Experimental Simulation of the Exploitation of Natural Gas Hydrate
Abstract
:1. Introduction
2. Distribution of Global Natural Gas Hydrate Resource
2.1. Amount and Distribution Area of Global Natural Gas Hydrate Resource
| Classification | Geographic location | Distribution characteristics | Reference |
|---|---|---|---|
| Terrestrial hydrate zone | Messoyakha River basin to the north and northeast of Russia | The distribution area is 1700 × 104 km2 and the depth of hydrate layer is underground 300–1000 m | [16,17] |
| Prudhoe Bay to the north slope of Alaska | The depth of hydrate layer is underground 210–950 m and the amount of natural gas hydrate in the north slope of Alaska is about 1.0–1.2 × 1012 m3 | [18,19] | |
| Mackenzie Delta to North Pole | The depth of the hydrate layer is 200 m underground | [20] | |
| Qinghai-Tibet Plateau permafrost region | Below the permafrost layer 133–396 m | [21] | |
| Marine hydrate zone | Gas hydrate formation zone in the Arctic Ocean | It is estimated that the region from 90 m water depth to mainland in the Arctic continental shelf is the permafrost zone. The hydrate distribution in this region is similar to that in terrestrial permafrost zone. | [22,23] |
| Gas hydrate formation zone in the Atlantic | Black Ridge (natural gas hydrate present between 190–450 m in sediment column, and the amount is at least 67 × 1015 g), Gulf of Mexico (approximately 500–1000 m below the mudline), Gulf of Guinea, Spitzbergen Margin | [24,25,26,27] | |
| Gas hydrate formation zone in the Pacific | Hydrate Ridge (the volume of methane gas in hydrate reservoirs is 6.4 × 1010 m3); in the Nankai Trough off Japan (the natural gas hydrate resource is 4–20 trillion cubic meters); in the Okhotsk Sea [the methane preserved in hydrate is (15 ± 12) × 1013 m3]; in the South China Sea (the top of the hydrate layers are located 155–229 m below the seafloor, and the thickness varies from 10 to 43 m); Middle America Trench; Hikurangi Trough off New Zealand; the Bering Sea | [28,29,30,31,32,33,34,35,36] | |
| Gas hydrate formation zone in the Indian Ocean | The Arabian Sea gas hydrate deposits area is about 80,000 km2, The Gulf of Oman gas hydrate layer is stable within the uppermost 350–700 m of sediment | [18,37,38,39] | |
| Gas hydrate formation zone in inland seas | The Black Sea (the thickness of natural gas hydrate is between 160–500 m based on the depth of seawater, the distribution area is 3.0 × 104 km2, and the amount of natural gas hydrate is about 42 × 1012 m3), The Caspian Sea (the top of the hydrate layers are located 390–480 m below the seafloor, and the thickness of hydrate layer is 134–152 m), The Azov Sea Basin | [15,40] |
2.2. Discovered Natural Gas Hydrate Deposits in the World
| No. | Natural gas hydrate deposits | Evidence of hydrate samples |
|---|---|---|
| 1 | Pacific Ocean off Panama | BSR |
