Effect of Lignocellulose Related Compounds on Microalgae Growth and Product Biosynthesis: A Review
Abstract
:1. Microalgae: A Source of Valuable Compounds
| Taxonomy | Microalgae | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Domain | Eucaryota | ||||||||||
| Division | Chlorophyta | ||||||||||
| Class | Chlorophyceae | Trebouxiophyceae | |||||||||
| Order | Sphaeropleales | Volvocaes 1 | Chlamydomonadales | Chlorellales | |||||||
| Family | Scenedesmaceae | Haematococcaceae | Dunaliellaceae | Chlamydomonadaceae | Chlorellaceae | ||||||
| Genus | Acutodesmus | Haematococcus | Dunaliella | Chlamydomonas | Chlorella | ||||||
| Species | Scenedesmus obliquus | Haematococcus pluvialis | Dunaliella salina | Chlamydomonas reinhardtii | Chlorella prothotecoides | Chlorella zoofingiensis | Chlorella vulgaris | ||||
| Content | Proteins 51% | Pigments 1.5% | Pigments 11% | Lipids 65% | Lipids 62% | Lipids 54% | Proteins 46% | ||||
| Reference | [19] | [20] | [21] | [22] | [23] | [24] | [25] | ||||
| Domain | Eucaryota | ||||||||||
| Division | Heterokontophyta | Dinophyta 2 | Euglenophyta | Heterokontophyta | Chlorophyta | ||||||
| Class | Bacillarophyceae | Dinophyceae | Euglenophyceae | Eustigmatophyceae | Chlorophyceae | Chlorophyceae 3 | |||||
| Order | Naviculales | Dinotrichales 2 | Euglenales | Eustigmatales | Sphaeropleales | Chlorococcales | Chlorococcales 3 | ||||
| Family | Phaeodactylaceae | Crypthecodiniaceae | Euglenaceae | Monodopsidaceae | Selenastraceae | Chlorococcaceae | Dictyosphaeriaceae 3 | ||||
| Genus | Phaeodactylum | Crypthecodinium | Euglena | Nannochloropsis | Monoraphidium | Neochloris | Botryococcus | ||||
| Species | Phaeodyctylum tricornutum | Crypthecodinium cohnii | Euglena gracilis | Nannochloropsis oculata | Monoraphidium contortum | Neochloris oleoabundans | Botryococcus braunii | ||||
| Content | Lipids 20% | Lipids 20% | Lipids 29% | Lipids 32% | Lipids 30% | Lipids 52% | Lipids 65% | ||||
| Reference | [26] | [27] | [28] | [29] | [30] | [31] | [32] | ||||
| Domain | Procaryota | ||||||||||
| Division | Cyanobacteria | ||||||||||
| Class | Cyanophyceae | ||||||||||
| Order | Nostocales 3 | Chroococcales 4 | Chroococcales | Chroococcales 5 | Oscillatoriales 6 | ||||||
| Family | Nostocaceae 3 | Spirulinaceae 4 | Microcystaceae | – | Oscillatoriaceae | ||||||
| Genus | Anabaena | Spirulina | Microcystis | Thermosynechococcus | Oscillatoria | ||||||
| Species | Anabaena azollae | Spirulina platensis | Microcystis aeruginosa | Thermosynechococcus elongates | Oscillatoria acuminata | ||||||
| Content | Proteins 40% | Proteins 67% | Lipids 28% | Lipids 20% | Lipids 25% | ||||||
| Reference | [33] | [34] | [35] | [36] | [35] | ||||||
2. Composition and Treatment of Lignocellulose Materials
| Component * | Triticum Aestivum Bran | Corn Stover | Cynodon Dactylon Grass | Hordeum Vulgare Brewer’s Spent Grain | Oryza Sativa Straw | Picea Abies Softwood | Saccharum Officinarum Bagasse | Salix Hardwood |
|---|---|---|---|---|---|---|---|---|
| Glucan 1 | 10.5 | 36.1 | 30.4 | 16.7 | 35.9 | 40.9 | 35.8 | 43.0 |
| Xylan | 18.3 | 21.4 | 22.6 | 19.9 | 19.0 | 5.1 | 21.2 | 14.9 |
| Mannan | – | 1.8 | 0.0 | – | – | 10.1 | 0.79 | 3.2 |
| Galactan | 1.1 | 2.5 | 1.8 | – | – | 1.9 | 0.74 | 2.0 |
| Arabinan | 10.1 | 3.5 | 4.9 | 8.4 | 3.1 | 1.0 | 1.94 | 1.2 |
| Klason lignin | 5.0 | 17.2 3 | 18.8 | 22.9 | 13.6 | 27.7 3 | 16.6 | 24.2 |
| AS lignin 2 | – | 4.4 | 4.8 | 3.3 | 1.6 | 2.4 | ||
| Reference | [41] | [56] | [57] | [58] | [59] | [60] | [61] | [62] |
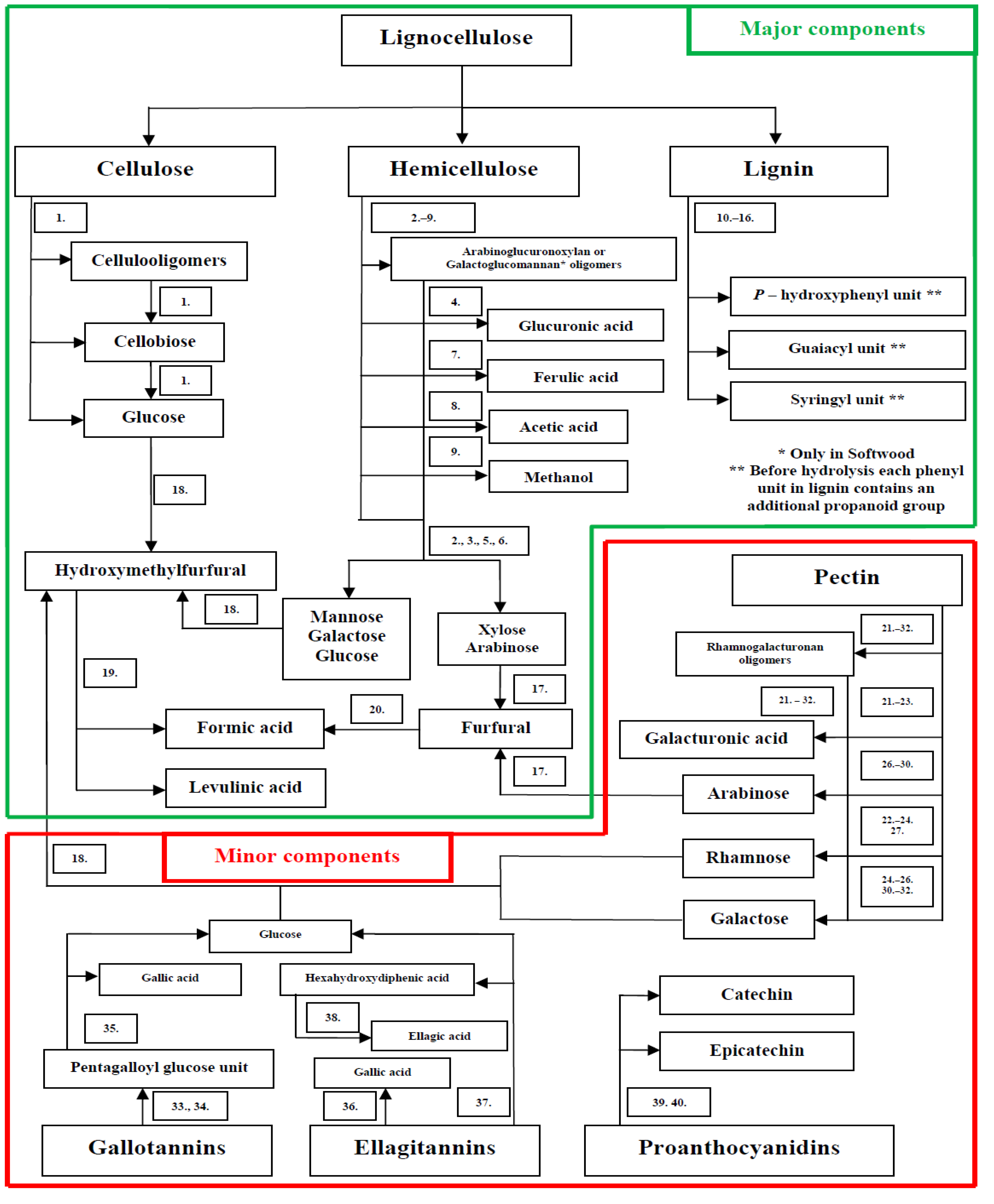
3. Effect of Lignocellulose Components on Microalgae Cultures
3.1. Sugars
3.2. Acetates
3.3. Methanol
3.4. Sugar Acids
3.5. Phenolics
| Phenolic Compound | Concentration in Hydrolysate (mg/L) | Treatment Method | Material | References |
|---|---|---|---|---|
| Vanillin | 36, 430 | Dilute acid treatment | Spruce | [68,98] |
| Steam explotion + SO2 impregnation | Willow | |||
| Vanilic acid | 3, 33 | Alkaline hydrolysis Dilute acid treatment | Brewer’s spent grain Spruce | [68,99] |
| Catechol | 440 | Steam explotion + SO2 impregnation | Willow | [98] |
| Ferulic acid | 145 | Alkaline hydrolysis | Brewer’s spent grain | [99] |
| p-Hydroxybenzoic acid | 27, 81 | Alkaline hydrolysis Dilute acid treatment | Brewer’s spent grain Spruce | [68,99] |
| p-Coumaric acid | 139 | Alkaline hydrolysis | Brewer’s spent grain | [99] |
| Syringic acid | 8 | Alkaline hydrolysis | Brewer’s spent grain | [99] |
3.6. Furans
3.7. Levulinic Acid
3.8. Fatty Acids
3.9. Terpenoids
| Name | Group | Source | Extraction Solvent | Content | Ref. |
|---|---|---|---|---|---|
| α–Pinene (1) | Monoterpenoids | Abies alba wood | Water | 0.2% A | [46] |
| Abies alba knots | 26.4% A | ||||
| β-Pinene | Monoterpenoids | Abies balsamea knots | Water | 0.4% A | [46] |
| Abies alba knots | 2.3% A | ||||
| Limonene | Monoterpenoids | Abies alba knots | Water | 2.1% A | [46] |
| Abietic acid | Diterpenoids | Pinus sylvestris wood | Acetone | 0.65%–1.43% A | [47] |
| Pinus sylvestris knots | 2.1%–3.9% A | ||||
| Picea abies wood | 0.017% A | ||||
| Picea abies knots | 0% A | ||||
| Palustric acid | Diterpenoids | Pinus sylvestris wood | Acetone | 0.25%–0.67% A | [47] |
| Pinus sylvestris knots | 0.43%–1.7% A | ||||
| Picea abies wood | 0.045% A | ||||
| Picea abies knots | 0.014% A | ||||
| Betulin | Triterpenoids | Betula papyrifera bark | EtOAC— Ethanol-Water | 15.4% A | [48] |
| Gallic acid (2) | Gallotannins | Terminalia paniculata bark | Water-Chloroform | 0.068% B | [112] |
| Ellagic acid (3) | Ellagitannins | Terminalia paniculata bark | Water-Chloroform | 0.061% B | [112] |
| Catechin (4) | Proanthocyanidins | Acacia catechu wood | Water | 4.5% A | [113] |
| Quercetin (5) | Flavonoids | Terminalia paniculata bark | Water-Chloroform | 0.019% B | [112] |
| Rutin (6) | Flavonoids | Terminalia paniculata bark | Water-Chloroform | 0.049% B | [112] |
| Pinosylvin | Stilbenes | Pinus sylvestris wood | Acetone | 0.12%–0.98% A | [47] |
| Pinus sylvestris knots | 0.91%–3.5% A | ||||
| Resveratrol | Stilbenes | Picea mariana bark | Water | 0.01% A | [114] |
| Pterostilbene (7) | Stilbenes | Pterocarpus marsupium wood | EtOAc | No data | [115] |
| Secoisolariciresinol | Lignans | Araucaria araucana wood | Methanol | 32.99% C | [116] |
| Lariciresinol | Lignans | Araucaria araucana wood | Methanol | 10.09% C | [116] |
