Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass
Abstract
:1. Introduction
2. Macroalgal Production Process Operations
- cultivation including “seed” production;
- harvesting;
- post-harvest treatments including cleaning, size reduction, preservation and storage;
- energy extraction.
3. Energy Extraction from Macroalgal Biomass
- 1)
- Energy extraction methods requiring dry macroalgae
- direct combustion;
- pyrolysis;
- gasification (conventional);
- trans-esterification to biodiesel.
- 2)
- Energy extraction methods for wet macroalgae
- hydrothermal treatments;
- fermentation to bioethanol or biobutanol;
- anaerobic digestion.
| Method | Utilises entire organic biomass | Requires biomass drying after harvesting | Primary energy product |
|---|---|---|---|
| Direct combustion | Yes | Yes | Heat |
| Pyrolysis | Yes | Yes | Primarily liquid by fast pyrolysis |
| Gasification | Yes | Yes b (conventional) | Primarily Gas |
| Biodiesel production | No | Yes c | Liquid |
| Hydrothermal treatments | Yes | No | Primarily Liquid |
| Bioethanol production | No a | No | Liquid |
| Biobutanol production | No a | No | Liquid |
| Anaerobic digestion | Yes | No | Gas |
3.1. Energy Extraction Methods Requiring Dry Macroalgae
3.1.1. Dewatering and Drying Macroalgae
3.1.2. Direct Combustion of Macroalgae
Co-Combustion of Macroalgae
3.1.3. Pyrolysis
Effect of Metals in Seaweed Pyrolysis
Refining of Oils from Pyrolysis
Energy Return on Investment Considerations
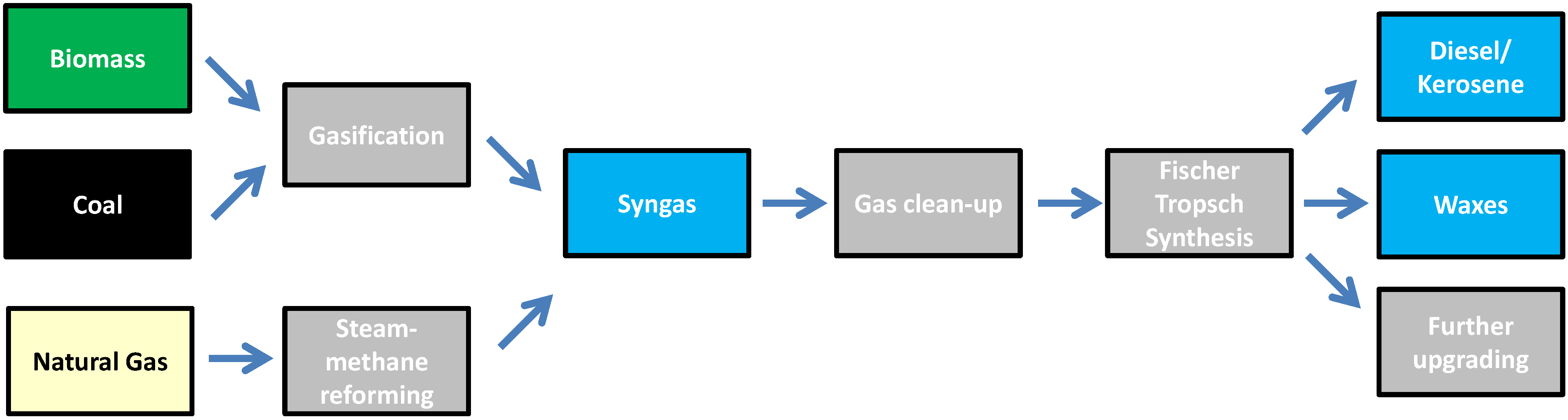
3.1.4. Gasification
Fischer-Tropsch Synthesis
3.1.5. Fatty Acid Ester-Derived Fuels via Trans-Esterification
3.2. Energy Extraction Methods for Wet Macroalgae
3.2.1. Hydrothermal Liquefaction
3.2.2. Bioethanol
3.2.3. Biobutanol Production from Seaweed
3.2.4. Anaerobic Digestion of Macroalgae
4. Discussion
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Sheehan, J.; Dunahay, T.; Benemann, J.; Roessler, P. A Look Back at the us Department of Energy’s Aquatic Species Program—Biodiesel from Algae; NREL/TP-580–24190; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 1998; Volume 7. [Google Scholar]
- Harvey, P.J.; Psycha, M.; Kokossis, A.; Abubakar, A.L.; Trivedi, V.; Swamy, R.; Cowan, A.K.; Schroeder, D.; Highfield, A.; Reinhardt, G.; et al. Glycerol production by halophytic microalgae: Strategy for producing industrial quantities in saline water. In Proceedings of the 20th European Biomass Conference and Exhibition, Milan, Italy, 18–22 June 2012; pp. 85–90.
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Hughes, A. Scottish seaweed aquaculture; research, development and commercialisation. In Proceedings of the Seaweed for Biofuel: Towards a Sustainable Seaweed Supply Chain, Oban, UK, 21 May 2014.
