Assessment of the Effects of Flow Rate and Ionic Strength on the Performance of an Air-Cathode Microbial Fuel Cell Using Electrochemical Impedance Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
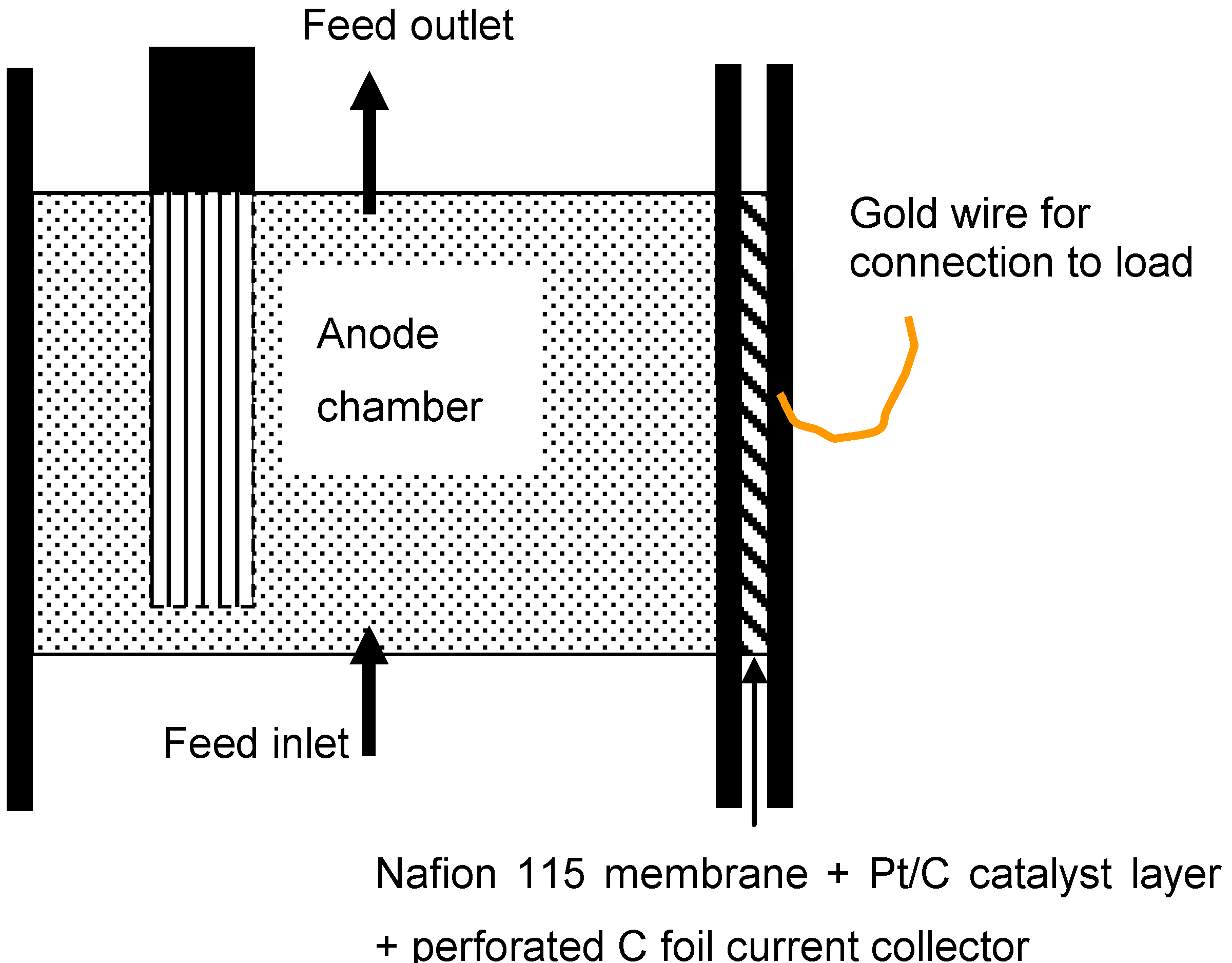
2.1. MFC design and operation
2.2. Electrochemical impedance spectroscopy
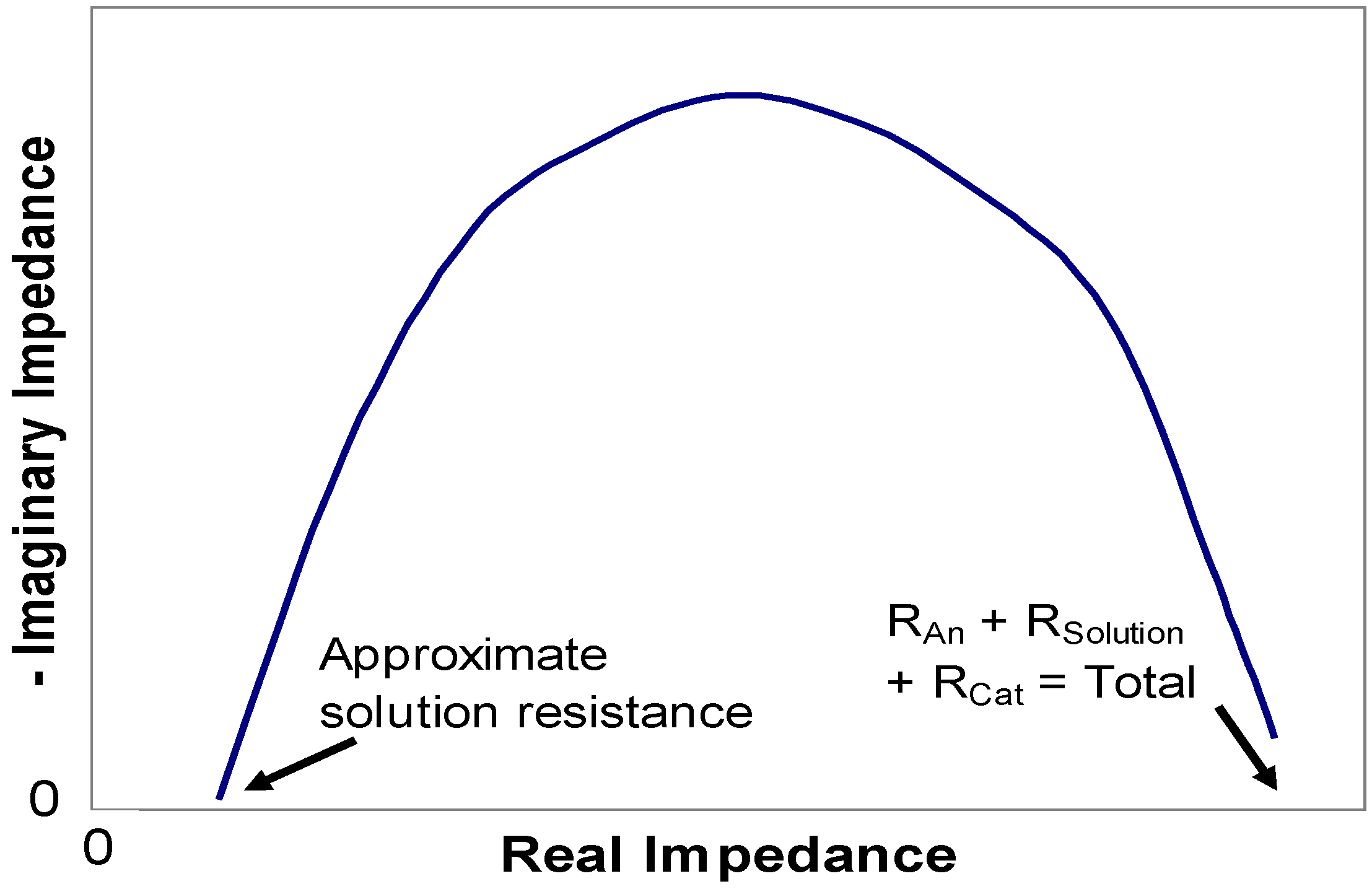
2.3. Equivalent circuit model

| Anode | Resistor | RAn |
| Constant phase element-coefficient | Yo_An | |
| Constant phase element-exponent | A_An | |
| Solution | Resistor | RSolution |
| Cathode | Resistor | RCat |
| Constant phase element-coefficient | Yo_Cat | |
| Constant phase element-exponent | ACat |
2.4. MFC variables
3. Results and discussion
3.1. Model fitting and justification
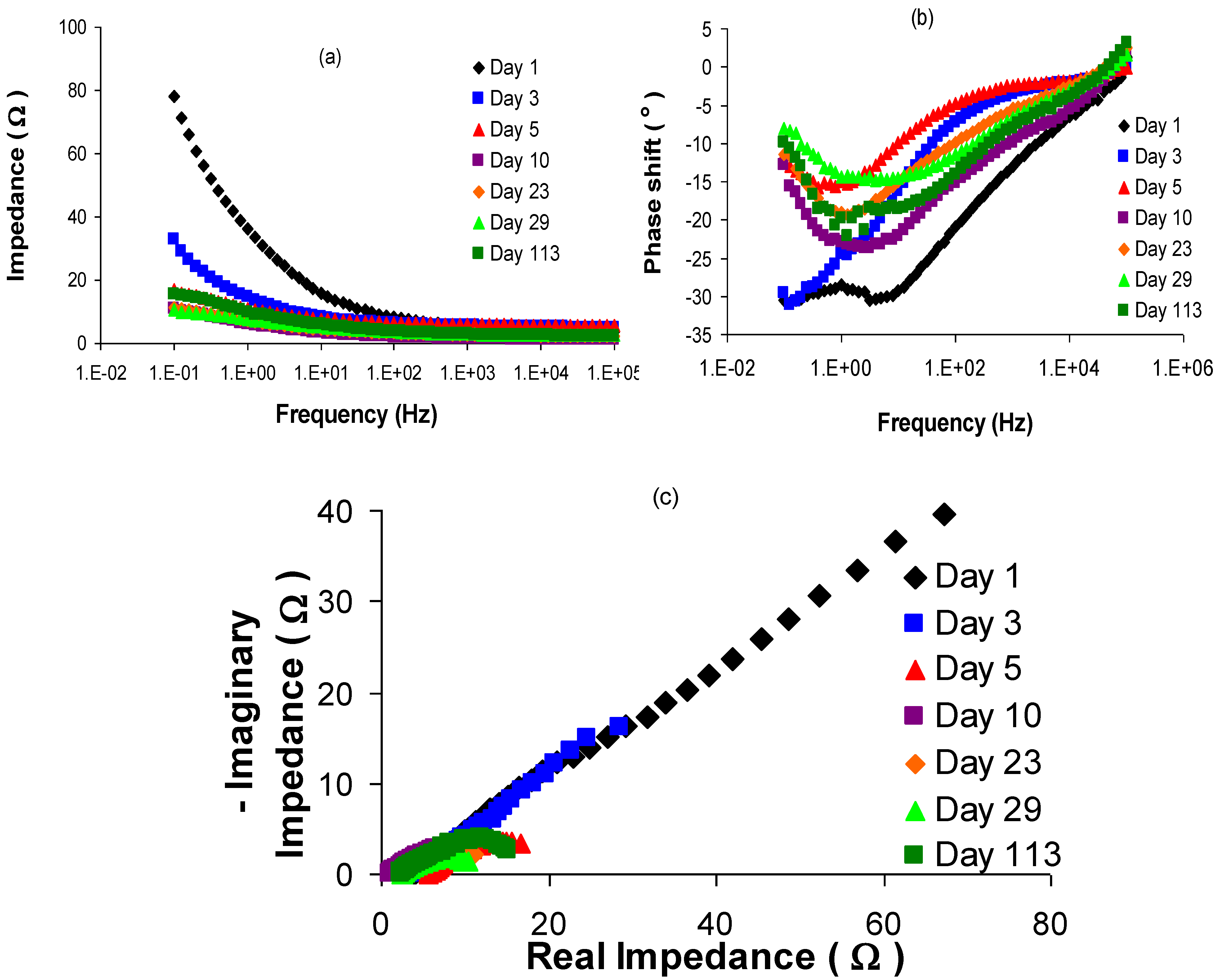

| 5 | 10 | 23 | 29 | 113 | |
| Yo_An | 0.181 | 0.122 | 0.053 | 0.225 | 0.116 |
| A_An | 0.124 | 0.308 | 0.397 | 0.667 | 0.975 |
| Yo_Cat | 0.056 | 0.068 | 0.055 | 0.058 | 0.038 |
| A_Cat | 0.557 | 0.550 | 0.683 | 0.532 | 0.407 |
