The textile bioreactor used in this work is a novel bioreactor for producing bioethanol. It is made from a backbone of textile which is coated with several layers of polymers to make it resistant to chemicals, gas and liquid leakage. It is flexible, long lasting and can withstand temperatures up to 150 °C. Some of the advantages of using a textile bioreactor for bioethanol production include: it does not corrode, it can withstand the tough environmental conditions encountered during fermentation, it is a far cheaper alternative than the currently used bioethanol bioreactors, it is light, and designed for easy and safe transportation, installation and operation, and it is ultraviolet irradiations (UV) resistant, it can be sterilized with steam at 121 °C and with chemicals. It was originally developed for biogas production [
13], but it was never examined for any other fermentation products. In this work, this new textile bioreactor was developed for bioethanol production, its performance was examined and the results are presented here.
2.1. Textile vs. Other Materials for Construction of Bioreactors
The materials used for constructing bioreactors must be able to withstand the physiochemical conditions encountered while running the bioreactor and during clean-up and sterilization [
14]. Apart from stainless steel, other materials that could be used for making bioreactors include carbon steel, borosilicate glass, polytetrafluoroethylene (PTFE) plastic, and ceramics [
14]. Only stainless steel 304 and to a lesser extent reinforced carbon steel are currently being used to make industrial ethanol bioreactors. The other materials are normally added to stainless steel bioreactors at specific points for certain purposes (e.g., borosilicate glass used in sight glasses) [
14].
Bioethanol is produced by fermentation. Bioethanol fermentation takes place under slightly acidic conditions (pH between 4 and 6), temperatures ranging from 25 to 38 °C, generally without oxygen, and in a liquid medium. Ensuring that only the desired microorganism is what grows in the bioreactor is necessary to ensure the fermentable sugars are converted to the product of interest [
15]. It is essential that the material used for constructing bioreactors for producing bioethanol does not affect the fermentation process and can be sterilized when needed. For all the experiments performed in the textile bioreactor, it was autoclaved for sterilization at 121 °C for 20 min and 2 bar pressure. This created a sterile working condition for the textile bioreactor. There were no incidences of bacterial contamination in all the experiments performed in the textile bioreactor, as there were no areas for harbouring unwanted microorganisms, which is one of the main reasons why stainless steel is used as the current material for making bioreactors [
16]. The material of construction of the textile bioreactor has been proven to resist diverse environmental conditions (pH 3–12) [
13]. In addition the material when burnt does not ignite, but rather forms a semi-solid composite which recoils inward, making the bioreactor fire resistant. The material was designed to have high tensile strength with high flexibility to make its assembly and disassembly easy.
Table 2 shows some advantages and disadvantages of using certain materials of construction for ethanol bioreactors. Considering these features and the comparison in
Table 2, as a bioreactor material of construction, the textile bioreactor is an excellent choice for bioethanol production.
Table 2.
Advantages and disadvantages of possible materials for construction for ethanol bioreactors [
14,
17,
18].
Table 2.
Advantages and disadvantages of possible materials for construction for ethanol bioreactors [14,17,18].
