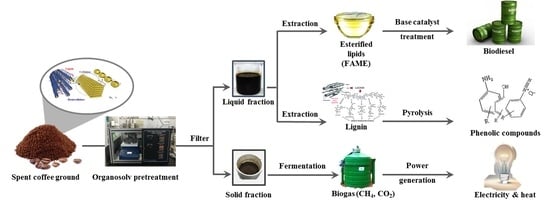
Sequential Production of Lignin, Fatty Acid Methyl Esters and Biogas from Spent Coffee Grounds via an Integrated Physicochemical and Biological Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock
2.2. Organosolv Pretreatment
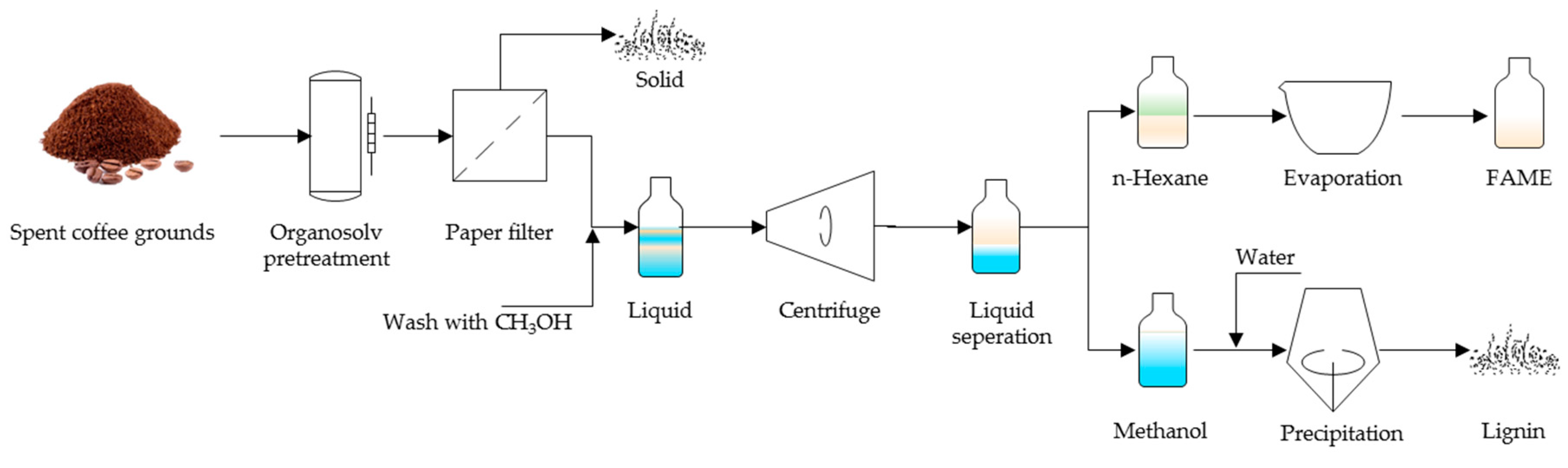
2.3. Recovery of Lignin and Esterified Lipid
2.4. Experimental Design and Statistical Analysis
2.5. Lignin Characterization and FAME Quantification
2.6. Biochemical Methane Potential Test
3. Results and Discussion
3.1. Feedstock Characterization
3.2. Effects of Treatment Conditions and Optimization
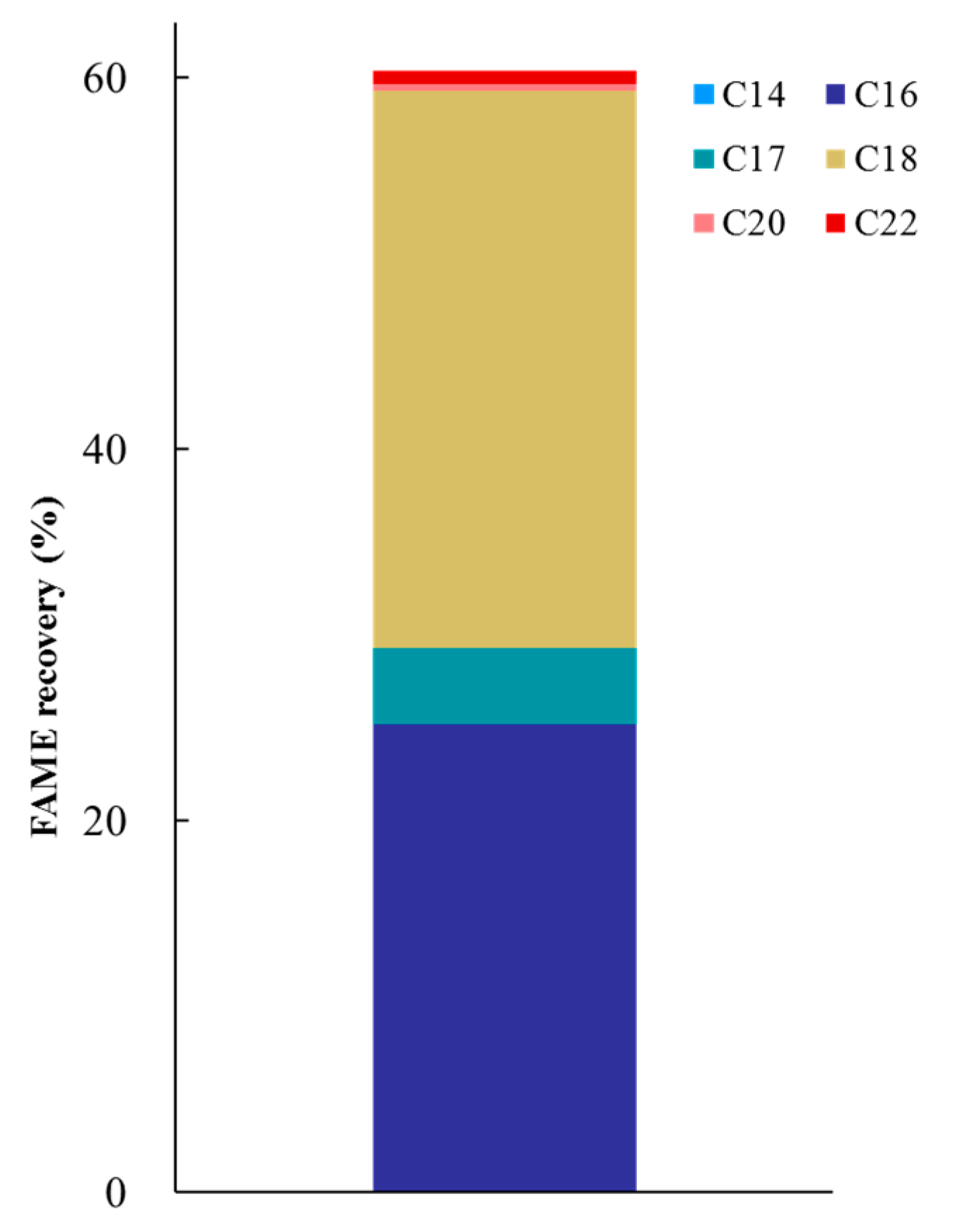
3.3. Structural Characterization of Lignin and FAME
3.4. Digestibility of SCG Residues in a Batch Anaerobic Digester
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Njoroge, J.; Agwanda, C.; Kingori, P.; Karanja, A.; Gathaara, M. Coffee. In Handbook of Industrial Crops; The Haworth Press: New York, NY, USA, 2005; pp. 295–333. [Google Scholar]
- Murthy, P.S.; Naidu, M.M. Recovery of phenolic antioxidants and functional compounds from coffee industry by-products. Food Bioprocess Technol. 2012, 5, 897–903. [Google Scholar] [CrossRef]
- Leifa, F.; Pandey, A.; Soccol, C.R. Solid state cultivation—An efficient method to use toxic agro-industrial residues. J. Basic Microbiol. Int. J. Biochem. Phys. Genet. Morphol. Ecol. Microorg. 2000, 40, 187–197. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Gominho, J.; Olivella, M.; Fiol, N.; Villaescusa, I.; Pereira, H. The chemical composition of exhausted coffee waste. Ind. Crop. Prod. 2013, 50, 423–429. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2013, 61, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Pacioni, T.R.; Soares, D.; Di Domenico, M.; Rosa, M.F.; Moreira, R.d.F.P.M.; José, H.J. Bio-syngas production from agro-industrial biomass residues by steam gasification. Waste Manag. 