Nanofibrillated Cellulose (NFC): A High-Value Co-Product that Improves the Economics of Cellulosic Ethanol Production
Abstract
: Cellulosic ethanol is a sustainable alternative to petroleum as a transportation fuel, which could be made biologically from agricultural and forestry residues, municipal waste, or herbaceous and woody crops. Instead of putting efforts on steps overcoming the natural resistance of plants to biological breakdown, our study proposes a unique pathway to improve the outcome of the process by co-producing high-value nanofibrillated cellulose (NFC), offering a new economic leverage for cellulosic ethanol to compete with fossil fuels in the near future. In this study, glucose has been produced by commercial enzymes while the residual solids are converted into NFC via sonification. Here, we report the morphology of fibers changed through the process and yield of glucose in the enzymatic hydrolysis step.1. Introduction
In response to concerns about the long–term adequacy and accessibility of fossil fuels, researchers and governments around the World are searching for alternative energy sources for transportation [1]. Biofuel is one possibility, although neither starch nor sucrose can meet societal demands. Breakdown of woody and herbaceous cellulose leads to simple sugars, such as glucose and cellobiose. Subsequent cellulosic ethanol production by fermentation of those sugars is projected to be capable of displacing more than 30% of gasoline used for transportation in the United States [2,3]. For this to happen, however, the economics of cellulosic ethanol production must be improved significantly in the future [4]. Two commonly cited key steps in the production of cellulosic ethanol are: (1) removal of lignin [5,6], and (2) conversion of cellulose into simple sugars [7,8].
One major factor inhibiting the commercialization of cellulosic ethanol production is the high cost of both materials, such as enzymes, and processing, specifically the pretreatment of raw materials and the recovery of ethanol from the fermented mixture [9]. Considerable research has been done on the production of enzymes capable of the higher efficiencies needed to increase the amount of ethanol released per ton of biomass, as one way to reduce the costs [10,11]. However, the highest tolerance for ethanol by the currently used microorganisms is 10% (w/v) at 30 °C [12]. Fewer people are studying revenue-increasing process alternatives to cost reduction studies. One alternative strategy, previously proposed by us, is the simultaneous production of higher valued co-products during cellulosic ethanol production, thereby increasing total revenue and enhancing profitability [13]. In this paper, we will discuss how nanocellulose (NCC) production can help unlock low-cost cellulosic ethanol by providing an additional revenue stream.
Nanocellulose (NCC) is extractable from diverse sources, including wood, cotton, grass, algae, bacteria, municipal solid waste and even animals, such as tunicates. It has attracted considerable attention due to its unique optical properties, high strength, large surface area and sustainable raw material origin [14]. To provide material for establishing NCC's potential in various applications, at least four pilot plants have been established or are under construction. These include one in Maine, funded through a joint venture of the University of Maine with the USDA Forest Service; a second in Madison, Wisconsin, at the US Forest Products Laboratory; a third, CelluForce, located in Windsor, Québec, is funded through a joint venture of Domtar Corporation and FPInnovations; and the fourth involves a collaboration between Innventia and the Royal Institute of Technology (KTH), both in Stockholm, Sweden.
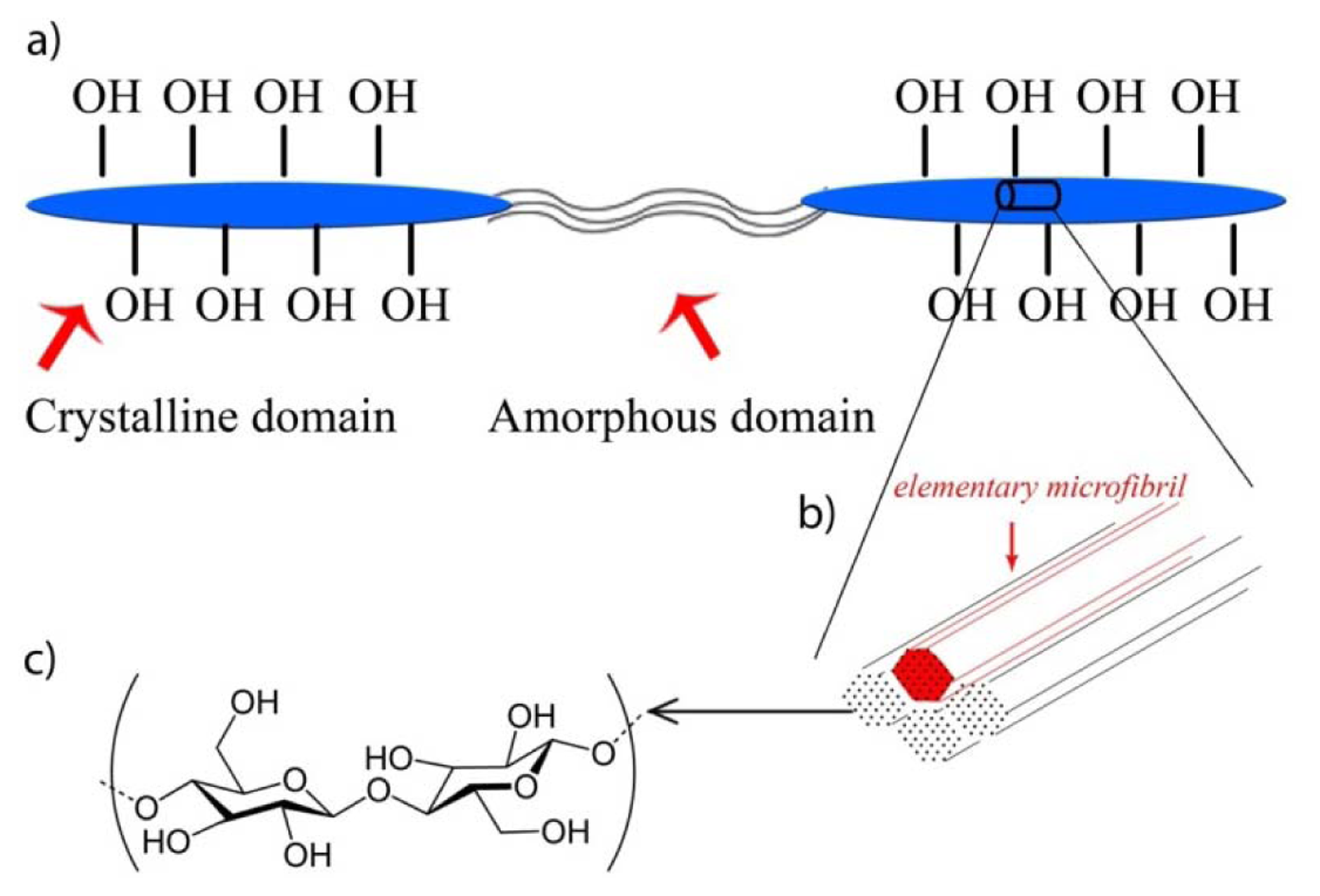
