Polyethylene Glycol Drilling Fluid for Drilling in Marine Gas Hydrates-Bearing Sediments: An Experimental Study
Abstract
:1. Introduction
- (1)
- The relative density (ρ) of the drilling fluid must have an appropriate changing range. The drilling fluid can supply a definite pressure to counteract the stratums and prevent hydrates around the borehole from decomposing to keep the borehole wall stable. For a practical situation of hydrates sediment, results of previous experiments in our lab indicate the optimum relative density is in the range of 1.05–1.2 [18], according to the safe density window of drilling fluid.
- (2)
- The drilling fluid should be able to effectively inhibit shale hydration and gas-hydrate aggregations in the drilling pipe and blowout preventer.
- (3)
- The drilling fluid should have a low temperature that can effectively prevent the dissociation of hydrates around the borehole.
- (4)
- The drilling fluid should have good rheological properties and stability at low temperatures.
- (5)
- The drilling fluid should prevent calcium- and magnesium-ion pollution. Normally, the concentration of calcium and magnesium ions in the ocean is about 0.40 g/kg and 1.28 g/kg, respectively [19]. Though these percentages are very small, they can greatly influence the performance of the drilling fluid.
- (6)
- The drilling fluid must have sufficient lubrication and low filtration.
2. Experimental
2.1. Apparatus

2.2. Materials
2.3. Principles and Procedure
3. Results and Analysis
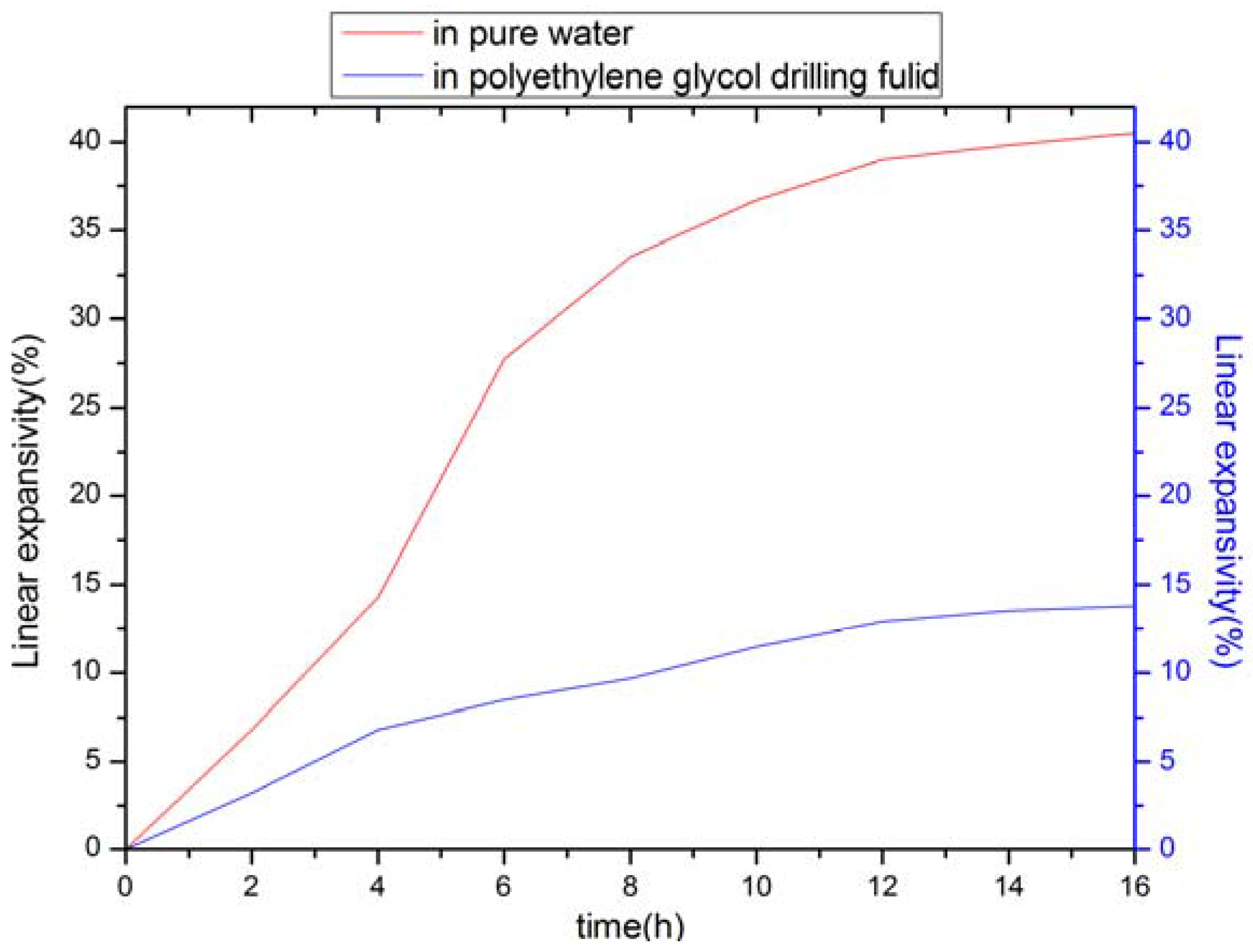
3.1. Shale Inhibition
| Test System | Linear Expansivity (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 4 h | 6 h | 8 h | 10 h | 12 h | 14 h | 16 h | |