| Material | Modification | Advantage | Disadvantage |
|---|
| Textile | Layered with some polymers and UV filter | Portable. | Currently a horizontal vessel. |
| Corrosion proof. |
| Good sterility. |
| More cost effective than stainless steel. |
| Can withstand high temperature. |
| Leak proof. |
| Long life span. |
| Can be designed to have regions that are transparent, for easy process monitoring. |
| Stainless steel 304 | – | Cheapest of all the stainless steel. | Quite expensive. |
| Leak proof. |
| Good sterility. |
| Can withstand high temperature and pressure. |
| Corrosion proof. |
| Long life span. |
| Carbon steel | Reinforced with stainless steel | Cheaper than 304 stainless steel. | Corrosion and contamination. |
| Leak proof. |
| Borosilicate glass | – | Transparent. | Very fragile. |
| Inert to chemicals. |
| Plastic | – | Very portable. | Leaks and short life span. |
| Cheap. | High chances of contamination. |
| Ceramics | – | Chemically stable. | Brittle. |
| Wear resistant. | Prone to thermal shock. |
2.2. Reactor Cost Comparison
A major challenge facing biofuel production is its economic feasibility [
19]. Bioethanol production consists of the collection of feedstock, pre-treatment of feedstock (if the feedstock is starch or lignocellulosic based), fermentation, distillation and possibly dehydration [
20]. The fermentation cost of a 100,000 m
3/y ethanol production facility contributes 11% of the total plant cost, while the bioreactor cost makes up 32% of the fermentation cost [
8]. In this section a comparison is made between the investment cost of stainless steel bioreactors and textile bioreactors excluding operation cost (maintenance and installation cost). Typically, the installed cost (investment and operation cost) of a stainless steel reactor is 1.7 times its purchase cost [
21], while that of a textile bioreactor is 1.5 times its purchase cost [
13].
The purchase cost for a 1000 m
3 textile bioreactor is $100,000.
Table 3 shows the purchasing cost of different reactor sizes, for both the developed textile bioreactor and stainless steel reactors. The purchasing cost of stainless steel reactor was estimated using Equations (1) and (2) (see
Section 3.5). For all reactor volumes considered, the purchasing cost of the developed textile bioreactor was far less than half the purchasing cost of the stainless steel bioreactor. Considering a 100,000 m
3/y ethanol production facility using sucrose as its raw material and having a fermentation time between 10 and 15 h [
22,
23], this plant will require a bioreactor volume between 1000 and 1500 m
