Influence of Chemical Blends on Palm Oil Methyl Esters’ Cold Flow Properties and Fuel Characteristics
Abstract
:1. Introduction
2. Biodiesel Fuel Properties
3. Methodology
3.1. Materials
| Property | Ethanol | Butanol | Diethyl ether | POME |
|---|---|---|---|---|
| Chemical Formula | C2H5OH | C4H10O | C4H10O | - |
| Molecular Weight (g/mole) | 46.07 | 74.12 | 74.12 | - |
| Carbon weight% | 52.2 | 64.8 | 64.8 | 76.2 |
| Hydrogen weight% | 13.1 | 13.6 | 13.6 | 12.6 |
| Oxygen weight% | 34.7 | 21.6 | 21.6 | 11.2 |
| Specific Gravity @ 20 °C | 0.790 | 0.8100 | 0.714 | 0.880 * |
| Boiling point, °C | 78 | 116 | 34.6 | - |
| Freezing point, °C | −114.1 | −89.5 | −116 | - |
| Viscosity (cSt) @ 20 °C | 1.52 | 3.64 | 0.34 | 4.61 ** |
| Flash point, °C | 16.6 | 35 | −45 | 135 |
| Auto ignition temperature, °C | 363 | 343 | 160 | - |
| Vapour Density, (Air = 1) | 1.59 | 2.6 | 2.55 | - |
| Heating value (MJ/kg) | 29.7 | 33 | 34 | 38.6 |
3.2. Fatty Acid Composition
3.3. Preparation of POME-Chemicals Blends
| Fuel | POME (vol%) | E (vol%) | BU (vol%) | DE (vol%) |
|---|---|---|---|---|
| B100 | 100 | 0 | 0 | 0 |
| B-E1 | 99 | 1 | 0 | 0 |
| B-E3 | 97 | 3 | 0 | 0 |
| B-E5 | 95 | 5 | 0 | 0 |
| B-BU1 | 99 | 0 | 1 | 0 |
| B-BU3 | 97 | 0 | 3 | 0 |
| B-BU5 | 95 | 0 | 5 | 0 |
| B-DE1 | 99 | 0 | 0 | 1 |
| B-DE3 | 97 | 0 | 0 | 3 |
| B-DE5 | 95 | 0 | 0 | 5 |
3.4. Fuel Properties Measurements
3.4.1. Density Measurement
3.4.2. Kinematic Viscosity Measurement
3.4.3. Acid Value Measurement
3.4.4. Energy Content Measurement
3.4.5. Cloud Point and Pour Point Measurement