| Pinoresinol | Lignans | Araucaria araucana wood | Methanol | 7.32% C | [116] |
| Eudesmin (8) | Lignans | Araucaria araucana wood | Methanol | 18.24% C | [116] |
| Gramine (9) | Alkaloids | Hordeum vulgare shoots Phalaris arundinacea samples | No data Chloroform | 0.7% A 0.011% A | [117] [52] |
| Berberine (10) | Alkaloids | Phellodendron bark | Water or Methanol | No data | [118] |
| Flindersine (11) | Alkaloids | Flindersia australis wood Hortia colombiana wood | No data Ethanol | No data 0.009% A | [119] [120] |

3.10. Polyphenols
3.11. Alkaloids
3.12. Impurities
3.12.1. Heavy Metal Ions
3.12.2. Ionic Liquids
4. Effect of Lignocellulose Hydrolysates on Microalgae
| Compound | Concentration | Microalgae | Light | Cultivation Time | Effect on Microalgae | Ref. |
|---|---|---|---|---|---|---|
| Glucose Mannose Galactose | 50 g/L a | Chlorella zofingiensis | No | Not mentioned | Growth confirmed Astaxanthin synthesis confirmed | [74] |
| Glucose | 10 g/L | Chlorella vulgaris | No | 6 days | Increased growth 1 Decreased lipid content 1 | [75] |
| Glucose Cellobiose | 10 g/L a | Neochloris oleoabundans | No | 5 days | Growth confirmed | [31] |
| Xylose Arabinose | 10 g/L a | Neochloris oleoabundans | No | 5 days | No effect on growth | [31] |
| Glucose | 8 g/L 8 g/L | Chlorella sorokiniana | Yes No | 6 days 6 days | Growth acceleration 1 Increased total fatty acid content 1 Growth acceleration 1 Increased total fatty acid content 1 | [76] |
| Glucose | 18 g/L | Chlorella sorokiniana | Yes | 10 days | Increased biomass density 1 Decreased lutein content 1 | [77] |
| Glucose | 0.5–1 g/L | Phaeodactylum tricornutum | Yes | 10 days | Increased growth 1 Increased lipid content 1 | [78] |
| Xylose | 0.15 g/L | Chlorella | Yes | 2 weeks | Increased growth 2 | [79] |
| Glucose Rhamnose Xylose | 1.8 g/L a 1.64 g/L a 1.5 g/L a | Chlorella vulgaris | No | 15 days | Growth confirmed | [80] |
| Acetate | 2.46 g/L over 4.1 g/L | Haematococcus pluvialis | Yes | 8 days | Growth confirmed Decreased growth 3 | [81] |
| Acetate | 2.5 g/L 10–20 g/L | Haematococcus pluvialis | Yes | 10 days | Increased growth 1 Increased carotenoid content 1 Decreased growth 1 Increased carotenoid content 1 | [82] |
| Acetate | up to 3.28 g/L 4.1–4.9 g/L | Chlorella sorokiniana | Yes | 10 days | Increased biomass concentration 1 Increased lutein content 1 Increased biomass concentration 1 Decreased lutein content 1 | [77] |
| Acetate | 1 g/L | Chlamydomonas reinhardtii | Yes | 2 days | Increased growth 1 Chlorophyll content increased 1 Cell size increased 1 Oxygen production increased 1 Increased growth 4 Chlorophyll content decreased 4 Cell size unchanged 4 Oxygen production decreased 4 | [84] |
| Methanol | 7.9 g/L + 5% CO2 7.9 g/L without 5% CO2 | Chlorella sp. | Yes Yes | 45 days 45 days | Increased biomass growth 4 Increased lipid content 4 Decreased biomass growth 4 Decreased lipid content 4 | [85] |
| Methanol | 3.9 g/L | Scenedesmus obliquus | Yes No | 40 h 24 h | Biomass growth enhancement 1 No growth enhancement 1 | [86] |
| Glucuronic acid | 2.5 g/L b | Ochromonas danica | Yes | 6 h | No increase in ascorbic acid synthesis 5 | [89] |
| Glucuronic acid | 2.5 g/L | Euglena gracilis | Yes | 4 h | Enhanced ascorbic acid synthesis 6 | [90] |
| Galacturonic acid | 2.5 g/L b | Ochromonas danica | Yes | 6 h | Enhanced ascorbic acid synthesis 5 | [89] |
| Galacturonic acid | 2.5 g/L | Euglena gracilis | Yes | 4 h | Enhanced ascorbic acid synthesis 6 | [90] |
| Catechol | 0.05 μg c | Chlorella zofingiensis Coelastrum microporum Mesotaenium caldarorium | Yes | Not mentioned | Growth inhibition 7a | [92] |
| Catechol | 0.05 μg c | Chlorella saccharophila Scenedesmus quadricauda | Yes | Not mentioned | No effect on growth 7b | [92] |
| Catechol P-hydroxybenzoic acid P-coumaric acid Caffeic acid Ferulic acid | 0.4 g/L a | Scenedesmus quadricauda | Yes | 5 or 10 days | Removal of compounds from growth medium | [92] |
| O-hydroxybenzoic acid P-hydroxybenzoic acid | 13.8 mg/L a | Chlorella vulgaris | Yes | 6–9 days | Growth stimulation 8 Increased pigment content 8 Increased protein content 8 Increased RNA and DNA content 8 | [93] |
| M-hydroxybenzoic acid | 13.8 mg/L | Chlorella vulgaris | Yes | 6–9 days | Growth inhibition 8 | [93] |
| P-hydroxybenzoic acid | 13.8–55 mg/L | Chlorella pyrenoidosa | Yes | 16 days | Growth stimulation 8 | [94] |
| Vanillic acid | 16.8–67 mg/L | Chlorella pyrenoidosa | Yes | 16 days | Growth stimulation 8 | [94] |
| Syringic acid | 19.8–79 mg/L 99 mg/L | Chlorella pyrenoidosa | Yes | 16 days | Growth stimulation 8 Culture death | [94] |
| P-hydroxybenzoic acid | 13.8–138 mg/L 1.36 g/L | Pseudokirchneriella subcapitata+ | Yes | 72 h | Growth stimulation 8 Growth inhibition 8 | [95] |
| O-hydroxybenzoic acid | 13.8–138 mg/L | Pseudokirchneriella subcapitata+ | Yes | 72 h | Growth inhibition 8 | [95] |
| 2-Furfural | 0.67 g/L | Spirulina maxima | Yes | 144 h | Growth inhibition 8 Photosynthesis inhibition 8 | [102] |
| 2-Furfural | 0.6 g/L + acetate | Chlamydomonas reinhardtii | Yes | Not mentioned | Growth inhibition 9 | [103] |
| 5-HMF | 1.13 g/L | Spirulina maxima | Yes | 144 h | Growth inhibition 8 Photosynthesis inhibition 8 | [102] |
| Levulinic acid | 1.16–11.6 g/L | Sceletonema costatum | Yes | 96 h | Growth inhibition 8 Aminolevulinic acid accumulation 8 Chlorophyll synthesis inhibited 8 | [105] |
| Levulinic acid | 1.16–5.8 g/L | Chlorella vulgaris | Yes | 24 h | Growth inhibition 8 Aminolevulinic acid accumulation8 Chlorophyll synthesis inhibited 8 | [104] |
| Levulinic acid | 6.96 g/L | Agmenellum quadruplicatum | Yes | 14 h | Growth inhibition 8 Aminolevulinic acid accumulation 8 Chlorophyll synthesis inhibited 8 | [106] |
| Palmitic acid C16:0 | 3.87 mg/L | Selenastrum capricornutum | Yes | 72 h | Growth inhibition 8 | [108] |
| Palmitic acid C16:0 | 59.1 mg/L | Chlorella vulgaris | Yes | 24 h | Growth inhibition 8 K+ leakage from cells | [109] |
| Palmitic acid C16:0 | 9.2 mg/L | Monoraphidium contortum | Yes | 24 h | Growth inhibition 8 K+ leakage from cells | [109] |
| Oleic acid C18:1 | 0.47 mg/L | Selenastrum capricornutum | Yes | 72 h | Growth inhibition 8 | [108] |
| Oleic acid C18:1 | 12.4 mg/L | Chlorella vulgaris | Yes | 24 h | Growth inhibition 8 K+ leakage from cells | [109] |
