Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstocks Preparation
2.2. Fluidized Bed Gasification
2.3. Property Analysis of Biomass and Char
3. Results and Discussion
3.1. Physical and Chemical Properties
3.1.1. Proximate Analysis and Char Yields
| Content | Switchgrass | Sorghum | Red cedar |
|---|---|---|---|
| Moisture (w.b.) | 9.70 | 9.39 | 8.50 |
| Volatile matter (w.b.) | 70.36 | 68.1 | 71.79 |
| Ash (w.b.) | 4.62 | 5.05 | 4.09 |
| Fixed carbon (w.b.) | 15.02 | 17.46 | 15.62 |
| Nitrogen (d.b.) | 0.57 | 0.51 | 0.37 |
| Hydrogen (d.b.) | 5.74 | 6.4 | 6.27 |
| Sulfur (d.b.) | 0.30 | 0.20 | 1.07 |
| Carbon (d.b.) | 43.19 | 40.68 | 47.51 |
| Oxygen (d.b.) | 50.20 | 52.2 | 44.79 |
| Feedstock | ER | Moisture (wt% on w.b.) | Volatile (wt% on w.b.) | Ash (wt% on w.b.) | Fixed carbon (wt% on w.b.) | HHV (MJ/Kg) | BET surface area (m²/g) | Micropore volume (10−3 mL/g) |
|---|---|---|---|---|---|---|---|---|
| Switchgrass | 0.20 | 0.69 ± 0.09 | 12.69 ± 1.48 | 51.61 ± 2.21 | 34.99 ± 0.57 | 7.40 | 1.3 | 0.63 |
| 0.25 | 2.01 ± 0.18 | 16.86 ± 0.89 | 57.70 ± 2.67 | 23.42 ± 1.39 | 4.03 | 5.2 | 2.84 | |
| 0.28 | 1.83 ± 0.37 | 12.11 ± 0.71 | 64.07 ± 1.29 | 21.98 ± 0.67 | 6.70 | 20.8 | 11.88 | |
| Sorghum | 0.20 | 1.99 ± 0.20 | 14.24 ± 0.71 | 50.89 ± 0.59 | 33.76 ± 0.34 | 4.18 | 1.0 | 0.45 |
| 0.25 | 1.94 ± 0.13 | 20.01 ± 2.12 | 45.94 ± 2.49 | 32.10 ± 0.35 | 9.42 | 0.7 | 0.44 | |
| 0.28 | 1.1 ± 0.11 | 11.36 ± 1.06 | 54.87 ± 1.17 | 32.67 ± 0.16 | 4.63 | 5.6 | 2.14 | |
| Red cedar | 0.20 | 3.4 ± 0.27 | 15.72 ± 1.41 | 40.41 ± 1.00 | 40.49 ± 0.10 | 9.09 | 2.1 | 1.57 |
| 0.25 | 3.1 ± 0.17 | 15.68 ± 0.81 | 43.89 ± 3.65 | 37.33 ± 2.13 | 5.87 | 60.8 | 31.33 | |
| 0.28 | 2.7 ± 0.14 | 14.14 ± 1.70 | 47.52 ± 0.81 | 35.66 ± 0.89 | 4.07 | 30.6 | 16.34 |
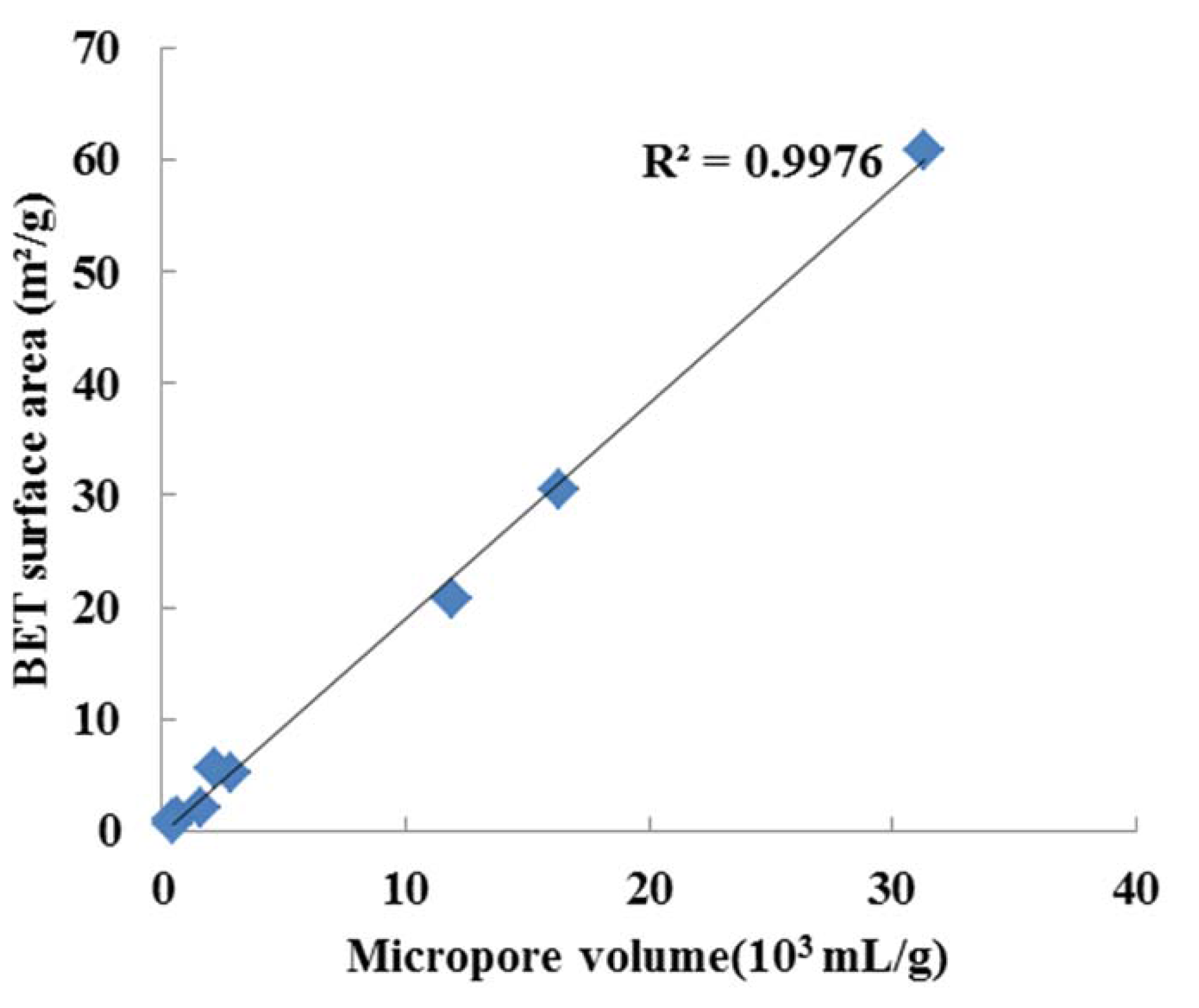
3.1.2. Heating Value and BET Surface Area
3.1.3. SEM Morphology

3.2. Elemental (Proximate) Analysis
| Feedstock | ER | Carbon (wt%, d.b.) | Hydrogen (wt%, d.b.) | Nitrogen (wt%, d.b.) | Sulfur (wt%, d.b.) |
|---|---|---|---|---|---|
| Switchgrass | 0.20 | 48.29 ± 0.80 | 1.21 ± 0.30 | 0.67 ± 0.06 | 0.22 ± 0.09 |
| 0.25 | 34.73 ± 2.35 | 0.65 ± 0.01 | 0.65 ± 0.05 | 0.07 ± 0.01 | |
| 0.28 | 38.55 ± 1.59 | 0.82 ± 0.04 | 0.66 ± 0.08 | 0.12 ± 0.01 | |
| Sorghum | 0.20 | 38.5 ± 13.13 | 0.80 ± 0.05 | 1.46 ± 0.17 | 0.14 ± 0.01 |
| 0.25 | 40.11 ± 0.16 | 0.94 ± 0.02 | 1.48 ± 0.04 | 0.13 ± 0.00 | |
| 0.28 | 40.69 ± 1.23 | 0.79 ± 0.03 | 0.92 ± 0.13 | 0.10 ± 0.01 | |
| Red cedar | 0.20 | 45.14 ± 0.83 | 1.12 ± 0.06 | 0.26 ± 0.08 | 0.13 ± 0.01 |
| 0.25 | 44.89 ± 0.76 | 1.05 ± 0.07 | 0.51 ± 0.03 | 0.20 ± 0.02 | |
| 0.28 | 43.71 ± 2.40 | 0.99 ± 0.42 | 0.61 ± 0.15 | 0.19 ± 0.07 |

3.3. Mineral Content