| 2 | Middle America Trench (MAT) | BSR |
| 3 | MAT off Nicaragua | BSR |
| 4 | MAT off Guatemala | BSR |
| 5 | MAT off Mexico | core sampling |
| 6 | Mexico(Gulf of California, Guaymas Basin) | BSR |
| 7 | Eel River basin off California | BSR |
| 8 | Oregon USA (Cascadia Basin) | BSR |
| 9 | Vancouver Island(Cascadia Basin) | BSR |
| 10 | E. Aleutian Trench off Alaska | BSR |
| 11 | Mid Aleutian Trench | BSR |
| 12 | Bering Sea Alaska | vertical velocity abnormal |
| 13 | Beringian margin off Alaska | BSR |
| 14 | Shirshov Ridge (Russia) | BSR |
| 15 | Paramushir Island (Okhotsk Sea) | core sampling |
| 16 | Japan (Japan Sea) | core sampling |
| 17 | Japan (Japan Trench) | chlorine abnormal |
| 18 | Nankai Trough off Japan | BSR |
| 19 | Hikurangi Trough off New Zealand | BSR |
| 20 | Peru-Chile Trench off Chile | BSR |
| 21 | Peru-Chile Trench off Peru | BSR |
| 22 | Sahkalin Island (Russia) (Okhotsk Sea) | core sampling |
| 23 | Argentina (Central Argentine Basin) | BSR |
| 24 | Brazil(Amazon Fan) | BSR |
| 25 | Barbados Ridge Complex off Barbados | BSR |
| 26 | S. Caribbean Sea | BSR |
| 27 | Colombia Basin off Panama & Colombia | BSR |
| 28 | Gulf of Mexico off Mexico | BSR |
| 29 | Gulf of Mexico off S. USA | core sampling |
| 30 | Blake Outer Ridge off SE USA | BSR |
| 31 | Carolina Rise | BSR |
| 32 | Continental Rise off E. USA | BSR |
| 33 | Labrador Shelf off Newfoundland | BSR |
| 34 | Norway (Cont. Slope) | BSR |
| 35 | Crimea, Ukraine Black Sea (Russia) | core sampling |
| 36 | Caucasus, Russia Black Sea | BSR |
| 37 | Makran Margin, Gulf of Oman | BSR |
| 38 | Beaufort Sea off Alaska | BSR |
| 39 | Beaufort Sea off Canada | well logging |
| 40 | Svedrup Basin off Canada | well logging |
| 41 | Norway (Barents Sea) | BSR |
| 42 | Svalbard(Fram Strait) | BSR |
| 43 | Wilkes Land Margin off Antarctica | BSR |
| 44 | W. Ross Sea off Antarctica | gas |
| 45 | Weddell Sea off Antarctica | BSR |
| 47 | Lake Baikal, Russia | BSR |
| 48 | North slope, Alaska | well logging |
| 49 | Mackenzie Delta, Canada | well logging |
| 50 | Arctic Island, Canada | well logging |
| 51 | Timan-Pechora Province, USSR | gas |
| 52 | Messokayha Field, USSR | core sampling |
| 53 | E. Siberian Craton, USSR | gas |
| 54 | NE Siberia, USSR | gas |
| 55 | Kamchatka, USSR | gas |
| 56 | the volcanoes in the eastern Mediterranean Sea | gas and isotope of oxygen |
| 57 | Isla Mocha across the southern Chile margin | BSR |
| 58 | The northwestern Sea of Okhotsk | BSR |
| 59 | Santa Barbara Basin | gas |
| 60 | Manon site at the outer edge of the Barbados | chlorine abnormal |
| 61 | Congo-Angola | core sampling |
| 62 | Hakon Mosby mud volcano in the Norwegiian Sea | core sampling |
| 63 | The Ormen Lange area of the Storegga Slide | seismic data |
| 64 | The northern part of South China Sea (Xisha region), Dongsha region, and the edge of Manila | BSR, core sampling |
