Effects of Pretreatment Methods on Electrodes and SOFC Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of Anode Supported Cell
2.2. Different Pretreatments and Testing on the Anode Supported Cell with LSM Cathode
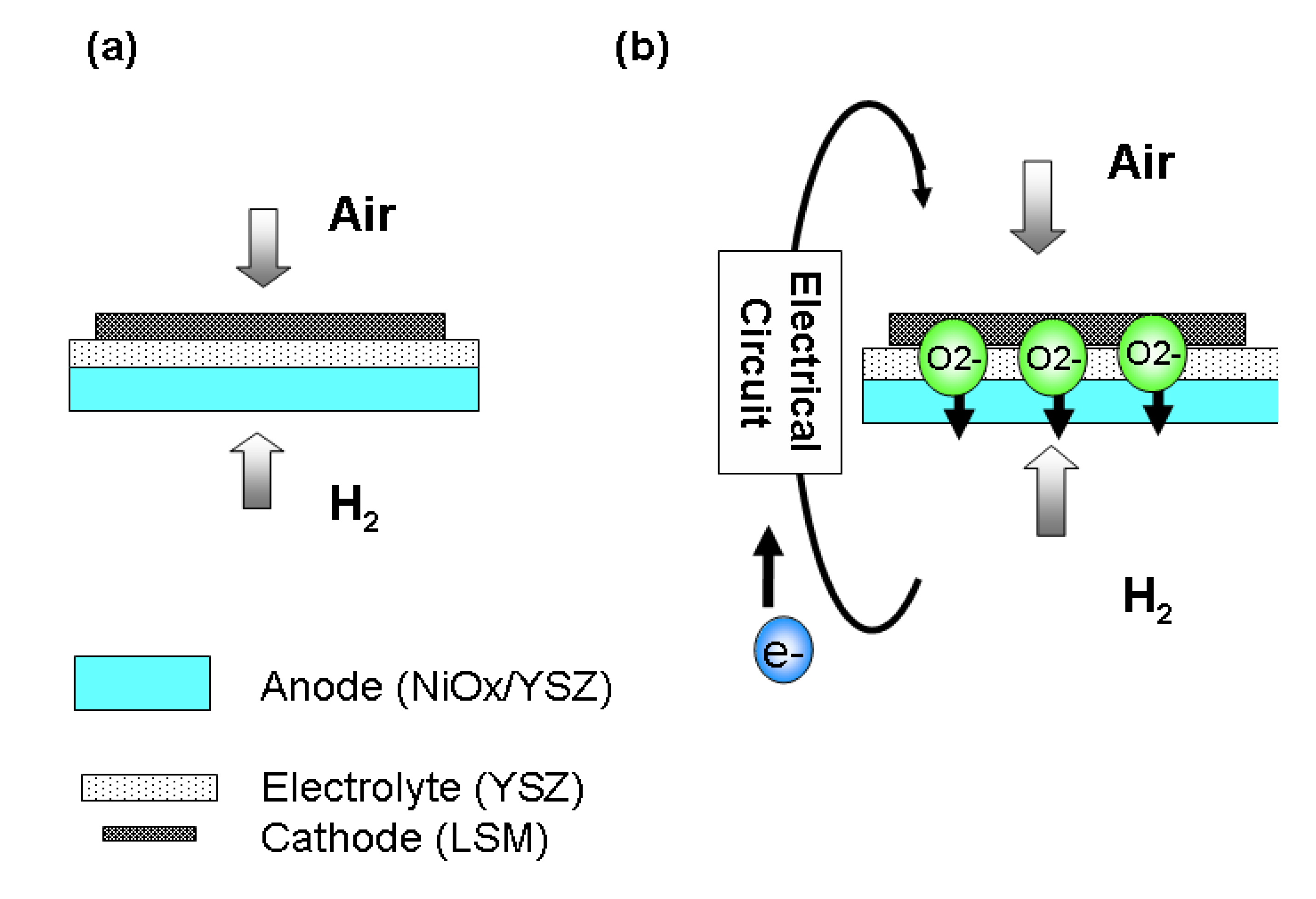
2.3. Different Pretreatments and Testing on Anode Supported Cell with Pt Cathode
3. Results and Discussion
3.1. Effect of Pretreatments on NiO-YSZ/YSZ/LSM

3.1.1. Characterization by AC Impedance Spectroscopy- NiO-YSZ/YSZ/LSM

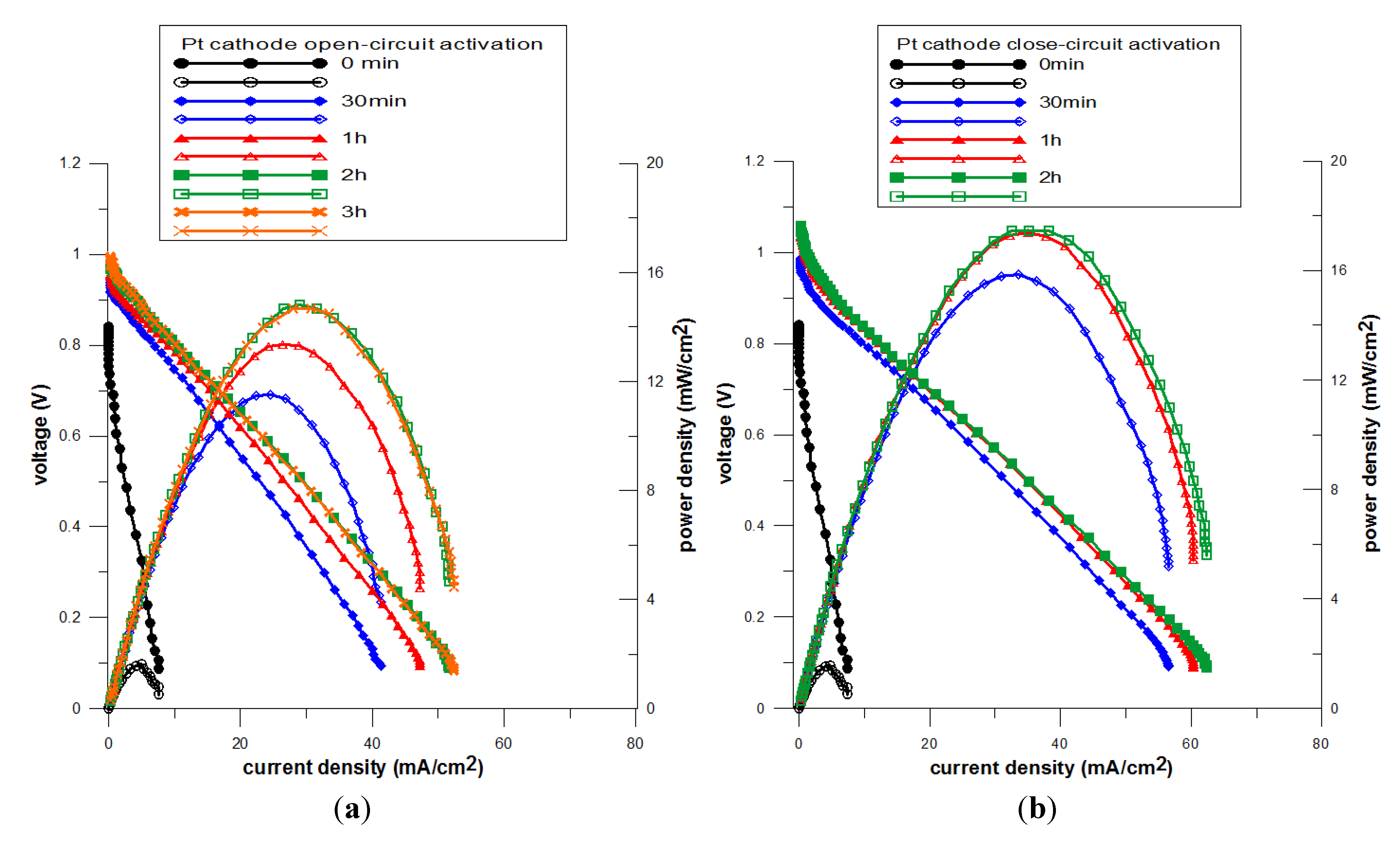
3.2. Effect of Pretreatments on NiO-YSZ/YSZ/Pt
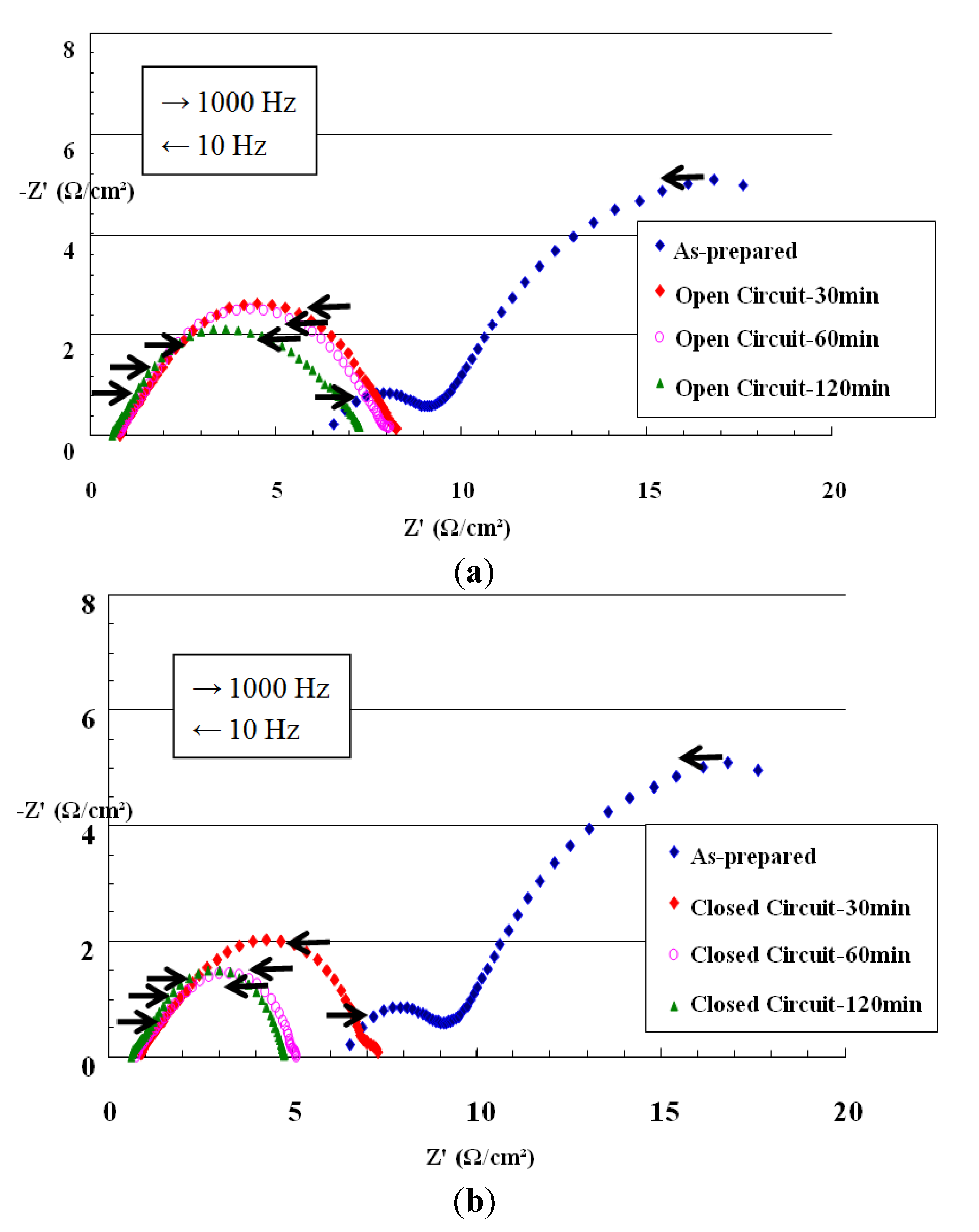
3.2.1. Characterization by AC Impedance Spectroscopy- NiO-YSZ/YSZ/Pt
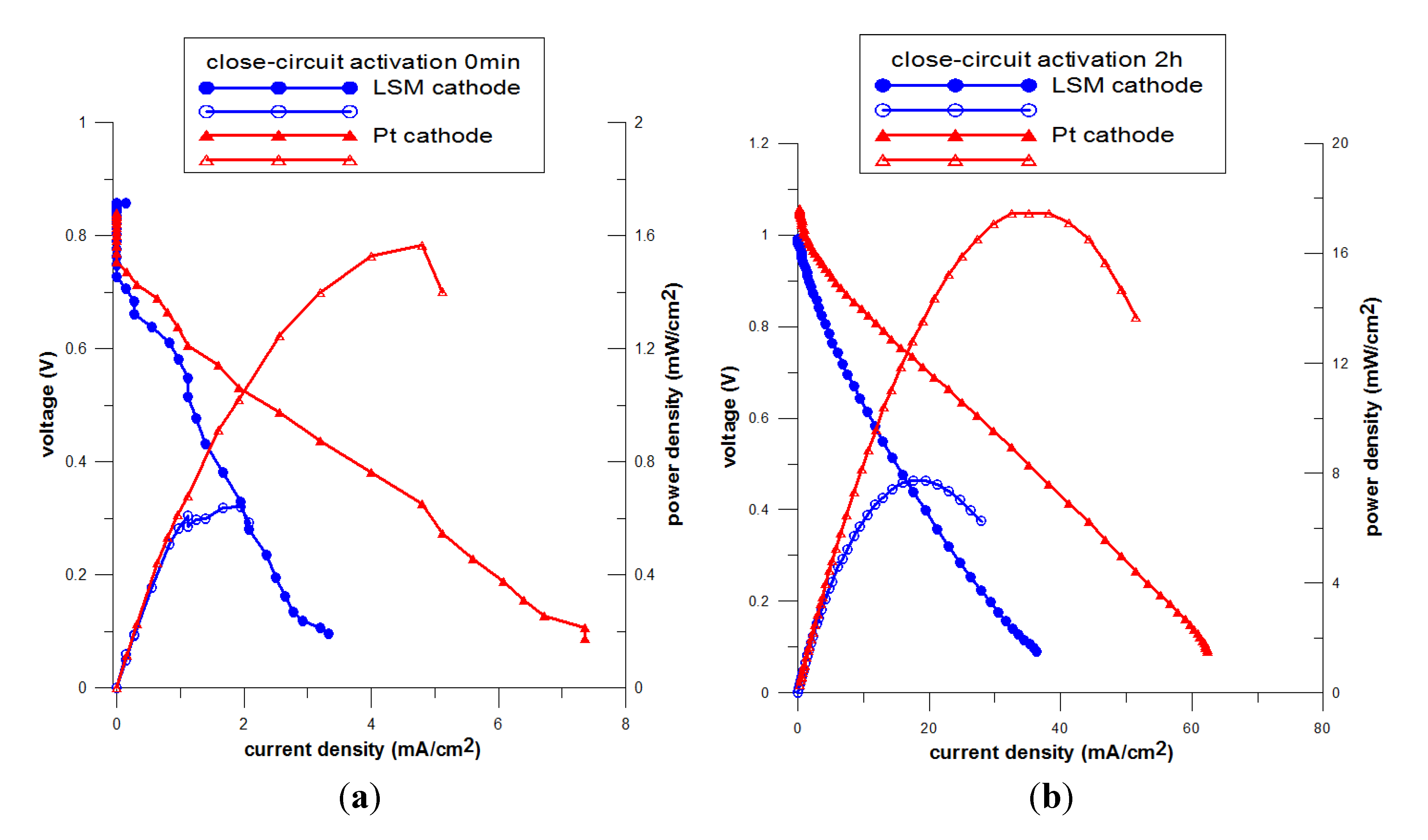
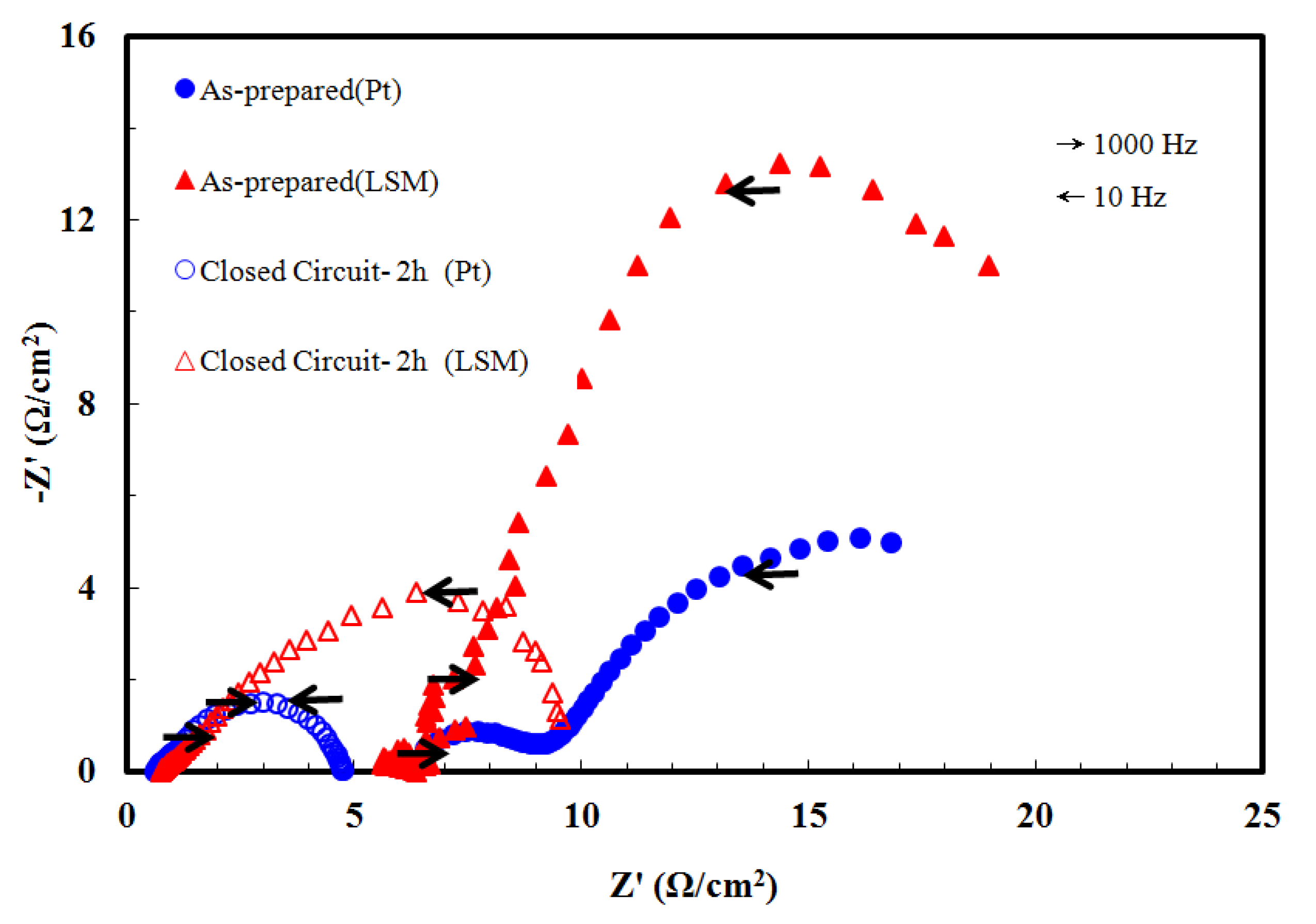
