Tri-Axial Shear Tests on Hydrate-Bearing Sediments during Hydrate Dissociation with Depressurization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Apparatus
2.2. Experimental Procedure and Test Conditions
2.2.1. Hydrate Formation and Water Substitution Process
2.2.2. Shear Tests and Hydrate Dissociation Processes
2.2.3. Saturation Calculation of Hydrates
3. Results and Discussion
3.1. The Geotechnics of Methane Hydrate-Bearing Sediments Dissociated by the Depressurization Method
3.2. Influence of the Stress States at the Start of Depressurization
3.3. Influence of Hydrate Saturation
3.4. Particle Size of the Sediments after the Shear Tests
4. Conclusions
- (1)
- During the shear process, pore pressure decreases may result in the failure of the hydrate-bearing specimen, but it did not collapse completely due to the high effective confining pressure.
- (2)
- The contraction tendency of volumetric strain was observed during depressurization and the ultimate deformation showed no difference from prior to depressurization.
- (3)
- The degree of stress reduction was greater during depressurization for the high saturation hydrate-bearing sediments. This could be because the methane hydrate acted as a skeleton structure when the pore hydrate saturation is large.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kvenvolden, K.A. Methane hydrate—A major reservoir of carbon in the shallow geosphere? Chem. Geol. 1988, 71, 41–51. [Google Scholar] [CrossRef]
- Lee, S.Y.; Holder, G.D. Methane hydrates potential as a future energy source. Fuel Process. Technol. 2001, 71, 181–186. [Google Scholar] [CrossRef]
- Chong, Z.R.; Yang, S.H.B.; Babu, P.; Linga, P.; Li, X.-S. Review of natural gas hydrates as an energy resource: Prospects and challenges. Appl. Energy 2016, 162, 1633–1652. [Google Scholar] [CrossRef]