| 65 | Costa Rica forearc | core sampling |
| 66 | Barkley Canyon | carbon and deuterium isotope abnormal |
| 67 | Congo Basin, offshore southwestern Africa | core sampling |
| 68 | The Makassar Strait, between the islands of Borneo and Sulawesi, offshore Indonesia | core sampling |
| 69 | Sado Island in the eastern Japan Sea | core sampling |
| 70 | The Storegga Slide and at the southern edge of the Vǿring Plateau | BSR |
| 71 | The Makran continental margin | BSR |
| 72 | Qilian Mountains, Qinghai-Tibet Plateau permafrost region | core sampling |
3. Classification of Gas Hydrate Reservoirs
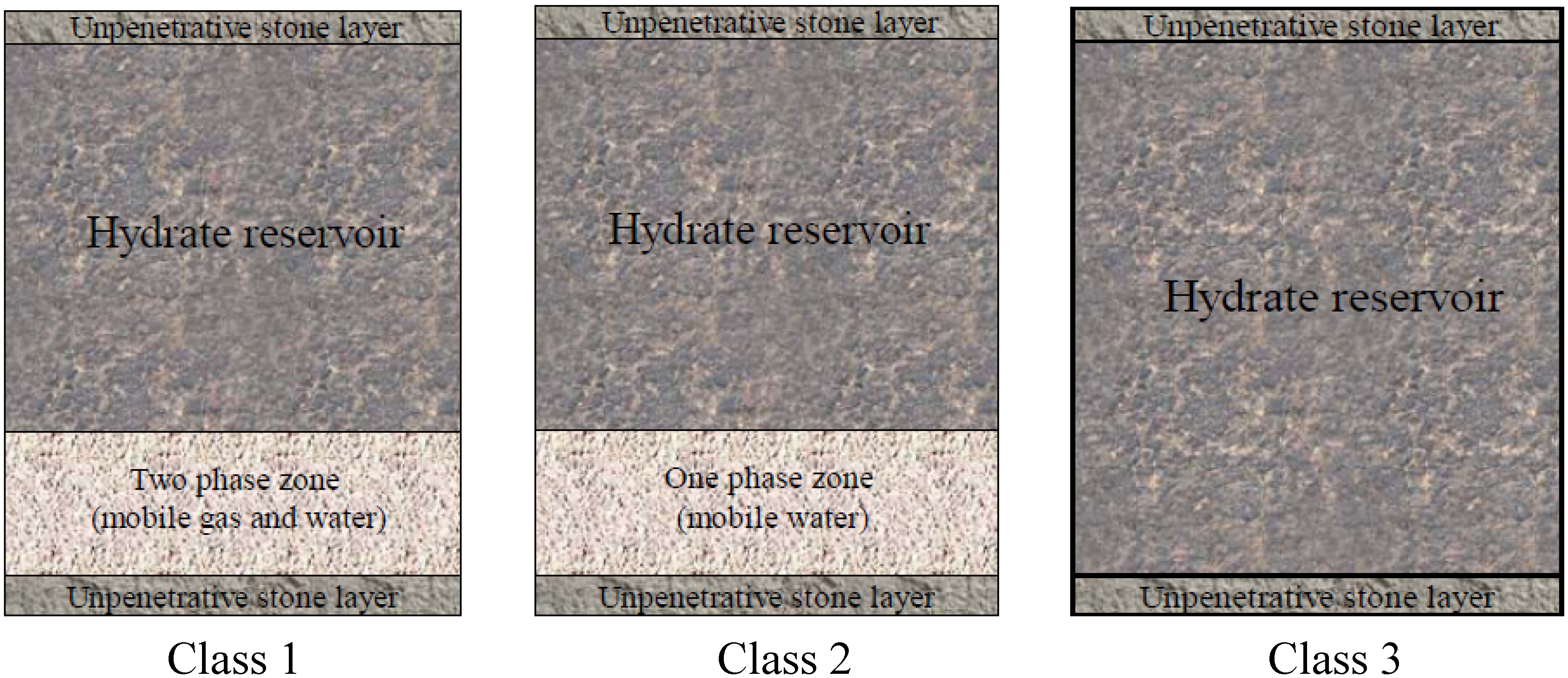
4. Techniques for Exploitation of Natural Gas Hydrate
4.1. Depressurization Method

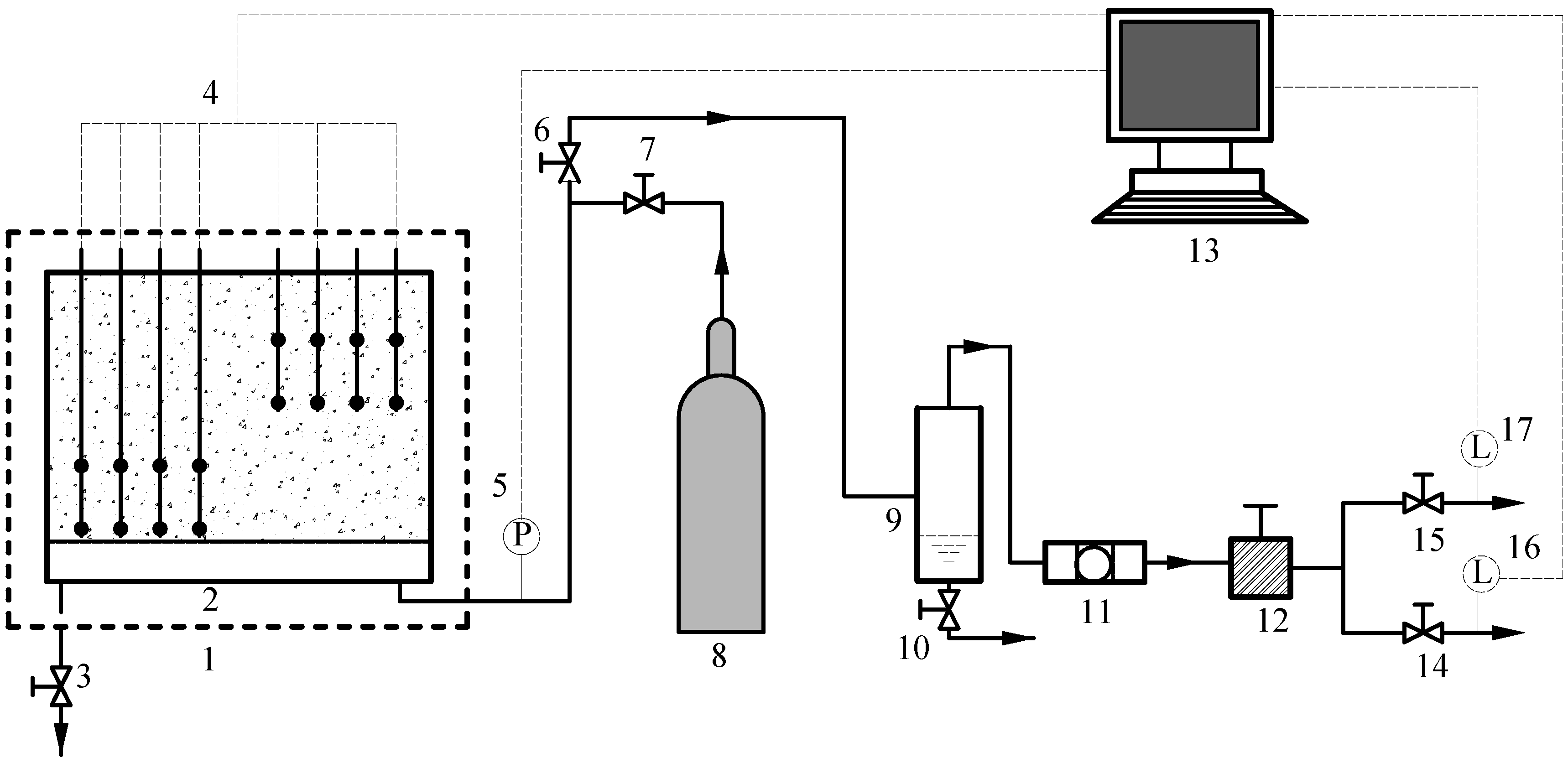

4.2. Thermal Stimulation Methods
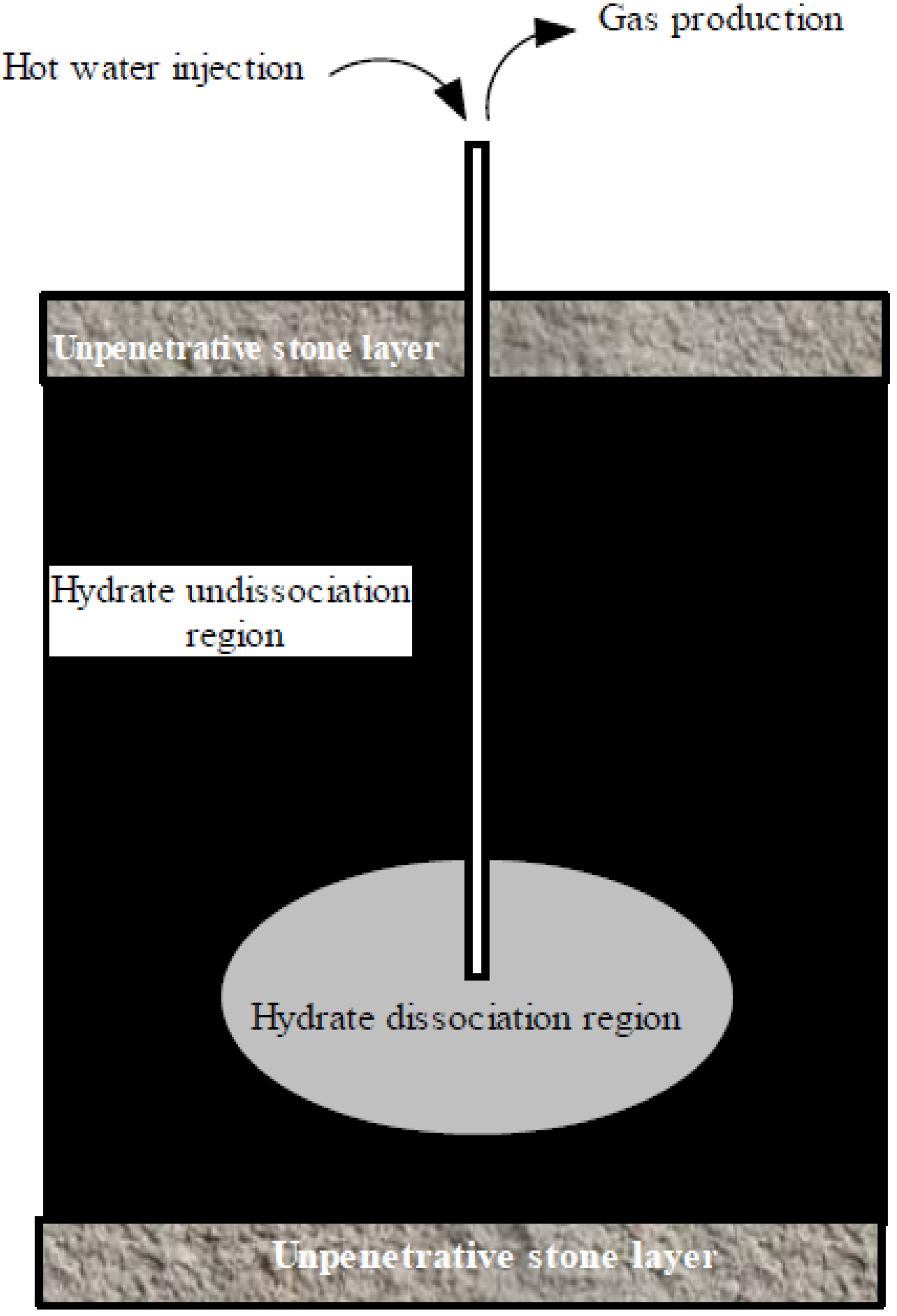
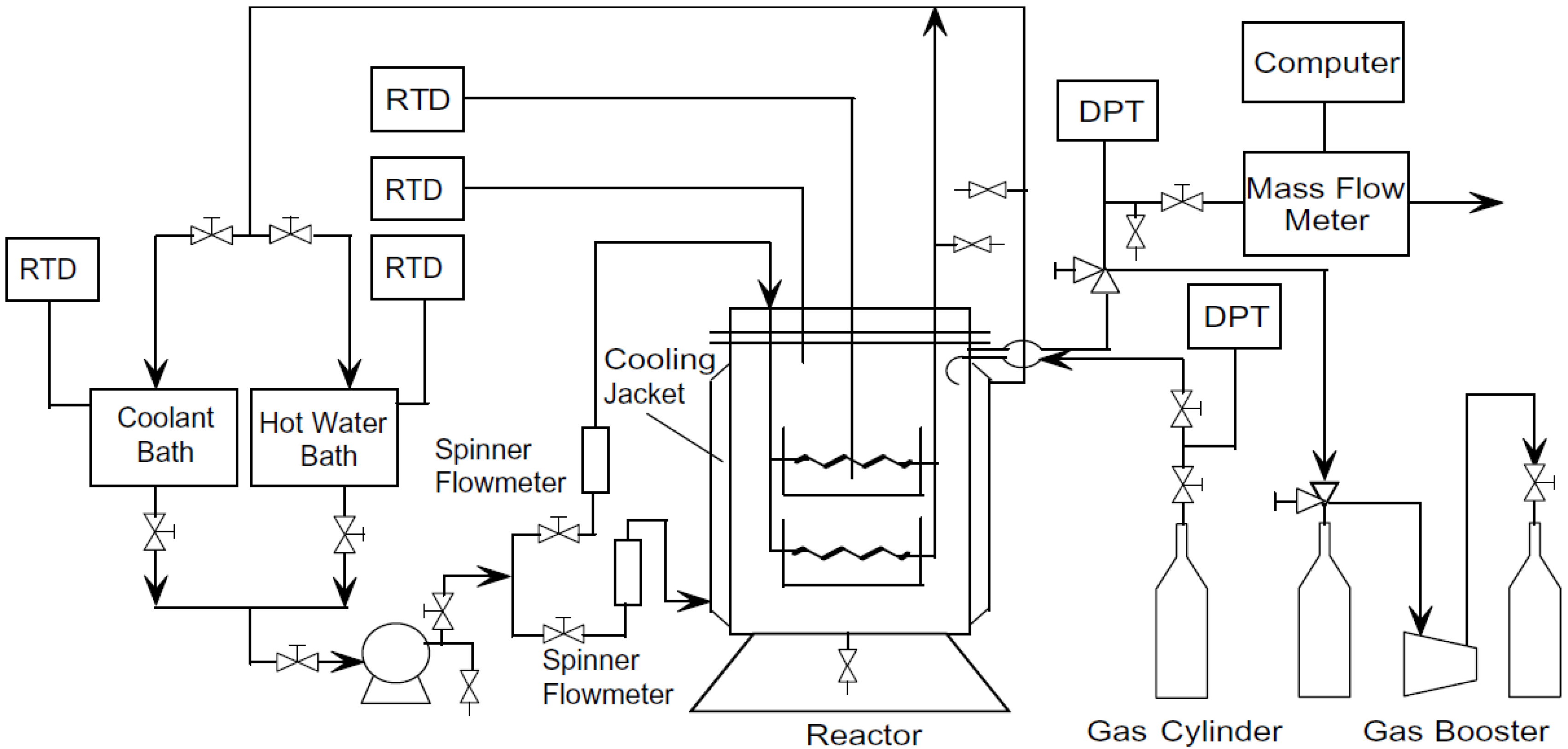
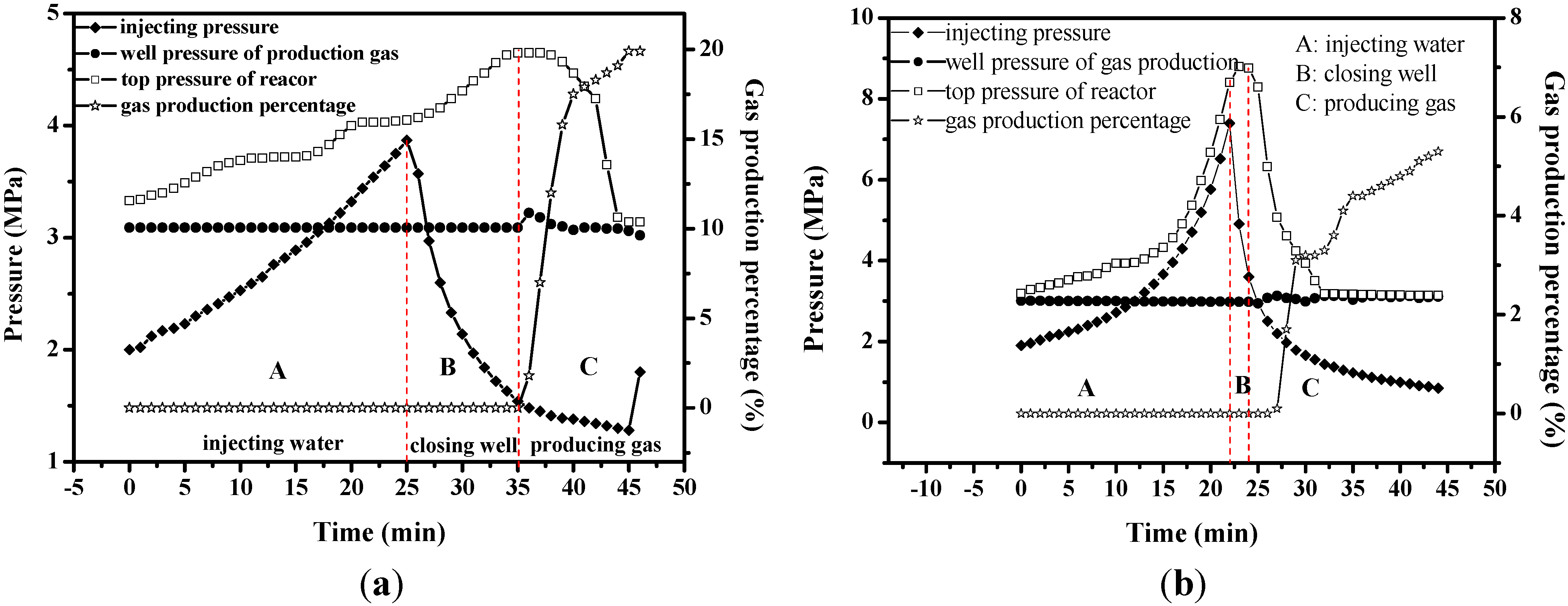
4.3. Chemical Injection Method
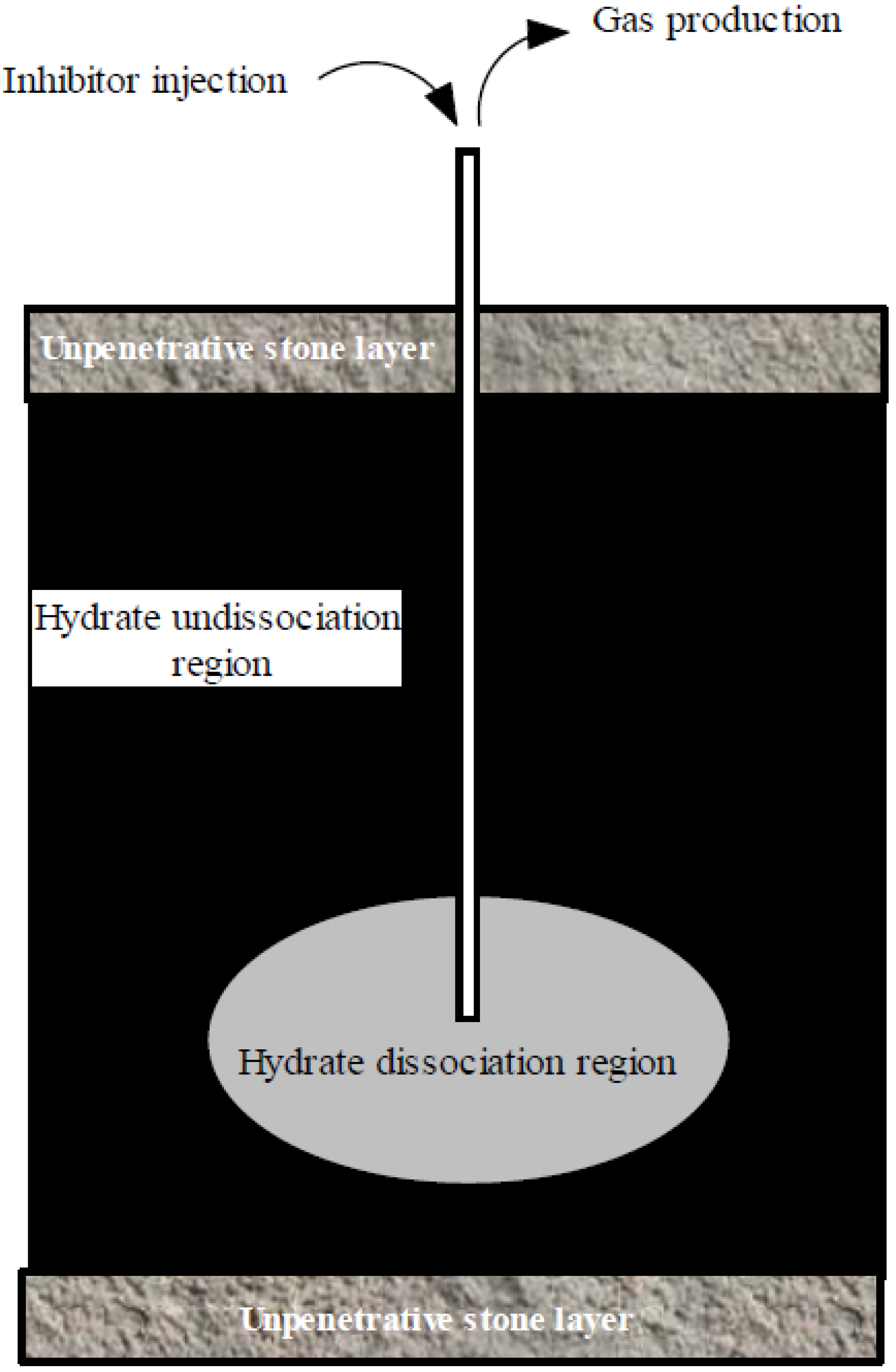
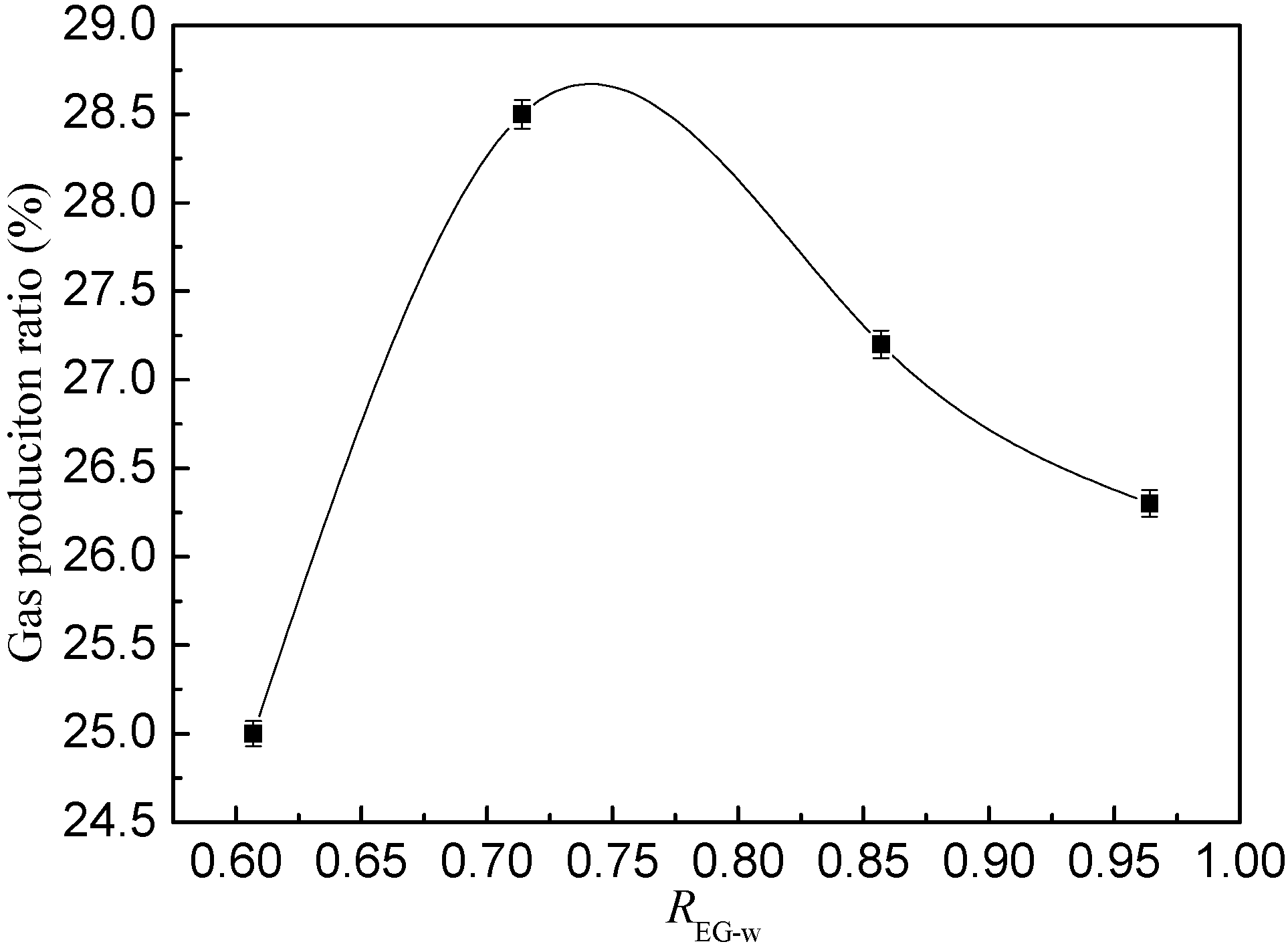
4.4. Other Exploitation Methods
4.5. The Current Status of the Industrial Exploitation of Natural Gas Hydrates
| Thickness of hydrate reservoir | 84 m |
|---|---|
| porosity | 16–38% (average value 25%) |
| residual water saturation | 29–50% (average value 40%) |
| initial pressure of hydrate reservoir | 7.8 MPa |
| temperature range of hydrate reservoir | 281–285 K |
| water salinity of hydrate reservoir | <1.5 wt% |
| composition of free gas | 98.6% CH4, 0.1% C2H6, 0.1% C3H8, 0.5% CO2, 0.7% N2 |
| Well No. | Type of inhibitor | Volume of inhibitor, m3 | Gas flow before treatment, 1000 m3/day | Gas flow after treatment, 1000 m3/day |
|---|---|---|---|---|
| 2 | 96 wt% methanol | 3.5 | did not reach the expected results | did not reach the expected results |
| 129 | 96 wt% methanol | 3.5 | 30 | 150 |
| 131 | 96 wt% methanol | 3.0 | 175 | 275 |
| 133 | methanol | — | 25 | 50 |
| 50 | 50 | |||
| 100 | 150 | |||
| 150 | 200 | |||
| 138 | 10% methanol + 90% CaCl2 (30 wt%) | 4.8 | 200 | 300 |
| 139 | same as well 138 | 2.8 | — | — |
| 141 | same as well 138 | 4.8 | 150 | 200 |
| 142 | methanol | — | 5 | 50 |
| 10 | 100 | |||
| 25 | 150 | |||
| 50 | 200 |
| Area | Depth of Stratum, m | Average porosity, % | Gas hydrate saturation | Permeability (excluding hydrate), mD | Permeability (including hydrate), mD |
|---|---|---|---|---|---|
| A | 892–930 | 32–38 | ~0.8 | 100–1000 | 0.1 |
| B | 942–993 | 30–40 | 0.4–0.8 | 1 | 0.01–0.1 |
| C | 1070–1107 | 30–40 | 0.8–0.9 | 1 | 0.01–0.1 |
5. Conclusions and Prospects
Acknowledgments
References
- Demirbas, A. Methane hydrates as potential energy resource: Part 1—Importance, resource and recovery facilities. Energy Convers. Manag. 2010, 51, 1547–1561. [Google Scholar] [CrossRef]