3 for the fermentation only. If this plant has just one 1300 m
3 stainless steel bioreactor, replacing this with a 1300 m
3 textile bioreactor will reduce the fermentation investment cost by 19%, and the total plant investment cost of the facility by 2.1%.
Table 3.
Purchasing cost of developed textile bioreactors and 304 stainless steel reactors.
Table 3.
Purchasing cost of developed textile bioreactors and 304 stainless steel reactors.
| Reactor Size (m3) | Purchasing Cost of Developed Textile Bioreactor ($) | Purchasing Cost of 304 Stainless Steel Reactor ($) |
|---|
| 500 | 66,000 | 201,000 |
| 1,000 | 100,000 | 282,000 |
| 1,300 | 130,000 | 325,000 |
| 1,500 | 150,000 | 352,000 |
2.3. Mixing and Temperature Control in the Textile Bioreactor
The mass transfer in a bioreactor affects the net productivity of the system [
11,
24]. The two crucial aspects of mass transfer in a bioreactor are the uniform distribution of the product and substrate in the bulk liquid, and the transfer of substrate into the cells and the products out of the cells. Mixing helps to minimize local variation of concentration and temperature in a bioreactor [
11]. Mixing in liquid media can be achieved by agitation, or with the aid of a stirrer, or by the use of a pump for recirculation, depending on the viscosity of the liquid and if it media is single- or multi-phased [
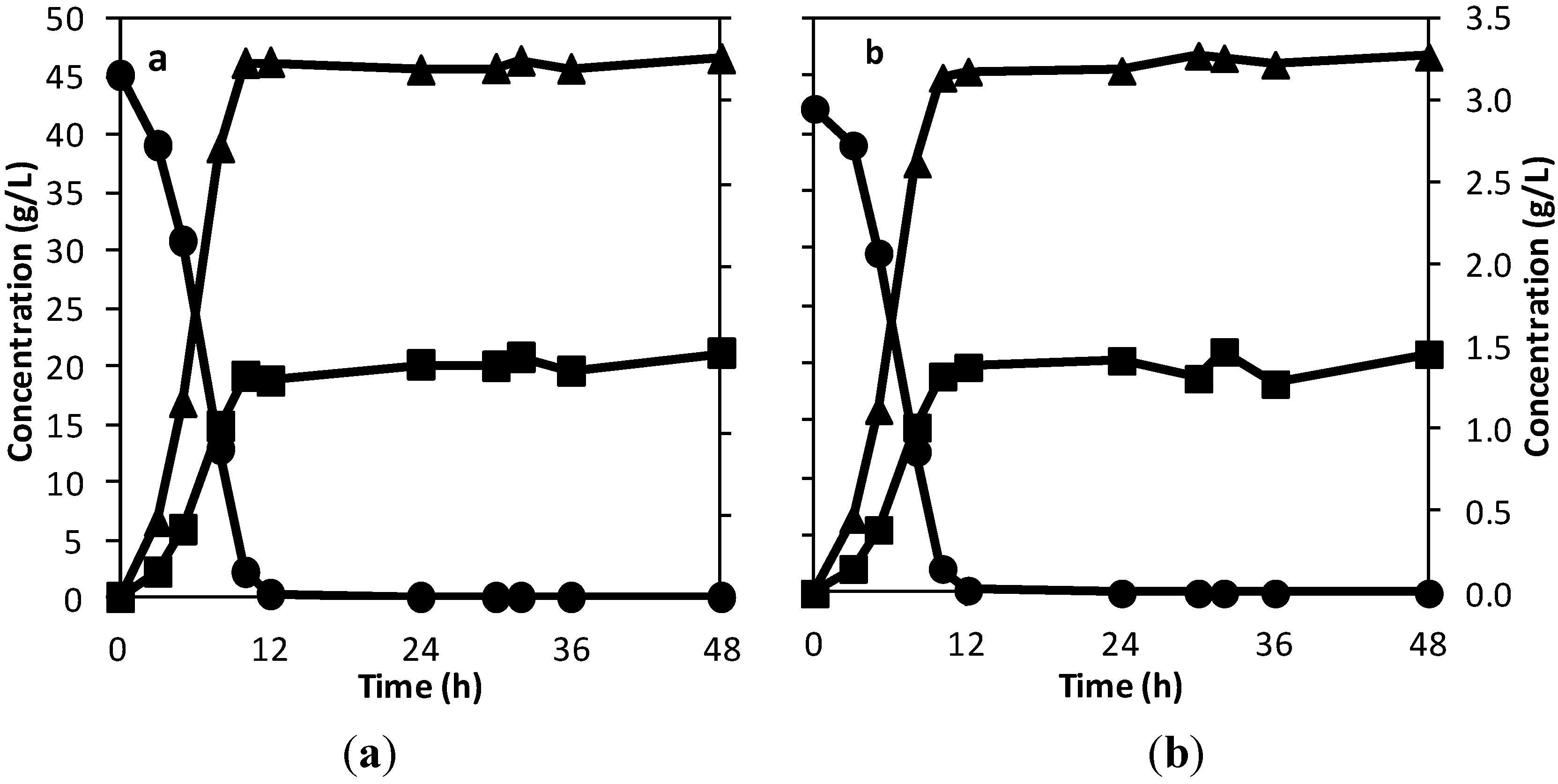
25]. For the textile bioreactor, mixing was performed using a recirculation pump. To determine the effectiveness of the mixing in the bioreactor and the possibility of it being used for continuous fermentation, experiments were performed where samples were collected from the sampling point at the centre of the textile bioreactor and from the exit pipes from the bioreactor. One of the basics of a continuous stirred tank reactor (CSTR) is having same concentration in the reactor as what leaves the reactor. From
Figure 1, it is clearly observed that there is no significance difference between the concentration in the bioreactor (samples from the centre of the reactor) and that leaving the bioreactor (samples from the exit pipe). This shows that there is the possibility of the textile bioreactor being used for batch, fed-batch, and continuous fermentation. In addition to ensuring uniform substrate and product distribution in the textile bioreactor, the mixing also helped to provide a good transfer of substrate into and products out of the yeast (
Figure 1), as the sugar was fully consumed about the same time as peak ethanol concentration was reached. Thus the mixing by recirculation in the textile bioreactor is effective.
