Enzymatic Biofuel Cells—Fabrication of Enzyme Electrodes
Abstract
:1. Introduction
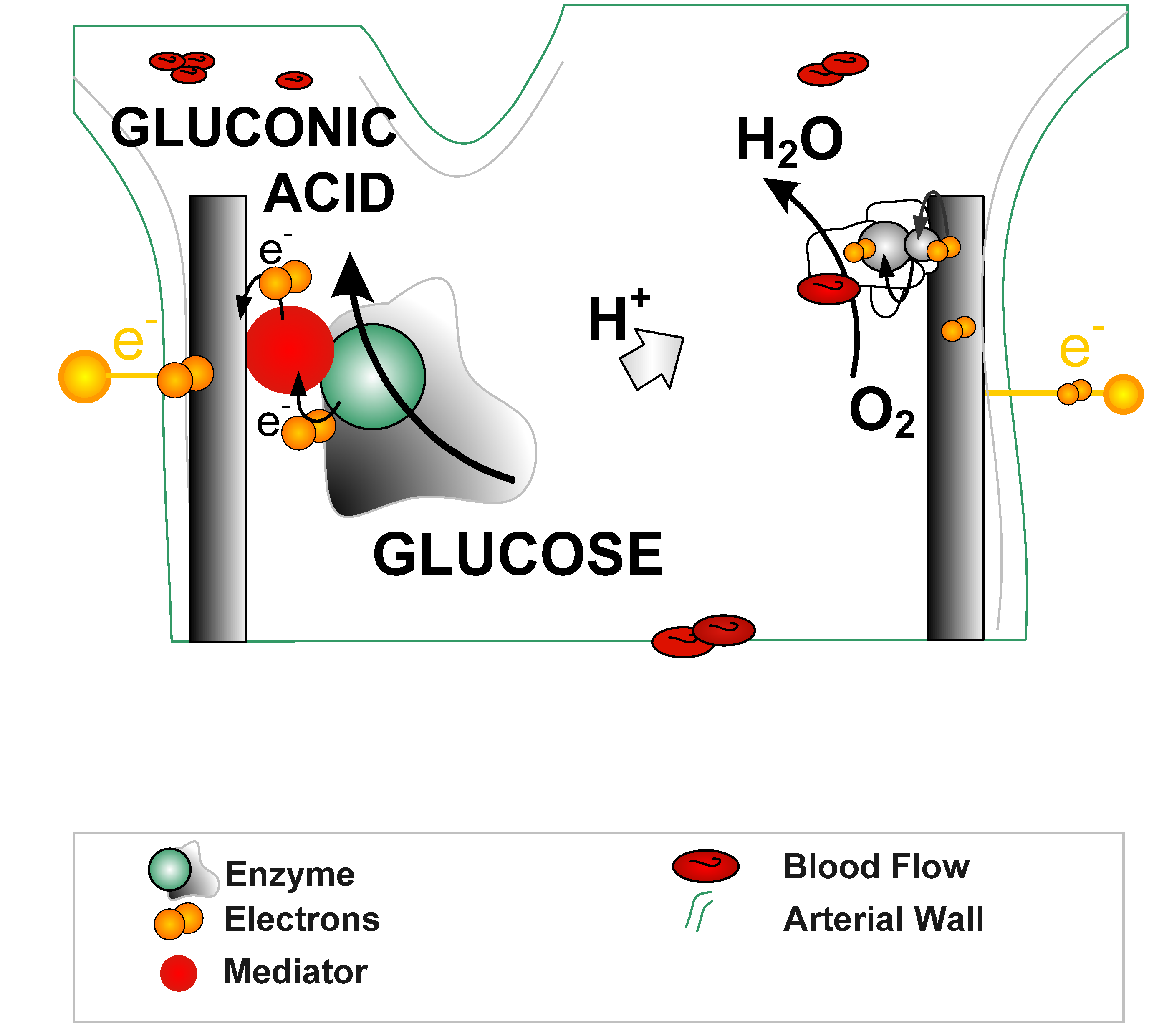
2. Types of Biofuel Cells and Enzymes
2.1. Types of biofuel cells
- Product type. In this type of fuel cells enzymes are not involved in direct energy generation, and the energy generation is realised by a conversional fuel cell. Enzymes generate the fuel substrate for fuel cell by a biocatalytic transformation or metabolic process. There have been several studies that have demonstrated the use of hydrogenase to produce hydrogen from glucose for conventional hydrogen-oxygen fuel cells [36,37]. This type of biofuel cell is less common in enzymatic fuel cells.
- Direct energy production type. In this type of biofuel cells, enzymes are directly involved in the bioreactions for energy production. Enzymes participate in the electron transfer chain between the fuel and the anode. On the anode, enzymes oxidise organic matters and produce electrons, and on the cathode, enzymes act as catalysts for oxidant reduction and accept electrons, the same principle as the conventional fuel cells. The performance of the fuel cell is mainly dependant on the enzyme activity. Most enzymatic fuel cells are this type. One of the main challenges for this type of fuel cells is to establish efficient electron transfer between enzymes and electrode supports.
2.2. Types of enzymes based on electron transfer methods

- (1)
- Enzymes with nicotinamide adenine dinucleotide (NADH/NAD+) or nicotinamide adenine dinucleotide phosphate (NADPH/NADP+) redox centres, which are often weakly bound to the protein of the enzyme. Glucose dehydrogenase and alcohol dehydrogenase belong to this group.
- (2)
- Enzymes in which at least part of the redox centre is conveniently located at or near the periphery of the protein shell. Peroxidases, laccase and other multi copper enzymes fall into this category. Peroxidases, such as horseradish peroxidises and cytochrome c peroxidise have been commonly used enzyme reactions and immunoassay.
- (3)
- Enzymes with a strongly bound redox centre deeply bound in a protein or glycoprotein shell. Glucose oxidase is the most studied enzyme example of this type.
