The Effects of Particle Size, Different Corn Stover Components, and Gas Residence Time on Torrefaction of Corn Stover
Abstract
:1. Introduction
2. Materials and Methods
2.1. Corn Stover Samples
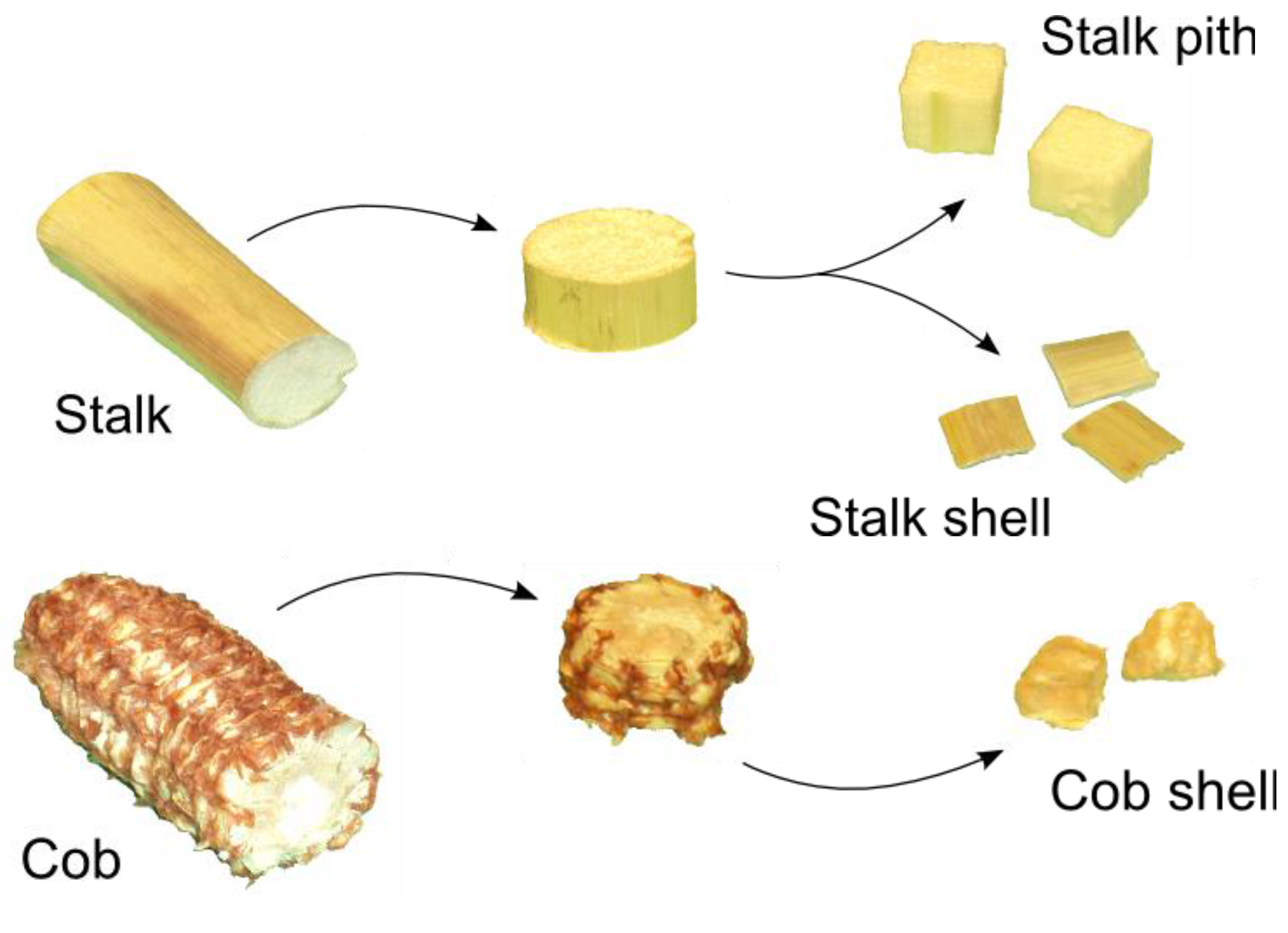
| Factor | Level | Dimensions (mm) | Shape |
|---|---|---|---|
| Particle size | Ground stover | <0.85 | Spherical |
| Whole stalk | 19 × 16 × 100 | Elliptic cylinder | |
| Corn stover component | Stalk shell | 5 × 5 × 2 | Prism |
| Stalk pith | 5 × 5 × 5 | Cube | |
| Cob shell | 5 × 5 × 5 | Cube | |
| Gas residence time | 1.2 s | ||
| 12 s | |||
| 60 s |
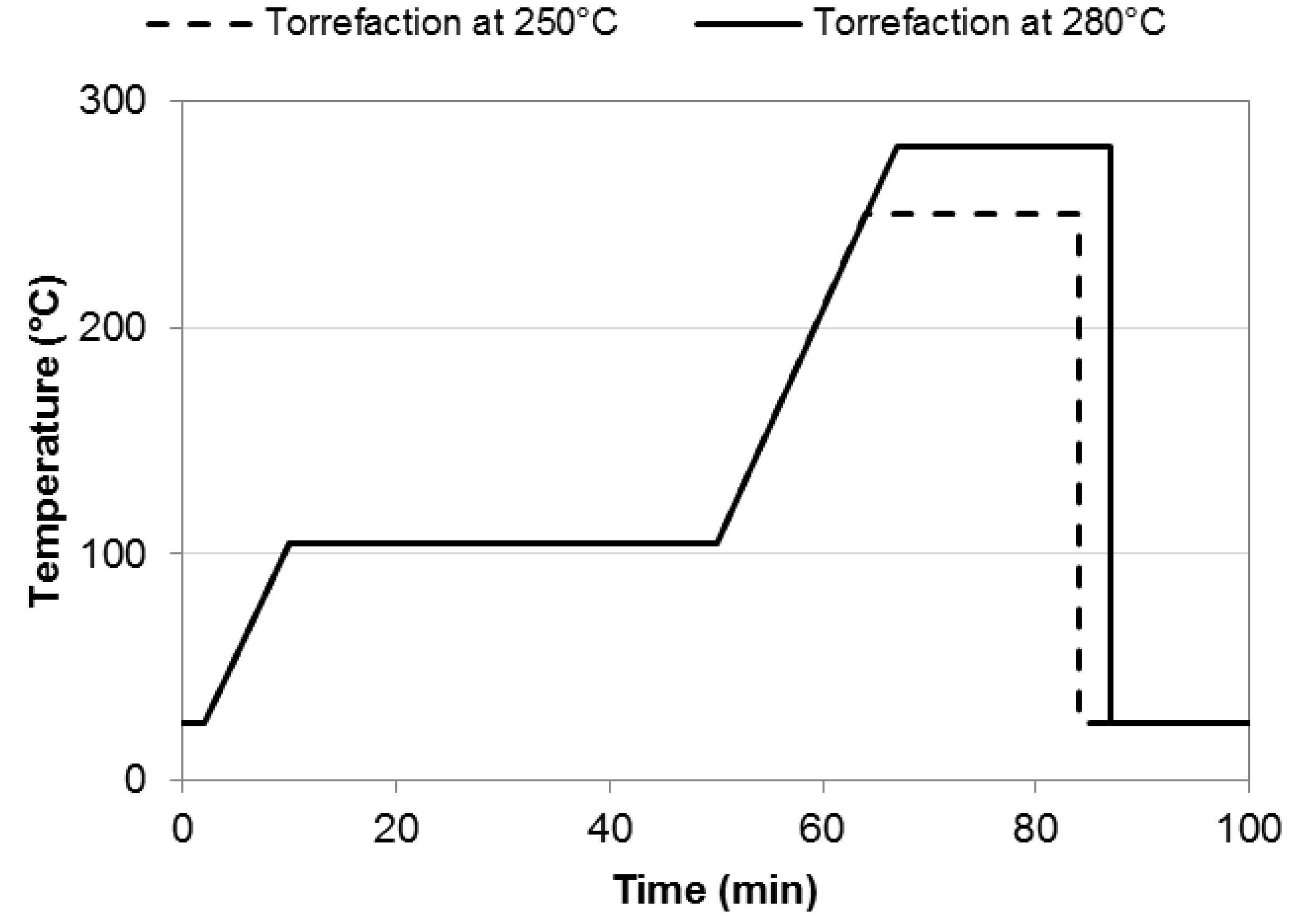
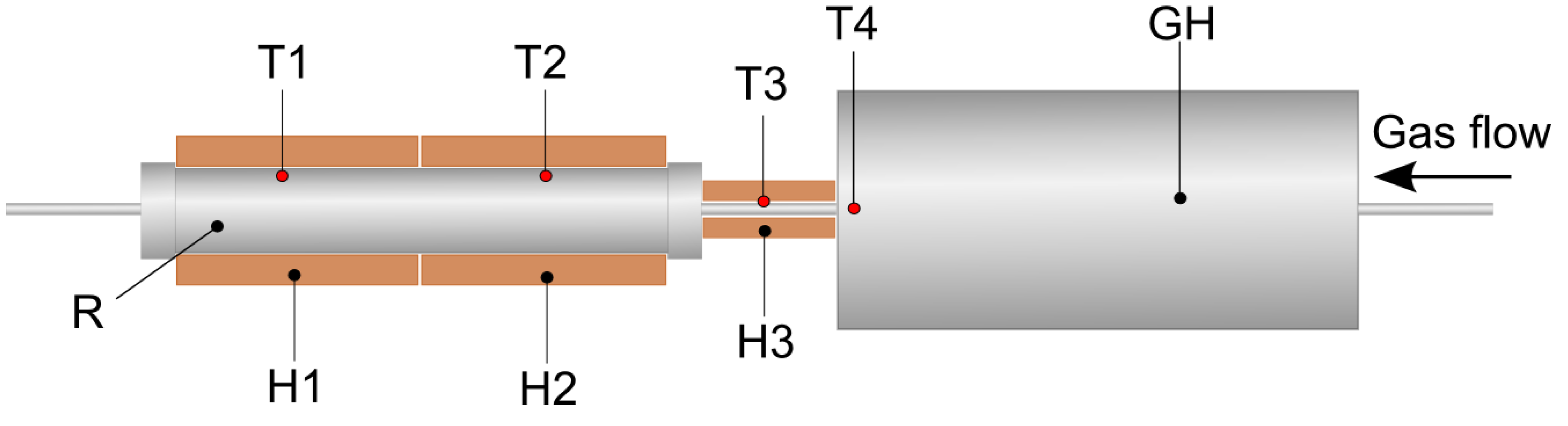
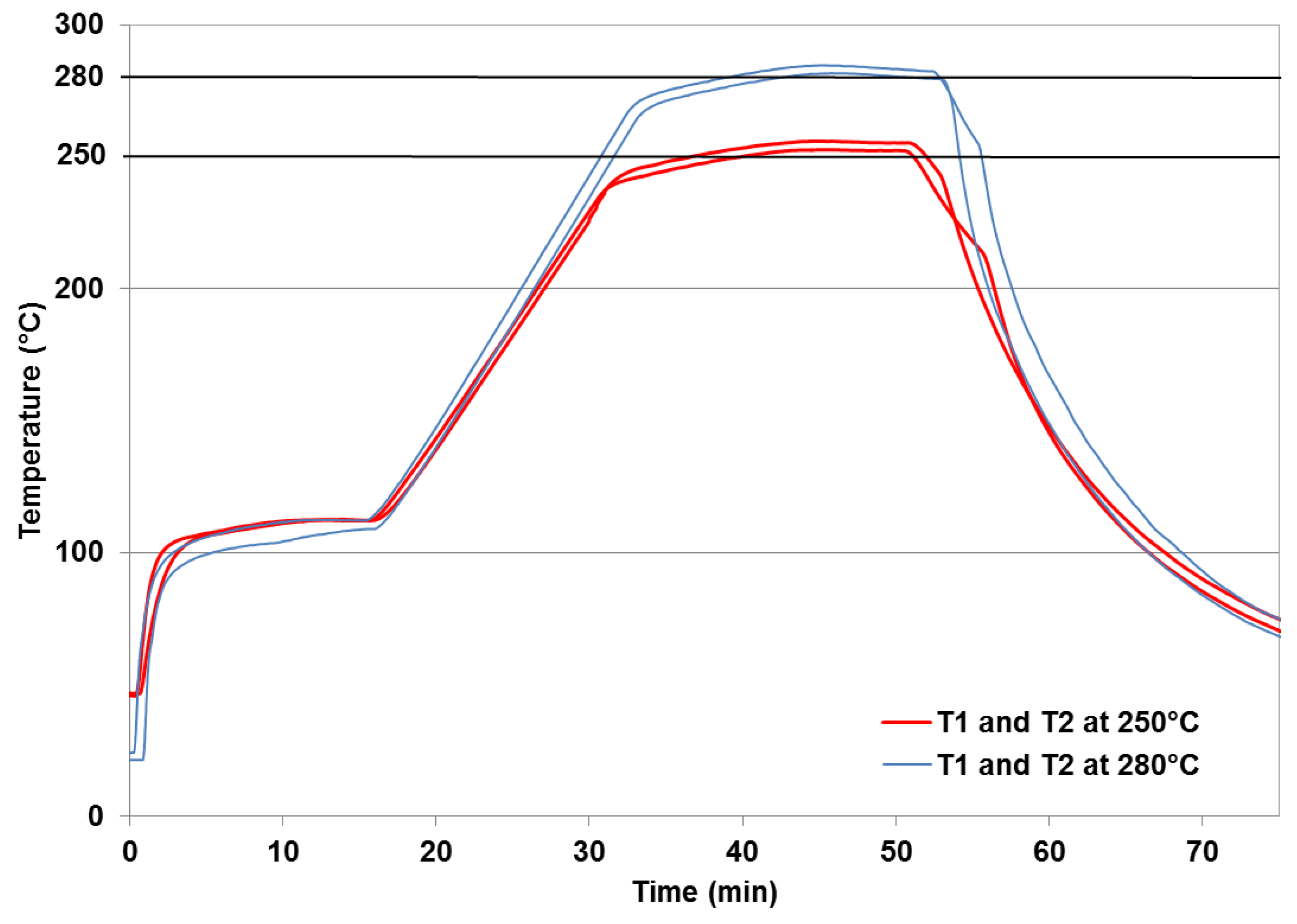
2.2. Torrefaction Experiments
2.3. Chemical Analysis of Raw and Torrefied Biomass
2.4. Statistical Analysis
3. Results and Discussion
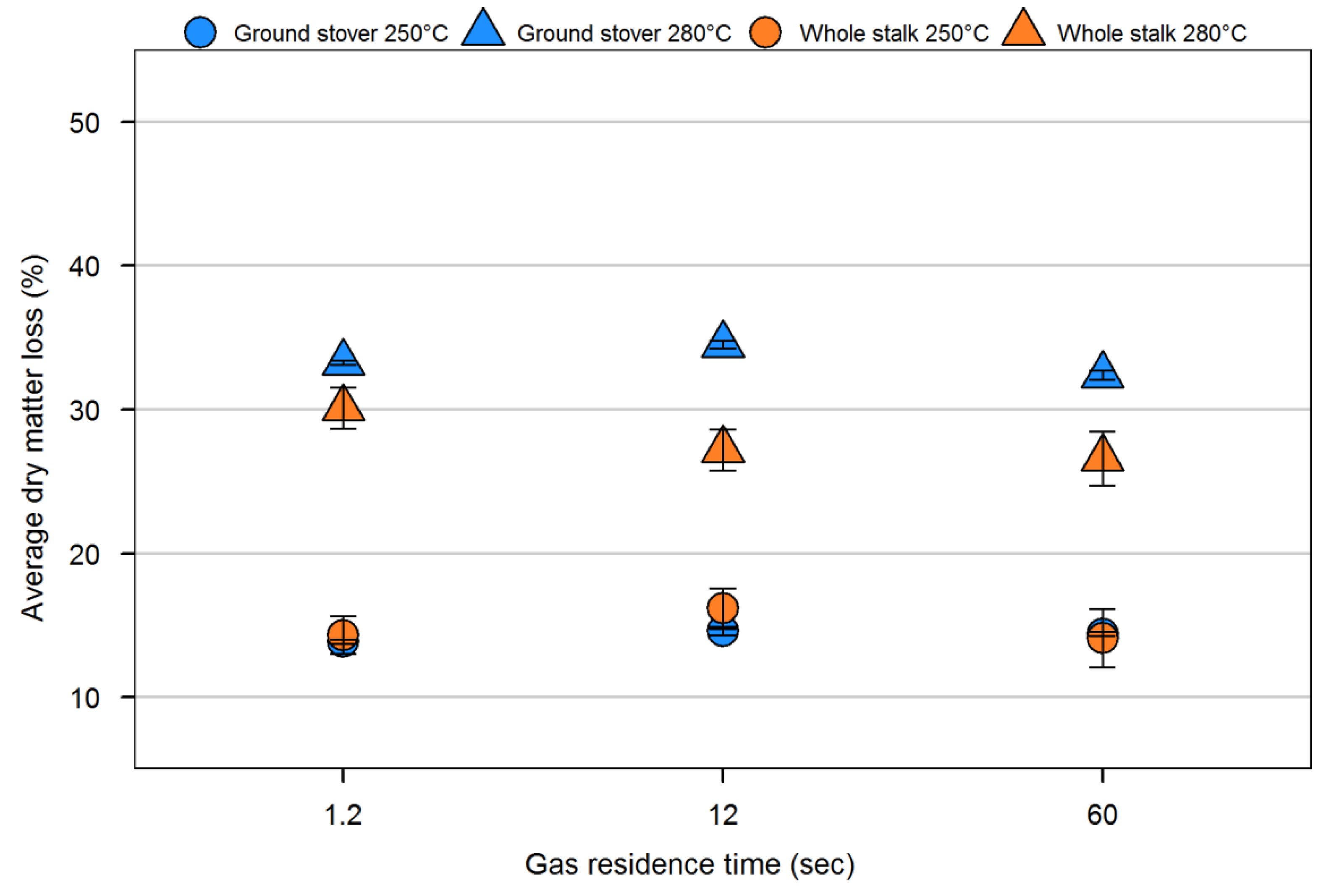
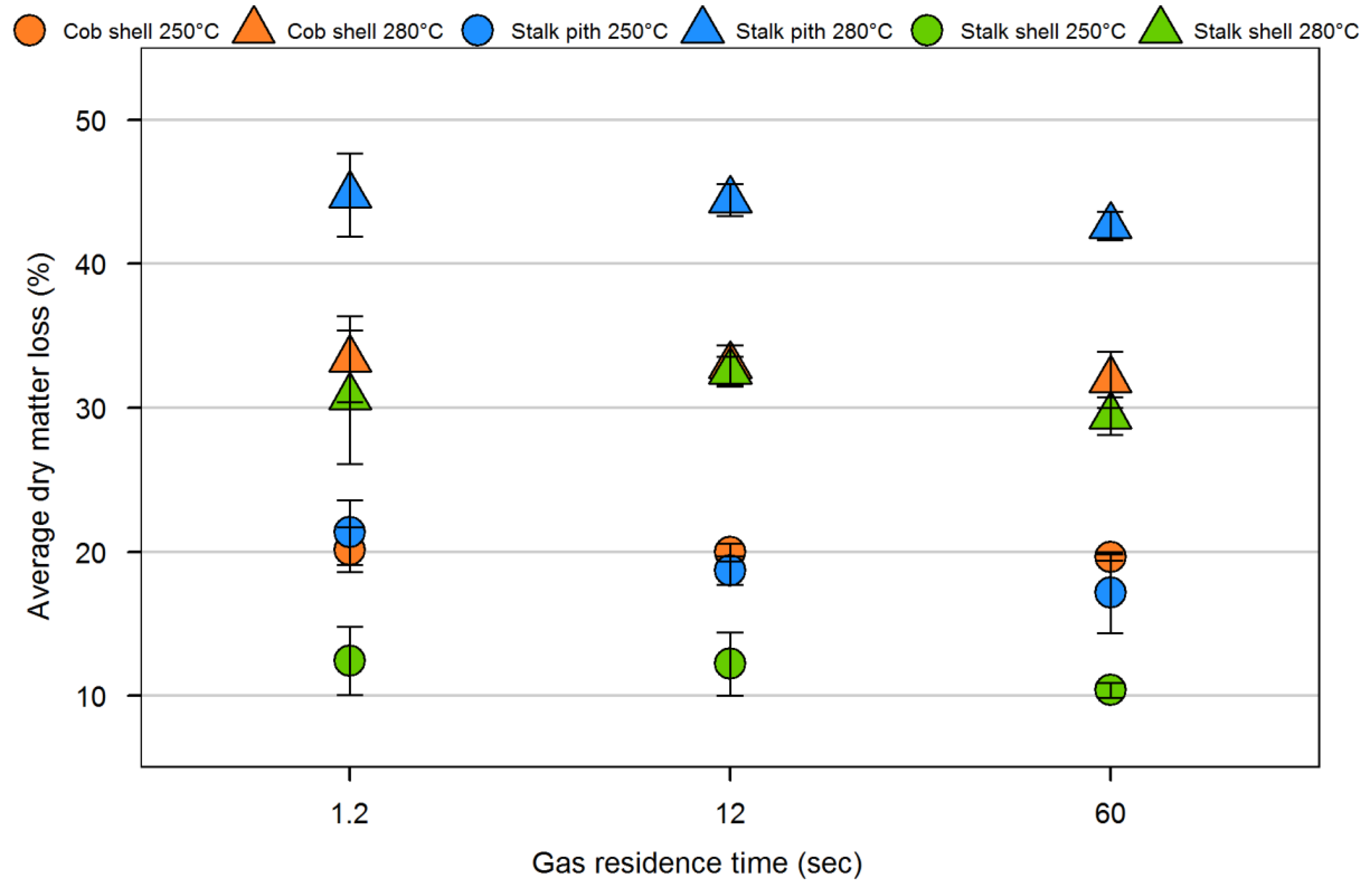
| Sample | Volatiles (%) | Fixed carbon (%) | Ash (%) | Particle density (kg/m3) | Fiber composition (%) | |||
|---|---|---|---|---|---|---|---|---|
| Cellulose | Hemicellulose | Lignin | Other | |||||
| Ground stover | 77.05 ± 0.22 | 17.56 ± 0.01 | 5.45 ± 0.33 | - | 43.3 ± 0.5 | 32.3 ± 0.1 | 6.3 ± 0.4 | 18.03 ± 0.0 |
| Stalk shell | 75.43 ± 0.20 | 21.31 ± 0.00 | 3.18 ± 0.06 | 444.61 ± 48.39 | 56.6 ± 0.2 | 15.2 ± 1.0 | 13.5 ± 0.1 | 15.62 ± 1.3 |
| Stalk pith | 80.57 ± 1.27 | 13.89 ± 1.13 | 5.49 ± 0.12 | 34.93 ± 6.66 | 57.6 ± 0.4 | 21.3 ± 1.8 | 6.1 ± 0.6 | 14.40 ± 0.8 |