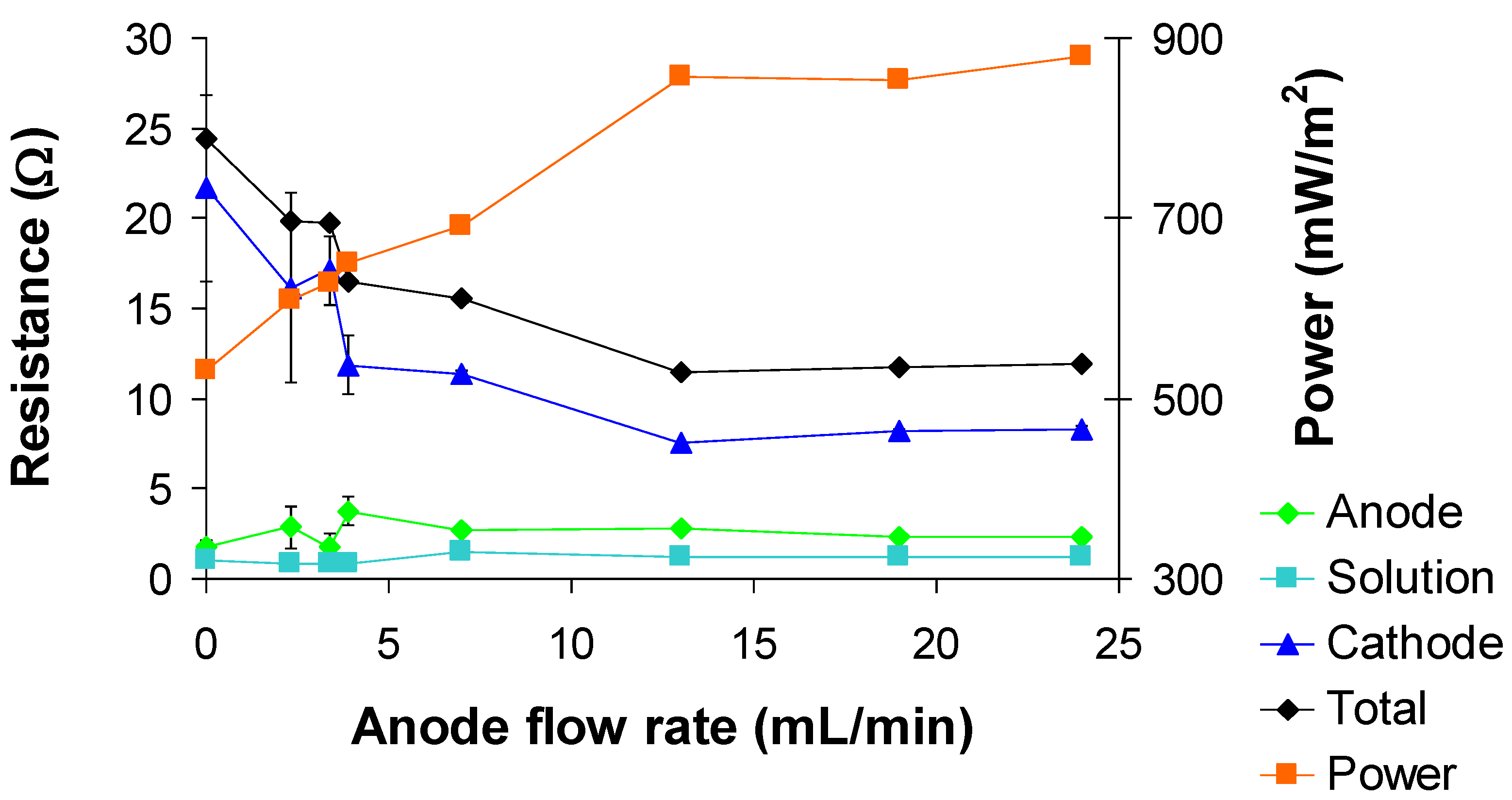
3.2. Effect of anode circulation flow rate
3.3. Effect of anode fluid ionic strength
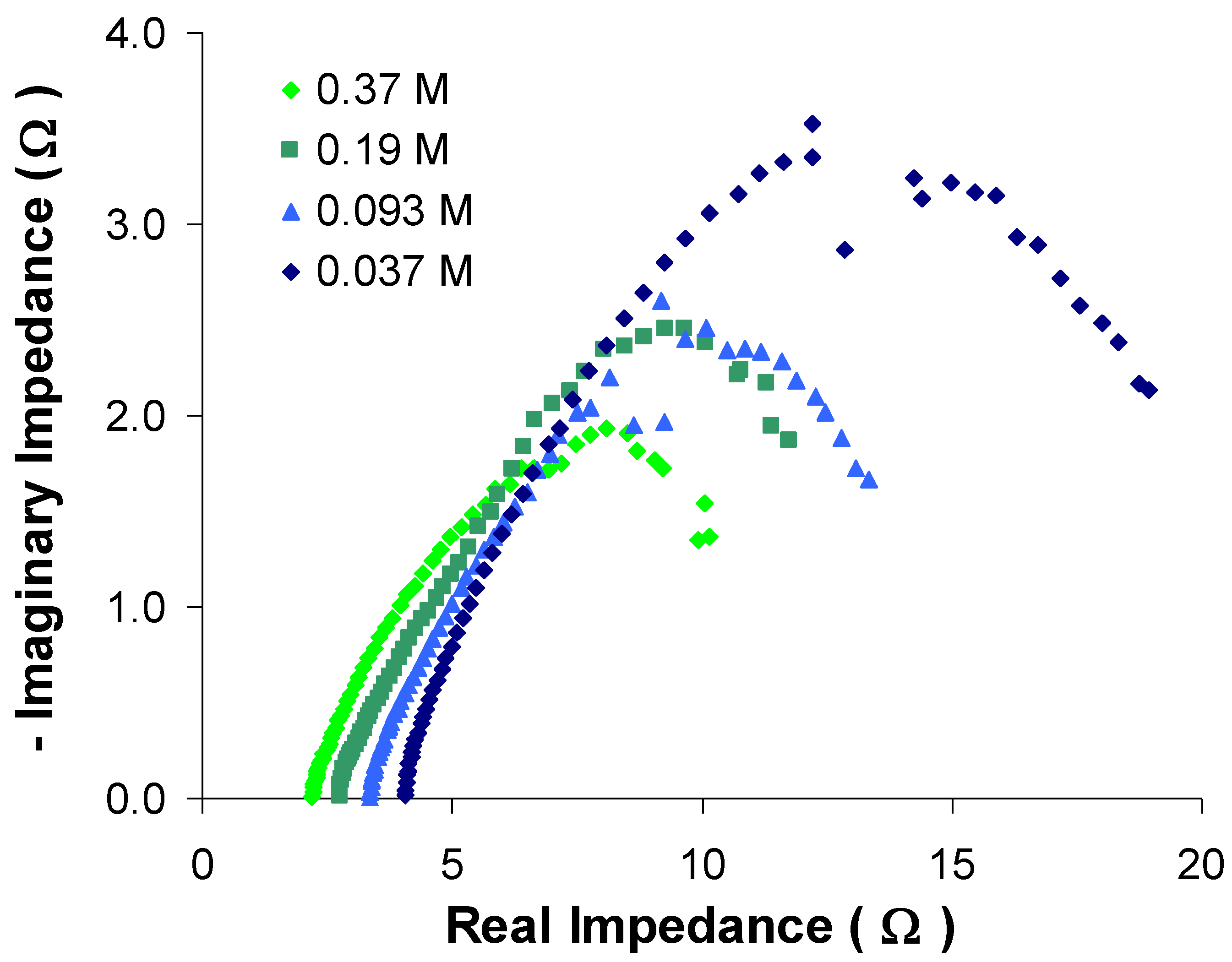
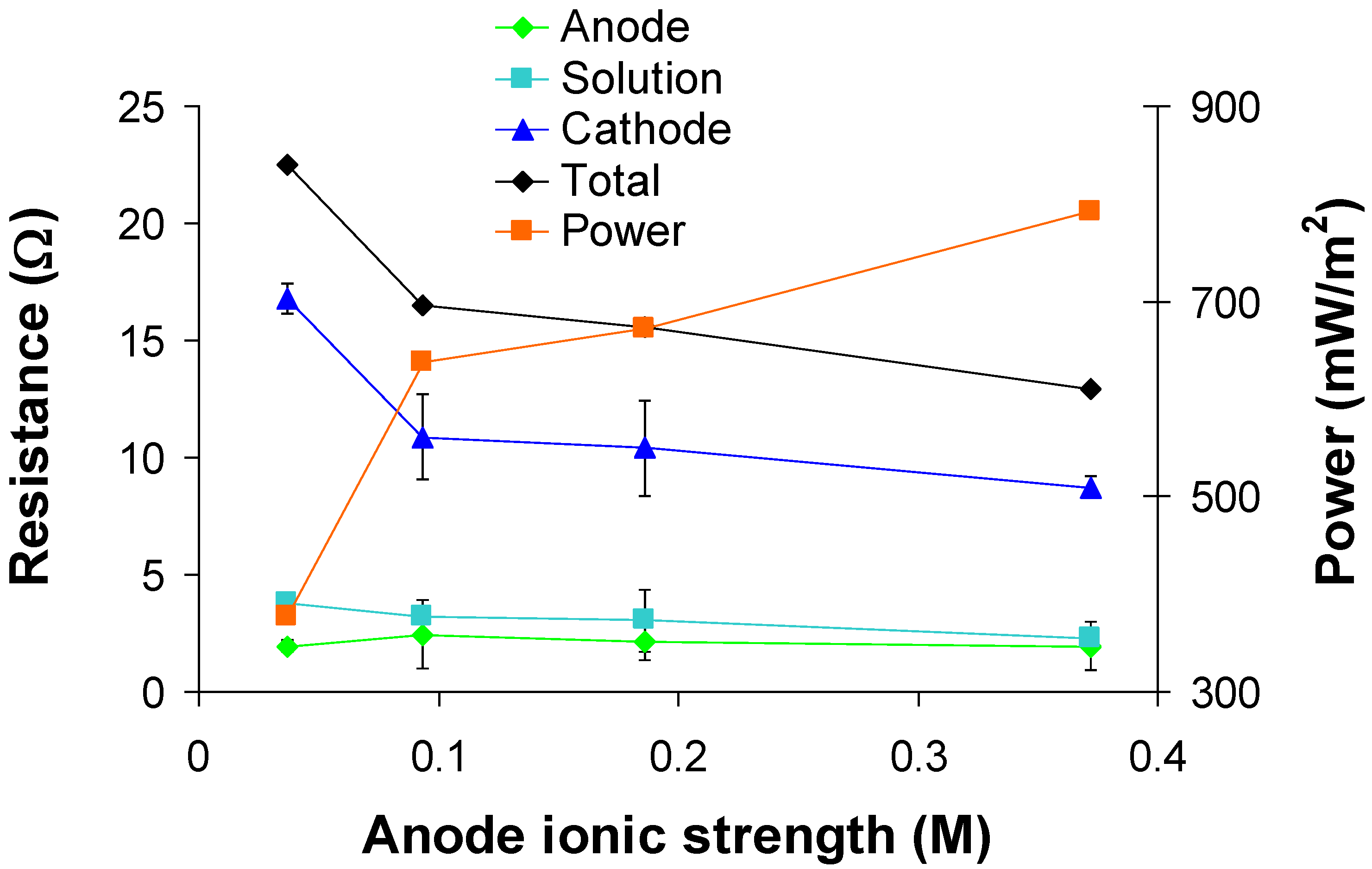
4. Conclusions
Acknowledgments
References and Notes
- Borole, A.P.; Hamilton, C.Y.; Vishnivetskaya, T.A.; Leak, D.; Andras, C.; Morrell-Falvey, J.; Davison, B.H.; Keller, M. Integrating engineering design improvements with exoelectrogen enrichment process to increase power output from microbial fuel cells. J. Power Sources 2009, 191, 520–527. [Google Scholar] [CrossRef]
- Borole, A.P.; Hamilton, C.Y.; Vishnivetskaya, T.A.; Leak, D.; Andreas., C. Improving power production from acetate-fed microbial fuel cells via enrichment of exoelectrogenic organisms in continuous flow systems. Biochem. Eng. J. 2009, 48, 71–80. [Google Scholar] [CrossRef]