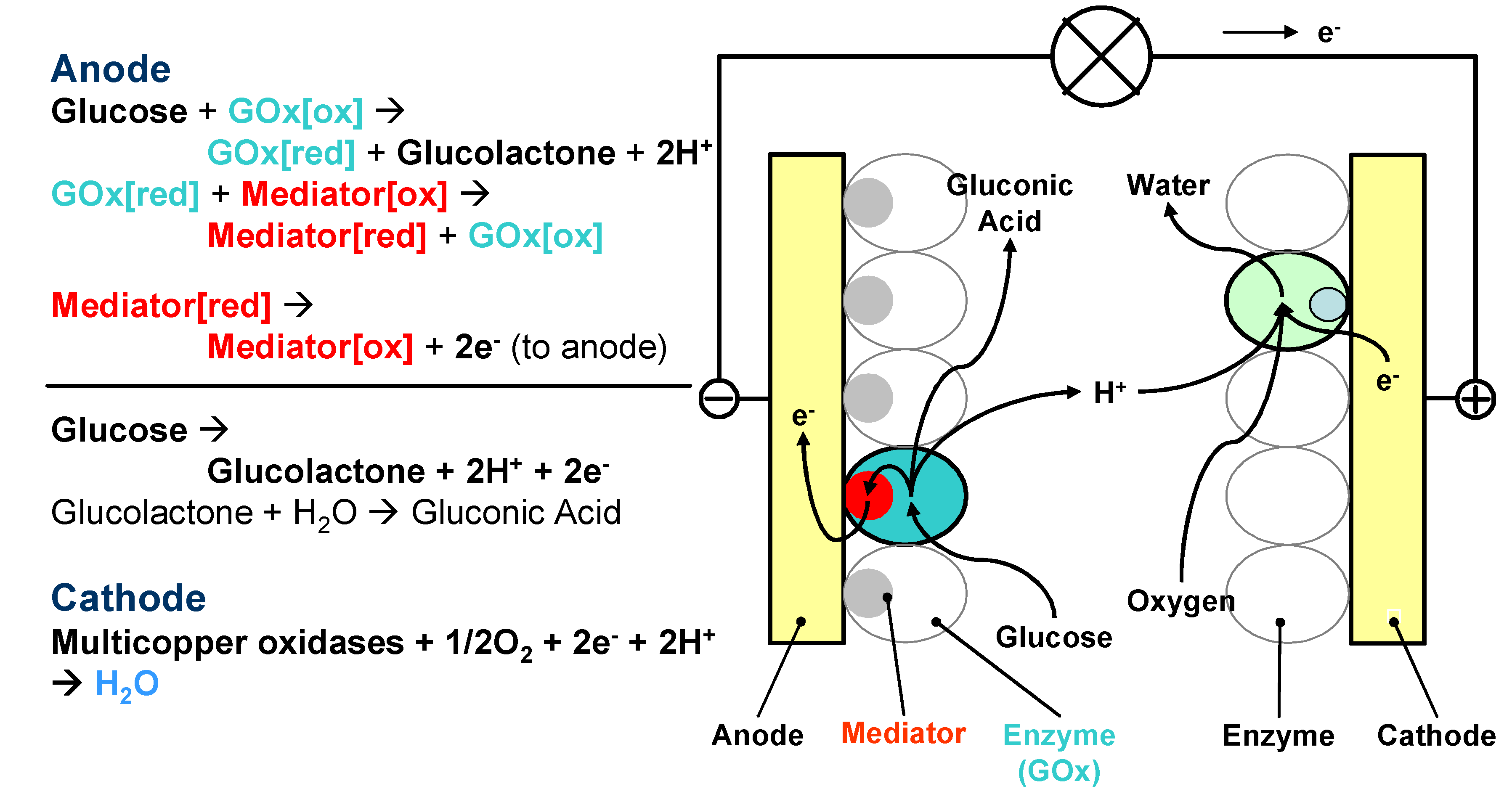
3. Fabrication of Enzyme Electrodes
3.1. Enzyme electrodes with layered structures
3.2. Enzyme electrodes with polymer matrix
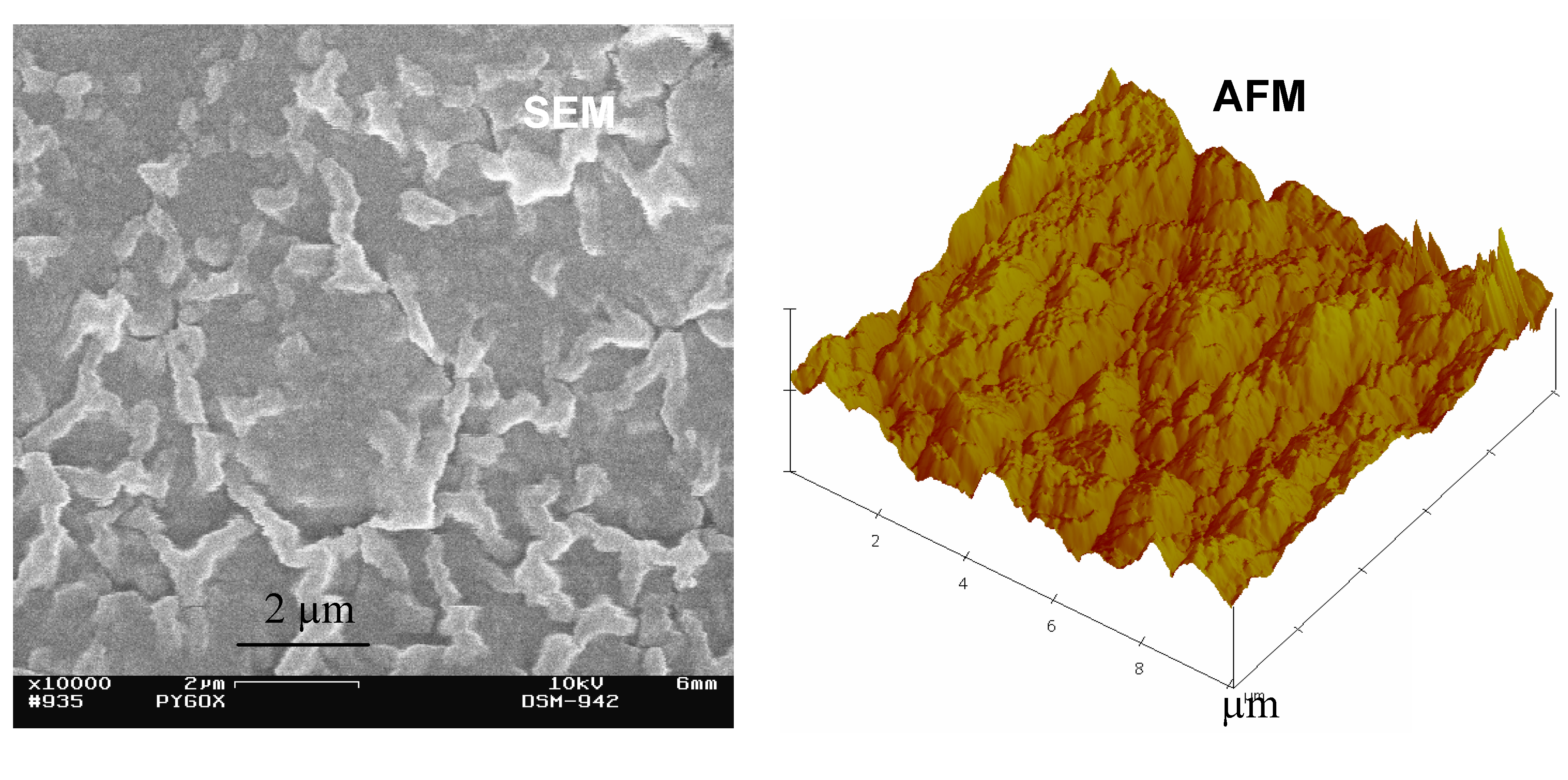
4. Performance of Enzymatic Biofuel Cells
5. Conclusions and Outlook
| Anode | Cathode | Open circuit voltage/mV | Short-circuit current | Power/power density | Ref. |
|---|---|---|---|---|---|
| PQQ monolayer-functionalized-Au-electrode Fuel: 1,4-dihydronicotinamide adenine dinucleotide, NADH | microperoxidase-11 (MP-11)-modified Au-electrode Oxidant: H2O2 | 320 | 30 µA cm−2 | 8 µW | [44] |
| reconstituted glucose oxidase monolayer on gold Fuel: glucose | reconstituted cytochrome c/cytochrome oxidase couple Oxidant: O2 | 130 | transduced current density at a glucose concentration of 80 mM corresponds to ca. 200 μA cm−2 | 4 µW | [93] |
| Fuel: glucose | Oxidant: O2 | 120 | 550 µA cm−2 | 4.3 µW | [94] |
| Os derivatives polymer mediators Fuel: glucose | Os derivatives polymer mediators Oxidant: O2 | 600 | 4.8 W mm−2 | [82] | |
| glucose oxidase and using 1,1'-dicarboxyferrocene as mediator Fuel: fruit juices (orange, banana and grape juice) | Laccase and using 1,1'-dicarboxyferrocene as mediator Oxidant: O2 | 220 without mediator 195 with ferrocen mediator Grape juice as fuel: 191 Banana juice as fuel: 202 Orange juice as fuel: 360 six as-prepared BFCs by using orange juice as fuels in series have reached Voc value of 2.2 V | 109.8 μA cm−2 with glucose 56 μA (current density ca. 136.6 μA cm−2) 60 μA, current density ca. 146.3 μA cm−2) 72 μA, current density ca. 175.6 μA cm−2) | 11.66 μW (power density ca. 28.4 μW cm−2) | [9] |
| poly-L-lysine (PLL-VK3) as mediator for diaphorase (Dp) oxidation of NADH and then coated with glucose dehydrogenase Fuel: NADH and glucose | PDMS-coated Pt Oxidant: O2 | 550 | 0.13 mA cm−2 | 32 µW cm−2 at 0.29 V when a pH 7.0 buffered fuel containing 5.0 mM glucose and 1.0 mM NAD(+) | [96] |