| Cob shell | 76.88 ± 2.43 | 18.96 ± 2.24 | 4.13 ± 0.27 | 357.40 ± 47.84 | 45.2 ± 0.3 | 38.3 ± 1.1 | 10.3 ±0.0 | 7.17 ± 1.4 |
| Whole stover | 76.74 ± 0.87 | 17.20 ± 0.73 | 3.86 ± 0.17 | 116.48 ± 18.02 | 56.2 ± 1.8 | 17.6 ± 1.0 | 11.6 ± 0.8 | 14.60 ± 0.0 |
| Sample | Torrefaction temperature (°C) | Gas residence time (s) | C (wt %) | H (wt %) | N (wt %) | S (wt %) | O (wt %) | O/C | H/C | HHV (MJ/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Ground stover | 250 | 1.2 | 45.76 ± 0.37 | 5.99 ± 0.13 | 0.62 ± 0.06 | 0.36 ± 0.10 | 47.28 ± 0.43 | 1.03 ± 0.02 | 0.13 ± 0.01 | 18.68 ± 0.14 |
| 12 | 45.78 ± 1.08 | 5.27 ± 1.20 | 0.46 ± 0.08 | 0.32 ± 0.08 | 48.17 ± 2.42 | 1.05 ± 0.08 | 0.11 ± 0.00 | 18.85 ± 1.10 | ||
| 60 | 46.27 ± 0.22 | 5.81 ± 0.24 | 0.63 ± 0.06 | 0.56 ± 0.03 | 46.73 ± 0.13 | 1.01 ± 0.01 | 0.12 ± 0.02 | 18.70 ± 0.11 | ||
| 280 | 1.2 | 49.59 ± 0.25 | 5.56 ± 0.08 | 0.65 ± 0.10 | 0.33 ± 0.08 | 43.86 ± 0.31 | 0.88 ± 0.02 | 0.11 ± 0.00 | 19.48 ± 0.07 | |
| 12 | 50.21 ± 0.10 | 5.57 ± 0.07 | 0.70 ± 0.07 | 0.39 ± 0.05 | 43.13 ± 0.09 | 0.86 ± 0.00 | 0.13 ± 0.00 | 19.66 ± 0.02 | ||
| 60 | 50.35 ± 0.31 | 5.45 ± 0.08 | 0.71 ± 0.05 | 0.36 ± 0.03 | 43.14 ± 0.29 | 0.85 ± 0.01 | 0.11 ± 0.00 | 19.61 ± 0.10 | ||
| Raw | - | 44.84 ± 0.70 | 6.32 ± 0.07 | 0.46 ± 0.15 | 0.12 ± 0.03 | 48.26 ± 0.67 | 1.08 ± 0.03 | 0.14 ± 0.00 | 18.66 ± 0.24 | |
