An Experimental Investigation of Thermal Characteristics of Phase Change Material Applied to Improve the Isothermal Operation of a Refrigerator
Abstract
:1. Introduction
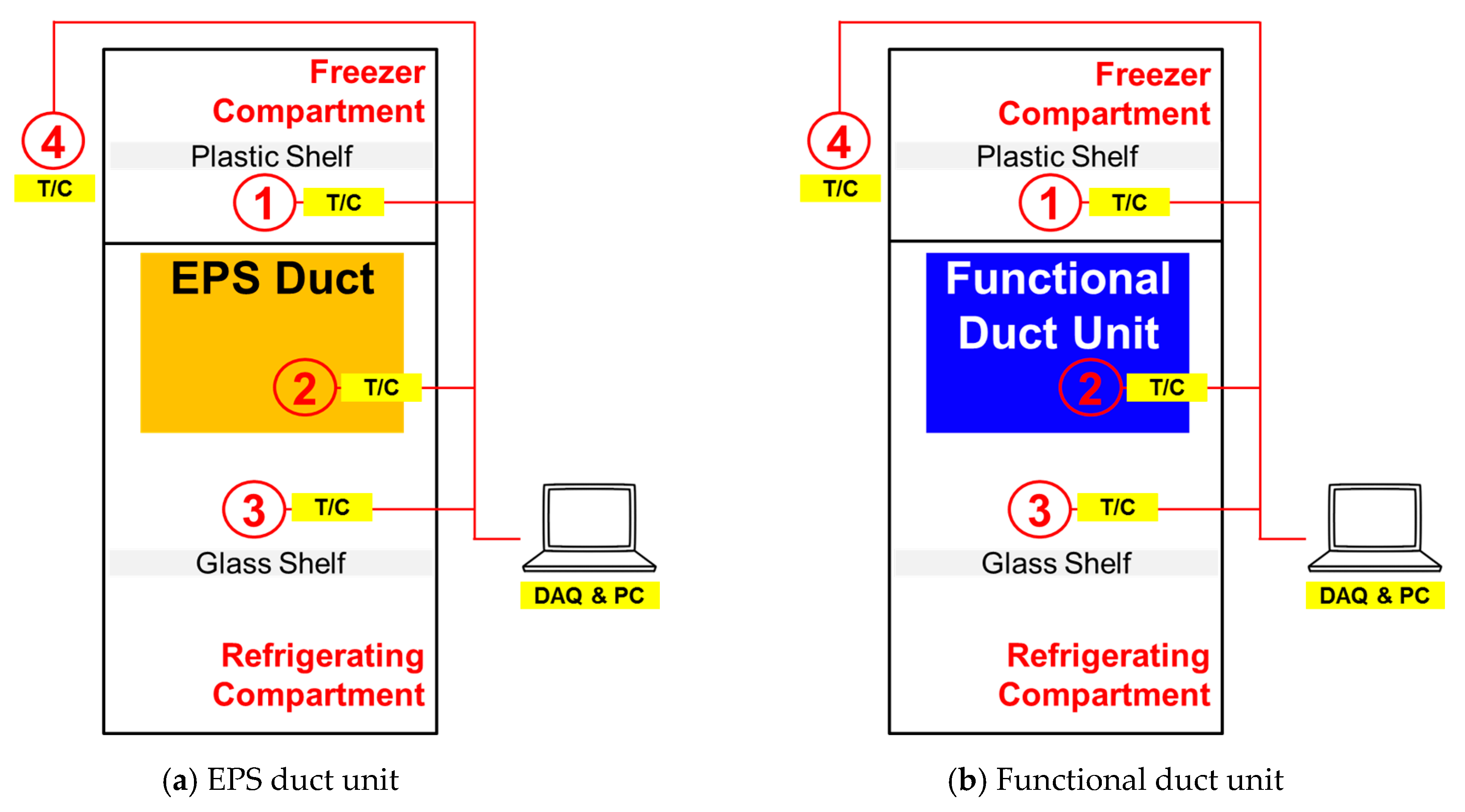
2. Experimental Setup and Method
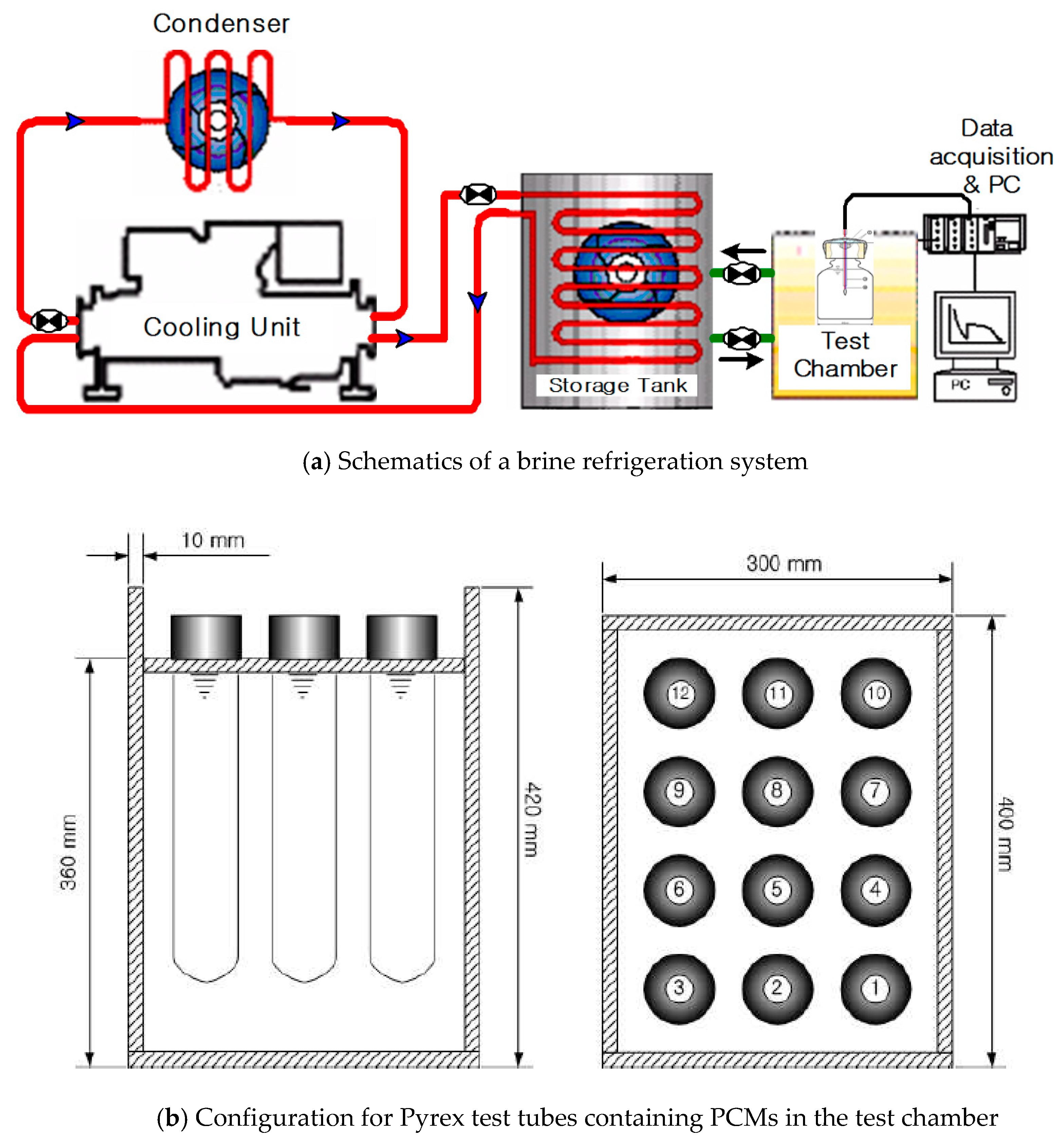