| Oleic acid C18:1 | 12.1 mg/L | Monoraphidium contortum | Yes | 24 h | Growth inhibition 8 K+ leakage from cells | [109] |
| Linoleic acid C18:2 | 1.55 mg/L | Selenastrum capricornutum | Yes | 72 h | Growth inhibition 8 | [108] |
| Linoleic acid C18:2 | 9.4 mg/L | Chlorella vulgaris | Yes | 24 h | Growth inhibition 8 K+ leakage from cells | [109] |
| Linoleic acid C18:2 | 8.0 mg/L | Monoraphidium contortum | Yes | 24 h | Growth inhibition 8 K+ leakage from cells | [109] |
| α–Pinene β–Pinene Limonene | 10 g/L d | Chlorella pyrenoidosa | Yes | 2 days | No effect on growth 7b | [110] |
| α–Pinene | Analytical grade | Chlorella vulgaris stored as dried paste | – | 7–8 h of extraction | Extraction of lipids from Chlorella | [111] |
| Gallic acid | 10 mg/L | Nostoc sp. | Yes | 5 days | Growth inhibition 8 Protein content reduction 8 Chlorophyll content reduction 8 Inhibition of glutamine synthetase activity 8 Inhibition of nitrate reductase activity 8 | [128] |
| Gallic acid | 1 mg/L | Microcystis aeruginosa | Yes | 15 days | Growth inhibition 8 | [131] |
| Ellagic acid | 5 mg/L | Microcystis aeruginosa | Yes | 15 days | Growth inhibition 8 | [131] |
| Quercetin | 6 mg/L | Thalassiosira pseudonana Phaeodactylum tricornutum Thalassiosira weissflogii | Yes | Not mentioned | Photosynthetic mechanism inhibited 8 | [132] |
| Quercetin | 12 mg/L | Chlamydomonas sp. Dunaliella tetriolecta | Yes | Not mentioned | No inhibition of photosynthetic mechanism 8 | [132] |
| Rutin | 0.4 mg/L | Sceletonema costatum | Yes | 3 days | Growth inhibition 8 | [133] |
| Catechin | 25–100 mg/L | Microcystis aeruginosa Pseudokirchneriella subcapitata | Yes No Yes No | 2 h 2 h 2 h 2 h | Formation of ROS~ in cells Formation of ROS~ in cells Formation of ROS~ in cells Formation of ROS~ in cells | [134] |
| Pinosylvin | 21.2 mg/L 21.2 mg/L | Selenastrum capricornutum Oscillatoria perornata | Yes Yes | 4 days 4 days | No effect on growth 8 No effect on growth 8 | [135] |
| Resveratrol | 22.8 mg/L 22.8 mg/L | Selenastrum capricornutum Oscillatoria perornata | Yes Yes | 4 days 4 days | No effect on growth 8 No effect on growth 8 | [135] |
| Pterostilbene | 2.5 mg/L 25.6 mg/L | Selenastrum capricornutum Oscillatoria perornata | Yes Yes | 4 days 4 days | Growth inhibition 8 Growth inhibition 8 | [135] |
| Eudesmin | 3.8 mg/L 38.6 mg/L 38.6 mg/L | Oscillatoria perornata Oscillatoria agardhii Selenastrum capricornutum | Yes Yes Yes | 4 days 4 days 4 days | Growth inhibition 8 No effect on growth 8 Growth inhibition 8 | [136] |
| Gramine | 2 mg/L 1 mg/L 8 mg/L | Microcystis aeruginosa | Yes | 24–60 h 5 days 1 day or 5 days | Breakage of cell wall structure 8 DNA fragmentation 8 DNA fragmentation 8 | [138] |
| Gramine | 65 mg/L | Chlorella vulgaris | Yes | 10 days | Growth inhibition 8 | [139] |
| Berberine | 1 mg/L 1 mg/L 0.75 mg/L 0.27 mg/L 0.57 mg/L 0.64 mg/L | Pseudokirchneriella subcapitata+ Chlorella vulgaris Scenedesmus quadricauda Microcystis aeruginosa Synechococcus nidulans Aphanothece clathrata | Yes Yes Yes Yes Yes Yes | 4 days 4 days 4 days 4 days 4 days 4 days | Not stated Not stated Growth inhibition 8 Growth inhibition 8 Growth inhibition 8 Growth inhibition 8 | [140] |
| Berberine | 0.2 g/L | Microcystis aeruginosa | Yes | 3 days | Inhibition of SOD activity 8 Increased O2− content in cells 8 | [141] |
| Flindersine | 3.6 mg/L 22.7 mg/L 4 mg/L | Oscillatoria perornata Oscillatoria agardhii Selenastrum capricornutum | Yes Yes Yes | 4 days 4 days 4 days | Growth inhibition 8 No effect on growth 8 Growth inhibition 8 | [136] |
| Lead Pb (added as PbCl2) | 0.5 mg/L | Selenastrum capricornutum Chlorella pyrenoidosa Chlorella ellipsoidea Chlorella vulgaris | Yes | 7 days | Growth inhibition 8 | [144] |
| Cadmium Cd (added as CdCl2) | 17 mg/L | Scenedesmus armatus | Yes | 24 h | Growth inhibition 10a Inhibition of photosynthetic mechanism 10a Growth inhibition 10b Inhibition of photosynthetic mechanism 10b | [146] |
| Nickel Ni (added as NiCl2) | 10 mg/L | Synechococcus sp. | Yes | 10 days | Growth inhibition 8 | [145] |
| Chromium Cr (added as K2CrO4) | 0.97 mg/L | Chlorella vulgaris | Yes | 96 h | Growth inhibition 8 Photosynthetic mechanism inhibited 8 | [147] |
| EMIM Cl | 1.46 g/L | Chlorella vulgaris | Yes | 72 h | Growth inhibition 8 | [151] |
| EMIM Cl | 1.83 g/L | Oocystis submarina | Yes | 72 h | Growth inhibition 8 | [151] |
| EMIM Cl | 14.6 mg/L | Cyclotella meneghiniana | Yes | 72 h | Growth inhibition 8 | [151] |
| BMIM Cl | 0.17 g/L | Chlorella vulgaris | Yes | 72 h | Growth inhibition 8 | [151] |
| BMIM Cl | 0.26 g/L | Oocystis submarina | Yes | 72 h | Growth inhibition 8 | [151] |
| BMIM Cl | 1.74 mg/L | Cyclotella meneghiniana | Yes | 72 h | Growth inhibition 8 | [151] |
| Rice straw hydrolysate | 11 g/L sugars e | Chlorella pyrenoidosa | Yes | 60 h | Increased growth 11 Increased lipid content 11 | [152] |
| Wheat bran hydrolysate | 0.25%–1.5% f | Chlorella vulgaris | Yes | 6 days | Increased biomass growth 12 Increased protein content 12 Increased pigment content 12 | [19] |
| Wheat bran hydrolysate | 0.25%–1.5% f | Chlorella vulgaris | No | 6 days | Increased biomass growth 13 Increased protein content 13 Increased pigment content 13 | [19] |
| Wheat bran hydrolysate | 0.25%–1.5% f | Scenedesmus obliquus | Yes | 8 days | Increased biomass growth 12 Increased protein content 12 Decreased pigment content 12 | [19] |
| Wheat bran hydrolysate | 0.25%–1.5% f | Scenedesmus obliquus | No | 8 days | Increased biomass growth 13 Increased protein content 13 Decreased pigment content 13 | [19] |
5. Strategies for Implementing Lignocellulose Extracts into Microalgae Cultivation Systems
6. Conclusions
Acknowledgments
Conflict of Interests
References
- Hallmann, A. Algal transgenics and biotechnology. Trans. Plant. J. 2007, 1, 81–98. [Google Scholar]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Sakthivel, R.; Elumalai, S.; Mohommadarif, M. Microalgae lipid research, past, present: A critical review for biodiesel production, in the future. J. Exp. Sci. 2011, 2, 29–49. [Google Scholar]
- Khozin-Goldberg, I.; Iskandarov, U.; Cohen, Z. LC-PUFA from photosynthetic microalgae: Occurrence, biosynthesis, and prospects in biotechnology. Appl. Microbiol. Biotechnol. 2011, 91, 905–915. [Google Scholar] [CrossRef]