As linear polymers consisting of 1–4 linked β-d-glucopyranosyl residues, cellulose chains exist in the plant cell wall in a hierarchical structure: single cellulose chains aggregated, under biological control, into arrays of parallel chains called elementary microfibrils stabilized by hydrogen bonds [15]. These aggregate further into larger macroscopic fibers. Within the microfibrils some regions are highly organized while others are relatively unorganized. Thus, the macroscopic fibers contain two domains: crystalline domain and amorphous domain (Figure 1). Crystalline domains have lower accessibility than amorphous domains, due to the impenetrability of the tight crystalline structure of the former versus the open, unorganized nature of the latter. Accessibility of the amorphous domains makes them more susceptible to hydrolysis by acids or enzymes while the ordered regions tend to be highly crystalline and less accessible. Following the National Nanotechnology Initiative [16] such particles having at least one dimension smaller than 100 nm are referred to as cellulose nanocrystals (CNC) or nanofibrillated cellulose (NFC), depending upon the morphology of the particles [17].
CNC production via sulfuric acid hydrolysis (AH, 64%, w/v, 45 °C, 1 g cellulose/10 mL acid) has been under continuous research for three decades [19–22]. This technique has been chosen for pilot plant operations. The acid hydrolysis produced CNC (CNC-AH) has been shown to have unique chiral nematic orientation in nanoscale [23] and their reinforcement ability when mixed with other polymers has been documented [24]. However, as a large amount of corrosive acid is used in the process, high-cost, acid-resistant equipment and additional downstream processes are required to handle and remove the acid and byproducts, which increases the capital investment and operation costs. For example, sugar degradation products are produced in this process, lowering yields and contaminating products unless completely removed.
In the present paper, we will show that enzymatic hydrolysis under conditions appropriate to a cellulosic ethanol biorefinery coupled with sonification (SF) is a promising process for the production of fermentable sugar and NFC.
2. Experimental Section
2.1. Raw Materials
Air-dried hardwood pulp, northern bleached hardwood kraft (NBHK) using dense northern maple hardwood as its major fiber components, SAPPI (Cloquet Pulp Mill, ECF, Cloquet, MN, USA); air-dried softwood pulp, northern bleached softwood kraft pulp (NBSK, Weyerhaeuser, Kamloops, BC, Canada); each of these two pulps was used without further treatment. Enzymes employed in cellulose destructuring were Cellic® CTec2 (a mixture of aggressive cellulases with a high level of β-glucosidase) (Novozymes, Franklinton, NC, USA) and Cellic® HTec2 (endoxylanase) (Novozymes). The activity of the as received Cellic CTec2, measured in “Filter Paper Units” or FPUs per mL [25], was 148 FPU/mL. When a 2:1 (v/v) mixture of CTec2 to HTec2 was assayed in an identical manner, the activity increased to 206 FPU/mL of CTec2.
2.2. Sulfuric Acid (H2SO4) Hydrolysis (Acid Hydrolysis, AH)
Hydrolysis was performed at 45 °C with 64% H2SO4 at 1-to-10 (w:v) pulp-to-acid ratio. The reaction was stopped by diluting ten times with distilled water. The suspension of CNC was filtered on a glass fiber circle with a pore size of 1.2 μm to remove the larger particles. Centrifugation of the solution at 10,000 rpm for 10 min separated the solids from the acidic liquid in the suspension. The remaining CNC was washed with distilled water three times (15,000 rpm, 20 m), until further centrifugation was incapable of further precipitation of the solid content from water (pH∼2.5). Finally, the CNC suspension was placed in a Spectra/por® dialysis tube (50,000 molecular weight cut-off, Spectrum Laboratories, Inc., Rancho Dominguez, CA, USA), dialyzed against flowing distilled water until the pH did not change. The resulting CNC from NBSK was labeled as CNC-SW-AH.