- Walker, D. Biofuels, facts, fantasy, and feasibility. J. Appl. Phycol. 2009, 21, 509–517. [Google Scholar] [CrossRef]
- Tredici, M.R. Photobiology of microalgae mass cultures: Understanding the tools for the next green revolution. Biofuels 2010, 1, 143–162. [Google Scholar] [CrossRef]
- Williams, P.J.L.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Rajkumar, R.; Yaakob, Z.; Takriff, M.S. Potential of the micro and macro algae for biofuel production: A brief review. BioResources 2013, 9, 1606–1633. [Google Scholar]
- Leu, S.; Boussiba, S. Advances in the production of high-value products by microalgae. Ind. Biotechnol. 2014, 10, 169–183. [Google Scholar] [CrossRef]
- Menetrez, M. An overview of algae biofuel production and potential environmental impact. Environ. Sci. Technol. 2012, 46, 7073–7085. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Algae’s second try. Science 2011, 333, 1238–1239. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Barberio, G. Utilization of macro-algae for enhanced CO2 fixation and biofuels production: Development of a computing software for an LCA study. Fuel Process. Technol. 2005, 86, 1679–1693. [Google Scholar] [CrossRef]
- Milledge, J.J.; Heaven, S. Methods of energy extraction from microalgal biomass: A review. Rev. Environ. Sci. Biotechnol. 2014, 13, 301–320. [Google Scholar] [CrossRef]
- Liu, X.; Saydah, B.; Eranki, P.; Colosi, L.M.; Greg Mitchell, B.; Rhodes, J.; Clarens, A.F. Pilot-scale data provide enhanced estimates of the life cycle energy and emissions profile of algae biofuels produced via hydrothermal liquefaction. Bioresour. Technol. 2013, 148, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, L.; Zhang, S.; Fu, H.; Chen, J. Hydrothermal liquefaction of macroalgae enteromorpha prolifera to bio-oil. Energy Fuels 2010, 24, 4054–4061. [Google Scholar] [CrossRef]
- Lundquist, T.J.; Woertz, I.C.; Quinn, N.W.T.; Benemann, J.R. A Realistic Technology and Engineering Assessment of Algae Biofuel Production; Energy Biosciences Institution: Berkeley, CA, USA, 2010. [Google Scholar]
- Smit, A. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Kelly, M.S.; Dworjanyn, S. The Potential of Marine Biomass for Anaerobic Biogas Production a Feasibility Study with Recommendations for Further Research; The Crown Estate on behalf of the Marine Estate: Scotland, UK, 2008. [Google Scholar]
- Roesijadi, G.; Copping, A.E.; Huesemann, M.H.; Foster, J.; Benemann, J.R. Techno-Economic Feasibility Analysis of Offshore Seaweed Farming for Bioenergy and Biobased Products; PNNL-19944; U.S. Department of Energy: Washington, DC, USA, 2010. [Google Scholar]
- Kraan, S. Mass-cultivation of carbohydrate rich macroalgae, a possible solution for sustainable biofuel production. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 27–46. [Google Scholar] [CrossRef]
- Milledge, J.J. Microalgae—Commercial potential for fuel, food and feed. Food Sci. Technol. 2012, 26, 26–28. [Google Scholar]
- Holdt, S.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed; Pomin, V.H., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2011. [Google Scholar]
- Murphy, F.; Devlin, G.; Deverell, R.; McDonnell, K. Biofuel production in ireland—An approach to 2020 targets with a focus on algal biomass. Energies 2013, 6, 6391–6412. [Google Scholar] [CrossRef]
- Roesijadi, G.; Jones, S.B.; Snowden-Swan, L.J.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysis; U.S. Department of Energy: Washington, DC, USA, 2010. [Google Scholar]
- Fei, X.; Bao, Y.; Lu, S. Seaweed cultivation: Traditional way and its reformation. Chin. J. Ocean. Limnol. 1999, 17, 193–199. [Google Scholar] [CrossRef]
- Horn, S.V. Bioenergy from Brown Seaweeds; Norwegian University of Science and Technology (NTNU): Trondheim, Norway, 2000. [Google Scholar]
- Black, W.A.P. The preservation of seaweed by ensiling and bactericides. J. Sci. Food Agric. 1955, 6, 14–23. [Google Scholar] [CrossRef]
- Uchida, M.; Miyoshi, T. Algal fermentation-the seed for a new fermentation industry of foods and related products. Jpn. Agric. Res. Q. 2013, 47, 53–63. [Google Scholar] [CrossRef]
- Wout, R.; Greenwell, H.; Davies, D.; Theodorou, M. Methods of Ensiling Algae, Ensiled Algae and Uses of Ensiled Algae. WO2013045931-A1, 4 April 2013. [Google Scholar]
- McLaren, J. Sugarcane as a Feedstock for Biofuels: An Analytical White Paper; National Corn Growers Association: Chesterfield, MO, USA, 2009. [Google Scholar]
- Eurostat Handbook for Annual Crop Statistics; European Commission Eurostat: Luxembourg, 2014.