| Stalk shell | 250 | 1.2 | 48.76 ± 0.18 | 5.75 ± 0.21 | 0.19 ± 0.02 | 0.23 ± 0.16 | 45.06 ± 0.30 | 0.92 ± 0.01 | 0.12 ± 0.00 | 19.39 ± 0.18 |
| 12 | 48.35 ± 0.68 | 5.77 ± 0.20 | 0.19 ± 0.01 | 0.31 ± 0.17 | 45.38 ± 1.00 | 0.94 ± 0.03 | 0.10 ± 0.00 | 19.45 ± 0.30 | ||
| 60 | 48.60 ± 0.37 | 5.67 ± 0.06 | 0.14 ± 0.02 | 0.36 ± 0.44 | 45.22 ± 0.83 | 0.93 ± 0.02 | 0.12 ± 0.00 | 19.29 ± 0.12 | ||
| 280 | 1.2 | 53.97 ± 1.30 | 5.54 ± 0.19 | 0.16 ± 0.05 | 0.66 ± 0.71 | 39.67 ± 1.13 | 0.74 ± 0.04 | 0.09 ± 0.01 | 20.70 ± 0.25 | |
| 12 | 55.83 ± 0.38 | 5.29 ± 0.14 | 0.32 ± 0.06 | 0.24 ± 0.14 | 38.32 ± 0.32 | 0.69 ± 0.01 | 0.12 ± 0.01 | 21.07 ± 0.20 | ||
| 60 | 53.78 ± 0.62 | 5.37 ± 0.04 | 0.15 ± 0.04 | 0.40 ± 0.43 | 40.30 ± 1.01 | 0.75 ± 0.03 | 0.11 ± 0.01 | 20.54 ± 0.14 | ||
| Raw | - | 47.87 ± 0.06 | 6.28 ± 0.07 | 0.04 ± 0.03 | 0.10 ± 0.04 | 45.72 ± 0.15 | 0.95 ± 0.01 | 0.13 ± 0.00 | 19.50 ± 0.05 | |
| Stalk pith | 250 | 1.2 | 46.40 ± 0.06 | 5.48 ± 0.05 | 0.33 ± 0.12 | 0.15 ± 0.02 | 47.64 ± 0.04 | 1.03 ± 0.00 | 0.12 ± 0.00 | 18.54 ± 0.06 |
| 12 | 45.61 ± 0.54 | 5.58 ± 0.14 | 0.31 ± 0.04 | 0.18 ± 0.02 | 48.32 ± 0.45 | 1.06 ± 0.02 | 0.10 ± 0.01 | 18.46 ± 0.14 | ||
| 60 | 45.18 ± 0.08 | 5.34 ± 0.09 | 0.26 ± 0.05 | 0.08 ± 0.05 | 49.14 ± 0.08 | 1.09 ± 0.00 | 0.12 ± 0.01 | 18.10 ± 0.08 | ||
| 280 | 1.2 | 49.99 ± 0.67 | 4.77 ± 0.18 | 0.46 ± 0.07 | 0.11 ± 0.07 | 44.67 ± 0.57 | 0.90 ± 0.02 | 0.10 ± 0.01 | 19.07 ± 0.12 | |