- Myers, R.A.; Worm, B. Rapid worldwide depletion of predatory fish communities. Nature 2003, 423, 280–283. [Google Scholar] [CrossRef]
- Reitan, K.I. Digestion of lipids and carbohydrates from microalgae (Chaetoceros muelleri Lemmermann and Isochrysis aff. galbana clone T-ISO) in juvenile scallops (Pecten maximus L.). Aquac. Res. 2011, 42, 1530–1538. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Jin, E.; Polle, J.E.W.; Lee, H.K.; Hyun, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Mortensen, A. Carotenoids and other pigments as natural colorants. Pure Appl. Chem. 2006, 8, 1477–1491. [Google Scholar]
- Stahl, W.; Sies, H. Bioactivity and protective effects of natural carotenoids. Biochim. Biophys. Acta 2005, 1740, 101–107. [Google Scholar] [CrossRef]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Chojnacka, K.; Noworyta, A. Evaluation of Spirulina sp. growth in photoautotrophic, heterotrophic and mixotrophic cultures. Enzyme Microb. Technol. 2003, 34, 461–465. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Park, K.C.; Whitney, C.; McNichol, J.C.; Dickinson, K.E.; MacQuarrie, S.; Skrupski, B.P.; Zou, J.; Wilson, K.E.; O’Leary, S.J.B.; McGinn, P.J. Mixotrophic and photoautotrophic cultivation of 14 microalgae isolates from Saskatchewan, Canada: Potential applications for wastewater remediation for biofuel production. J. Appl. Phycol. 2012, 24, 339–348. [Google Scholar] [CrossRef]
- Running, J.A.; Huss, R.J.; Olson, P.T. Heterotrophic production of ascorbic acid by microalgae. J. Appl. Phycol. 1994, 6, 99–104. [Google Scholar] [CrossRef]
- De Vrije, T.; Bakker, R.R.; Budde, M.A.W.; Lai, M.H.; Mars, A.E.; Claassen, P.A.M. Efficient hydrogen production from the lignocellulosic energy crop Miscanthus by the extreme thermophilic bacteria Caldicellulosiruptor saccharolyticus and Thermotoga neapolitana. Biotechnol. Biofuels 2009, 2. [Google Scholar] [CrossRef]
- Villarreal, M.L.M.; Prata, A.M.R.; Felipe, M.G.A.; Almeida, E.; Silva, J.B. Detoxification procedures of eucalyptus hemicellulose hydrolysate for xylitol production by Candida guilliermondii. Enzyme Microb. Technol. 2006, 40, 17–24. [Google Scholar] [CrossRef]
- Klement, T.; Milker, S.; Jäger, G.; Grande, P.M.; de María, P.D.; Büchs, J. Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb. Cell. Fact. 2012, 11. [Google Scholar] [CrossRef] [Green Version]
- EL-Sheekh, M.M.; Bedaiwy, M.Y.; Osman, M.E.; Ismail, M.M. Mixotrophic and heterotrophic growth of some microalgae using extract of fungal-treated wheat bran. Int. J. Rec. Org. Waste Agric. 2012, 1, 121–129. [Google Scholar]
- García-Malea, M.C.; Brindley, C.; del Río, E.; Acien, F.G.; Fernandez, J.M.; Molina, E. Modelling of growth and accumulation of carotenoids in Haematococcus pluvialis as a function of irradiance and nutrients supply. Biochem. Eng. J. 2005, 26, 107–114. [Google Scholar] [CrossRef]
- El-Baky, A.; El Baz, F.K.; El-Baroty, G.S. Production of antioxidant by the green alga Dunaliella salina. Int. J. Agric. Biol. 2004, 6, 49–57. [Google Scholar]
- James, G.O.; Hocart, C.H.; Hillier, W.; Chen, H.; Kordbacheh, F.; Price, G.D.; Djordjevic, M.A. Fatty acid profiling of chlamydomonas reinhardtii under nitrogen deprivation. Bioresour. Technol. 2011, 102, 3343–3351. [Google Scholar] [CrossRef]
- Chen, Y.H.; Walker, T.H. Biomass and lipid production of heterotrophic microalgae Chlorella protothecoides by using biodiesel-derived crude glycerol. Biotechnol. Lett. 2011, 33, 1973–1983. [Google Scholar] [CrossRef]
- Feng, P.; Deng, Z.; Hu, Z.; Fan, L. Lipid accumulation and growth of Chlorella zofingiensis in flat plate photobioreactors outdoors. Bioresour. Technol. 2011, 102, 10577–10584. [Google Scholar] [CrossRef]
- Seyfabadi, J.; Ramezanpour, Z.; Khoeyi, Z.A. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Gatenby, C.M.; Orcutt, D.M.; Kreeger, D.A.; Parker, B.C. Biochemical composition of three algal species proposed as food for captive freshwater mussels. J. Appl. Phycol. 2003, 15, 1–11. [Google Scholar] [CrossRef]
- Couto, R.M.; Simoes, P.C.; Reis, A.; da Silva, T.L.; Martins, V.H.; Sanchez-Vicente, Y. Supercritical fluid extraction of lipids from the heterotrophic microalga Crypthecodinium cohnii. Eng. Life Sci. 2010, 10, 158–164. [Google Scholar]
- Rezić, T.; Filipović, J.; Šantek, B. Photo-mixotrophic cultivation of algae Euglena gracilis for lipid production. Agric. Conspec. Sci. 2013, 78, 65–69. [Google Scholar]
- Gu, N.; Lin, Q.; Li, G.; Tan, Y.; Huang, L.; Lin, J. Effect of salinity on growth, biochemical composition, and lipid productivity of Nannochloropsis oculata CS 179. Eng. Life Sci. 2012, 12, 631–637. [Google Scholar] [CrossRef]
- Sathya, S.; Srisudha, S. Isolation and identification of freshwater microalgal strains—Potential for biofuel production. Int. J. Rec. Sci. Res. 2013, 4, 1432–1437. [Google Scholar]
- Morales-Sánchez, D.; Tinoco-Valencia, R.; Kyndt, J.; Martinez, A. Heterotrophic growth of Neochloris oleoabundans using glucose as a carbon source. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef]
- Tran, H.L.; Kwon, J.S.; Kim, Z.H.; Oh, Y.; Lee, C.G. Statistical optimization of culture media for growth and lipid production of Botryococcus braunii LB572. Biotechnol. Bioprocess. Eng. 2010, 15, 277–284. [Google Scholar] [CrossRef]
- Venugopal, V.; Prasanna, R.; Sood, A.; Jaiswal, P.; Kaushik, B.D. Stimulation of pigment accumulation in Anabaena azollae strains: Effect of light intensity and sugars. Folia Microbiol. 2006, 1, 50–56. [Google Scholar]
- Chauhan, U.K.; Pathak, N. Effect of different conditions on the production of chlorophyll by Spirulina platensis. J. Algal Biomass Utln. 2010, 1, 89–99. [Google Scholar]
- Sharathchandra, K.; Rajashekhar, M. Total lipid and fatty acid composition in some freshwater cyanobacteria. J. Algal Biomass Utln. 2011, 2, 83–97. [Google Scholar]
- Eberly, J.O.; Ely, R.L. Photosynthetic accumulation of carbon storage compounds under CO2 enrichment by the thermophilic cyanobacterium thermosynechococcus elongates. J. Ind. Microbiol. Biotechnol. 2012, 39, 843–850. [Google Scholar] [CrossRef] [Green Version]
- Hamelinck, C.N.; van Hooijdonk, G.; Faaij, A.P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Lee, J. Biological conversion of lignocellulosic biomass to ethanol. J. Biotechnol. 1997, 56, 1–24. [Google Scholar] [CrossRef]