| Feedstock | ER | P (%) | Ca (%) | K (%) | Mg (%) | Na (%) | S (%) | Fe (ppm) | Zn (ppm) | Cu (ppm) | Mn (ppm) | Ni (ppm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Switchgrass | 0* | 0.10 | 0.25 | 0.89 | 0.26 | 0.002 | 0.05 | 134 | 25 | 2 | 38 | 3 |
| 0.20 | 0.53 | 1.70 | 1.52 | 0.82 | 0.05 | 0.08 | 10,692 | 184 | 15 | 602 | 51 | |
| 0.25 | 0.68 | 1.90 | 2.08 | 1.05 | 0.06 | 0.10 | 24,292 | 180 | 33 | 785 | 56 | |
| 0.28 | 0.29 | 0.77 | 0.75 | 0.40 | 0.03 | 0.04 | 6,838 | 78 | 9 | 248 | 27 | |
| Sorghum | 0* | 0.04 | 0.22 | 0.42 | 0.09 | 0.04 | 0.05 | 640 | 10 | 2 | 38 | 1 |
| 0.20 | 0.47 | 1.08 | 4.12 | 0.81 | 0.02 | 0.11 | 3,191 | 73 | 10 | 161 | 8 | |
| 0.25 | 0.71 | 1.63 | 6.25 | 1.34 | 0.02 | 0.13 | 8,207 | 90 | 17 | 278 | 11 | |
| 0.28 | 0.56 | 1.75 | 3.87 | 0.69 | 0.15 | 0.09 | 2,249 | 52 | 6 | 107 | 7 | |
| Red Cedar | 0* | 0.02 | 0.65 | 0.12 | 0.04 | 0.002 | 0.03 | 294 | 9 | 1 | 87 | 1 |
| 0.20 | 0.04 | 0.91 | 0.22 | 0.08 | 0.005 | 0.02 | 2,552 | 40 | 3 | 143 | 9 | |
| 0.25 | 0.12 | 2.64 | 0.71 | 0.26 | 0.018 | 0.07 | 35,592 | 59 | 35 | 529 | 74 | |
| 0.28 | 0.15 | 2.46 | 1.36 | 0.33 | 0.025 | 0.07 | 30,610 | 63 | 21 | 495 | 61 |
3.4. ATR FT-IR Analysis
| Functional group | Characteristic absorption (cm−1) | Feedstocks and chars |
|---|---|---|
| Alkyl C-H Stretch [28] | 2950–2850 | Red cedar, switch grass, sorghum straw, char with 0.2 ER |
| Aromatic C-H Bending [28] | 860–680 | Red cedar, switch grass, sorghum straw and respective chars |
| Aromatic C=C Bending [28] | 1600–1500 | Red cedar, switch grass, sorghum straw |
| aromatic C, indicative of lignin C=C [29] | 1440, 1510 | Red cedar, switchgrass, sorghum straw and sorghum char |
| Alcohol/Phenol O-H Stretch [28] | 3550–3200 | Red cedar, sorghum, sorghum char, switchgrass |
| Aldehyde, Ketone, Ester, Carboxylic Acid [10] | 1780–1700 | Red cedar, switch grass, sorghum straw |
| Phenol O-H bending [28] | 1375 | Raw biomass, switchgrass and sorghum char |
| C-O stretching C-O-C groups and aryl ethers; phenolic C-O associated with lignin [29] | 1270–1250 | Red cedar, switch grass, sorghum straw, switchgrass and sorghum char of ER 0.2 |
| Phosphines and phosphine oxides, Silican oxid, C-O-C stretching [10,30] | 1100–950 | All |

4. Conclusions
Acknowledgements
Conflict of Interest
References
- Zhang, A.; Bian, R.; Pan, G.; Cui, L.; Hussain, Q.; Li, L.; Zheng, J.; Zheng, J.; Zhang, X.; Han, X.; et al. Effects of biochar amendment on soil quality, crop yield and greenhouse gas emission in a Chinese rice paddy: A field study of 2 consecutive rice growing cycles. Field Crop Res. 2012, 127, 153–160. [Google Scholar]