- Valderrama, D.; Cai, J.; Hishamunda, N.; Ridler, N. Social and Economic Dimensions of Carrageenan Seaweed Farming; FAO Fisheries and Aquaculture Technical Paper 580; Food and Agriculture Organisation of the United Nations (FAO): Rome, Italy, 2014. [Google Scholar]
- Fudholi, A.; Sopian, K.; Othman, M.Y.; Ruslan, M.H. Energy and exergy analyses of solar drying system of red seaweed. Energy Build. 2014, 68, 121–129. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae—A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Oswald, W.J. Large-scale algal culture systems (engineering aspects). In Micro-Algal Biotechnology; Borowitzka, M.A., Borowitzka, L.J., Eds.; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Bruton, T.; Lyons, H.; Lerat, Y.; Stanley, M.; Rasmussen, M.B. A Review of the Potential of Marine Algae as a Source of Biofuel in Ireland; Sustainable Energy Ireland: Dublin, Ireland, 2009. [Google Scholar]
- Demirbas, A. Biomass resource facilities and biomass conversion processing for fuels and chemicals. Energy Convers. Manag. 2001, 42, 1357–1378. [Google Scholar] [CrossRef]
- Yu, L.J.; Wang, S.; Jiang, X.M.; Wang, N.; Zhang, C.Q. Thermal analysis studies on combustion characteristics of seaweed. J. Therm. Anal. Calorim. 2008, 93, 611–617. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, X.M.; Wang, Q.; Han, X.X.; Ji, H.S. Experiment and grey relational analysis of seaweed particle combustion in a fluidized bed. Energy Convers. Manag. 2013, 66, 115–120. [Google Scholar] [CrossRef]
- Ross, A.B.; Jones, J.M.; Kubacki, M.L.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Varfolomeev, S.D.; Wasserman, L.A. Microalgae as source of biofuel, food, fodder, and medicines. Appl. Biochem. Microbiol. 2011, 47, 789–807. [Google Scholar] [CrossRef]
- Yantovski, E.I. Solar energy conversion through seaweed photosynthesis and zero emissions power generation. Surface Eng. Appl. Electrochem. 2008, 44, 138–145. [Google Scholar] [CrossRef]
- Saidur, R.; Abdelaziz, E.A.; Demirbas, A.; Hossain, M.S.; Mekhilef, S. A review on biomass as a fuel for boilers. Renew. Sustain. Energy Rev. 2011, 15, 2262–2289. [Google Scholar] [CrossRef]
- Misra, M.K.; Ragland, K.W.; Baker, A.J. Wood ash composition as a function of furnace temperature. Biomass Bioenergy 1993, 4, 103–116. [Google Scholar] [CrossRef]
- Alvarado-Morales, M.; Boldrin, A.; Karakashev, D.B.; Holdt, S.L.; Angelidaki, I.; Astrup, T. Life cycle assessment of biofuel production from brown seaweed in nordic conditions. Bioresour. Technol. 2013, 129, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Olesen, B.; Arias, C.; Jensen, P.D. Bioenergy potential of ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Nikolaison, L.; Dahl, J.; Bech, K.S.; Bruhn, A.; Rasmussen, M.B.; Bjerre, A.B.; Nielsen, H.B.; Ambus, P.; Rost, K.A.; Kadar, Z.; et al. Energy production fom macroalgae. In Proceedings of the 20th European Biomass Conference, Milan, Italy, 18–22 June 2012.
- Adams, J.M.M.; Ross, A.B.; Anastasakis, K.; Hodgson, E.M.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [PubMed]
- Milledge, J.J.; Staple, A.; Harvey, P. Pyrolysis of Invasive Seaweed Species. In Proceedings of the British Phycological Society Annual Meeting, Galway, Ireland, 25–27 June 2014.
- Salter, A.; Banks, C. Anaerobic digestion: Overall energy balances—parasitic inputs & beneficial outputs. In Proceedings of the Sustainable Organic Resources Partnership—Advances in Biological Processes for Organics and Energy Recycling, Birmingham, UK, 15 May 2008.
- Belosevic, S. Modeling approaches to predict biomass co-firing with pulverized coal. Open Thermodyn. J. 2010, 4, 50–70. [Google Scholar]
- Kadam, K.L. Environmental implications of power generation via coal-microalgae cofiring. Energy 2002, 27, 905–922. [Google Scholar] [CrossRef]
- Li, L.; Rowbotham, J.S.; Greenwell, C.H.; Dyer, P.W. An introduction to pyrolysis and catalytic pyrolysis: Versatile techniques for biomass conversion. In New and Future Developments in Catalysis : Catalytic Biomass Conversion; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 173–208. [Google Scholar]
- Ghasemi, Y.; Rasoul-Amini, S.; Naseri, A.T.; Montazeri-Najafabady, N.; Mobasher, M.A.; Dabbagh, F. Microalgae biofuel potentials (review). Appl. Biochem. Microbiol. 2012, 48, 126–144. [Google Scholar]
- Peacocke, C.; Joseph, S. Notes on Terminology and Technology in Thermal Conversion. Available online: http://www.biochar-international.org/publications/IBI#Pyrolysis_guidelines (accessed on 15 April 2014).