- Durham, W.B.; Kirby, S.H.; Stern, L.A.; Zhang, W. The strength and rheology of methane clathrate hydrate. J. Geophys. Res. Solid Earth 2003, 108. [Google Scholar] [CrossRef] [Green Version]
- Yun, T.S.; Narsilio, G.A.; Santamarina, J.C.; Ruppel, C. Instrumented pressure testing chamber for characterizing sediment cores recovered at in situ hydrostatic pressure. Mar. Geol. 2006, 229, 285–293. [Google Scholar] [CrossRef]
- Masui, A.; Miyazaki, K.; Haneda, H.; Ogata, Y.; Aoki, K. Mechanical Properties of Natural Gas Hydrate Bearing Sediments Retrieved from Eastern Nankai Trough. In Proceedings of the Offshore Technology Conference, Houston, Texas, USA, 5–8 May 2008. [Google Scholar]
- Hyodo, M.; Nakata, Y.; Yoshimoto, N.; Orense, R.; ISOPE. Shear behaviour of methane hydrate-bearing sand. In Proceedings of the Seventeenth International Offshore and Polar Engineering Conference, Lisbon, Portugal, 1–6 July 2007. [Google Scholar]
- Yoneda, J.; Hyodo, M.; Yoshimoto, N.; Nakata, Y.; Kato, A. Development of high-pressure low-temperature plane strain testing apparatus for methane hydrate-bearing sand. Soils Found. 2013, 53, 774–783. [Google Scholar] [CrossRef]
- Yun, T.S.; Santamarina, J.C.; Ruppel, C. Mechanical properties of sand, silt, and clay containing tetrahydrofuran hydrate. J. Geophys. Res.-Solid Earth 2007, 112. [Google Scholar] [CrossRef] [Green Version]
- Hyodo, M.; Nakata, Y.; Yoshimoto, N.; Yoneda, J. Shear strength of methane hydrate bearing sand and its deformation during dissociation of methane hydrate. Deform. Charact. Geomater. 2008, 1–2, 549–556. [Google Scholar]
- Luo, T.; Song, Y.; Zhu, Y.; Liu, W.; Liu, Y.; Li, Y.; Wu, Z. Triaxial experiments on the mechanical properties of hydrate-bearing marine sediments of South China Sea. Mar. Petrol. Geol. 2016, 77, 507–514. [Google Scholar] [CrossRef]
