Comparative Analysis of CO2, N2, and Gas Mixture Injection on Asphaltene Deposition Pressure in Reservoir Conditions
Abstract
:1. Introduction
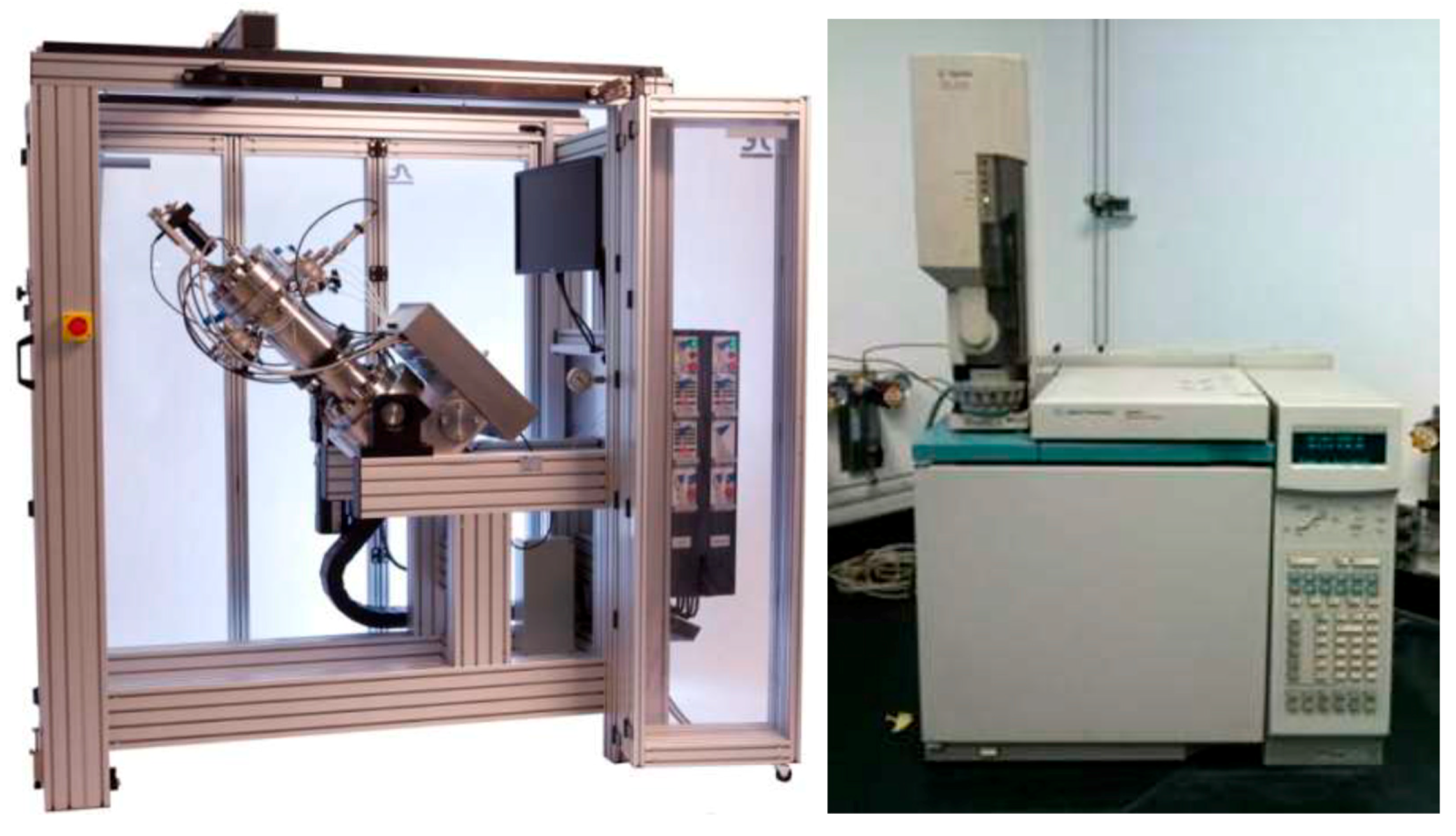
2. Experiments
2.1. Flash Separation Experiments
2.2. Asphaltene Deposition Simulation in the PVTsim Nova
3. Results and Discussion
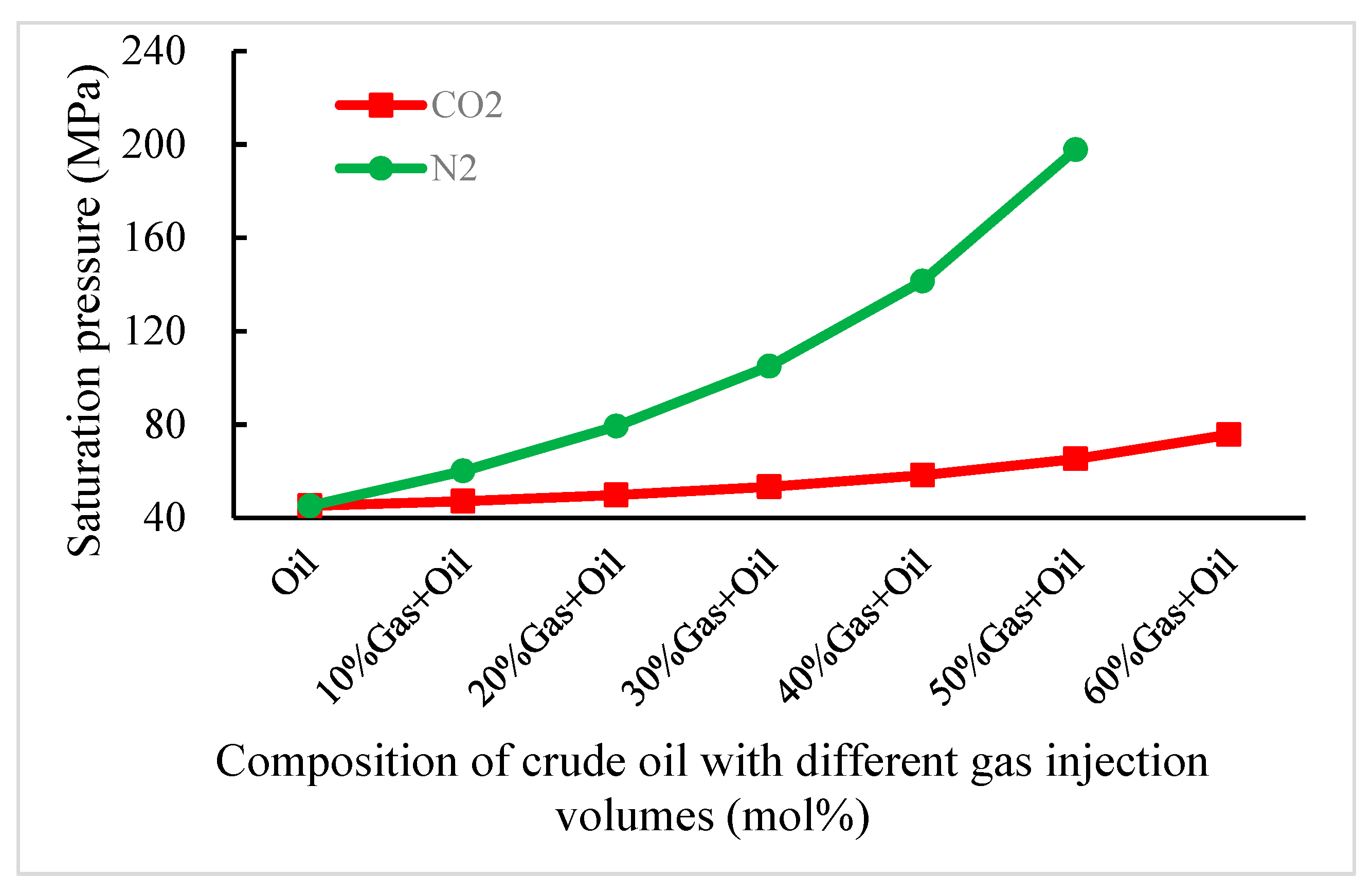
3.1. Effect on the Composition Change of CO2 and N2 Injection in the Shallow Reservoir
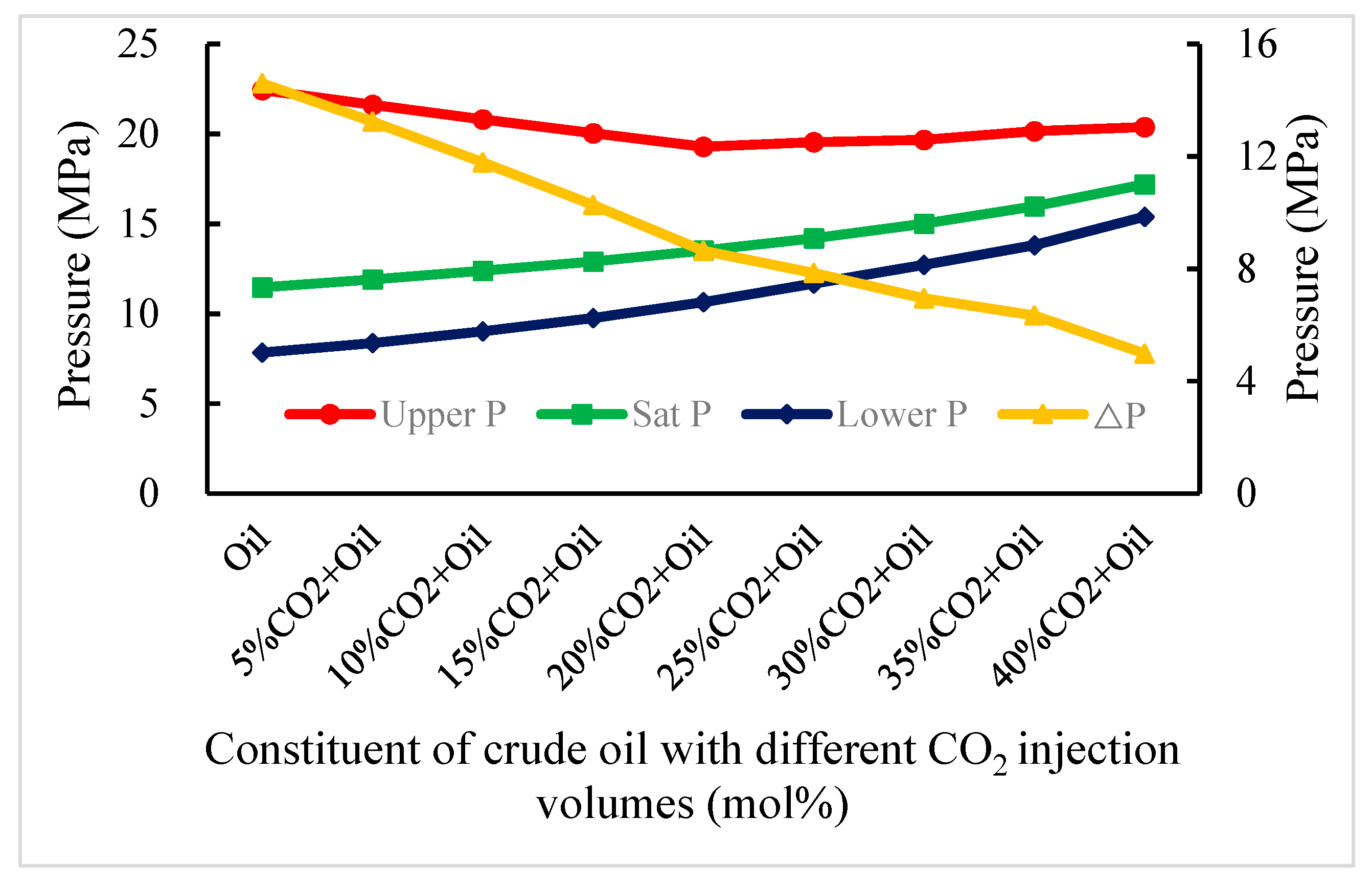
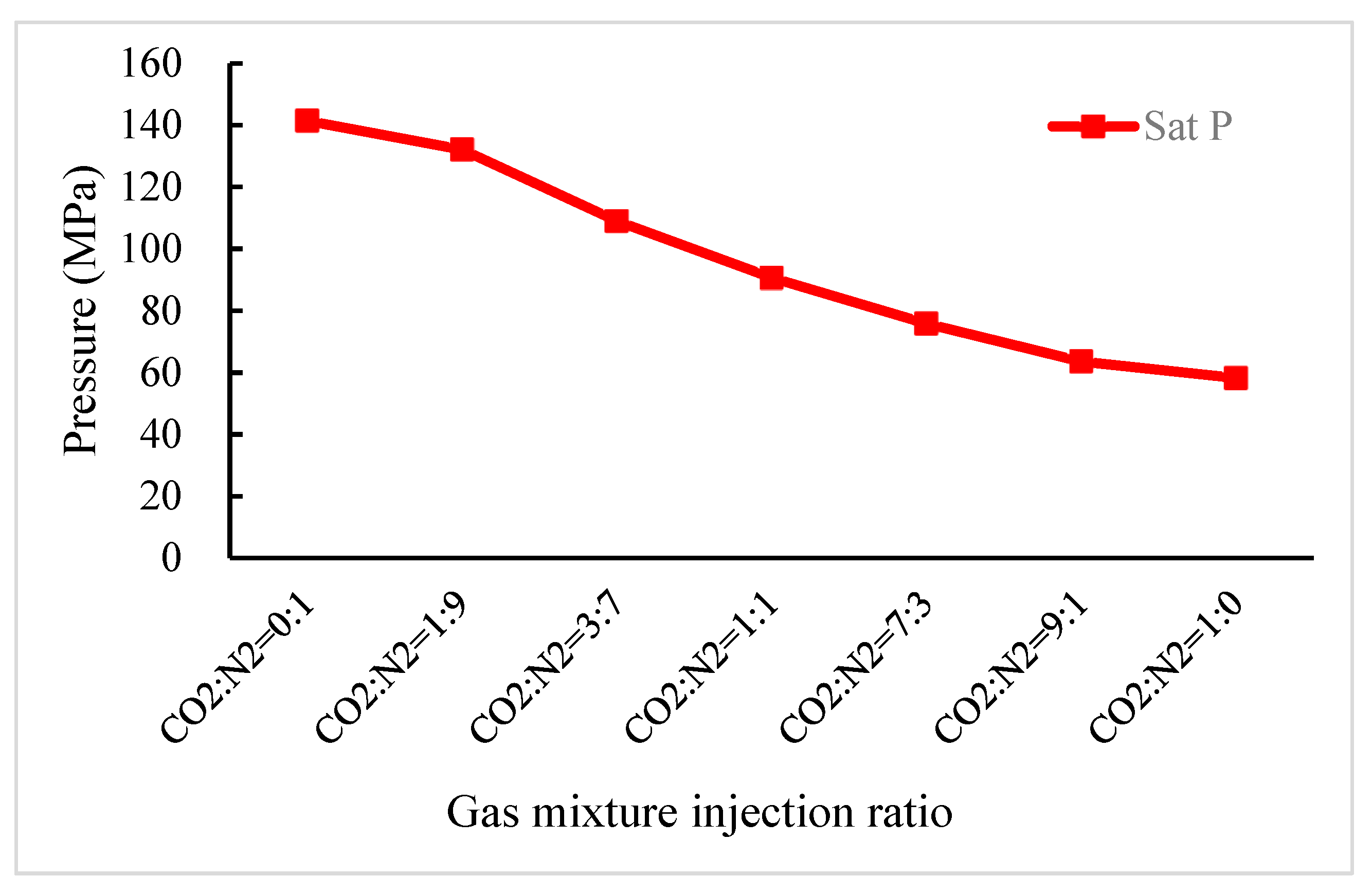
3.2. Effect on Asphaltene Deposition Pressure of CO2, N2 and Gas Mixture Injection in a Shallow Reservoir
3.3. Effect of CO2 and N2 Injection on the Composition Change in a Buried-Hill Reservoir
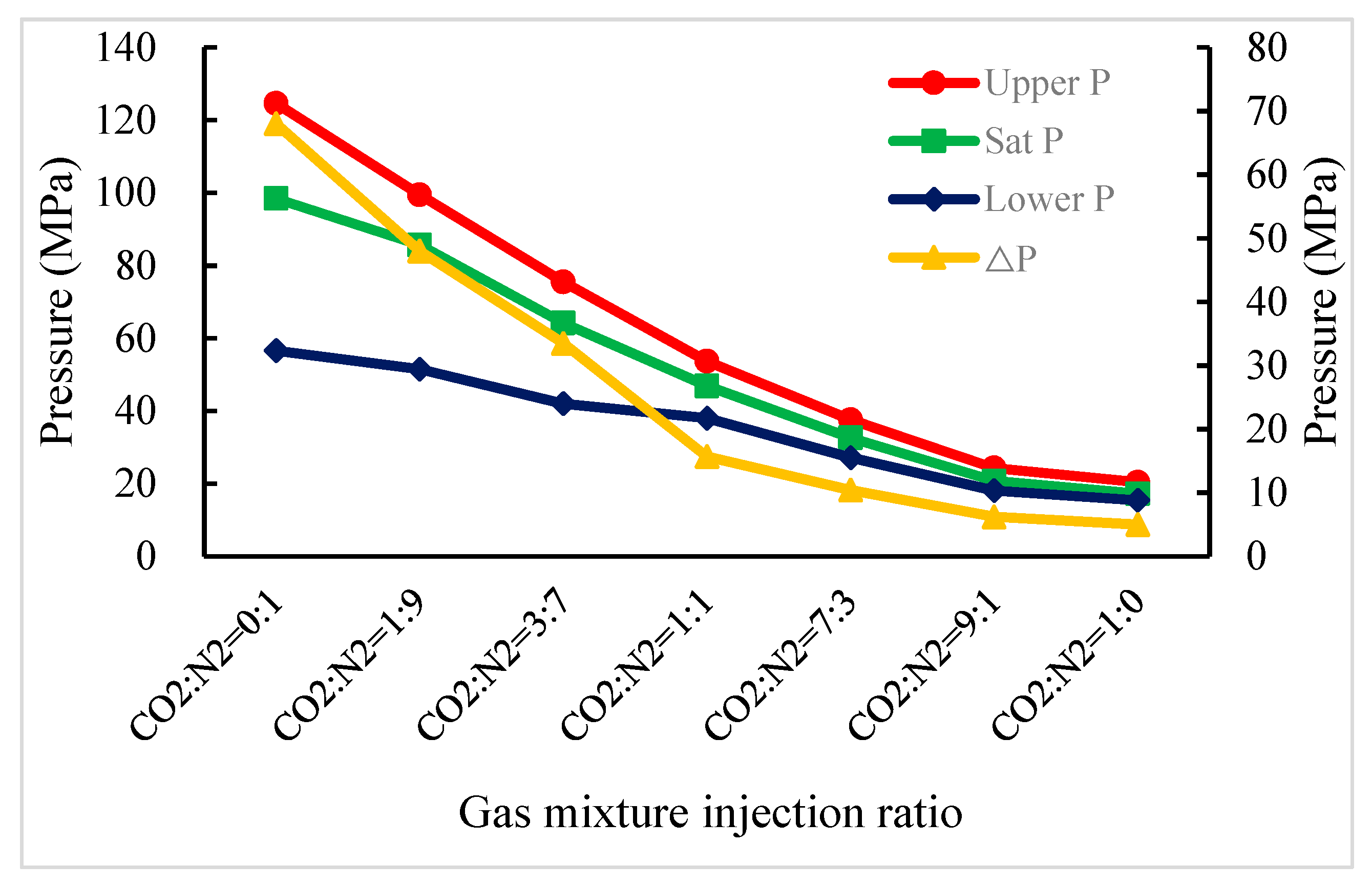
3.4. Effect of CO2, N2 and Gas Mixture Injection on the Asphaltene Deposition Pressure in a Buried-Hill Reservoir
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Srivastava, R.K.; Huang, S.S.; Dong, M. Asphaltene deposition during CO2 flooding. SPE Prod. Facil. 1999, 14, 235–245. [Google Scholar] [CrossRef]
- Ju, B.; Qiu, X.; Qin, J.; Chen, X.; Fan, T. Asphaltene deposition and its effects on production performances in the development of oil field by CO2 flooding: A numerical simulation assessment. In Proceedings of the SPE EUROPE/EAGE Annual Conference and Exhibition, Barcelona, Spain, 14–17 June 2010. [Google Scholar]
- Soroush, S.; Pourafshary, P.; Vafaie-Sefti, M. A comparison of asphaltene deposition in miscible and immiscible carbon dioxide flooding in porous media. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 31 March–2 April 2014. [Google Scholar]
- Einstein, M.A.; Castillo, Y.C.G.; Gil, J.C. A novel improved condensate-recovery method by cyclic supercritical CO2 injection. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 15–18 April 2007. [Google Scholar]
