Effects of Fuel Type and Operation Parameters on Combustion and NOx Emission of the Iron Ore Sintering Process
Abstract
:1. Introduction
2. Mathematical Model
2.1. Conservation Equations
2.1.1. Equations of the Gas Phase
2.1.2. Equations of the Solid Phase
2.2. Model of Solid Fuel Combustion
2.2.1. Coke Combustion and Gasification
2.2.2. Gaseous Combustion
2.3. Model of NOx Formation
2.3.1. NO Production Originating from Fuel
2.3.2. Thermal NO Formation
2.3.3. NO Reduction by Coke Particles
2.3.4. NO Reduction by CO
2.4. Other Models and Thermo-Physical Properties
2.5. Solution of the Model
2.5.1. Initial and Boundary Conditions
2.5.2. Numerical Methods
3. Results and Discussion
3.1. Model Validation
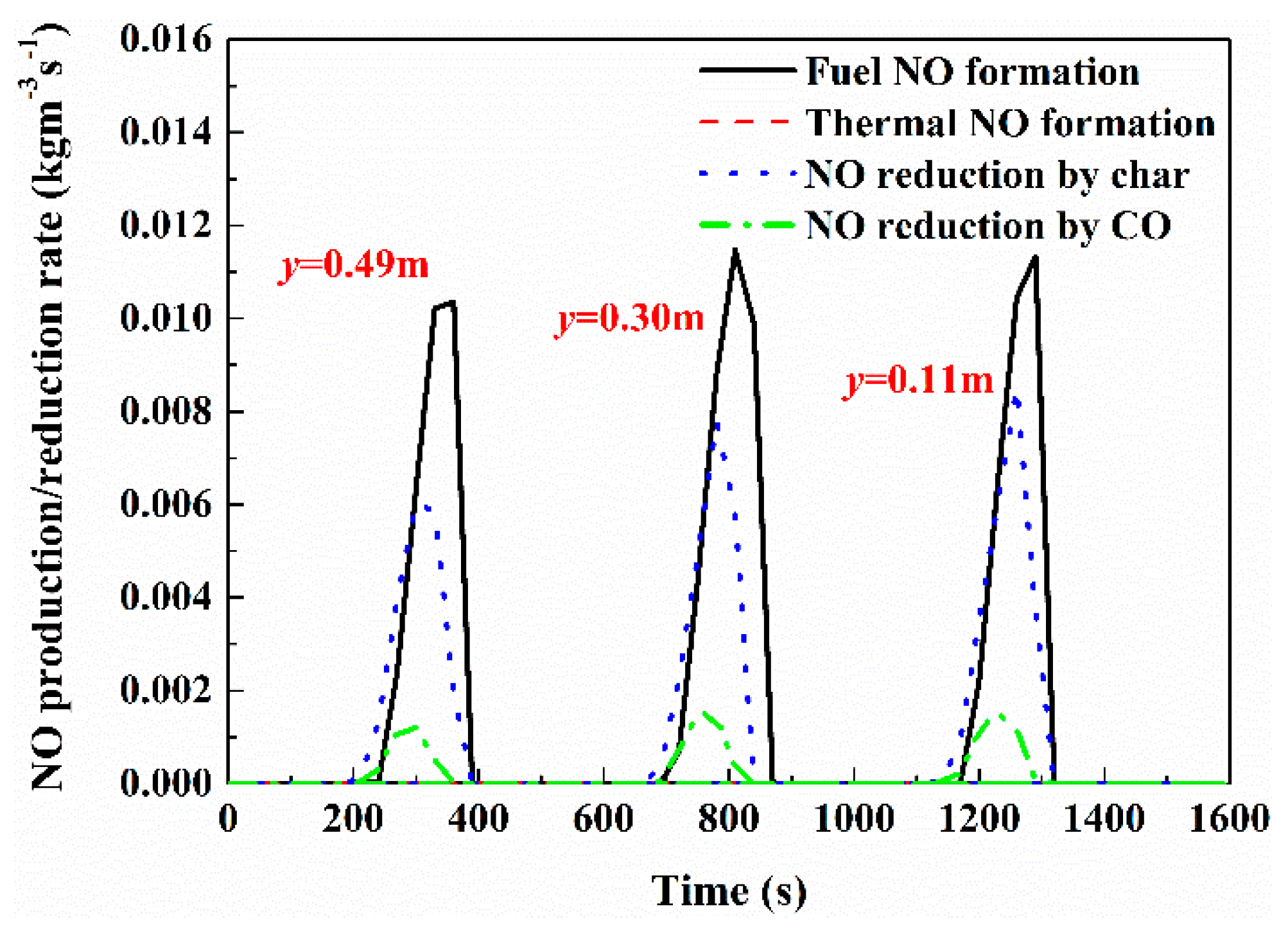
3.2. Quantification of NOx Formation and Reduction for Overall NOx Emission
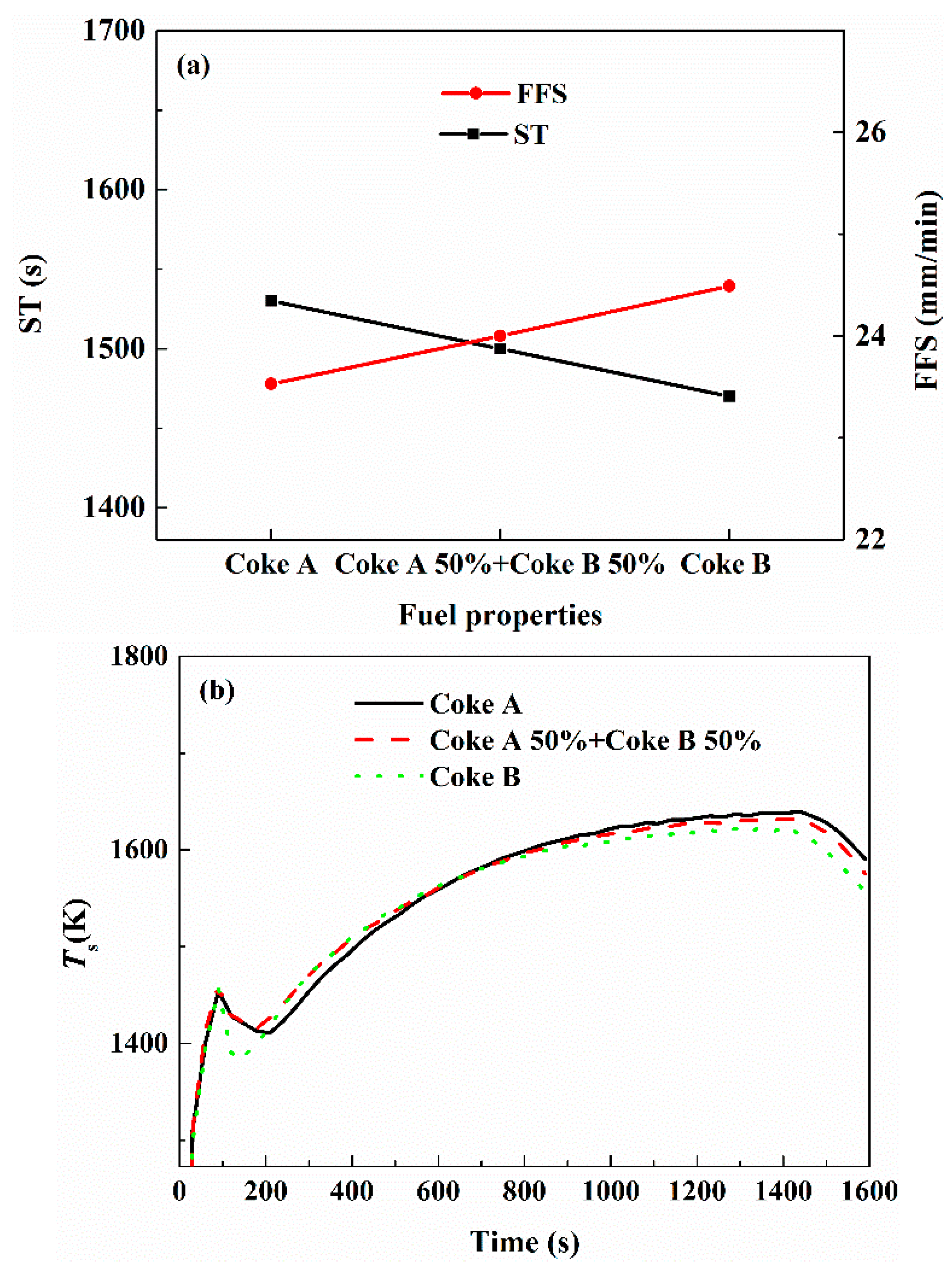
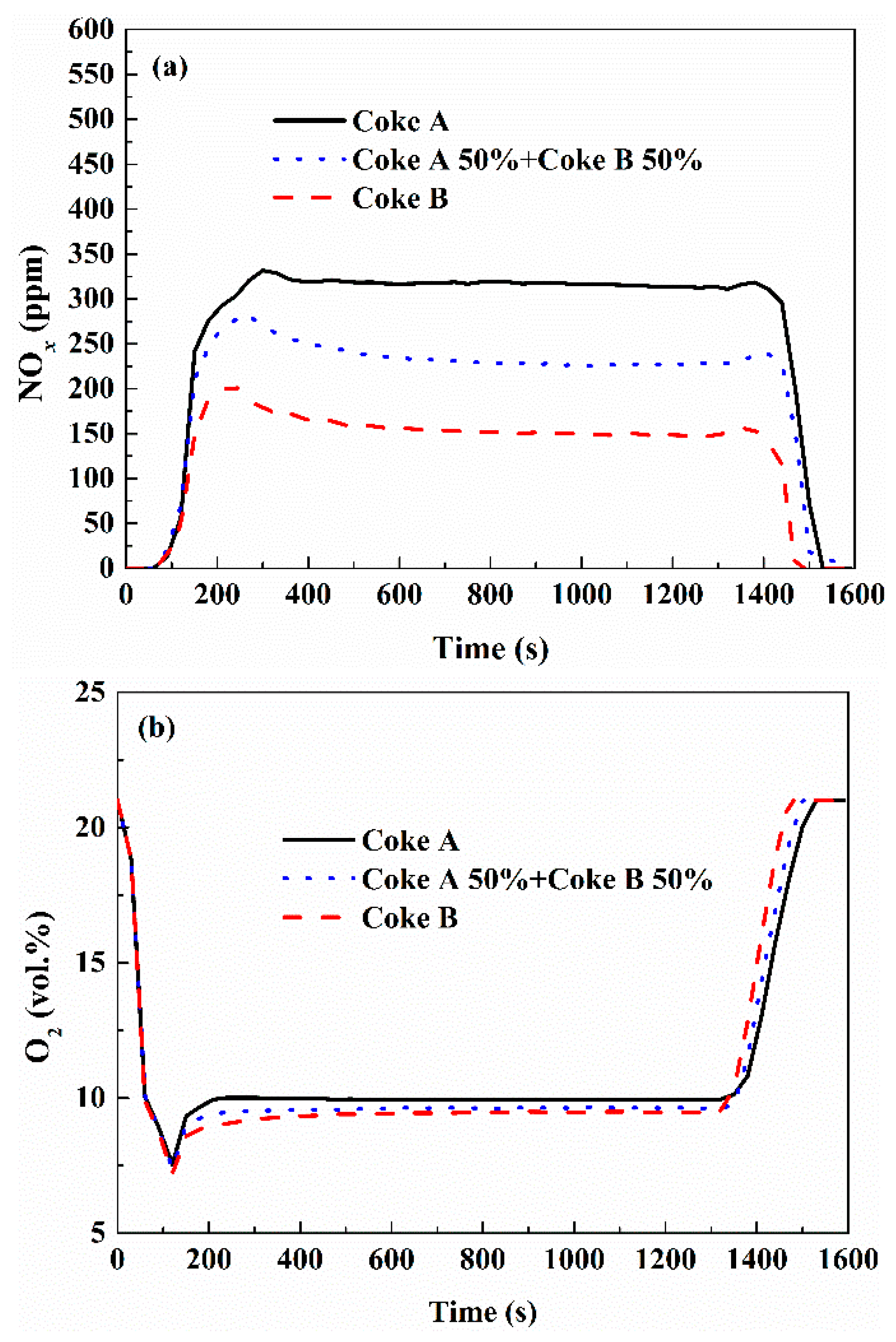
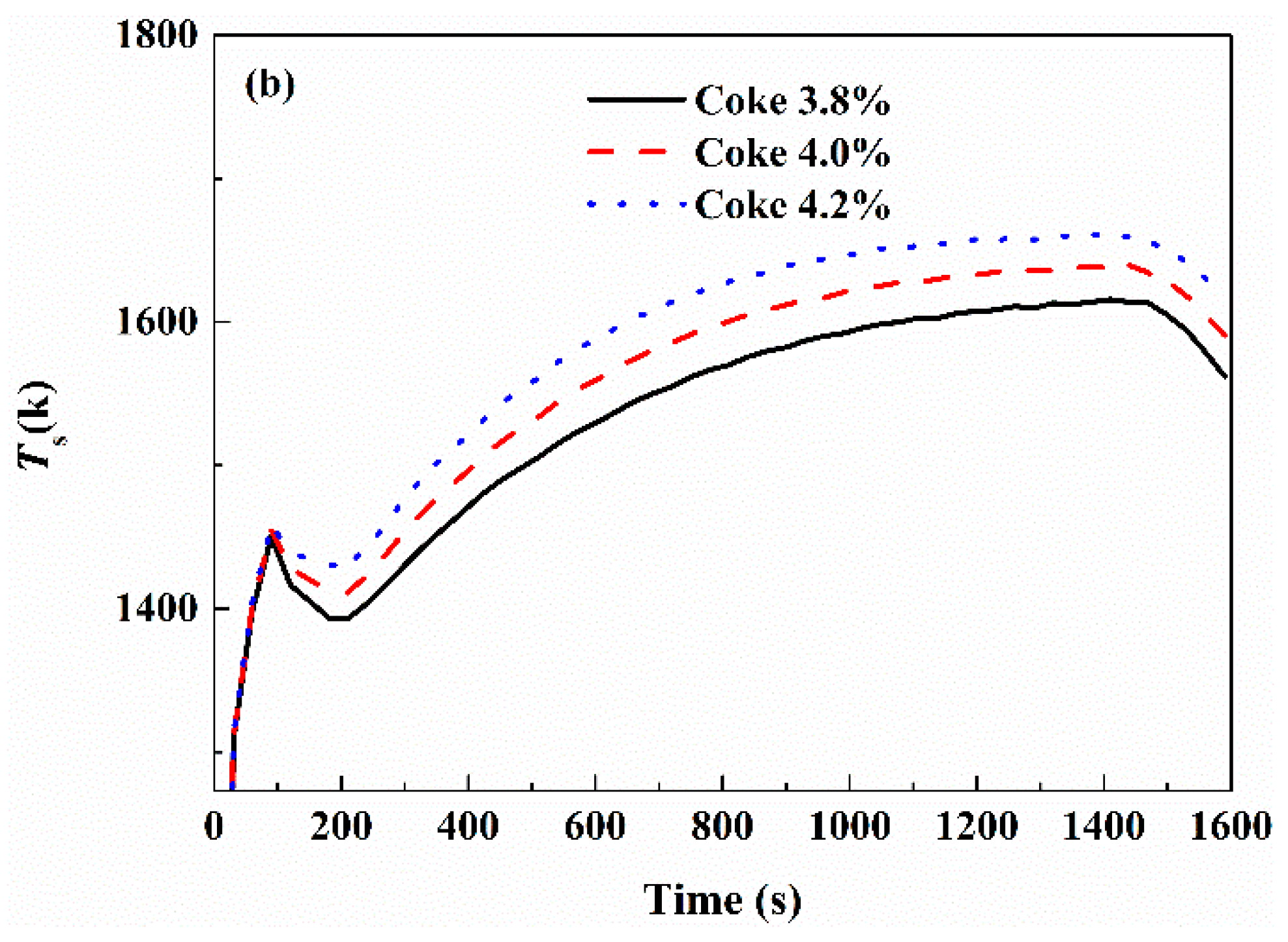
3.3. Effect of Fuel Type
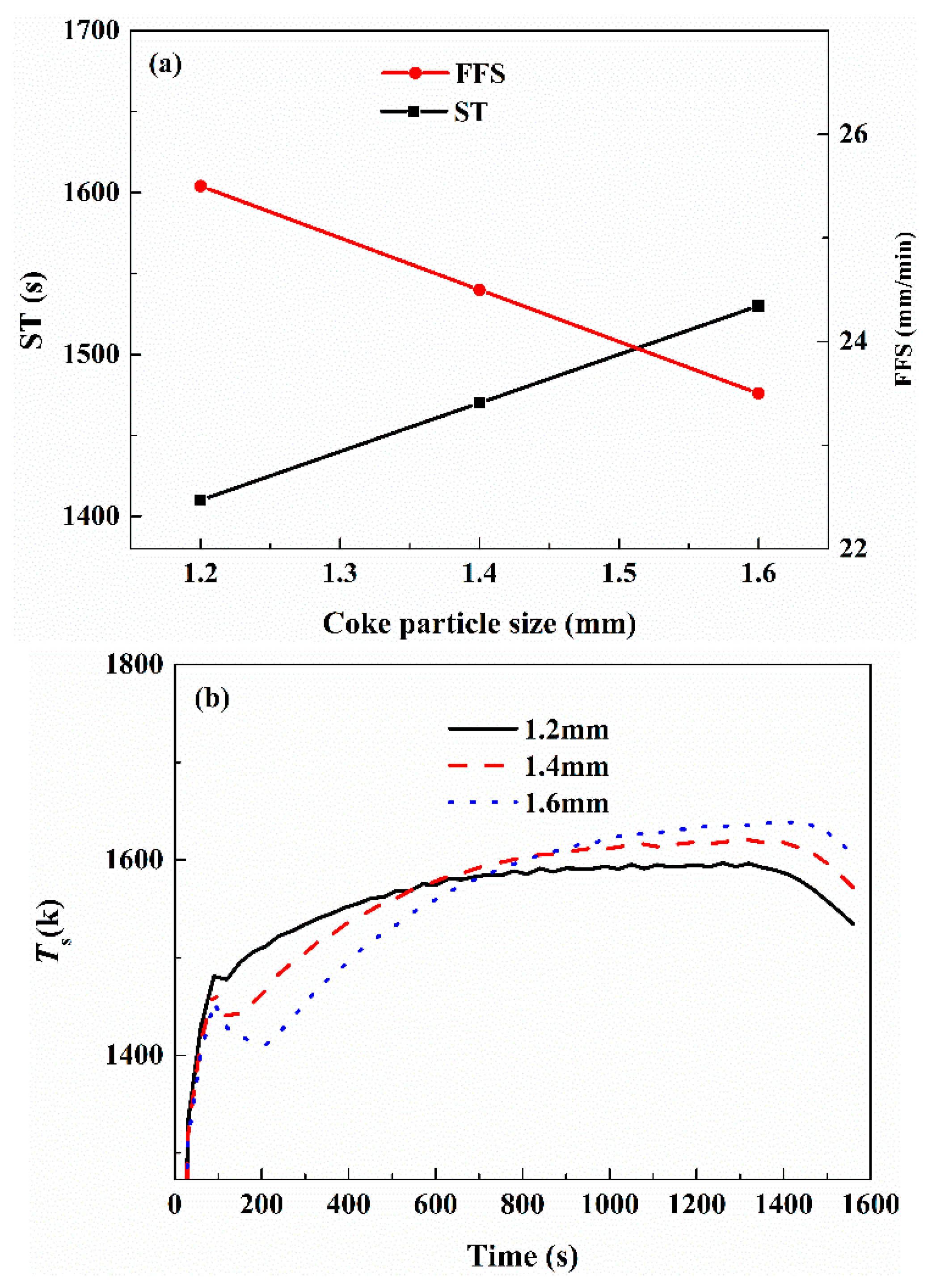
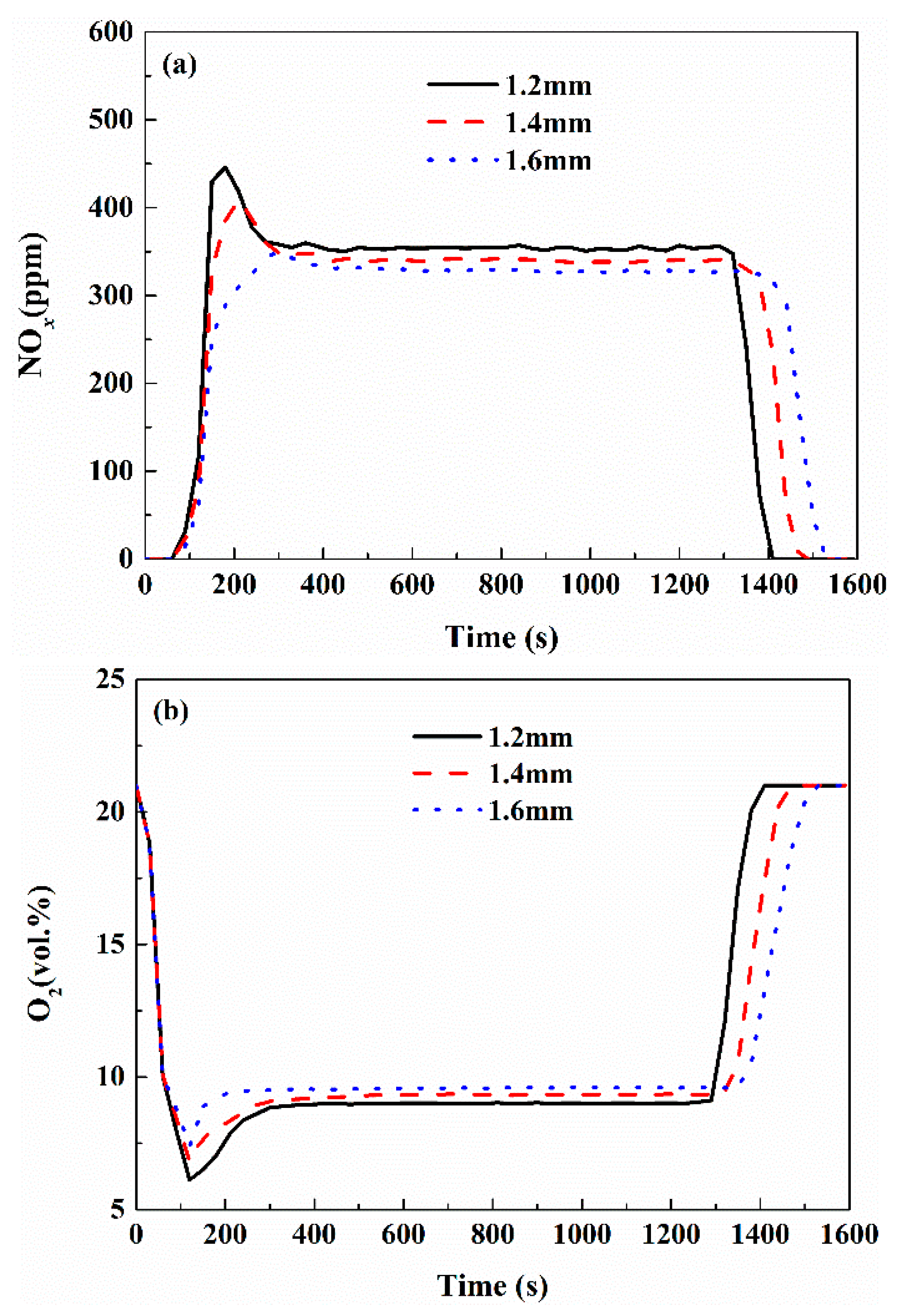
3.4. Effect of Operation Parameters
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Assa | specific surface area, m2/m3 |
| C | inertial resistance coefficient, - |
| Ci | molar concentration, kmol/m3 |
| Cp,g, Cp,s | specific heat capacity of the gas and solid phases, J/(kg·K) |
| Di,m, Dk | effective diffusion coefficient of the ith gaseous species and diffusion coefficient of the kth gaseous species in the bulk gas, m2/s |
| dp | hydraulic diameter of particles in a material layer, m |
| Hs | carrying enthalpy of gas produced by gas–solid reaction, J/(m3·s) |
| hg-w | heat convection coefficients for the gas–wall interface, W/(m2·K) |
| hs-w | heat convection coefficients for the solid–wall interface, W/(m2·K) |
| hair | heat convection coefficients for the outside pot wall, W/(m2·K) |