3.5. Engine Test
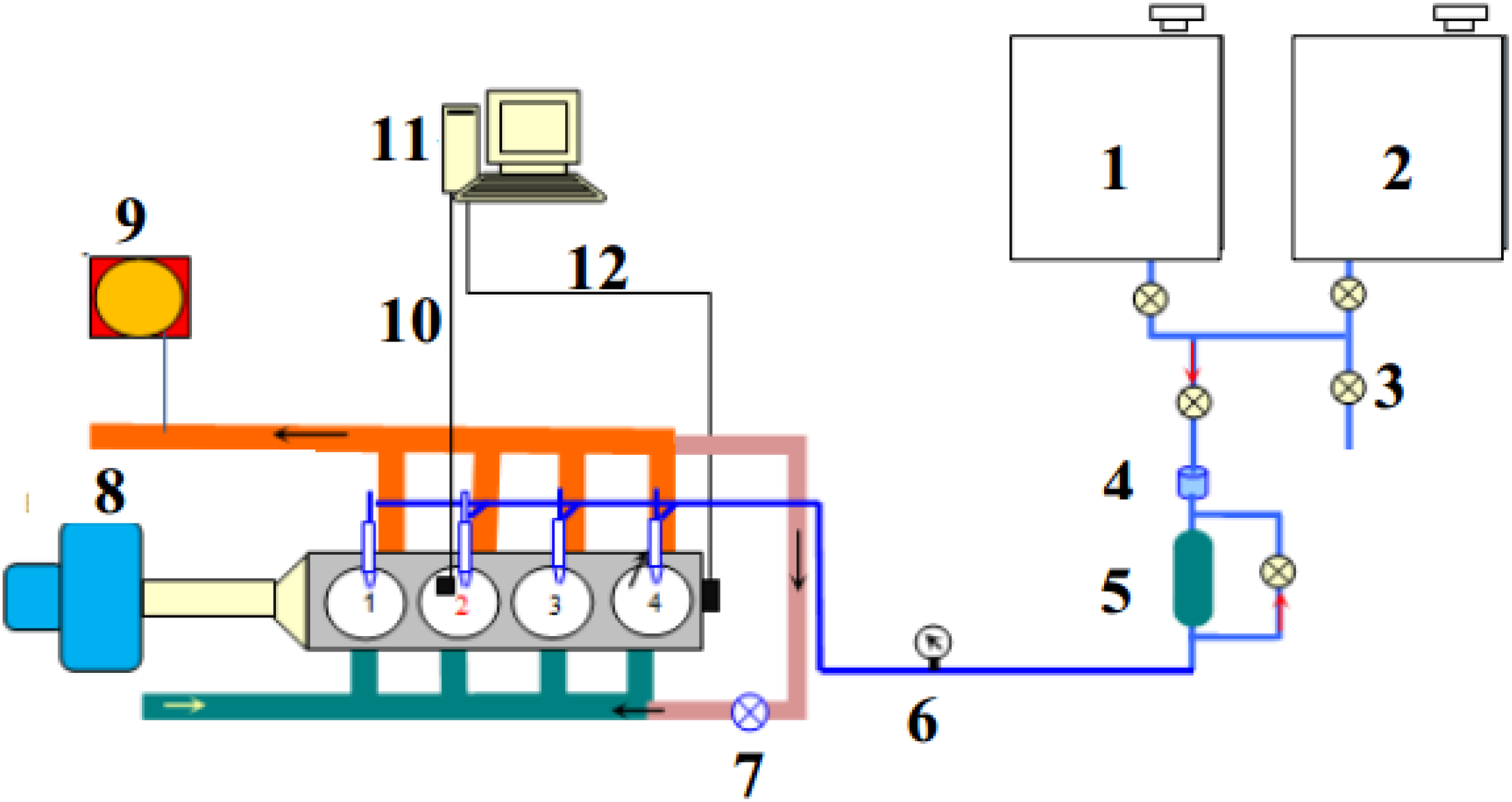
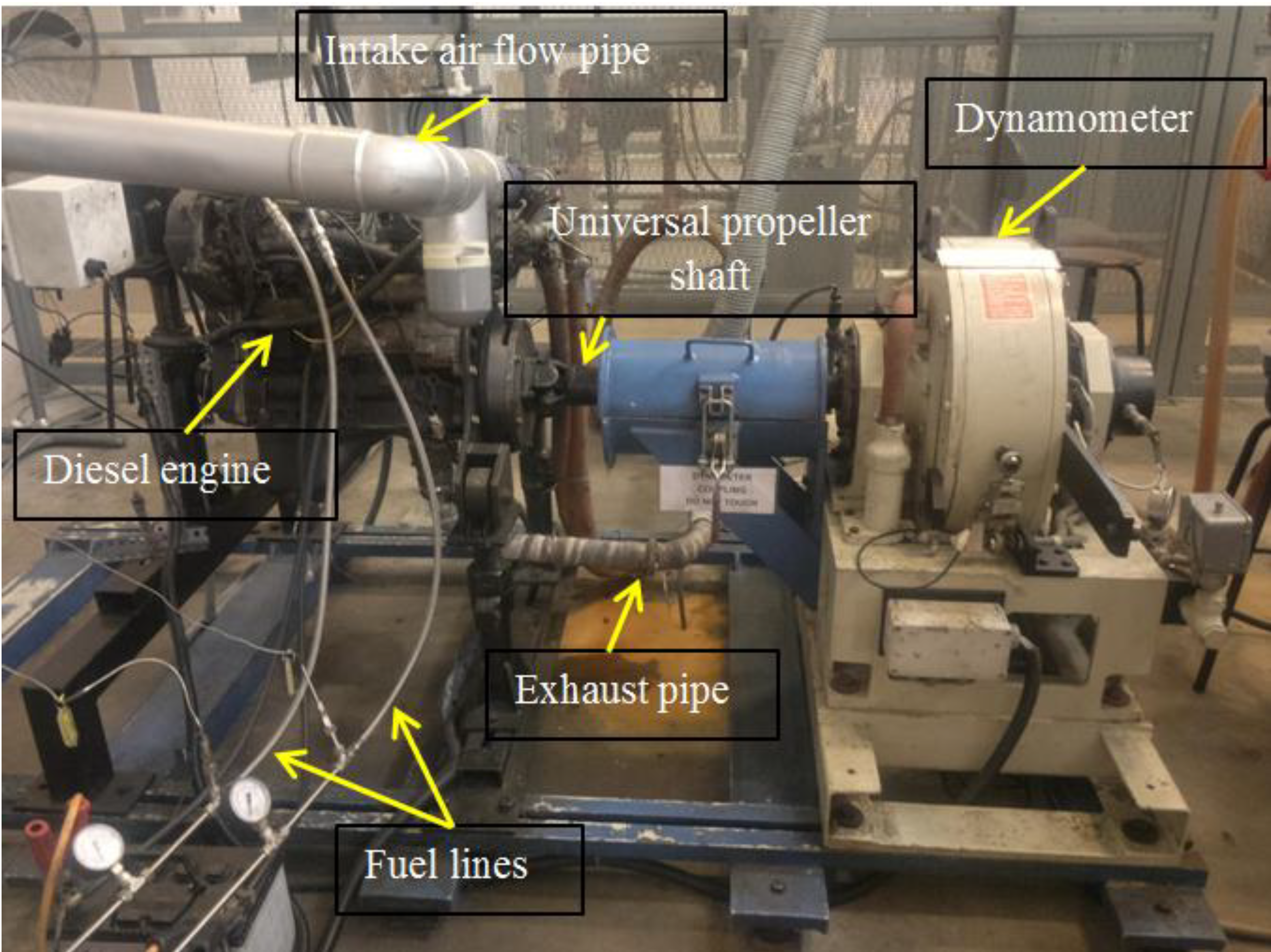
4. Results and Discussion
| No. | Fatty Acid | Structure | Formula | Molecular Mass | POME (%) |
|---|---|---|---|---|---|
| 1 | Lauric | 12:0 | C12H24O2 | 200 | 0.3 |
| 2 | Myristic | 14:0 | C14H28O2 | 228 | 1.0 |
| 3 | Palmitic | 16:0 | C16H32O2 | 256 | 43.3 |
| 4 | Palmitioleic | 16:1 | C16H30O2 | 254 | 0.1 |
| 5 | Margaric | 17:0 | C17H34O2 | 270 | 0.1 |
| 6 | Stearic | 18:0 | C18H36O2 | 284 | 5.4 |
| 7 | Oleic | 18:1 | C18H34O2 | 282 | 49.2 |
| 8 | Arachidic | 20:0 | C20H40O2 | 312 | 0.4 |
| 9 | Eicosenoic | 20:1 | C20H38O2 | 310 | 0.1 |
| Saturation | - | - | - | 50.6 | |
| Unsaturation | - | - | - | 49.4 | |
| Total | - | - | - | 100.0 | |

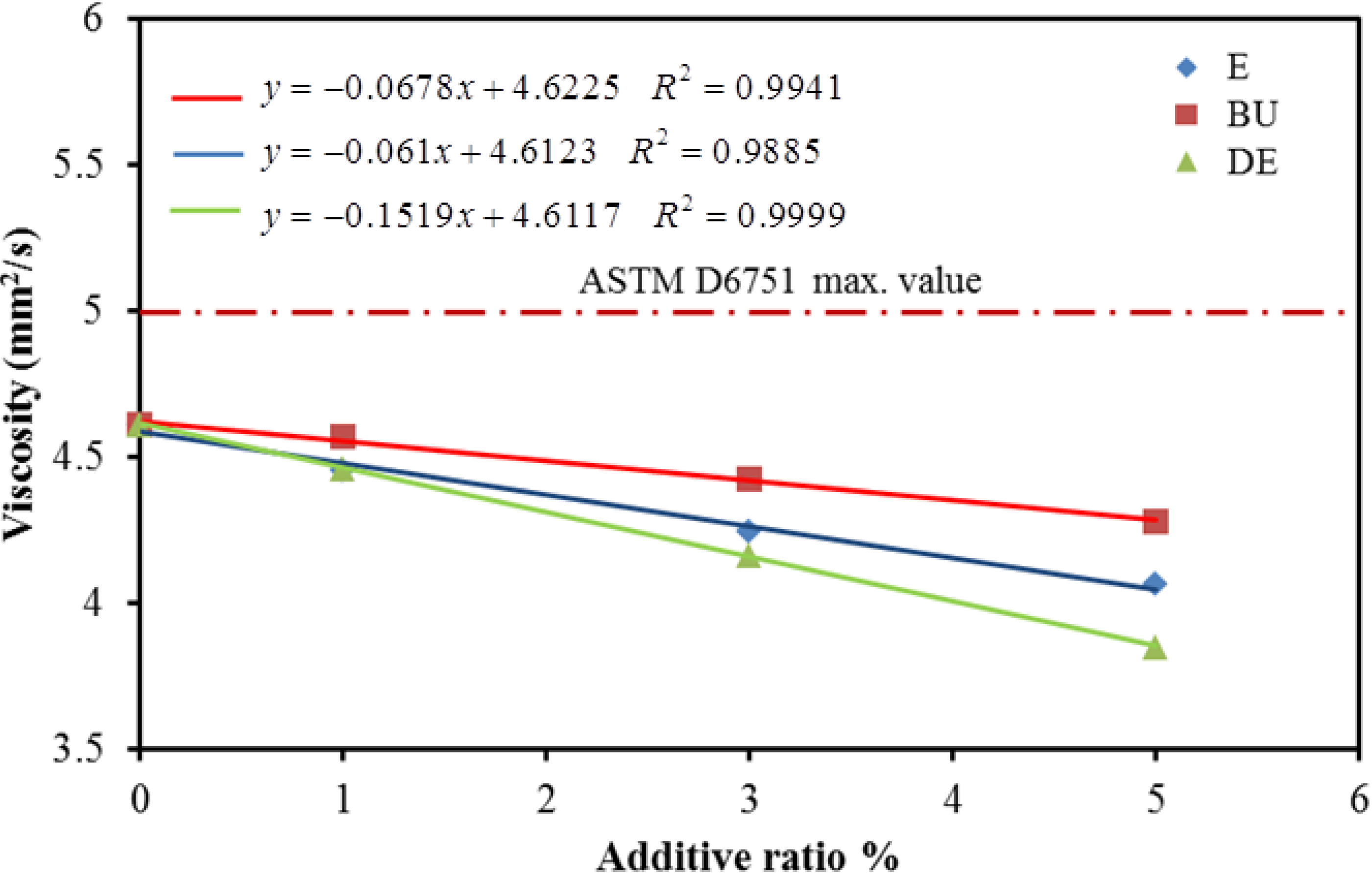

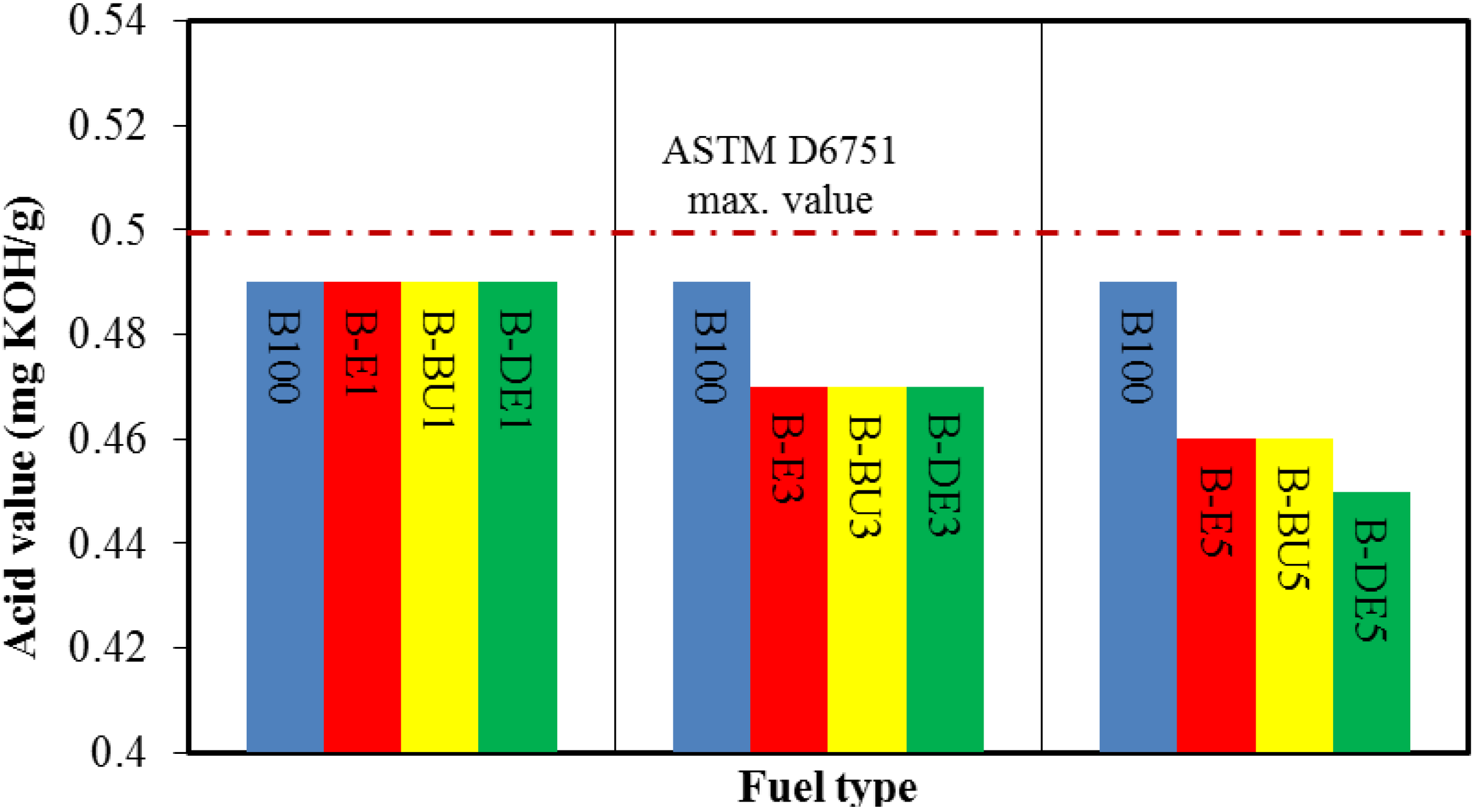
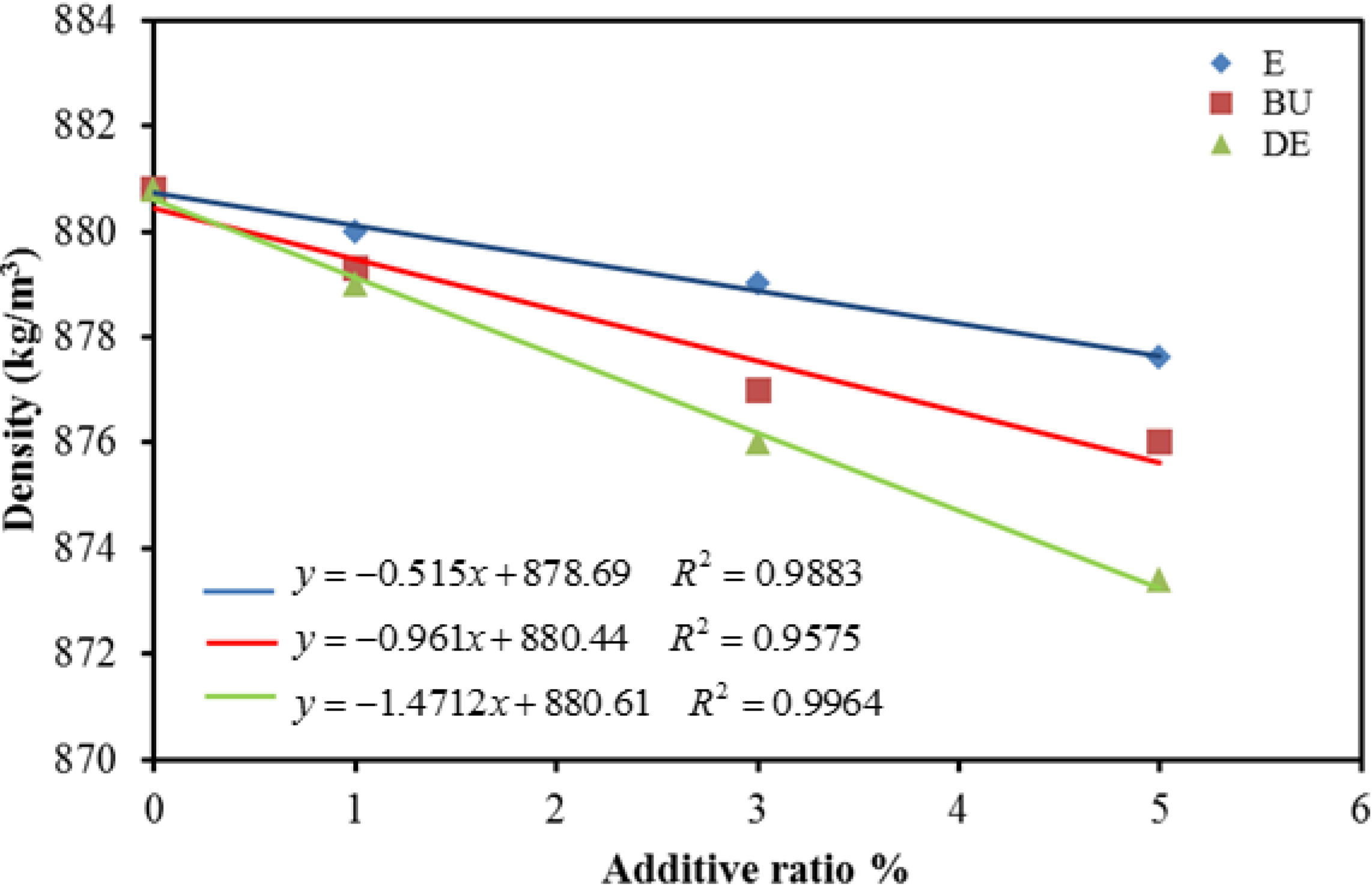
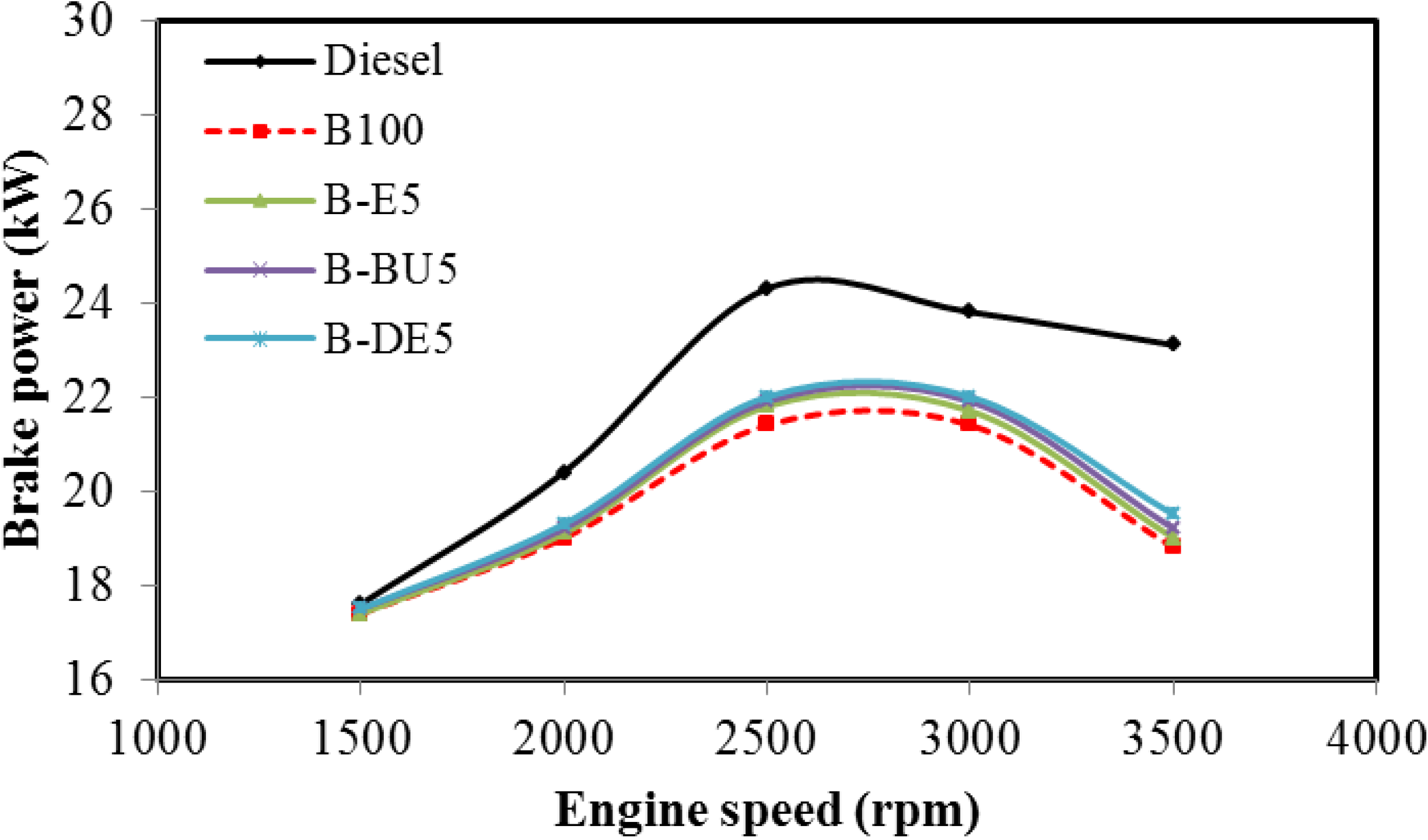
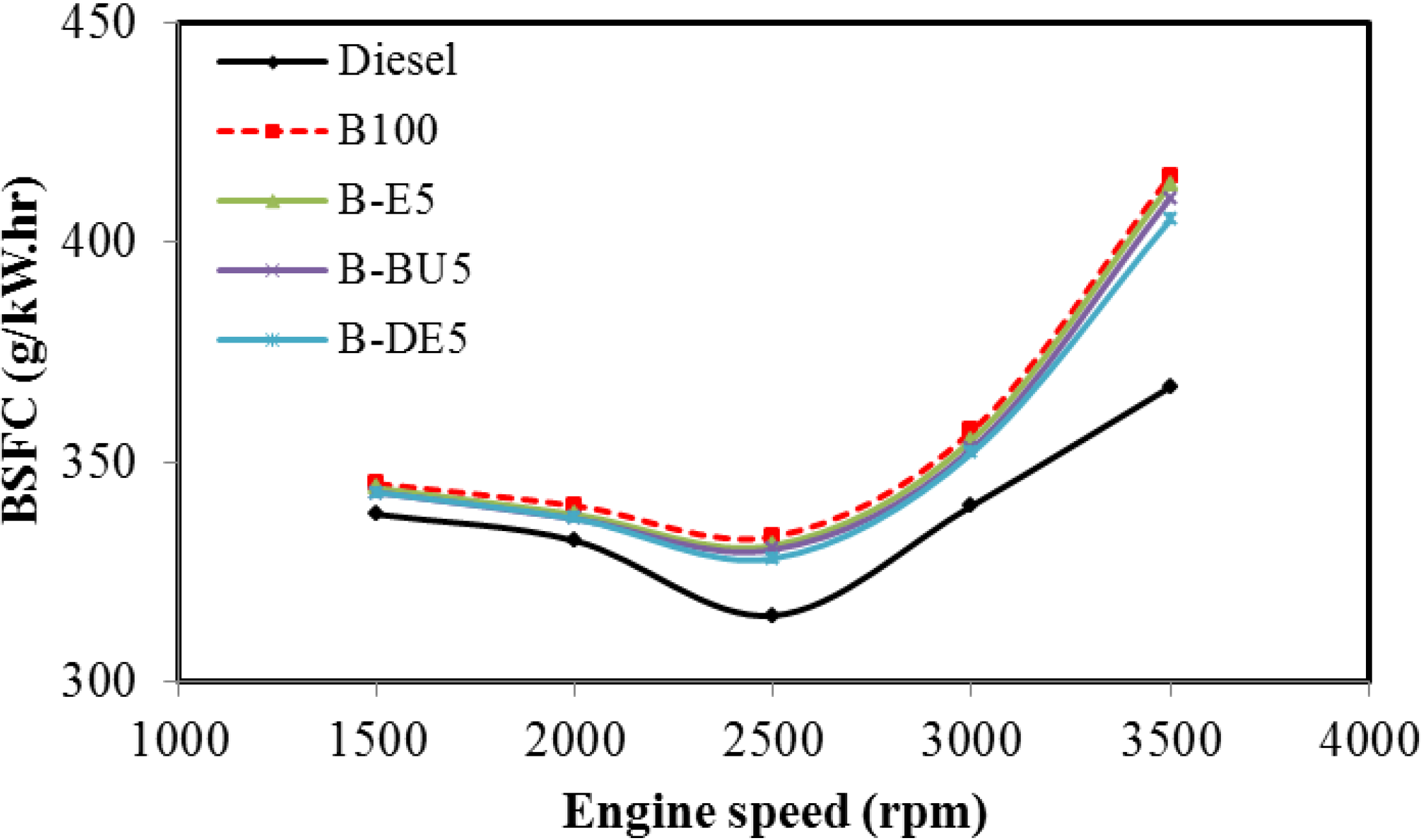
5. Conclusions
Abbreviations
| POME | Palm oil methyl ester |
| FAME | Fatty acid methyl esters |
| CP | Cloud point |
| PP | Pour point |
| DE | Diethyl ether |
| E | Ethanol |
| BU | Butanol |
| B100 | Pure biodiesel |
| B-E | Biodiesel-ethanol blend |
| B-DE | Biodiesel-diethyl ether blend |
| B-BU | Biodiesel-butanol blend |
| AV | Acid value |
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Demirbas, A. Biodiesel: A Realistic Fuel Alternative for Diesel Engines; Springer: London, UK, 2008. [Google Scholar]