- Miao, X.L.; Wu, Q.Y.; Yang, C.Y. Fast pyrolysis of microalgae to produce renewable fuels. J. Anal. Appl. Pyrolysis 2004, 71, 855–863. [Google Scholar] [CrossRef]
- Song, L.; Hu, M.; Liu, D.; Zhang, D.; Jiang, C. Thermal cracking of enteromorpha prolifera with solvents to bio-oil. Energy Convers. Manag. 2014, 77, 7–12. [Google Scholar] [CrossRef]
- Jena, U.; Das, K.C. Comparative evaluation of thermochemical liquefaction and pyrolysis for bio-oil production from microalgae. Energy Fuels 2011, 25, 5472–5482. [Google Scholar] [CrossRef]
- Marcilla, A.; Catalá, L.; García-Quesada, J.C.; Valdés, F.J.; Hernández, M.R. A review of thermochemical conversion of microalgae. Renew. Sustain. Energy Rev. 2013, 27, 11–19. [Google Scholar] [CrossRef]
- Bhola, V.; Desikan, R.; Santosh, S.K.; Subburamu, K.; Sanniyasi, E.; Bux, F. Effects of parameters affecting biomass yield and thermal behaviour of chlorella vulgaris. J. Biosci. Bioeng. 2011, 111, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Demirbas, A. Hydrogen from mosses and algae via pyrolysis and steam gasification. Energy Sources Part A-Recovery Util. Environ. Eff. 2010, 32, 172–179. [Google Scholar] [CrossRef]
- Miao, X.L.; Wu, Q.Y. High yield bio-oil production from fast pyrolysis by metabolic controlling of chlorella protothecoides. J. Biotechnol. 2004, 110, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ozkurt, I. Qualifying of safflower and algae for energy. Energy Educ. Sci. Technol.-Part A 2009, 23, 145–151. [Google Scholar]
- Du, Z.Y.; Li, Y.C.; Wang, X.Q.; Wan, Y.Q.; Chen, Q.; Wang, C.G.; Lin, X.Y.; Liu, Y.H.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.N.; Jensen, P.A.; Dam-Johansen, K.; Knudsen, N.O.; Sorensen, H.R.; Hvilsted, S. Comparison of lignin, macroalgae, wood, and straw fast pyrolysis. Energy Fuels 2013, 27, 1399–1409. [Google Scholar] [CrossRef]
- Yanik, J.; Stahl, R.; Troeger, N.; Sinag, A. Pyrolysis of algal biomass. J. Anal. Appl. Pyrolysis 2013, 103, 134–141. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Jiang, X.M.; Han, X.X.; Ji, H.S. Compositional analysis of bio-oil derived from pyrolysis of seaweed. Energy Convers. Manag. 2013, 68, 273–280. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Dyer, P.W.; Greenwell, H.C.; Selby, D.; Theodorou, M.K. Copper(II)-mediated thermolysis of alginates: A model kinetic study on the influence of metal ions in the thermochemical processing of macroalgae. Interface Focus 2013, 3. [Google Scholar] [CrossRef]
- Stratford, J.P.; Hutchings, T.R.; de Leij, F.A.A.M. Intrinsic activation: The relationship between biomass inorganic content and porosity formation during pyrolysis. Bioresour. Technol. 2014, 159, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Choi, J.-W.; Suh, D.J.; Ha, J.-M.; Hwang, J.W.; Jung, H.W.; Lee, K.-Y.; Woo, H.-C. Production of brown algae pyrolysis oils for liquid biofuels depending on the chemical pretreatment methods. Energy Convers. Manag. 2014, 86, 371–378. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of fast pyrolysis of biomass and product upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Peng, W.M.; Wu, Q.Y.; Tu, P.G. Effects of temperature and holding time on production of renewable fuels from pyrolysis of chlorella protothecoides. J. Appl. Phycol. 2000, 12, 147–152. [Google Scholar] [CrossRef]
- Rowbotham, J.S.; Dyer, P.W.; Greenwell, H.C.; Theodorou, M.K. Thermochemical processing of macroalgae: A late bloomer in the development of third-generation biofuels? Biofuels 2012, 3, 441–461. [Google Scholar] [CrossRef]
- Williams, P.T.; Besler, S. The influence of temperature and heating rate on the pyrolysis of biomass. Renew. Energy 1996, 7, 233–250. [Google Scholar] [CrossRef]
- Zhao, H.; Yan, H.X.; Liu, M.; Sun, B.B.; Zhang, Y.; Dong, S.S.; Qi, L.B.; Qin, S. Production of bio-oil from fast pyrolysis of macroalgae enteromorpha prolifera powder in a free-fall reactor. Energy Sources Part A-Recovery Util. Environ. Eff. 2013, 35, 859–867. [Google Scholar] [CrossRef]
- Lee, H.W.; Choi, S.J.; Park, S.H.; Jeon, J.-K.; Jung, S.-C.; Joo, S.H.; Park, Y.-K. Catalytic conversion of laminaria japonica over microporous zeolites. Energy 2014, 66, 2–6. [Google Scholar] [CrossRef]
- Maddi, B.; Viamajala, S.; Varanasi, S. Comparative study of pyrolysis of algal biomass from natural lake blooms with lignocellulosic biomass. Bioresour. Technol. 2011, 102, 11018–11026. [Google Scholar] [CrossRef] [PubMed]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic pyrolysis of microalgae to high-quality liquid bio-fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Bird, K.T.; Benson, P.H. Seaweed Cultivation for Renewable Resources; Elsevier: Bergen, Norway, 1987. [Google Scholar]
- Viger, M.; Hancock, R.D.; Miglietta, F.; Taylor, G. More plant growth but less plant defence? First global gene expression data for plants grown in soil amended with biochar. GCB Bioenergy 2014. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 3): Gasification technologies. Bioresour. Technol. 2002, 83, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Production of Bio-Methanol-Technology Brief; International Renewable Energy Agency (IRENA): Abu Dhabi, UAE, 2013.