Figure 1.
Concentration of sucrose (●) and ethanol (■) on the primary axis (left side), and glycerol (▲) on the secondary axis (right side), with samples taken from the exit pipe (a) and centre of the reactor (b) with time, to determine the effectiveness of mixing by recirculation.
Figure 1.
Concentration of sucrose (●) and ethanol (■) on the primary axis (left side), and glycerol (▲) on the secondary axis (right side), with samples taken from the exit pipe (a) and centre of the reactor (b) with time, to determine the effectiveness of mixing by recirculation.
Temperature control is essential for optimal product formation as every microorganism has a temperature range in which it functions optimally. For anaerobic condition that for
S. cerevisiae is around 30 °C [
15]. For lab scale production heat is normally added to the system while for the large industrial bioreactors with low surface to volume ratio, cooling is necessary [
11]. Because of the nature of the material used for the developed textile bioreactor, cooling can easily be achieved by recirculation of chilled water, while heating can be achieved with hot water. The area to volume ratio of a 1000 m
3 textile bioreactor is 0.96, while that of a conventional 1000 m
3 bioreactor having a height to diameter ratio of 3 it is 0.62. The higher area to volume ratio of the textile bioreactor makes cooling (or heating) easily achievable because the heat loss (or gained) by evaporation and radiation increases with increasing area to volume ratio. Temperature control was achieved in the textile bioreactor as described in