- Salamanca, M.; Mondragón, F.; Agudelo, J.R.; Santamaría, A. Influence of palm oil biodiesel on the chemical and morphological characteristics of particulate matter emitted by a diesel engine. Atmos. Environ. 2012, 62, 220–227. [Google Scholar] [CrossRef]
- Ali, Y.; Hanna, M.A.; Cuppett, S.L. Fuel properties of tallow and soybean oil esters. J. Am. Oil Chem. Soc. 1995, 72, 1557–1564. [Google Scholar] [CrossRef]
- Yilmaz, N.; Vigil, F.M.; Burl Donaldson, A.; Darabseh, T. Investigation of CI engine emissions in biodiesel-ethanol-diesel blends as a function of ethanol concentration. Fuel 2014, 115, 790–793. [Google Scholar] [CrossRef]
- Franco, V.; Kousoulidou, M.; Muntean, M.; Ntziachristos, L.; Hausberger, S.; Dilara, P. Road vehicle emission factors development: A review. Atmos. Environ. 2013, 70, 84–97. [Google Scholar] [CrossRef]
- Schröder, O.; Bünger, J.; Munack, A.; Knothe, G.; Krahl, J. Exhaust emissions and mutagenic effects of diesel fuel, biodiesel and biodiesel blends. Fuel 2013, 103, 414–420. [Google Scholar] [CrossRef]
- Randazzo, M.L.; Sodré, J.R. Cold start and fuel consumption of a vehicle fuelled with blends of diesel oil-soybean biodiesel-ethanol. Fuel 2011, 90, 3291–3294. [Google Scholar] [CrossRef]
- Moser, B.R. Impact of fatty ester composition on low temperature properties of biodiesel-petroleum diesel blends. Fuel 2014, 115, 500–506. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R.; Faizal, C.K.M. Review of the effects of additives on biodiesel properties, performance, and emission features. J. Renew. Sustain. Energy 2013, 5, 012701. [Google Scholar] [CrossRef]