2.3. Enzymatic Hydrolysis (EH)
Air-dried NBHK/NBSK pulp (5 g) and citrate buffer (0.05 N, pH 4.8, 100 mL) were added into a 250 mL Erlenmeyer flask. The pH of the mixture was adjusted to 5.0 using a saturated NaOH solution. The slurries were equilibrated at 50 °C and 90 oscillations/m in a heating/reciprocal shaking water bath (Precision Scientific 2870, Thermo Scientific, Waltham, MA, USA) for 1 h before enzyme addition. Enzyme loading was 0.2 mL Cellic® CTec2 and 0.1 mL Cellic® HTec2/5g NBHK (NBSK). Samples (3 mL) were collected at 1, 2, 4, 6, 8, 10, 14, 18, 24, 36, 48, 72 h and the sugar content of each sample was analyzed by HPLC, with Waters IC-Pak™ ion exclusion column and following the procedure of Sluiter et al. [26] The NBHK pulp and NBSK pulp after 72 h enzymatic treatment were designated as MFC-SW-EH or MFC-HW-EH (where SW and HW respectively stand for softwood, NBSK) and MFC-HW-EH (HW stands for hardwood pulp, NBHK).
2.4. Sonification (SF)
After enzymatic hydrolysis treatment, the samples of MFC-SW-EH/MFC-HW-EH were centrifuged at 15,000 rpm for 10 min. Supernatants, which contained primarily glucose and citrate buffer, were removed. Residual solid fibers were washed twice with distilled water by centrifugation (15,000 rpm, 10 min) and then dispersed in distilled water (concentration 0.2%–1.7% w/v basis). 40 mL aliquots containing enzymatic hydrolysis treated fibers were then placed in a Q700 sonicator (QSonica, Newtown, CT, USA) operating at 20–25 KHz frequency and equipped with a 0.5 inch (diameter) flat tipped titanium probe. The output level was 90% providing an indicated output power of 80–99 W. The solutions, in HDPE containers, were kept in an ice bath during the entire sonification process. The temperature of the solutions, under sonification, was monitored and maintained below 90 °C. The sonification time, 120 min (total pulse-on time), with 20 s pulse-on time followed by 20 s pulse-off time. The resulted nanofibrillated cellulose (NFC) preparations were labeled as NFC-HW-EH-SF and NFC-SW-EH-SF for NBHK and NBSK pulps, respectively.
2.5. Scanning Electron Microscopy (SEM)
Morphology of enzymatic hydrolysis treated fibers was observed by scanning electron microscopy (JSM-5800LV, JEOL, Peabody, MA, USA). Air-dried MFC-SW-EH and MFC-HW-EH fibers were mounted on a metal stubs with a graphite conductive adhesive 112 (Cat.#12693-30, Electron Microscopy Sciences, Hatfield, PA, USA). All samples were platinum coated using a sputter coater (Denton Vacuum, Desk II, Moorestown, NJ, USA).
2.6. Transmission Electron Microscopy (TEM)
Nanoparticle morphology was observed by transmission electron microscopy (JEM-2000EX) operated at 100 KV. For each sample, 20 μL of a suitably diluted CNC or NFC suspension was applied to a TEM grid (FCF 300-Cu, Formvar® carbon film on a 300 mesh copper grid, Electron Microscopy Sciences, Hatfield, PA, USA). Samples were stained with 4% (w/v) uranyl acetate.
3. Results and Discussion
3.1. Sugar Releasing in the Enzymatic Hydrolysis Process
Enzymatic hydrolysis is an environmentally friendly alternative to acid hydrolysis. It preferentially removes the amorphous domains from macroscopic native cellulose fibers. Generally speaking, three kinds of cellulolytic enzymes, endoglucanases, exoglucanases (typically cellobiohydrolases), and β-glucosidases are required to degrade cellulose to glucose [27]. However, native cellulose macroscopic fibers have inherent resistance as the crystalline domains limit accessibility. Thus, the amorphous domains are removed while the crystalline domains are largely unperturbed during enzymatic hydrolysis.
Cellic® CTec2 is a blend of cellulases, a high level of β-glucosidase, a hemicellulase, and a proprietary compound that the manufacturer describes as a unique performance booster. Cellic®HTec2 is an endoxylanase, which increases the performance of Cellic® CTec2. After 72 h reaction, the glucose concentrations were 20.2 g/L (enzyme loading 0.2 mL Cellic™ CTec2/5 g NBSK) and 28.6 g/L (enzyme loading 0.2 mL Cellic® CTec2 and 0.1 mL Cellic® HTec2/5g NBSK). This confirms that Cellic® HTec2 improves the digestibility of NBSK by Cellic®.CTec2, even though only a trace amount of xylose was detected by HPLC. The goal of this work is to remove the easy-to-react amorphous part of cellulose fibers as much as possible and take advantage of the more-resistant crystalline domains in cellulose fibers to produce nanocellulose. Thus, even though hemicelluloses were not targeted, Cellic® HTec2 was tested as the removal of hemicelluloses might facilitate cellulose hydrolysis and, in practice, it did increase glucose release. Moreover, only a trace amount of cellobiose was detected, which is consistent with the high level of β-glucosidase in Cellic® CTec2 as described by Novozymes in their product literature [28].