- Davy, H. The bakerian lecture: On some of the combinations of oxymuriatic gas and oxygene, and on the chemical relations of these principles to inflammable bodies. Phil. Trans. R. Soc. Lond. 1811, 101, 1–35. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrate of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kvenvolden, K.A. Methane hydrate—A major reservoir of carbon in the shallow geosphere. Chem. Geol. 1998, 71, 41–51. [Google Scholar] [CrossRef]
- Zhang, G.X.; Huang, Y.X.; Chen, B.Y. Seismology of Marine Natural Gas Hydrate; Ocean Press: Beijing, China, 2003. (in Chinese) [Google Scholar]
- Bonnefoy, O.; Herri, J.M. Formation & dissociation of methane hydrates in sediments Part I: A new experimental set-up for measurements. In Proceedings of the 4th International Conference on Gas Hydrates, Yokohama, Japan, 19–23 May 2002; pp. 797–801.
- Fatykhov, M.A.; Bagautdinov, N.Y. Experimental investigations of decomposition of gas hydrate in a pipe under the impact of a microwave electromagnetic field. High Temp. 2005, 43, 614–619. [Google Scholar] [CrossRef]
- Li, D.L.; Liang, D.Q.; Fan, S.S.; Li, X.S.; Tang, L.G.; Huang, N.S. In situ hydrate dissociation using microwave heating: Preliminary study. Energy Convers. Manag. 2008, 49, 2207–2213. [Google Scholar] [CrossRef]
- Dou, B.; Jiang, G.S.; Wu, X.; Zhang, L.; Ning, F.L. Exploitation of natural gas hydrate by ground decomposition method. Nat. Gas. Ind. 2008, 28, 123–125. (in Chinese). [Google Scholar]
- Milkov, A.V. Global estimates of hydrate-bound gas in marine sediments: How much is really out there? Earth Sci. Rev. 2004, 66, 183–197. [Google Scholar] [CrossRef]
- Yao, B.C. The gas hydrate in the south China sea. J. Trop. Oceanogr. 2001, 20, 20–28. (in Chinese). [Google Scholar]
- Xu, S.S.; Wang, T.; Liu, T.J.; Wang, D.; Cao, D.Y. Resource quantity estimation of gas hydrate in Muli coalfield, Qinghai Province. Coal. Geol. China 2009, 21, 1–3. (in Chinese). [Google Scholar]
- Makogon, Y.F. Natural gas hydrates—A promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—A potential energy source for the 21st Century. J. Petrol. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Sloan, E.D. Clathrate Hydrates of Nature Gas; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Nikitin, S.P. Introduction of natural gas hydrate distribution zone in USSR. Oil Gas Geol. 1987, 2, 7–10. (in Russian). [Google Scholar]
- Chersky, N.V.; Tsarev, V.P.; Nikitin, S.P. Natural gas hydrate—The prospect natural gas resource in north east USSR. Magadan 1984, 1, 105–108. (in Russian). [Google Scholar]
- Shi, D.; Zheng, J.W. The status and prospects of research and exploitation of natural gas hydrate in the world. Adv. Earth Sci. 1999, 14, 330–339. (in Chinese). [Google Scholar]
- Collett, T.S. Natural-gas hydrates of the Prudhoe Bay and Kuparuk River area, North Slope, Alaska. AAPG Bull. 1993, 77, 793–812. [Google Scholar]
