Recent Approaches to Controlling the Nanoscale Morphology of Polymer-Based Bulk-Heterojunction Solar Cells
Abstract
:1. Introduction
2. Operational Principles of OPVs
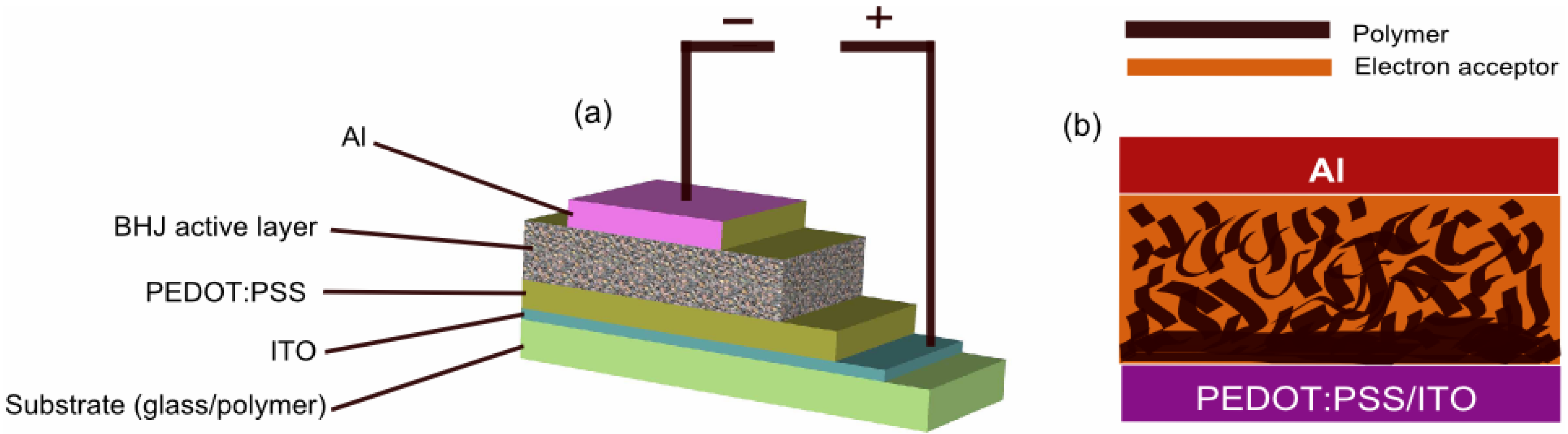

3. BHJ Solar Cells
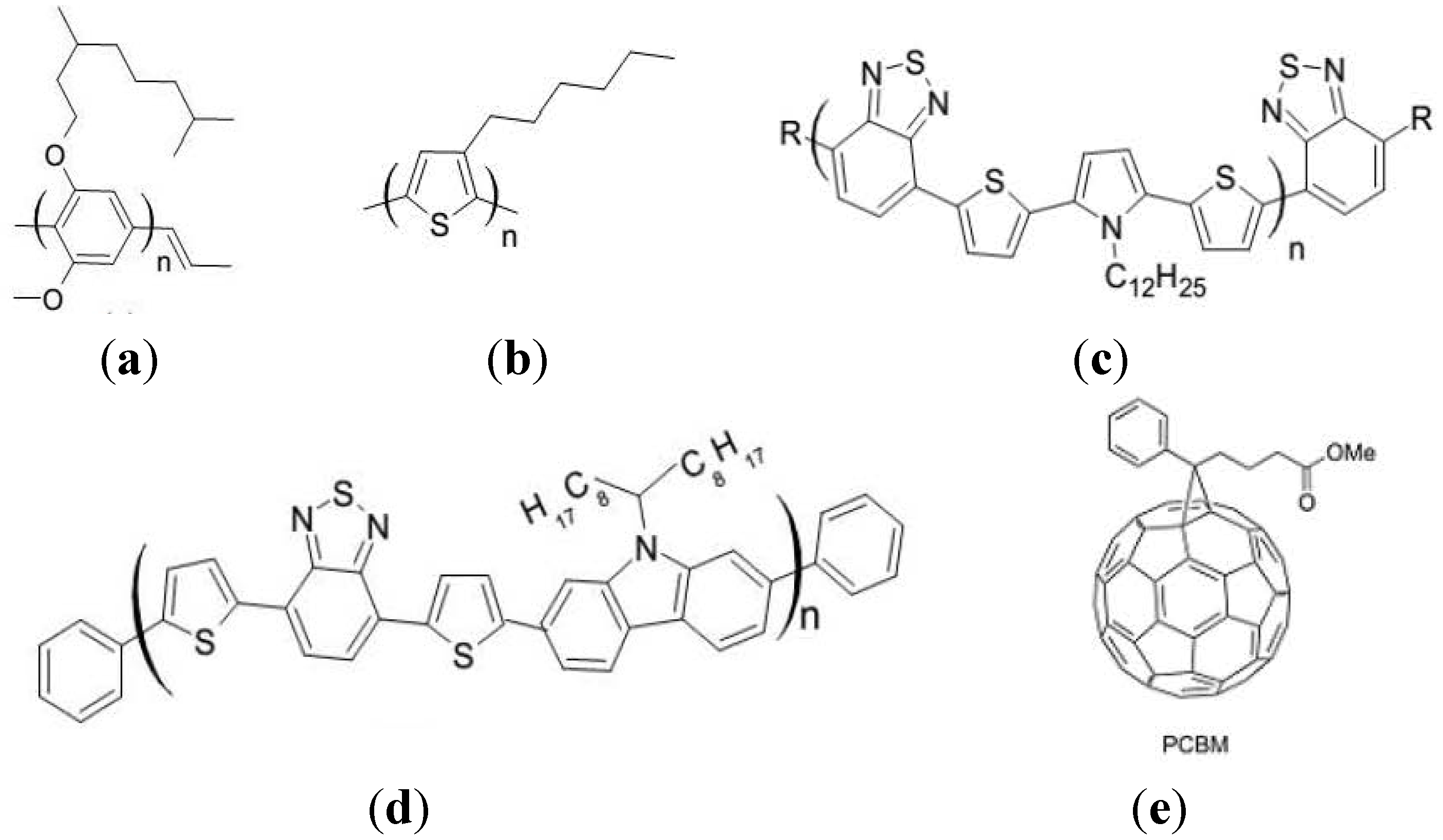
4. Morphology of BHJs
5. Strategies for Controlling the Active-Layer Morphology in BHJs
5.1. Roles of Solvent in Controlling the Active-Layer Morphologies
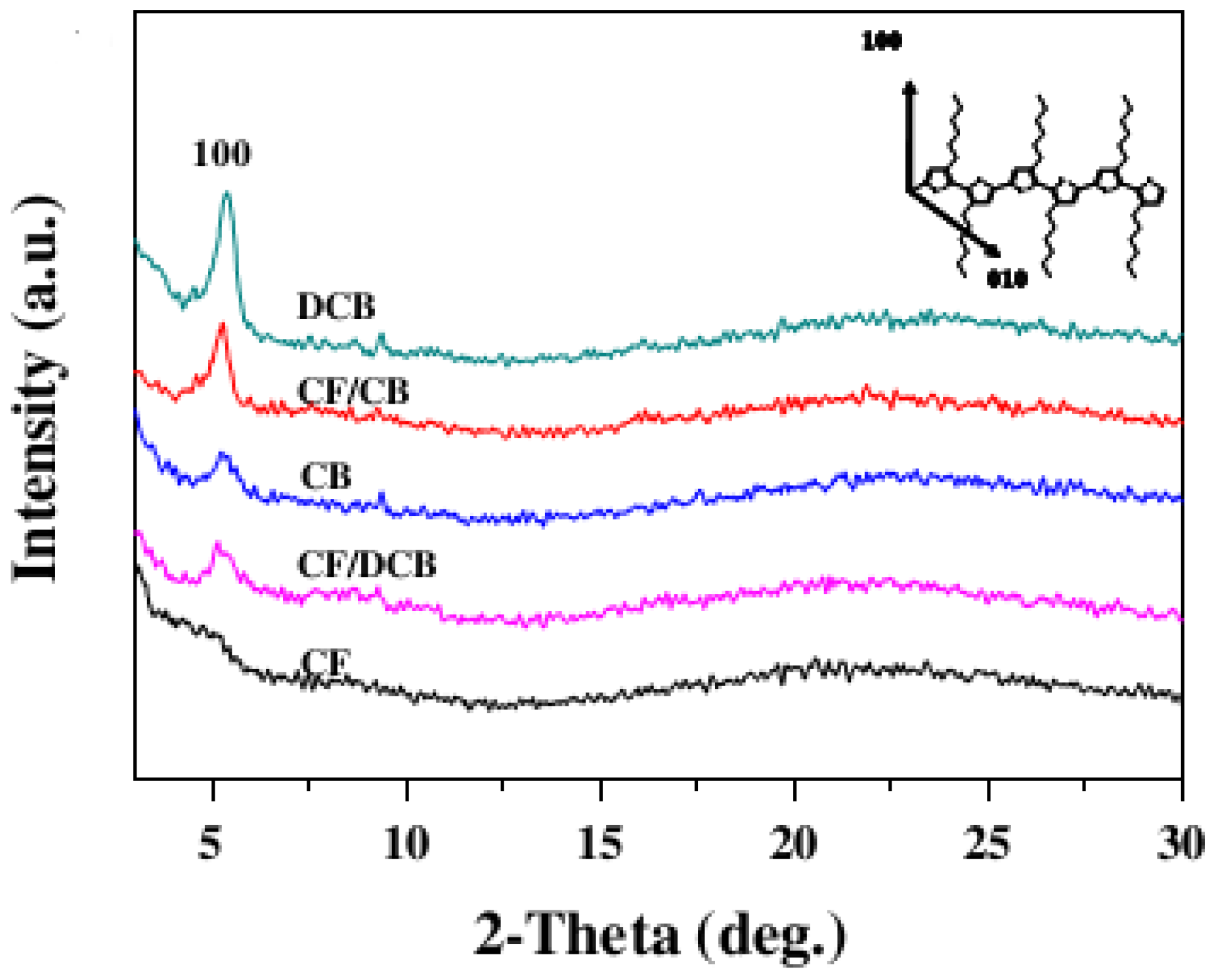
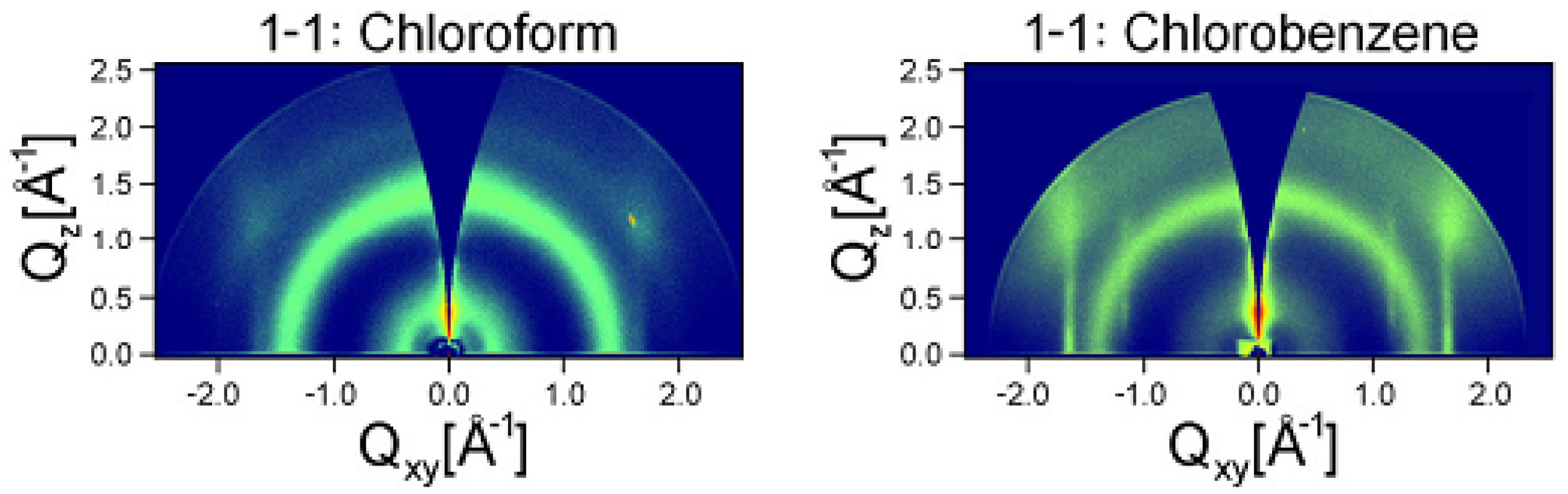
5.2. Use of Chemical Additives
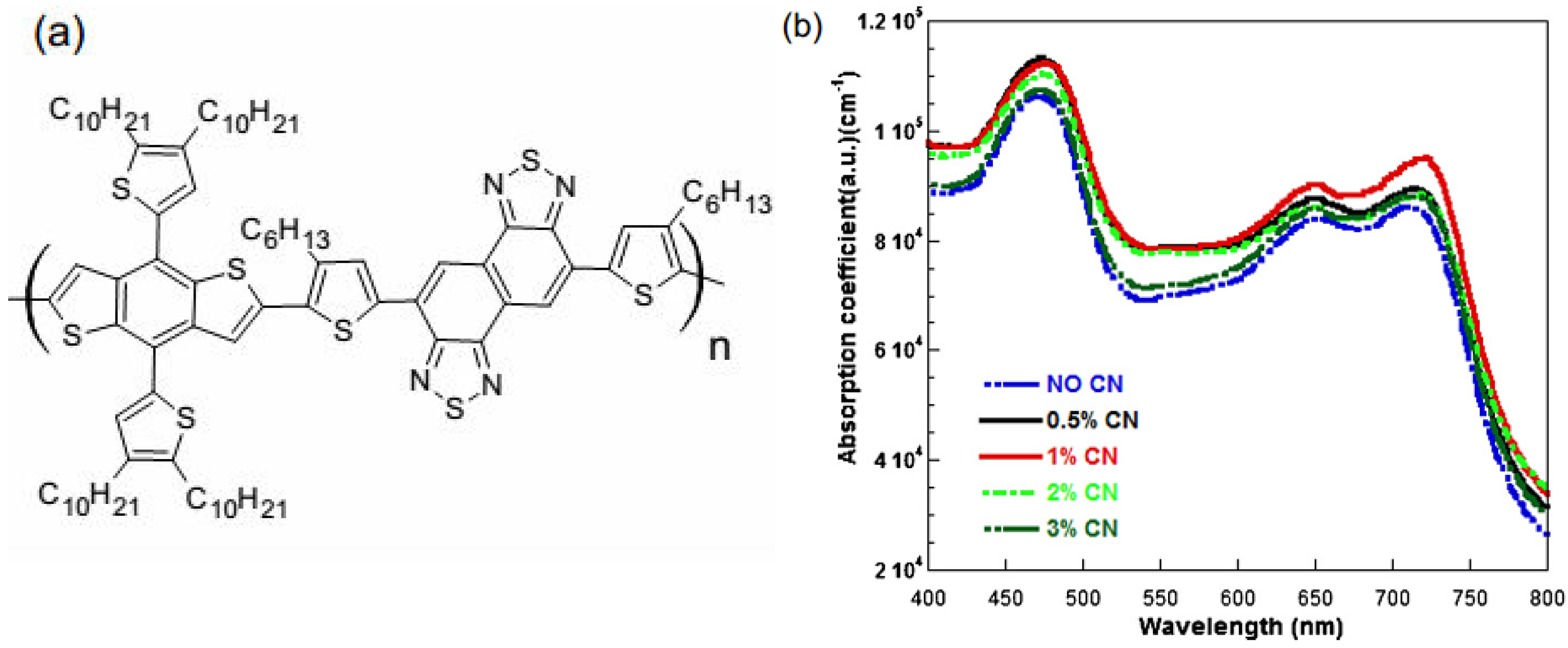
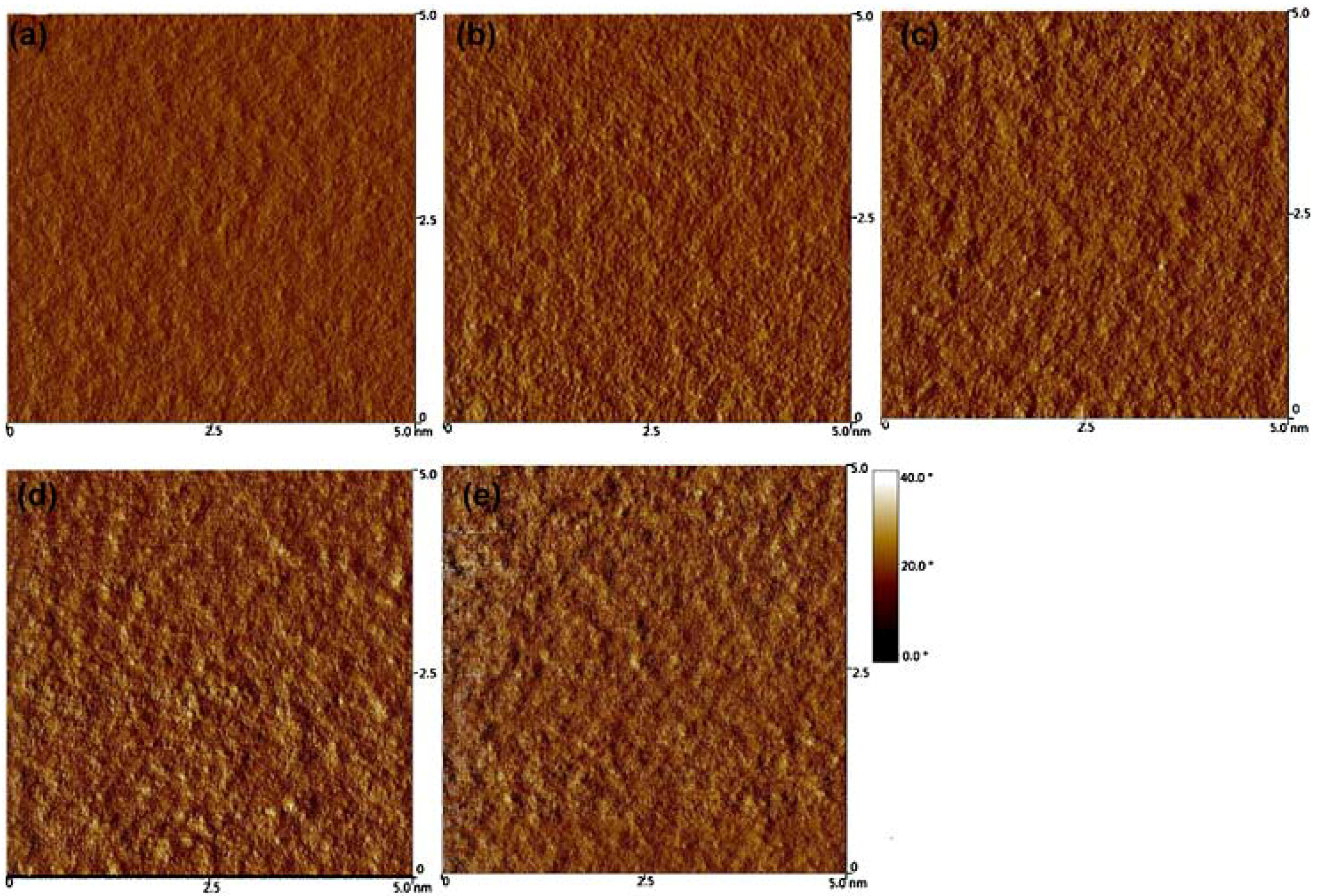
5.3. Donor–Acceptor-Blend Ratio Method
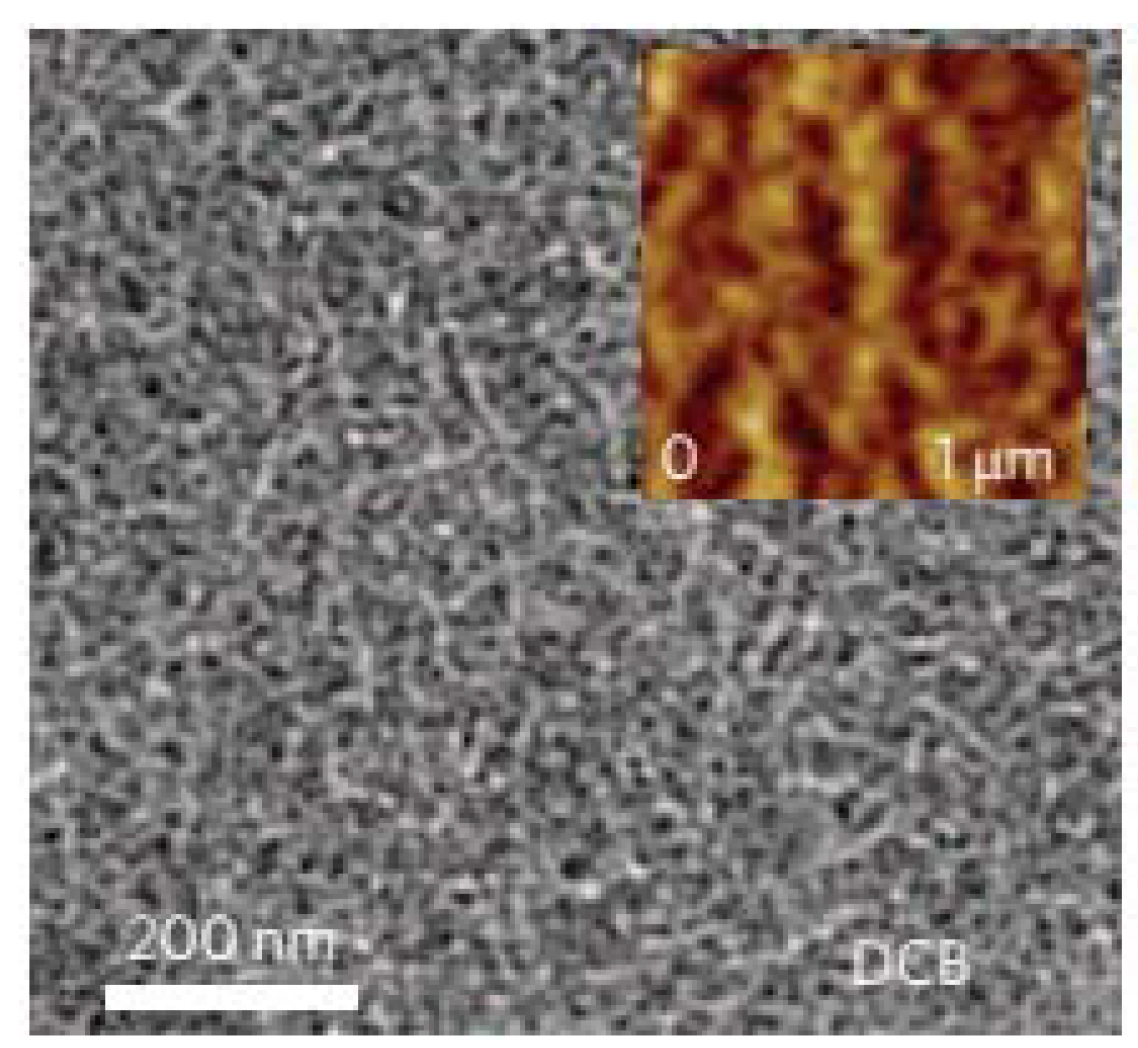
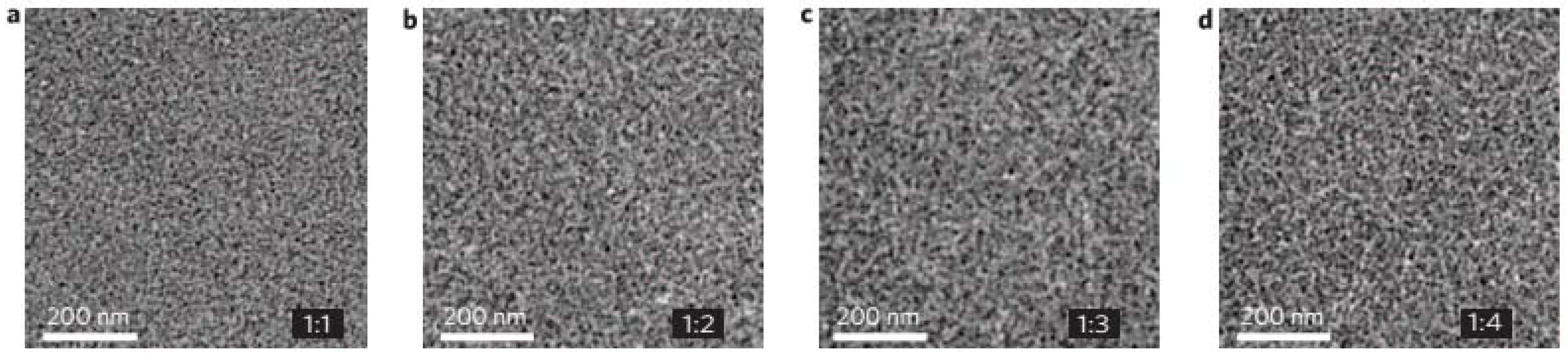
5.4. Effects of Thermal and Solvent Annealing on the Active-Layer Morphology