| 12 | 51.27 ± 1.31 | 5.02 ± 0.29 | 0.47 ± 0.23 | 0.12 ± 0.03 | 43.13 ± 1.41 | 0.84 ± 0.05 | 0.12 ± 0.00 | 19.60 ± 0.53 | ||
| 60 | 49.89 ± 0.40 | 4.75 ± 0.12 | 0.54 ± 0.30 | 0.02 ± 0.01 | 44.79 ± 0.57 | 0.90 ± 0.02 | 0.10 ± 0.01 | 19.04 ± 0.03 | ||
| Raw | - | 44.69 ± 0.77 | 5.93 ± 0.12 | 0.12 ± 0.01 | 0.16 ± 0.07 | 49.10 ± 0.83 | 1.10 ± 0.04 | 0.13 ± 0.00 | 18.36 ± 0.30 | |
| Cob shell | 250 | 1.2 | 52.74 ± 0.13 | 5.80 ± 0.01 | 0.51 ± 0.25 | 0.18 ± 0.03 | 40.77 ± 0.26 | 0.77 ± 0.01 | 0.11 ± 0.00 | 20.54 ± 0.04 |
| 12 | 53.23 ± 0.83 | 6.07 ± 0.14 | 0.29 ± 0.01 | 0.19 ± 0.02 | 40.22 ± 0.94 | 0.76 ± 0.03 | 0.10 ± 0.00 | 20.67 ± 0.33 | ||
| 60 | 52.42 ± 0.40 | 5.68 ± 0.05 | 0.31 ± 0.06 | 0.10 ± 0.02 | 41.49 ± 0.41 | 0.79 ± 0.01 | 0.11 ± 0.01 | 20.37 ± 0.10 | ||
| 280 | 1.2 | 56.52 ± 0.30 | 5.48 ± 0.09 | 0.40 ± 0.04 | 0.05 ± 0.03 | 37.56 ± 0.20 | 0.66 ± 0.01 | 0.10 ± 0.01 | 21.39 ± 0.03 | |
| 12 | 56.44 ± 0.69 | 5.86 ± 0.10 | 0.41 ± 0.03 | 0.18 ± 0.04 | 37.11 ± 0.62 | 0.66 ± 0.02 | 0.11 ± 0.00 | 21.62 ± 0.15 | ||
| 60 | 56.12 ± 0.48 | 5.41 ± 0.05 | 0.37 ± 0.07 | 0.05 ± 0.04 | 38.05 ± 0.54 | 0.68 ± 0.02 | 0.10 ± 0.01 | 21.23 ± 0.14 | ||
| Raw | - | 47.15 ± 0.14 | 6.25 ± 0.02 | 0.10 ± 0.04 | 0.08 ± 0.04 | 46.41 ± 0.21 | 0.98 ± 0.01 | 0.13 ± 0.00 | 19.28 ± 0.04 | |
| Whole stover | 250 | 1.2 | 47.96 ± 0.41 | 5.83 ± 0.35 | 0.30 ± 0.08 | 0.26 ± 0.04 | 45.65 ± 0.67 | 0.95 ± 0.02 | 0.12 ± 0.01 | 19.21 ± 0.33 |
| 12 | 48.36 ± 1.27 | 5.73 ± 0.11 | 0.41 ± 0.03 | 0.25 ± 0.02 | 45.25 ± 1.33 | 0.94 ± 0.06 | 0.11 ± 0.00 | 19.25 ± 0.39 | ||