| K | permeability, - |
| Ks,eff | effective thermal conductivity of solid phase, W/(m·K) |
| kg,eff | effective thermal conductivity of gas phase, W/(m·K) |
| Mi,g, Mi,s | mass of i from the homogeneous gas reactions and the gas–solid heterogeneous reaction, kg/kmol |
| P | phase pressure, Pa |
| Qs,g | heat of gas–solid heterogeneous reaction into the gas phase, J/(m3·s) |
| Qs,s | heat of gas–solid heterogeneous reaction into the solid phase, J/(m3·s) |
| Qs,ms | melting and solidification heat, J/(m3·s) |
| Qg | heat of homogeneous combustion reactions in gas phase, J/(m3·s) |
| Qconv | heat of gas–solid convection, J/(m3·s) |
| R | gas constant, J/(mol·K) |
| Ri | reaction rate of the ith species, kmol/(m3·s) |
| Ss | mass transfer between gas and solid as a result of heterogeneous gas–solid reaction, kg/(m3∙s) |
| Sg | mass transfer of homogeneous gas reaction, kg/(m3·s) |
| Si | source term of momentum equation, kg/(m2·s2) |
| Ts, Tg | solid and gas temperature, K |
| Tair | environment temperature around the sintering pot, K |
| Ui,g | superficial velocity, m/s |
| xj | spatial coordinates, m |
| Yi,g, Yi,s | mass fraction of the ith gaseous species and mass fraction of the ith solid species, - |
| ε | sintering bed porosity, - |
| μg | dynamic coefficient of viscosity, Pa·s |
| ρg, ρs | gas and solid densities, kg/m3 |
| ρb | sintering bed density, kg/m3 |
| δw | thickness of the sintering pot wall, m |
| λw | thermal conductivity of the sintering pot wall, W/(m2·K) |
References
- Kawaguchi, T.; Hara, M. Utilization of Biomass for Iron Ore Sintering. ISIJ Int. 2013, 53, 1599–1606. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Wang, J.; Wei, S.; Guo, Z.; Yang, J.; Wang, Q. Optimization of gaseous fuel injection for saving energy consumption and improving imbalance of heat distribution in iron ore sintering. Appl. Energy 2017, 207, 230–242. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, X.; Gan, M.; Chen, X.; Lv, W. NOx Reduction in the Iron Ore Sintering Process with Flue Gas Recirculation. JOM 2017, 69, 1570–1574. [Google Scholar] [CrossRef]
- Yanguang, C.; Zhancheng, G.; Gensheng, F. NOx reduction by coupling combustion with recycling flue gas in iron ore sintering process. Int. J. Miner. Metall. Mater. 2011, 18, 390–396. [Google Scholar]
- Cen, K.F.; Yao, Q. Advanced Combustion; Zhejiang University Press: Hangzhou, China, 2002. [Google Scholar]
- Kasai, E.; Wu, S.; Sugiyama, T.; Inaba, S.; Omori, Y. Combustion rate and NO emission during combustion of coke granules in packed beds. Tetsu Hagane 1992, 78, 1005–1012. [Google Scholar] [CrossRef]
- Zhou, H.; Cheng, M.; Zhou, M.; Liu, Z.; Liu, R.; Cen, K. Influence of sintering parameters of different sintering layers on NOx emission in iron ore sintering process. Appl. Therm. Eng. 2016, 94, 786–798. [Google Scholar] [CrossRef]
- Mo, C.-L.; Teo, C.-S.; Hamilton, I.; Morrison, J. Admixing Hydrocarbons in Raw Mix to Reduce NOx Emission in Iron Ore Sintering Process. ISIJ Int. 1997, 37, 350–357. [Google Scholar] [CrossRef]
- Pan, J. Theoretical and Process Studies of the Abatement of Flue Gas Emissions during Iron Ore Sintering; Central South University of Technology: Changsha, China, 2007. [Google Scholar]
- Zhou, H.; Liu, Z.; Cheng, M.; Zhou, M.; Liu, R. Influence of Coke Combustion on NOx Emission during Iron Ore Sintering. Energy Fuels 2015, 29, 974–984. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, M.; Liu, Z.; Cheng, M.; Chen, J. Modeling NOx emission of coke combustion in iron ore sintering process and its experimental validation. Fuel 2016, 179, 322–331. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, J.P.; Loo, C.E.; Ellis, B.G.; Cen, K.F. Numerical Modeling of the Iron Ore Sintering Process. ISIJ Int. 2012, 52, 1550–1558. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Ryu, C.; Choi, S.; Choi, E.; Lee, D.; Huh, W. Modeling of Combustion and Heat Transfer in an Iron Ore Sintering Bed with Considerations of Multiple Solid Phases. ISIJ Int. 2004, 44, 492–499. [Google Scholar] [CrossRef] [Green Version]
- De Castro, J.A.; Nogami, H.; Yagi, J.-I. Three-dimensional Multiphase Mathematical Modeling of the Blast Furnace Based on the Multifluid Model. ISIJ Int. 2002, 42, 44–52. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, J.; Li, M. Prediction of sinter yield and strength in iron ore sintering process by numerical simulation. Appl. Therm. Eng. 2018, 131, 70–79. [Google Scholar] [CrossRef]
- Young, R.W. Dynamic mathematical model of sintering process. Ironmak. Steelmak. 1977, 6, 321–328. [Google Scholar]
- Pahlevaninezhad, M.; Davazdah Emami, M.; Panjepour, M. The effects of kinetic parameters on combustion characteristics in a sintering bed. Energy 2014, 73, 160–176. [Google Scholar] [CrossRef]
- Hashimoto, N.; Watanabe, H.; Kurose, R.; Shirai, H. Effect of different fuel NO models on the prediction of NO formation/reduction characteristics in a pulverized coal combustion field. Energy 2017, 118, 47–59. [Google Scholar] [CrossRef]
- Oyama, N.; Iwami, Y.; Yamamoto, T.; Machida, S.; Higuchi, T.; Sato, H.; Sato, M.; Takeda, K.; Watanabe, Y.; Shimizu, M.; et al. Development of Secondary-fuel Injection Technology for Energy Reduction in the Iron Ore Sintering Process. ISIJ Int. 2011, 51, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.P.; Zhang, J.L.; Zhang, C.; Wang, Y.Z.; Liu, Z.J.; Wang, G.W.; Ma, F.T.; Zhao, Z.X. Modelling and visual verification of combustion zone transfer in ultra-thick bed sintering process. Ironmak. Steelmak. 2016, 44, 304–310. [Google Scholar] [CrossRef]