- Silitonga, A.S.; Ong, H.C.; Masjuki, H.H.; Mahlia, T.M.I.; Chong, W.T.; Yusaf, T.F. Production of biodiesel from Sterculia foetida and its process optimization. Fuel 2013, 111, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Steiner, S.; Czerwinski, J.; Comte, P.; Popovicheva, O.; Kireeva, E.; Müller, L.; Heeb, N.; Mayer, A.; Fink, A.; Rothen-Rutishauser, B. Comparison of the toxicity of diesel exhaust produced by bio- and fossil diesel combustion in human lung cells in vitro. Atmos. Environ. 2013, 81, 380–388. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R. Improving engine performance and low temperature properties of blended palm biodiesel using additives. A review. Appl. Mech. Mater. 2013, 315, 68–72. [Google Scholar] [CrossRef]
- Vallinayagam, R.; Vedharaj, S.; Yang, W.M.; Saravanan, C.G.; Lee, P.S.; Chua, K.J.E.; Chou, S.K. Emission reduction from a diesel engine fueled by pine oil biofuel using SCR and catalytic converter. Atmos. Environ. 2013, 80, 190–197. [Google Scholar] [CrossRef]
- Fang, Q.; Fang, J.; Zhuang, J.; Huang, Z. Effects of ethanol–diesel–biodiesel blends on combustion and emissions in premixed low temperature combustion. Appl. Therm. Eng. 2013, 54, 541–548. [Google Scholar] [CrossRef]
- Shadidi, B.; Yusaf, T.; Alizadeh, H.H.A.; Ghobadian, B. Experimental investigation of the tractor engine performance using diesohol fuel. Appl. Energy 2013, 114. [Google Scholar] [CrossRef]
- Rahimi, H.; Ghobadian, B.; Yusaf, T.; Najafi, G.; Khatamifar, M. Diesterol: An environment-friendly IC engine fuel. Renew. Energy 2009, 34, 335–342. [Google Scholar] [CrossRef]