| Pure water | 6.8 | 14.3 | 27.7 | 33.5 | 36.7 | 39.0 | 39.8 | 40.5 |
| The drilling fluid system | 3.2 | 6.8 | 8.5 | 9.7 | 11.5 | 12.9 | 13.5 | 13.8 |
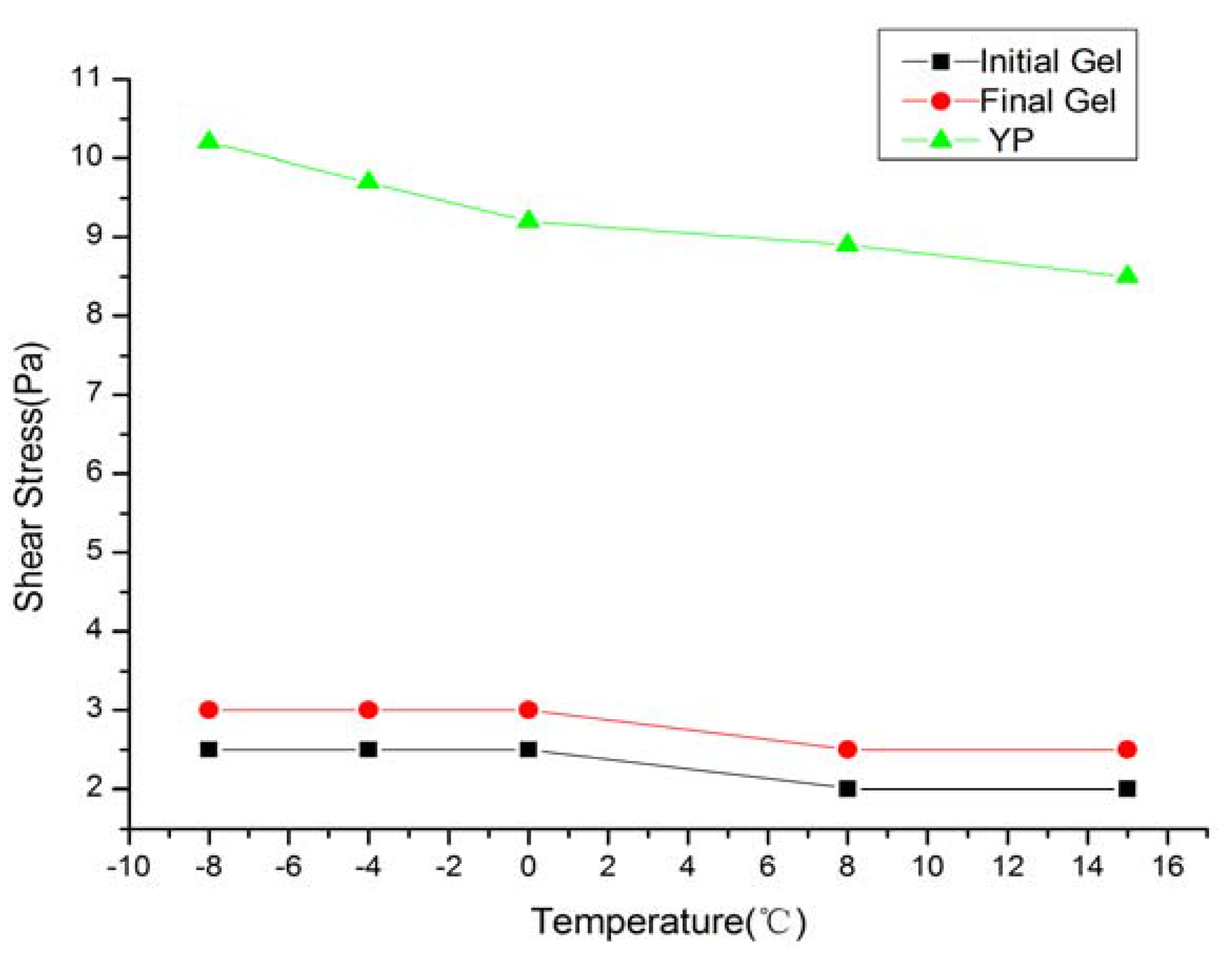
3.2. Properties at Low Temperature
| T (°C) | ρ (g/cm3) | Gel (Pa/Pa) | PV (mPa·s) | YP (Pa) | FL (mL) |
|---|---|---|---|---|---|
| 15 | 1.15 | 2/2.5 | 18 | 8.5 | 5.5 |
| 8 | 1.15 | 2/2.5 | 20 | 8.9 | 5.5 |
| 0 | 1.16 | 2.5/3 | 21 | 9.2 | 5.5 |
| −4 | 1.16 | 2.5/3 | 22 | 9.7 | 5.7 |
| −8 | 1.16 | 2.5/3 | 25 | 10.2 | 5.8 |
3.3. Prevention of the Pollution Caused by Calcium and Magnesium Ions
| Formula | PV (mPa.s) | YP (Pa) | Gel (Pa/Pa) | FL (mL) |
|---|---|---|---|---|
| The drilling fluid | 18.0 | 8.5 | 2/2.5 | 5.5 |
| The drilling fluid + 0.3% MgCl2 + 0.1% CaCl2 | 18.5 | 9.2 | 2.5/3 | 5.5 |
| The drilling fluid + 0.51% MgCl2 + 0.18% CaCl2 | 20.0 | 9.0 | 3/3.5 | 5.8 |
| The drilling fluid + 0.8% MgCl2 + 0.4% CaCl2 | 21.5 | 9.0 | 3/3.5 | 5.8 |
3.4. Hydrate Inhibition
| PVP Content | ρ (g/cm3) | Gel (Pa/Pa) | PV (mPa.s) | YP (Pa) | FL (mL) |
|---|---|---|---|---|---|
| 0.4% | 1.165 | 1.5/2.0 | 12.0 | 5.1 | 11.0 |
| 0.5% | 1.165 | 1.5/2.0 | 13.0 | 5.1 | 10.8 |
| 0.6% | 1.165 | 1.0/2.0 | 14.5 | 6.6 | 10.4 |
| 0.7% | 1.166 | 1.5/2.5 | 16.8 | 3.6 | 9.5 |
| 0.8% | 1.166 | 1.5/2.0 | 18.0 | 4.6 | 7.8 |
| 0.9% | 1.167 | 2.0/2.5 | 18.5 | 8.7 | 6.5 |
| 1.0% | 1.168 | 2.0/2.5 | 20.0 | 9.2 | 5.5 |
| 1.1% | 1.168 | 2.0/3.0 | 22.5 | 8.7 | 5.5 |
| 1.2% | 1.168 | 2.5/4.5 | 23.0 | 9.7 | 5.5 |
4. Conclusions and Suggestions
Acknowledgements
References
- Makogon, Y.F. Natural gas hydrates—a promising source of energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Kvenvolden, K.A. Gas hydrate—geological perspective and global change. Rev. Geophys. 1993, 31, 173–187. [Google Scholar] [CrossRef]