2016, 58, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S. Renewable resource-based green composites of surface-treated spent coffee grounds and polylactide: Characterisation and biodegradability. Polym. Degrad. Stab. 2015, 121, 51–59. [Google Scholar] [CrossRef]
- Rocha, M.V.P.; de Matos, L.J.B.L.; de Lima, L.P.; da Silva Figueiredo, P.M.; Lucena, I.L.; Fernandes, F.A.N.; Gonçalves, L.R.B. Ultrasound-assisted production of biodiesel and ethanol from spent coffee grounds. Bioresour. Technol. 2014, 167, 343–348. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Baek, G.; Lee, C. Anaerobic co-digestion of spent coffee grounds with different waste feedstocks for biogas production. Waste Manag. 2017, 60, 322–328. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Carneiro, L.M.; Silva, J.P.; Roberto, I.C.; Teixeira, J.A. A study on chemical constituents and sugars extraction from spent coffee grounds. Carbohydr. Polym. 2011, 83, 368–374. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Ballesteros, L.F.; Martins, S.; Teixeira, J.A. Extraction of antioxidant phenolic compounds from spent coffee grounds. Sep. Purif. Technol. 2011, 83, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Cruz, R.; Cardoso, M.M.; Fernandes, L.; Oliveira, M.; Mendes, E.; Baptista, P.; Morais, S.; Casal, S. Espresso coffee residues: A valuable source of unextracted compounds. J. Agric. Food Chem. 2012, 60, 7777–7784. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, J.; Lee, C. Effect of mild-temperature thermo-alkaline pretreatment on the solubilization and anaerobic digestion of spent coffee grounds. Energies 2018, 11, 865. [Google Scholar] [CrossRef]
- Mousavioun, P.; Doherty, W.O. Chemical and thermal properties of fractionated bagasse soda lignin. Ind. Crops Prod. 2010, 31, 52–58. [Google Scholar] [CrossRef] [Green Version]
