability of direct heat-to-electricity energy conversion. Currently, Silicon-Germanium (
SiGe) alloys are the only thermoelectric materials that have found applications in power generation in the temperature range of 900 K <
T < 1300 K [
1,
2]. Many efforts have been exerted to enhance the dimensionless thermoelectric figure of merit
of
SiGe compounds [
3,
4], where
is the Seebeck coefficient,
the electrical conductivity,
the thermal conductivity,
T the absolute temperature, and
the power factor. The
p-type (
Boron, B-doped) and
n-type (
Phosphorus,
P-doped)
SiGe material used in NASA’s radioisotope thermoelectric generators (RTGs) possess a
ZT of 0.5 and 0.9, respectively [
2]. In the past few decades, there have been many theoretical [
5,
6,
7] and experimental efforts [
8,
9,
10,
11] toward further enhancing the
ZT of
SiGe via an enhancement of the
PF and/or a reduction in
. Some results are noteworthy. For example, particle size distribution was found to be crucial to achieve dense homogeneous samples [
12]; polycrystalline
SiGe possesses a better thermoelectric performance than single crystalline
SiGe [
13]; mechanical ball milling is effective in producing single phased
SiGe powder [
14,
15]. A significant improvement in
ZT with peak values about 1.3 and 0.95 at 1200 K in
n-type and
p-type respectively were achieved via reducing the lattice thermal conductivity
using a high energy ball milling method to produce nanostructured SiGe [
11,
16]. Recently we have employed the spark plasma sintering (SPS) technique to synthesize high relative density
SiGe directly from single elemental (SE)
Si and
Ge powders, which possess thermoelectric performance comparable to those used in NASA’s RTGs [
17].
Doping plays a central role in optimizing the thermoelectric performance of
SiGe as dopants optimize the carrier concentration while simultaneously acting as point defects which can strongly scatter the heat-carrying phonons at elevated temperatures. Previously the effects of different dopants, including
P,
Ga,
B,
GaP,
In,
Sb, and
InSb, on the thermoelectric properties of
Si80Ge20 alloys have been investigated [
18,
19]. Doping typically governs the
PF, which is at the core and often the bottle-neck of enhancing
ZT in many thermoelectric materials. It is generally accepted that a strongly energy-dependent differential electrical conductivity leads to enhancement of the
PF [
20]. Such strong energy dependence arises from the density of states and/or from the relaxation time of charge carriers [
21,
22]. In addition to doping, the “compositing or nanostructuring” process is another way to enhance the
PF. Bergman and Fel showed that the
PF could be enhanced by making a two-phase composite in a parallel slab micromorphology or a core-shell micromorphology [
23]. Doping and nanostructuring combined in this modulation-doping approach to enhance the
PF. For example, the
PF of
p-type
Si80Ge20B1.5 was improved by a factor of 40% by embedding 30 vol. %
B-doped
SiGe nanoparticles in the intrinsic
SiGe host matrix to form
(Si80Ge20)0.7(Si100B5)0.3 composites [
24]. Furthermore, grain boundary engineering is another effective approach to enhancing the
PF in several cases. For example, the alkali metal salt
NaBH4 processing appears to lead to thermoelectrically-favorable grain boundaries in
p-type
Bi2Te3 [
25] and
Pbo.75Sn0.25Te [
26].
In this work, we have simultaneously achieved Na-doping, B-doping, formation of Na2B29 nanoparticles, and sample densification via the decomposition of NaBH4 in a single-step synergistic “doping-nanostructuring-sintering” process. As a result, the PF is enhanced while the total thermal conductivity, , is reduced, thereby leading to a significantly enhanced ZT.
2. Experimental Results and Data Analyses
In this work, doping is achieved via decomposition of NaBH4 in the SPS process. Three samples were treated with sodium boron hydride (NaBH4)x (x = 0.7, 1.0, and 2.7), while two samples were treated with single element boron (B) in addition to sodium boron hydride B1.7-y(NaBH4)y (y = 0.2 and 0.7). The purpose of preparing the Si80Ge20B1,5(NaBH4)0.2 and Si80Ge20B1(NaBH4)0.7 sample with increasing percentage of NaBH4 and decreasing B, is to investigate the extent of the NaBH4 decomposition with the same total amount of B.
In general, the thermal decomposition of NaBH
4 follows:
where the products can be single elemental
Na,
B,
H2, or binary phases, e.g.,
Na–B such as
Na2B29,
Na2B30,
Na3B20 or
Na–H, or ternary phases,
Na–B–H [
27].