- Morales, J.B.; Jiménez, L.A.P.; Chiang, F. Seasonal fluctuations of starch in wood and bark of trees from a tropical deciduous forest in Mexico. Anales. Inst. Biol. Univ. Nac. Auton. Mexico Ser. Bot. 1997, 68, 7–19. [Google Scholar]
- Linde, M.; Galbe, M.; Zacchi, G. Simultaneous saccharification and fermentation of steam-pretreated barley straw at low enzyme loadings and low yeast concentration. Enzyme Microb. Technol. 2007, 40, 1100–1107. [Google Scholar] [CrossRef]
- Palmarola-Adrados, B.; Choteborska, P.; Galbe, M.; Zacchi, G. Ethanol production from non-starch carbohydrates of wheat bran. Bioresour. Technol. 2005, 96, 843–850. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant. Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Ragland, K.W.; Aerts, D.J.; Baker, A.J. Properties of wood for combustion analysis. Bioresour. Technol. 1991, 37, 161–168. [Google Scholar] [CrossRef]
- Sarnklong, C.; Cone, J.W.; Pellikaan, W.; Hendriks, W.H. Utilization of rice straw and different treatments to improve its feed value for ruminants: A review. Asian Aust. J. Anim. Sci. 2010, 23, 680–692. [Google Scholar] [CrossRef]
- Gutiérrez, A.; del Río, J.C.; González-Vila, F.J.; Martín, F. Chemical composition of lipophilic extractives from eucalyptus globulus labill wood. Holzforschung 1999, 53, 481–486. [Google Scholar]
- Sekine, N.; Shibutani, S.; Yatagai, M. Chemical composition of the terpenoids in wood and knots of Abies species. Eur. J. Wood. Prod. 2013, 71, 679–682. [Google Scholar] [CrossRef]
- Hovelstad, H.; Leirset, I.; Oyaas, K.; Fiksdahl, A. Screening analyses of pinosylvin stilbenes, resin acids and lignans in norwegian conifers. Molecules 2006, 11, 103–114. [Google Scholar]
- Lugemwa, F.N. Extraction of betulin, trimyristin, eugenol and carnosic acid using water-organic solvent mixtures. Molecules 2012, 17, 9274–9282. [Google Scholar] [CrossRef]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems—A review. Plant Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Luostarinen, K.; Mottonen, V. Effects of log storage and drying on birch (Betula pendula) wood proanthocyanidin concentration and discoloration. J. Wood Sci. 2004, 50, 151–156. [Google Scholar]
- Conde, E.; Fang, W.; Hemming, J.; Willfor, S.; Moure, A.; Dominguez, H.; Parajo, J.C. Water-soluble components of Pinus pinaster wood. Bioresources 2013, 8, 2047–2063. [Google Scholar]
- Majak, W.; McDiarmid, R.E.; Ryswyk, A.L.; Broersma, K.; Bonin, S.G. Alkaloid levels in reed canarygrass grown on wet meadows in British Columbia. J. Range Manag. 1979, 32, 322–326. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New cytotoxic alkaloids from the wood of Vepris punctata from the madagascar rainforest. J. Nat. Prod. 2003, 66, 532–534. [Google Scholar] [CrossRef]
- Shafiei, M.; Zilouei, H.; Zamani, A.; Taherzadeh, M.J.; Karimi, K. Enhancement of ethanol production from spruce wood chips by ionic liquid pretreatment. Appl. Energy 2013, 102, 163–169. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Cotta, M.A. Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess. Biosyst. Eng. 2007, 30, 419–427. [Google Scholar] [CrossRef]
- Ohgren, K.; Bura, R.; Lesnicki, G.; Saddler, J.; Zacchi, G. A comparison between simultaneous saccharification and fermentation and separate hydrolysis and fermentation using steam-pretreated corn stover. Process. Biochem. 2007, 42, 834–839. [Google Scholar] [CrossRef]
- Lee, J.M.; Shi, J.; Venditti, R.A.; Jameel, H. Autohydrolysis pretreatment of Coastal Bermuda grass for increased enzyme hydrolysis. Bioresour. Technol. 2009, 100, 6434–6441. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Chemical characterization and liberation of pentose sugars from brewer’s spent grain. J. Chem. Technol. Biotechnol. 2006, 81, 268–274. [Google Scholar] [CrossRef]
- Yang, L.; Cao, J.; Jin, Y.; Chang, H.; Jameel, H.; Phillips, R.; Li, Z. Effects of sodium carbonate pretreatment on the chemical compositions and enzymatic saccharification of rice straw. Bioresour. Technol. 2012, 124, 283–291. [Google Scholar] [CrossRef]
- Kallioinen, A.; Hakola, M.; Riekkola, T.; Repo, T.; Leskelä, M.; von Weymarn, N.; Siika-aho, M. A novel alkaline oxidation pretreatment for spruce, birch and sugar cane bagasse. Bioresour. Technol. 2013, 140, 414–420. [Google Scholar] [CrossRef]
- De Carvalho, D.M.; Perez, A.; Garcia, J.C.; Colodette, J.L.; Lopez, F.; Diaz, M.J. Ethanol-soda pulping of sugarcane bagasse and straw. Cellul. Chem. Technol. 2014, 48, 355–364. [Google Scholar]
- Sassner, P.; Galbe, M.; Zacchi, G. Bioethanol production based on simultaneous saccharification and fermentation of steam-pretreated Salix at high dry-matter content. Enzyme Microb. Technol. 2006, 39, 756–762. [Google Scholar] [CrossRef]
- Yan, Q.; Modigell, M. Mechanical pretreatment of lignocellulosic biomass using a screw press as an essential step in the biofuel production. Chem. Eng. Trans. 2012, 29, 601–606. [Google Scholar]
- Hu, R.; Lin, L.; Liu, T.; Liu, S. Dilute sulfuric acid hydrolysis of sugar maple wood extract at atmospheric pressure. Bioresour. Technol. 2010, 101, 3586–3594. [Google Scholar]
- Sun, Y.; Cheng, J.J. Dilute acid pretreatment of rye straw and bermudagrass for ethanol production. Bioresour. Technol. 2005, 96, 1599–1606. [Google Scholar] [CrossRef]
- Silveira, F.Q.P.; Ximenes, F.A.; Cacais, A.O.G.; Milagres, A.M.F.; Medeiros, C.L.; Puls, J.; Filho, E.X.F. Hydrolysis of xylans by enzyme systems from solid cultures of Trichoderma harzianum strains. Braz. J. Med. Biol. Res. 1999, 32, 947–952. [Google Scholar]
- Lee, J.W.; Gwak, K.S.; Park, J.Y.; Park, M.J.; Choi, D.H.; Kwon, M.; Choi, I.-G. Biological pretreatment of softwood Pinus densiflora by three white rot fungi. J. Microbiol. 2007, 45, 485–491. [Google Scholar]
- Miyafuji, H.; Danner, H.; Neureiter, M.; Thomasser, C.; Bvochora, J.; Szolar, O.; Braun, R. Detoxification of wood hydrolysates with wood charcoal for increasing the fermentability of hydrolysates. Enzyme Microb. Technol. 2003, 32, 396–400. [Google Scholar] [CrossRef]
- Simon, M.; Brostaux, Y.; Vanderghem, C.; Jourez, B.; Paquot, M.; Richel, A. Optimization of a formic/acetic acid delignification treatment on beech wood and its influence on the structural characteristics of the extracted lignins. Chem. Technol. Biotechnol. 2013, 89. [Google Scholar] [CrossRef]