- Li, Y.H.; Song, Y.C.; Liu, W.G.; Yu, F. Experimental Research on the Mechanical Properties of Methane Hydrate-Ice Mixtures. Energies 2012, 5, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Aoki, K.; Masui, A.; Haneda, H.; Ogata, Y. Compaction behavior of Toyoura sand during methane hydrate dissociation. In Proceedings of the Seventh ISOPE Ocean Mining Symposium, Lisbon, Portugal, 1–6 July 2007. [Google Scholar]
- Hyodo, M.; Li, Y.H.; Yoneda, J.; Nakata, Y.; Yoshimoto, N.; Nishimura, A. Effects of dissociation on the shear strength and deformation behavior of methane hydrate-bearing sediments. Mar. Petrol. Geol. 2014, 51, 52–62. [Google Scholar] [CrossRef]
- Priest, J.A.; Rees, E.V.L.; Clayton, C.R.I. Influence of gas hydrate morphology on the seismic velocities of sands. J. Geophys. Res. Solid Earth 2009, 114. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, K.; Masui, A.; Sakamoto, Y.; Aoki, K.; Tenma, N.; Yamaguchi, T. Triaxial compressive properties of artificial methane-hydrate-bearing sediment. J. Geophys. Res. Solid Earth 2012, 116. [Google Scholar] [CrossRef]
- Dai, S.; Seol, Y. Impacts of Hydrate Distribution on the Hydro-Thermo-Mechanical Properties of Hydrate-Bearing Sediments; AGU Fall Meeting Abstracts; AGU: Washington, DC, USA, 2015. [Google Scholar]
- Kimoto, S.; Oka, F.; Fushita, T.; Fujiwaki, M. A chemo-thermo-mechanically coupled numerical simulation of the subsurface ground deformations due to methane hydrate dissociation. Comput. Geotech. 2007, 34, 216–228. [Google Scholar] [CrossRef]
- Rutqvist, J.; Moridis, G.J.; Grover, T.; Collett, T. Geomechanical response of permafrost-associated hydrate deposits to depressurization-induced gas production. J. Petrol. Sci. Eng. 2009, 67, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ghiassian, H.; Grozic, J.L.H. Strength behavior of methane hydrate bearing sand in undrained triaxial testing. Mar. Petrol. Geol. 2013, 43, 310–319. [Google Scholar] [CrossRef]