The thermal decomposition of
NaBH4 occurs between 600 °C and 700 °C [
27], significantly below our SPS temperature of 1020 °C. Indeed, we observed the presence of
B-rich
Na–B compounds namely
Na2B29 by SEM, EDX, and XRD measurements. Importantly, degasing was observed in spark plasma sintering
NaBH4-added
SiGe samples, but not in
NaBH4-free samples, confirming the formation of
H2. An immediate question arises as to where exactly the
Na resides after this decomposition.
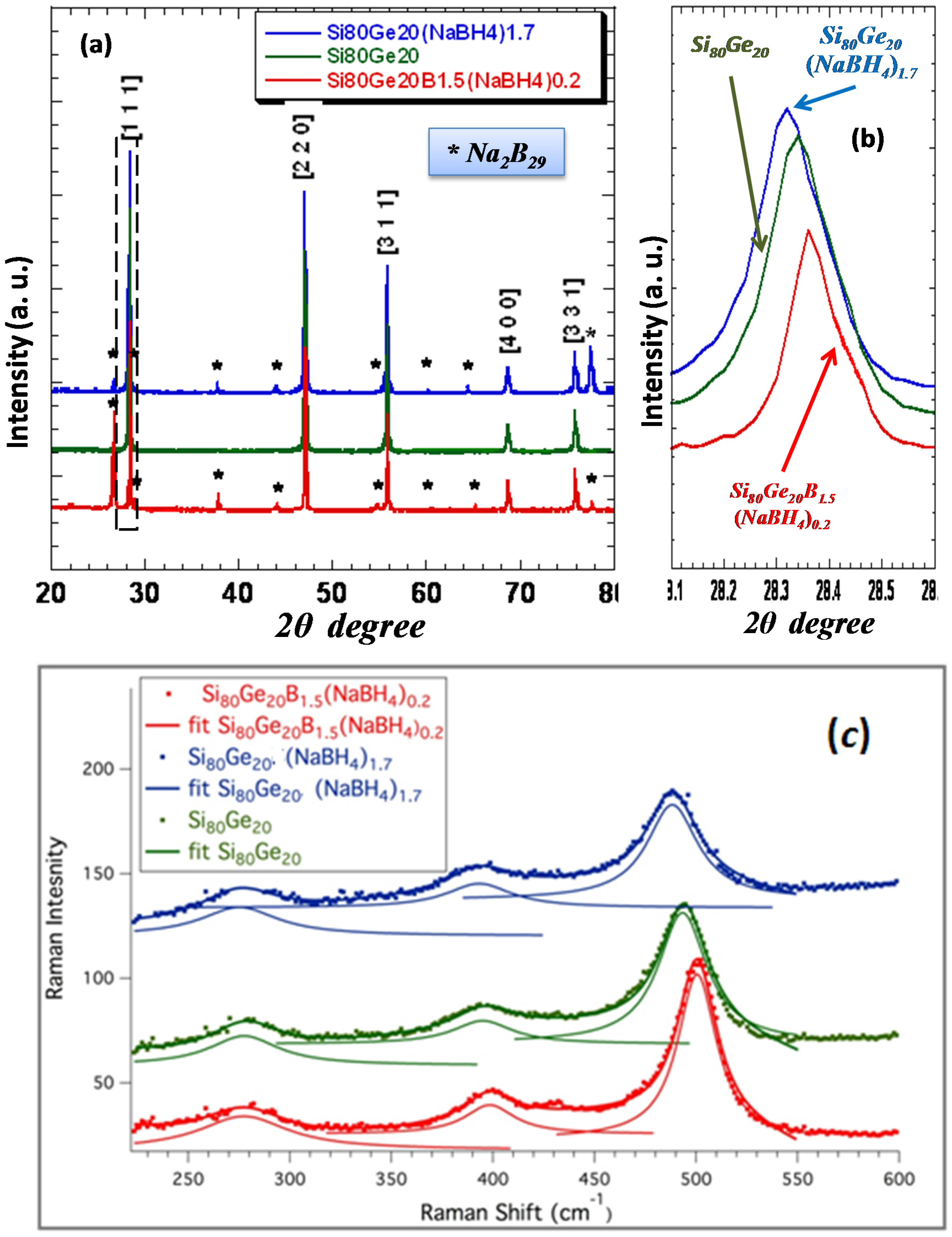
Figure 1a shows the XRD pattern of
NaBH4 –added
Si80Ge20 alloys after the SPS process. The lattice constant was determined using the (111) and (220) peaks by Bragg’s law. The peaks from
Na2B29 were observed and marked with asterisk in
Figure 1a. The ICSD PDF file number of
Na2B29 is 01-071-2824, and the space group is I 1 m 1 [
27]. No other secondary phases were observed.