| 60 | 47.78 ± 1.00 | 5.98 ± 0.12 | 0.30 ± 0.04 | 0.29 ± 0.12 | 45.65 ± 0.93 | 0.95 ± 0.04 | 0.12 ± 0.00 | 19.26 ± 0.34 | ||
| 280 | 1.2 | 52.57 ± 0.95 | 5.42 ± 0.23 | 0.40 ± 0.11 | 0.32 ± 0.10 | 41.30 ± 0.95 | 0.79 ± 0.03 | 0.11 ± 0.00 | 20.23 ± 0.22 | |
| 12 | 51.83 ± 0.82 | 5.68 ± 0.14 | 0.35 ± 0.01 | 0.23 ± 0.03 | 41.90 ± 0.94 | 0.81 ± 0.03 | 0.12 ± 0.01 | 20.20 ± 0.31 | ||
| 60 | 50.94 ± 0.31 | 5.72 ± 0.13 | 0.29 ± 0.04 | 0.27 ± 0.04 | 42.77 ± 0.35 | 0.84 ± 0.01 | 0.11 ± 0.01 | 19.98 ± 0.17 | ||
| Raw | - | 47.95 ± 0.23 | 6.18 ± 0.02 | 0.14 ± 0.06 | 0.16 ± 0.02 | 45.63 ± 0.25 | 0.95 ± 0.01 | 0.13 ± 0.00 | 19.45 ± 0.08 |
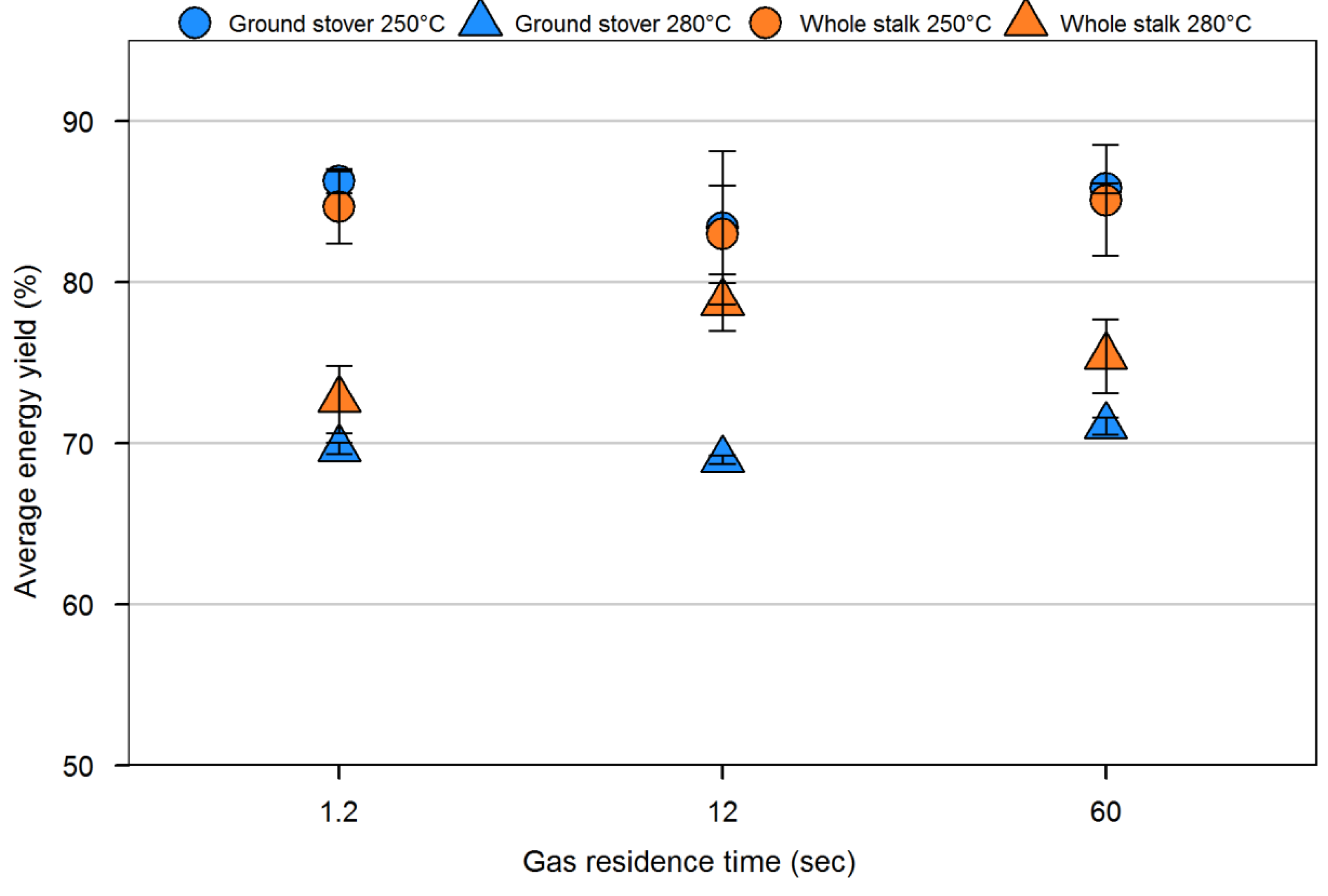
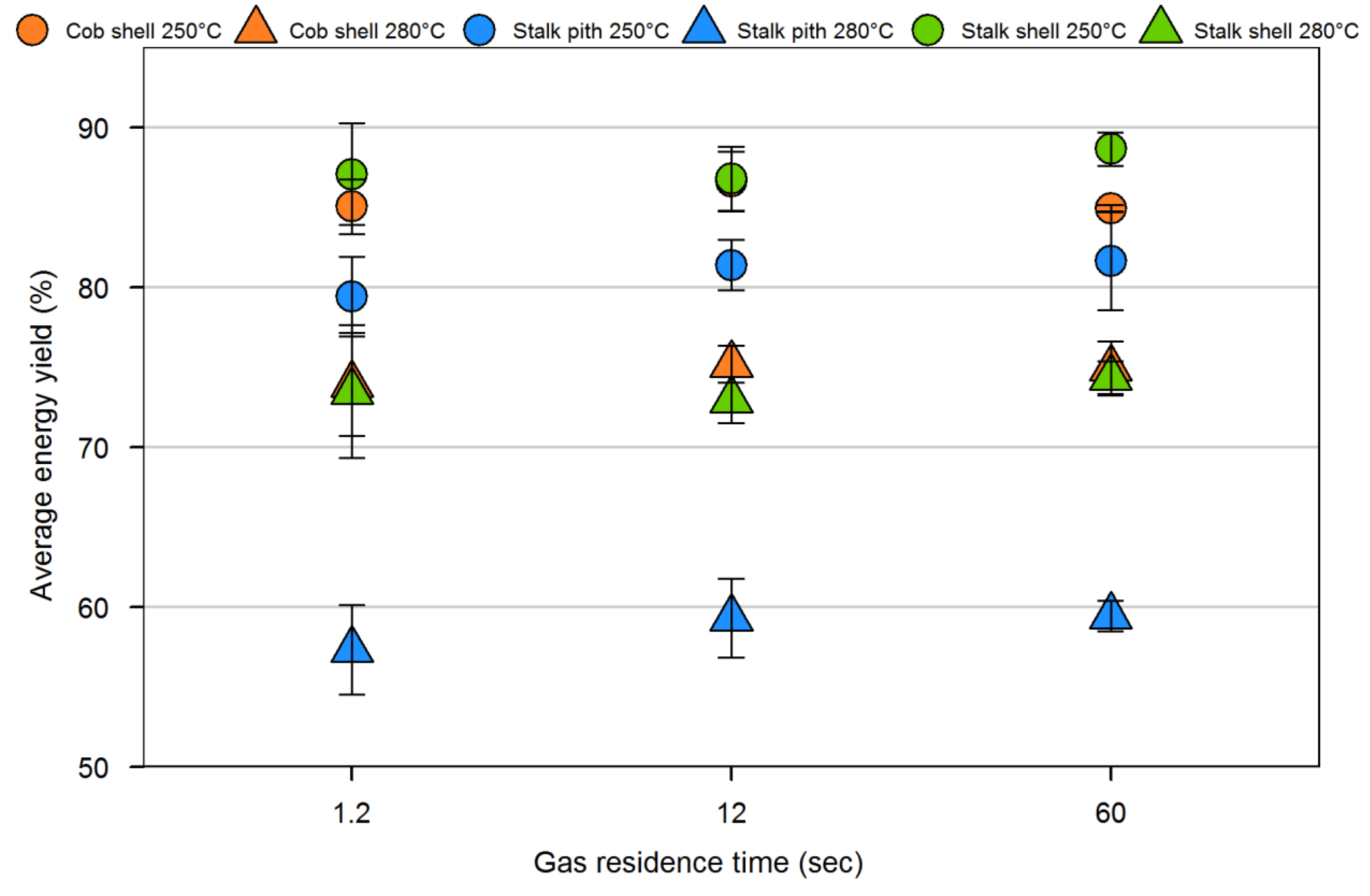
4. Conclusions
Acknowledgments
References
- The Energy Independence and Security Act of 2007. Available online: http://www.energy.wsu.edu/documents/EnergyIndependenceAndSecurityActOf2007.pdf (accessed on 22 April 2012).
- Feng, Y.; Xiao, B.; Goerner, K.; Cheng, G.; Wang, J. Influence of particle size and temperature on gasification performance in externally heated gasifier. Smart Grid Renew. Energy 2011, 2, 158–164. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Guo, X.; Hu, Z.; Liu, S.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of particle size on gasification performance. Int. J. Hydrog. Energy 2009, 34, 1260–1264. [Google Scholar] [CrossRef]