- Zekri, A.Y.; Almehaideb, R.A.; Shedid, S.A. Displacement efficiency of supercritical CO2 flooding in tight carbonate rocks under immiscible and miscible conditions. In Proceedings of the SPE EUROPE/EAGE Annual Conference and Exhibition, Vienna, Austria, 12–15 June 2006. [Google Scholar]
- Zekri, A.Y.; Shedid, S.A.; Almehaideb, R.A. An experimental investigation of interactions between supercritical CO2, asphaltenic crude oil, and reservoir brine in carbonate cores. In Proceedings of the SPE International Symposium on Oilfield Chemistry, Houston, TX, USA, 28 February–2 March 2007. [Google Scholar]
- Piyarat, W. Precipitation and Characterization of Petroleum Asphaltenes. Ph.D. Thesis, University of Michigan, Ann Arbor, MI, USA, 2004. [Google Scholar]
- Speight, J.G. On the molecular nature of petroleum asphaltenes. In Chemistry of Asphaltenes; Bunger, J.W., Li, N.C., Eds.; American Chemical Society: Washington, DC, USA, 1981. [Google Scholar]
- Ting, P.D. Thermodynamic Stability and Phase Behavior of Asphaltenes in Oil and Other Highly Asymmetric Mixtures. Ph.D. Thesis, RICE University, Houston, TX, USA, 2003. [Google Scholar]
- Speight, J.G. The Chemistry and Technology of Petroleum, 5th ed.; Taylor & Francis (Reprint): Boca Raton, FL, USA, 2014. [Google Scholar]
- Long, R.B. The concept of asphaltenes. In Chemistry of Asphaltenes; Bunger, J.W., Li, N.C., Eds.; American Chemical Society: Washington, DC, USA, 1981; pp. 17–27. [Google Scholar]
- Speight, J.G. The Chemistry and Technology of Petroleum; Marcel Dekker: New York, NY, USA, 1999. [Google Scholar]
- Yin, Y.R.; Yen, A.T. Asphaltene deposition and chemical control in CO2 floods. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, Tulsa, OK, USA, 3–5 April 2000. [Google Scholar]