- Nath, N.K.; Mitra, K. Mathematical Modeling and Optimization of Two-Layer Sintering Process for Sinter Quality and Fuel Efficiency Using Genetic Algorithm. Mater. Manuf. Process. 2005, 20, 335–349. [Google Scholar] [CrossRef]
- Ohno, K.I.; Noda, K.; Nishioka, K.; Maeda, T.; Shimizu, M. Effect of Coke Combustion Rate Equation on Numerical Simulation of Temperature Distribution in Iron Ore Sintering Process. ISIJ Int. 2013, 53, 1642–1647. [Google Scholar] [CrossRef] [Green Version]
- Muchi, I. Theoretical Analysis on the Operation of Sintering. Tetsu Hagane 1970, 56, 371–381. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, X.-M. Model of iron ore sintering based on melt and mineral formation. J. Iron Steel Res. Int. 2015, 22, 288–296. [Google Scholar] [CrossRef]
- Junichiro, Y. Blast Furnace Phenomena and Modelling; Elsevier Applied Science Publishers LTD: London, UK; New York, NY, USA, 1982. [Google Scholar]
- Lockwood, F.C.; Romo-Millares, C.A. Mathematical modelling of fuel-NO emissions from PF burners. J. Inst. Energy 1992, 65, 144–152. [Google Scholar]
- Nelson, P.F.; Nancarrow, P.C.; Bus, J.; Prokopiuk, A. Fractional conversion of char N to no in an entrained flow reactor. Proc. Combust. Inst. 2002, 29, 2267–2274. [Google Scholar] [CrossRef]
- Song, Y.H.; Beér, J.M.; Sarofim, A.F. Reduction of Nitric Oxide by Coal Char at Temperatures of 1250–1750 K. Combust. Sci. Technol. 1981, 25, 237–240. [Google Scholar] [CrossRef]
- Levy, J.M.; Chan, L.K.; Sarofim, A.F.; Beér, J.M. NO/Char Reactions at Pulverized Coal Flame Conditions. Symp. (Int.) Combust. 1981, 18, 111–120. [Google Scholar] [CrossRef]
- Garijo, E.G.A.; Jensen, A.D.; Glarborg, P. Kinetic study of NO reduction over Biomass char under dynamic conditions. Energy Fuels 2003, 17, 1429–1436. [Google Scholar] [CrossRef]