- Meyer, S.; Glaser, B.; Quicker, P. Technical, economical, and climate-related aspects of biochar production technologies: A literature review. Environ. Sci. Technol. 2011, 45, 9473–9483. [Google Scholar]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar]
- Oleszczuk, P.; Hale, S.E.; Lehmann, J.; Cornelissen, G. Activated carbon and biochar amendments decrease pore-water concentrations of polycyclic aromatic hydrocarbons (PAHs) in sewage sludge. Bioresour. Technol. 2012, 111, 84–91. [Google Scholar]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous. Mat. 2008, 110, 413–421. [Google Scholar]
- Lima, I.M.; Marshall, W.E. Adsorption of selected environmentally important metals by poultry manure-based granular activated carbons. J. Chem. Technol Biotechnol. 2005, 80, 1054–1061. [Google Scholar]
- Wang, D.; Yuan, W.; Ji, W. Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Appl. Energy 2011, 88, 1656–1663. [Google Scholar]
- Abu El-Rub, Z.; Bramer, E.A.; Brem, G. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 2008, 87, 2243–2252. [Google Scholar]
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar]
- Kumar, A.; Jones, D.; Hanna, M. Thermochemical biomass gasification: A review of the current status of the technology. Energies 2009, 2, 556–581. [Google Scholar]
- Dąbrowski, A.; Podkościelny, P.; Hubicki, Z.; Barczak, M. Adsorption of phenolic compounds by activated carbon—A critical review. Chemosphere 2005, 58, 1049–1070. [Google Scholar]
- Brewer, C.E.; Schmidt-Rohr, K.; Satrio, J.A.; Brown, R.C. Characterization of biochar from fast pyrolysis and gasification systems. Environ. Prog. Sustain. 2009, 28, 386–396. [Google Scholar]
- Melligan, F.; Auccaise, R.; Novotny, E.H.; Leahy, J.J.; Hayes, M.H.B.; Kwapinski, W. Pressurised pyrolysis of Miscanthus using a fixed bed reactor. Bioresour. Technol. 2011, 102, 3466–3470. [Google Scholar]
- Sharma, A.; Kumar, A.; Patil, K.; Huhnke, R. Performance evaluation of a lab-scale fluidized bed gasifier using switchgrass as feedstock. Trans. ASABE 2011, 54, 2259–2266. [Google Scholar]
- ASTM International. Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke; ASTM Standard D3175-11; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- ASTM International. Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Base Materials; ASTM Standard D4442-07; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM International. Standard Test Method for Ash in Biomass; ASTM Standard E1755-01(2007); ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- ASTM International. Standard Practice for Proximate Analysis of Coal and Coke; ASTM Standard D3172-07a; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Bruun, E.W. Application of Fast Pyrolysis Biochar to a Loamy Soil-Effects on Carbon and Nitrogen Dynamics and Potential for Carbon Sequestration. Ph.D. Thesis, Technical University of Denmark, Roskilde, Denmark, 2011. [Google Scholar]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology; Earthscan: London, UK, 2009. [Google Scholar]