- Fernando, S.; Hanna, M. Development of a novel biofuel blend using ethanol-biodiesel-diesel microemulsions: EB-diesel. Energy Fuels 2004, 18, 1695–1703. [Google Scholar] [CrossRef]
- Satge de Caro, P.; Mouloungui, Z.; Vaitilingom, G.; Berge, J.C. Interest of combining an additive with diesel-ethanol blends for use in diesel engines. Fuel 2001, 80, 565–574. [Google Scholar] [CrossRef]
- Li, D.; Zhen, H.; Lv, X.; Zhang, W.; Yang, J. Physico-chemical properties of ethanol-diesel blend fuel and its effect on performance and emissions of diesel engines. Renew. Energy 2005, 30, 967–976. [Google Scholar] [CrossRef]
- Joshi, H.; Moser, B.R.; Toler, J.; Smith, W.F.; Walker, T. Effects of blending alcohols with poultry fat methyl esters on cold flow properties. Renew. Energy 2010, 35, 2207–2210. [Google Scholar] [CrossRef]
- Bhale, P.V.; Deshpande, N.V.; Thombre, B.S. Improving the low temperature properties of biodiesel fuel. Renew. Energy 2009, 34, 794–800. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Effects of blending ethanol with palm oil methyl esters on low temperature flow properties and fuel characteristics. Int. J. Adv. Sci. Technol. 2013, 59, 85–96. [Google Scholar] [CrossRef]
- Qi, D.H.; Chen, H.; Geng, L.M.; Bian, Y.Z. Effect of diethyl ether and ethanol additives on the combustion and emission characteristics of biodiesel-diesel blended fuel engine. Renew. Energy 2011, 36, 1252–1258. [Google Scholar] [CrossRef]