| quino-hemoprotein-alcohol dehydrogenase (QH-ADH) Fuel: ethanol | alcohol oxidase (AOx) and microperoxidase (MP-8) Oxidant: H2O2 | 240 | 30 μA cm−2 With 25 mM ethanol | 1.5 µW cm−2 | [97] |
| PQQ-ADH, PQQ-AldDH and oxalate oxidase immobilised within a tetrabutylammonium-modified Nafion membrane Fuel: glycerol | Oxidant: O2 | 3.5 ± 0.48 mA cm−2 with 5 M glycerol | 1.32 mW cm−2 | [98] | |
| packed enzymes (GDH) and mediator on carbon-fiber Fuel: glucose | PDMS-coated Pt Oxidant: O2 | 800 | 11 mA cm−2 | 100 mW with the volume of 80 cm3 with multi-stacking 1.45 ± 0.24 mW cm−2 at 0.3 V. | [99] |
| Anodic glucose oxidase (GOx) immobilized on the porous silicon/SWNT substrates Fuel: glucose | cathodic laccase (Lac) immobilized on the porous silicon/SWNT substrates Oxidant: Air/O2 | 30 μA cm−2 | 1.38 µW cm2 down to 0.3 µW cm−2 in 24 h was obtained in 4 mM glucose solution | [100] |
- (1)
- Protein engineering to engineering native enzyme molecules with desired properties tailored for specific applications. There two main strategies in protein engineering are rational design, which combines site-directed mutagenesis with the detailed knowledge of enzyme structures and functions or computational models, and directed evolution, which is based on the random synthesis of a pool of mutated enzymes and the subsequent selection by an iterative process [101]. Mutant glucose oxidase obtained from directed evolution has shown improved pH and thermal stability. The biological catalytic kinetics and affinity between mutant enzyme and substrate were improved [21]. Moreover, electrochemical characterisation of mutant enzymes also showed improved electrochemical catalytic activity and electron transfer rate from the mutant glucose oxidase comparing to wild type enzyme [102].