2.1. Experiment for Determining a Refrigerator PCM
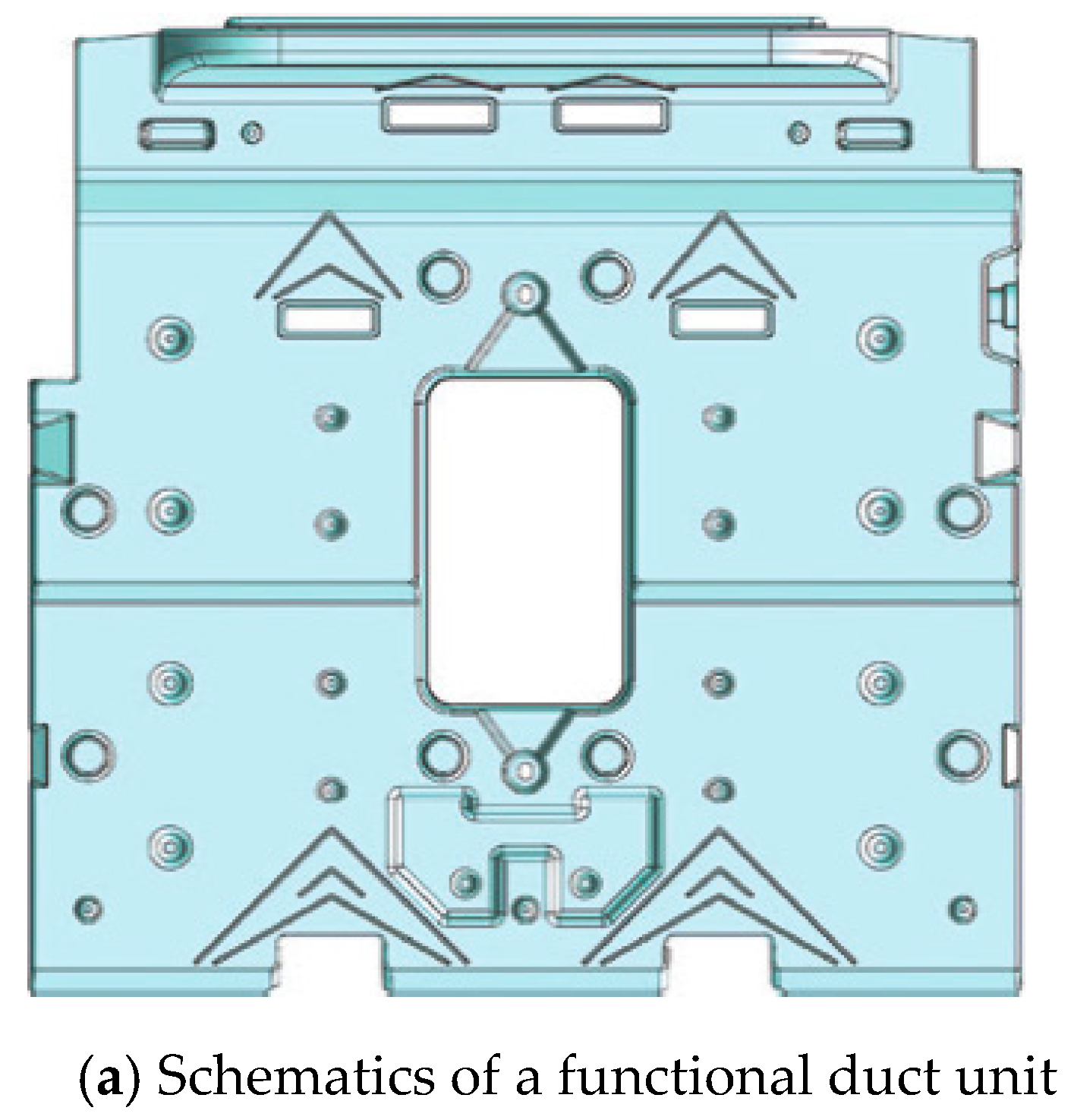
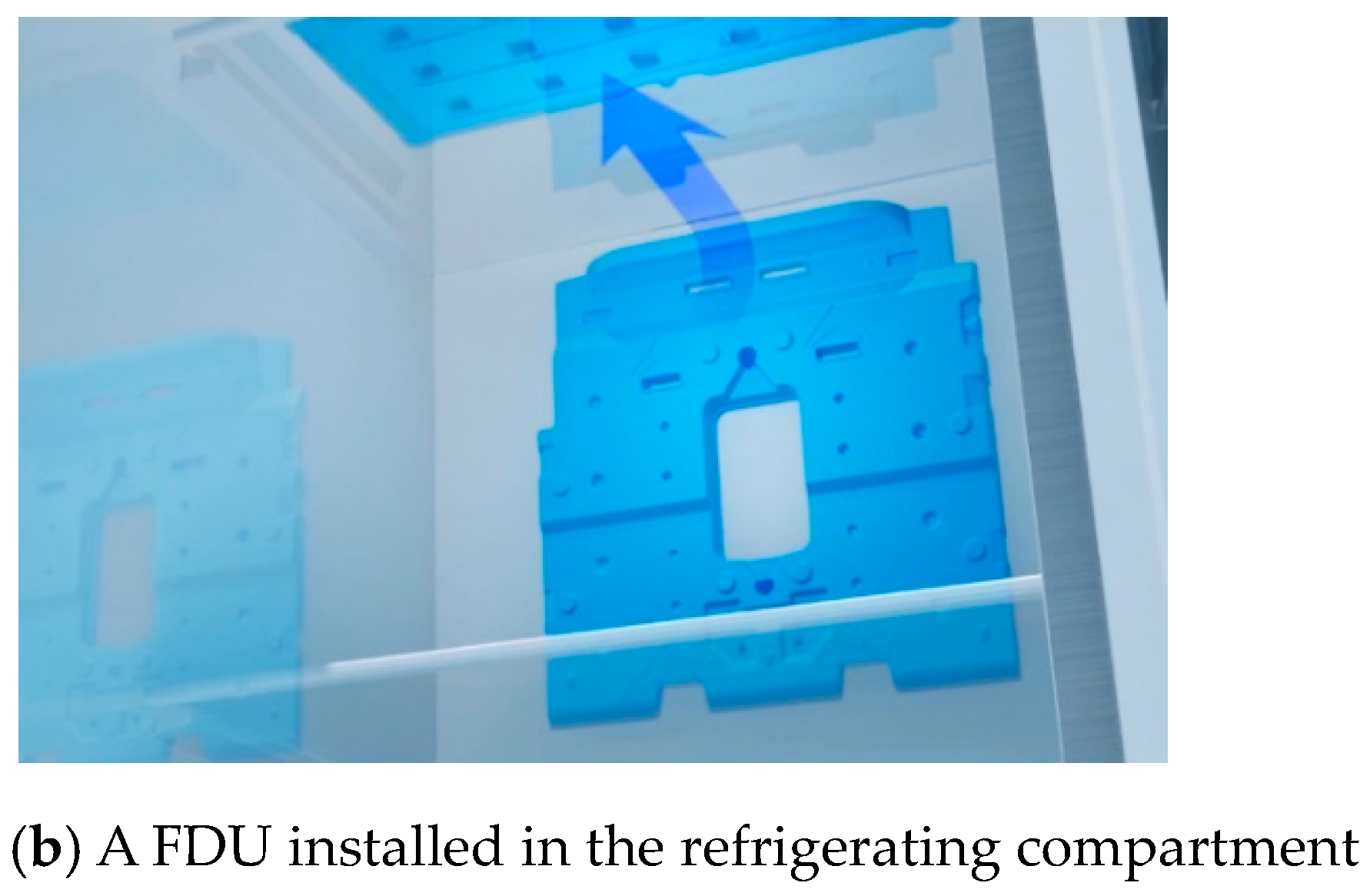
2.2. Performance Experiment of a Refrigerator with PCM
3. Experimental Results and Discussion
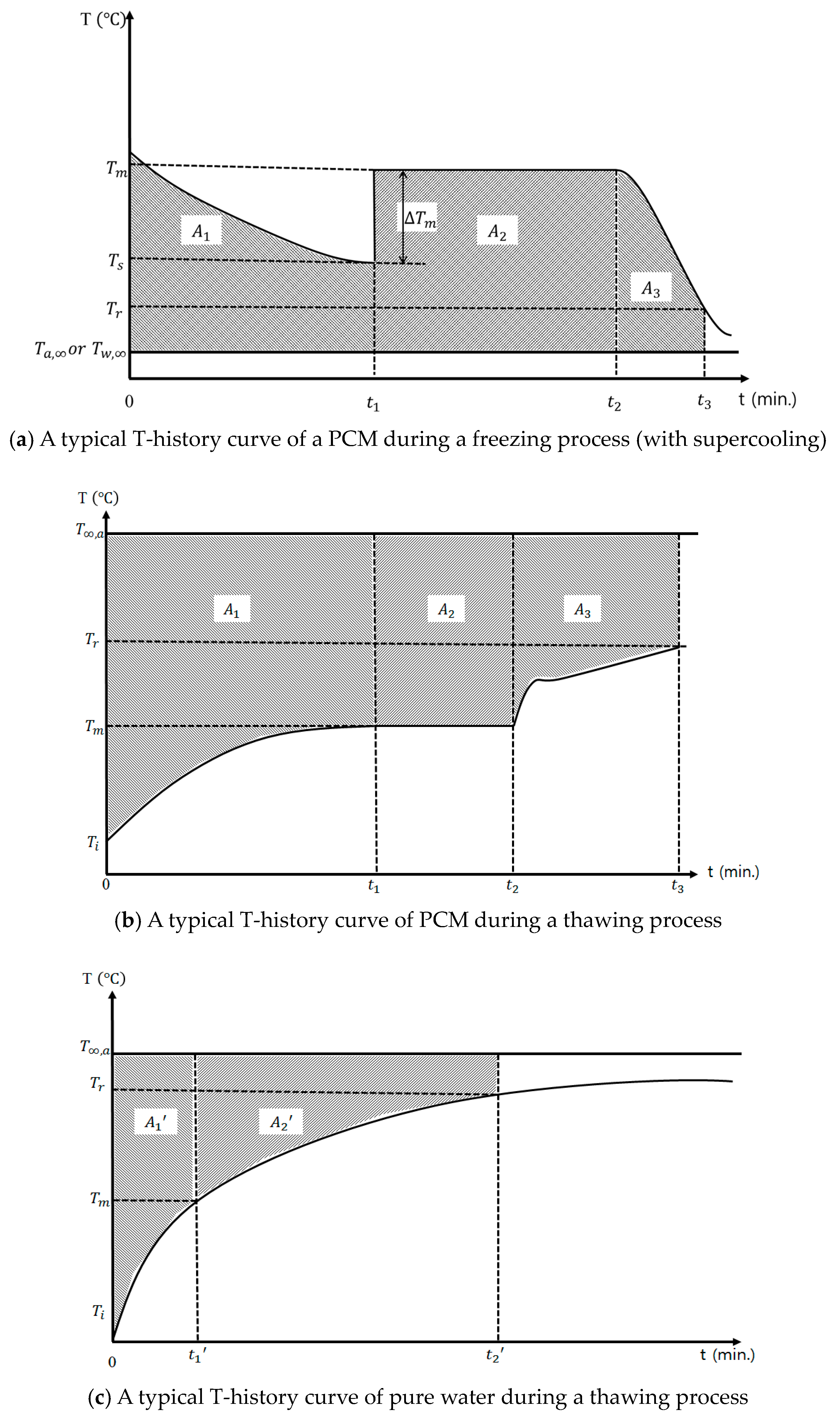