- Randazzo, M.L.; Sodré, J.R. Exhaust emissions from a diesel powered vehicle fuelled by soybean biodiesel blends (B3–B20) with ethanol as an additive (B20E2–B20E5). Fuel 2011, 90, 98–103. [Google Scholar] [CrossRef]
- Aydin, H.; İlkılıç, C. Effect of ethanol blending with biodiesel on engine performance and exhaust emissions in a CI engine. Appl. Therm. Eng. 2010, 30, 1199–1204. [Google Scholar] [CrossRef]
- Zhu, L.; Cheung, C.S.; Zhang, W.G.; Huang, Z. Emissions characteristics of a diesel engine operating on biodiesel and biodiesel blended with ethanol and methanol. Sci. Total Environ. 2010, 408, 914–921. [Google Scholar] [CrossRef]
- Miller Jothi, N.K.; Nagarajan, G.; Renganarayanan, S. LPG fueled diesel engine using diethyl ether with exhaust gas recirculation. Int. J. Therm. Sci. 2008, 47, 450–457. [Google Scholar] [CrossRef]
- Subramanian, K.A.; Ramesh, A. Use of Diethyl ether along with Water Diesel Emulsion in a DI diesel Engine. In Proceedings of the SAE Powertrain & Fluid Systems Conference & Exhibition, San Diego, CA, USA, 21–24 October 2002. [CrossRef]
- Demirbas, A. Progress and recent trends in biodiesel fuels. Energy Convers. Manag. 2009, 50, 14–34. [Google Scholar] [CrossRef]
- Lam, M.K.; Tan, K.T.; Lee, K.T.; Mohamed, A.R. Malaysian palm oil: Surviving the food versus fuel dispute for a sustainable future. Renew. Sustain. Energy Rev. 2009, 13, 1456–1464. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E.; Natarajan, M. Review of biodiesel composition, properties, and specifications. Renew. Sustain. Energy Rev. 2012, 16, 143–169. [Google Scholar] [CrossRef]