Interestingly, the XRD peaks of the sample treated with
B and
NaBH4 {
Si80Ge20B1.5(NaBH4)0.2} shifted to higher angle, contrary to those samples treated with only
NaBH4 {
Si80Ge20(NaBH4)1.7}, indicating that the dominant dopant is
B. The peak of the sample treated with only
NaBH4 {
Si80Ge20(NaBH4)1.7}, on the other hand, is shifted to lower angle, consistent with a dominant
Na doping (
Figure 1b). This opposite shifting is attributed to the ionic radii of the
Na (99 pm) and
B (35 pm) comparing to Si or Ge (39 pm) [
28]. Shifts of Raman peaks track with those of XRD peaks, confirming the doping by
Na and
B in the
SiGe host matrix (
Figure 1c). The decomposition of
NaBH4 in the SPS process opens a new way to dope
Na into the
SiGe host matrix, which is actually a (
Si80Ge20) composition.
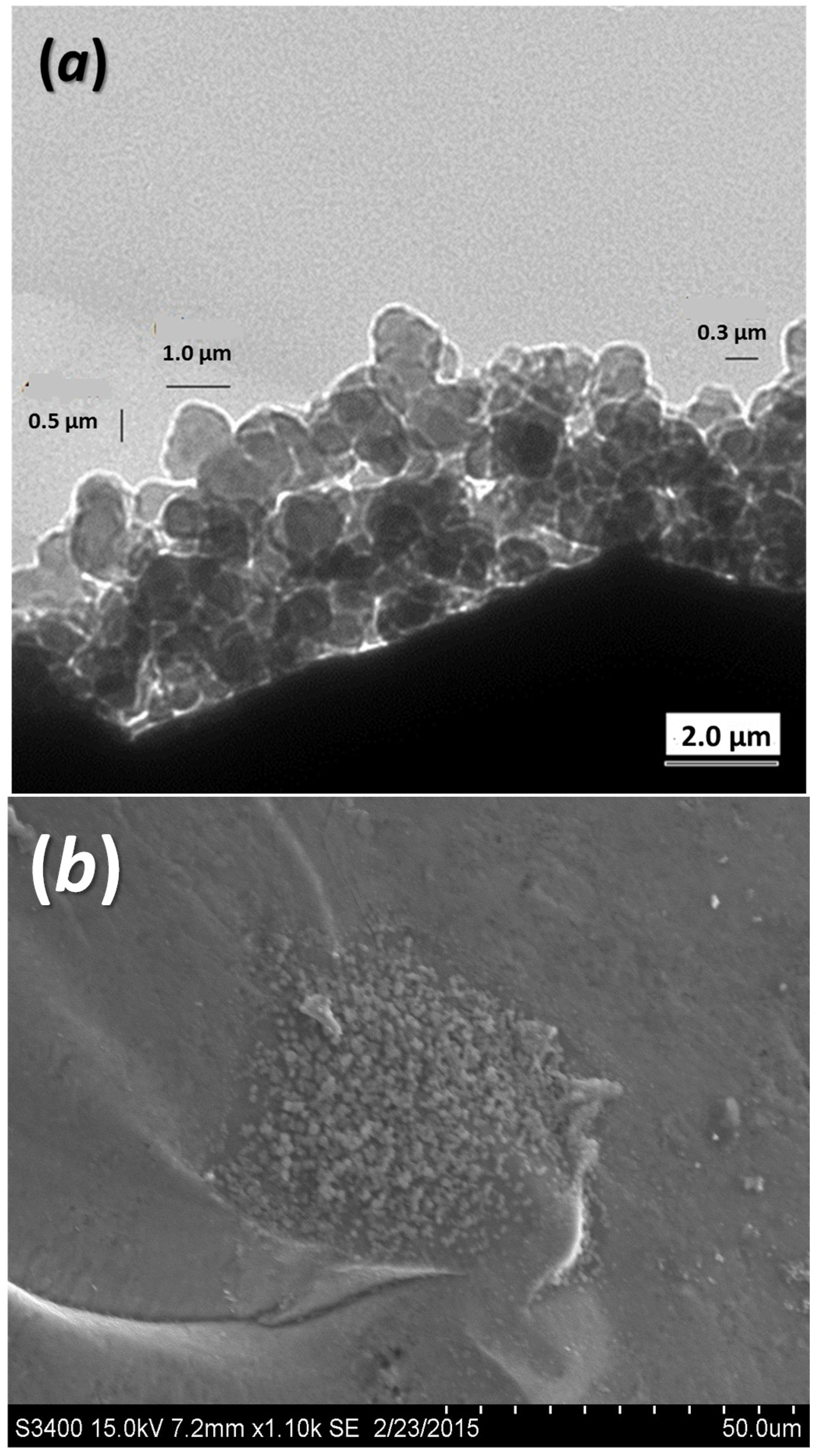
The TEM image (
Figure 2a) shows that the ball milled admixture of
Si and
Ge powders has grain size from 0.3 to 1.0 μm, compared to 1–20 μm and 44 μm for pristine
Si and
Ge powders before the ball milling process. Despite the grain coarsening during the SPS process in each sample, we adopted the same ball milling and SPS conditions for all samples; it is, thus, plausible to assume the grain size of
SiGe coarse grains should be nearly the same for all samples after SPS process, which is a fixed parameter in our study of the doping effects.