- Fan, Y.; Hu, H.; Liu, H. Enhanced coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 2007, 171, 348–354. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, S.; Huang, L.P.; Logan, B.E. Scale-up of membrane-free single-chamber microbial fuel cells. J. Power Sources 2008, 179, 274–279. [Google Scholar] [CrossRef]
- Liang, P.; Huang, X.; Fan, M.Z.; Cao, X.X.; Wang, C. Composition and distribution of internal resistance in three types of microbial fuel cells. Appl. Microbiol. Biotechnol. 2007, 77, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Aelterman, P.; Rabaey, K.; Pham, H.T.; Boon, N.; Verstraete, W. Continuous electricity generation at high voltages and currents uding stacked microbial fuel cells. Environ. Sci. Technol. 2006, 40, 3388–3394. [Google Scholar] [CrossRef] [PubMed]
- Gomadam, P.M.; Weidner, J.W. Analysis of electrochemical impedance spectroscopy in proton exchange membrane fuel cells. Int. J. Energy Res. 2005, 29, 1133–1151. [Google Scholar] [CrossRef]
- He, Z.; Mansfeld, F. Exploring the use of electrochemical impedance spectroscopy (EIS) in microbial fuel cell studies. Energy Environ. Sci. 2009, 2, 215–219. [Google Scholar] [CrossRef]