- (2)
- New immobilisation method and biomaterials to improve the stability of enzymes. Immobilisation of enzymes in hydrophilic sol-gel matrixes and ionic liquids has yielded desired stability and direct electron transfer from enzyme electrodes [103,104,105,106,107]. Biomaterials based on novel phospholipid polymers with unit imitating biomembrane have been developed. They have excellent biocompatibility and the ability of maintaining enzyme activity [20,108]. These new methods and materials provide new means in developing enzyme electrode with required stability.
- (3)
- Nanomaterials integrated in the enzyme electrode structure to improve the electron transfer and enzyme catalytic activity. Gold nano particles and nano carbon materials have been utilised to establish and improve the electron transfer between enzyme active centre and electrodes because of their excellent electronic properties [100,109,110,111]. Mesoporous materials can be another substrate for enzyme immobilisation. The ordered porous structure with high surface area can increase the enzyme loading and improve the electrode activity.
- (4)
- Novel design on fuel cell configuration to improve the cell voltage and power out put. For conventional fuel cells, fuel cell stacks are normally developed to achieve high cell voltage and power output. The same principles can be also applied to biofuel cells. Modular microfluidics makes it possible to develop miniaturised biofuel cell stacks with substantial power output.
Acknowledgements
References
- Rigla, M.; Hernando, M.E.; Gomez, E.J.; Brugues, E.; Garcia-Saez, G.; Capel, I.; Pons, B.; de Leiva, A. Real-time continuous glucose monitoring together with telemedical assistance improves glycemic control and glucose stability in pump-treated patients. Diabetes Technol. Therapeut. 2008, 10, 194–199. [Google Scholar] [CrossRef]
- Heller, A. Implanted electrochemical glucose sensors for the management of diabetes. Annu. Rev. Biomed. Eng. 1999, 1, 153–175. [Google Scholar] [CrossRef] [PubMed]
- Barton, C.S.; Gallaway, J.; Atanassov, P. Enzymatic biofuel cells for implantable and microscale devices. Chem. Rev. 2004, 104, 4867–4886. [Google Scholar] [CrossRef] [PubMed]
- Heller, A. Miniature biofuel cells. Phys. Chem. Chem. Phys. 2004, 6, 209–216. [Google Scholar] [CrossRef]
- Itamar, W. Biofuel cells: Harnessing biomass or body fluids for the generation of electrical power. Fuel Cells 2009, 9, 5. [Google Scholar] [CrossRef]
- Suwansa-Ard, S.; Kanatharana, P.; Asawatreratanakul, P.; Limsakul, C.; Wongkittisuksa, B.; Thavarungkul, P. Semi disposable reactor biosensors for detecting carbamate pesticides in water. Biosens. Bioelectron. 2005, 21, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Tizzard, A.C.; Lloyd-Jones, G. Bacterial oxygenases: In vivo enzyme biosensors for organic pollutants. Biosens. Bioelectron. 2007, 22, 2400–2407. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Chen, Z.C.; Yang, S.N.; Jin, X.; Lin, X.F. A novel inhibition biosensor constructed by layer-by-layer technique based on biospecific affinity for the determination of sulfide. Sens. Actuators B: Chem. 2008, 129, 218–224. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, S.J. A biofuel cell harvesting energy from glucose-air and fruit juice-air. Biosens. Bioelectron. 2007, 23, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Cracknell, J.A.; Vincent, K.A.; Armstrong, F.A. Enzymes as working or inspirational electrocatalysts for fuel cells and electrolysis. Chem. Rev. 2008, 108, 2439–2461. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Biofuel cell based on direct bioelectrocatalysis. Biosens. Bioelectron. 2005, 20, 1962–1967. [Google Scholar] [CrossRef] [PubMed]
- The bioeconomy at work: Sony develops most efficient biofuel cell ever, powered by sugar. Available online: http://news.mongabay.com/bioenergy/2007/08/bioeconomy-at-work-sony-develops-most.html (accessed on 17 December 2009).
- Yahiro, A.T.; Lee, S.M.; Kimble, D.O. Bioelectrochemistry: I. Enzyme utilizing bio-fuel cell studies. BBA – Biophys. 1964, 88, 375–383. [Google Scholar]
- Rusling, J.F.; Ito, K. Voltammetric determination of electron-transfer rate between an enzyme and a mediator. Anal. Chim. Acta 1991, 252, 23–27. [Google Scholar] [CrossRef]
- Gallaway, J.W.; Calabrese Barton, S.A. Kinetics of redox polymer-mediated enzyme electrodes. J. Am. Chem. Soc. 2008, 130, 8527–8536. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Lioubashevsky, O.; Willner, I. Electromechanics of a redox-active rotaxane in a monolayer assembly on an electrode. J. Am. Chem. Soc. 2004, 126, 15520–15532. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Oshida, H.; Endo, Y.; Ueda, T.; Watanabe, A.; Nakabayashi, N. Hemocompatibility of human whole blood on polymers with a phospholipid polar group and its mechanism. J. Biomed. Mater. Res. 1992, 26, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Iwasaki, Y.; Nakabayashi, N. Novel biomedical polymers for regulating serious biological reactions. Ma. Sci. Eng. C-Biomim. Supram. S. 1998, 6, 253–259. [Google Scholar] [CrossRef]