- Buriro, M.A.; Shuker, M.T. Asphaltene prediction and prevention: A strategy to control asphaltene precipitation. In Proceedings of the SPE/PAPG Annual Technical Conference, Islamabad, Pakistan, 3–5 December 2012. [Google Scholar]
- Adyani, W.N.; Daud, W.A.W.; Darman, N.B.; Memon, A.I.; Khan, I.A.; Jamaluddin, A.K.M. A systematic approach to evaluate asphaltene precipitation during CO2 injection. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–21 July 2011. [Google Scholar]
- Novosad, Z.; Costain, T.G. Experimental and modeling studies of asphaltene equilibria for a reservoir under CO2 injection. In Proceedings of the 65th Annual Technical Conference and Exhibition of the Society of Petroleum Engineers, New Orleans, LA, USA, 23–26 September 1990. [Google Scholar]
- Hirschberg, A.; Dejone, L.N.J.; Schipper, B.A.; Meijer, J.G. Influence of temperature and pressure onasphaltene flocculation. SPE J. 1984, 24, 283–293. [Google Scholar]
- Leontaritis, K.J.; Amaefule, J.O.; Charles, R.E. A systematic approach for the prevention and treatment of formation damage caused by asphaltene deposition. SPE Prod. Facil. 1994, 9, 157–164. [Google Scholar] [CrossRef]
- Nghiem, L.X.; Coombe, D.A. Modelling asphaltene precipitation during primary depletion. SPE J. 1997, 2, 170–176. [Google Scholar] [CrossRef]
- Paricaud, P.; Galindo, A.; Jackson, G. Recent advances in the use of the SAFT approach in describing electrolytes, interfaces, liquid crystals and polymers. Fluid Phase Equilib. 2002, 33, 87–96. [Google Scholar] [CrossRef]
- Chapman, W.G.; Sauer, S.G.; Ting, D.; Ghosh, A. Phase behavior applications of SAFT based equations of state—From associating fluids to polydisperse, polar copolymers. Fluid Phase Equilib. 2004, 217, 137–143. [Google Scholar] [CrossRef]