- Chan, L.K.; Sarofim, A.F.; Beér, J.M. Kinetics of the NO-carbon reaction at fluidized bed combustor conditions. Combust. Flame 1983, 52, 37–45. [Google Scholar] [CrossRef]
- De Soete, G.G.; Croiset, E.; Richard, J.R. Heterogeneous formation of nitrous oxide from char-bound nitrogen. Combust. Flame 1999, 117, 140–154. [Google Scholar] [CrossRef]
- Schönenbeck, C.; Gadiou, R.; Schwartz, D. A kinetic study of the high temperature NO–char reaction. Fuel 2004, 83, 443–450. [Google Scholar] [CrossRef]
- Mitra, N.K.; Mitra, K. Optimisation of suction pressure for iron ore sintering by genetic algorithm. Ironmak. Steelmak. 2004, 31, 199–206. [Google Scholar]
- Wang, G.; Wen, Z.; Lou, G.; Dou, R.; Li, X.; Liu, X.; Su, F. Mathematical modeling and combustion characteristic evaluation of a flue gas recirculation iron ore sintering process. Int. J. Heat Mass Transf. 2016, 97, 964–974. [Google Scholar] [CrossRef]
- Zhao, J.P.; Loo, C.E.; Dukino, R.D. Modelling fuel combustion in iron ore sintering. Combust. Flame 2015, 162, 1019–1034. [Google Scholar] [CrossRef]
- Pahlevaninezhad, M.; Emami, M.D.; Panjepour, M. Identifying major zones of an iron ore sintering bed. Appl. Math. Model. 2016, 40, 8475–8492. [Google Scholar] [CrossRef]
- Ramos, M.V.; Kasai, E.; Kano, J.; Nakamura, T. Numerical Simulation Model of the Iron Ore Sintering Process Directly Describing the Agglomeration Phenomenon of Granules in the Packed Bed. ISIJ Int. 2000, 40, 448–454. [Google Scholar] [CrossRef]
- Zhao, J.; Loo, C.E.; Zhou, H.; Yuan, J.; Li, X.; Zhu, Y.; Yang, G. Modelling and analysis of the combustion behaviour of granulated fuel particles in iron ore sintering. Combust. Flame 2018, 189, 257–274. [Google Scholar] [CrossRef]