- Konno, T.; Watanabe, J.; Ishihara, K. Conjugation of enzymes on polymer nanoparticles covered with phosphorylcholine groups. Biomacromolecules 2004, 5, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, K.; Konno, T.; Takai, M.; Ishihara, K. Bioconjugated phospholipid polymer biointerface for enzyme-linked immunosorbent assay. Biomacromolecules 2008, 9, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.W.; Gautam, A.; Nazor, J.; Momeu, C.; Prodanovic, R.; Schwaneberg, U. Directed evolution of glucose oxidase from Aspergillus niger for ferrocenemethanol-mediated electron transfer. Biotechnol. J. 2007, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.H.; Sundmacher, K. Enzyme electrodes for glucose oxidation prepared by electropolymerization of pyrrole. Process Saf. Environ. Prot. 2007, 85, 489–493. [Google Scholar] [CrossRef]
- Willner, I.; Blonder, R.; Katz, E.; Stocker, A.; Buckmann, A.F. Reconstitution of apo-glucose oxidase with a nitrospiropyran-modified FAD cofactor yields a photoswitchable biocatalyst for amperometric transduction of recorded optical signals. J. Am. Chem. Soc. 1996, 118, 5310–5311. [Google Scholar] [CrossRef]
- Xiao, Y.; Patolsky, F.; Katz, E.; Hainfeld, J.F.; Willner, I. "Plugging into enzymes": Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar] [CrossRef] [PubMed]
- Degani, Y.; Heller, A. Direct electrical communication between chemically modified enzymes and metal-electrodes .2. Methods for bonding electron-transfer relays to glucose-oxidase and d-amino-acid oxidase. J. Am. Chem. Soc. 1988, 110, 2615–2620. [Google Scholar] [CrossRef]
- Chen, Q.; de LumleyWoodyear, T.; Kenausis, G.; Schmidtke, D.; Heller, A. Developments in ''wired'' enzyme sensors. Abstr. Pap. Am. Chem. Soc. 1997, 213, 183-ANYL. [Google Scholar]
- Zhu, Z.W.; Momeu, C.; Zakhartsev, M.; Schwaneberg, U. Making glucose oxidase fit for biofuel cell applications by directed protein evolution. Biosens. Bioelectron. 2006, 21, 2046–2051. [Google Scholar] [CrossRef] [PubMed]
- Gooding, J.J.; Mearns, F.; Yang, W.R.; Liu, J.Q. Self-assembled monolayers into the 21(st) century: Recent advances and applications. Electroanalysis 2003, 15, 81–96. [Google Scholar] [CrossRef]
- Dieter, T.; Rennebergb, R. Encapsulation of glucose oxidase microparticles within a nanoscale layer-by-layer film: immobilization and biosensor applications. Biosens. Bioelectron. 2003, 18, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Yagi, T.; Chu, M.; Saito, H.; Morimoto, N.; Iwasaki, Y.; Akiyoshi, K.; Mitsubayashi, K. Glucose sensor using a phospholipid polymer-based enzyme immobilization method. Anal. Bioanal. Chem. 2008, 391, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Raitman, O.A.; Katz, E.; Buckmann, A.F.; Willner, I. Integration of polyaniline/poly(acrylic acid) films and redox enzymes on electrode supports: an in situ electrochemical/surface plasmon resonance study of the bioelectrocatalyzed oxidation of glucose or lactate in the integrated bioelectrocatalytic systems. J. Am. Chem. Soc. 2002, 124, 6487–6496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yang, W.; Niu, Y.; Sun, C. Multilayered construction of glucose oxidase on gold electrodes based on layer-by-layer covalent attachment. Anal. Chim. Acta 2004, 523, 209–217. [Google Scholar] [CrossRef]
- Gooding, J.J.; Erokhin, P.; Losic, D.; Yang, W.; Policarpio, V.; Liu, J.; Ho, F.M.; Situmorang, M.; Hibbert, D.B.; Shapter, J.G. Parameters important in fabricating enzyme electrodes using self-assembled monolayers of alkanethiols. Anal. Sci. 2001, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Moehlenbrock, M.J.; Minteer, S.D. Extended lifetime biofuel cells. Chem. Soc. Rev. 2008, 37, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Cooney, M.J.; Svoboda, V.; Lau, C.; Martin, G.; Minteer, S.D. Enzyme catalysed biofuel cells. Energy Environ. Sci. 2008, 1, 320–337. [Google Scholar] [CrossRef]
- Woodward, J.; Mattingly, S.M.; Danson, M.; Hough, D.; Ward, N.; Adams, M. In vitro hydrogen production by glucose dehydrogenase and hydrogenase. Nat. Biotechnol. 1996, 14, 872–874. [Google Scholar] [CrossRef] [PubMed]
- Mertens, R.; Liese, A. Biotechnological applications of hydrogenases. Curr. Opin. Biotechnol. 2004, 15, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Heller, A. Electrical connection of enzyme redox centers to electrodes. J. Phys. Chem. 1992, 96, 3579–3587. [Google Scholar] [CrossRef]
- Bullen, R.A.; Arnot, T.C.; Lakeman, J.B.; Walsh, F.C. Biofuel cells and their development. Biosens. Bioelectron. 2006, 21, 2015–2045. [Google Scholar] [CrossRef] [PubMed]
- Katz, E.; Shipway, A.N.; Willner, I. Handbook of Fuel Cells; Vielstich, W., Gasteriger, H.A., Lamm, A., Eds.; John Wiley and Sons, Ltd.: London, UK, 2003. [Google Scholar]
- Degani, Y.; Heller, A. Direct electrical communication between chemically modified enzymes and metal-electrodes .1. electron-transfer from glucose-oxidase to metal-electrodes via electron relays, bound covalently to the enzyme. J. Phys. Chem. 1987, 91, 1285–1289. [Google Scholar] [CrossRef]
- Schumacher, D.; Vogel, J.; Lerche, U. Construction and applications of an enzyme electrode for determination of galactose and galactose-containing saccharides. Biosens. Bioelectron. 1994, 9, 85–90. [Google Scholar] [CrossRef]