- Ahmed, I.I.; Gupta, A.K. Pyrolysis and gasification of food waste: Syngas characteristics and char gasification kinetics. Appl. Energy 2010, 87, 101–108. [Google Scholar] [CrossRef]
- Hayashi, J.-I.; Kudo, S.; Kim, H.-S.; Norinaga, K.; Matsuoka, K.; Hosokai, S. Low-temperature gasification of biomass and lignite: Consideration of key thermochemical phenomena, rearrangement of reactions, and reactor configuration. Energy Fuels 2013, 28, 4–21. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Savage, P.E.; Wei, C.H. Gasification of alga nannochloropsis sp in supercritical water. J. Supercrit. Fluids 2012, 61, 139–145. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Wei, C.H.; Savage, P.E. Kinetic model for supercritical water gasification of algae. Phys. Chem. Chem. Phys. 2012, 14, 3140–3147. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.; Lehmann, J.; Fisher, E.M.; Angenent, L.T. Biofuels from pyrolysis in perspective: Trade-offs between energy yields and soil-carbon additions. Environ. Sci. Technol. 2014, 48, 6492–6499. [Google Scholar] [CrossRef] [PubMed]
- Cherad, R.; Onwudili, J.A.; Ekpo, U.; Williams, P.T.; Lea-Langton, A.R.; Carmargo-Valero, M.; Ross, A.B. Macroalgae supercritical water gasification combined with nutrient recycling for microalgae cultivation. Environ. Prog. Sustain. Energy 2013, 32, 902–909. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Lea-Langton, A.R.; Ross, A.B.; Williams, P.T. Catalytic hydrothermal gasification of algae for hydrogen production: Composition of reaction products and potential for nutrient recycling. Bioresour. Technol. 2013, 127, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.E.; Jeon, Y.J.; Yi, H. New candidate for biofuel feedstock beyond terrestrial biomass for thermo-chemical process (pyrolysis/gasification) enhanced by carbon dioxide (CO2). Bioresour. Technol. 2012, 123, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Anastasakis, K.; Kubacki, M.; Jones, J.M. Investigation of the pyrolysis behaviour of brown algae before and after pre-treatment using PY-GC/MS and TGA. J. Anal. Appl. Pyrolysis 2009, 85, 3–10. [Google Scholar] [CrossRef]
- Kaewpanha, M.; Guan, G.; Hao, X.; Wang, Z.; Kasai, Y.; Kusakabe, K.; Abudula, A. Steam co-gasification of brown seaweed and land-based biomass. Fuel Process. Technol. 2014, 120, 106–112. [Google Scholar] [CrossRef]
- Rizkiana, J.; Guan, G.Q.; Widayatno, W.B.; Hao, X.G.; Huang, W.; Tsutsumi, A.; Abudula, A. Effect of biomass type on the performance of cogasification of low rank coal with biomass at relatively low temperatures. Fuel 2014, 134, 414–419. [Google Scholar] [CrossRef]
- Singh, J.; Gu, S. Biomass conversion to energy in India—A critique. Renew. Sustain. Energy Rev. 2010, 14, 1367–1378. [Google Scholar] [CrossRef]
- Ventura, J.-R.S.; Yang, B.; Lee, Y.-W.; Lee, K.; Jahng, D. Life cycle analyses of CO2, energy, and cost for four different routes of microalgal bioenergy conversion. Bioresour. Technol. 2013, 137, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Delrue, F.; Seiter, P.A.; Sahut, C.; Cournac, L.; Roubaud, A.; Peltier, G.; Froment, A.K. An economic, sustainability, and energetic model of biodiesel production from microalgae. Bioresour. Technol. 2012, 111, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Dry, M.E. The fischer-tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- De Klerk, A.; Furimsky, E. Fischer-tropsch synthesis. In Catalysis in the Refining of Fischer-Tropsch Syncrude; The Royal Society of Chemistry: Cambridge, UK, 2010; pp. 11–23. [Google Scholar]
- Affordable, Low-Carbon Diesel Fuel from Domestic Coal and Biomass; DOE/NETL-2009/1349; National Energy Technology Laboratory NETL: Morgantown, WV, USA, 2009.
- Van Steen, E.; Claeys, M. Fischer-tropsch catalysts for the biomass-to liquid process. Chem. Eng. Technol. 2008, 31, 655–666. [Google Scholar] [CrossRef]
- Jahangiri, H.; Bennett, J.; Mahjoubi, P.; Wilson, K.; Gu, S. A review of advanced catalyst development for fischer-tropsch synthesis of hydrocarbons from biomass derived syn-gas. Catal. Sci. Technol. 2014, 4, 2210–2229. [Google Scholar] [CrossRef] [Green Version]
- Triantafyllidis, K.; Lappas, A.; Stöcker, M. The Role of Catalysis for the Sustainable Production of Bio-Fuels and Bio-Chemicals; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Derouane, E.G.; Parmon, V.; Lemos, F.; Ribeiro, F.R. Sustainable Strategies for the Upgrading of Natural Gas: Fundamentals, Challenges, and Opportunities; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Lapidus, A.L. The mechanism of hydrocarbon synthesis from Co and H2 on cobalt catalysts. Solid Fuel Chem. 2013, 47, 315–328. [Google Scholar] [CrossRef]
- Leckel, D. Hydrocracking of iron-catalyzed fischer-tropsch waxes. Energy Fuels 2005, 19, 1795–1803. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Faaij, A.P.C.; den Uil, H.; Boerrigter, H. Production of FT transportation fuels from biomass; technical options, process analysis and optimisation, and development potential. Energy 2004, 29, 1743–1771. [Google Scholar] [CrossRef]
- Maitlis, P.M.; de Klerk, A. Greener Fischer-Tropsch Processes for Fuels and Feedstocks; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Tijmensen, M.J.A.; Faaij, A.P.C.; Hamelinck, C.N.; van Hardeveld, M.R.M. Exploration of the possibilities for production of fischer tropsch liquids and power via biomass gasification. Biomass Bioenergy 2002, 23, 129–152. [Google Scholar] [CrossRef]
- Huang, G.; Chen, F.; Wei, D.; Zhang, X.; Chen, G. Biodiesel production by microalgal biotechnology. Appl. Energy 2010, 87, 38–46. [Google Scholar] [CrossRef]
- Bahadar, A.; Bilal Khan, M. Progress in energy from microalgae: A review. Renew. Sustain. Energy Rev. 2013, 27, 128–148. [Google Scholar] [CrossRef]
- Lenstra, W.J.; Hal, J.W.V.; Reith, J.H. Economic aspects of open ocean seaweed cultivation. In Proceedings of the Alg’n Chem 2011, Algae, New Resources for Industry, Montpellier, France, 7–10 November 2011.