- Reddy, N.; Yang, Y. Biofibers from agricultural byproducts for industrial applications. Trends Biotechnol. 2005, 23, 22–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norgren, M.; Edlund, H. Lignin: Recent advances and emerging applications. Curr. Opin. Colloid Interface Sci. 2014, 19, 409–416. [Google Scholar] [CrossRef]
- Kim, S.; Holtzapple, M.T. Lime pretreatment and enzymatic hydrolysis of corn stover. Bioresour. Technol. 2005, 96, 1994–2006. [Google Scholar] [CrossRef] [Green Version]
- Carrapiso, A.I.; Timón, L.M.; Petrón, J.M.; Tejeda, J.F.; García, C. In situ transesterification of fatty acids from Iberian pig subcutaneous adipose tissue. Meat Sci. 2000, 56, 159–164. [Google Scholar] [CrossRef]
- Lee, H.; Shin, J.; Park, Y.; Kim, Y.M. Lignin isolation during recycling of waste wood in an urban locale. Desalin. Water Treat. 2018, 120, 234–240. [Google Scholar] [CrossRef]
- Bolonio, D.; García-Martínez, M.J.; Ortega, M.F.; Lapuerta, M.; Rodríguez-Fernández, J.; Canoira, L. Fatty acid ethyl esters (FAEEs) obtained from grapeseed oil: A fully renewable biofuel. Renew. Energy 2019, 132, 278–283. [Google Scholar] [CrossRef]
- Vardon, D.R.; Moser, B.R.; Zheng, W.; Witkin, K.; Evangelista, R.L.; Strathmann, T.J.; Rajagopalan, K.; Sharma, B.K. Complete utilization of spent coffee grounds to produce biodiesel, bio-oil, and biochar. ACS Sustain. Chem. Eng. 2013, 1, 1286–1294. [Google Scholar] [CrossRef]
- Jang, H.M.; Shin, J.; Choi, S.; Shin, S.G.; Park, K.Y.; Cho, J.; Kim, Y.M. Fate of antibiotic resistance genes in mesophilic and thermophilic anaerobic digestion of chemically enhanced primary treatment (CEPT) sludge. Bioresour. Technol. 