- Chung, T.H. Thermodynamic modelling for organic solid precipitation. SPE Pap. 1992, 24851, 869–878. [Google Scholar]
- Anderson, S.I.; Speight, J.G. Thermodynamic models for asphaltene solubility and precipitation. J. Pet. Sci. Eng. 1999, 22, 53–56. [Google Scholar] [CrossRef]
- Leontaritis, K.J.; Mansoori, G.A. Asphaltene deposition: A comprehensive description of petroleum manifestations and modelling approaches. SPE Pap. 1989, 18892. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Huang, S.S. Asphaltene deposition during CO2 flooding: A laboratory assessment. In Proceedings of the SPE Production Operations Symposium, Oklahoma City, OK, USA, 9–11 March 1997. [Google Scholar]
- Guerrero-Martin, C.A.; Montes-Páez, E.; Oliveira, M.C.K.; Campos, J.; Lucas, E.F. Calculating asphaltene precipitation onset pressure by using cardanol as precipitation inhibitor: A strategy to increment the oil well production. In Proceedings of the SPE Trinidad and Tobago Section Energy Resources Conference, Port of Spain, Trinidad and Tobago, 25–26 June 2018. [Google Scholar]
- Ratnakar, R.R.; Mantilla, C.A.; Dindoruk, B. Experimental and numerical investigation of the impact of asphaltene stability on interfacial properties and the hysteresis behavior at the interfaces. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 26–28 March 2018. [Google Scholar]
- Davudov, D.; Moghanloo, R.G.; Akita, E.; Karami, H. A diagnostic approach to predict asphaltene deposition in reservoir and wellbore. In Proceedings of the SPE Western Regional Meeting, Garden Grove, CA, USA, 22–27 April 2018. [Google Scholar]
- Afra, S.; Alrashidi, H.G.; Nasr-EL-Din, H.A. Interrelationship between asphaltene precipitation methods and asphaltene characteristics and self-association behavior. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Buenos Aires, Argentina, 18–19 May 2017. [Google Scholar]