- Lee, S.H.; Doherty, T.V.; Linhardt, R.J.; Dordick, J.S. Ionic liquid-mediated selective extraction of lignin from wood leading to enhanced enzymatic cellulose hydrolysis. Biotechnol. Bioeng. 2009, 102, 1368–1376. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.I. A review of lignocellulose bioconversion using enzymatic hydrolysis and synergistic cooperation between enzymes—Factors affecting enzymes, conversion and synergy. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; de Luise, V.; Licursi, D.; di Nasso, N.N. Levulinic acid production from waste biomass. BioResources 2012, 7, 1824–1834. [Google Scholar]
- Sun, N.; Wang, Y.; Li, Y.-T.; Huang, J.-C.; Chen, F. Sugar-based growth, astaxanthin accumulation and carotenogenic transcription of heterotrophic Chlorella zofingiensis (Chlorophyta). Process. Biochem. 2008, 43, 1288–1292. [Google Scholar] [CrossRef]
- Kim, D.G.; Hur, S.B. Growth and fatty acid composition of three heterotrophic Chlorella species. Algae 2013, 28, 101–109. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Cordero, B.F.; Obraztsova, I.; Couso, I.; Leon, R.; Vargas, M.A.; Rodriguez, H. Enhancement of lutein production in Chlorella sorokiniana (Chorophyta) by improvement of culture conditions and random mutagenesis. Mar. Drugs 2011, 9, 1607–1624. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Fu, R.; Pei, G. A study on lipid production of the mixotrophic microalgae Phaeodactylum tricornutum on various carbon sources. Afr. J. Microbiol. Res. 2012, 6, 1041–1047. [Google Scholar]
- Hawkins, R.L. Utilization of xylose for growth by the eukaryotic alga, Chlorella. Curr. Microbiol. 1999, 38, 360–363. [Google Scholar] [CrossRef]
- Gim, G.H.; Kim, J.K.; Kim, H.S.; Kathiravan, M.N.; Yang, H.; Jeong, S.H.; Kim, S.W. Comparison of biomass production and total lipid content of freshwater green microalgae cultivated under various culture conditions. Bioprocess. Biosyst. Eng. 2013. [Google Scholar] [CrossRef]
- Jeon, Y.-C.; Cho, C.-W.; Yun, Y.-S. Combined effects of light intensity and acetate concentration on the growth of unicellular microalga Haematococcus pluvialis. Enzyme Microb. Technol. 2006, 39, 490–495. [Google Scholar] [CrossRef]
- Orosa, M.; Franqueira, D.; Cid, A.; Abalde, J. Carotenoid accumulation in Haematococcus pluvialis in mixotrophic growth. Biotechnol. Lett. 2001, 23, 373–378. [Google Scholar] [CrossRef]
- Zhang, X.W.; Chen, F.; Johns, M.R. Kinetic models for heterotrophic growth of Chlamydomonas reinhardtii in batch and fed-batch cultures. Proc. Biochem. 1999, 35, 385–389. [Google Scholar] [CrossRef]
- Fischer, B.B.; Wiesendanger, M.; Eggen, R.I.L. Growth condition-dependent sensitivity, photodamage and stress response of Chlamydomonas reinhardtii exposed to high light conditions. Plant. Cell. Physiol. 2006, 47, 1135–1145. [Google Scholar] [CrossRef]
- Choi, W.Y.; Oh, S.H.; Seo, Y.C.; Kim, G.B.; Kang, D.H.; Lee, S.Y.; Jung, K.H.; Cho, J.S.; Ahn, J.H.; Choi, G.P.; et al. Effects of methanol on cell growth and lipid production from mixotrophic cultivation of Chlorella sp. Biotechnol. Bioprocess. Eng. 2011, 16, 946–955. [Google Scholar] [CrossRef]
- Theodoridou, A.; Dornemann, D.; Kotzabasis, K. Light-dependent induction of strongly increased microalgal growth by methanol. Biochim. Biophys. Acta 2002, 1573, 189–198. [Google Scholar]
- Ferrari, M.D.; Neirotti, E.; Albornoz, C.; Saucedo, E. Ethanol production from eucalyptus wood hemicellulose hydrolysate by Pichia stipitis. Biotechnol. Bioeng. 1992, 40, 753–759. [Google Scholar]
- Cruz-Rus, E.; Amaya, I.; Sanchez-Sevilla, J.F.; Botella, M.A.; Valpuesta, V. Regulation of l-ascorbic acid content in strawberry fruits. J. Exp. Bot. 2011, 1–11. [Google Scholar] [CrossRef]
- Helsper, J.P.; Kagan, L.; Hilby, C.L.; Maynard, T.M.; Loewus, F.A. l-Ascorbic acid biosynthesis in Ochromonas Danica. Plant. Physiol. 1982, 69, 465–468. [Google Scholar] [CrossRef]
- Shigeoka, S.; Nakano, Y.; Kitaoka, S. The biosynthetic pathway of l-ascorbic acid in Euglena gracilis Z. J. Nutr. Sci. Vitaminol. (Tokyo) 1979, 25, 299–307. [Google Scholar] [CrossRef]
- Delgenes, J.P.; Moletta, R.; Navarro, J.M. Effects of lignocellulose degradation products on ethanol fermentations of glucose and xylose by Saccharomyces cerevisiae, Zymomonas mobilis, Pichia stipitis, and Candida shehatae. Enzyme Microb. Technol. 1996, 19, 220–225. [Google Scholar] [CrossRef]
- Pinto, G.; Pollio, A.; Previtera, L.; Temussi, F. Biodegradation of phenols by microalgae. Biotechnol. Lett. 2002, 24, 2047–2051. [Google Scholar] [CrossRef]
- Bajguz, A.; Czerpak, R.; Piotrowska, A.; Polecka, M. Effect of isomers of hydroxybenzoic acid on the growth and metabolism of Chlorella vulgaris Beijerinck (Chlorophyceae). Acta Soc. Bot. Pol. 2001, 70, 253–259. [Google Scholar]
- Larson, L.J. Effect of phenolic acids on growth of Chlorella pyrenoidosa. Hydrobiologia 1989, 183, 217–222. [Google Scholar] [CrossRef]
- Kamaya, Y.; Tsuboi, S.; Takada, T.; Suzuki, K. Growth stimulation and inhibition effects of 4-hydroxybenzoic acid and some related compounds on the freshwater green alga Pseudokirchneriella subcapitata. Arch. Environ. Contam. Toxicol. 2006, 51, 537–541. [Google Scholar] [CrossRef]
- Lika, K.; Papadakis, I.A. Modeling the biodegradation of phenolic compounds by microalgae. J. Sea Res. 2009, 62, 135–146. [Google Scholar]
- Nichols, N.N.; Sharma, L.N.; Mowery, R.A.; Chambliss, C.K.; van Walsum, G.P.; Dien, B.S.; Iten, L.B. Fungal metabolism of fermentation inhibitors present in corn stover dilute acid hydrolysate. Enzyme Microb. Technol. 2008, 42, 624–630. [Google Scholar] [CrossRef]
- Jonsson, L.J.; Palmqvist, E.; Nilvebrant, N.O.; Hahn-Hagerdal, B. Detoxification of wood hydrolysates with laccase and peroxidase from the white-rot fungus Trametes versicolor. Appl. Microbiol. Biotechnol. 1998, 49, 691–697. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Ferulic and p-coumaric acids extraction by alkaline hydrolysis of brewer’s spent grain. Ind. Crops Prod. 2007, 25, 231–237. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Acid-based hydrolysis processes for ethanol from lignocellulosic materials: A review. Bioresources 2007, 3, 472–499. [Google Scholar]
- Taherzadeh, M.J.; Niklasson, C.; Liden, G. Conversion of dilute-acid hydrolyzates of spruce and birch to ethanol by fed-batch fermentation. Bioresour. Technol. 1999, 69, 59–66. [Google Scholar] [CrossRef]