- Streefland, M. Report on Biofuel Production Processes from Micro, Macroalgae and Other Aquatic Biomass; AquaFUELs: Brussels, Belgium, 2010. [Google Scholar]
- Van der Wal, H.; Sperber, B.L.H.M.; Houweling-Tan, B.; Bakker, R.R.C.; Brandenburg, W.; López-Contreras, A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013, 128, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Heaven, S.; Milledge, J.; Zhang, Y. Comments ‘on anaerobic digestion of microalgae as a necessary step to make microalgal biodiesel sustainable’. Biotechnol. Adv. 2011, 29, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Aresta, M.; Dibenedetto, A.; Carone, M.; Colonna, T.; Fragale, C. Production of biodiesel from macroalgae by supercritical CO2 extraction and thermochemical liquefaction. Environ. Chem. Lett. 2005, 3, 136–139. [Google Scholar] [CrossRef]
- Suganya, T.; Renganathan, S. Optimization and kinetic studies on algal oil extraction from marine macroalgae Ulva lactuca. Bioresour. Technol. 2012, 107, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Suganya, T.; Nagendra Gandhi, N.; Renganathan, S. Production of algal biodiesel from marine macroalgae enteromorpha compressa by two step process: Optimization and kinetic study. Bioresour. Technol. 2013, 128, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Anastasakis, K.; Ross, A.B. Hydrothermal liquefaction of the brown macro-alga laminaria saccharina: Effect of reaction conditions on product distribution and composition. Bioresour. Technol. 2011, 102, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Florentinus, A.; Harmelinck, C.; Lint, S.D.; Iersel, S.V. Worldwide Potential of Aquatic Biomass; Ecofys: Utrecht, The Netherlands, 2008. [Google Scholar]
- Neveux, N.; Yuen, A.K.L.; Jazrawi, C.; Magnusson, M.; Haynes, B.S.; Masters, A.F.; Montoya, A.; Paul, N.A.; Maschmeyer, T.; de Nys, R. Biocrude yield and productivity from the hydrothermal liquefaction of marine and freshwater green macroalgae. Bioresour. Technol. 2014, 155, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Minowa, T.; Yokoyama, S.; Kishimoto, M.; Okakura, T. Oil production from algal cells of dunaliella-tertiolecta by direct thermochemical liquefaction. Fuel 1995, 74, 1735–1738. [Google Scholar] [CrossRef]
- Sawayama, S.; Minowa, T.; Yokoyama, S.Y. Possibility of renewable energy production and CO2 mitigation by thermochemical liquefaction of microalgae. Biomass Bioenergy 1999, 17, 33–39. [Google Scholar] [CrossRef]
- Brown, T.M.; Duan, P.; Savage, P.E. Hydrothermal liquefaction and gasification of Nannochloropsis sp. Energy Fuels 2010, 24, 3639–3646. [Google Scholar] [CrossRef]
- Torri, C.; Alba, L.G.; Samori, C.; Fabbri, D.; Brilman, D.W.F. Hydrothermal treatment (HTT) of microalgae: Detailed molecular characterization of HTT oil in view of htt mechanism elucidation. Energy Fuels 2012, 26, 658–671. [Google Scholar] [CrossRef]
- Vardon, D.R.; Sharma, B.K.; Blazina, G.V.; Rajagopalan, K.; Strathmann, T.J. Thermochemical conversion of raw and defatted algal biomass via hydrothermal liquefaction and slow pyrolysis. Bioresour. Technol. 2012, 109, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Alba, L.G.; Torri, C.; Samori, C.; van der Spek, J.; Fabbri, D.; Kersten, S.R.A.; Brilman, D.W.F. Hydrothermal treatment of microalgae: Evaluation of the process as conversion method in an algae biorefinery concept. Energy Fuels 2012, 26, 642–657. [Google Scholar] [CrossRef]
- Bach, Q.-V.; Sillero, M.V.; Tran, K.-Q.; Skjermo, J. Fast hydrothermal liquefaction of a norwegian macro-alga: Screening tests. Algal Res. 2014. [Google Scholar] [CrossRef]
- Jin, B.; Duan, P.; Xu, Y.; Wang, F.; Fan, Y. Co-liquefaction of micro- and macroalgae in subcritical water. Bioresour. Technol. 2013, 149, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xu, M.; Zhang, X.Z.; Hu, Q.A.; Sommerfeld, M.; Chen, Y.S. Life-cycle analysis on biodiesel production from microalgae: Water footprint and nutrients balance. Bioresour. Technol. 2011, 102, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Balat, H.; Oz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- U.S. Department of Energy (DOE). National Algal Biofuels Technology Roadmap; U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Biomass Program: Washington, DC, USA, 2010. [Google Scholar]