- Vincent, K.A.; Cracknell, J.A.; Lenz, O.; Zebger, I.; Friedrich, B.; Armstrong, F.A. Electrocatalytic hydrogen oxidation by an enzyme at high carbon monoxide or oxygen levels. Proc. Nat. Acad. Sci. USA 2005, 102, 16951–16954. [Google Scholar] [CrossRef] [PubMed]
- Willner, I.; Arad, G.; Katz, E. A biofuel cell based on pyrroloquinoline quinone and microperoxidase-11 monolayer-functionalized electrodes. Bioelectrochem. Bioenerget. 1998, 44, 209–214. [Google Scholar] [CrossRef]
- Willner, I.; HelegShabtai, V.; Blonder, R.; Katz, E.; Tao, G.L. Electrical wiring of glucose oxidase by reconstitution of FAD-modified monolayers assembled onto Au-electrodes. J. Am. Chem. Soc. 1996, 118, 10321–10322. [Google Scholar] [CrossRef]
- Katz, E.; Riklin, A.; Heleg-Shabtai, V.; Willner, I.; Buckmann, A.F. Glucose oxidase electrodes via reconstitution of the apo-enzyme: tailoring of novel glucose biosensors. Anal. Chim. Acta 1999, 385, 45–58. [Google Scholar] [CrossRef]
- Willner, V.; Blonder, R.; Katz, E.; Tao, G.; Buckmann, A.F.; Heller, A. Electrical Wiring of Glucose Oxidase by Reconstitution of FAD-Modified Monolayers Assembled onto Au-Electrodes. J. Am. Chem. Soc. 1996, 118, 10321. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Li, J. Electrochemical study of 4-ferrocene thiophenol monolayers assembled on gold nanoparticles. Microelectron. Eng. 2003, 66, 91–94. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Tada, K.; Shimazu, K. Redox and mass transport characteristics of domain-free mixed ferrocenyloctanethiol/alkanethiol monolayers on gold. J. Electroanal. Chem. 2003, 543, 41–49. [Google Scholar] [CrossRef]
- Felgenhauer, T.; Rong, H.T.; Buck, M. Electrochemical and exchange studies of self-assembled monolayers of biphenyl based thiols on gold. J. Electroanal. Chem. 2003, 550-551, 309–319. [Google Scholar]
- Schlereth, D.D.; Kooyman, R.P.H. Self-assembled monolayers with biospecific affinity for lactate dehydrogenase for the electroenzymatic oxidation of lactate. J. Electroanal. Chem. 1997, 431, 285–295. [Google Scholar] [CrossRef]
- Sato, Y.; Mizutani, F. Electrochemical responses of cytochrome c on a gold electrode modified with mixed monolayers of 3-mercaptopropionic acid and n-alkanethiol. J. Electroanal. Chem. 1997, 438, 99–104. [Google Scholar] [CrossRef]
- Dong, S.; Li, J. Self-assembled monolayers of thiols on gold electrodes for bioelectrochemistry and biosensors. Bioelectrochem. Bioenerg. 1997, 42, 7–13. [Google Scholar] [CrossRef]
- Krysinski, P.; Brzostowska-Smolska, M. Capacitance characteristics of self-assembled monolayers on gold electrode. Bioelectrochem. Bioenerg. 1998, 44, 163–168. [Google Scholar] [CrossRef]
- Bourdillon, C.; Demaille, C.; Moiroux, J.; Saveant, J.M. Step-by-step immunological construction of a fully active multilayer enzyme electrode. J. Am. Chem. Soc. 1994, 116, 10328–10329. [Google Scholar] [CrossRef]
- Shoham, B.; Migron, Y.; Riklin, A.; Willner, I.; Tartakovsky, B. A bilirubin biosensor based on a multilayer network enzyme electrode. Biosens. Bioelectron. 1995, 10, 341–352. [Google Scholar] [CrossRef]
- Lowy, D.A.; Finklea, H.O. Gold electrodes with polyion multilayers and electrostatically bound redox couples. Electrochim. Acta 1997, 42, 1325–1335. [Google Scholar] [CrossRef]
- Yoon, H.C.; Kim, H.S. Multilayered assembly of dendrimers with enzymes on gold: Thickness-controlled biosensing interface. Anal. Chem. 2000, 72, 922–926. [Google Scholar] [CrossRef] [PubMed]
- Calvo, E.J.; Battaglini, F.; Danilowicz, C.; Wolosiuk, A.; Otero, M. Layer-by-layer electrostatic deposition of biomolecules on surfaces for molecular recognition, redox mediation and signal generation. Faraday Discussions 2000, 116, 47–65. [Google Scholar] [CrossRef] [PubMed]
- Ramanavicius, A.; Ramanaviciene, A.; Malinauskas, A. Electrochemical sensors based on conducting polymer- polypyrrole. Electrochim. Acta 2006, 51, 6025–6037. [Google Scholar] [CrossRef]
- Sung, W.J.; Bae, Y.H. A Glucose Oxidase Electrode Based on Electropolymerized Conducting Polymer with Polyanion-Enzyme Conjugated Dopant. Anal. Chem. 2000, 72, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Serge, C.G.; Watelet, J.C. A Polypyrrole-bienzyme electrode (salicylate hydroxylase-polyphenol oxidase) for the interference-free determination of salicylate. Electroanalysis 2001, 13, 906–910. [Google Scholar] [CrossRef]
- Yasuzawa, M.; Nieda, T.; Hirano, T.; Kunugi, A. Properties of glucose sensors based on the immobilization of glucose oxidase in N-substituted polypyrrole film. Sens. Actuators B: Chem. 2000, 66, 77–79. [Google Scholar] [CrossRef]
- Gondran, C.; Cosnier, S. Fabrication of biosensors by attachment of biological macromolecules to electropolymerized conducting films. Analusis 1999, 27, 558. [Google Scholar] [CrossRef]