Figure 2b shows the
Na2B29 nanoparticles on the grain boundaries of
SiGe host matrix grains.
Figure 1.
(a) XRD patterns of the sample dominantly doped with B, the sample doped dominantly with Na, and pristine SiGe; (b) magnification of the (111) peak shift upon the Na and B doping, note that the peaks shift to the opposite direction; and (c) Raman peak shifting tracks with XRD peak shifting.
Figure 1.
(a) XRD patterns of the sample dominantly doped with B, the sample doped dominantly with Na, and pristine SiGe; (b) magnification of the (111) peak shift upon the Na and B doping, note that the peaks shift to the opposite direction; and (c) Raman peak shifting tracks with XRD peak shifting.
Figure 2.
(a) TEM image shows the grain size of ball milled SiGe powder before SPS; and (b) SEM image of a fracture surface shows the agglomerate of Na2B29 nanoparticles at the grain boundary.
Figure 2.
(a) TEM image shows the grain size of ball milled SiGe powder before SPS; and (b) SEM image of a fracture surface shows the agglomerate of Na2B29 nanoparticles at the grain boundary.
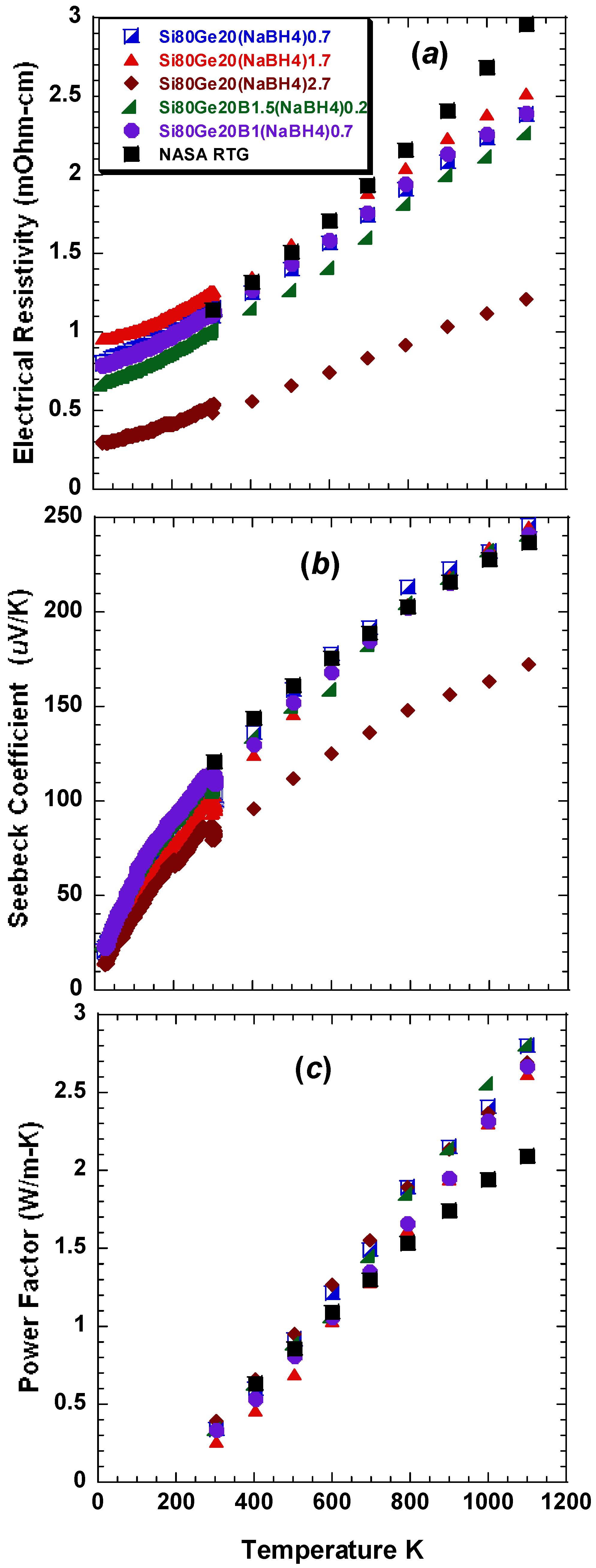
Figure 3a–c present the temperature dependence of the electrical resistivity Seebeck coefficient, and power factor
, respectively, of the dense bulk
NaBH4-treated
p-type
Si80Ge20 in comparison to the
p-leg material used in NASA’s RTGs [
2]. All of the samples exhibit similar trends in the temperature dependence of their physical properties. The sign of the Seebeck coefficient and that of the Hall coefficient confirm a
p-type conduction. As shown in
Figure 3b, the Seebeck coefficient, of most of the as-prepared samples, rivals that of the reference (
i.e., the
p-leg material used in NASA RTGs), except the 2.7%
NaBH4 sample that shows a lower α. Since the Seebeck coefficient is positively correlated to the effective mass
m*, and inversely proportional to the carrier concentration
n, one possible explanation for the decreased
in the sample with 2.7%
NaBH4 is the increasing of
n. A significant enhancement in the
PF, compared to the reference, is attained for all the samples (see
Figure 3c).