- Pinto, A.C.; Guarieiro, L.L.N.; Rezende, M.J.C.; Ribeiro, N.M.; Torres, E.A.; Lopes, W.A.; Pereira, P.A.P.; de Andrade, J.B.J. Biodiesel: An overview. Chem. Soc. 2005, 16, 1313–1330. [Google Scholar]
- Krahl, J.; Munack, A.; Schroder, O.; Stein, H.; Bunger, J. Influence of Biodiesel and Different Designed Diesel Fuels on the Exhaust Gas Emissions and Health Effects; Technical Paper 2003-01-31 2003; SAE International: Warrendale, PA, USA, 2003. [Google Scholar] [CrossRef]
- Knothe, G. Determining the blend level of mixtures of biodiesel with conventional diesel fuel by fiber-optic near-infrared spectroscopy and 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc. 2001, 78, 1025–1028. [Google Scholar] [CrossRef]
- Jenkins, C.; Mastbergen, D.S.R. Measurement of the percentage of biodiesel in blends with a commercial dielectric fuel sensor. In Proceedings of the ASME Internal Combustion Engine Division 2006 Fall Technical Conference, Sacramento, CA, USA, 5–8 November 2006; pp. 109–115.
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Atabani, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar]
- Basha, S.A.; Raja Gopal, K. A review of the effects of catalyst and additive on biodiesel production, performance, combustion and emission characteristics. Renew. Sustain. Energy Rev. 2012, 16, 711–717. [Google Scholar] [CrossRef]
- Van Gerpen, J.H.; Peterson, C.L.; Goering, C.E. Biodiesel: An alternative fuel for compression ignition engines. In Proceedings of the Agricultural Equipment Technology Conference, Louisville, KY, USA, 11–14 February 2007; pp. 1–22.