- Shen, Z.; Sheng, J.J. Experimental study of asphaltene aggregation during CO2 and CH4 injection in shale oil reservoirs. In Proceedings of the SPE Improved Oil Recovery Conference, Tulsa, OK, USA, 11–13 April 2016. [Google Scholar]

| Components | Molar Volume Content (mol %) | Components | Molar Volume Content (mol %) |
|---|---|---|---|
| CO2 | 0.00 | C17 | 2.55 |
| N2 | 1.78 | C18 | 2.36 |
| C1 | 27.45 | C19 | 2.08 |
| C2 | 1.04 | C20 | 1.77 |
| C3 | 0.18 | C21 | 1.67 |
| iC4 | 0.06 | C22 | 1.53 |
| nC4 | 0.04 | C23 | 1.42 |
| iC5 | 0.00 | C24 | 1.31 |
| nC5 | 0.00 | C25 | 1.38 |
| C6 | 0.00 | C26 | 1.52 |
| C7 | 0.15 | C27 | 1.78 |
| C8 | 0.24 | C28 | 1.94 |
| C9 | 0.83 | C29 | 1.86 |
| C10 | 0.45 | C30 | 1.62 |
| C11 | 0.88 | C31 | 1.15 |
| C12 | 1.42 | C32 | 0.90 |
| C13 | 1.97 | C33 | 0.72 |
| C14 | 2.35 | C34 | 0.61 |
| C15 | 2.17 | C35 | 0.56 |
| C16 | 2.33 | C36+ | 27.93 |
| Component | mol % | Mol wt.% | Liquid Density (g/cm3) | Critical Temperature (°C) | Critical Pressure (bara) | Critical Volume (cm3/mol) |
|---|---|---|---|---|---|---|
| N2 | 1.78 | 28.014 | −146.95 | 33.94 | 89.8 | |
| C1 | 27.45 | 16.043 | −82.55 | 46 | 99 | |
| C2 | 1.04 | 30.07 | 32.25 | 48.84 | 148 | |
| C3 | 0.18 | 44.097 | 96.65 | 42.46 | 203 | |
| iC4 | 0.06 | 58.124 | 134.95 | 36.48 | 263 | |
| nC4 | 0.04 | 58.124 | 152.05 | 38 | 255 | |
| C7 | 0.15 | 96 | 0.738 | 263.338 | 29.45 | 475.55 |
| C8 | 0.24 | 107 | 0.765 | 284.893 | 27.42 | 487.84 |
| C9 | 0.83 | 121 | 0.781 | 308.928 | 25.06 | 529.18 |
| C10–C19 | 18.56 | 211.075 | 0.8399 | 435.872 | 17.61 | 908.95 |
| C20–C26 | 10.6 | 315.691 | 0.8766 | 542.232 | 14.62 | 1364.5 |
| C27–C31 | 8.35 | 399.351 | 0.8995 | 618.622 | 13.49 | 1759.02 |