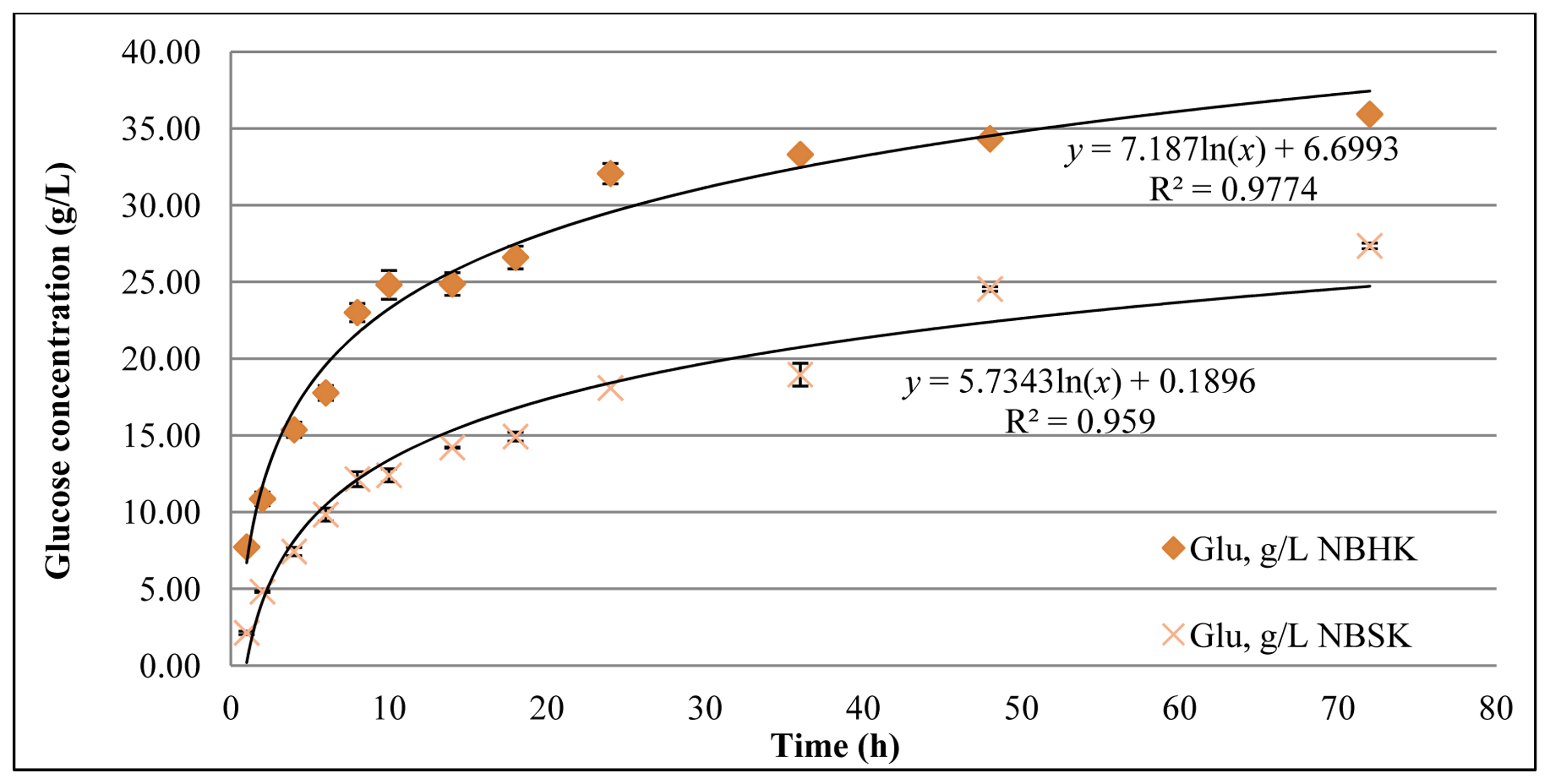
As shown in Figure 2, the final glucose concentration from enzymatic hydrolysis of NBHK pulp and NBSK pulp was 35.9 g/L and 27.3 g/L, respectively. The yield of glucose in the enzymatic hydrolysis of NBHK pulp and NBSK pulp is 75.6% and 57.5%, respectively. This result indicates that hardwood pulp (NBHK) is more readily digested than softwood pulp (NBSK) by a mixture of Cellic® CTec2 and Cellic® HTec2. This difference is consistent with shorter fibers having a higher density of initially accessible chain ends/gram of solids and, therefore, somewhat higher reactivity compared to softwood pulp.
As shown in Figure 3, the glucose release rate (rG) for softwood pulp was 2.7 (g/L) h−1 at 2 h. Two factors favoring rG increase in the first 2 h are: (1) The enzymes might require some time to equilibrate to the environment (temperature, pH); (2) The endoglucanase is expected to randomly cleave the cellulose chain, creating more free ends for the exoglucanase to digest. For these reasons we use an open asterisk instead of the solid asterisk used for the remaining dots to indicate that the first point has not been considered in the power function curve. That the rG decrease to 0.5 (g/L) h−1 after 24 h, may be the result of: (1) The deactivation of enzymes and (2) the physical barrier of the crystalline domains. Industrial economic considerations might suggest stopping before completion of hydrolysis. If we choose a target completion point of 80%–90% of the maximum as is observed, the time requirement for hardwood is 24–30 h and for softwood is 40–60 h.