- Puri, D.J.; Heaven, S.; Banks, C.J. Improving the performance of enzymes in hydrolysis of high solids paper pulp derived from MSW. Biotechnol. Biofuels 2013, 6. [Google Scholar] [CrossRef] [PubMed]
- Gressel, J. Transgenics are imperative for biofuel crops. Plant Sci. 2008, 174, 246–263. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Kawai, S.; Murata, K. Strategies for the production of high concentrations of bioethanol from seaweeds: Production of high concentrations of bioethanol from seaweeds. Bioengineered 2013, 4, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Golberg, A.; Vitkin, E.; Linshiz, G.; Khan, S.A.; Hillson, N.J.; Yakhini, Z.; Yarmush, M.L. Proposed design of distributed macroalgal biorefineries: Thermodynamics, bioconversion technology, and sustainability implications for developing economies. Biofuels Bioprod. Biorefining 2014, 8, 67–82. [Google Scholar] [CrossRef]
- Borines, M.G.; de Leon, R.L.; Cuello, J.L. Bioethanol production from the macroalgae Sargassum spp. Bioresour. Technol. 2013, 138, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.A.; Lim, S.R.; Kim, Y.; Park, J.M. Potentials of macroalgae as feedstocks for biorefinery. Bioresour. Technol. 2013, 135, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Quarterman, J.; Jin, Y.S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Yoza, B.A.; Masutani, E.M. The analysis of macroalgae biomass found around Hawaii for bioethanol production. Environ. Technol. 2013, 34, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.; Hong, Y.-K.; Jeong, G.-T. Detoxification of acidic catalyzed hydrolysate of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Huesemann, M.H.; Kuo, L.J.; Urquhart, L.; Gill, G.A.; Roesijadi, G. Acetone-butanol fermentation of marine macroalgae. Bioresour. Technol. 2012, 108, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Meinita, M.D.N.; Marhaeni, B.; Winanto, T.; Jeong, G.T.; Khan, M.N.A.; Hong, Y.K. Comparison of agarophytes (Gelidium, Gracilaria, and Gracilariopsis) as potential resources for bioethanol production. J. Appl. Phycol. 2013, 25, 1957–1961. [Google Scholar] [CrossRef]
- Wargacki, A.J.; Leonard, E.; Win, M.N.; Regitsky, D.D.; Santos, C.N.S.; Kim, P.B.; Cooper, S.R.; Raisner, R.M.; Herman, A.; Sivitz, A.B.; et al. An engineered microbial platform for direct biofuel production from brown macroalgae. Science 2012, 335, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, M.; Asaoka, K.; Atsumi, M.; Sakou, T. Seaweed Bioethanol Production in Japan—The Ocean Sunrise Project. In Proceedings of the OCEANS 2007, Vancouver, BC, Canada, 29 September–4 October 2007; pp. 1–5.
- Beal, C.M. Algal Biofuels: Energy and Water. In WEG Symposium; University of Texas: Austin, TX, USA, 2011. [Google Scholar]
- Walker, D.A. Biofuels—for better or worse? Ann. Appl. Biol. 2010, 156, 319–327. [Google Scholar] [CrossRef]
- Philippsen, A. Energy Input, Carbon Intensity, and Cost for Ethanol Produced from Brown Seaweed; University of Victoria: Victoria, BC, Canada, 2013. [Google Scholar]
- Huesemann, M.; Roesjadi, G.; Benemann, J.; Metting, F.B. Biofuels from Microalgae and Seaweeds. In Biomass to Biofuels; Blackwell Publishing Ltd.: Oxford, UK, 2010; pp. 165–184. [Google Scholar]
- Horn, S.J.; Aasen, I.M.; Ostgaard, K. Ethanol production from seaweed extract. J. Ind. Microbiol. Biotechnol. 2000, 25, 249–254. [Google Scholar] [CrossRef]
- Parliamentary Office of Science & Technology. Biofuels from Algae; Postnote: London, UK, 2011. [Google Scholar]
- Potts, T.; Du, J.; Paul, M.; May, P.; Beitle, R.; Hestekin, J. The production of butanol from Jamaica bay macro algae. Environ. Prog. Sustain. Energy 2012, 31, 29–36. [Google Scholar] [CrossRef]
- Sutherland, A.; Varela, J. Comparison of various microbial inocula for the efficient anaerobic digestion of Laminaria hyperborea. BMC Biotechnol. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Biomara. A Short History of Seaweed Exploitation in the Western British Isles. Available online: http://www.biomara.org/understanding-seaweed/the-importance-of-seaweed-across-the-ages (accessed on 27 January 2014).
- Discover Tiree. Brown Gold. Available online: http://www.isleoftiree.com/about-tiree/the-land/ (accessed on 27 January 2014).