| C32–C37 | 6.389 | 487.292 | 0.9197 | 695.091 | 12.78 | 2201.57 |
| C38–C42 | 7.192 | 554.183 | 0.934 | 750.134 | 12.42 | 2543.06 |
| C43–C47 | 5.196 | 624.183 | 0.9475 | 806.373 | 12.15 | 2910.8 |
| C48–C53 | 3.806 | 700.352 | 0.9606 | 827.809 | 11.55 | 3319.78 |
| C48–C53-A | 0.563 | 700.352 | 0.9606 | 1125.35 | 14.95 | 3319.78 |
| C54–C63 | 3.471 | 807.543 | 0.9768 | 902.584 | 11.01 | 3911.42 |
| C54–C63-A | 0.906 | 807.543 | 0.9768 | 1125.35 | 14.95 | 3911.42 |
| C64–C80 | 2.535 | 982.587 | 0.9993 | 1068.026 | 10.72 | 4904.15 |
| C64–C80-A | 0.662 | 982.587 | 0.9993 | 1125.35 | 14.95 | 4904.15 |
| C1 (mol %) | C2 (mol %) | C3 (mol %) | iC4 (mol %) | nC4 (mol %) | C36+ (mol %) | |
|---|---|---|---|---|---|---|
| Oil | 27.45 | 1.04 | 0.18 | 0.06 | 0.04 | 27.93 |
| 5% CO2 + Oil | 26.08 | 0.99 | 0.17 | 0.06 | 0.04 | 26.53 |
| 10% CO2 + Oil | 24.71 | 0.94 | 0.16 | 0.05 | 0.04 | 25.14 |
| 15% CO2 + Oil | 23.33 | 0.88 | 0.15 | 0.05 | 0.03 | 23.74 |
| 20% CO2 + Oil | 21.96 | 0.83 | 0.14 | 0.05 | 0.03 | 22.34 |
| 25% CO2 + Oil | 20.59 | 0.78 | 0.14 | 0.05 | 0.03 | 20.95 |
| 30% CO2 + Oil | 19.22 | 0.73 | 0.13 | 0.04 | 0.03 | 19.55 |
| 35% CO2 + Oil | 17.84 | 0.68 | 0.12 | 0.04 | 0.03 | 18.16 |
| 40% CO2 + Oil | 16.47 | 0.62 | 0.11 | 0.04 | 0.02 | 16.76 |
| 5% N2 + Oil | 26.55 | 1.01 | 0.17 | 0.06 | 0.04 | 27.01 |
| 10% N2 + Oil | 25.15 | 0.95 | 0.17 | 0.06 | 0.04 | 25.59 |
| 15% N2 + Oil | 23.76 | 0.90 | 0.16 | 0.05 | 0.04 | 24.17 |
| 20% N2 + Oil | 22.36 | 0.85 | 0.15 | 0.05 | 0.03 | 22.75 |
| 25% N2 + Oil | 20.96 | 0.79 | 0.14 | 0.05 | 0.03 | 21.33 |
| 30% N2 + Oil | 19.56 | 0.74 | 0.13 | 0.04 | 0.03 | 19.91 |
| 35% N2 + Oil | 18.17 | 0.69 | 0.12 | 0.04 | 0.03 | 18.48 |
| 40% N2 + Oil | 16.77 | 0.64 | 0.11 | 0.04 | 0.02 | 17.06 |