- Hyodo, M.; Yoneda, J.; Yoshimoto, N.; Nakata, Y. Mechanical and dissociation properties of methane hydrate-bearing sand in deep seabed. Soils Found. 2013, 53, 299–314. [Google Scholar] [CrossRef]
- Yan, C.; Cheng, Y.; Li, M.; Han, Z.; Zhang, H.; Li, Q.; Teng, F.; Ding, J. Mechanical experiments and constitutive model of natural gas hydrate reservoirs. Int. J. Hydrogen Energy 2017, 42, 19810–19818. [Google Scholar] [CrossRef]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, 465–484. [Google Scholar] [CrossRef]
- Berge, L.I.; Jacobsen, K.A.; Solstad, A. Measured acoustic wave velocities of R11 (CCl 3 F) hydrate samples with and without sand as a function of hydrate concentration. J. Geophys. Res. Solid Earth 1999, 104, 15415–15424. [Google Scholar] [CrossRef]
- Yun, T.S.; Francisca, F.M.; Santamarina, J.C.; Ruppel, C. Compressional and shear wave velocities in uncemented sediment containing gas hydrate. Geophys. Res. Lett. 2005, 32, 153–174. [Google Scholar] [CrossRef]
- Zhang, X.H.; Luo, D.S.; Lu, X.B.; Liu, L.L.; Liu, C.L. Mechanical properties of gas hydrate-bearing sediments during hydrate dissociation. Acta Mech. Sin. 2018, 34, 266–274. [Google Scholar] [CrossRef]
- Shun, U. Numerical Investigation of Geomechanical Behaviour of Hydrate-Bearing Sediments. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2013. [Google Scholar]

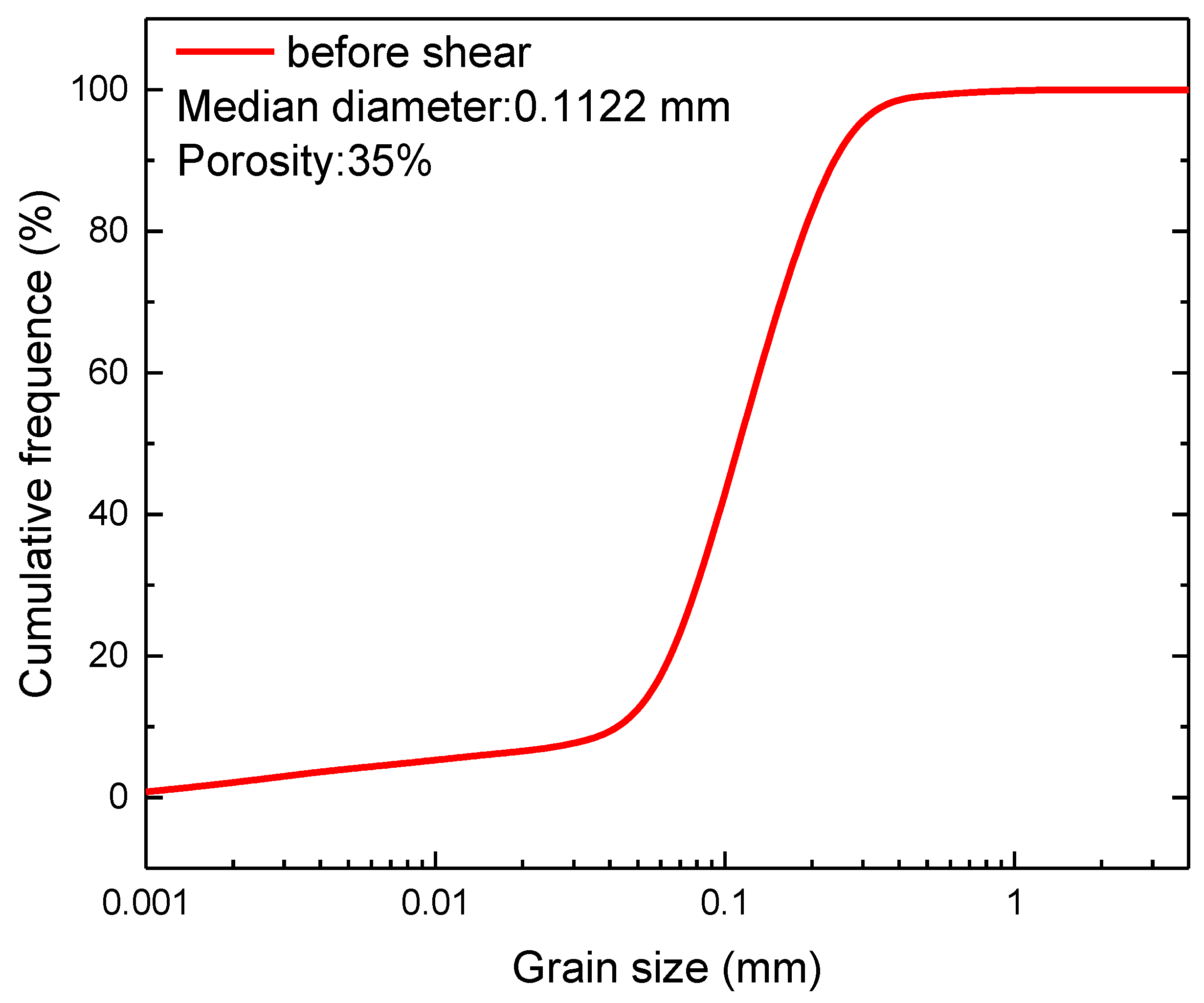



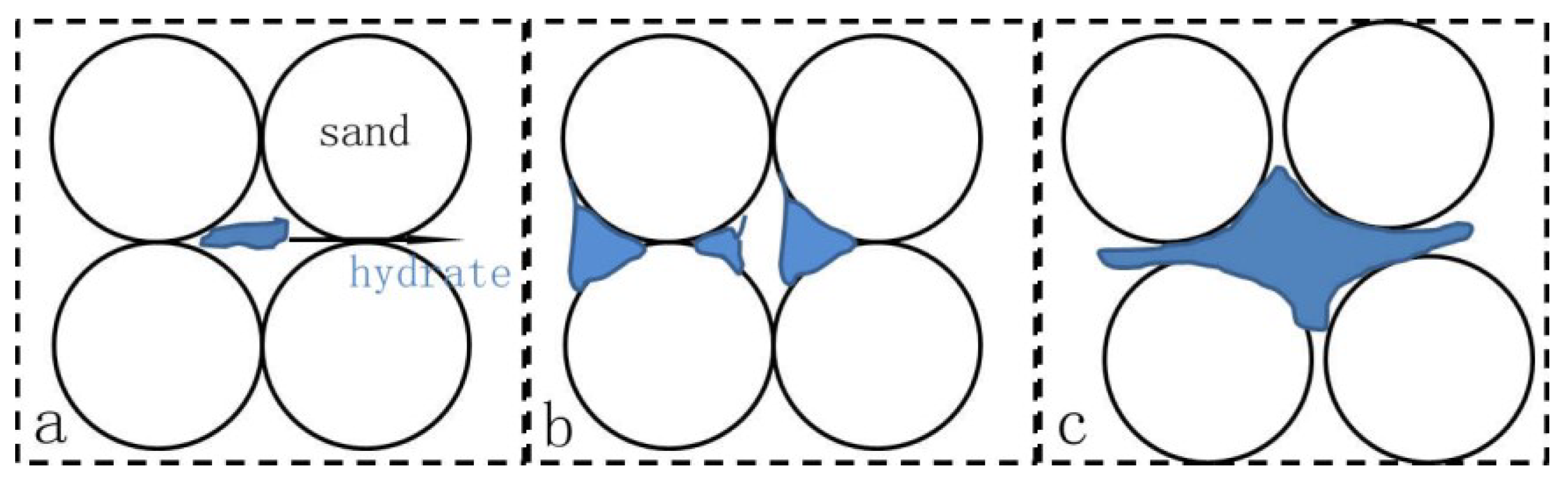
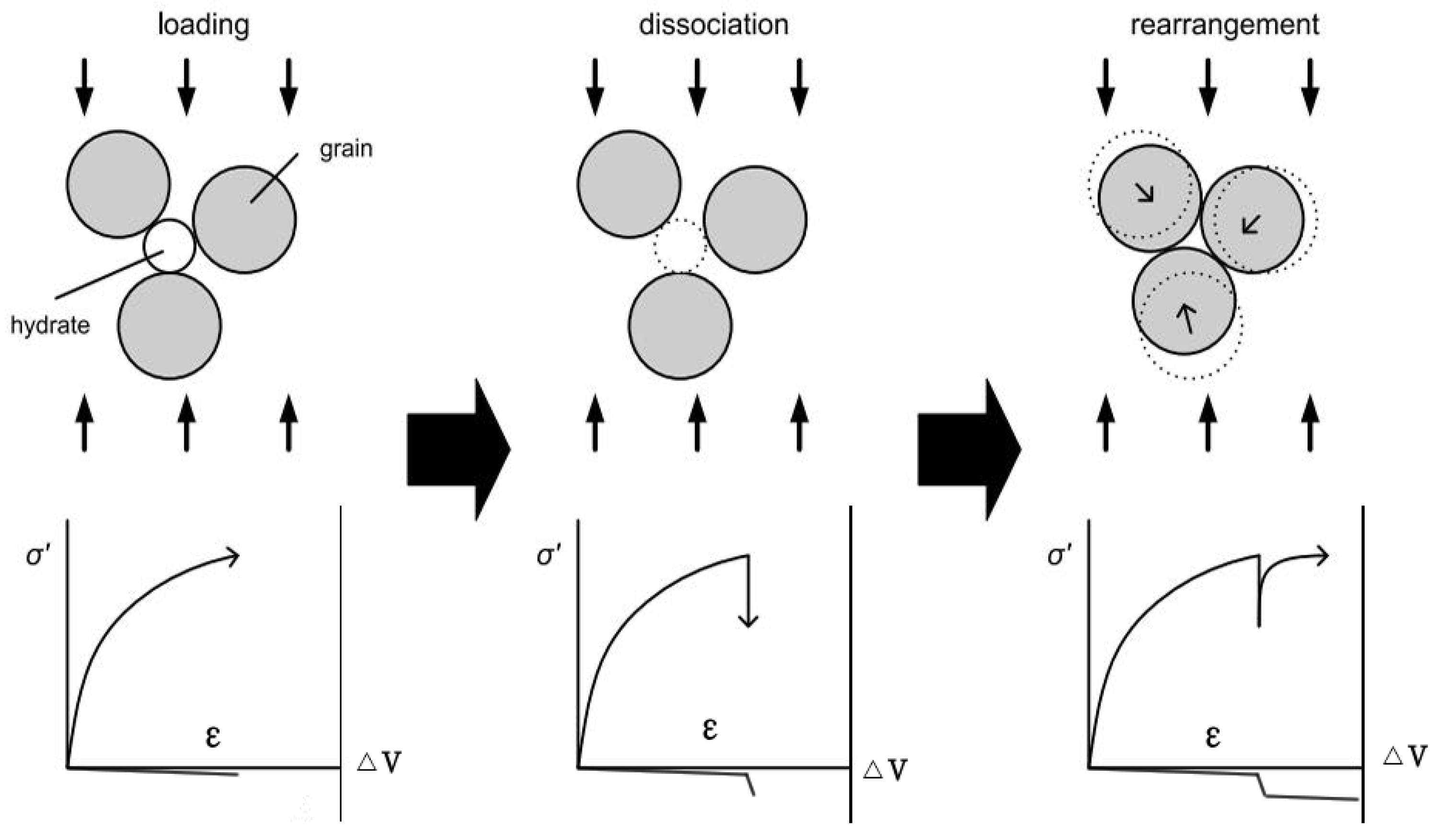

| Test Name | Temperature (K) | Confining Pressure (MPa) | Pore Pressure (MPa) | Saturation (%) | Remarks |
|---|---|---|---|---|---|
| P1 | 279.15 | 10 | 8 | 0 | Partially water saturated sand |