- Wang, G.; Wen, Z.; Lou, G.; Dou, R.; Li, X.; Liu, X.; Su, F. Mathematical modeling of and parametric studies on flue gas recirculation iron ore sintering. Appl. Therm. Eng. 2016, 102, 648–660. [Google Scholar] [CrossRef]
- Yang, W.; Choi, S.; Choi, E.S.; Ri, D.W.; Kim, S. Combustion characteristics in an iron ore sintering bed-evaluation of fuel substitution. Combust. Flame 2006, 145, 447–463. [Google Scholar] [CrossRef]
- Morioka, K.; Inaba, S.; Shimizu, M.; Ano, K.; Sugiyama, T. Primary Application of the “In-Bed-deNOx” Process Using Ca-Fe Oxides in Iron Ore Sintering Machines. ISIJ Int. 2000, 40, 280–285. [Google Scholar] [CrossRef]







| Physio-Chemical Phenomena | Source of Mathematical Model |
|---|---|
| Convection heat transfer | Zhou Hao [12] |
| Conduction and radiation | Zhou Hao [12] |
| Melting and solidification | Young [16] |
| Geometric changes | Wang Gan [35] |
| Limestone calcination | Zhao Jiapei [36] |
| Reduction and oxidation of iron oxides | Pahlevaninezhad [37] |
| Water evaporation | Maximiano [38] |
| Parameters | Gas Phase | Solid Phase |
|---|---|---|
| Heat capacities | ||
| Density | ||
| Diffusion coefficient | ||
| Viscosity | ||
| Thermal conductivity |
| Parameters | Value | Parameters | Value |
|---|---|---|---|
| Ignition time (s) | 90 | Iron ore (%) | 83.2 |
| Gas inlet velocity during ignition (m/s) | 4 | Limestone (%) | 13.0 |
| Gas inlet velocity after ignition (m/s) | 0.43 | Coke (%) | 3.8 |
| Negative pressure during ignition (Pa) | −10,000 | Moisture (%) | 7.0 |
| Negative pressure after ignition (Pa) | −15,000 | Coke diameters (m) | 0.0016 |
| Initial temperature of solid (K) | 300 | Dolomite diameters (m) | 0.0016 |
| Initial temperature of gas (K) | 300 | Limestone diameters (m) | 0.0016 |
| Ignition temperature (K) | 1400 | Iron ore diameters (m) | 0.0032 |
| Initial porosity of the bed | 0.4 | Average diameter of particles (m) | 0.0032 |