- Park, J.B.K.; Craggs, R.J.; Shilton, A.N. Wastewater treatment high rate algal ponds for biofuel production. Bioresour. Technol. 2011, 102, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.; LeDuy, A. Improved performance of anaerobic digestion of spirulina maxima algal biomass by addition of carbon-rich wastes. Biotechnol. Lett. 1983, 5, 677–682. [Google Scholar] [CrossRef]
- Gonzalez-Fernandez, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Impact of microalgae characteristics on their conversion to biofuel. Part II: Focus on biomethane production. Biofuels Bioprod. Biorefining 2012, 6, 205–218. [Google Scholar] [CrossRef]
- Park, S.; Li, Y.B. Evaluation of methane production and macronutrient degradation in the anaerobic co-digestion of algae biomass residue and lipid waste. Bioresour. Technol. 2012, 111, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Zamalloa, C.; Vulsteke, E.; Albrecht, J.; Verstraete, W. The techno-economic potential of renewable energy through the anaerobic digestion of microalgae. Bioresour. Technol. 2011, 102, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Buswell, A.M.; Mueller, H.F. Mechanism of methane fermentation. Ind. Eng. Chem. 1952, 44, 550–552. [Google Scholar] [CrossRef]
- Symons, G.E.; Buswell, A.M. The methane fermentation of carbohydrates. J. Am. Chem. Soc. 1933, 55, 2028–2036. [Google Scholar] [CrossRef]
- Golueke, C.G.; Oswald, W.J.; Gotaas, H.B. Anaerobic digestion of algae. Appl. Microbiol. 1957, 5, 47–55. [Google Scholar] [PubMed]
- Hilton, M.G.; Archer, D.B. Anaerobic digestion of a sulfate-rich molasses wastewater: Inhibition of hydrogen sulfide production. Biotechnol. Bioeng. 1988, 31, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Hierholtzer, A.; Akunna, J.C. Modelling sodium inhibition on the anaerobic digestion process. Water Sci. Technol. 2012, 66, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, O.; Moletta, R. Treatment of organic pollution in industrial saline wastewater: A literature review. Water Res. 2006, 40, 3671–3682. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.H.; Han, S.K.; Sung, S. Sodium inhibition of thermophilic methanogens. J. Environ. Eng.-ASCE 2003, 129, 506–512. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Kumaraswamy, S.; Mallick, K.; Adhya, T.K.; Rao, V.R.; Sethunathan, N. Effect of various anionic species on net methane production in flooded rice soils. World J. Microbiol. Biotechnol. 1998, 14, 743–749. [Google Scholar] [CrossRef]
- El-Dessouky, H.T.; Ettouney, H.M. Fundamentals of Salt Water Desalination; Elsevier: Amsterdam, The Netherlands, 2002. [Google Scholar]
- Tedesco, S.; Barroso, T.M.; Olabi, A.G. Optimization of mechanical pre-treatment of Laminariaceae spp. Biomass-derived biogas. Renew. Energy 2014, 62, 527–534. [Google Scholar] [CrossRef]
- Ter Veld, F. Beyond the fossil fuel era: On the feasibility of sustainable electricity generation using biogas from microalgae. Energy Fuels 2012, 26, 3882–3890. [Google Scholar] [CrossRef]
- Milledge, J.J. Micro-algal biorefineries. In Towards Establishing Value Chains for Bioenergy; The African, Caribbean and Pacific Group of States (ACP) Science and Technology Programme: Swakopmund, Namibia, 2013. [Google Scholar]
- Lenstra, W.J.; Reith, J.H.; Hal, J.W.V. Economic Perspectives of Seaweed. In Proceedings of the 4th International Algae Congress, Amsterdam, The Netherlands, 1–2 December 2010.
- Milledge, J.J. Commercial application of microalgae other than as biofuels: A brief review. Rev. Environ. Sci. Biotechnol. 2011, 10, 31–41. [Google Scholar] [CrossRef]
- Milledge, J.J. Micro-Algal Process Flow-Sheet Energy Balance Optimisation: Initial Software Evaluation. In Algal Biotechnology: Biofuels and Beyond; University College (UCL): London, UK, 2012. [Google Scholar]
- Milledge, J.J. The Challenge of Algal Fuel: Economic Processing of the Entire Algal Biomass. In Condensed Matter—Materials Engineering Newsletter; McMaster University: Hamilton, PA, USA, 2010; Volume 1, pp. 4–6. [Google Scholar]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Biofuels and the biorefinery concept. Energy Policy 2008, 36, 4406–4409. [Google Scholar] [CrossRef]
- Olguin, E.J. Dual purpose microalgae-bacteria-based systems that treat wastewater and produce biodiesel and chemical products within a biorefinery. Biotechnol. Adv. 2012, 30, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Delgado, A.D.; Kafarov, V. Microalgae based biorefinery: Issues to consider. A review. CT F Cienc. Tecnol. Futuro 2011, 4, 5–21. [Google Scholar]
- Rawat, I.; Ranjith Kumar, R.; Mutanda, T.; Bux, F. Biodiesel from microalgae: A critical evaluation from laboratory to large scale production. Appl. Energy 2013, 103, 444–467. [Google Scholar] [CrossRef]
- Singh, U.; Ahluwalia, A. Microalgae: A promising tool for carbon sequestration. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 73–95. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Alvim-Ferraz, M.C.M.; Martins, F.G.; Simoes, M. Carbon dioxide capture from flue gases using microalgae: Engineering aspects and biorefinery concept. Renew. Sustain. Energy Rev. 2012, 16, 3043–3053. [Google Scholar] [CrossRef]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milledge, J.J.; Smith, B.; Dyer, P.W.; Harvey, P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies 2014, 7, 7194-7222. https://doi.org/10.3390/en7117194
Milledge JJ, Smith B, Dyer PW, Harvey P. Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass. Energies. 2014; 7(11):7194-7222. https://doi.org/10.3390/en7117194
Chicago/Turabian StyleMilledge, John J., Benjamin Smith, Philip W. Dyer, and Patricia Harvey. 2014. "Macroalgae-Derived Biofuel: A Review of Methods of Energy Extraction from Seaweed Biomass" Energies 7, no. 11: 7194-7222. https://doi.org/10.3390/en7117194