Figure 3.
Temperature dependence of (a) the electrical conductivity; (b) Seebeck coefficient; and (c) power factor , of five SPS prepared nanostructured dense bulk Si80Ge20 alloy samples doped with (NaBH4)x (x = 0.7,1.7 and 2.7) and B1.7-y(NaBH4)y (y = 0.2 and 0.7) in comparison to the p-leg material used in NASA’s RTGs.
Figure 3.
Temperature dependence of (a) the electrical conductivity; (b) Seebeck coefficient; and (c) power factor , of five SPS prepared nanostructured dense bulk Si80Ge20 alloy samples doped with (NaBH4)x (x = 0.7,1.7 and 2.7) and B1.7-y(NaBH4)y (y = 0.2 and 0.7) in comparison to the p-leg material used in NASA’s RTGs.
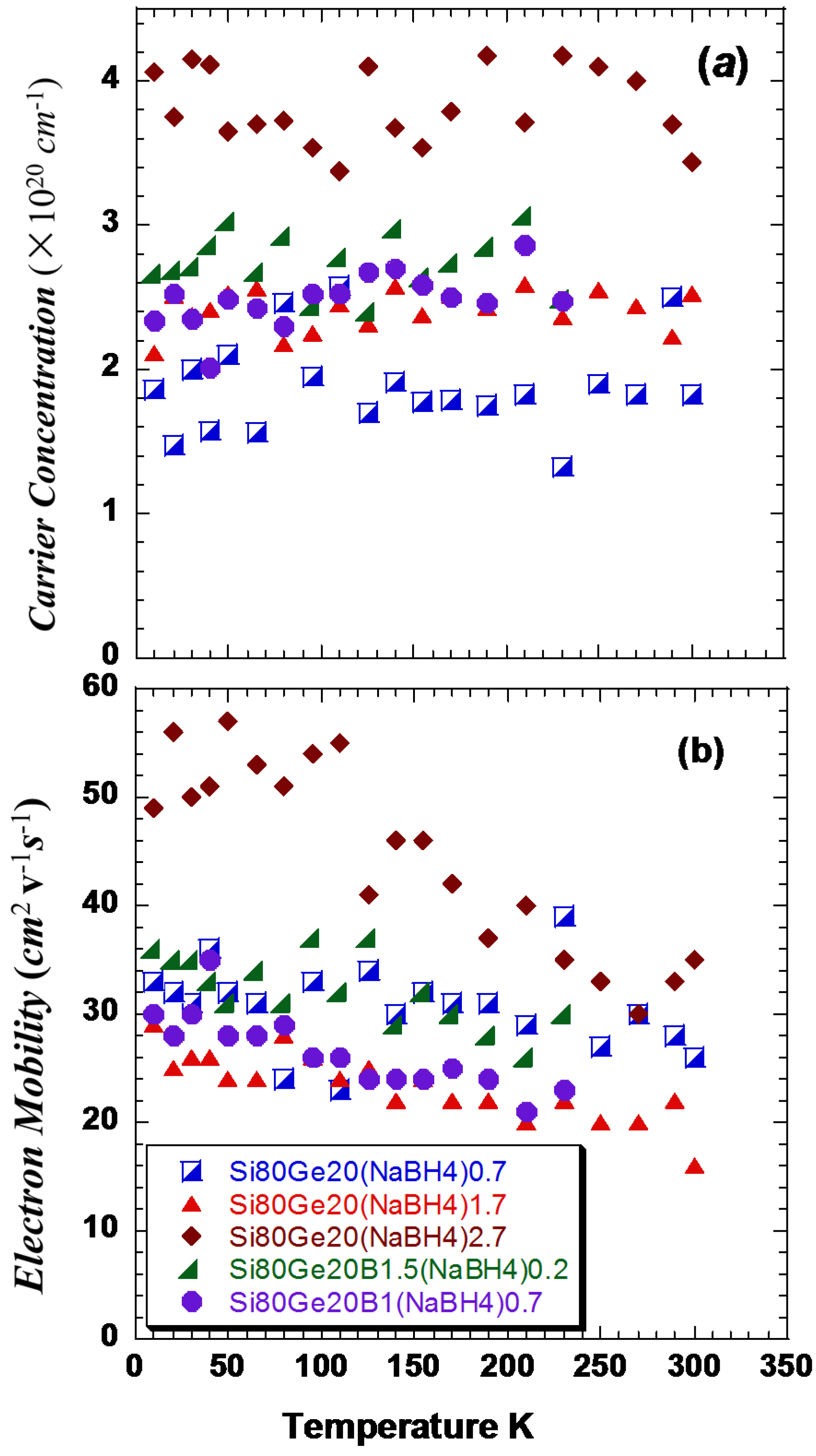
The carrier concentration of
p-type
Si80Ge20 is about 1.67 × 10
20 cm
−3 [
11,
13]. The decrease of the electrical resistivity
for the samples can be explained in terms of the increasing of both the carrier concentration
n and the Hall mobility (
n ~ 2.5 × 10
20 cm
−3 and μ ~ 34 cm
2·V
−1·s
−1), especially for the sample treated with 2.7%
NaBH4 that has higher
n and μ (
n ~ 4×10
20 cm
−3 and μ ~ 40 cm
2·V
−1·s
−1 at room temperature), as shown in
Figure 4a,b
. The carrier concentration exhibits an essentially temperature-independent behavior, while the mobility shows a weak, but well-discerned, negative temperature coefficient. Among the conventional scattering mechanisms, such as the charge carrier-phonon scattering, charge carrier-charge neutral point defect scattering, charge carrier-charged point defect scattering, and charge carrier-grain boundary, only the charge carrier-phonon scattering mechanism gives rises to a negative temperature coefficient [
29]. Hence, we conclude that the charge carrier-phonon scattering is the dominant carrier scattering mechanism, though the charged and charge neutral point defects may coexist. Note that the magnitude change of the carrier concentration agrees with a hole-doping scenario, as expected for the Na-doping and B-doping.
Figure 4.
(a) Temperature dependent of carrier concentration, n; and (b) Hall mobility, μ.
Figure 4.
(a) Temperature dependent of carrier concentration, n; and (b) Hall mobility, μ.