3.1. T-History Method-Based Measurement of Thermal Properties of PCM
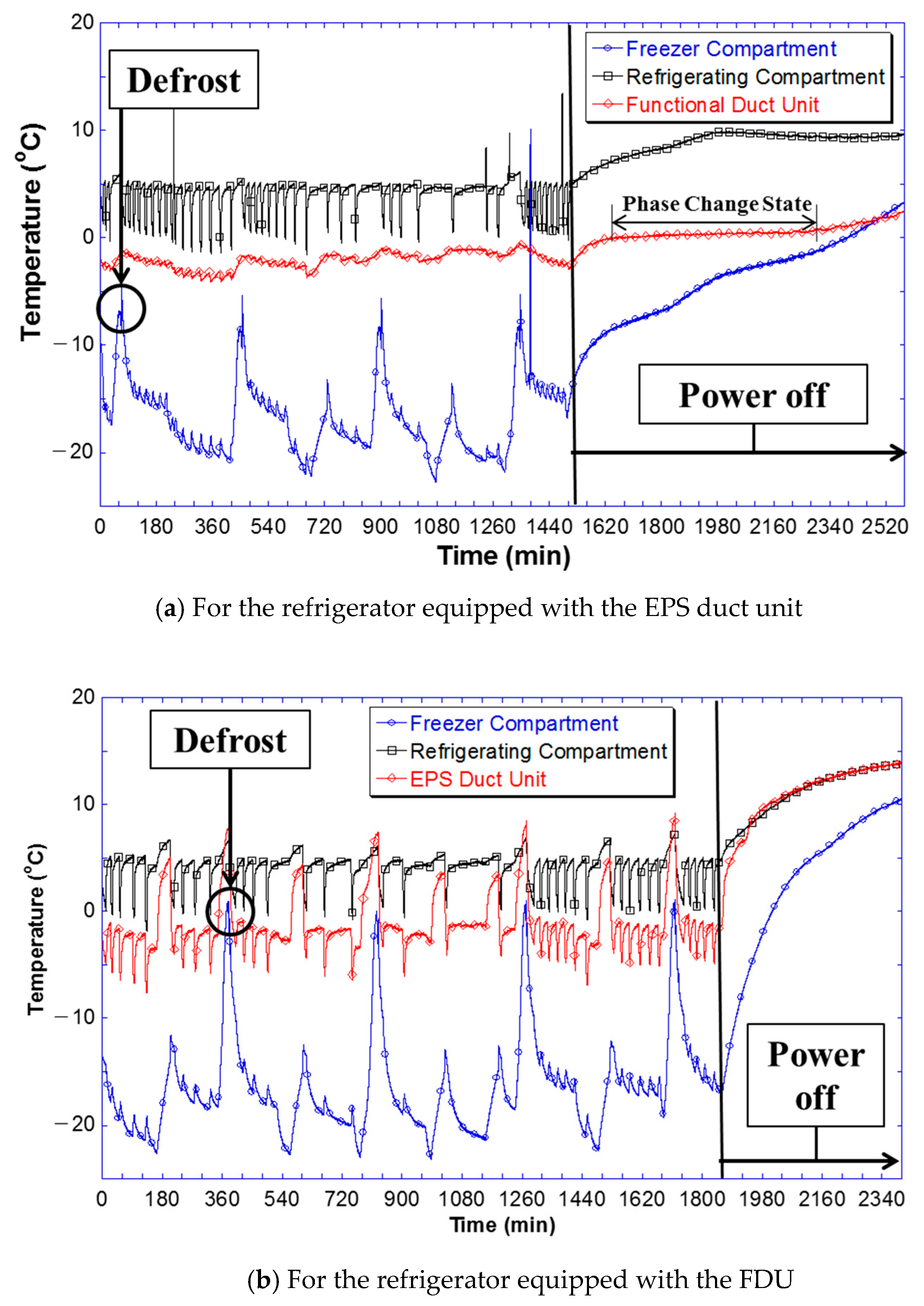
3.2. Operational Characteristics of a Refrigerator with PCM Installed in the Refrigerating Compartment
4. Conclusions
- When the viscosity agent (SAP) was added to a PCM based on the thermal property data of the T-history method, the PCM improved the supercooling temperature by 0.3 °C–2.3 °C as compared to a PCM without the viscosity agent. The latent heat of the PCM with the viscosity agent decreased by approximately 2 kJ/kg depending on the specimens.
- The PCM with 1 wt.% eutectic salt compounds had a phase change temperature of −0.5 °C, supercooling temperature of −2.9 °C, cold retention time of 510 min, and latent heat of 304.9 kJ/kg. These were the best thermal properties among all the PCMs with different mass concentrations. Accordingly, the PCM with 1 wt.% eutectic salt compounds was selected for the FDU.
- Choosing the PCM containing 1 wt.% eutectic salt compounds can reduce the total energy added to the refrigerator before the liquid–solid phase change period by lowering the supercooling temperature and thus eventually increasing the energy efficiency.