2017, 244, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, K.; Jang, H.M.; Shin, J.; Park, K.Y.; Cho, J.; Kim, Y.M. Biomethanation and anaerobic co-digestion via microbial communities of microalgal Hydrodictyon reticulatum biomass residues with sewage sludge. Desalin. Water Treat. 2017, 77, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Caetano, N.S.; Silva, V.F.M.; Melo, A.C.; Martins, A.A.; Mata, T.M. Spent coffee grounds for biodiesel production and other applications. Clean Technol. Environ. Policy 2014, 16, 1423–1430. [Google Scholar] [CrossRef] [Green Version]
- Obruca, S.; Benesova, P.; Kucera, D.; Petrik, S.; Marova, I. Biotechnological conversion of spent coffee grounds into polyhydroxyalkanoates and carotenoids. New Biotechnol. 2015, 32, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.Z.; Vinjamur, M. Parametric effects on kinetics of esterification for biodiesel production: A Taguchi approach. Chem. Eng. Sci. 2014, 110, 94–104. [Google Scholar] [CrossRef]
- Liu, Y.; Tu, Q.; Knothe, G.; Lu, M. Direct transesterification of spent coffee grounds for biodiesel production. Fuel 2017, 199, 157–161. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Russo-Profili, A.; Eveleigh, A.; Aliev, A.; Kay, A.; Mills-Lamptey, B. Influence of solvent selection and extraction temperature on yield and composition of lipids extracted from spent coffee grounds. Ind. Crops Prod. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- Johansson, A.; Aaltonen, O.; Ylinen, P. Organosolv pulping-methods and pulp properties. Biomass 1987, 13, 45–65. [Google Scholar] [CrossRef]
- Delgado, N.; Ysambertt, F.; Chávez, G.; Bravo, B.; García, D.E.; Santos, J. Valorization of Kraft Lignin of Different Molecular Weights as Surfactant Agent for the Oil Industry. Waste Biomass Valoriz. 2018, 1–13. [Google Scholar] [CrossRef]