- Dallimore, S.R.; Collett, T.S. Intrapermafrost gas hydrates from a deep core hole in the Mackenzie Delta, Northwest Teritories, Canada. Geology 1995, 23, 527–530. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhang, Y.Q.; Wen, H.J.; Lu, Z.Q.; Wang, P.K. Gas hydrate in the Qilian Mountain permafrost and their basic characteristics. Acta Geol. Sin. 2010, 31, 7–16. (in Chinese). [Google Scholar]
- Ginsburg, G.D.; Soloviev, V.A.; Telepnev, E.V. Evaluation of subsea natural gas hydrate in Arctic zone. Abstr. All Union Conf. 1986, Part 1, 17–18. (in Russian). [Google Scholar]
- Andreessen, K.; HART, P.E.; GRANTZ, A. Seismic studies of a BSR related to gas hydrate beneath the continental margin of the Beaufort Sea. J. Geophys. Res. 1995, 100, 12659–12673. [Google Scholar] [CrossRef]
- Borowiski, W.S. A review of methane and gas hydrates in the dynamic stratified system of the Blake Ridge region, offshore southeastern North America. Chem. Geol. 2004, 205, 311–346. [Google Scholar] [CrossRef]
- Sassen, R.; Sweet, S.T.; Milkov, A.V.; DeFreitas, D.A.; Salata, G.G.; McDade, E.C. Geology and geochemistry of gas hydrates, central Gulf of Mexico continental slope. Trans. Gulf Coast Assoc. Geol. 1999, 49, 462–468. [Google Scholar]
- Panaev, V.A. Natural gas hydrate in the ocean [Russia]. Dep. Geol. Mosc. 1987, 62, 66–72. [Google Scholar]
- Ginsburg, G.D.; Gramberg, I.S.; Ivanov, V.L.; Soloviev, V.A. Achievement and mission for research on subsea natural gas hydrate. Geology of the oceans and seas. Abstr. Rep. Ocean Geol. Coll. USSR 1986, 3, 183–184. (in Russian). [Google Scholar]
- Kvenvolden, K.A.; Mc. Donald, T.J. Gas hydrates of the middle American trench—DSDP/IPOD, Leg 84. Initial Rep. DSDP 1985, 84, 517–520. [Google Scholar]
- Satoh, M. Distribution and resources of marine natural gas hydrates around Japan. Nat. Gas. Geosci. 2003, 14, 512–513. [Google Scholar]
- Antje, B.; Erwin, S. Hydrate Ridge: A natural laboratory for the study of microbial life fueled by methane from near-surface gas hydrates. Chem. Geol. 2004, 205, 291–310. [Google Scholar] [CrossRef]
- Ludmann, T.; Wong, H.K. Characteristics of gas hydrate occurrences associated with mud diapirism and gas escape structures in the northwestern Sea of Okhotsk. Mar. Geol. 2003, 201, 269–289. [Google Scholar] [CrossRef]
- Li, G.; Moridis, G.J.; Zhang, K.; Li, X.S. Evaluation of gas production potential from marine gas hydrate deposits in Shenhu Area of South China Sea. Energy Fuels 2010, 24, 6018–6033. [Google Scholar] [CrossRef]
- Chertkov, L.; Bilichenko, A.A.; Stunzhas, P.A. The detection of methane hydrate in Okhotsk Sea. In Proceedings of the 3rd Conference of Ocean Scientist in USSR, Part in Ocean Geology, Geophysics, Geochemistry, 14–19 December 1987; Lenin Spengler: Bayreuth, Germany; pp. 172–173. (in Russian).