3.3. Effect of Closed-Circuit Pre-Treatment on NiO-YSZ/YSZ/LSM and NiO-YSZ/YSZ/Pt
4. Conclusions
Acknowledgments
Nomenclature
| A | current |
| I | current density, A/cm2 |
| P | power density, W/cm2 |
| Z’ | resistance, Ω/cm2 |
| −Z | resistance, Ω/cm2 |
| Vo | oxygen vacancy |
| T | temperature, °C |
| t | time, min |
| V | voltage |
Conflicts of Interest
References
- Najafi, B.; Shirazi, A.; Aminyavari, M.; Rinaldi, F.; Taylor, R.A. Exergetic, economic and environmental analyses and multi-objective optimization of an SOFC-gas turbine hybrid cycle coupled with an MSF desalination system. Desalination 2014, 334, 46–59. [Google Scholar] [CrossRef]
- Shirazi, A.; Aminyavari, M.; Najafi, B.; Rinaldi, F.; Razaghi, M. Thermal-economic-environmental analysis and multi-objective optimization of an internal-reforming solid oxide fuel cell-gas turbine hybrid system. Int. J. Hydrog. Energy 2012, 37, 19111–19124. [Google Scholar] [CrossRef]
- Saribog, V.; Öksüzömer, F. The investigation of active Ni/YSZ interlayer for Cu-based direct-methane solid oxide fuel cells. Appl. Energy 2012, 93, 707–721. [Google Scholar]
- Yan, W.M.; Wang, X.D.; Lee, D.J.; Zhang, X.X.; Guo, Y.F.; Su, A. Experimental study of commercial size proton exchange membrane fuel cell performance. Appl. Energy 2011, 88, 392–396. [Google Scholar] [CrossRef]
- Ha, S.; Rice, C.A.; Masel, R.I.; Wieckowski, A. Methanol conditioning for improved performance of formic acid fuel cells. J. Power Sources 2002, 112, 605–609. [Google Scholar] [CrossRef]