- Moghiman, M.; Hashemi, T.; Zahmatkesh, I.; Daghighi, Y. Effects of particle size and equivalence ratio on cyclone gasification of wood powder. J. Energy Inst. 2007, 80, 29–34. [Google Scholar] [CrossRef]
- Shen, J.; Wang, X.-S.; Garcia-Perez, M.; Mourant, D.; Rhodes, M.J.; Li, C.-Z. Effects of particle size on the fast pyrolysis of oil mallee woody biomass. Fuel 2009, 88, 1810–1817. [Google Scholar] [CrossRef]
- Gaston, K.R.; Jarvis, M.W.; Pepiot, P.; Smith, K.M.; Frederick, W.J.; Nimlos, M.R. Biomass pyrolysis and gasification of varying particle sizes in a fluidized-bed reactor. Energy Fuel 2011, 25, 3747–3757. [Google Scholar] [CrossRef]
- Zhu, W.; Song, W.; Lin, W. Effect of the coal particle size on pyrolysis and char reactivity for two types of coal and demineralized coal. Energy Fuel 2008, 22, 2482–2487. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Kiel, J.H.A.; Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction for entrained-flow gasification of biomass. ECN-C-05-067; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Paulrud, S.; Mattson, J.E.; Nilsson, C. Particle and handling characteristics of wood fuel powder: Effects of different mills. Fuel Process. Technol. 2002, 76, 23–39. [Google Scholar] [CrossRef]
- Lu, H.; Ip, E.; Scott, J.; Foster, P.; Vickers, M.; Baxter, L.L. Effects of particle shape and size on devolatilization of biomass particle. Fuel 2010, 89, 1156–1168. [Google Scholar] [CrossRef]