Figure 5a shows the temperature dependent total thermal conductivity of all the samples. With the exception of the
Si80Ge20(NaBH4)2.7 sample the composited samples have lower total thermal conductivity than the reference. The sample treated with 2.7%
NaBH4 shows a higher thermal conductivity than that of the reference, which is attributed to the large electronic thermal conductivity
(
Figure 5b). The electronic thermal conductivity is estimated by the Wiedemann-Franz relation and subtracted from the total thermal conductivity to derive the lattice thermal conductivity. The lattice thermal conductivity
as a function of temperature is plotted in
Figure 5c. Once again, the reference has the highest
, while the
Si80Ge20(NaBH4)1.7 sample shows the lowest lattice thermal conductivity. The lattice thermal conductivity is reduced due to an increase in the number of grain boundaries [
30] by ball milling and the formation of nanoparticles, and the extra point defects introduced by the Na and B dopants. The best result was attained by
(NaBH4)1.7 treatment whose thermal conductivity is ~20%–25% lower than the reference over a wide temperature range between 300 K and 1100 K.
Figure 5.
Temperature dependence of (a) the total thermal conductivity; (b) the electronic thermal conductivity; and (c) the lattice thermal conductivity.
Figure 5.
Temperature dependence of (a) the total thermal conductivity; (b) the electronic thermal conductivity; and (c) the lattice thermal conductivity.
As mentioned earlier, the purpose of treating two samples with
B and
NaBH4 {
Si80Ge20B1,5(NaBH4)0.2 and
Si80Ge20B1(NaBH4)0.7} with increased percentage of
NaBH4 and decreased
B, is to investigate whether
NaBH4 completely decomposes to only
B,
Na, and
H2 or also to boron-rich sodium phase. We found that increasing the
NaBH4 percentage leads to a larger reduction in the total and lattice thermal conductivities without a degradation of the electrical properties, which can be correlated with the increasing amount of
Na2B29 nanoparticles at the grain boundaries. For example, the lowest thermal conductivity of
Si80Ge20(NaBH4)1.7 sample that has most
Na2B29 has the lowest lattice thermal conductivity.