3.2. Morphology of Fibers
3.2.1. Morphology of CNC from AH Process
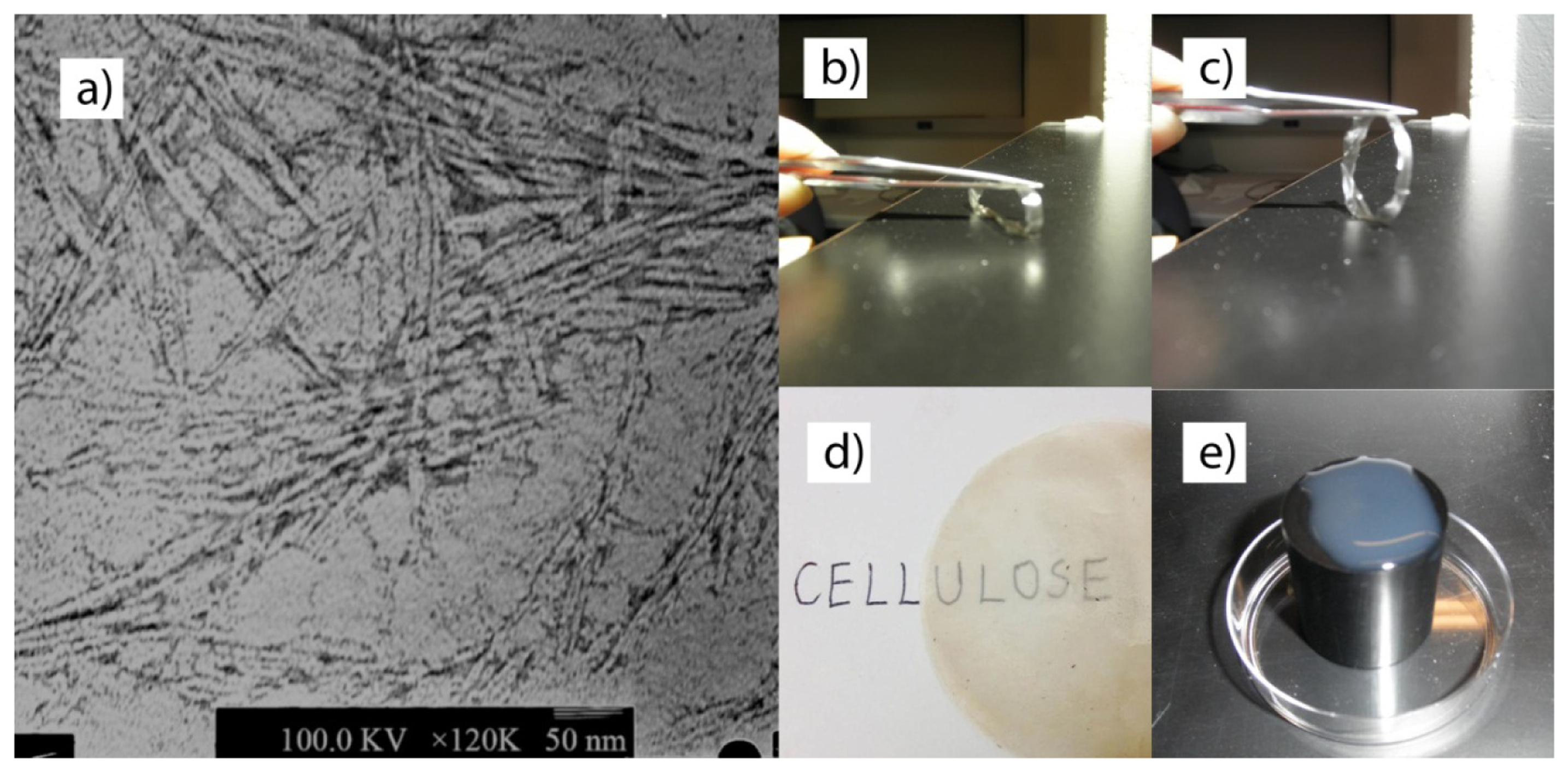
As shown in Figure 4a, CNC-SW-AH nanoparticles were characterized by a width of 5–8 nm and a length of 200–400 nm. Figure 4e depicts a milky suspension of CNC-SW-AH on the surface of a black plastic rod. It demonstrates the liquid crystal status of CNC-SW-AH suspension. Figure 4b,c was obtained from an air-dried membrane (the ring) made from CNC-SW-AH. Figure 4b shows that if the ring is compressed with forceps, the shape of the ring will be changed without breaking the ring, thus demonstrating a certain level of flexibility of the membrane. In Figure 4d, “cellulose” was written on a paper, which was then placed so that “ulose” was beneath the membrane. The word “ulose” portion of the word cellulose was still readable through the membrane. In other words, the membrane was relatively transparent.
3.2.2. Morphology of NFC after Enzymatic Hydrolysis and Sonication Processes
As discussed in the sugar release Section 2.1, native cellulose macroscopic fibers have inherent resistance to degradation, as the crystalline domains reduce accessibility. As a result, the residue of enzymatic hydrolysis treatments is characterized by particles whose width is about three orders of magnitude larger than that of nanoparticles. The enzymatically hydrolyzed fibers, thus, need further mechanical treatment to reduce them to nanoparticle size.
Various mechanical treatments have been reported to produce nanofibrillated cellulose including grinding, microfluidization [29], and sonification [30,31]. Tang et al. [32] reported that, based on energy density for certain volumes of solution, sonification has a ten times higher efficiency than microfluidization in the nanoemulsification process.