- Hage, R.E.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- Tejado, A.; Peña, C.; Labidi, J.; Echeverria, J.M.; Mondragon, I. Physico-chemical characterization of lignins from different sources for use in phenol-formaldehyde resin synthesis. Bioresour. Technol. 2007, 98, 1655–1663. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, R.; Moorthy, I.G.; Kumar, R.V.; Sivasubramanian, V. Microwave mediated production of FAME from waste cooking oil: Modelling and optimization of process parameters by RSM and ANN approach. Fuel 2019, 237, 40–49. [Google Scholar] [CrossRef]
- Özgül-Yücel, S.; Proctor, A. Rice Bran FFA Determination by Diffuse Reflectance IR Spectroscopy. JAOCS J. Am. Oil Chem. Soc. 2004, 81, 221–224. [Google Scholar] [CrossRef]
- Park, J.; Kim, B.; Lee, J.W. In-situ transesterification of wet spent coffee grounds for sustainable biodiesel production. Bioresour. Technol. 2016, 221, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.U.; Kim, Y.M.; Choi, Y.N.; Kim, H.G.; Park, J.M. Influence of temperature on volatile fatty acid production and microbial community structure during anaerobic fermentation of microalgae. Bioresour. Technol. 2015, 191, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamamre, Z.; Foerster, S.; Hartmann, F.; Kröger, M.; Kaltschmitt, M. Oil extracted from spent coffee grounds as a renewable source for fatty acid methyl ester manufacturing. Fuel 2012, 96, 70–76. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Vergara-Castañeda, H.A.; Oomah, B.D. Spent coffee grounds: A review on current research and future prospects. Trends Food Sci. Technol. 2015, 45, 24–36. [Google Scholar] [CrossRef]
- Atabani, A.E.; Mercimek, S.M.; Arvindnarayan, S.; Shobana, S.; Kumar, G.; Cadir, M.; Al-Muhatseb, A.H. Valorization of spent coffee grounds recycling as a potential alternative fuel resource in Turkey: An experimental study. J. Air Waste Manag. Assoc. 2018, 68, 196–214. [Google Scholar] [CrossRef] [Green Version]