- Neathery, J.K. Biomass Gasification. In Thermochemical Conversion of Biomass to Liquid Fuels and Chemicals; Crocker, M., Ed.; Blackwell Publishing Professional: Ames, IA, USA, 2007; pp. 67–94. [Google Scholar]
- Antal, M.J.; Gronli, M. The art, science, and technology of charcoal production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Antal, M.J.; Croiset, E.; Dai, X.; DeAlmeida, C.; Shu-lai Mok, W.; Norberg, N. High-yield biomass charcoal. Energy Fuels 1996, 10, 652–658. [Google Scholar] [CrossRef]
- Klass, D.L. Biomass for Renewable Energy, Fuels and Chemicals; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Arias, B.; Pevida, C.; Fermoso, J.; Plaza, M.G.; Rubeira, F.; Pis, J.J. Influence of torrefaction on the grindability and reactivity of woody biomass. Fuel Process. Technol. 2008, 89, 169–175. [Google Scholar] [CrossRef]
- Yan, W.; Acharjee, T.C.; Coronella, C.J.; Vaquez, R.V. Thermal pretreatment of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2009, 28, 435–440. [Google Scholar] [CrossRef]
- Bergman, P.C.A.; Boersma, A.R.; Zwart, R.W.R.; Kiel, J.H.A. Torrefaction for biomass co-firing in existing coal-fired power stations. ECN-C-05-013; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Biomass Research and Development Initiative (BRDI). Roadmap for Bioenergy and Biobased Products in the United States. Available online: http://www1.eere.energy.gov/biomass/pdfs/obp_roadmapv2_web.pdf (accessed on 22 April 2012).