Figure 2b shows the
Na2B29 nanoparticles imbedded in the fracture surface of the sample after SPS, where numerous nanoparticles with a size ~20 nm have emerged at the grain boundary. As the percentage of
NaBH4 is increased (consequently increased
Na2B29) the peak of the lattice thermal conductivity decreased especially for the
Si80Ge20(NaBH4)1.7 sample that has a higher concentration of boron-rich sodium nanoparticles.
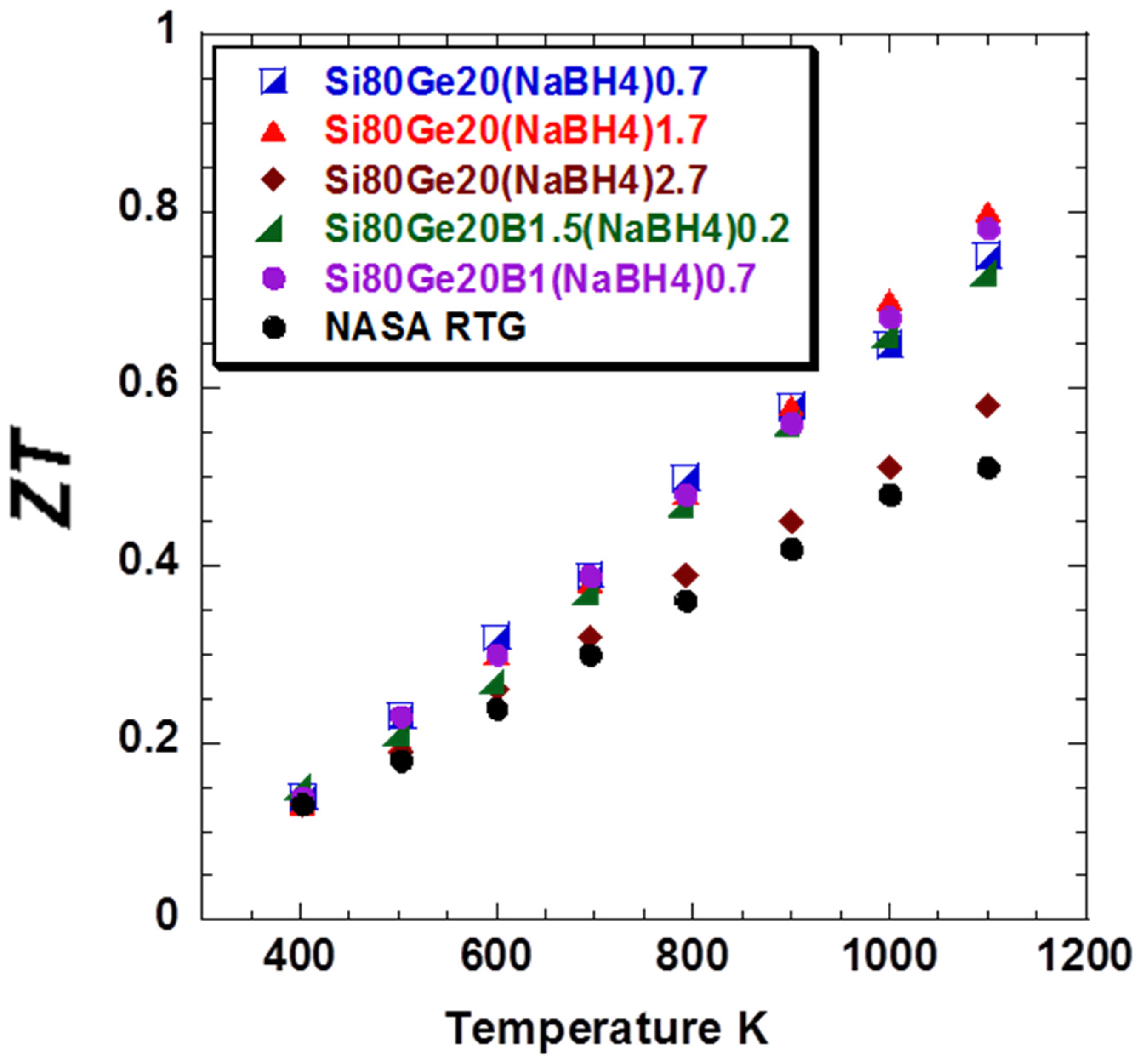
Dimensionless figure of merit
ZT values higher than those of NASA’s RTG materials were attained (
Figure 6) for all of the samples studied. This enhancement can be attributed to the enhancement in the
PF and also the reduction of thermal conductivity, especially the lattice component. The
ZT value of
Si80Ge20-(NaBH4)1.7 and
Si80Ge20-B1.5(NaBH4)0.2 shows a maximum of about 0.8 at 1100 K, which is about 45% higher than that of the
p-leg material of NASA’s RTG (
ZT ~ 0.5).