- Cassidy, M.; Lindsay, G.; Kendall, K. The reduction of nickel-zirconia cermet anodes and the effects on supported thin electrolytes. J. Power Sources 1996, 6, 189–192. [Google Scholar] [CrossRef]
- Wang, Y.; Walter, M.E.; Sabolsky, K.; Seabaugh, M.M. Effects of power sizes and reduction parameters on the strength of Ni-YSZ anodes. Solid States Ion. 2006, 177, 1517–1527. [Google Scholar] [CrossRef]
- Jiang, S.P.; Love, J.G.; Zhang, J.P.; Hoang, M.; Ramprakash, Y.; Hughes, A.E.; Badwal, S.P.S. The electrochemical performance of LSM/zirconia–yttria interface as a function of A-site non-stoichiometry and cathodic current treatment. Solid State Ion. 1999, 121, 1–10. [Google Scholar] [CrossRef]
- Lee, H.Y.; Cho, W.S.; Oh, S.M.; WiemhVofer, H.D.; Gvopel, W. Active reaction sites for oxygen reduction in La0.9Sr0.1MnO3/YSZ electrodes. J. Electrochem. Soc. 1995, 142, 2659–2664. [Google Scholar] [CrossRef]
- Jiang, S.P.; Love, J.G. Origin of the initial polarization behavior of Sr-doped LaMnO3 for O2 reduction in solid oxide fuel cells. Solid State Ion. 2001, 138, 183–190. [Google Scholar] [CrossRef]
- Haanappel, V.A.C.; Mai, A.; Mertens, J. Electrode activation of anode-supported SOFCs with LSM- or LSCF-type cathodes. Solid State Ion. 2006, 177, 2033–2037. [Google Scholar] [CrossRef]
- Jung, G.B.; Lo, K.F.; Chan, S.H. Effect of pretreatments on the anode structure of solid oxide fuel cells. J. Solid State Electrochem. 2007, 11, 1435–1440. [Google Scholar] [CrossRef]
- Cai, Z.; Lan, T.N.; Wang, S.; Dokiy, M. Supported Zr(Sc)O2 SOFCs for reduced temperature prepared by slurry coating and co-firing. Solid State Ion. 2002, 152, 583–590. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jung, G.-B.; Fang, L.-H.; Chiou, M.-J.; Nguyen, X.-V.; Su, A.; Lee, W.-T.; Chang, S.-W.; Kao, I.-C.; Yu, J.-W. Effects of Pretreatment Methods on Electrodes and SOFC Performance. Energies 2014, 7, 3922-3933. https://doi.org/10.3390/en7063922
Jung G-B, Fang L-H, Chiou M-J, Nguyen X-V, Su A, Lee W-T, Chang S-W, Kao I-C, Yu J-W. Effects of Pretreatment Methods on Electrodes and SOFC Performance. Energies. 2014; 7(6):3922-3933. https://doi.org/10.3390/en7063922
Chicago/Turabian StyleJung, Guo-Bin, Li-Hsing Fang, Min-Jay Chiou, Xuan-Vien Nguyen, Ay Su, Win-Tai Lee, Shu-Wei Chang, I-Cheng Kao, and Jyun-Wei Yu. 2014. "Effects of Pretreatment Methods on Electrodes and SOFC Performance" Energies 7, no. 6: 3922-3933. https://doi.org/10.3390/en7063922