- Bridgeman, T.G.; Jones, J.M.; Shield, I.; Williams, P.T. Torrefaction of reed canary grass, wheat straw and willow to enhance solid fuel qualities and combustion properties. Fuel 2008, 87, 844–856. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Potter, B.; Zimmerrnan, J. Effects of torrefaction process parameters on biomass feedstock upgrading. Fuel 2011, 91, 147–154. [Google Scholar] [CrossRef]
- Shah, A.; Darr, M.; Medic, D.; Anex, R.; Khanal, S.; Maski, D. Techno-economic analysis of a production-scale torrefaction system for cellulosic biomass upgrading. Biofuels Bioprod. Biorefin. 2012, 6, 45–57. [Google Scholar] [CrossRef]
- Medic, D.; Darr, M.; Shah, A.; Rahn, S. Effect of torrefaction on water vapor adsorption properties and resistance to microbial degradation of corn stover. Energy Fuels 2012. [Google Scholar] [CrossRef]
- Chen, W.-H.; Kuo, P.-C. Torrefaction and co-torrefaction characterization of hemicellulose, cellulose and lignin as well as of some basic constituents in biomass. Energy 2011, 36, 803–811. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood. Part 2. Analysis of products. J. Anal. Appl. Pyrol. 2006, 77, 35–40. [Google Scholar] [CrossRef]
- Ciolkosz, D.; Wallace, R. A review of torrefaction for bioenergy feedstock production. Biofuels, Bioprod. Biorefin. 2011, 5, 317–329. [Google Scholar] [CrossRef]
- van der Stelt, M.J.C.; Gerhauser, H.; Kiel, J.H.A.; Ptasinski, K.J. Biomass upgrading by torrefaction for the production of biofuels: A review. Biomass. Bioenergy 2011, 35, 3748–3762. [Google Scholar]
- Bergman, P.C.A. Combined Torrefaction and Pelletization: ECN-C-05-073; ECN: Petten, The Netherlands, 2005. [Google Scholar]
- Couhert, C.; Salvador, S.; Commandre, J.-M. Impact of torrefaction on sygas production from wood. Fuel 2009, 88, 2286–2290. [Google Scholar] [CrossRef]
- Prins, M.J.; Ptasinski, K.J.; Janssen, F.J.J.G. Torrefaction of wood. Part 1. Weight Loss Kinetics. J. Anal. Appl. Pyrol. 2006, 77, 28–34. [Google Scholar] [CrossRef]
- ASAE standard D358.2 (R2008). In Moisture Measurement—Forages; American Society of Agricultural Engineers: St. Joseph, MI, USA, 1998.
- ASTM D 5142-04. In Standard Test Method for Proximate Analysis of the Analysis Sample of Coal and Coke by Instrumental Procedures; ASTM International: West Conshohocken, PA, USA, 2008.
- ASTM D 5373-08. In Standard Test Method for Instrumental Determination of Carbon, Hydrogen and Nitrogen in Laboratory Samples of Coal; ASTM International: West Conshohocken, PA, USA, 2008.
- Sheng, C.; Azevedo, J.L.T. Estimating the higher heating value of biomass fuels from basic analysis data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- AOAC Standard 973.18. In Fiber (Acid Detergent) and Lignin in Animal Feeds; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 1990.
- AOAC Standard 2002.04. In Gravimetric Determination of Amylase-Treated Neutral Detergent Fiber in Feeds Using Refluxing in Beaker or Crucibles; Association of Official Analytical Chamists: Gaithersburg, MD, USA, 2002.
- Krull, L.H.; Inglett, G.E. Analysis of neutral carbohydrate in agricultural residues by gas-liquid chromatography. J. Agric. Food Chem. 1980, 28, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Garlock, R.J.; Chundawat, S.P.; Balan, V.; Dale, B.E. Optimizing harvest of cont stover fractions based on overall sugar yields following ammonia fiber expansion pretreatment and enzymatic hydrolysis. Biotechnol. Biofuels 2009, 2, 29. [Google Scholar] [CrossRef] [PubMed]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Medic, D.; Darr, M.; Shah, A.; Rahn, S. The Effects of Particle Size, Different Corn Stover Components, and Gas Residence Time on Torrefaction of Corn Stover. Energies 2012, 5, 1199-1214. https://doi.org/10.3390/en5041199
Medic D, Darr M, Shah A, Rahn S. The Effects of Particle Size, Different Corn Stover Components, and Gas Residence Time on Torrefaction of Corn Stover. Energies. 2012; 5(4):1199-1214. https://doi.org/10.3390/en5041199
Chicago/Turabian StyleMedic, Dorde, Matthew Darr, Ajay Shah, and Sarah Rahn. 2012. "The Effects of Particle Size, Different Corn Stover Components, and Gas Residence Time on Torrefaction of Corn Stover" Energies 5, no. 4: 1199-1214. https://doi.org/10.3390/en5041199