- In comparison with the refrigerator equipped with an EPS duct, the FDU-mounted refrigerator, which was filled with the PCM with 1 wt.% eutectic salt compounds restrained a drastic temperature rise inside the freezer compartment during defrosting. Furthermore, in the case of a power shutdown, the PCM in the FDU-mounted refrigerator reached the phase change temperature and released latent heat, thereby effectively restraining a drastic temperature rise inside the refrigerating compartment.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kim, C.O. A Study on Cooling Characteristics of TMA-Water Clathrate Compound for Low Temperature Latent Heat Storage. Ph.D. Thesis, Chosun University, Gwangju, Korea, 2008. [Google Scholar]
- Peck, J.H. A Study on Measurement of the Latent Heat for Phase Change Material. Ph.D. Thesis, Chonbuk National University, Jeonju, Korea, 2015. [Google Scholar]
- Tyagi, V.V.; Buddhi, D. PCM thermal storage in buildings: A state of the art. Renew. Sustain. Energy Rev. 2007, 11, 1146–1166. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.V.; Chen, C.R.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, Y.; Yuan, Y.; Zhang, Z.; Lu, J. Energy-Saving Analysis of Solar Heating System with PCM Storage Tank. Energies 2018, 11, 237. [Google Scholar] [CrossRef]
- Siyabi, I.A.; Khanna, S.; Mallick, T.; Sundaram, S. Multiple Phase Change Material (PCM) Configuration for PCM-Based Heat Sinks-An Experimental Study. Energies 2018, 11, 1629. [Google Scholar] [CrossRef]
- Mazzeo, D.; Oilveti, G.; Arcuri, N. Definition of a new set of parameters for the dynamic thermal characterization of PCM layers in the presence of one or more liquid–solid interfaces. Energy Build. 2017, 141, 379–396. [Google Scholar] [CrossRef]
- Mazzeo, D.; Oilveti, G.; Arcuri, N. A Method for Thermal Dimensioning and for Energy Behavior Evaluation of a Building Envelope PCM Layer by Using Characteristic Days. Energies 2017, 10, 659. [Google Scholar] [CrossRef]
- Oró, E.; Miró, L.; Farid, M.M.; Cabeza, L.F. Improving thermal performance of freezers using phase change materials. Int. J. Refrig. 2012, 35, 984–991. [Google Scholar] [CrossRef]
- Azzouz, K.; Leducq, D.; Gobin, D. Enhancing the performance of household refrigerators with latent heat storage: An experimental investigation. Int. J. Refrig. 2009, 32, 1634–1644. [Google Scholar] [CrossRef]
- Tulapurkar, C.; Subramaniam, P.R.; Thagamani, G.; Thiyagarajan, R. Phase change materials for domestic refrigerators to improve food quality and prolong compressor off time. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 12–15 July 2010. [Google Scholar]
- Gin, B.; Farid, M.M.; Bansal, P.K. Effect of door opening and defrost cycle on a freezer with phase change panels. Energy Convers. Manag. 2010, 51, 2698–2709. [Google Scholar] [CrossRef]
- Cheng, W.L.; Mei, B.J.; Liu, Y.N.; Huang, Y.H.; Yuan, X.D. A novel household refrigerator with shape-stabilized PCM (Phase Change Material) heat storage condenser: An experimental investigation. Energy 2011, 36, 5797–5804. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, Y.; Jiang, Y. A simple method, the T-history method, of determining the heat of fusion, specific heat and thermal conductivity of phase-change materials. Meas. Sci. Technol. 1999, 10, 201–205. [Google Scholar] [CrossRef]