- Manohar, A.K.; Mansfeld, F. The internal resistance of a microbial fuel cell and its dependence on cell design and operating conditions. Electrochim. Acta 2009, 54, 1664–1670. [Google Scholar] [CrossRef]
- He, Z.; Huang, Y.L.; Manohar, A.K.; Mansfeld, F. Effect of electrolyte pH on the rate of the anodic and cathodic reactions in an air-cathode microbial fuel cell. Bioelectrochemistry 2008, 74, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Ouitrakul, S.; Sriyudthsak, M.; Charojrochkul, S.; Kakizono, T. Impedance analysis of bio-fuel cell electrodes. Biosens. Bioelectron. 2007, 23, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.I.; Lee, H.S.; Rittmann, B.E. Carbonate species as OH- carriers for decreasing the pH gradient between cathode and anode in biological fuel cells. Environ. Sci. Technol. 2008, 42, 8773–8777. [Google Scholar] [CrossRef] [PubMed]
- Manohar, A.K.; Bretschger, O.; Nealson, K.H.; Mansfeld, F. The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 2008, 72, 149–154. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wagner, N.; Minteer, S.D.; Angenent, L.T. An upflow microbial fuel cell with an interior cathode: Assessment of the internal resistance by impedance spectroscopy. Environ. Sci.Technol. 2006, 40, 5212–5217. [Google Scholar] [CrossRef] [PubMed]
- You, S.J.; Zhao, Q.L.; Zhang, J.; Liu, H.; Jiang, J.Q.; Zhao, S.Q. Increased sustainable electricity generation in up-flow air-cathode microbial fuel cells. Biosens. Bioelectron. 2008, 23, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.P.; Gadhamshetty, V.; Nadeau, L.; Johnson, G.R. Impedance spectroscopy as a tool for non-intrusive detection of extracellular mediators in microbial fuel cells. Biotechnol. Bioeng. 2009, 104, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Borole, A.P.; Hamilton, C.Y. Energy production from food industry wastewaters using bioelectrochemical cells. In Emerging Environmental Technologies; Vishal, Shah, Ed.; Springer: Dordrecht, the Netherlands, 2010; Volume II, pp. 97–113. [Google Scholar]