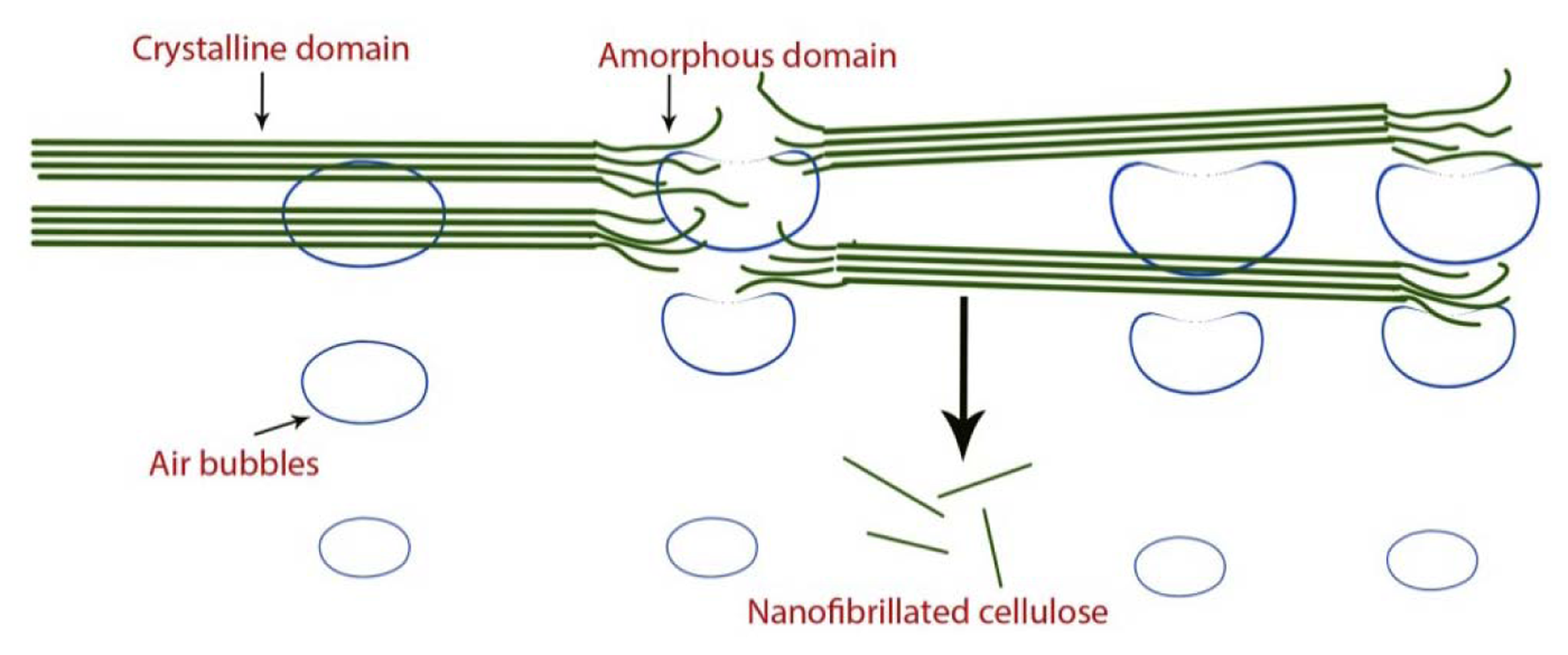
NFC production via sonification is believed to occur through the following mechanism [33]: The air-bubble forms, grows, and collapses during the acoustic cavitation, releasing energy to break the weak spots in the fibers including hydrogen bonds between the elementary microfibrils (Figure 5).
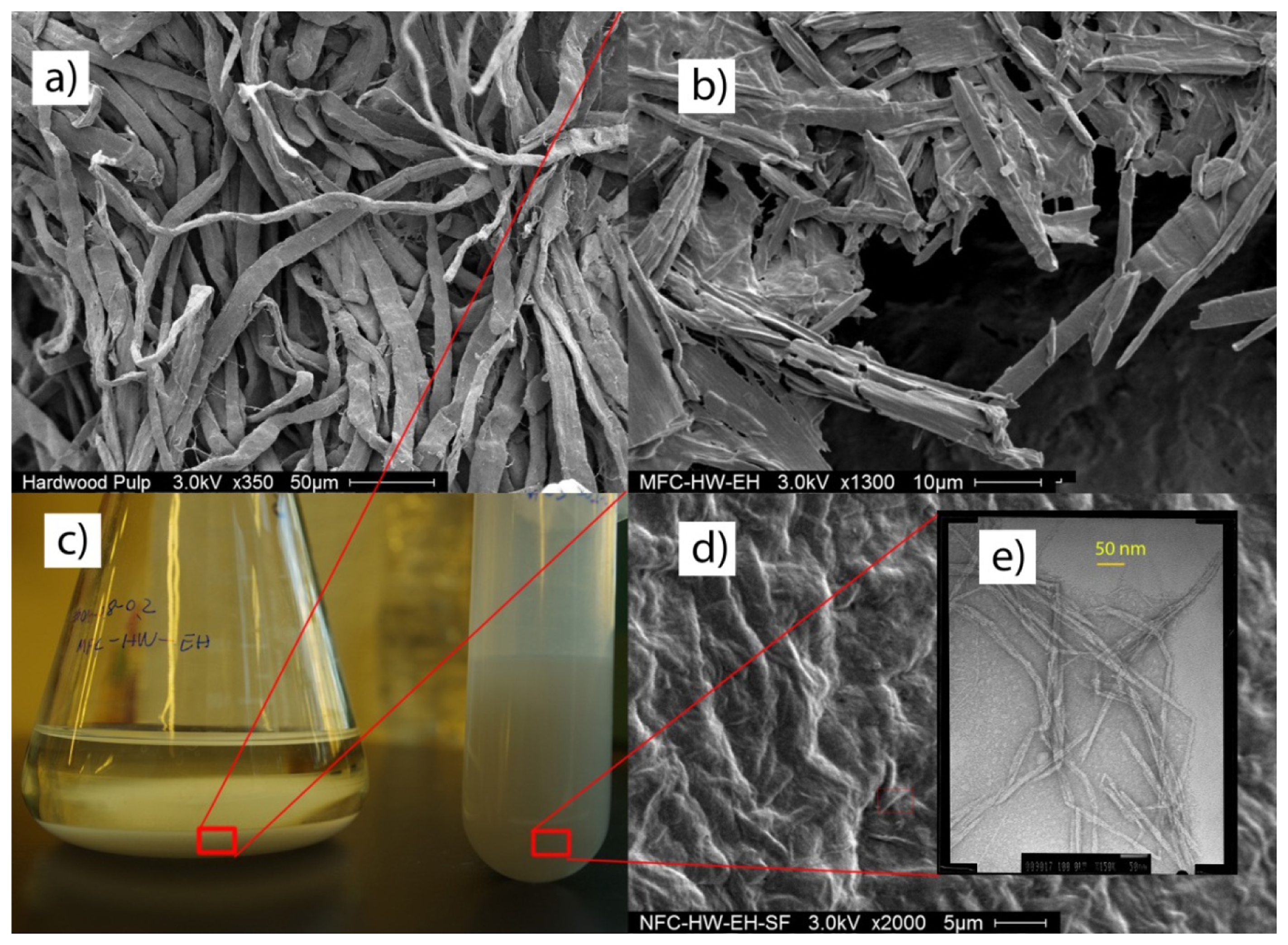
The change of fibers' morphology during the enzymatic hydrolysis and sonification was recorded by SEM and TEM. The width of raw material NBHK and NBSK was observed to be 20–30 μm by SEM, and the length was greater than 1 mm, typical of intact pulp fibers (Figures 6a,b and 7a). After enzymatic hydrolysis, the length of the treated fibers significantly decreased. However, the width was still several micrometers, demonstrating that enzymatic hydrolysis produced MFC (Figures 6c and 7b). Upon sonification, however, the width of the MFC decreased to 5–10 nm and NFC was produced (Figures 6d and 7e). The length of the resulting NFC was over 1μm. It is worthwhile noting that, based on conditions used in this study (80–99 W, 2 h), sonification may not lead to 100% conversion from macrofiber to nanocellulose. The SEM micrograph (Figure 7d) shows that some larger particles persist after sonification for 120 min. However, such particles were removable by centrifugation (2000 rpm, 10 min).
4. Conclusions and Future Work
Nanofibrillated cellulose (NFC) was produced by sonification of enzymatically-hydrolyzed, bleached softwood and hardwood pulps. NFC produced in this manner can be a high-value co-product coupled to bioethanol production. This additional revenue stream will reduce the materials and processing costs attributed to the fuel product thereby reducing the cost per liter of fuel thus increasing the economic feasibility of cellulosic ethanol. The width of the resultant NFC particles is 5–10 nm, while the typical length is greater than 1 μm, see Figures 6d and 7e, essentially the same values reported for NFC produced by acid hydrolysis, Figure 4a. Our particles are likely to have significant applications as reinforcing filler particles for various sustainable composites, including specialty papers, polyvinyl alcohol/acetate, biobased polyesters such as polyhydroxyalkanoates and polylactic acid, as well as biodegradable polycaprolactone. The glucose yields in the