- Zonenshain, L.P.; Murdmaa, I.O.; Baranov, B.V.; Kuznetsov, A.P.; Kuzin, B.S.; Kuzmin, M.I.; Avdeiko, G.P.; Stunzhas, P.A.; Lukashin, V.N.; Barash, M.S.; et al. The gas source in Okhotsk Sea of west Paramushir. Oceanology 1987, 27, 795–800. (in Russian). [Google Scholar]
- Hammond, R.D.; Gaither, J.R. Anomalous seismic character-bearing sea shelf. Geophysics 1983, 48, 590–605. [Google Scholar] [CrossRef]
- Carlson, P.R.; Golan, B.M.; Karl, H. Seismic and geochemical evidence for shallow gas in sediment on Navarin continental margin, Bering Sea. AAPG Bull. 1985, 69, 422–436. [Google Scholar]
- White, R.S. Gas hydrate layers trapping free gas in the Gulf of Oman. Earth. Planet. Sci. Lett. 1979, 42, 114–120. [Google Scholar] [CrossRef]
- Michael, D.M. Gas hydrate potential of the Indian Sector of the NE Arabian Sea and Northern Indian Ocean. In Natural Gas Hydrate in Oceanic and Permafrost Environments; Springer: Berlin, Germany, 2000; pp. 213–224. [Google Scholar]
- Jeffery, B.K.; Stanley, I.S. Global distribution of methane hydrate in ocean sediment. Energy Fuels 2005, 19, 459–470. [Google Scholar] [CrossRef]
- Shi, D. Evaluating the resources of hydrate gas in the Black Sea. Nat. Gas Geosci. 2003, 14, 519–524. [Google Scholar]
- Moridis, G.J.; Kowalsky, M.B.; Pruess, K. Depressurization-induced gas production from Class 1 hydrate deposits. SPE Reserv. Eval. Eng. 2007, 10, 458–481. [Google Scholar] [CrossRef]
- Makogon, Y.F. Hydrates of Natural Gas; PennWell: Tulsa, OK, USA, 1974. [Google Scholar]
- Krason, J.; Ciesnik, M. Geological Evolution and Analysis of Confirmed or Suspected Gas Hydrate Localities: Gas Hydrates in the Russian Literature; U.S. Department of Energy: Washington, DC, USA, 1985; Volume 5, DOE/MC/21181-1950. [Google Scholar]
- Ahmadi, G.; Ji, C.A.; Smith, D.H. Production of natural gas from methane hydrate by a constant downhole pressure well. Energy Convers. Manag. 2007, 48, 2053–2068. [Google Scholar] [CrossRef]
- Yousif, M.H.; Li, P.M.; Selim, M.S.; Sloan, E.D. Depressurization of natural-gas hydrates in Berea sandstone cores. J. Incl. Phenom. Mol. Recognit. Chem. 1990, 8, 71–88. [Google Scholar] [CrossRef]
- Yousif, M.H.; Abass, H.H.; Selim, M.S.; Sloan, E.D. Experimental and theoretical investigation of methane-gas-hydrate dissociation in porous media. SPE Reserv. Eng. 1991, 6, 69–76. [Google Scholar] [CrossRef]
- Kono, H.O.; Narasimhan, S.; Song, F.; Smith, D.H. Synthesis of methane gas hydrate in porous sediments and its dissociation by depressurizing. Powder Technol. 2002, 122, 239–246. [Google Scholar] [CrossRef]
- Kneafsey, T.J.; Tomutsa, L.; Moridis, G.J.; Seol, Y.; Freifeld, B.M.; Taylor, C.E.; Gupta, A. Methane hydrate formation and dissociation in a partially saturated core-scale sand sample. J. Petrol. Sci. Eng. 2007, 56, 108–126. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Sung, W. An experimental study on the productivity of dissociated gas from gas hydrate by depressurization scheme. Energy Convers. Manag. 2010, 51, 2510–2515. [Google Scholar] [CrossRef]
- Davidson, D.W.; Garg, S.K.; Guogh, S.R.; Handa, Y.P.; Ratcliffe, C.I.; Tse, J.S.; Ripmeester, J.A. Some structural and thermodynamic studies of clathrate hydrates. J. Incl. Phenom. 1984, 2, 231–238. [Google Scholar] [CrossRef]
- John, V.T.; Holder, C.D. Hydrate of methane + n-butane below the ice point. J. Chem. Eng. Data 1982, 27, 18–27. [Google Scholar] [CrossRef]
- Dharma-wardana, M.W.C. Thermal conductivity of the ice polymorphs and the ice clathrates. J. Phys. Chem. 1983, 87, 4185–4190. [Google Scholar] [CrossRef]
- Tang, L.G.; Li, X.S.; Huang, C.; Feng, Z.P.; Li, G.; Fan, S.S. Control mechanisms for gas hydrate production by depressurization in different scale hydrate reservoirs. Energy Fuels 2007, 21, 227–233. [Google Scholar] [CrossRef]
- Li, X.S.; Zhang, Y. Study on dissociation behaviors of methane hydrate in porous media based on experiments and fractional dimension shrinking-core model. Ind. Eng. Chem. Res. 2011, 50, 8263–8271. [Google Scholar] [CrossRef]
- Haligva, C.; Linga, P.; Ripmeester, J.A.; Englezos, P. Recovery of methane from a variable-volume bed of silica sand/hydrate by depressurization. Energy Fuels 2010, 24, 2947–2955. [Google Scholar] [CrossRef]
- Phelps, T.J.; Peters, D.J.; Marshall, S.L.; West, O.R.; Liang, L.; Blencoe, J.G.; Alexiades, V.; Jacobs, G.K.; Naney, M.T.; Heck, J.L., Jr. A new experimental facility for investigating the formation and properties of gas hydrates under simulated seafloor conditions. Rev. Sci. Instrum. 2001, 72, 1514–1521. [Google Scholar] [CrossRef]
- Zhou, Y.; Castaldi, M.J.; Yegulalp, T.M. Experimental investigation of methane gas production from methane hydrate. Ind. Eng. Chem. Res. 2009, 48, 3142–3149. [Google Scholar] [CrossRef]
- Li, X.S.; Zhang, Y.; Li, G.; Chen, Z.Y.; Wu, H.J. Experimental investigation into the production behavior of methane hydrate in porous sediment by depressurization with a novel three-dimensional cubic hydrate simulator. Energy Fuels 2011, 25, 4497–4505. [Google Scholar] [CrossRef]
- Sun, C.Y.; Chen, G.J. Methane hydrate dissociation above 0 °C and below 0 °C. Fluid Phase Equilib. 2006, 242, 123–128. [Google Scholar] [CrossRef]
- Lin, W.; Chen, G.J.; Sun, C.Y.; Guo, X.Q.; Wu, Z.K.; Liang, M.Y.; Chen, L.T.; Yang, L.Y. Effect of surfactant on the formation and dissociation kinetic behavior of methane hydrate. Chem. Eng. Sci. 2004, 59, 4449–4455. [Google Scholar] [CrossRef]
- Su, K.H.; Sun, C.Y.; Yang, X.; Chen, G.J.; Fan, S.S. Experimental investigation of methane hydrate decomposition by depressurizing in porous media with 3-Dimension device. J. Nat. Gas Chem. 2010, 19, 210–216. [Google Scholar] [CrossRef]
- Yang, X.; Sun, C.Y.; Su, K.H.; Yuan, Q.; Li, Q.P.; Chen, G.J. A three-dimensional study on the formation and dissociation of methane hydrate in porous sediment by depressurization. Energy Convers. Manag. 2012, 56, 1–7. [Google Scholar] [CrossRef]