Section 3.2. In addition, the recirculation of the fluid also helped to ensure temperature uniformity in every part of the reactor.
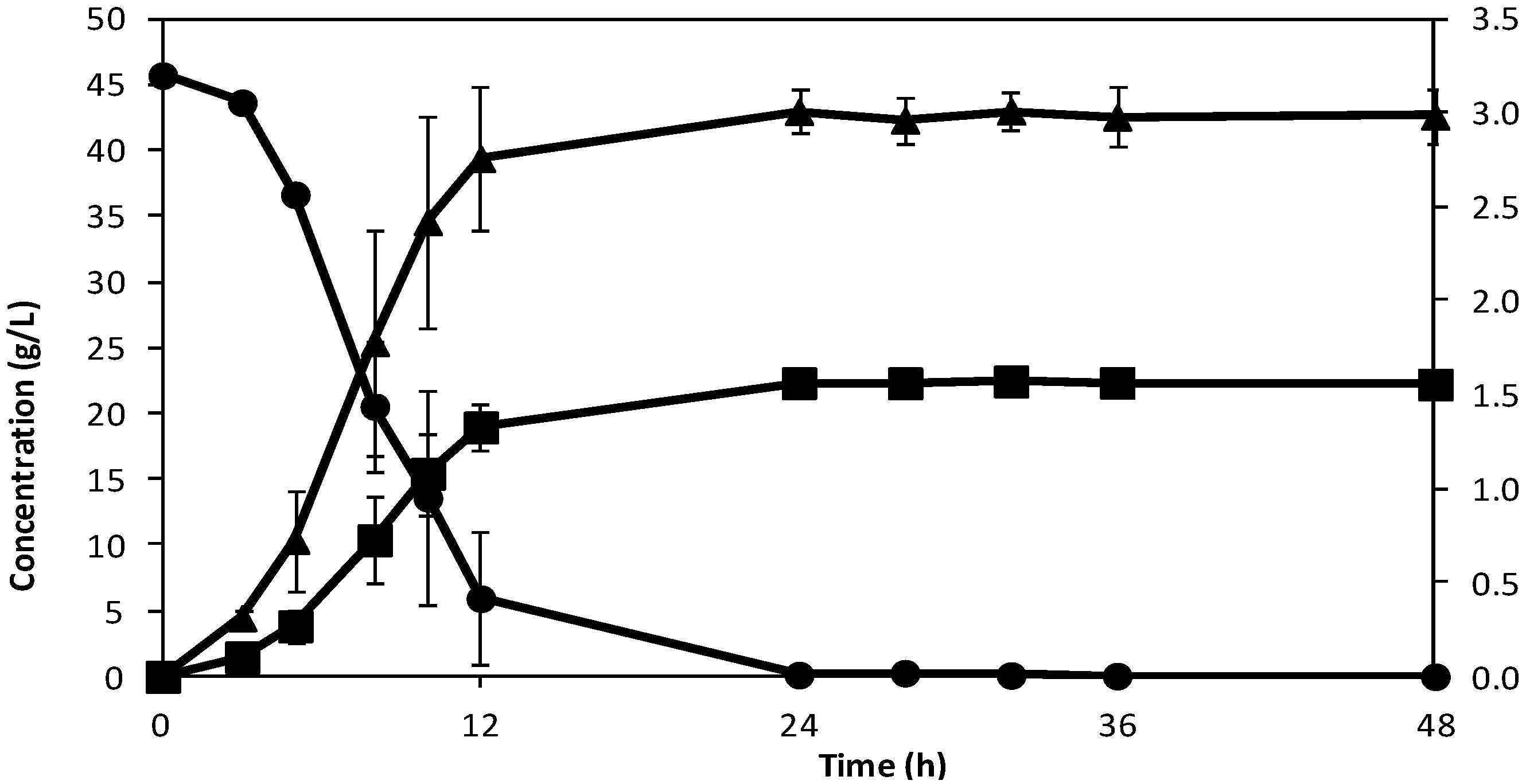
Figure 2 and
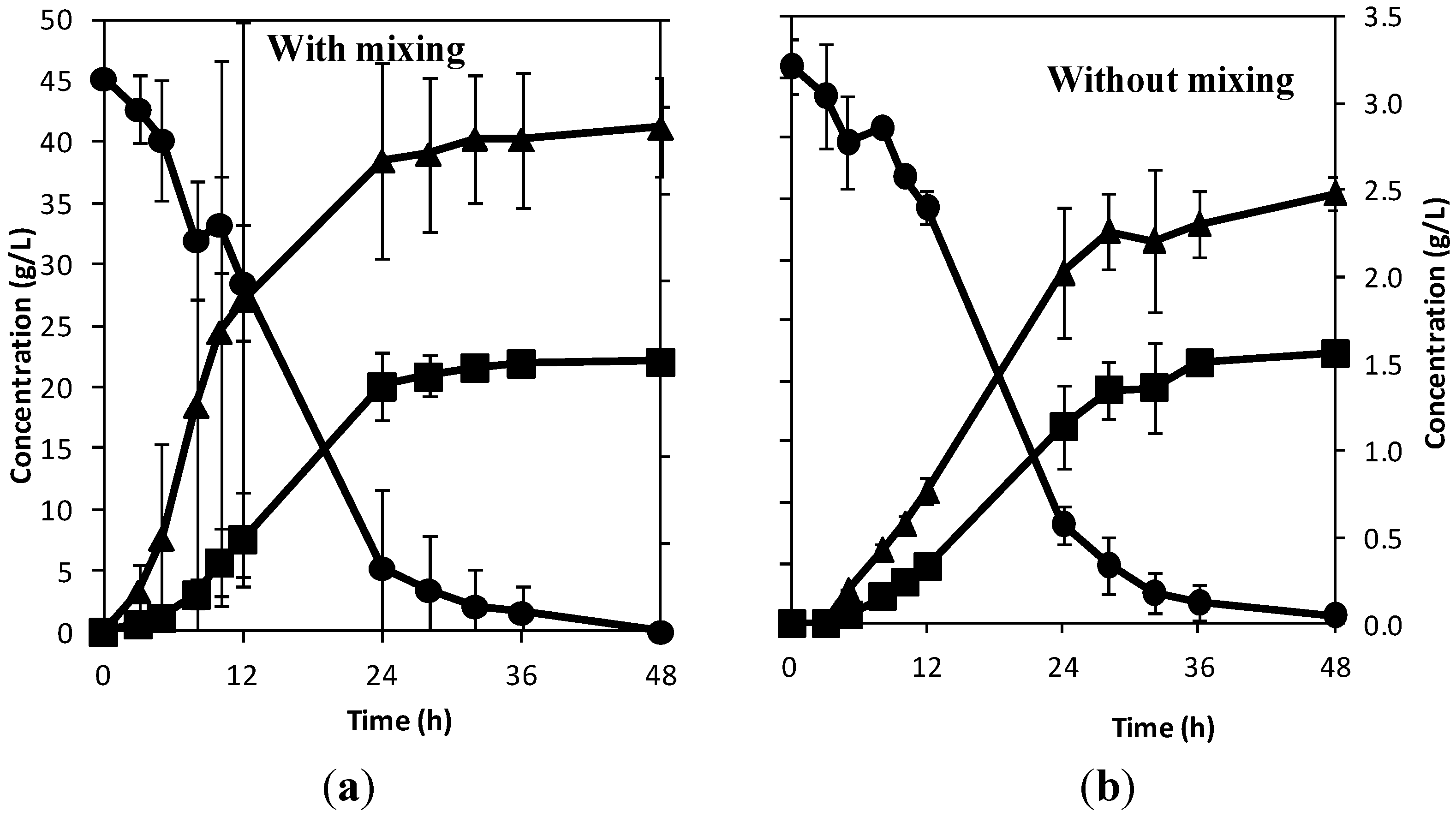
Figure 3 shows the result of the experiments performed with temperature control, while
Figure 4 shows the result of the experiments without temperature control. For both cases, ethanol yields greater than 87% of the theoretical values were reached, while the experiments where temperature was maintained at 30 °C had higher fermentation rates and peak product concentrations were reached in less than 24 h. This shows that the temperature control developed for the textile bioreactor is effective.
Figure 2.
Concentration of sucrose (●) and ethanol (■) on the primary axis, and glycerol (▲) on the secondary axis with time, at 30 °C and with mixing.
Figure 2.
Concentration of sucrose (●) and ethanol (■) on the primary axis, and glycerol (▲) on the secondary axis with time, at 30 °C and with mixing.
Figure 3.
Concentration of sucrose (●) and ethanol (■) on the primary axis, and glycerol (▲) on the secondary axis with time, at 30 °C without mixing.
Figure 3.
Concentration of sucrose (●) and ethanol (■) on the primary axis, and glycerol (▲) on the secondary axis with time, at 30 °C without mixing.
Figure 4.