- Paola, M.G.D.; Arcuri, N.; Calabró, V.; Simone, M.D. Thermal and Stability Investigation of Phase Change Material Dispersions for Thermal Energy Storage by T-history and Optical Methods. Energies 2017, 10, 354. [Google Scholar] [CrossRef]
- Hong, H.; Kim, S.K.; Kim, Y.S. Accuracy improvement of T-history method for measuring heat of fusion of various materials. Int. J. Refrig. 2004, 27, 360–366. [Google Scholar] [CrossRef]
- Peak, J.H.; Kim, J.J.; Kang, C.; Hong, H. A study of accurate latent heat measurement for a PCM with a low melting temperature using T-history method. Int. J. Refrig. 2006, 29, 1225–1232. [Google Scholar] [CrossRef]
- Park, C.H.; Peck, J.H.; Kang, C.D.; Hong, H.K. Accuracy Improvement for Measurement of Heat of Fusion by T-history Method. Korean J. Air-Cond. Refrig. Eng. 2003, 15, 652–661. [Google Scholar]
- Incropera, F.P.; Bergman, T.L.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer, 4th ed.; John Wily & Sons, Inc.: Hoboken, NJ, USA, 1966; pp. 212–217. [Google Scholar]


| Materials Name | Chemistry Symbols | Chemical Abstract Service (CAS) Number |
|---|---|---|
| Sodium sulfate | Na2SO4 | 7757-82-6 |
| Sodium phosphate | Na3PO4 | 7632-05-5 |
| Potassium sulfate | K2SO4 | 7778-18-9 |
| Carbamide | CO(NH2)2 | 57-13-6 |
| Silica | SiO2 | 10279-57-9 |
| Water | H2O | 7732-18-5 |
| Thermal Properties | 1 wt.% | 3 wt.% | 5 wt.% | Pure Water (Reference) |
|---|---|---|---|---|
| Phase change temperature (°C) | −0.1 | −0.5 | −1.1 | 0 |
| Liquid retention time (min) | 40.5 | 48.5 | 53.6 | 47 |
| Supercooling temperature (°C) | −4.6 | −7.4 | −8.9 | −8.3 |
| Liquid specific heat (kJ/kg·K) | 3.94 | 3.89 | 3.81 | 4.18 |
| Solid specific heat (kJ/kg·K) | 1.93 | 1.88 | 1.84 | 2.04 |
| Latent heat (kJ/kg) | 306.2 | 288.6 | 260.4 | 333 |
| Thermal Properties | 1 wt.% + SAP | 3 wt.% + SAP | 5 wt.% + SAP | Pure Water (Reference) |
|---|---|---|---|---|
| Phase change temperature (°C) | −0.5 | −0.8 | −1.2 | 0 |
| Liquid retention time (min) | 36.5 | 45.5 | 49.2 | 47 |
| Supercooling temperature (°C) | −2.9 | −6.8 | −8.6 | −8.3 |
| Liquid specific heat (kJ/kg·K) | 3.91 | 3.85 | 3.82 | 4.18 |
| Solid specific heat (kJ/kg·K) | 1.89 | 1.84 | 1.80 | 2.04 |
| Latent heat (kJ/kg) | 304.9 | 286.1 | 258.7 | 331 |
| Location | EPS Duct Unit | Functional Duct Unit |
|---|---|---|
| Freezer compartment (1) | 1.8 °C | −5.4 °C |
| Duct surface (2) | 8.8 °C | −1.5 °C |
| Refrigerating compartment (3) | 7.4 °C | 5.9 °C |
| Duration | With EPS Duct Unit (°C) | With FDU (°C) |
|---|---|---|
| At power off | 0.0 | 0.0 |
| 60 min after power off | 7.5 | 6.0 |
| 120 min after power off | 9.1 | 6.9 |
| ∆T/min | 0.076 | 0.058 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-J.; Park, S.-H. An Experimental Investigation of Thermal Characteristics of Phase Change Material Applied to Improve the Isothermal Operation of a Refrigerator. Energies 2018, 11, 2017. https://doi.org/10.3390/en11082017
Lee S-J, Park S-H. An Experimental Investigation of Thermal Characteristics of Phase Change Material Applied to Improve the Isothermal Operation of a Refrigerator. Energies. 2018; 11(8):2017. https://doi.org/10.3390/en11082017
Chicago/Turabian StyleLee, Seok-Joon, and Seul-Hyun Park. 2018. "An Experimental Investigation of Thermal Characteristics of Phase Change Material Applied to Improve the Isothermal Operation of a Refrigerator" Energies 11, no. 8: 2017. https://doi.org/10.3390/en11082017