- Li, X.S.; Wan, L.H.; Li, G.; Li, Q.P.; Chen, Z.Y.; Yan, K.F. Experimental investigation into the production behavior of methane hydrate in porous sediment with hot brine stimulation. Ind. Eng. Chem. Res. 2008, 47, 9696–9702. [Google Scholar] [CrossRef]
- Makogon, T.Y.; Larsen, R.; Knight, C.A.; Sloan, E.D. Melt growth of tetrahydrofuran clathrate hydrate and its inhibition: Method and first results. J. Cryst. Growth 1997, 179, 258–262. [Google Scholar] [CrossRef]
- Holder, G.D.; Angert, P.F.; Godbole, S.P. Simulation of gas production from a reservoir containing both gas hydrates and free natural gas. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 26–29 September 1982. [CrossRef]
- McGuire, P.L. Methane hydrate gas production by thermal stimulation. In Proceedings of the 4th National Research Council of Canada Permafrost Conference, Calgary, Canada, 2–6 March 1981; pp. 356–362.
- Kamath, V.A.; Holder, G.D. Dissociation heat transfer characteristics of methane hydrates. AIChE J. 1987, 33, 347–350. [Google Scholar] [CrossRef]
- Ullerich, J.W.; Selim, M.S.; Sloan, E.D. Theory and measurement of hydrate dissociation. AIChE J. 1987, 33, 747–752. [Google Scholar] [CrossRef]
- Pang, W.X.; Xu, W.Y.; Sun, C.Y.; Zhang, C.L.; Chen, G.J. Methane hydrate dissociation experiment in a middle-sized quiescent reactor using thermal method. Fuel 2009, 88, 497–503. [Google Scholar] [CrossRef]
- Selim, M.S.; Sloan, E.D. Hydrate decomposition in sediment. SPE Reserv. Eng. 1990, 5, 245–251. [Google Scholar] [CrossRef]
- Kamata, Y.; Ebinuma, T.; Omura, R.; Minagawa, H.; Narita, H.; Masuda, Y.; Konno, Y. Decomposition experiment of methane hydrate sediment by thermal recovery method. In Proceedings of the 5th International Conference on Gas Hydrates, Trondheim, Norway, 13–16 June 2005; pp. 81–85.
- Tang, L.G.; Xiao, R.; Huang, C.; Feng, Z.P.; Fan, S.S. Experimental investigation of production behavior of gas hydrate under thermal stimulation in unconsolidated sediment. Energy Fuels 2005, 19, 2402–2407. [Google Scholar] [CrossRef]
- Kwon, T.H.; Kim, H.S.; Cho, G.C. Dissociation behavior of CO2 hydrate in sediments during isochoric heating. Environ. Sci. Technol. 2008, 42, 8571–8577. [Google Scholar] [CrossRef] [PubMed]
- Linga, P.; Haligva, C.; Nam, S.C.; Ripmeester, J.A.; Englezos, P. Recovery of methane from hydrate formed in a variable volume bed of silica sand particles. Energy Fuels 2009, 23, 5508–5516. [Google Scholar] [CrossRef]
- Yang, X.; Sun, C.Y.; Yuan, Q.; Ma, P.C.; Chen, G.J. Experimental study on gas production from methane hydrate-bearing sand by hot-water cyclic injection. Energy Fuels 2010, 24, 5912–5920. [Google Scholar] [CrossRef]
- Li, X.S.; Wang, Y.; Li, G.; Zhang, Y.; Chen, Z.Y. Experimental investigation into methane hydrate decomposition during three-dimensional thermal huff and puff. Energy Fuels 2011, 25, 1650–1658. [Google Scholar] [CrossRef]
- Li, G.; Li, X.S.; Wang, Y.; Zhang, Y. Production behavior of methane hydrate in porous media using huff and puff method in a novel three-dimensional simulator. Energy 2011, 25, 3170–3178. [Google Scholar] [CrossRef]
- Li, X.S.; Yang, B.; Li, G.; Li, B.; Zhang, Y.; Chen, Z.Y. Experimental study on gas production from methane hydrate in porous media by huff and puff method in pilot-scale hydrate simulator. Fuel 2011. [Google Scholar] [CrossRef]
- Li, G.; Li, X.S.; Tang, L.G.; Zhang, Y. Experimental investigation of production behavior of methane hydrate under ethylene glycol injection in unconsolidated sediment. Energy Fuels 2007, 21, 3388–3393. [Google Scholar] [CrossRef]
- Ross, M.J.; Toczylkin, L.S. Hydrate dissociation pressures for methane or ethane in the presence of aqueous solutions of triethylene glycol. J. Chem. Eng. Data 1992, 37, 488–491. [Google Scholar] [CrossRef]
- Servio, P.; Englezos, P. Incipient equilibrium propane hydrate formation conditions in aqueous triethylene glycol solutions. J. Chem. Eng. Data 1997, 42, 800–801. [Google Scholar] [CrossRef]
- Englezos, P.; Bishnoi, P.R. Prediction of gas hydrate formation conditions in aqueous electrolyte solutions. AIChE J. 1988, 34, 1718–1721. [Google Scholar] [CrossRef]
- Englezos, P. Computation of the incipient equilibrium carbon dioxide hydrate formation conditions in aqueous electrolyte solutions. Ind. Eng. Chem. Res. 1992, 31, 2232–2237. [Google Scholar] [CrossRef]
- Hutz, U.; Englezos, P. Measurement of structure H hydrate phase equilibrium and the effect of electrolytes. Fluid Phase Equilib. 1996, 117, 178–185. [Google Scholar] [CrossRef]
- Atik, Z.; Windmeier, C.; Oellrich, L.R. Experimental gas hydrate dissociation pressures for pure methane in aqueous solutions of MgCl2 and CaCl2 and for a (methane + ethane) gas mixture in an aqueous solution of (NaCl + MgCl2). J. Chem. Eng. Data 2006, 51, 1862–1867. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Estimating the hydrate safety margin in the presence of salt or organic inhibitor using refractive index data of aqueous solution. Ind. Eng. Chem. Res. 2006, 45, 8207–8212. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Estimating the hydrate safety margin using surface tension data of salt aqueous solution. Ind. Eng. Chem. Res. 2006, 45, 8154–8157. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Determination of gas hydrate safety margin using specific gravity data of salt or organic inhibitor aqueous solution. Ind. Eng. Chem. Res. 2007, 46, 3852–3857. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Use of boiling point elevation data of aqueous solutions for estimating hydrate stability zone. Ind. Eng. Chem. Res. 2007, 46, 987–989. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Methane hydrate phase equilibrium in the presence of salt (NaCl, KCl, or CaCl2) + ethylene glycol or salt (NaCl, KCl, or CaCl2) + methanol aqueous solution: Experimental determination of dissociation condition. J. Chem. Thermodyn. 2009, 41, 1374–1377. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Gas hydrate phase equilibrium in the presence of ethylene glycol or methanol aqueous solution. Ind. Eng. Chem. Res. 2010, 49, 8865–8869. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Richon, D. Phase equilibria of methane hydrates in the presence of methanol and/or ethylene glycol aqueous solutions. Ind. Eng. Chem. Res. 2010, 49, 925–928. [Google Scholar] [CrossRef]