- Liu, H.; Cheng, S.A.; Logan, B.E. Power generation in fed-batch microbial fuel cells as a function of ionic strength, temperature, and reactor configuration. Environ. Sci. Technol. 2005, 39, 5488–5493. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.; Logan, B.E.; Lee, H. Brewery wastewater treatment using air-cathode microbial fuel cells. Environmental Biotechnology, 2008, 78, 873–880. [Google Scholar] [CrossRef]
- Rosenbaum, M.; Zhao, F.; Quaas, M.; Wulff, H.; Shroder, U.; Scholz, F. Evaluation of catalytic properties of tungsten carbide for the anode of microbial fuel cells. Appl. Catal. B. 2007, 74, 262–270. [Google Scholar]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schroder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef] [PubMed]
- Borole, A.P.; Hamilton, C.Y.; Aaron, D.S.; Tsouris, C. Investigating microbial fuel cell bioanode performance under different cathode conditions. Biotechnol. Prog. 2009, 25, 1630–1636. [Google Scholar] [PubMed]
- Gamry Instruments Application Note. Basics of Electrochemical Impedance Spectroscopy. 2006.
- Ramasamy, R.P.; Ren, Z.Y.; Mench, M.M.; Regan, J.M. Impact of initial biofilm growth on the anode impedance of microbial fuel cells. Biotechnol. Bioeng. 2008, 101, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Orazem, M.E.; Tribollet, B. Electrochemical Impedance Spectroscopy; John Wiley and Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Dlugolecki, P.; Anet, B.; Metz, S.J.; Nijmeijer, K.; Wessling, M. Transport limitations in ion exchange membranes at low salt concentrations. J. Membr. Sci. 2010, 346, 163–171. [Google Scholar] [CrossRef]
- Aaron, D.; Yiacoumi, S.; Tsouris, C. Effects of proton-exchange membrane fuel-cell operating conditions on charge transfer resistances measured by electrochemical impedance spectroscopy. Sep. Sci.Technol. 2008, 43, 2307–2320. [Google Scholar] [CrossRef]