- Yu, S.; Forsberg, A.; Kral, K.; Pedersen, M. Furfural and hydroxymethylfurfural inhibition of growth and photosynthesis in Spirulina. Brit. Phys. J. 1990, 25, 141–148. [Google Scholar]
- Liang, Y.; Zhao, X.; Chi, Z.; Rover, M.; Johnston, P.; Brown, R.; Jarboe, L.; Wen, Z. Utilization of acetic acid-rich pyrolytic bio-oil by microalga Chlamydomonas reinhardtii: Reducing bio-oil toxicity and enhancing algal toxicity tolerance. Bioresour. Technol. 2013, 133, 500–506. [Google Scholar] [CrossRef]
- Beale, S.I. Studies on the biosynthesis and metabolism of δ-aminolevulinic acid in Chlorella. Plant Physiol. 1971, 48, 316–319. [Google Scholar] [CrossRef]
- Owens, T.G.; Riper, D.M.; Falkowski, P.G. Studies of delta-aminolevulinic acid dehydrase from Skeletonema costatum, a marine plankton diatom. Plant Physiol. 1978, 62, 516–521. [Google Scholar]
- Kipe-Nolt, J.A.; Stevens, S.E., Jr. Effect of levulinic acid on pigment biosynthesis in Agmenellum quadruplicatum. J. Bacteriol. 1979, 137, 146–152. [Google Scholar]
- Rencoret, J.; Gutierrez, A.; del Rio, J.C. Lipid and lignin composition of woods from different eucalypt species. Holzforschung 2007, 61, 165–174. [Google Scholar]
- Kamaya, Y.; Kurogi, Y.; Suzuki, K. Acute toxicity of fatty acids to the freshwater green alga Selenastrum capricornutum. Inc. Environ. Toxicol. 2003, 18, 289–294. [Google Scholar] [CrossRef]
- Wu, J.T.; Chiang, Y.R.; Huang, W.Y.; Jane, W.N. Cytotoxic effects of free fatty acids on phytoplankton algae and cyanobacteria. Aquat. Toxicol. 2006, 80, 338–345. [Google Scholar] [CrossRef]
- Ikawa, M.; Mosley, S.P.; Barbero, L.J. Inhibitory effects of terpene alcohols and aldehydes on growth of green alga Chlorella pyrenoidosa. J. Chem. Ecol. 1992, 18, 1755–1760. [Google Scholar] [CrossRef]
- Tanzi, C.D.; Vian, M.A.; Ginies, C.; Elmaataoui, M.; Chemat, F. Terpenes as green solvents for extraction of oil from microalgae. Molecules 2012, 17, 8196–8205. [Google Scholar] [CrossRef]
- Talwar, S.; Jagani, H.V.; Nayak, P.G.; Kumar, N.; Kishore, A.; Bansal, P.; Shenoy, R.R.; Nandakumar, K. Toxicological evaluation of Terminalia paniculata bark extract and its protective effect against CCl4-induced liver injury in rodents. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef]
- Hye, M.A.; Taher, M.A.; Ali, M.Y.; Ali, M.U.; Zaman, S. Isolation of (+)-catechin from Acacia Catechu (Cutch Tree) by a convenient method. J. Sci. Res. 2009, 1, 300–305. [Google Scholar]
- García-Pérez, M.E.; Royer, M.; Herbette, G.; Desjardins, Y.; Pouliot, R.; Stevanovic, T. Picea mariana bark: A new source of trans-resveratrol and other bioactive polyphenols. Food Chem. 2012, 135, 1173–1182. [Google Scholar] [CrossRef]
- Maurya, R.; Ray, A.B.; Duah, F.K.; Slatkin, D.J.; Schiff, P.L., Jr. Constituents of Pterocarpus Marsupium. J. Nat. Prod. 1984, 47, 179–181. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Avila, J.G.; Garcıa, A.M.; Becerra, J.; Flores, C. Antifungal and antibacterial activities of Araucaria araucana (Mol.) K. Koch heartwood lignans. Z. Naturforsch. 2006, 61, 35–43. [Google Scholar]
- Hanson, A.D.; Ditz, K.M.; Singletary, G.W.; Leland, T.J. Gramine accumulation in leaves of barley grown under high-temperature stress. Plant. Physiol. 1983, 71, 896–904. [Google Scholar] [CrossRef]
- Ahn, C.; Zeng, X.; Obendorf, S.K. Analysis of dye extracted from Phellodendron bark and its identification in archaeological textiles. Text. Res. J. 2012, 82, 1645–1658. [Google Scholar] [CrossRef]
- Mathes, H.; Schreiber, E. Ber dert pharm ges. 1914, 24, 385.
- Cuca, S.L.E.; Martínez, V.J.C.; Monache, F.D. Alcaloides presentes en Hortia colombiana. Rev. Colomb. Quím. 1998, 27, 23–29. (In Spanish) [Google Scholar]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar] [CrossRef]
- He, F.; Pan, Q.H.; Shi, Y.; Duan, C.Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef]
- Okuda, T.; Ito, H. Tannins of constant structure in medicinal and food plants—Hydrolyzable tannins and polyphenols related to tannins. Molecules 2011, 16, 2191–2217. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Vieira, M.C.; Lelis, R.C.C.; da Silva, B.C.; Oliveira, G.L. Tannin extraction from the bark of Pinus oocarpa var. oocarpa with sodium carbonate and sodium bisulfite. Florest. Ambient. 2011, 18, 1–8. [Google Scholar] [CrossRef]
- Janceva, S.; Dizhbite, T.; Telisheva, G.; Spulle, U.; Klavinsh, L.; Dzenis, M. Tannins of deciduous trees bark as a potential source for obtaining ecologically safe wood adhesives. Environ. Technol. Res. Proceedings of the 7th International Scientific and Practical Conference 2011, 1, 265–270. [Google Scholar]
- Chiarini, A.; Micucci, M.; Malaguti, M.; Budriesi, R.; Ioan, P.; Lenzi, M.; Fimognari, C.; Toschi, T.G.; Comandini, P.; Hrelia, S. Sweet Chestnut (Castanea sativa Mill.) bark extract: Cardiovascular activity and myocyte protection against oxidative damage. Oxid. Med. Cell. Longev. 2013, 2013. [Google Scholar] [CrossRef]
- Zhao, G.; Watson, J.; Crowder, C.; Stevens, S.E., Jr. Changes in biological production of the cyanobacterium, Nostoc sp. strain MAC, under subinhibitory concentrations of tannic acid and related compounds. J. Appl. Phycol. 1998, 10, 1–7. [Google Scholar] [CrossRef]
- Flores, E.; Frıas, J.E.; Rubio, L.R.; Herrero, A. Photosynthetic nitrate assimilation in cyanobacteria. Photosynt. Res. 2005, 83, 117–133. [Google Scholar] [CrossRef]
- Moat, A.G.; Foster, J.W.; Spector, M.P. Biosynthesis and metabolism of amino acids. Microb. Physiol. 2002, 15, 503–544. [Google Scholar]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Myriophyllum Spicatum-released allelopathic polyphenols inhibiting growth of blue-green algae Microcystis Aeruginosa. Water Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- McGinn, P.J.; Morel, F.M.M. Expression and inhibition of the carboxylating and decarboxylating enzymes in the photosynthetic C4 pathway of marine diatoms. Plant Physiol. 2008, 146, 300–309. [Google Scholar] [CrossRef]
- Jiang, D.; Huang, L.F.; Lin, Y.Q.; Nie, L.L.; Lv, S.L.; Kuang, T.Y.; Li, Y.X. Inhibitory effect of Salicornia europaea on the marine alga Skeletonema costatum. Sci. China Life Sci. 2012, 55, 551–558. [Google Scholar]
- Wang, J.; Zhu, J.; Liu, S.; Liu, B.; Gao, Y.; Wu, Z. Generation of reactive oxygen species in cyanobacteria and green algae induced by allelochemicals of submerged macrophytes. Chemosphere 2011, 85, 977–982. [Google Scholar] [CrossRef]