- Karmakar, A.; Karmakar, S.; Mukherjee, S. Properties of various plants and animals feedstocks for biodiesel production. Bioresour. Technol. 2010, 101, 7201–7210. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Naik, M.K.; Pradhan, S.; Naik, S.N.; Das, L.M. High quality biodiesel from Thevetia peruviana Juss: Physico-chemical, emission and fuel performance characteristics. J. Biobased Mater. Bioenergy 2012, 6, 269–275. [Google Scholar] [CrossRef]
- Bahadur, N.P.; Boocock, D.G.B.; Konar, S.K. Liquid hydrocarbons from catalytic pyrolysis of sewage sludge lipid and canola oil: Evaluation of fuel properties. Energy Fuels 1995, 9, 248–256. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Ejim, C.E.; Fleck, B.A.; Amirfazli, A. Analytical study for atomization of biodiesels and their blends in a typical injector: Surface tension and viscosity effects. Fuel 2007, 86, 1534–1544. [Google Scholar] [CrossRef]
- Satyanarayana, M.; Muraleedharan, C. Biodiesel production from vegetable oils: A comparative optimization study. J. Biobased Mater. Bioenergy 2009, 3, 335–341. [Google Scholar] [CrossRef]
- Knothe, G.; Steidley, K.R. Kinematic viscosity of biodiesel fuel components and related compounds, influence of compound structure and comparison to petrodiesel fuel components. Fuel 2005, 84, 1059–1065. [Google Scholar] [CrossRef]
- Agarwal, A.K. Biofuels (alcohols and biodiesel) applications as fuels for internal combustion engines. Prog. Energy Combust. Sci. 2007, 33, 233–271. [Google Scholar] [CrossRef]
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Robbins, C.; Hoekman, S.K.; Ceniceros, E.; Natarajan, M. Effects of Biodiesel Fuels upon Criteria Emissions; SAE Technical Paper 2011-01-1943; SAE International: Warrendale, PA, USA, 2011. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C.; Ceniceros, E. Investigation of Biodiesel Chemistry, Carbon Footprint and Regional Fuel Quality; Clean Technologies and Renewable Energy Center: Reno, NV, USA, 2011. [Google Scholar]
- Rawat, D.S.; Joshi, G.; Lamba, B.Y.; Tiwari, A.K.; Mallick, S. Impact of additives on storage stability of Karanja (Pongamia Pinnata) biodiesel blends with conventional diesel sold at retail outlets. Fuel 2014, 120, 30–37. [Google Scholar] [CrossRef]
- Smith, P.C.; Ngothai, Y.; Nguyen, Q.D.; O’Neill, B.K. Improving the low-temperature properties of biodiesel: Methods and consequences. Renew. Energy 2010, 35, 1145–1151. [Google Scholar] [CrossRef]
- Chandler, J.E.; Horneck, F.G.; Brown, G.I. The Effect of Cold Flow Additives on Low Temperature Operability Of Diesel Fuels; SAE Technical Paper 922186; SAE International: Warrendale, PA, USA, 1992. [Google Scholar] [CrossRef]
- Dunn, R.O.; Shockley, M.W.; Bagby, M.O. Improving the low-temperature properties of alternative diesel fuels: Vegetable oil-derived methyl esters. J. Am. Oil Chem. Soc. 1996, 73, 1719–1728. [Google Scholar] [CrossRef]
- Knothe, G.; Krahl, J.; van Gerpen, J. The Biodiesel Handbook; AOCS Press: Champaign, IL, USA, 2004. [Google Scholar]
- Merck Material Safety Data Sheets (MSDS). Available online: http://www.merckmillipore.my/chemicals/msds-search/c_r_ab.s1O_d4AAAEl7otx3CaA (accessed on 22 January 2014).
- Swaminathan, C.; Sarangan, J. Performance and exhaust emission characteristics of a CI engine fueled with biodiesel (fish oil) with DEE as additive. Biomass Bioenergy 2012, 39, 168–174. [Google Scholar] [CrossRef]
- ASTM Biodiesel Specifications. Available online: http://www.afdc.energy.gov/fuels/biodiesel_specifications.html (accessed on 7 July 2014).
- Akcay, H.T.; Karslioglu, S. Optimization process for biodiesel production from some Turkish vegetable oils and determination fuel properties. Energy Educ. Sci. Technol. 2012, 28, 861–868. [Google Scholar]
- Filemon, A. Biofuels from Plant Oils; ASEAN Foundation: Jakarta, Indonesia, 2010; p. 47. [Google Scholar]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of biodiesel obtained from cotton-seed oil. Fuel Process. Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]
- European Committee for Standardization. European Standard; EN 14214; CEN: Brussels, Belgium, 2008. [Google Scholar]
- Buyukkaya, E. Effects of biodiesel on a DI diesel engine performance, emission and combustion characteristics. Fuel 2010, 89, 3099–3105. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ali, O.M.; Yusaf, T.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Influence of Chemical Blends on Palm Oil Methyl Esters’ Cold Flow Properties and Fuel Characteristics. Energies 2014, 7, 4364-4380. https://doi.org/10.3390/en7074364
Ali OM, Yusaf T, Mamat R, Abdullah NR, Abdullah AA. Influence of Chemical Blends on Palm Oil Methyl Esters’ Cold Flow Properties and Fuel Characteristics. Energies. 2014; 7(7):4364-4380. https://doi.org/10.3390/en7074364
Chicago/Turabian StyleAli, Obed M., Talal Yusaf, Rizalman Mamat, Nik R. Abdullah, and Abdul Adam Abdullah. 2014. "Influence of Chemical Blends on Palm Oil Methyl Esters’ Cold Flow Properties and Fuel Characteristics" Energies 7, no. 7: 4364-4380. https://doi.org/10.3390/en7074364