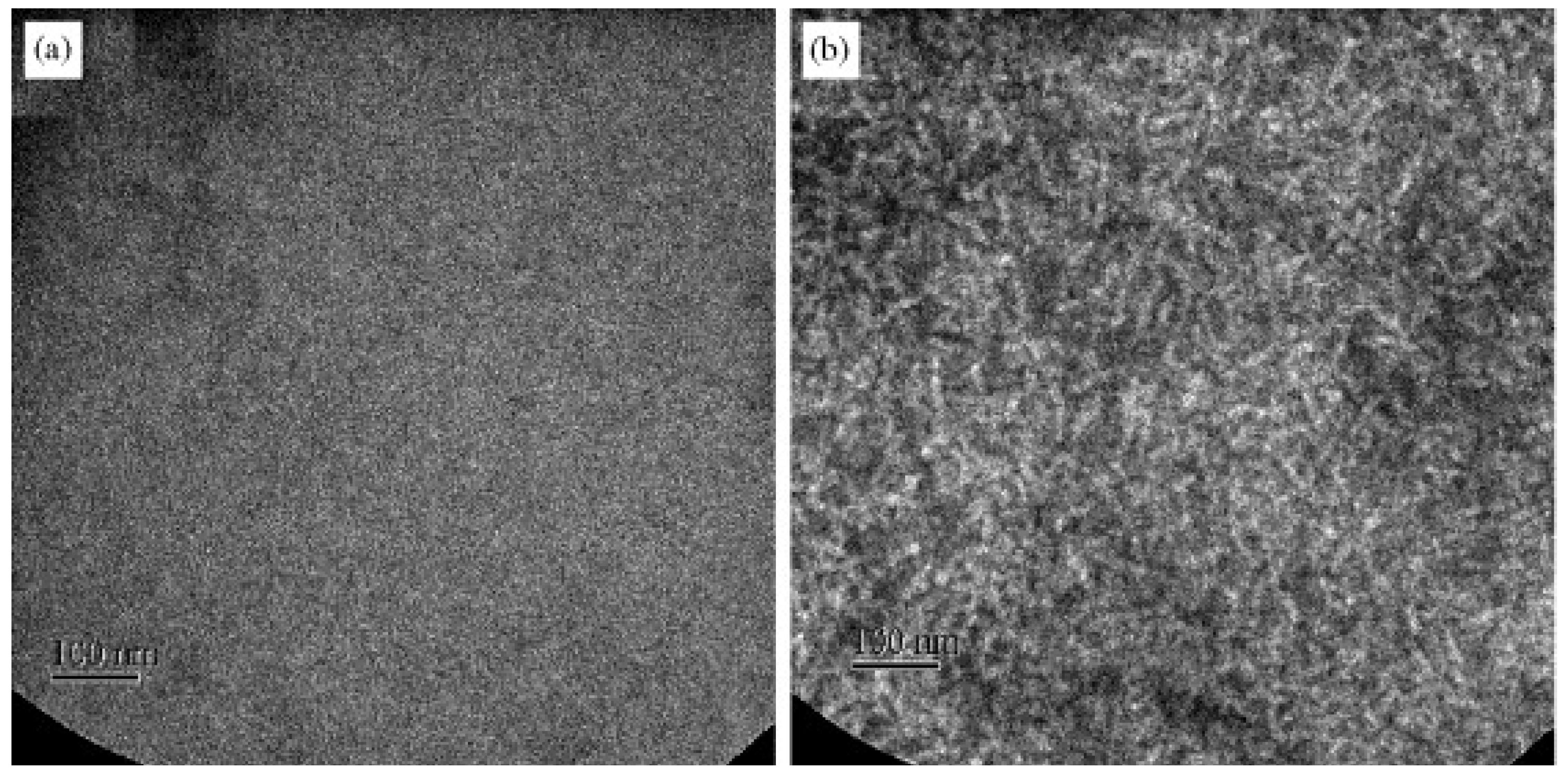
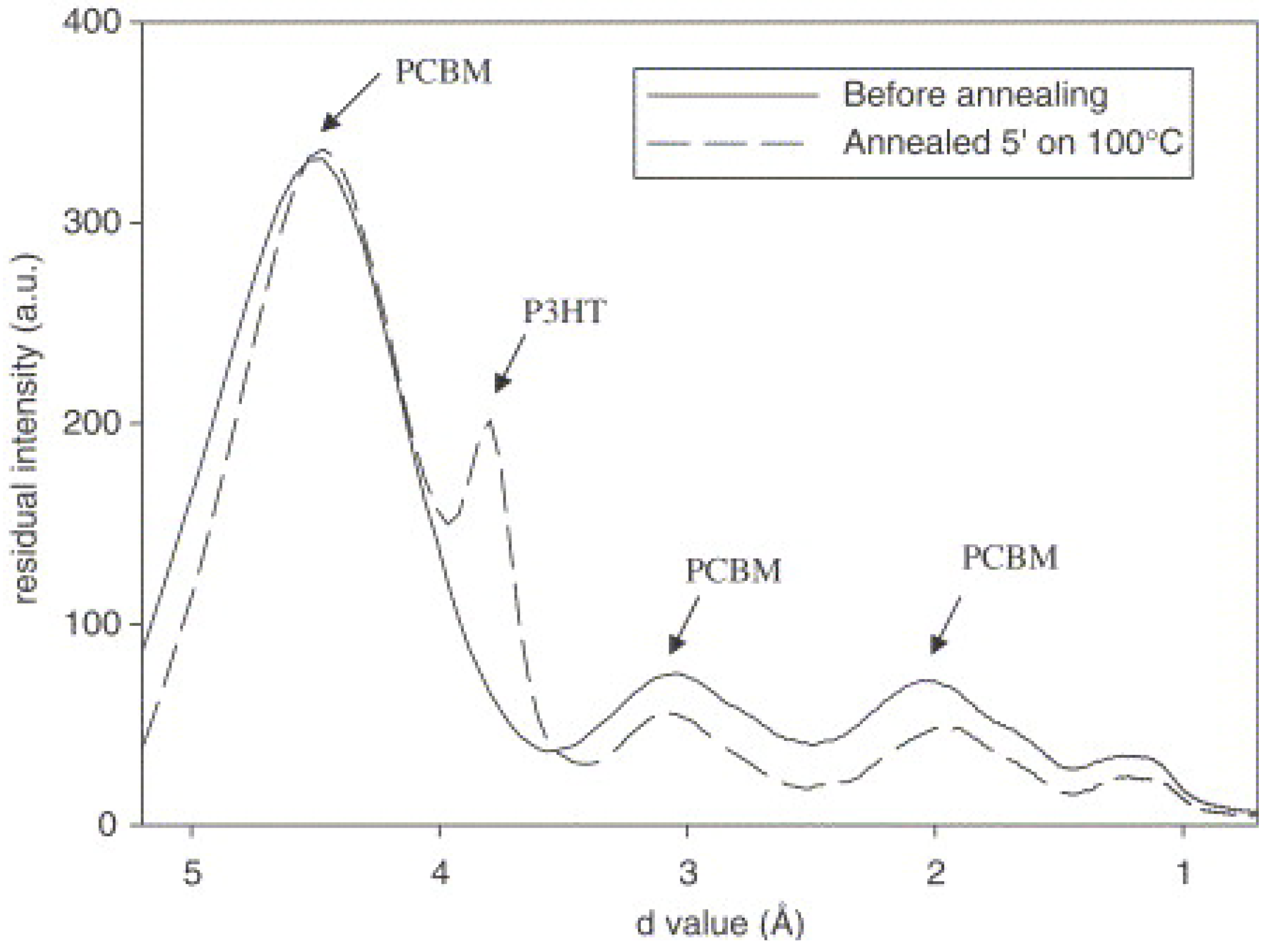
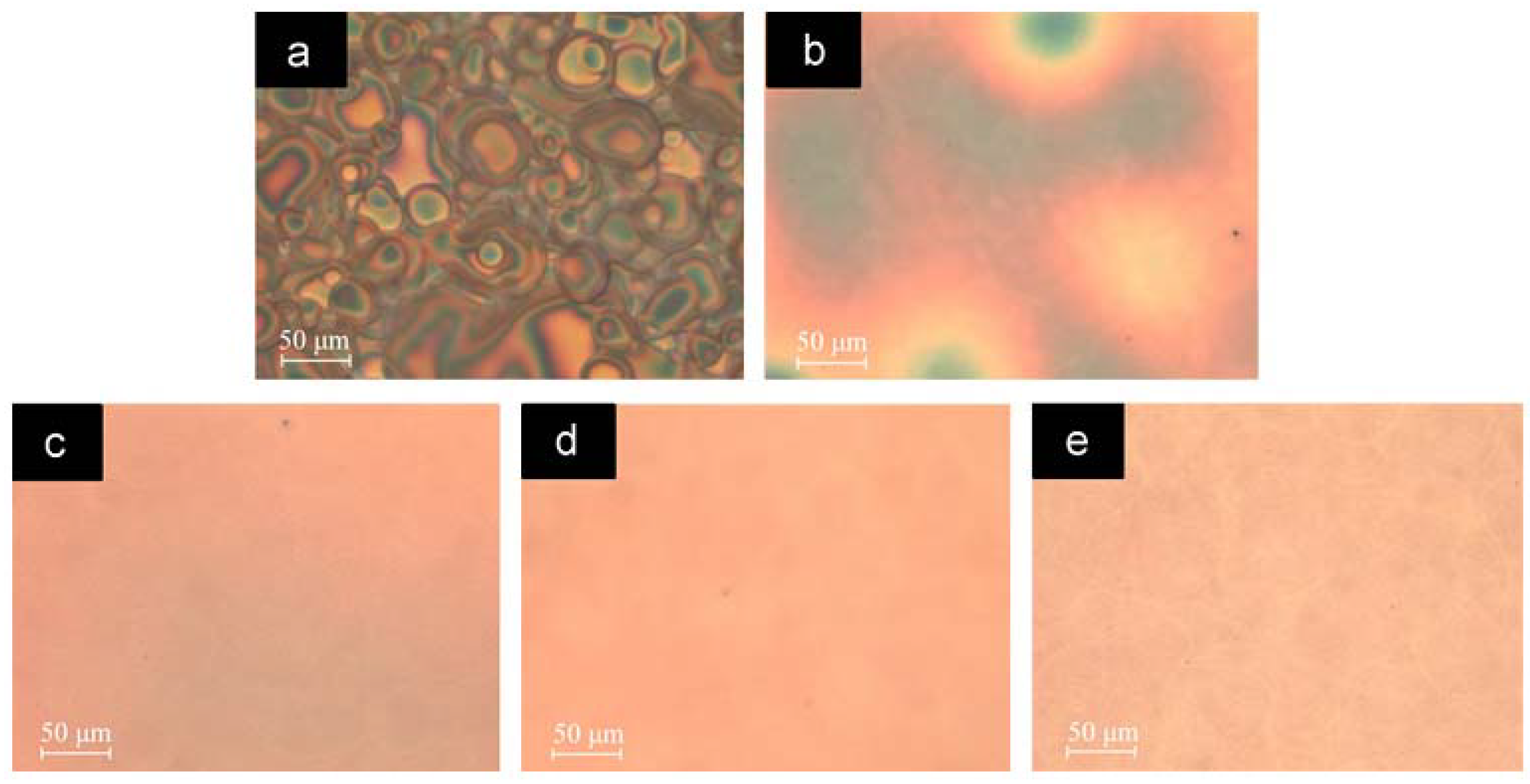
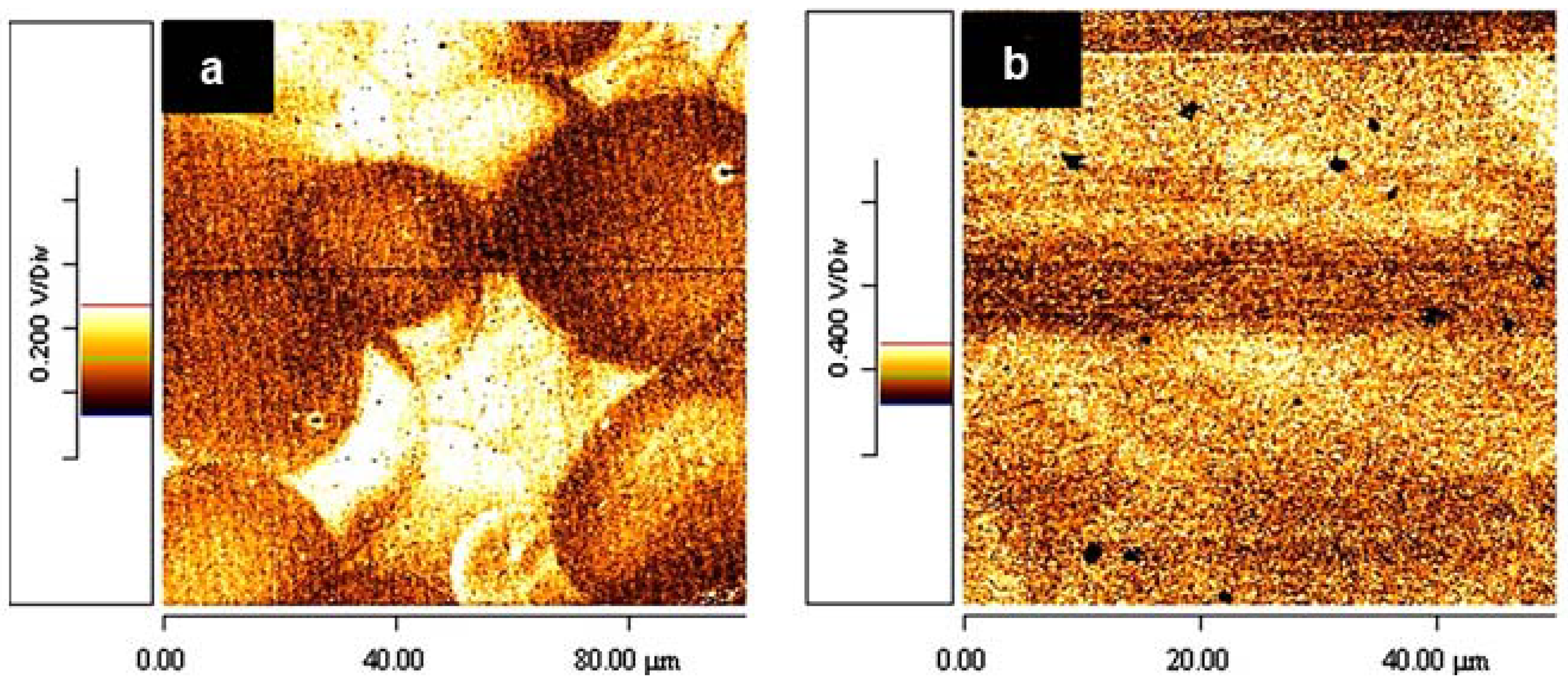
5.5. Use of NWs to Control the Active-Layer Morphology
6. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Bundgaard, E.; Krebs, F.C. Low band gap polymers for organic photovoltaics. Solar Energy Mater. Solar Cells 2007, 91, 954–985. [Google Scholar] [CrossRef]
- Irfan, U.; Wire, C. Novel Methods Store Sunshine as Fuel. Scientific American, 6 October 2011. [Google Scholar]
- Rothwarf, A.; Böer, K. Direct conversion of solar energy through photovoltaic cells. Prog. Solid State Chem. 1975, 10, 71–102. [Google Scholar] [CrossRef]