| P2 | 279.15 | 12 | 10 | 0 | Partially water saturated sand |
| P3 | 279.15 | 14 | 12 | 0 | Partially water saturated sand |
| P4 | 279.15 | 10 | 3 | 0 | Water saturated sand |
| P5 | 279.15 | 12 | 3 | 0 | Water saturated sand |
| M0 | 279.15 | 12 | 10 | 35.23 | Shear without dissociation |
| M1 | 279.15 | 10 | 8→3 | 38.05 | Shear 2.5%→shear under dissociation |
| M2 | 279.15 | 12 | 10→3 | 33.82 | Shear 0.5%→shear under dissociation |
| M3 | 279.15 | 12 | 10→3 | 33.82 | Shear 1.0%→shear under dissociation |
| M4 | 279.15 | 12 | 10→3 | 37.48 | Shear 2.5%→shear under dissociation |
| M5 | 279.15 | 12 | 10→3 | 56.14 | Shear 2.5%→shear under dissociation |
| M6 | 279.15 | 14 | 12→3 | 37.58 | Shear 2.5%→shear under dissociation |
| Specimens | Test Condition | Median Diameter (mm) | Variation (mm) | |
|---|---|---|---|---|
| Confining Pressure (MPa) | Pore Pressure (MPa) | |||
| P1 | 10 | 8 | 0.1107 | −0.0014 |
| P2 | 12 | 10 | 0.1087 | −0.0034 |
| P3 | 14 | 12 | 0.0969 | −0.0152 |
| M1 | 10 | 8→3 | 0.1112 | −0.0009 |
| M4 | 12 | 10→3 | 0.1040 | −0.0081 |
| M6 | 14 | 12→3 | 0.0965 | −0.0156 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Wu, Q.; Wang, Z.; Lu, J.; Liang, D.; Li, X. Tri-Axial Shear Tests on Hydrate-Bearing Sediments during Hydrate Dissociation with Depressurization. Energies 2018, 11, 1819. https://doi.org/10.3390/en11071819
Li D, Wu Q, Wang Z, Lu J, Liang D, Li X. Tri-Axial Shear Tests on Hydrate-Bearing Sediments during Hydrate Dissociation with Depressurization. Energies. 2018; 11(7):1819. https://doi.org/10.3390/en11071819
Chicago/Turabian StyleLi, Dongliang, Qi Wu, Zhe Wang, Jingsheng Lu, Deqing Liang, and Xiaosen Li. 2018. "Tri-Axial Shear Tests on Hydrate-Bearing Sediments during Hydrate Dissociation with Depressurization" Energies 11, no. 7: 1819. https://doi.org/10.3390/en11071819