- Cosnier, S.; Senillou, A.; Gratzel, M.; Comte, P.; Vlachopoulos, N.; Jaffrezic Renault, N.; Martelet, C. A glucose biosensor based on enzyme entrapment within polypyrrole films electrodeposited on mesoporous titanium dioxide. J. Electroanal. Chem. 1999, 469, 176–181. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Matuszewski, W.; Podsiadla, M. Enzyme entrapped polypyrrole modified electrode for flow-injection determination of glucose. Biosens. Bioelectron. 1990, 5, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Cosnier, S.; Deronzier, A.; Roland, J.F. Electrocatlatic oxidation of alcohols on carbon electrodes modified by functionalised polypyrrole RuO2 films. J. Molecular Cata. 1992, 71, 303–315. [Google Scholar] [CrossRef]
- Cosnier, S. Fabrication of amperometric biosensors by entrapment of enzymes in functionalized polypyrrole films. Can. J. Chem. Eng. 1998, 76, 1000–1007. [Google Scholar] [CrossRef]
- Cosnier, S.; Stoytcheva, M.; Senillou, A.; Perrot, H.; Furriel, R.P.M.; Leone, F.A. A biotinylated conducting polypyrrole for the spatially controlled construction of an amperometric biosensor. Anal. Chem. 1999, 71, 3692–3697. [Google Scholar] [CrossRef] [PubMed]
- Ouerghi, C.; Touhami, A.; Jaffrezic-Renault, N.; Martelet, C.; Ben Ouada, H.; Cosnier, S. Electrodeposited biotinylated polypyrrole as an immobilization method for impedimetric immunosensors. IEEE Sens. J. 2004, 4, 559–567. [Google Scholar] [CrossRef]
- Cooper, J.C.; Hall, E.H. Catalytic Reduction of Benzoquinone at Polyaniline and Polyaniline Enzyme Films. Electroanalysis 1993, 5, 385–397. [Google Scholar] [CrossRef]
- Raitman, O.E.; Buckmann, A.F.; Willner, I. Integration of polyaniline/poly(acrylic acid) films and redox enzymes on electrode supports: An in situ electrochemical/surface plasmon resonance study of the bioelectrocatalyzed oxidation of glucose or lactate in the integrated bioelectrocatalytic systems. J. Am. Chem. Soc. 2002, 124, 6487. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.G.; Shen, Z.Q.; Li, C.M. Improved selectivity and stability of glucose biosensor based on in situ electropolymerized polyaniline-polyacrylonitrile composite film. Biosens. Bioelectron. 2005, 20, 2330–2334. [Google Scholar] [CrossRef] [PubMed]
- Raitman, O.A.; Patolsky, F.; Katz, E.; Willner, I. Electrical contacting of glucose dehydrogenase by the reconstitution of a pyrroloquinoline quinone-functionalized polyaniline film associated with an Au-electrode: an in situ electrochemical SPR study. Chem. Commun. 2002, 8, 1936–1937. [Google Scholar] [CrossRef]
- Shi, L.X.; Xiao, Y.; Willner, I. Electrical contacting of glucose oxidase by DNA-templated polyaniline wires on surfaces. Electrochem. Commun. 2004, 6, 1057–1060. [Google Scholar] [CrossRef]
- Gregg, B.A.; Heller, A. Redox polymer-films containing enzymes .2. glucose-oxidase containing enzyme electrodes. J. Phys. Chem. 1991, 95, 5976–5980. [Google Scholar] [CrossRef]
- Gregg, B.A.; Heller, A. Redox polymer-films containing enzymes .1. A redox-conducting epoxy cement—synthesis, characterization, and electrocatalytic oxidation of hydroquinone. J. Phys. Chem. 1991, 95, 5970–5975. [Google Scholar] [CrossRef]
- Barton, S.C.; Kim, H.H.; Binyamin, G.; Zhang, Y.C.; Heller, A. The "wired" laccase cathode: High current density electroreduction of O-2 to water at+0.7 V (NHE) at pH 5. J. Am. Chem. Soc. 2001, 123, 5802–5803. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.C.; Kim, H.H.; Binyamin, G.; Zhang, Y.C.; Heller, A. Electroreduction of O-2 to water on the "Wired" laccase cathode. J. Phys. Chem. B 2001, 105, 11917–11921. [Google Scholar] [CrossRef]
- Kang, C.; Shin, H.; Heller, A. On the stability of the "wired" bilirubin oxidase oxygen cathode in serum. Bioelectrochemistry 2006, 68, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Soukharev, V.; Mano, N.; Heller, A. A four-electron O-2-electroreduction biocatalyst superior to platinum and a biofuel cell operating at 0.88 V. J. Am. Chem. Soc. 2004, 126, 8368–8369. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Mao, F.; Heller, A. A miniature membrane-less biofuel cell operating at +0.60 V under physiological conditions. Chembiochemistry 2004, 5, 1703–1705. [Google Scholar] [CrossRef]
- Kim, H.H.; Zhang, Y.C.; Heller, A. Bilirubin oxidase label for an enzyme-linked affinity assay with O-2 as substrate in a neutral pH NaCl solution. Anal. Chem. 2004, 76, 2411–2414. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Mao, F.; Shin, W.; Chen, T.; Heller, A. A miniature biofuel cell operating at 0.78 V. Chem. Commun. 2003, 7, 518–519. [Google Scholar] [CrossRef]
- Mano, N.; Fernandez, J.L.; Kim, Y.; Shin, W.; Bard, A.J.; Heller, A. Oxygen is electroreduced to water on a "wired" enzyme electrode at a lesser overpotential than on platinum. J. Am. Chem. Soc. 2003, 125, 15290–15291. [Google Scholar] [CrossRef] [PubMed]
- Mano, N.; Kim, H.H.; Zhang, Y.C.; Heller, A. An oxygen cathode operating in a physiological solution. J. Am. Chem. Soc. 2002, 124, 6480–6486. [Google Scholar] [CrossRef] [PubMed]