- Chen, J.-T.; Hsu, C.-S. Conjugated polymer nanostructures for organic solar cell applications. Polym. Chem. 2011, 2, 2707–2722. [Google Scholar] [CrossRef]
- Lewis, N.S. Toward cost-effective solar energy use. Science 2007, 315, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Zoombelt, A.P.; Mathijssen, S.G.J.; Turbiez, M.G.R.; Wienk, M.M.; Janssen, R.A.J. Small band gap polymers based on diketopyrrolopyrrole. J. Mater. Chem. 2010, 20, 2240–2246. [Google Scholar] [CrossRef]
- Rahimi, R.; Roberts, A.; Narang, V.; Korakakis, D. Investigate the role of the active layers’ structures and morphology in the performance of the organic solar cell devices. Appl. Phys. Lett. 2013, 102, 073105:1–073105:3. [Google Scholar] [CrossRef]
- Narayanan, S.; Wenham, S.R.; Green, M.A. 17.8-percent efficiency polycrystalline silicon solar cells. IEEE Trans. Electron Devices 1990, 37, 382–384. [Google Scholar] [CrossRef]
- Soga, T. Nanostructured Materials for Solar Energy Conversion; Elsevier Science: Philadelphia, PA, USA, 2006; pp. 1–614. [Google Scholar]
- Forrest, S.R. The path to ubiquitous and low-cost organic electronic appliances on plastic. Nature 2004, 428, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liang, Y.; Szarko, J.; Lee, B.; Son, H.J.; Rolczynski, B.S.; Yu, L.; Chen, L.X. Structure, dynamics, and power conversion efficiency correlations in a new low bandgap polymer: PCBM solar cell. J. Phys. Chem. B 2010, 114, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Yu, L. Development of semiconducting polymers for solar energy harvesting. Polym. Rev. 2010, 50, 454–473. [Google Scholar] [CrossRef]
- Delgado, J.L.; Bouit, P.-A.; Filippone, S.; Herranz, M.Á.; Martín, N. Organic photovoltaics: A chemical approach. Chem. Commun. 2010, 46, 4853–4865. [Google Scholar] [CrossRef] [Green Version]
- Service, R.F. Outlook brightens for plastic solar cells. Science 2011, 332, 293. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.-C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, J.H.; Park, J.H.; Shim, C.; Sim, M.; Cho, K. High‐efficiency organic solar cells based on preformed poly(3‐hexylthiophene) nanowires. Adv. Funct. Mater. 2011, 21, 480–486. [Google Scholar] [CrossRef]
- Ray, B.; Nair, P.R.; Alam, M.A. Unraveling the Role of Morphology on Organic Solar Cell Performance. 2012. arXiv:1011.0956. arXiv.org e-Print archive. Available online: http://arxiv.org/abs/1011.0956 (accessed on 30 June 2013).