- Afzal, W.; Mohammadi, A.H.; Richon, D. Experimental measurements and predictions of dissociation conditions for carbon dioxide and methane hydrates in the presence of triethylene glycol aqueous solutions. J. Chem. Eng. Data 2007, 52, 2053–2055. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Afzal, W.; Richon, D. Experimental data and predictions of dissociation conditions for ethane and propane simple hydrates in the presence of distilled water and methane, ethane, propane, and carbon dioxide simple hydrates in the presence of ethanol aqueous solutions. J. Chem. Eng. Data 2008, 53, 73–76. [Google Scholar] [CrossRef]
- Afzal, W.; Mohammadi, A.H.; Richon, D. Experimental measurements and predictions of dissociation conditions for methane, ethane, propane, and carbon dioxide simple hydrates in the presence of diethylene glycol aqueous solutions. J. Chem. Eng. Data 2008, 53, 663–666. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Afzal, W.; Richon, D. Experimental data and predictions of dissociation conditions for ethane and propane simple hydrates in the presence of methanol, ethylene glycol, and triethylene glycol aqueous solutions. J. Chem. Eng. Data 2008, 53, 683–686. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Afzal, W.; Richon, D. Gas hydrates of methane, ethane, propane, and carbon dioxide in the presence of single NaCl, KCl, and CaCl2 aqueous solutions: Experimental measurements and predictions of dissociation conditions. J. Chem. Thermodyn. 2008, 40, 1693–1697. [Google Scholar] [CrossRef]
- Mohammadi, A.H.; Laurens, S.; Richon, D. Experimental study of methane hydrate phase equilibrium in the presence of polyethylene glycol-400 aqueous solution. J. Chem. Eng. Data 2009, 54, 3118–3120. [Google Scholar] [CrossRef]
- Katz, D.L.; Cornell, D.; Poettmann, F.H.; Vary, J.A.; Elenbaas, J.R.; Weinaug, C.F. Handbook of Natural Gas Engineering; McGraw-Hill Book Co.,Inc.: New York, NY, USA, 1959; p. 802. [Google Scholar]
- Elgibaly, A.; Elkamel, A. Optimal hydrate inhibition policies with the aid of neural networks. Energy Fuels 1999, 13, 105–113. [Google Scholar] [CrossRef]
- Makogon, Y.F. Hydrates of Natural Gas; PennWell: Tulsa, OK, USA, 1981; pp. 131–132. [Google Scholar]
- Sira, J.H.; Patil, S.L.; Kamath, V.A. Study of hydrate dissociation by methanol and glycol injection. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 23–26 September 1990. [CrossRef]
- Kawamura, T.; Sakamoto, Y.; Ohtake, M.; Yamamoto, T.; Komai, T.; Haneda, H. Dissociation behavior of pellet-shaped methane hydrate in ethylene glycol and silicone oil. Part 1: Dissociation above ice point. Ind. Eng. Chem. Res. 2006, 45, 360–364. [Google Scholar] [CrossRef]
- Fan, S.S.; Zhang, Y.Z.; Tian, G.L.; Liang, D.Q.; Li, D.L. Natural gas hydrate dissociation by presence of ethylene glycol. Energy Fuels 2006, 20, 324–326. [Google Scholar] [CrossRef]
- Dong, F.H.; Zang, X.Y.; Li, D.L.; Fan, S.S.; Liang, D.Q. Experimental investigation on propane hydrate dissociation by high concentration methanol and ethylene glycol solution injection. Energy Fuels 2009, 23, 1563–1567. [Google Scholar] [CrossRef]
- Lee, J. Experimental study on the dissociation behavior and productivity of gas hydrate by brine injection scheme in porous rock. Energy Fuels 2010, 24, 456–463. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, C.Y.; Yang, X.; Ma, P.C.; Ma, Z.W.; Li, Q.P.; Chen, G.J. Gas production from methane-hydrate-bearing sands by ethylene glycol injection using a three-dimensional reactor. Energy Fuels 2011, 25, 3108–3115. [Google Scholar] [CrossRef]
- Hirohama, S.; Shimoyama, Y.; Wakabayashi, A.; Tatsuta, S.; Nishida, N. Conversion of CH4-hydrate to CO2-hydrate in liquid CO2. J. Chem. Eng. Jpn. 1996, 29, 1014–1020. [Google Scholar] [CrossRef]
- Jadhawar, P.; Mohammadi, A.H.; Yang, J.; Tohidi, B. Subsurface carbon dioxide storage through clathrate hydrate formation. In Advances in the Geological Storage of Carbon Dioxide; Lombardi, S., Altunina, L.K., Beaubien, S.E., Eds.; Springer: Dordrecht, the Netherlands, 2006; pp. 111–126. [Google Scholar]
- Castaldi, M.J.; Zhou, Y.; Yegulalp, T.M. Down-hole combustion method for gas production from methane hydrates. J. Pet. Sci. Eng. 2007, 56, 176–185. [Google Scholar] [CrossRef]
- Ning, F.L.; Jiang, G.S.; Tang, F.L.; Xiang, W.; Pan, X.Y. Utilizing geothermal energy to exploit marine gas hydrate. Nat. Gas Ind. 2006, 26, 136–138. (in Chinese). [Google Scholar]
- Islam, M.R. A new recovery technique for gas production from Alaskan gas hydrates. J. Pet. Sci. Eng. 1994, 11, 267–281. [Google Scholar] [CrossRef]
- Rachit, G.; Konark, O.; Arpit, C. New horizons in gas hydrate recovery using fluorine and microwave technology. In Proceedings of the Europec/EAGE Conference and Exhibition, Rome, Italy, 9–12 June 2008. [CrossRef]
- Li, X.S.; Wang, Yi.; Li, G.; Zhang, Y. Experimental investigations into gas production behaviors from methane hydrate with different methods in a cubic hydrate simulator. Energy Fuels 2011. [CrossRef]
- Makogon, Y.F. Natural Gas Hydrates: The state of study in the USSR and perspectives for its use. In Proceedings of the Third Chemical Congress of North America, Toronto, Canada, 5–10 June 1988.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liu, B.; Yuan, Q.; Su, K.-H.; Yang, X.; Wu, B.-C.; Sun, C.-Y.; Chen, G.-J. Experimental Simulation of the Exploitation of Natural Gas Hydrate. Energies 2012, 5, 466-493. https://doi.org/10.3390/en5020466
Liu B, Yuan Q, Su K-H, Yang X, Wu B-C, Sun C-Y, Chen G-J. Experimental Simulation of the Exploitation of Natural Gas Hydrate. Energies. 2012; 5(2):466-493. https://doi.org/10.3390/en5020466
Chicago/Turabian StyleLiu, Bei, Qing Yuan, Ke-Hua Su, Xin Yang, Ben-Cheng Wu, Chang-Yu Sun, and Guang-Jin Chen. 2012. "Experimental Simulation of the Exploitation of Natural Gas Hydrate" Energies 5, no. 2: 466-493. https://doi.org/10.3390/en5020466