- Barton, S.C.; Pickard, M.; Vazquez-Duhalt, R.; Heller, A. Electroreduction of O-2 to water at 0.6 V (SHE) at pH 7 on the 'wired' Pleurotus ostreatus laccase cathode. Biosens. Bioelectron. 2002, 17, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Pishko, M.V.; Michael, A.C.; Heller, A. Amperometric glucose microelectrodes prepared through immobilisation of glucose-oxidase in redox hydrogels. Anal. Chem. 1991, 63, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Barton, S.C.; Binyamin, G.; Gao, Z.Q.; Zhang, Y.C.; Kim, H.H.; Heller, A. A miniature biofuel cell. J. Am. Chem. Soc. 2001, 123, 8630–8631. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, N.; Williams, D.F. Preparation of non-thrombogenic materials using 2-methacryloyloxyethyl phosphorylcholine. Biomaterials 2003, 24, 2431–2435. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.; Himuro, Y.; Takai, M.; Ishihara, K. Feasibility study of introducing redox property by modification of PMBN polymer for biofuel cell applications. Appl. Biochem. Biotech. 2009. [Google Scholar] [CrossRef]
- Himuro, Y.; Takai, M.; Ishihara, K. Poly(vinylferrocene-co-2-hydroxyethyl methacrylate) mediator as immobilized enzyme membrane for the fabrication of amperometric glucose sensor. Sens. Actuators B: Chem. 2009, 136, 122–127. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I.; Kotlyar, A.B. A non-compartmentalized glucose/O2 biofuel cell by bioengineered electrode surfaces. J. Electroanal. Chem. 1999, 479, 64–68. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. A biofuel cell with electrochemically switchable and tunable power output. J. Am. Chem. Soc. 2003, 125, 6803–6813. [Google Scholar] [CrossRef] [PubMed]
- Amir, L.; Tam, T.K.; Pita, M.; Meijler, M.M.; Alfonta, L.; Katz, E. Biofuel cell controlled by enzyme logic systems. J. Am. Chem. Soc. 2009, 131, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Togo, M.; Takamura, A.; Asai, T.; Kaji, H.; Nishizawa, M. An enzyme-based microfluidic biofuel cell using vitamin K-3-mediated glucose oxidation. Electrochim. Acta 2007, 52, 4669–4674. [Google Scholar] [CrossRef]
- Ramanavicius, A.; Kausaite, A.; Ramanaviciene, A. Enzymatic biofuel cell based on anode and cathode powered by ethanol. Biosens. Bioelectron. 2008, 24, 761–766. [Google Scholar] [CrossRef]
- Arechederra, R.L.; Minteer, S.D. Complete oxidation of glycerol in an enzymatic biofuel cell. Fuel Cells 2009, 9, 63–69. [Google Scholar] [CrossRef]
- Sakai, H.; Nakagawa, T.; Tokita, Y.; Hatazawa, T.; Ikeda, T.; Tsujimura, S.; Kano, K. A high-power glucose/oxygen biofuel cell operating under quiescent conditions. Energy Environ. Sci. 2009, 2, 133–138. [Google Scholar] [CrossRef]
- Wang, S.C.; Yang, F.; Silva, M.; Zarow, A.; Wang, Y.B.; Iqbal, Z. Membrane-less and mediator-free enzymatic biofuel cell using carbon nanotube/porous silicon electrodes. Electrochem. Commun. 2009, 11, 34–37. [Google Scholar] [CrossRef]
- Wong, T.S.; Schwaneberg, U. Protein engineering in bioelectrocatalysis. Curr. Opin. Biotechnol. 2003, 14, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Yu, E.H.; Ostafe, R.; Schwaneberg, U. Electrochemical oxidation of glucose using mutant glucose oxidase from directed protein evolution. In Proceedings of the 214th ECS Meeting., Honolulu, HI, USA, October, 2008.
- Kandimalla, V.B.; Tripathi, V.S.; Ju, H.X. Immobilization of biomolecules in sol-gels: Biological and analytical applications. Cri. Rev. Anal. Chem. 2006, 36, 73–106. [Google Scholar] [CrossRef]
- Pierre, A.C. The sol-gel encapsulation of enzymes. Biocatal. Biotrans. 2004, 22, 145–170. [Google Scholar] [CrossRef]
- Xiang, C.L.; Zou, Y.J.; Sun, L.X.; Xu, F. Direct electron transfer of cytochrome c and its biosensor based on gold nanoparticles/room temperature ionic liquid/carbon nanotubes composite film. Electrochem. Commun. 2008, 10, 38–41. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of ionic liquids in electrochemical sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Pauliukaite, R.; Doherty, A.P.; Murnaghan, K.D.; Brett, C.M.A. Characterisation and application of carbon film electrodes in room temperature ionic liquid media. J. Electroanal. Chem. 2008, 616, 14–26. [Google Scholar] [CrossRef]
- Goto, Y.; Matsuno, R.; Konno, T.; Takai, M.; Ishihara, K. Polymer nanoparticles covered with phosphorylcholine groups and immobilized with antibody for high-affinity separation of proteins. Biomacromolecules 2008, 9, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.M.; Deng, W.F.; Ge, B.; Xie, Q.J.; Huang, J.H.; Yao, S.Z. Biofuel cell and phenolic biosensor based on acid-resistant laccase-glutaraldehyde functionalized chitosan-multiwalled carbon nanotubes nanocomposite film. Biosens. Bioelectron. 2009, 24, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.; Chikkaveeraiah, B.V.; Patel, V.; Gutkind, J.S.; Rusling, J.F. Ultrasensitive immunosensor for cancer biomarker proteins using gold nanoparticle film electrodes and multienzyme-particle amplification. ACS Nano 2009, 3, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Pingarron, J.M.; Yanez-Sedeno, P.; Gonzalez-Cortes, A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hao Yu, E.; Scott, K. Enzymatic Biofuel Cells—Fabrication of Enzyme Electrodes. Energies 2010, 3, 23-42. https://doi.org/10.3390/en3010023
Hao Yu E, Scott K. Enzymatic Biofuel Cells—Fabrication of Enzyme Electrodes. Energies. 2010; 3(1):23-42. https://doi.org/10.3390/en3010023
Chicago/Turabian StyleHao Yu, Eileen, and Keith Scott. 2010. "Enzymatic Biofuel Cells—Fabrication of Enzyme Electrodes" Energies 3, no. 1: 23-42. https://doi.org/10.3390/en3010023