- Klauda, J.B.; Sandler, S.I. Global distribution of methane hydrate in ocean sediment. Energy Fuels 2005, 19, 459–470. [Google Scholar] [CrossRef]
- Makogon, Y.F.; Holditch, S.A.; Makogon, T.Y. Natural gas-hydrates—a potential energy source for the 21st Century. J. Pet. Sci. Eng. 2007, 56, 14–31. [Google Scholar] [CrossRef]
- Ning, F.; Wu, N.; Jiang, G.; Zhang, L.; Guan, J.; Yu, Y.; Tang, F. A Method to Use Solar Energy for the Production of Gas from Marine Hydrate-Bearing Sediments: A Case Study on the Shenhu Area. Energies 2010, 3, 1861–1879. [Google Scholar] [CrossRef]
- Moridis, G.J.; Sloan, E.D. Gas production potential of disperse low-saturation hydrate accumulations in oceanic sediments. Energy Convers. Manag. 2007, 48, 1834–1849. [Google Scholar] [CrossRef]
- Jiang, G.; Wang, D.; Tang, F.; Ye, J.; Zou, H.; Ni, X. Natural Gas Hydrates Exploration and Development, 1st ed.; China University of Geoscience Press: Wuhan, China, 2002. [Google Scholar]
- Tan, C.P.; Freij-Ayoub, R.; Clennell, M.B.; Tohidi, B.; Yang, J. Managing Wellbore instability Risk in Gas-Hydrate-Bearing Sediments. In Proceedings of Asia Pacific Oil & Gas Conference and Exhibition, Jakarta, Indonesia, April 2005.
- Freij-Ayoub, R.; Tan, C.P.; Clennell, M.B.; Tohidi, B.; Yang, J. A wellbore stability model for hydrate bearing sediments. J. Pet. Sci. Eng. 2007, 57, 209–220. [Google Scholar] [CrossRef]
- Ning, F.; Jiang, G.; Zhang, L.; Dou, B.; Wu, X. Analysis on Characteristics of Drilling Fluids Invading into Gas Hydrates-Bearing Formation. In Proceedings of the 6th International Conference on Gas Hydrates (ICGH 2008), Vancouver, Canada, July 2008.