- Hesami, S.M.; Zilouei, H.; Karimi, K.; Asadinezhad, A. Enhanced biogas production from sunflower stalks using hydrothermal and organosolv pretreatment. Ind. Crop. Prod. 2015, 76, 449–455. [Google Scholar] [CrossRef]
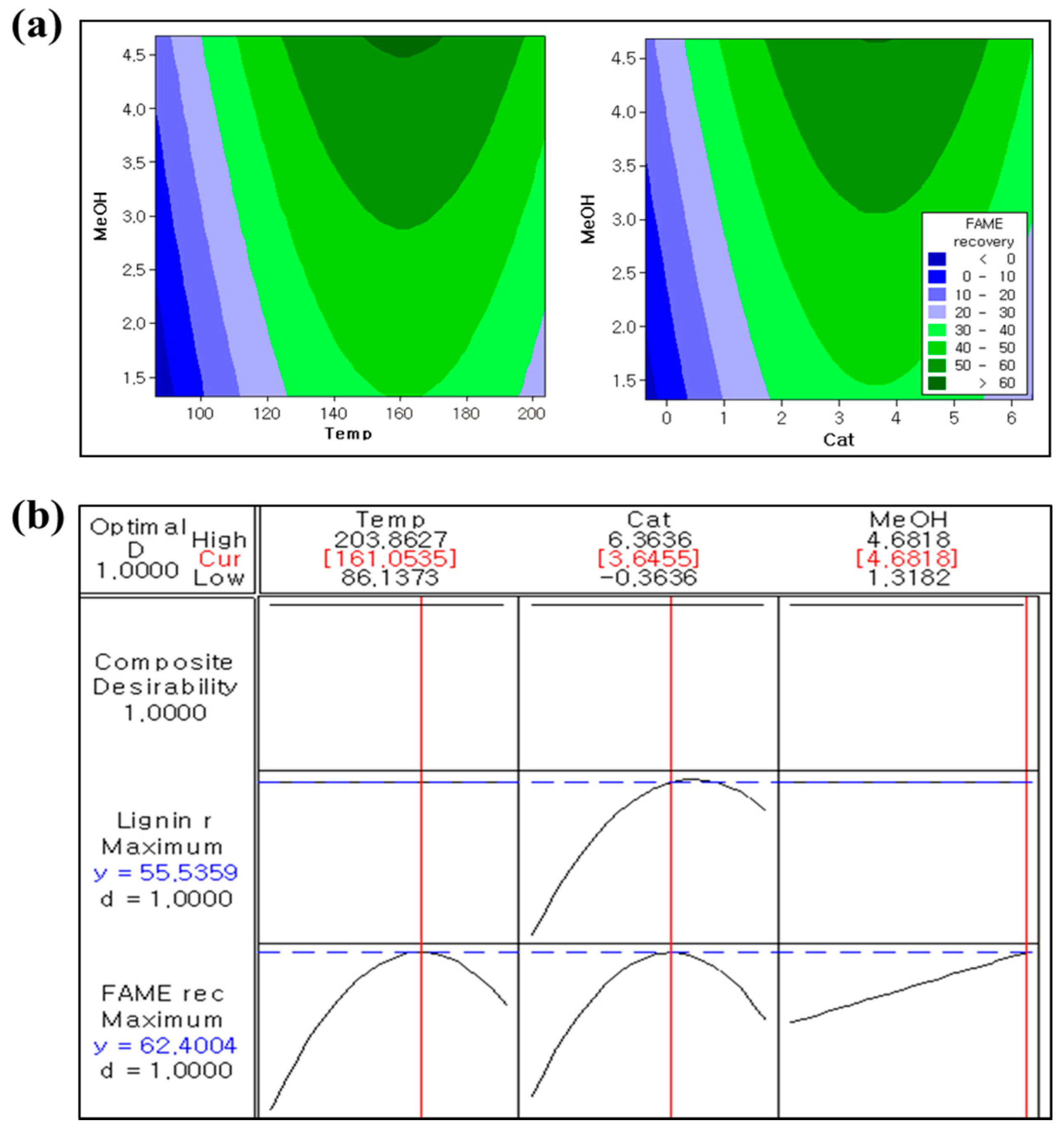

| Level | Independent Variables | |||
|---|---|---|---|---|
| (X1) Reaction Temperature (°C) | (X2) Concentration of Sulfuric Acid (%v/v) | (X3) Ratio of Methanol to Spent Coffee Grounds (SCG) (mL/g) | ||
| +Alpha | +1.68 | 203.9 | 6.4 | 4.7 |
| Max | +1 | 180.0 | 5.0 | 4.0 |
| Central level | 0 | 145.0 | 3.0 | 3.0 |
| Min | −1 | 110.0 | 1.0 | 2.0 |
| −Alpha | −1.68 | 86.1 | 0 | 1.3 |
| Serial Number | Independent Variables | Dependent Variables | ||||||
|---|---|---|---|---|---|---|---|---|
| Reaction Temperature (°C) | Concentration of Sulfuric Acid (%v/v) | Ratio of Methanol to SCG (mL/g) | Recovery of Lignin (%) | Recovery of FAME (%) | ||||
| X1 | X2 | X3 | Y1 | Y2 | ||||
| 1 | 110.0 | −1 | 1.0 | −1 | 2.0 | −1 | 38.18 | 4.76 |
| 2 | 180.0 | 1 | 1.0 | −1 | 2.0 | −1 | 29.01 | 40.46 |
| 3 | 110.0 | −1 | 5.0 | 1 | 2.0 | −1 | 60.47 | 19.66 |
| 4 | 180.0 | 1 | 5.0 | 1 | 2.0 | −1 | 45.51 | 41.27 |
| 5 | 110.0 | −1 | 1.0 | −1 | 4.0 | 1 | 28.71 | 13.52 |
| 6 | 180.0 | 1 | 1.0 | −1 | 4.0 | 1 | 34.21 | 39.34 |
| 7 | 110.0 | −1 | 5.0 | 1 | 4.0 | 1 | 62.30 | 40.46 |
| 8 | 180.0 | 1 | 5.0 | 1 | 4.0 | 1 | 46.42 | 53.40 |
| 9 | 86.1 | −1.68 | 3.0 | 0 | 3.0 | 0 | 38.48 | 4.03 |
| 10 | 203.9 | 1.68 | 3.0 | 0 | 3.0 | 0 | 56.20 | 23.15 |
| 11 | 145.0 | 0 | 0.0 | −1.68 | 3.0 | 0 | 4.89 | 0.60 |
| 12 | 145.0 | 0 | 6.4 | 1.68 | 3.0 | 0 | 50.39 | 23.88 |
| 13 | 145.0 | 0 | 3.0 | 0 | 1.3 | −1.68 | 61.08 | 24.28 |
| 14 | 145.0 | 0 | 3.0 | 0 | 4.7 | 1.68 | 59.25 | 51.04 |
| 15 | 145.0 | 0 | 3.0 | 0 | 3.0 | 0 | 51.31 | 55.46 |
| 16 | 145.0 | 0 | 3.0 | 0 | 3.0 | 0 | 52.22 | 53.89 |