| Components | Molar Volume Content (mol %) | Components | Molar Volume Content (mol %) |
|---|---|---|---|
| CO2 | 11.72 | C17 | 1.34 |
| N2 | 0.00 | C18 | 1.16 |
| C1 | 55.47 | C19 | 1.12 |
| C2 | 3.11 | C20 | 1.06 |
| C3 | 0.80 | C21 | 1.04 |
| iC4 | 0.21 | C22 | 0.98 |
| nC4 | 0.17 | C23 | 0.93 |
| iC5 | 0.00 | C24 | 0.87 |
| nC5 | 0.00 | C25 | 0.83 |
| C6 | 0.00 | C26 | 0.78 |
| C7 | 0.04 | C27 | 0.75 |
| C8 | 0.10 | C28 | 0.67 |
| C9 | 0.15 | C29 | 0.59 |
| C10 | 0.37 | C30 | 0.49 |
| C11 | 0.70 | C31 | 0.41 |
| C12 | 1.14 | C32 | 0.33 |
| C13 | 1.42 | C33 | 0.28 |
| C14 | 1.70 | C34 | 0.23 |
| C15 | 1.40 | C35 | 0.20 |
| C16 | 1.43 | C36+ | 6.02 |
| Component | mol % | Mol wt.% | Liquid Density (g/cm3) | Critical Temperature (°C) | Critical Pressure (bara) | Critical Volume (cm3/mol) |
|---|---|---|---|---|---|---|
| CO2 | 11.719 | 44.01 | 31.05 | 73.76 | 94 | |
| C1 | 55.464 | 16.043 | −82.55 | 46 | 99 | |
| C2 | 3.11 | 30.07 | 32.25 | 48.84 | 148 | |
| C3 | 0.8 | 44.097 | 96.65 | 42.46 | 203 | |
| iC4 | 0.21 | 58.124 | 134.95 | 36.48 | 263 | |
| nC4 | 0.17 | 58.124 | 152.05 | 38 | 255 | |
| C7 | 0.04 | 96 | 0.738 | 263.338 | 29.45 | 475.55 |
| C8 | 0.1 | 107 | 0.765 | 284.893 | 27.42 | 487.84 |
| C9 | 0.15 | 121 | 0.781 | 308.928 | 25.06 | 529.18 |
| C10–C15 | 6.729 | 177.7 | 0.8256 | 392.459 | 19.43 | 748.78 |
| C16–C18 | 3.93 | 235.674 | 0.8473 | 458.417 | 16.58 | 989.37 |
| C19–C22 | 4.2 | 282.762 | 0.8655 | 508.3 | 15.27 | 1202.86 |
| C23–C25 | 2.63 | 330.821 | 0.8809 | 555.086 | 14.36 | 1424.79 |
| C26–C29 | 2.79 | 379.09 | 0.8945 | 600.208 | 13.71 | 1658.63 |
| C30–C36 | 3.354 | 467.399 | 0.9184 | 679.217 | 12.94 | 2107.8 |
| C37 | 1.082 | 514 | 0.9385 | 717.487 | 12.72 | 2338.2 |
| C38–C40 | 1.945 | 539.531 | 0.9554 | 739.603 | 12.67 | 2480.55 |