- Collett, T.S. Energy resource potential of natural gas hydrates. AAPG Bullutin 2002, 86, 1971–1992. [Google Scholar]
- Maurer, W. Gas Hydrate Drilling Problems. In Proceedings of Gulf of Mexico Hydrates R&D Workshop, Houston, TX, USA, 2000; pp. 40–45.
- Barker, J.W.; Gomez, R.K. Formation of Hydrates during Deepwater Drilling Operations. J. Petrol. Tech. 1989, 41, 297–301. [Google Scholar] [CrossRef]
- Østergaard, K.K.; Tohidi, B.; Danesh, A.; Todd, A.C. Gas hydrates and offshore drilling: predicting the hydrate free zone. Ann. N. Y. Acad. Sci. 2000, 912, 411–419. [Google Scholar]
- Reyna, E.M.; Stewart, S.R. Case history of the removal of a hydrate plug formed during deep water well testing. In Proceedings of the SPE/IADC Drilling Conference, Amsterdam, The Netherlands, 2001.
- Birchwood, R.A.; Noeth, S.; Tjengdrawira, M.A.; Kisra, S.M.; Elisabeth, F.L.; Sayers, C.M.; Singh, R.; Hooyman, P.J.; Plumb, R.A.; Jones, E.; Bloys, J.B. Modeling the Mechanical and Phase Change Stability of Wellbores Drilled in Gas Hydrates; Technical Report for Joint Industry Participation Program (JIPP) Gas Hydrates Project, Phase II: Anaheim, CA, USA, November 2007. [Google Scholar]
- Ning, F.; Wu, X.; Zhang, L.; Cai, J.; Tu, Y.; Jiang, G.S. Experimental study on performance of water based drilling fluid used to drill formations with gas hydrate (in Chinese). Nat. Gas Ind. 2006, 26, 52–55. [Google Scholar]
- Cohen, J.H.; Williams, T.E.; Kadaster, A.G.; Liddell, B.V. Hydrate Core Drilling Tests. Available online: http://www.netl.doe.gov/technologies/oil-gas/publications/Hydrates/pdf/hyd-core-drill-maurer.pdf (accessed on January 7, 2011).