| Regression | Source | Sum of Squares | Degrees of Freedom | Mean of Square | F-Value | Prob > F | Remarks |
|---|---|---|---|---|---|---|---|
| Lignin | Model | 2921.48 | 2 | 1460.74 | 28.64 | <0.001 | Significant |
| X2 | 1901.08 | 1 | 1902.95 | 37.30 | <0.001 | Significant | |
| X22 | 1020.40 | 1 | 1020.40 | 20.00 | 0.001 | Significant | |
| Residual | 663.14 | 13 | 51.01 | ||||
| Lack of fit | 32.74 | 2 | 16.37 | 0.29 | 0.757 | Not significant | |
| Pure Error | 630.40 | 11 | 57.31 | ||||
| R2 | 0.82 | ||||||
| FAME | Model | 4348.03 | 5 | 869.61 | 9.05 | 0.002 | Significant |
| X1 | 1203.93 | 1 | 1203.93 | 12.54 | 0.005 | Significant | |
| X2 | 672.89 | 1 | 672.89 | 7.01 | 0.024 | Significant | |
| X3 | 536.22 | 1 | 536.22 | 5.58 | 0.040 | Significant | |
| X12 | 624.71 | 1 | 1196.90 | 12.46 | 0.005 | Significant | |
| X22 | 1310.29 | 1 | 1310.29 | 13.64 | 0.004 | Significant | |
| Residual | 960.39 | 10 | 96.04 | ||||
| Lack of fit | 959.16 | 9 | 106.57 | 86.47 | 0.083 | Not significant | |
| Pure Error | 1.23 | 1 | 1.23 | ||||
| R2 | 0.82 |
| Peak (cm−1) | Assignment | |
|---|---|---|
| SCG-lignin | SCG-FAME | |
| 3200 | - | Phenolic and aliphatic O–H stretch |
| - | 3008 | C–H stretching alkene |
| 2927 | - | C–H stretch |
| - | 2922 | C–H stretching of CH2 and CH3 |
| - | 2852 | C–H stretching of CH2 and CH3 |
| - | 1741 | C=O stretching of ester |
| 1703 | - | C=O stretch unconjugated |
| 1608 | - | C–C stretch (aromatic skeletal vibration) |
| 1512 | - | C–C stretch (aromatic skeletal vibration), G |
| - | 1460 | C–H deformation of methyl and methylene |
| 1439 | - | C–H deformation (aromatic skeletal vibration) |
| - | 1436 | Aromatic C–H deformation |
| 1366 | - | Phenolic O–H and aliphatic C–H stretch methyl groups |
| - | 1362 | O–CH2 deformation in glycerol moiety |
| - | 1242 | Phenolic O–H deformation |
| - | 1194 | O–CH3 stretching |