enzymatic hydrolysis are 75.6% and 57.5% for NBHK and NBSK, respectively, and the yields of higher oligomers at their expected retention volumes are, in all cases, below the detection threshold. Additionally, the resultant glucose from the enzyme based product stream is free of fermentation inhibitors, such as H3O+ from acid and acetyl groups from xylan, as the hemicellulose is appreciably reduced in the enzymatic hydrolysis step by the Cellic®HTec2. Thus, the sugars should be readily fermented, a significant advantage compared to the low pH associated with commonly used acid hydrolysis methods. As we progress, commercially bleached wood input streams will be replaced with wood pretreated by hot-water extraction of hemicelluloses and organosolv delignification. For this process to compete with ongoing scale-up by the groups noted in the introduction, both cost-effectiveness and the ability to control the process and the relative amounts of generated particles, fuel, and other products will be essential. Other areas that will require attention include the matrix of factors that influence of degree of fibrillation, the optimization of sonication conditions, and the identification of areas that do not scale readily. Finally, the reinforcement effect of NFC with various polymer matrixes and improvement of compatibilization with polymer matrices will be explored.
Acknowledgments
This work was financially supported by the National Science Foundation (PFI: BIC 1237815). We thank the N.C. Brown Center for Ultrastructure Studies and particularly its manager, Robert Smith, for valuable assistance in the SEM and TEM experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wyman, C.E. What is (and is not) vital to advancing cellulosic ethanol. Trends Biotechnol. 2007, 25, 153–157. [Google Scholar]
- Perlack, R.D.; Wright, L.L.; Turhollow, A.F.; Graham, R.L.; Stokes, B.J.; Erbach, D.C. Biomass as Feedstock for a Bioenergy and Bioproducts Industry: The Technical Feasibility of a Billion-Ton Annual Supply; U.S. Department of Energy: Oak Ridge, TN, USA, U.S. Department of Agriculture: Oak Ridge, TN, USA; 2005. [Google Scholar]
- National Research Council (US) Committee on Assessing the Importance and Impact of Glycomics and Glycosciences. In Transforming Glycoscience: A Roadmap for the Future; National Academies Press: Washington, DC, USA, 2012.