Concentration of sucrose (●) and ethanol (■) on the primary axis, and glycerol (▲) on the secondary axis with time, at room temperature of 22 °C (a) with and (b) without mixing.
Figure 4.
Concentration of sucrose (●) and ethanol (■) on the primary axis, and glycerol (▲) on the secondary axis with time, at room temperature of 22 °C (a) with and (b) without mixing.
2.4. Fermentation in the Textile Bioreactor and Its Economics
To determine how effective the textile bioreactor was for producing bioethanol, lab scale experiments were performed under different operating conditions. Temperature and mixing were varied, while the pH of all the experiments performed was around 6.0 ± 0.2.
Figure 2 shows the result of the experiment performed in the textile bioreactor at 30 °C and with mixing. For this experiment, the yield of ethanol from the experiment (using the initial sucrose concentration of 44.2 g/L) was 0.48 ± 0.01 g/g, which is 88% of the theoretical value, and it took an average of 15 h for the yeast to consume the sugar. Comparing this fermentation time with that from a similar work were 10 g/L of yeast was used and it required a fermentation time of 10 h [
22], shows that the fermentation time is good. Thus fermentation takes place effectively well and at a good rate in the textile bioreactor. From
Figure 2, the average of the peak ethanol concentration was 20.04 ± 0.53 g/L, using the average fermentation time gave the specific productivity to be 1.34 ± 0.02 g L
−1·h
−1.