| 1164 | - | C–O stretch in ester group |
| - | 1163 | C–O stretching |
| - | 1117 | O–CH2–C stretching in triglyceride |
| 1044 | - | Aromatic C–H deformation G + S |
| - | 1016 | C–O stretching of ester groups |
| 874 | - | C–H glycosidic linkage and G |
| - | 844 | C–O stretching of ester groups |
| 763 | - | CH alkene |
| - | 722 | aromatic compounds |
| Parameter | Unit | Value | |
|---|---|---|---|
| Original SCG | The Solid Remaining SCG | ||
| Total solids (TS) | g-TS/g | 0.977 ± 0.001 | 0.958 ± 0.010 |
| Volatile solids (VS) | g-VS/g | 0.960 ± 0.005 | 0.720 ± 0.010 |
| Total COD (tCOD) | g-COD/g | 1.030 ± 0.034 | 0.250 ± 0.014 |
| Soluble COD (sCOD) | g-COD/g | 0.038 ± 0.001 | 0.053 ± 0.002 |
| Lignin fraction | % (w/w) | 28.200 ± 0.300 | 36.500 ± 0.081 |
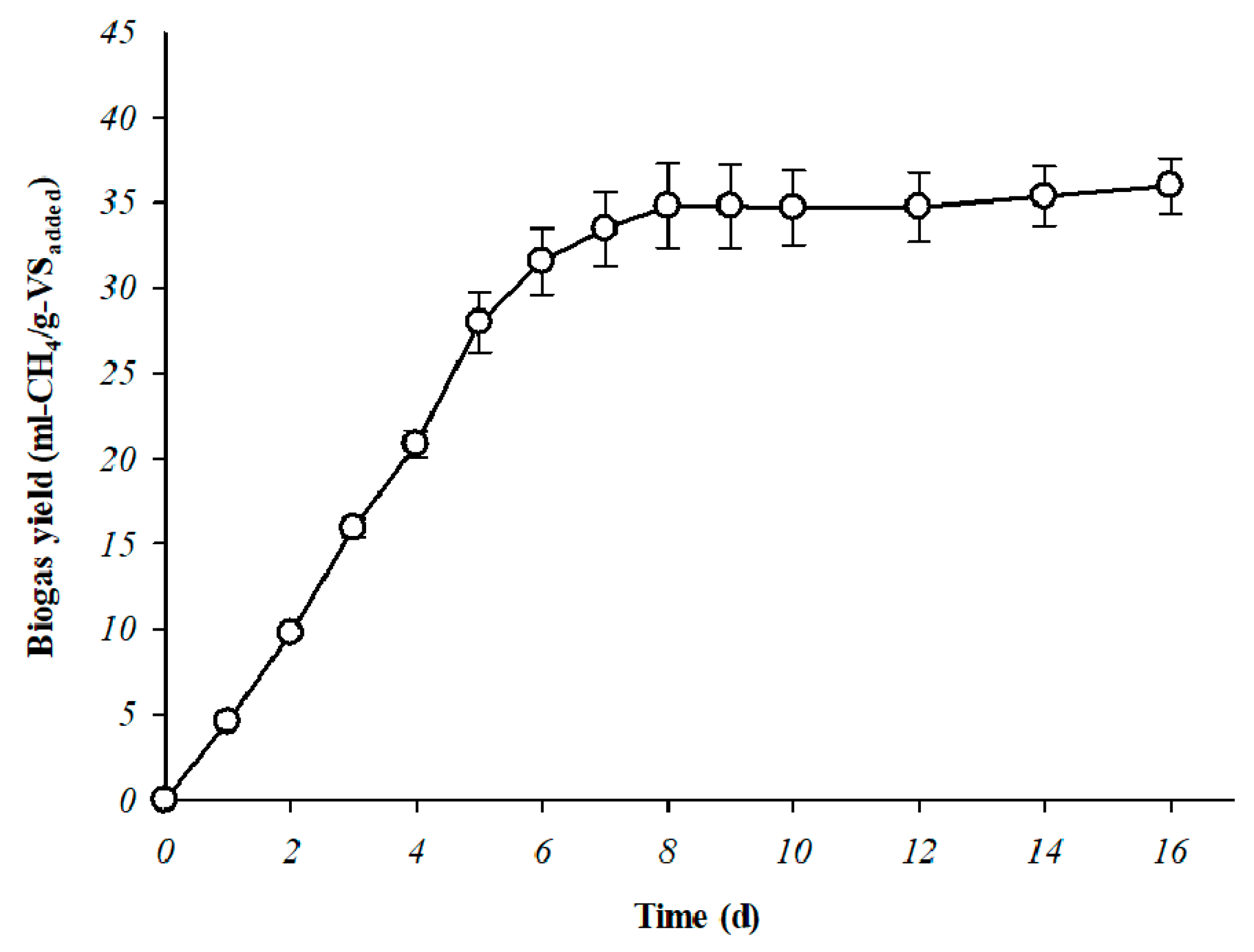
| Theoretical BMP a | mL-CH4/g-VSadded | 248.480 | 40.000 |
| Specific BMP | mL-CH4/g-VSadded | - | 36.000 ± 1.150 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Yang, M.; Choi, S.; Shin, J.; Park, C.; Cho, S.-K.; Kim, Y.M. Sequential Production of Lignin, Fatty Acid Methyl Esters and Biogas from Spent Coffee Grounds via an Integrated Physicochemical and Biological Process. Energies 2019, 12, 2360. https://doi.org/10.3390/en12122360
Lee M, Yang M, Choi S, Shin J, Park C, Cho S-K, Kim YM. Sequential Production of Lignin, Fatty Acid Methyl Esters and Biogas from Spent Coffee Grounds via an Integrated Physicochemical and Biological Process. Energies. 2019; 12(12):2360. https://doi.org/10.3390/en12122360
Chicago/Turabian StyleLee, Minjeong, Minseok Yang, Sangki Choi, Jingyeong Shin, Chanhyuk Park, Si-Kyung Cho, and Young Mo Kim. 2019. "Sequential Production of Lignin, Fatty Acid Methyl Esters and Biogas from Spent Coffee Grounds via an Integrated Physicochemical and Biological Process" Energies 12, no. 12: 2360. https://doi.org/10.3390/en12122360