- Moulé, A.J.; Meerholz, K. Morphology control in solution-processed bulk-heterojunction solar cell mixtures. Adv. Funct. Mater. 2009, 19, 3028–3036. [Google Scholar] [CrossRef]
- Schnabel, W. Photoconductivity. In Polymers and Light; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2007; pp. 49–71. [Google Scholar]
- Hoppe, H.; Niggemann, M.; Winder, C.; Kraut, J.; Hiesgen, R.; Hinsch, A.; Meissner, D.; Sariciftci, N.S. Nanoscale morphology of conjugated polymer/fullerene-based bulk-heterojunction solar cells. Adv. Funct. Mater. 2004, 14, 1005–1011. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated polymer-based organic solar cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Blom, P.W.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device physics of polymer: Fullerene bulk heterojunction solar cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef]
- Hoppe, H.; Glatzel, T.; Niggemann, M.; Schwinger, W.; Schaeffler, F.; Hinsch, A.; Lux-Steiner, M.C.; Sariciftci, N.S. Efficiency limiting morphological factors of MDMO-PPV:PCBM plastic solar cells. Thin Solid Films 2006, 511–512, 587–592. [Google Scholar] [CrossRef]
- Ratier, B.; Nunzi, J.-M.; Aldissi, M.; Kraft, T.M.; Buncel, E. Organic solar cell materials and active layer designs—Improvements with carbon nanotubes: A review. Polym. Int. 2012, 61, 342–354. [Google Scholar] [CrossRef]
- Thompson, B.C.; Fréchet, J.M. Polymer–Fullerene composite solar cells. Angew. Chem. Int. Ed. 2008, 47, 58–77. [Google Scholar] [CrossRef]
- Scharber, M.C.; Mühlbacher, D.; Koppe, M.; Denk, P.; Waldauf, C.; Heeger, A.J.; Brabec, C.J. Design rules for donors in bulk‐heterojunction solar cells—Towards 10% energy‐conversion efficiency. Adv. Mater. 2006, 18, 789–794. [Google Scholar] [CrossRef]
- Skompska, M. Hybrid conjugated polymer/semiconductor photovoltaic cells. Synth. Met. 2010, 160, 1–15. [Google Scholar] [CrossRef]
- Brabec, C.J.; Sariciftci, N.S.; Hummelen, J.C. Plastic solar cells. Adv. Funct. Mater. 2001, 11, 15–26. [Google Scholar] [CrossRef]
- Nishikitani, Y.; Uchida, S.; Kubo, T. Nanostructured Organic Bulk Heterojunction Solar Cells. In Nanostructured Materials for Solar Energy Conversion; Tetsuo, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 11; pp. 319–333. [Google Scholar]
- Cai, W.; Gong, X.; Cao, Y. Polymer solar cells: Recent development and possible routes for improvement in the performance. Solar Energy Mater. Solar Cells 2010, 94, 114–127. [Google Scholar] [CrossRef]
- Mozer, A.J.; Sariciftci, N.S. Conjugated polymer photovoltaic devices and materials. Comptes Rendus Chim. 2006, 9, 568–577. [Google Scholar] [CrossRef]
- Silvestri, F.; Irwin, M.D.; Beverina, L.; Facchetti, A.; Pagani, G.A.; Marks, T.J. Efficient squaraine-based solution processable bulk-heterojunction solar cells. J. Am. Chem. Soc. 2008, 130, 17640–17641. [Google Scholar] [CrossRef] [PubMed]
- Boudreault, P.-L.T.; Najari, A.; Leclerc, M. Processable low-bandgap polymers for photovoltaic applications. Chem. Mater. 2010, 23, 456–469. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Q.; Li, Z.; Pei, J.; Tian, W. Solution processable D–A small molecules for bulk-heterojunction solar cells. Energy Environ. Sci. 2010, 3, 1427–1436. [Google Scholar] [CrossRef]
- Mandoc, M.M.; Koster, L.J.A.; Blom, P.W.M. Optimum charge carrier mobility in organic solar cells. Appl. Phys. Lett. 2007, 90, 133504:1–133504:3. [Google Scholar] [CrossRef]
- Yong, X.; Zhang, J. Theoretical investigations for organic solar cells. Mater. Technol. Adv. Perform. Mater. 2013, 28, 1–2. [Google Scholar]
- Vardeny, Z.V. Spin-enhanced organic bulk heterojunction photovoltaic solar cells. Nat. Commun. 2012, 3, 1043. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.S.; Takacs, C.J.; Sun, Y.; Heeger, A.J. Spontaneous formation of bulk heterojunction nanostructures: Multiple routes to equivalent morphologies. Nano Lett. 2011, 11, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Wienk, M.M.; Kroon, J.M.; Verhees, W.J.; Knol, J.; Hummelen, J.C.; van Hal, P.A.; Janssen, R.A. Efficient methano [70] fullerene/MDMO‐PPV bulk heterojunction photovoltaic cells. Angew. Chem. 2003, 115, 3493–3497. [Google Scholar] [CrossRef]
- Moon, J.S.; Jo, J.; Heeger, A.J. Nanomorphology of PCDTBT:PC70BM bulk heterojunction solar cells. Adv. Energy Mater. 2012, 2, 304–308. [Google Scholar] [CrossRef]
- Tong, M.; Coates, N.E.; Moses, D.; Heeger, A.J.; Beaupré, S.; Leclerc, M. Charge carrier photogeneration and decay dynamics in the poly (2,7-carbazole) copolymer PCDTBT and in bulk heterojunction composites with PC70 BM. Phys. Rev. B 2010, 81, 125210:1–125210:6. [Google Scholar] [CrossRef]
- Cheung, D.L.; Troisi, A. Theoretical study of the organic photovoltaic electron acceptor PCBM: Morphology, electronic structure, and charge localization. J. Phys. Chem. C 2010, 114, 20479–20488. [Google Scholar] [CrossRef]
- Mayer, A.C.; Scully, S.R.; Hardin, B.E.; Rowell, M.W.; McGehee, M.D. Polymer-based solar cells. Mater. Today 2007, 10, 28–33. [Google Scholar] [CrossRef]
- Valentini, L.; Bagnis, D.; Marrocchi, A.; Seri, M.; Taticchi, A.; Kenny, J.M. Novel anthracene-core molecule for the development of efficient PCBM-based solar cells. Chem. Mater. 2007, 20, 32–34. [Google Scholar] [CrossRef]
- Wang, S.; Bian, Z.; Xia, X.; Huang, C. Heterojunction organic solar cells based on donor-π-acceptor small molecule layers with controlled interface morphology. Org. Electron. 2010, 11, 1909–1915. [Google Scholar] [CrossRef]
- Talgorn, E.; Gao, Y.; Aerts, M.; Kunneman, L.T.; Schins, J.M.; Savenije, T.; van Huis, M.A.; van der Zant, H.S.; Houtepen, A.J.; Siebbeles, L.D. Unity quantum yield of photogenerated charges and band-like transport in quantum-dot solids. Nat. Nanotechnol. 2011, 6, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Kovalenko, M.V.; Huang, J.; Chung, D.S.; Talapin, D.V. Band-like transport, high electron mobility and high photoconductivity in all-inorganic nanocrystal arrays. Nat. Nanotechnol. 2011, 6, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Colbert, A.E.; Janke, E.M.; Hsieh, S.T.; Subramaniyan, S.; Schlenker, C.W.; Jenekhe, S.A.; Ginger, D.S. Hole transfer from low band gap quantum dots to conjugated polymers in organic/inorganic hybrid photovoltaics. J. Phys. Chem. Lett. 2013, 4, 280–284. [Google Scholar] [CrossRef]