Figure 3 shows the result of the experiment where temperature was fixed at 30 °C without mixing. The yield of ethanol from the experiment was 0.49 ± 0.01 g/g, and it took an average of 20 h for the yeast to consume the sugar. Comparing
Figure 2 and
Figure 3 shows that mixing did not affect the fermentation rate that much when temperature is held at 30 °C without mixing, as the specific productivity for this case was 1.04 ± 0.01 g L
−1 h
−1 in comparison to 1.34 ± 0.02 g L
−1 h
−1 with mixing. To produce the same amount of ethanol as that which is produced when mixing is controlled; the textile bioreactor volume used has to be 1.29 times the one used with mixing. Taking a 1000 m
3 bioreactor operating with mixing and temperature control, this bioreactor will cost $282,000 but a 1300 m
3 textile bioreactor will cost $130,000 (see
Table 3). In addition, the cost of agitation and mixing in a bioreactor accounts for 24% of the fermentation cost of a 100,000 m
3 ethanol/y production facility [
8]. Using a textile bioreactor operated at 30 °C without mixing can eliminate the need for the cost of agitation and mixing, and it gives a bioreactor cost reduction of $152,000.
Figure 4 shows the result of the experiment that was performed at room temperature of 22 °C with and without mixing. The ethanol yield from the experiment with mixing was 0.49 ± 0.01 g/g, and it took an average of 40 h for the yeast to consume the sugar, while that without mixing had an ethanol yield of 0.49 ± 0.02 g/g, and it took an average of 42 h for peak ethanol concentration to be reached. Mixing did not affect the fermentation rate that much as the specific productivity with mixing was 0.55 ± 0.01 g L
−1 h
−1 while that without mixing was 0.53 ± 0.02 g L
−1 h
−1. This result shows that there is a possibility of running the textile bioreactor without temperature control and mixing. The slower fermentation rate from producing bioethanol at 22 °C can be accommodated by increasing the retention time and the bioreactor volume. Comparing
Figure 2 and
Figure 4, the same amount of ethanol per hour will be produced in both cases if the volume of the textile bioreactor for the production without temperature control and mixing is 2.53 times that with temperature control and mixing. Taking a 1000 m
3 bioreactor with temperature control and mixing, the purchasing cost of a 1000 m
3 bioreactor is $282,000 while that of a 2530 m
3 textile bioreactor (consisting of one 1000 m
3 reactor and one 1500 m
3 reactor) is $250,000 (see
Table 3). In addition, operating the textile bioreactor without temperature control and mixing also reduces the total fermentation cost, as the cost of temperature control, mixing and agitation accounts for 26% of the fermentation cost in a 100,000 m
3/y ethanol production plant [
8]. For the same bioethanol production rate it is more economical to use a larger volume of the textile reactor without temperature control and mixing than a smaller bioreactor volume with temperature control, mixing and agitation.
Comparing the scenario where there is mixing but the textile bioreactor is operated at 22 °C, the size of the reactor in this case would be 2.44 times the size of that operated at 30 °C. Using a 1000 m
3 bioreactor at 30 °C will cost $282,000 while a 2440 m
3 textile bioreactor running at 22 °C will cost $250,000. However running the textile bioreactor at 22 °C with mixing will only reduce the fermentation cost by 2%, which is not as economical as 26% cost reduction obtained by running it at 22 °C without mixing [
8].
For all experiments performed in the textile bioreactor there were no incidences of bacterial contamination. From the results, experiments performed at 30 °C had faster fermentation rates than the ones performed at room temperature. For a continuous process, both temperature control and mixing will be essential to achieve high dilution rate. The results of the experiments show that the textile bioreactor can be used for bioethanol production at different conditions of temperature and mixing.