- Wang, M.; Saricks, C.; Santini, D. Effects of Fuel Ethanol Use on Fuel-Cycle Energy and Greenhouse Gas Emissions; ANL/ESD-38; Argonne National Laboratory:Argonne: IL, USA, 1999. [Google Scholar]
- Gong, C.; Goundalkar, M.J.; Bujanovic, B.M.; Amidon, T.E. Evaluation of different sulfur-free delignification methods for hot-water extracted hardwood. J. Wood Chem. Technol. 2012, 32, 93–104. [Google Scholar]
- Ahmed, M.; Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into lignin degradation and its potential industrial applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar]
- Olson, D.G.; McBride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar]
- Horn, S.J.; Vaaje-Kolstad, G.; Westereng, B.; Eijsink, V.G.H. Novel enzymes for the degradation of cellulose. Biotechnol. Biofuels 2012, 5, 1–13. [Google Scholar]
- Sticklen, M.B. Plant genetic engineering for biofuel production: Towards affordable cellulosic ethanol. Nat. Rev. Genet. 2008, 9, 433–443. [Google Scholar]
- Wood, B.; Beall, D.; Ingram, L. Production of recombinant bacterial endoglucanase as a co-product with ethanol during fermentation using derivatives of escherichia coli KO11. Biotechnol. Bioeng. 1997, 55, 547–555. [Google Scholar]
- Nieves, R.; Ehrman, C.; Adney, W.; Elander, R.; Himmel, M. Survey and analysis of commercial cellulase preparations suitable for biomass conversion to ethanol. World J. Microbiol. Biotechnol. 1997, 14, 301–304. [Google Scholar]
- Hamelinck, C.N.; Hooijdonk, G.V.; Faaij, A.P. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar]
- Winter, W.T. Cellulose Nanocrystals: Can they be a Value-Added Coproduct to Bioethanol. Proceedings of the 234th ACS National Meeting, Boston, MA, USA, 19–23 August 2007.
- Peng, B.; Dhar, N.; Liu, H.; Tam, K. Chemistry and applications of nanocrystalline cellulose and its derivatives: A nanotechnology perspective. Can. J. Chem. Eng. 2011, 89, 1191–206. [Google Scholar]
- Guerriero, G.; Fugelstad, J.; Bulone, V. What do we really know about cellulose biosynthesis in higher plants? J. Integr. Plant Biol. 2010, 52, 161–175. [Google Scholar]
- National Science and Technology Council. The National Nanotechnology Initiative Supplement to the President's 2014 Budget. p. 3. Available online http://nano.gov/sites/default/files/pub_resource/nni_fy14_budget_supplement.pdf (accessed on 12 January 2014).
- Missoum, K.; Belgacem, M.N.; Bras, J. Nanofibrillated cellulose surface modification: A review. Materials 2013, 6, 1745–1766. [Google Scholar]
- Himmel, M.E.; Ding, S.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar]
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar]
- Roman, M.; Winter, W.T. Effect of sulfate groups from sulfuric acid hydrolysis on the thermal degradation behavior of bacterial cellulose. Biomacromolecules 2004, 5, 1671–1677. [Google Scholar]
- Beck-Candanedo, S.; Roman, M.; Gray, D.G. Effect of reaction conditions on the properties and behavior of wood cellulose nanocrystal suspensions. Biomacromolecules 2005, 6, 1048–1054. [Google Scholar]
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180. [Google Scholar]
- Gray, D. Chiral nematic ordering of polysaccharides. Carbohydr. Polym. 1994, 25, 277–284. [Google Scholar]
- Roohani, M.; Habibi, Y.; Belgacem, N.M.; Ebrahim, G.; Karimi, A.N.; Dufresne, A. Cellulose whiskers reinforced polyvinyl alcohol copolymers nanocomposites. Eur. Polym. J. 2008, 44, 2489–2498. [Google Scholar]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; Technical Report NREL/TP-510-42628; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; Technical Report NREL/TP-510-42623; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2008. [Google Scholar]
- Teeri, T.T. Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Trends Biotechnol. 1997, 15, 160–167. [Google Scholar]
- Novozymes A/S. Cellic® CTec2 and HTec2-Enzymes for Hydrolysis of Lignocellulosic Materials; Applications Report No. 2010-01668-01. Novozymes: Bagsvaerd, Denmark, 2010. Available online: http://bioenergy.novozymes.com/en/cellulosic-ethanol/CellicCTec3/Documents/AS_2010-01668-03.pdf (accessed on 12 January 2014).
- Zimmermann, T.; Bordeanu, N.; Strub, E. Properties of nanofibrillated cellulose from different raw materials and its reinforcement potential. Carbohydr. Polym. 2010, 79, 1086–1093. [Google Scholar]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar]
- Cheng, Q.; Wang, S.; Rials, T.G. Poly (vinyl alcohol) nanocomposites reinforced with cellulose fibrils isolated by high intensity ultrasonication. Compos. Part A Appl. Sci. Manuf. 2009, 40, 218–224. [Google Scholar]
- Tang, S.Y.; Shridharan, P.; Sivakumar, M. Impact of process parameters in the generation of novel aspirin nanoemulsions—Comparative studies between ultrasound cavitation and microfluidizer. Ultrason. Sonochem. 2013, 20, 485–497. [Google Scholar]
- Li, W.; Yue, J.; Liu, S. Preparation of nanocrystalline cellulose via ultrasound and its reinforcement capability for poly (vinyl alcohol) composites. Ultrason. Sonochem. 2012, 19, 479–485. [Google Scholar]


© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Song, Q.; Winter, W.T.; Bujanovic, B.M.; Amidon, T.E. Nanofibrillated Cellulose (NFC): A High-Value Co-Product that Improves the Economics of Cellulosic Ethanol Production. Energies 2014, 7, 607-618. https://doi.org/10.3390/en7020607
Song Q, Winter WT, Bujanovic BM, Amidon TE. Nanofibrillated Cellulose (NFC): A High-Value Co-Product that Improves the Economics of Cellulosic Ethanol Production. Energies. 2014; 7(2):607-618. https://doi.org/10.3390/en7020607
Chicago/Turabian StyleSong, Qiong, William T. Winter, Biljana M. Bujanovic, and Thomas E. Amidon. 2014. "Nanofibrillated Cellulose (NFC): A High-Value Co-Product that Improves the Economics of Cellulosic Ethanol Production" Energies 7, no. 2: 607-618. https://doi.org/10.3390/en7020607