Figure 6.
Dimensionless figure of merit ZT of all the samples compared to the p-leg material of NASA’s RTG.
Figure 6.
Dimensionless figure of merit ZT of all the samples compared to the p-leg material of NASA’s RTG.
3. Experimental Details
alloys were fabricated by ball milling (BM) process followed by spark plasma sintering (SPS) procedure, which is both time- and cost-efficient, and easy to scale up. Small grain size powders were selected: 1–20 μm silicon powder (99.9985% Alfa Aesar
®, 26 Parkridge Rd, Ward Hill, MA 01835, USA) and germanium powder −100 mesh (Alfa Aesar
® 99.999%) and used in this process. To avoid oxidation, the powders were loaded into a milling container inside a glove box. Then the powders were ball milled for 12 h to further refine the grains and thoroughly mix the powders. The ball milled powders were then divided into five batches, mixed with appropriate amount of
NaBH4 and B according to the nominal formulas
Si80Ge20(NaBH4)x and
Si80Ge20 B1.7-y(NaBH4)y, where x = 0.7, 1.7, 2.7, y = 0.2 and 0.7 in a 3-D mixer. These samples were sintered using a Dr. Sinter SPS-515S (Fuji Electronic Industrial Co.
®) (Tokyo, Japan) and characterized regarding their thermoelectric properties [
17]. The Archimedes method measurements showed that the densities of the as-pressed samples were least 98% of the theoretical density (2.99 ± 0.1 g/cc).
The phase purity and micromorphology of these samples before and after SPS were then characterized by X-ray diffraction (XRD) using a Rigaku
® Miniflex, The Woodlands, TX, USA) and Hitachi
® S-3400N (Hitachi America, Troy, NY USA) equipped with an Oxford X-act
® energy dispersive X-ray spectroscopy (EDX). Oxford Instruments, Raleigh, NC, USA) In order to investigate the effect of the dopants, Raman spectra were acquired with Dilor
® XY triple grating (Dilor
®, Lille, France) and Renishaw
® InVia Raman microscopes (Renishaw Inc.
®, Hoffman Estates, IL, USA) with
Elaser = 2.33 eV. The incident laser beam was focused using a 50× objective, and the laser power on the samples was kept to a minimum to avoid heating. The thermal diffusivity measurements were performed on the densified pellet with 12.7 mm diameter and 2 mm thickness before cutting to approximately 10 × 2 × 2 mm
3 bars for other transport measurements. High-temperature thermal diffusivity measurements were made on a Netzsch
® LFA 457 (Burlington, MA, USA) laser flash apparatus using the transient method from 300 K to 1100 K. The high-temperature thermal conductivity of the samples was calculated using κ =
d D , where
d is the thermal diffusivity,
D the density and
the specific heat at constant volume. The high-temperature specific heat at constant pressure,
, was measured on a Netzsch
® (Burlington, MA USA) differential scanning calorimeter 404 “Pegasus”. The specific heat at constant pressure,
, and the specific heat at constant volume,
, were considered to be essentially the same for the calculation of the total thermal conductivity. High-temperature resistivity (
) and Seebeck coefficient were measured using the commercially-available Ulvac
® ZEM-3 (Boston, MA, USA) from 300 K to 1100 K. The low temperature thermal conductivity was measured, on the same samples, from ~ 10 K to room temperature using a custom-designed steady-state technique [
31]. The lattice thermal conductivity (
) was obtained by applying the Wiedemann–Franz relationship,
, with the Lorenz number for a degenerate semiconductor,
W Ω/
,
the electrical conductivity, and the formula
The low temperature Seebeck coefficient and electrical resistivity were measured on a custom-designed system from 30 K to 300 K [
32]. Hall-effect measurements were performed on a Quantum Design
® (San Diego, CA, USA) physical properties measurement system (PPMS) using a five-probe configuration by sweeping the magnetic field between
Tesla, and the carrier concentration was calculated from the Hall data
, where
is the Hall coefficient,
e the electron charge, and
n the carrier concentration. The electron mobility was then calculated from μ
= RH/ρ. Additionally a thermal stability test was carried out by annealing the samples at 1100 K for 48 h in evacuated tubes before reinvestigating their thermoelectric properties. Importantly, no significant degradation of the physical properties was found.