| Case | Coke A (%mass) | Coke B (%mass) | Grain Diameter (m) | Oxygen Concentration (vol %) |
|---|---|---|---|---|
| Base case | 3.8 | 0 | 0.0016 | 21.0 |
| Case 0 | 1.9 | 1.9 | 0.0016 | 21.0 |
| Case 1 | 0 | 3.8 | 0.0016 | 21.0 |
| Case 2 | 3.8 | 0 | 0.0014 | 21.0 |
| Case 3 | 3.8 | 0 | 0.0012 | 21.0 |
| Case 4 | 4.0 | 0 | 0.0016 | 21.0 |
| Case 5 | 4.2 | 0 | 0.0016 | 21.0 |
| Case 6 | 3.8 | 0 | 0.0016 | 24.0 |
| Case 7 | 3.8 | 0 | 0.0016 | 27.0 |
| Case 8 | 3.8 | 0 | 0.0016 | 30.0 |
| Typical Parameter | MaxT (K) y = 0.49 m | MaxT (K) y = 0.30 m | MaxT (K) y = 0.11 m | FFS (mm/min) |
|---|---|---|---|---|
| Simulated | 1457.58 | 1603.09 | 1632.04 | 23.50 |
| Measured | 1437.44 | 1558.37 | 1628.90 | 23.53 |
| Error (%) | 1.40 | 2.87 | 0.192 | 0.127 |
| Elemental Analysis (%) | Proximate Analysis (%, Dry Base) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Volatile | Fixed Carbon | Ash | Heating Value (kJ/kg) | |
| Coke A | 86.04 | 1.34 | 10.85 | 1.55 | 0.21 | 2.99 | 81.69 | 15.32 | 25,993.72 |
| Coke B | 87.86 | 0.63 | 9.42 | 0.56 | 0.54 | 2.26 | 83.75 | 13.99 | 26,345.75 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, W.; Li, H.; Zhang, Y.; Zou, Z. Effects of Fuel Type and Operation Parameters on Combustion and NOx Emission of the Iron Ore Sintering Process. Energies 2019, 12, 213. https://doi.org/10.3390/en12020213
Ni W, Li H, Zhang Y, Zou Z. Effects of Fuel Type and Operation Parameters on Combustion and NOx Emission of the Iron Ore Sintering Process. Energies. 2019; 12(2):213. https://doi.org/10.3390/en12020213
Chicago/Turabian StyleNi, Wenjie, Haifeng Li, Yingyi Zhang, and Zongshu Zou. 2019. "Effects of Fuel Type and Operation Parameters on Combustion and NOx Emission of the Iron Ore Sintering Process" Energies 12, no. 2: 213. https://doi.org/10.3390/en12020213