| C41–C77 | 1.539 | 615.584 | 1.0017 | 798.062 | 12.51 | 2948.39 |
| C41–C77-A | 0.04 | 615.584 | 1.0017 | 1125.35 | 14.95 | 2948.39 |
| C1 (mol %) | C2 (mol %) | C3 (mol %) | iC4 (mol %) | nC4 (mol %) | C36+ (mol %) | |
|---|---|---|---|---|---|---|
| Oil | 55.47 | 3.11 | 0.80 | 0.21 | 0.17 | 6.02 |
| 10% CO2 + Oil | 49.19 | 2.76 | 0.71 | 0.19 | 0.15 | 5.34 |
| 20% CO2 + Oil | 42.91 | 2.41 | 0.62 | 0.16 | 0.13 | 4.66 |
| 30% CO2 + Oil | 36.63 | 2.05 | 0.53 | 0.14 | 0.11 | 3.98 |
| 40% CO2 + Oil | 30.35 | 1.70 | 0.44 | 0.12 | 0.09 | 3.29 |
| 50% CO2 + Oil | 24.06 | 1.35 | 0.35 | 0.09 | 0.07 | 2.61 |
| 60% CO2 + Oil | 17.78 | 1.00 | 0.26 | 0.07 | 0.05 | 1.93 |
| 10% N2 + Oil | 49.92 | 2.80 | 0.72 | 0.19 | 0.15 | 5.42 |
| 20% N2 + Oil | 44.37 | 2.49 | 0.64 | 0.17 | 0.14 | 4.82 |
| 30% N2 + Oil | 38.83 | 2.18 | 0.56 | 0.15 | 0.12 | 4.21 |
| 40% N2 + Oil | 33.28 | 1.87 | 0.48 | 0.13 | 0.10 | 3.61 |
| 50% N2 + Oil | 27.73 | 1.56 | 0.40 | 0.11 | 0.09 | 3.01 |
| 60% N2 + Oil | 22.19 | 1.24 | 0.32 | 0.08 | 0.07 | 2.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Zhao, F.; Hou, J.; Lu, G.; Zhang, M.; Wang, Z. Comparative Analysis of CO2, N2, and Gas Mixture Injection on Asphaltene Deposition Pressure in Reservoir Conditions. Energies 2018, 11, 2483. https://doi.org/10.3390/en11092483
Wang P, Zhao F, Hou J, Lu G, Zhang M, Wang Z. Comparative Analysis of CO2, N2, and Gas Mixture Injection on Asphaltene Deposition Pressure in Reservoir Conditions. Energies. 2018; 11(9):2483. https://doi.org/10.3390/en11092483
Chicago/Turabian StyleWang, Peng, Fenglan Zhao, Jirui Hou, Guoyong Lu, Meng Zhang, and Zhixing Wang. 2018. "Comparative Analysis of CO2, N2, and Gas Mixture Injection on Asphaltene Deposition Pressure in Reservoir Conditions" Energies 11, no. 9: 2483. https://doi.org/10.3390/en11092483