- Mizuno, C.S.; Schrader, K.K.; Rimando, A.M. Algicidal activity of stilbene analogues. J. Agric. Food Chem. 2008, 56, 9140–9145. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Schrader, K.K.; Mamonov, L.K.; Sitpaeva, G.T.; Kustova, T.S.; Dunbar, C.; Wedge, D.E. Isolation and identification of antifungal and antialgal alkaloids from Haplophyllum sieversii. J. Agric. Food Chem. 2005, 53, 7741–7748. [Google Scholar] [CrossRef]
- Cheeke, P.R. Endogenous toxins and mycotoxins in forage grasses and their effects on livestock. J. Anim. Sci. 1995, 73, 909–918. [Google Scholar]
- Hong, Y.; Hu, H.Y.; Sakoda, A.; Sagehashi, M. Effects of allelochemical gramine on metabolic activity and ultrastructure of cyanobacterium Microcystis aeruginosa. World Acad. Sci. Eng. Technol. 2010, 47, 826–830. [Google Scholar]
- Bravo, H.R.; Iglesias, M.J.; Copaja, S.V.; Argandoña, V.H. Phytotoxicity of indole alkaloids from cereals. Rev. Latinoam. Quím. 2010, 38, 123–129. [Google Scholar]
- Jancula, D.; Gregorova, J.; Marsalek, B. Algicidal and cyanocidal effects of selected isoquinoline alkaloids. Aquac. Res. 2010, 41, 598–601. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, B.; Dai, W.; Zhang, X. Oxidative damage and antioxidant responses in Microcystis aeruginosa exposed to the allelochemical berberine isolated from golden thread. J. Plant. Physiol. 2011, 168, 639–643. [Google Scholar] [CrossRef]
- Berggren, D.; Bertling, S.; Heijerick, D.; Herting, G.; Koundakjian, P.; Leygraf, C.; Wallinder, I.O. Release of Chromium, Nickel and Iron from Stainless Steel Exposed under Atmospheric Conditions and the Environmental Interaction of these Metals; European Confederation of Iron and Steel Industries: Brussel, Belgium, 2004. [Google Scholar]
- Malá, J.; Cvrčková, H.; Máchová, P.; Dostál, J.; Šíma, P. Heavy metal accumulation by willow clones in short-time hydroponics. J. For. Sci. 2010, 56, 28–34. [Google Scholar]
- Monahan, T.J. Lead inhibition of chlorophycean microalgae. J. Phycol. 1976, 12, 358–362. [Google Scholar]
- Nohomovich, B.; Nguyen, B.T.; Quintanilla, M.; Lee, L.H.; Murray, S.R.; Chu, T.C. Physiological effects of nickel chloride on the freshwater cyanobacterium Synechococcus sp. IU 625. Adv. Biosci. Biotechnol. 2013, 4, 10–14. [Google Scholar] [CrossRef]
- Tukaj, Z.; Bascik-Remisiewicz, A.; Skowronski, T.; Tukaj, C. Cadmium effect on the growth, photosynthesis, ultrastructure and phytochelatin content of green microalga Scenedesmus armatus: A study at low and elevated CO2 concentration. Environ. Exp. Botany 2007, 60, 291–299. [Google Scholar] [CrossRef]
- Ouyang, H.L.; Kong, X.Z.; He, W.; Qin, N.; He, Q.S.; Wang, Y.; Wang, R.; Xu, F.L. Effects of five heavy metals at sub-lethal concentrations on the growth and photosynthesis of Chlorella vulgaris. Chin. Sci. Bull. 2012, 57, 3363–3370. [Google Scholar] [CrossRef]
- Wei, L.; Li, K.; Ma, Y.; Hou, X. Dissolving lignocellulosic biomass in a 1-butyl-3-methylimidazolium chloride—Water mixture. Ind. Crops Prod. 2012, 37, 227–234. [Google Scholar] [CrossRef]
- Dee, S.; Bell, A.T. Effects of reaction conditions on the acid-catalyzed hydrolysis of miscanthus dissolved in an ionic liquid. Green Chem. 2011, 13, 1467–1475. [Google Scholar] [CrossRef]
- Engel, P.; Krull, S.; Seiferheld, B.; Spiess, C.A. Rational approach to optimize cellulase mixtures for hydrolysis of regenerated cellulose containing residual ionic liquid. Bioresour. Technol. 2012, 115, 27–34. [Google Scholar] [CrossRef]
- Latała, A.; Nedzi, M.; Stepnowski, P. Toxicity of imidazolium ionic liquids towards algae. Influence of salinity variations. Green. Chem. 2010, 12, 60–64. [Google Scholar] [CrossRef]
- Li, P.; Miao, X.; Li, R.; Zhong, J. In situ biodiesel production from fast-growing and high oil content Chlorella pyrenoidosa in rice straw hydrolysate. J. Biomed. Biotechnol. 2011, 2011. [Google Scholar] [CrossRef]
- Blifernez-Klassen, O.; Klassen, V.; Doebbe, A.; Kersting, K.; Grimm, P.; Wobbe, L.; Kruse, O. Cellulose degradation and assimilation by the unicellular phototrophic eukaryote Chlamydomonas reinhardtii. Nat. Commun. 2012, 3. [Google Scholar] [CrossRef]
- Suh, I.S.; Lee, S.B. A light distribution model for an internally radiating photobioreactor. Biotechnol. Bioeng. 2003, 82, 180–189. [Google Scholar] [CrossRef]
- Afiukwa, C.A.; Ogbonna, J.C. Effects of mixed substrates on growth and vitamin production by Euglena gracilis. Afr. J. Biotech. 2007, 6, 2612–2615. [Google Scholar]
- Stratton, G.W.; Smith, T.M. Interaction of organic solvents with the green alga Chlorella pyrenoidosa. Bull. Environ. Contam. Toxicol. 1988, 40, 736–742. [Google Scholar] [CrossRef]
- El Jay, A. Effects of organic solvents and solvent-atrazine interactions on two algae, Chlorella vulgaris and Selenastrum capricornutum. Arch. Environ. Contam. Toxicol. 1996, 31, 84–90. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Borde, X.; Guieysse, B.; Delgado, O.; Munoz, R.; Hatti-Kaul, R.; Nugier-Chauvin, C.; Patin, H.; Mattiasson, B. Synergistic relationships in algal–bacterial microcosms for the treatment of aromatic pollutants. Bioresour. Technol. 2003, 86, 293–300. [Google Scholar] [CrossRef]
- Munoz, R.; Guieysse, B. Algal–bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar]
- Collins, L.D.; Daugulis, A.J. Biodegradation of phenol at high initial concentrations in two-phase partitioning batch and fed-batch bioreactors. Biotechnol. Bioeng. 1997, 55, 155–162. [Google Scholar] [CrossRef]
- Semple, K.T.; Cain, R.B. Biodegradation of phenols by the alga Ochromonas Danica. Appl. Environ. Microbiol. 1996, 62, 1265–1273. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Effect of Lignocellulose Related Compounds on Microalgae Growth and Product Biosynthesis: A Review. Energies 2014, 7, 4446-4481. https://doi.org/10.3390/en7074446
Miazek K, Remacle C, Richel A, Goffin D. Effect of Lignocellulose Related Compounds on Microalgae Growth and Product Biosynthesis: A Review. Energies. 2014; 7(7):4446-4481. https://doi.org/10.3390/en7074446
Chicago/Turabian StyleMiazek, Krystian, Claire Remacle, Aurore Richel, and Dorothee Goffin. 2014. "Effect of Lignocellulose Related Compounds on Microalgae Growth and Product Biosynthesis: A Review" Energies 7, no. 7: 4446-4481. https://doi.org/10.3390/en7074446