- Gai, C.; Dong, Y.P. Experimental study on non-woody biomass gasification in a downdraft gasifier. Int. J. Hydrog. Energy 2012, 37, 4935–4944. [Google Scholar]
- Maroto-Valer, M.M.; Love, G.D.; Snape, C.E. Relationship between carbon aromaticities and HC ratios for bituminous coals. Fuel 1994, 73, 1926–1928. [Google Scholar]
- Kuhlbusch, T.A.J.; Crutzen, P.J. Toward a global estimate of black carbon in residues of vegetation fires representing a sink of atmospheric CO2 and a source of O2. Glob. Biogeochem. Cycles 1995, 9, 491–501. [Google Scholar]
- Kim, K.H.; Kim, J.-Y.; Cho, T.-S.; Choi, J.W. Influence of pyrolysis temperature on physicochemical properties of biochar obtained from the fast pyrolysis of pitch pine (Pinus rigida). Bioresour. Technol. 2012, 118, 158–162. [Google Scholar]
- Tangahu, B.V.; Abdullah, S.; Rozaimah, S.; Basri, H.; Idris, M.; Anuar, N.; Mukhlisin, M. A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. Int. J. Chem. Eng. 2011, 2011, 939161:1–939161:31. [Google Scholar]
- Okuno, T.; Sonoyama, N.; Hayashi, J.; Li, C.Z.; Sathe, C.; Chiba, T. Primary release of alkali and alkaline earth metallic species during the pyrolysis of pulverized biomass. Energy Fuel 2005, 19, 2164–2171. [Google Scholar]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley and Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar]
- Shokri, B.; Firouzjah, M.A.; Hosseini, S.I. FTIR Analysis of Silicon Dioxide Thin Film Deposited by Metal Organic-Based PECVD. In Proceedings of 19th International Symposium on Plasma Chemistry Society, Bochum, Germany, 26–31 August 2009.
- Pasangulapati, V.; Ramachandriya, K.D.; Kumar, A.; Wilkins, M.R.; Jones, C.L.; Huhnke, R.L. Effects of cellulose, hemicellulose and lignin on thermochemical conversion characteristics of the selected biomass. Bioresour. Technol. 2012, 114, 663–669. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R.L. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies 2013, 6, 3972-3986. https://doi.org/10.3390/en6083972
Qian K, Kumar A, Patil K, Bellmer D, Wang D, Yuan W, Huhnke RL. Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char. Energies. 2013; 6(8):3972-3986. https://doi.org/10.3390/en6083972
Chicago/Turabian StyleQian, Kezhen, Ajay Kumar, Krushna Patil, Danielle Bellmer, Donghai Wang, Wenqiao Yuan, and Raymond L. Huhnke. 2013. "Effects of Biomass Feedstocks and Gasification Conditions on the Physiochemical Properties of Char" Energies 6, no. 8: 3972-3986. https://doi.org/10.3390/en6083972