- Etgar, L.; Moehl, T.; Gabriel, S.; Hickey, S.G.; Eychmüller, A.; Grätzel, M. Light energy conversion by mesoscopic PbS quantum dots/TiO2 heterojunction solar cells. ACS Nano 2012, 6, 3092–3099. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, Z.; Zhang, X.; Yang, L.; Zhang, N.; Pan, G.; Yin, S.; Chen, Y.; Wei, J. Polymer photovoltaic cells based on solution‐processable graphene and P3HT. Adv. Funct. Mater. 2009, 19, 894–904. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Zhang, X.; Wang, Y.; Tian, J.; Huang, Y.; Ma, Y.; Zhang, X.; Chen, Y. A graphene hybrid material covalently functionalized with porphyrin: synthesis and optical limiting property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-S.; Tu, K.-H.; Lin, C.-C.; Chen, C.-W.; Chhowalla, M. Solution-processable graphene oxide as an efficient hole transport layer in polymer solar cells. ACS Nano 2010, 4, 3169–3174. [Google Scholar] [CrossRef] [PubMed]
- Stylianakis, M.M.; Spyropoulos, G.D.; Stratakis, E.; Kymakis, E. Solution-processable graphene linked to 3,5-dinitrobenzoyl as an electron acceptor in organic bulk heterojunction photovoltaic devices. Carbon 2012, 50, 5554–5561. [Google Scholar] [CrossRef]
- Bakhshi, A.; Kaur, A.; Arora, V. Molecular engineering of novel low band gap conducting polymers. Indian J. Chem. Part A Inorg. Phys. Theor. Anal. 2012, 51, 57–68. [Google Scholar]
- Roncali, J. Molecular engineering of the band gap of π‐conjugated systems: Facing technological applications. Macromol. Rapid Commun. 2007, 28, 1761–1775. [Google Scholar] [CrossRef]
- Roncali, J.; Frère, P.; Blanchard, P.; de Bettignies, R.; Turbiez, M.; Roquet, S.; Leriche, P.; Nicolas, Y. Molecular and supramolecular engineering of π-conjugated systems for photovoltaic conversion. Thin Solid Films 2006, 511, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Liu, F.; Wang, C.; Nakahara, A.; Russell, T.P. Bulk heterojunction photovoltaic active layers via bilayer interdiffusion. Nano Lett. 2011, 11, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Weerakoon, K.A.; Auad, M.; Horikawa, S.; Chin, B.A. Effect of active layer morphology on poly3-hexylthiophene phytochemical chemiresistor sensor performance. IEEE Sens. J. 2012, 12, 3062–3068. [Google Scholar] [CrossRef]
- Balderrama, V.; Estrada, M.; Formentin, P.; Viterisi, A.; Ferre-Borrull, J.; Pallares, J.; Palomares, E.; Marsal, L. Influence and Relationship of Film Morphology on Organic Solar Cells Manufactured with Different P3HT:PC[70]BM Blend Solutions. In Proceedings of the 2011 8th International Conference on Electrical Engineering Computing Science and Automatic Control (CCE), Merida, Mexico, 26–28 October 2011; pp. 1–5.
- Haung, S.H.; Lu, P.Y.; Chung, C.L.; Fu, S.L. Preparation of Active Layer of Solar Cells Device by F8T2 Blending with PCBM. In Proceedings of the 2010 IEEE CPMT Symposium Japan, Tokyo, Japan, 24–26 August 2010; pp. 1–4.
- Hau, S.K.; O’Malley, K.M.; You-Jung, C.; Yip, H.-L.; Ma, H.; Jen, A.K.Y. Optimization of active layer and anode electrode for high-performance inverted bulk-heterojunction solar cells. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 1665–1675. [Google Scholar] [CrossRef]
- Dang, M.T.; Wantz, G.; Bejbouji, H.; Urien, M.; Dautel, O.J.; Vignau, L.; Hirsch, L. Polymeric solar cells based on P3HT:PCBM: Role of the casting solvent. Solar Energy Mater. Solar Cells 2011, 95, 3408–3418. [Google Scholar] [CrossRef]
- Mihailetchi, V.D.; Xie, H.; de Boer, B.; Popescu, L.M.; Hummelen, J.C.; Blom, P.W.; Koster, L.J.A. Origin of the enhanced performance in poly(3-hexylthiophene): [6,6]-phenyl C61-butyric acid methyl ester solar cells upon slow drying of the active layer. Appl. Phys. Lett. 2006, 89, 012107:1–012107:3. [Google Scholar] [CrossRef]
- Li, G.; Shrotriya, V.; Huang, J.; Yao, Y.; Moriarty, T.; Emery, K.; Yang, Y. High-efficiency solution processable polymer photovoltaic cells by self-organization of polymer blends. Nat. Mater. 2005, 4, 864–868. [Google Scholar] [CrossRef]
- Xia, J.; Masaki, N.; Jiang, K.; Yanagida, S. The influence of doping ions on poly(3,4-ethylenedioxythiophene) as a counter electrode of a dye-sensitized solar cell. J. Mater. Chem. 2007, 17, 2845–2850. [Google Scholar] [CrossRef]
- Verploegen, E.; Mondal, R.; Bettinger, C.J.; Sok, S.; Toney, M.F.; Bao, Z. Effects of thermal annealing upon the morphology of polymer–fullerene blends. Adv. Funct. Mater. 2010, 20, 3519–3529. [Google Scholar] [CrossRef]
- Marsh, R.A.; Hodgkiss, J.M.; Albert-Seifried, S.; Friend, R.H. Effect of annealing on P3HT:PCBM charge transfer and nanoscale morphology probed by ultrafast spectroscopy. Nano Lett. 2010, 10, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Tang, C.W.; Chen, S.H. Effects of active layer thickness and thermal annealing on polythiophene: Fullerene bulk heterojunction photovoltaic devices. Appl. Phys. Lett. 2010, 97, 053305:1–053305:3. [Google Scholar]
- Verploegen, E.; Miller, C.E.; Schmidt, K.; Bao, Z.; Toney, M.F. Manipulating the morphology of P3HT-PCBM bulk heterojunction blends with solvent vapor annealing. Chem. Mater. 2012, 24, 3923–3931. [Google Scholar] [CrossRef]
- Tang, H.; Lu, G.; Li, L.; Li, J.; Wang, Y.; Yang, X. Precise construction of PCBM aggregates for polymer solar cells via multi-step controlled solvent vapor annealing. J. Mater. Chem. 2010, 20, 683–688. [Google Scholar] [CrossRef]
- Sharma, G.D.; Suresh, P.; Sharma, S.S.; Vijay, Y.K.; Mikroyannidis, J.A. Effect of solvent and subsequent thermal annealing on the performance of phenylenevinylene copolymer:PCBM solar cells. ACS Appl. Mater. Interfaces 2010, 2, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Truong, Q.N.N.; Truong, N.T.N.; Park, C.; Jung, J.H. Study of MEH–PPV/PCBM active layer morphology and its application for hybrid solar cell performance. Bull. Mater. Sci. 2012, 35, 277–281. [Google Scholar] [CrossRef]
- Shen, Y.-M.; Chen, C.-S.; Yang, P.-C.; Ma, S.-Y.; Lin, C.-F. Improvement of surface morphology of thin films and performance by applying electric field on P3HT:PCBM based solar cells. Solar Energy Mater. Solar Cells 2012, 99, 263–267. [Google Scholar] [CrossRef]
- Lou, S.J.; Szarko, J.M.; Xu, T.; Yu, L.; Marks, T.J.; Chen, L.X. Effects of additives on the morphology of solution phase aggregates formed by active layer components of high-efficiency organic solar cells. J. Am. Chem. Soc. 2011, 133, 20661–20663. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G.; Lee, J.; Lee, K. Morphology controlled bulk-heterojunction layers of fully electro-spray coated organic solar cells. Solar Energy Mater. Solar Cells 2012, 105, 272–279. [Google Scholar] [CrossRef]
- Rice, A.H.; Giridharagopal, R.; Zheng, S.X.; Ohuchi, F.S.; Ginger, D.S.; Luscombe, C.K. Controlling vertical morphology within the active layer of organic photovoltaics using poly(3-hexylthiophene) nanowires and phenyl-C61-butyric acid methyl ester. ACS Nano 2011, 5, 3132–3140. [Google Scholar] [CrossRef] [PubMed]