- Borole, A.P.; Aaron, D.; Hamilton, C.Y.; Tsouris, C. Understanding long-term changes in microbial fuel cells via electrochemical impedance spectroscopy. Environ. Sci. Technol. 2010. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Aaron, D.; Tsouris, C.; Hamilton, C.Y.; Borole, A.P. Assessment of the Effects of Flow Rate and Ionic Strength on the Performance of an Air-Cathode Microbial Fuel Cell Using Electrochemical Impedance Spectroscopy. Energies 2010, 3, 592-606. https://doi.org/10.3390/en3040592
Aaron D, Tsouris C, Hamilton CY, Borole AP. Assessment of the Effects of Flow Rate and Ionic Strength on the Performance of an Air-Cathode Microbial Fuel Cell Using Electrochemical Impedance Spectroscopy. Energies. 2010; 3(4):592-606. https://doi.org/10.3390/en3040592
Chicago/Turabian StyleAaron, Doug, Costas Tsouris, Choo Y. Hamilton, and Abhijeet P. Borole. 2010. "Assessment of the Effects of Flow Rate and Ionic Strength on the Performance of an Air-Cathode Microbial Fuel Cell Using Electrochemical Impedance Spectroscopy" Energies 3, no. 4: 592-606. https://doi.org/10.3390/en3040592