- Caenn, R.; Chillingar, G.V. Drilling fluids: state of the art. J. Pet. Sci. Eng. 1996, 14, 221. [Google Scholar] [CrossRef]
- Hu, Y.L.; Wang, J.H.; Zhang, Y.; Wu, B.; Xiang, X.J. Achievements of research on drilling fluid of deep water drilling (in Chinese). Offshore Oil 2004, 24, 83. [Google Scholar]
- Kelland, M.A.; Mønig, K.; Iversen, J.E.; Lekvam, K. Feasibility study for the use of kinetic hydrate inhibitors in deep-water drilling fluids. Energy Fuels 2008, 22, 2405–2410. [Google Scholar]
- Villano, L.D.; Kommedal, R.; Fijten, M.W.M.; Schubert, U.S.; Hoogenboom, R.; Kelland, M.A. A study of the kinetic hydrate inhibitor performance and seawater biodegradability of a series of poly(2-alkyl-2-oxazoline)s. Energy Fuels 2009, 23, 3665–3673. [Google Scholar] [CrossRef]
- Kelland, M.A.; Iversen, J.E. Kinetic hydrate inhibition at pressures up to 760 bar in deep water drilling fluids. Energy Fuels 2010, 24, 3003–3013. [Google Scholar] [CrossRef]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Fan, S.S.; Zhang, Y.Z.; Tian, G.L.; Liang, D.Q.; Li, D.L. Natural gas hydrate dissociation by presence of ethylene glycol. Energy Fuels 2006, 20, 324–326. [Google Scholar] [CrossRef]
- Ning, F.; Zhang, L.; Tu, Y.; Jiang, G.; Shi, M. Gas-hydrate formation, agglomeration and inhibition in oil-based drilling fluids for deep-water drilling. J. Nat. Gas Chem. 2010, 19, 234–240. [Google Scholar] [CrossRef]
- Bai, X.D.; Huang, J.; Hou, Q.L. The Analysis of Formation Causes and Prevention Measures of Natural Gas Hydrate in Deep Water Drilling Fluid (in Chinese). Fine Petrochem. Prog. 2004, 5, 52–54. [Google Scholar]
- Qiu, C.J.; Chen, L.Y.; Zhu, Z.P. The drilling fluid used in gas hydrates drilling (in Chinese). Exploit. Eng. 2002, 36, 36–37. [Google Scholar]
- Jiang, G.; Ning, F.; Zhang, L. Investigation of Drilling Fluids Techniques for Marine Gas Hydrate Drilling; Technical Report for Project 863 (2006AA09Z316): Wuhan, China, 30 September 2006. [Google Scholar]
- Liu, T.; Jiang, G.; Ning, F.; Zhang, L.; Tu, Y. Inhibition of polyethylene glycol drilling fluid with kinetic inhibitor for marine gas hydrates formation (in Chinese). Geol. Sci. Tech. Inf. 2010, 29, 116–120. [Google Scholar]
- Si, X.; Lu, Z. Lab evaluation and field application of polyglycol-based anti-sloughing drilling fluid system (in Chinese). Pet. Drill. Tech. 2001, 29, 45–46. [Google Scholar]
- Davie, M.K.; Zatsepina, O.Y.; Buffett, B.A. Methane solubility in marine hydrate environments. Mar. Geol. 2004, 203, 177–184. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, G.; Liu, T.; Ning, F.; Tu, Y.; Zhang, L.; Yu, Y.; Kuang, L. Polyethylene Glycol Drilling Fluid for Drilling in Marine Gas Hydrates-Bearing Sediments: An Experimental Study. Energies 2011, 4, 140-150. https://doi.org/10.3390/en4010140
Jiang G, Liu T, Ning F, Tu Y, Zhang L, Yu Y, Kuang L. Polyethylene Glycol Drilling Fluid for Drilling in Marine Gas Hydrates-Bearing Sediments: An Experimental Study. Energies. 2011; 4(1):140-150. https://doi.org/10.3390/en4010140
Chicago/Turabian StyleJiang, Guosheng, Tianle Liu, Fulong Ning, Yunzhong Tu, Ling Zhang, Yibing Yu, and Lixin Kuang. 2011. "Polyethylene Glycol Drilling Fluid for Drilling in Marine Gas Hydrates-Bearing Sediments: An Experimental Study" Energies 4, no. 1: 140-150. https://doi.org/10.3390/en4010140