- De Freitas, J.N.; Nogueira, A.F. Incorporation of Inorganic Nanoparticles into Bulk Heterojunction Organic Solar Cells. In Nanoenergy; Springer-Verlag: Heidelberg, Germany, 2013; pp. 1–47. [Google Scholar]
- Zhang, F.-Z.; Kato, T.; Fuji, M.; Takahashi, M. Gelcasting fabrication of porous ceramics using a continuous process. J. Eur. Ceram. Soc. 2006, 26, 667–671. [Google Scholar] [CrossRef]
- Vanlaeke, P.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; Manca, J.; Heremans, P.; Poortmans, J. Polythiophene based bulk heterojunction solar cells: Morphology and its implications. Thin Solid Films 2006, 511–512, 358–361. [Google Scholar] [CrossRef]
- Kim, Y.; Lee, Y.; Kim, J.K.; Seo, E.-O.; Lee, E.-W.; Lee, W.; Han, S.-H.; Lee, S.-H. Effect of solvents on the performance and morphology of polymer photovoltaic devices. Curr. Appl. Phys. 2010, 10, 985–989. [Google Scholar] [CrossRef]
- Lin, S.-H.; Lan, S.; Sun, J.-Y.; Lin, C.-F. Influence of mixed solvent on the morphology of the P3HT:Indene-C60 bisadduct (ICBA) blend film and the performance of inverted polymer solar cells. Org. Electron. 2013, 14, 26–31. [Google Scholar] [CrossRef]
- Jang, S.K.; Gong, S.C.; Chang, H.J. Effects of various solvent addition on crystal and electrical properties of organic solar cells with P3HT:PCBM active layer. Synth. Met. 2012, 162, 426–430. [Google Scholar] [CrossRef]
- Hu, X.; Wang, M.; Huang, F.; Gong, X.; Cao, Y. 23% Enhanced efficiency of polymer solar cells processed with 1-chloronaphthalene as the solvent additive. Synth. Met. 2013, 164, 1–5. [Google Scholar] [CrossRef]
- Moon, J.S.; Takacs, C.J.; Cho, S.; Coffin, R.C.; Kim, H.; Bazan, G.C.; Heeger, A.J. Effect of processing additive on the nanomorphology of a bulk heterojunction material. Nano Lett. 2010, 10, 4005–4008. [Google Scholar] [CrossRef] [PubMed]
- Salim, T.; Wong, L.H.; Brauer, B.; Kukreja, R.; Foo, Y.L.; Bao, Z.; Lam, Y.M. Solvent additives and their effects on blend morphologies of bulk heterojunctions. J. Mater. Chem. 2011, 21, 242–250. [Google Scholar] [CrossRef]
- Keawprajak, A.; Piyakulawat, P.; Klamchuen, A.; Iamraksa, P.; Asawapirom, U. Influence of crystallizable solvent on the morphology and performance of P3HT:PCBM bulk-heterojunction solar cells. Solar Energy Mater. Solar Cells 2010, 94, 531–536. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Vanlaeke, P.; Swinnen, A.; Haeldermans, I.; Vanhoyland, G.; Aernouts, T.; Cheyns, D.; Deibel, C.; D’Haen, J.; Heremans, P.; Poortmans, J.; et al. P3HT/PCBM bulk heterojunction solar cells: Relation between morphology and electro-optical characteristics. Solar Energy Mater. Solar Cells 2006, 90, 2150–2158. [Google Scholar] [CrossRef]
- Frank, C.W.; Rao, V.; Despotopoulou, M.M.; Pease, R.F.W.; Hinsberg, W.D.; Miller, R.D.; Rabolt, J.F. Structure in thin and ultrathin spin-cast polymer films. Science 1996, 273, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Hadj Romdhane, I.; Price, P.E.; Miller, C.A.; Benson, P.T.; Wang, S. Drying of glassy polymer films. Ind. Eng. Chem. Res. 2001, 40, 3065–3075. [Google Scholar] [CrossRef]
- Chang, L.; Lademann, H.W.; Bonekamp, J.B.; Meerholz, K.; Moulé, A.J. Effect of trace solvent on the morphology of P3HT:PCBM bulk heterojunction solar cells. Adv. Funct. Mater. 2011, 21, 1779–1787. [Google Scholar] [CrossRef]
- Nagarjuna, G.; Venkataraman, D. Strategies for controlling the active layer morphologies in OPVs. J. Polym. Sci. Part B Polym. Phys. 2012, 50, 1045–1056. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yu, C.-Y.; Fan, Y.-L.; Hung, L.-I.; Chen, C.-P.; Ting, C. Low-bandgap conjugated polymer for high efficient photovoltaic applications. Chem. Commun. 2010, 46, 6503–6505. [Google Scholar] [CrossRef]
- Miller, S.; Fanchini, G.; Lin, Y.-Y.; Li, C.; Chen, C.-W.; Su, W.-F.; Chhowalla, M. Investigation of nanoscale morphological changes in organic photovoltaics during solvent vapor annealing. J. Mater. Chem. 2008, 18, 306–312. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chia, H.-C.; Chuang, C.-M.; Tsao, C.-S.; Chen, C.-Y.; Su, W.-F. Facile hot solvent vapor annealing for high performance polymer solar cell using spray process. Solar Energy Mater. Solar Cells 2013, 114, 24–30. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kim, B.-G.; Kim, J. Effective variables to control the fill factor of organic photovoltaic cells. ACS Appl. Mater. Interfaces 2009, 1, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, Y.; Seifter, J.; Collins, S.D.; Luo, C.; Bazan, G.C.; Nguyen, T.-Q.; Heeger, A.J. High-efficiency polymer solar cells enhanced by solvent treatment. Adv. Mater. 2013, 25, 1646–1652. [Google Scholar] [CrossRef] [PubMed]
- Babel, A.; Jenekhe, S.A. Alkyl chain length dependence of the field-effect carrier mobility in regioregular poly(3-alkylthiophene)s. Synth. Met. 2005, 148, 169–173. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Hoppe, H.; Erb, T.; Günes, S.; Gobsch, G.; Sariciftci, N.S. Effects of annealing on the nanomorphology and performance of poly(alkylthiophene):Fullerene bulk-heterojunction solar cells. Adv. Funct. Mater. 2007, 17, 1071–1078. [Google Scholar] [CrossRef]
- Yang, X.; Loos, J.; Veenstra, S.C.; Verhees, W.J.H.; Wienk, M.M.; Kroon, J.M.; Michels, M.A.J.; Janssen, R.A.J. Nanoscale morphology of high-performance polymer solar cells. Nano Lett. 2005, 5, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Hiorns, R.C.; de Bettignies, R.; Leroy, J.; Bailly, S.; Firon, M.; Sentein, C.; Khoukh, A.; Preud’homme, H.; Dagron-Lartigau, C. High molecular weights, polydispersities, and annealing temperatures in the optimization of bulk-heterojunction photovoltaic cells based on poly(3-hexylthiophene) or poly(3-butylthiophene). Adv. Funct. Mater. 2006, 16, 2263–2273. [Google Scholar] [CrossRef]
- Xin, H.; Kim, F.S.; Jenekhe, S.A. Highly efficient solar cells based on poly(3-butylthiophene) nanowires. J. Am. Chem. Soc. 2008, 130, 5424–5425. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hammed, W.A.; Yahya, R.; Bola, A.L.; Mahmud, H.N.M.E. Recent Approaches to Controlling the Nanoscale Morphology of Polymer-Based Bulk-Heterojunction Solar Cells. Energies 2013, 6, 5847-5868. https://doi.org/10.3390/en6115847
Hammed WA, Yahya R, Bola AL, Mahmud HNME. Recent Approaches to Controlling the Nanoscale Morphology of Polymer-Based Bulk-Heterojunction Solar Cells. Energies. 2013; 6(11):5847-5868. https://doi.org/10.3390/en6115847
Chicago/Turabian StyleHammed, Wasiu Adebayo, Rosiyah Yahya, Abdulra'uf Lukman Bola, and Habibun Nabi Muhammad Ekramul Mahmud. 2013. "Recent Approaches to Controlling the Nanoscale Morphology of Polymer-Based Bulk-Heterojunction Solar Cells" Energies 6, no. 11: 5847-5868. https://doi.org/10.3390/en6115847




